Abstract
Objective:
Pregnant patients are vulnerable to both depression and sleep-disordered breathing (SDB), and both convey risks for maternal and fetal outcomes. Previous research has indicated that SDB is associated with depression, but further information related to the risk of depression based on timing of onset of snoring is needed.
Design:
When presenting to clinic for their initial prenatal visit, pregnant patients completed a packet of questionnaires, which included measures related to depression (Edinburgh Postnatal Depression Scale; EPDS) and snoring. Habitual snoring was defined as snoring three or more nights per week.
Results:
In total, 1367 women were included and 34.1% reported habitual snoring, either chronic (24.4%) or pregnancy-onset (9.8%), with increased frequency of pregnancy-onset habitual snoring in later stages of pregnancy. Unadjusted analyses suggested increased odds of depressive symptoms in chronic and pregnancy-onset habitual snoring groups relative to non-snorers (OR: 2.01, 95% CI: 1.39, 2.92, p<0.01; OR: 2.50, 95% CI: 1.54, 4.07, p<0.01, respectively). These findings were maintained after adjusting for maternal age, marital status, gestational age, and parity (chronic habitual snoring OR: 1.69, 95% CI: 1.14, 2.53, p<0.01; pregnancy-onset habitual snoring OR: 2.79, 95% CI: 1.35, 5.78, p<0.01)
Conclusions:
Maternal snoring may be a risk factor for prenatal depressive symptoms. Pregnancy-onset habitual snoring confers additional risk for depression compared to not snoring during pregnancy.
Keywords: Pregnancy, Snoring, Depression, Sleep-disordered breathing, Sleep
INTRODUCTION
Pregnancy induces a complex series of physiological, hormonal, and physical changes that impact maternal functioning and health. In particular, pregnancy is considered a vulnerable period for mental health. Indeed, as many as 20% of women experience prenatal depression,1 which is linked with poor pregnancy outcomes,2,3 and postpartum parenting and bonding challenges.4,5 Identification of modifiable risk factors is important to inform the development of new treatments, as current treatment paradigms leave most perinatal women untreated or symptomatic even with treatment.6
The incidence of specific sleep disorders such as restless legs syndrome, insomnia, and sleep disordered breathing (SDB) increases during pregnancy, which may also account for, at least in part, the high prevalence of sleep disturbances among pregnant women.7,8 Sleep disordered breathing is a spectrum of breathing disturbances during sleep ranging from habitual snoring (snoring three or more nights per week) to obstructive sleep apnea. Full or partial collapse of the airway occurs, and oxygen desaturation is often present, particularly in obstructive sleep apnea. Pregnant women are at increased risk for SDB because of physiological changes resulting from pregnancy including weight gain, edema, and hormonal shifts, which can lead to airway obstructions.9 Frequencies of habitual snoring, the cardinal symptom of SDB, are estimated at 10% in early pregnancy, rising to 35% later in pregnancy.7 The more severe form of SDB, obstructive sleep apnea, which requires overnight polysomnography to confirm, has been reported to occur in about 3% of healthy nulliparous women in early pregnancy, rising to 8% in mid-pregnancy.10 Obese women and those with pre-eclampsia have significantly higher frequencies of obstructive sleep apnea (15-25%11 and 60-80%12 respectively). Previous studies have shown that SDB in pregnancy is associated with other negative pregnancy outcomes such as hypertension,7 preeclampsia,7 and gestational diabetes7,10,13-16 as well as increased rates of cesarean delivery,12,13,17, low birthweight/growth restricted babies,17-19 preterm birth,20,21 low Apgar score at 5-minutes,20 neonatal intensive care admission20, accelerated fetal growth,20,22 assisted labor, and perinatal death.20 Furthermore, we have previously shown that the timing of SDB symptoms in relation to pregnancy is important as pregnancy-onset snoring had a greater impact on maternal and fetal wellbeing than chronic SDB.7,21,23
In non-pregnant populations, SDB has been shown to be a risk factor for the development of depression symptoms.24,25 In a national sample of women, odds of depression increase 3-fold when snoring/stopping breathing occurs at least five nights per week, and a diagnosis of sleep apnea increases depression odds by 5-fold.26 A recent study of pregnant women indicated that those with a diagnosis of obstructive sleep apnea have an 8-fold increase in the odds of experiencing depression.27
Despite the known links between SDB and depression in the general population, no data were available in the context of pregnancy until recently. We were the first to suggest that gestational habitual snoring is associated with prenatal depression,23 however, that study was limited by its relatively small (n=362) sample size and by the fact that depression and snoring were not screened at the same time. The goal of the present study, therefore, was to determine, in a large sample of pregnant women, whether the SDB symptom of habitual snoring is independently associated with depressive symptoms when screened for concurrently. This study is therefore distinct from our prior work in that it assessed the relationship between maternal snoring and depressive symptoms at the same time point and that these measures were predominantly assessed in early pregnancy. We hypothesized that pregnant women experiencing habitual snoring would be more likely to report clinically significant depression symptoms, and that women who reported pregnancy-onset habitual snoring would be at greater risk of depressive symptoms relative to those who reported not snoring.
PARTICIPANTS AND METHODS
Participants
This study used anonymous screening data from a registry of pregnant women who were interested in participating in research. Participants were pregnant women who presented for prenatal care in the obstetrics department of a large academic medical center. All partciipants provided informed consent to participate in the registry and for their anonymous information to be used.
Procedures
When presenting to the sampled clinic for their initial prenatal visit, pregnant patients completed a packet of questionnaires, which included a depression measure (the Edinburgh Postnatal Depression Scale; EPDS)28 and questions regarding their pregnancy, demographic characteristics, and snoring status both before pregnancy as well as at the time of evaluation. This questionnaire packet was anonymous and not directly linked to participants’ medical record or obstetrical care. An item assessing marital status (married or in a committed relationship; single, no partner; other) was added to the questionnaire after data collection began, meaning that marital status was assessed for most but not all participants. The EPDS is a valid, 10-item self-report depression questionnaire developed to identify depression in perinatal women (alpha=.88). The questionnaire asks women about symptoms of depression including anhedonia, worry, and sadness, as well sleep disturbance resulting from unhappiness, over the seven days prior to evaluation. Scores range from 0-30; higher scores indicate more severe depression. Habitual snoring items included the following: “Before your pregnancy, did you snore more than 3 nights a week?”; and “Do you currently snore 3 or more nights per week?". These questions were used because a single question is strongly and reliably associated with the PSG-derived apnea/hypopnea index;29 in women a report of “often” or “usually (always or almost always)” snoring is associated with PSG-confirmed SDB; its use provides an approach easily and immediately translated into clinical settings.29 This study was approved by the Institutional Review Board of the University of Michigan (IRB # HUM00028282).
Statistical Analysis
This was a cross-sectional study. Information about the presence and timing of habitual snoring onset was used to classify study participants into three groups: chronic habitual snoring (snored before and during pregnancy); pregnancy-onset habitual snoring (snored during pregnancy but not before); and non-snoring (never snored). Women were classified as chronic habitual snorers if they answered “yes” to both snoring items; pregnancy-onset habitual snorers if they answered “yes” only to current snoring; and non-snorers if they answered “no” to both questions. Chi-square and ANOVA tests were used to assess differences in demographic and pregnancy characteristics (i.e., marital status, maternal age, gestational age, and parity) across study groups. A cutoff of 10 on the EPDS was used to indicate clinically significant depression symptoms.30 To examine the robustness of our results we conducted a post-hoc analysis using a cutoff of 13 on the EPDS.
We compared EPDS scores against demographic variables using ANOVA and Chi-square tests to determine differences. As marital status information was missing for participants who completed an earlier version of the questionnaire, we imputed missing values of marital status using the SPSS multiple imputation algorithm. This algorithm creates five imputations of the data which are then pooled to reduce bias.31 Pooled results are reported. Logistic regression procedures were used to determine the odds of depression, with EPDS<10 as the reference category, by habitual snoring category, adjusted for demographic and maternal characteristics. The data were analyzed using a personal computer-based software package (IBM SPSS Statistics® 26.0, IBM Corp©, Armonk, NY).
Sensitivity Analyses
Most women who develop habitual snoring in pregnancy have onset in the later stages of pregnancy; thus, participants who do not report snoring in the first or second trimester may go on to develop habitual snoring later in their pregnancies. To account for possible misclassification of pregnant women who completed the questionnaire prior to the third trimester, we conducted sensitivity analyses and examined associations of habitual snoring (pregnancy-onset and chronic) and depressive symptoms with a reference group of non-snorers in a sample restricted to women in their third trimester (i.e., ≥ 28 weeks pregnant).
RESULTS
Overall 1,685 women completed the surveys. Six participants who reported habitual snoring prior to, but not during, pregnancy were excluded from analysis due to the small size of this group. An additional 312 participants were excluded because they had not completed the EPDS or measures related to their snoring status or had missing gestational age information. Thus, the final sample included 1,367 participants. The mean age was 30.7 ± 5.0 years. Of those that completed the marital status question, 94% reported being married or in a committed relationship (254 did not complete this question) and 43% were nulliparous. On average, gestational age was 16.4 ± 9.2 weeks. At the time of enrollment 58.4% of participants were in the first trimester (<14 weeks; n=779), 20.5% were in the second trimester (14-27 weeks; n=274), and 21.1% were in the third trimester (28+ weeks; n=282). The mean score on the EPDS was 4.4 ± 4.24; 11.6% scored above the depression cutoff (Table 1).
Table 1.
Demographic Information of Study Participants by Presence and Onset Timing of Habitual Snoring
| Frequency (n=1367) |
Chronic Habitual Snoring (n=333) |
Pregnancy Onset Habitual Snoring (n=134) |
Non-Snoring (n=900) |
P-Value | |
|---|---|---|---|---|---|
| Gestational Age (mean, SD) | 16.4 ±9.2 | 16.0 ± 9.1 | 23.4 ± 8.3 | 15.5 ± 9.0 | <.01 |
| Maternal Age (mean, SD) | 30.7 ± 5.0 | 30.2 ± 5.8 | 31.1 ± 5.5 | 30.8 ± 4.6 | .10 |
| EPDS Score | 4.4 ± 4.24 | 5.2 ± 4.9 | 5.3 ± 4.6 | 4.0 ± 3.9 | <.01 |
| EPDS Score ≥ 10 | 159 (11.6%) | ||||
| EPDS Score ≥ 13 | 62 (4.5%) | ||||
| Married/committed relationship | 1,046 (94.0%)* | 216 (89.6%) | 83 (98.8%) | 747 (94.8%) | .01 |
| Nulliparous | 586 (43.1) | 152 (45.8%) | 59 (44.4%) | 375 (41.9%) | .44 |
n is lower due to missing data
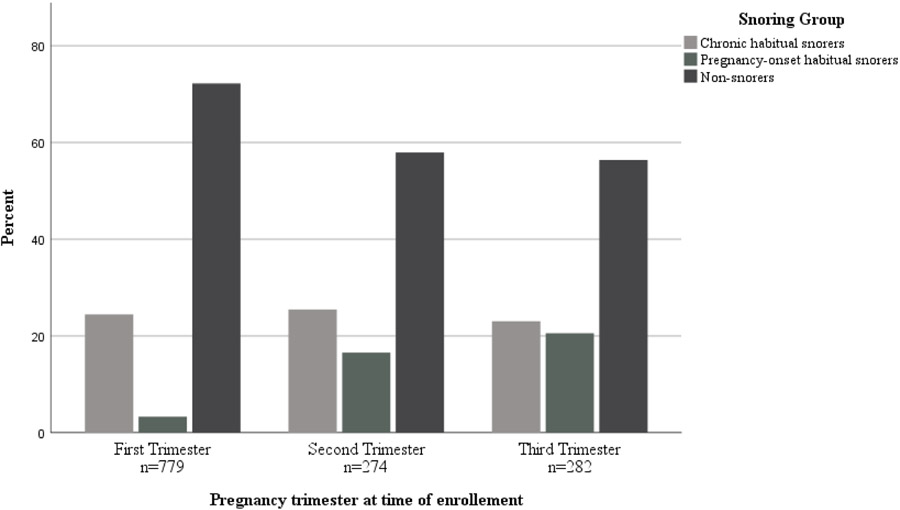
One third of the 1,367 participants reported habitual snoring, either chronic (24.4%) or pregnancy-onset (9.8%). As anticipated, women in the pregnancy-onset habitual snoring group reported a significantly advanced mean gestational age (23.4 ± 8.3 weeks) compared to chronic habitual snorers (16.0 ± 9.1 weeks) or non-snorers (15.5 ± 9 weeks). Of those in the first trimester, 25.0% reported chronic habitual snoring and 3.9% reported pregnancy-onset habitual snoring. For participants in their second trimester, 24.1% reported chronic habitual snoring and 16.4% reported pregnancy-onset habitual snoring. In the third trimester group, 23.0% reported chronic habitual snoring and 20.6% reported pregnancy-onset habitual snoring (See Figure 1).
Figure 1.
Participants reporting EPDS ≥10 were older on average (31.0 ± 4.83 years) than those with EPDS <10 (28.1 ± 5.7 years; p < .01). Only 9.5% of participants who reported being married or in a committed relationship scored above a cutoff for depression, while 36.7% of single participants had EPDS scores of ten or greater (p<.001).
Unadjusted analyses suggested increased odds of depressive symptoms in chronic and pregnancy-onset habitual snoring groups relative to non-snorers (OR: 2.01, 95% CI: 1.39, 2.92; OR: 2.50, 95% CI: 1.54, 4.07, p<.01, respectively). These findings were maintained after adjusting for maternal age, marital status, gestational age, and parity (chronic habitual snoring OR: 1.69, 95% CI: 1.14, 2.53, p=.01; pregnancy-onset habitual snoring OR: 2.79, 95% CI: 1.35, 5.78, p<.01) (Table 2). Other variables found to be significant predictors were maternal age (OR: 0.91, 95% CI: .87, .95, p<.01) and marital status (OR: 2.64, 95% CI: 1.06, 6.55, p<.05).
Table 2.
Depression Symptoms with EPDS threshold of 10 by Presence and Onset Timing of Habitual Snoring
| Snoring Status | na | Depression Symptoms (EPDS Cutoff 10; n=159) | |||||
|---|---|---|---|---|---|---|---|
| EPDS10 | Prevalence EPDS10 |
Unadjusted Model a | Adjusted Model b | ||||
| n | % | OR | 95% CI | OR | 95% CI | ||
| Chronic Snoring | 333 | 54 | 16.2 | 2.01 | [1.39, 2.92]** | 1.69 | [1.14, 2.53]** |
| Pregnancy Snoring | 134 | 26 | 19.4 | 2.50 | [1.54, 4.07]** | 2.79 | [1.35, 5.78]** |
| Non-Snoring | 900 | 79 | 8.8 | 1.0 | referent | 1.0 | referent |
Note. OR = odds ratio; CI = confidence interval
Bivariate model with snoring status as the independent variable.
Adjusted for marital status, maternal age, gestational age, and parity.
p ≤ 0.01
In comparison to associations between maternal snoring and depression using an EPDS cutoff of 10, the odds of depressive symptoms using the cutoff of 13 are similar in magnitude and direction in women with either chronic or pregnancy-onset snoring. While the odds of depressive symptoms were statistically significant among women who develop pregnancy-onset snoring, in those with chronic snoring odds did not remain significant. (Table 3).
Table 3.
Depression Symptoms with EPDS threshold of 13 by Presence and Onset Timing of Habitual Snoring
| Snoring Status | na | Depression Symptoms (EPDS Cutoff 13; n=62) | |||||
|---|---|---|---|---|---|---|---|
| EPDS13 | Prevalence EPDS13 |
Unadjusted Model a | Adjusted Model b | ||||
| n | % | OR | 95% CI | OR | 95% CI | ||
| Chronic Snoring | 333 | 29 | 9 | 2.13 | [1.20, 3.75]** | 1.63 | [0.89-3.00] |
| Pregnancy Snoring | 134 | 11 | 8 | 2.69 | [1.31, 5.51]** | 2.99 | [1.05-8.50]* |
| Non-Snoring | 900 | 22 | 2 | 1.0 | referent | 1.0 | referent |
Note. OR = odds ratio; CI = confidence interval
Based on model including snoring group.
Adjusted for marital status, maternal age, gestational age, and parity.
p ≤ 0.05
p ≤ 0.01
Sensitivity analyses restricted to 282 third trimester women increased the magnitude of the association between both habitual snoring groups and depressive symptoms (chronic habitual snoring OR: 3.04, 95% CI: 1.22, 7.55, p<.05; pregnancy-onset habitual snoring OR: 2.74, 95% CI: 1.05, 7.12, p<.05), as well as in a model adjusting for maternal age, marital status, gestational age, and parity (chronic habitual snoring OR: 2.75, 95% CI: 1.05, 7.2, p<.05; pregnancy-onset habitual snoring OR: 3.03, 95% CI: 1.11, 8.29, p<.05) (Table 4).
Table 4.
Depression symptoms in 282 Pregnant Third Trimester Women by Presence and Onset Timing of Habitual Snoring
| Snoring Status | n | Depression Symptoms | ||||
|---|---|---|---|---|---|---|
| Prevalence | Unadjusted Modela | Adjusted Modelb | ||||
| % | OR | 95% CI | OR | 95% CI | ||
| Chronic Snoring | 65 | 23.0 | 3.04 | [1.22, 7.55]* | 2.75 | [1.05, 7.2]* |
| Pregnancy-Onset Snoring | 58 | 20.6 | 2.74 | [1.05, 7.12]* | 3.03 | [1.11, 8.29]* |
| Non-Snoring | 159 | 56.4 | 1.0 | referent | 1.0 | referent |
Note. OR = odds ratio; CI = confidence interval
Bivariate model with snoring status as the independent variable.
Adjusted for marital status, maternal age, gestational age, and parity.
p < 0.05
Moreover, a separate sensitivity analysis that excluded the single sleep item from the EPDS (“I have been so unhappy that I have had difficulty sleeping”) did not appreciably alter the results. Using an EPDS cutoff of 9 (to account for the reduced number of items), a similar proportion of participants scored above threshold (12.2% vs 11.6%). Comparable findings were observed when excluding the sleep question item in the regression analysis (chronic habitual snoring OR: 1.68, 95% CI: 1.43, 2.00, p<.01; pregnancy-onset habitual snoring OR: 2.91, 95% CI: 2.35, 3.61, p<.01). When including the single-item sleep question as a separate covariate in the analyses, the results did not substantially change (chronic habitual snoring OR: 1.45, 95% CI: 1.20, 1.75, p<.01; pregnancy-onset habitual snoring OR: 2.41, 95% CI: 1.85, 3.14, p<.01), and the odds ratio for the sleep item was also significant (OR: 5.96, 95% CI: 5.33, 6.67, p<.01).
DISCUSSION
We have demonstrated that habitual snoring is associated with clinically significant symptoms of depression in a large sample of women in pregnancy and that this association remains when controlling for marital status, gestational age, parity, and maternal age. Moreover, those with pregnancy-onset habitual snoring had over twice the odds for depressive symptoms compared to non-snorers. These results support and extend our previous findings,23 and provide further evidence that snoring may be a risk factor for the development of depressive symptoms during pregnancy.
In non-pregnant adults, the association between SDB and depressive symptoms has been demonstrated in several studies.24,25 However, this relationship has been less studied in the perinatal population, even though women are uniquely vulnerable to both depression1 and SDB during pregnancy.7-9 Sleep-disordered breathing in pregnant women may result in poor sleep quality, which has been linked with prenatal and postpartum depression.32 At least one study has demonstrated that significant declines in sleep quality over the course of pregnancy, rather than poor sleep quality itself, may contribute to the development of depression symptoms,33 which is in line with the current findings that pregnancy-onset habitual snoring (a physiological challenge) may confer higher risks for depression than chronic habitual snoring, to which the body may have been able to somewhat adapt. Our findings suggest that snoring confers an additional yet independent risk for depression symptoms beyond that of just poor sleep, as evidenced by removal of the sleep-related item from the scoring paradigm. Additionally, recent data suggest that the prevalence of SDB within the four months post-delivery is the same of that during the third trimester of pregnancy, indicating that SDB does not immediately resolve postpartum.34
Untreated prenatal depression is associated with a host of negative outcomes for women and their children,7 and it is important to understand key modifiable risk factor for prenatal depression. Prenatal depression is a predictor of postpartum depression35 and intervening in the prenatal period is effective in preventing postpartum depression.36 Untreated prenatal depression is also associated with substance abuse,37 poor birth outcomes,38 impaired maternal-infant bonding,39 and increased risk for developmental delays5 and mental illness in infants.40 In severe cases, depression can lead to death by suicide. Improving detection and treatment for depression during the perinatal period is therefore crucial not only for improving maternal health and well-being, but for improving neonatal outcomes, and screening for SDB may be an important indicator of depression risk.
Traditional screening measures for SDB and obstructive sleep apnea have been shown to be poor predictors in the pregnant population indicating that accurate screening may be difficult.18,41,42 Updated screening measures specifically designed for pregnant women may better enable providers to assess for the presence of SDB and make informed decisions about referrals, evaluation, and treatment.18,41 Such interventions may be helpful for milder forms of SDB such as optimal sleep position or upper body elevation whereas for moderate-severe OSA CPAP therapy is likely needed. This study demonstrates the importance of continuing to explore traditional and innovative interventions in this unique population. A recent study of the impact of positional changes on OSA in pregnancy indicates that alternatives to CPAP may be effective for some patients,43 and further studies are needed to demonstrate the efficacy, safety, and feasibility of OSA treatment in pregnant patients.42 Several meta-analyses of randomized controlled trials that examined the effect of OSA treatments on depressive symptoms in the general population found that CPAP has a clinically relevant and modest improvement of depressive symptoms, particularly in those with the greatest burden of depression at baseline 44-46 Although the effects of CPAP on depression are not superior to sham CPAP when examined against only sham-controlled studies45,46 some relevant studies (e.g., Povitz et al., 2014) were not included in these analyses because they use a different scale for measuring depressive symptoms. Additionally, these meta-analyses did not specifically examine depression severity, which has been shown to be a predictor of treatment outcomes.44 Whether similar findings occur in pregnant women with depression remains to be seen.
In addition to treatment of SDB in pregnant women, this study highlights the importance of education about risk reduction and prevention in this population. Many factors can contribute to the development of SDB in pregnancy such as being overweight/obese at conception, excessive weight gain, fluid retention, hormonal changes, and smoking. Educating pregnant women about particular risks such as obesity and smoking may play an important part in encouraging overall wellness and reducing the likelihood that they will develop SDB and its associated problems.
More recent studies examining snoring and depressive symptoms in this population have had equivocal findings, but were limited by small sample sizes.47,48 A strength of the current study is the large sample size drawn from a general obstetrics clinic. Further strengths are the use of a validated depression measure; a more detailed characterization of habitual snoring symptoms by determining the timing of snoring in relation to pregnancy (e.g., chronic vs. pregnancy-onset); and the concurrent assessment of depressive symptoms and snoring unlike previous studies where snoring status was assessed after depression screening. This study is not, however, free of limitations including missing participant characteristics (e.g., race/ethnicity, weight/BMI). Since the surveys were completed anonymously, we were unable to link responses to medical records and thus were not able to account for these variables. Given the role of weight gain, BMI, and race on SDB, future studies specifically examining the effects of these factors would add greatly to our understanding of this phenomenom.14,49 This study is, however, an extension of previously published findings from the same medical center in which broader demographic characteristics were collected (i.e., the presence of pre-pregnancy obesity, race, maternal educational level, pre-eclampsia, and diabetes). In that study, snoring was found to be independently associated with a prenatal EPDS score ≥10.23 This indicates that the addition of these variables would not substantially impact the generalizability of these results.
This study also used cross-sectional data, however, prior studies have suggested SDB is a predictor of depressive symptoms rather than the inverse. Variation in the wording of questions related to frequency of snoring on the initial questionnaire meant that participants were asked whether they previously snored more than three nights per week, and if they currently snore three nights or more per week. This could potentially result in slight differences in frequencies. However, in our experience of querying thousands of pregnant women, we anticipate that this would not alter the frequencies enough to change the current findings. There is a potential for recall bias in any self-report study. Future studies would benefit from corroborating reports from family members or bed partners. Finally, women who were enrolled in the survey earlier in their pregnancy may not yet have developed SDB. As a result, the observed effect may be attenuated. We performed a sensitivity analysis by restricting data to women enrolling in their third trimester and observed a stronger effect of SDB on depressive symptoms in that analysis, further suggesting that SDB may play a role in depressive symptoms.
CONCLUSIONS
A hallmark symptom of SDB, habitual snoring is an important factor to disrupted sleep in pregnancy and may contribute to higher levels of depressive symptoms in pregnancy. This has clinical implications since obstetric healthcare providers can readily identify women who are at risk for SDB by asking patients and their bed partners about frequent snoring. Identification of women with SDB during pregnancy could offer a window of opportunity for treatment which could plausibly decrease the incidence of prenatal and postpartum depression since SDB can be treated. Further study is warranted to understand the temporal relationship between snoring and depressive symptoms as well as the implications of this study in non-pregnant adult populations.
ACKNOWLEDGEMENTS
This study was funded by a grant from the National Institutes of Health (K23 HL122461).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors have no competing interests to disclose.
REFERENCES
- 1.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. [DOI] [PubMed] [Google Scholar]
- 2.Gentile S Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–166. [DOI] [PubMed] [Google Scholar]
- 3.Kang-Yi CD, Kornfield SL, Epperson CN, Mandell DS. Relationship Between Pregnancy Complications and Psychiatric Disorders: A Population-Based Study With a Matched Control Group. Psychiatr Serv. 2018;69(3):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moehler E, Brunner R, Wiebel A, Reck C, Resch F. Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Arch Womens Ment Health. 2006;9(5):273–278. [DOI] [PubMed] [Google Scholar]
- 5.Hollins K Consequences of antenatal mental health problems for child health and development. Curr Opin Obstet Gynecol. 2007;19(6):568–572. [DOI] [PubMed] [Google Scholar]
- 6.Cox EQ, Sowa NA, Meltzer-Brody SE, Gaynes BN. The Perinatal Depression Treatment Cascade: Baby Steps Toward Improving Outcomes. J Clin Psychiatry. 2016;77(9):1189–1200. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487 e481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis JM, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521 e521–521 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pien GW, Schwab RJ. Sleep Disorders During Pregnancy. Sleep. 2004;27(7):1405–1417. [DOI] [PubMed] [Google Scholar]
- 10.Facco FL, Parker CB, Reddy UM, et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstetrics & Gynecology. 2017;129(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Brien LM, Bullough AS, Chames MC, et al. Hypertension, snoring, and obstructive sleep apnoea during pregnancy: a cohort study. BJOG. 2014;121(13):1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SK, Nehra A, Sinha S, et al. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep and Breathing. 2015;20(1):87–93. [DOI] [PubMed] [Google Scholar]
- 14.Wilson DL, Walker SP, Fung AM, et al. Sleep-disordered breathing in hypertensive disorders of pregnancy: a BMI-matched study. J Sleep Res. 2018;27(5):e12656. [DOI] [PubMed] [Google Scholar]
- 15.Dunietz GL, Shedden K, Lisabeth LD, Treadwell MC, O'Brien LM. Maternal Weight, Snoring, and Hypertension: Potential Pathways of Associations. Am J Hypertens. 2018;31(10):1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunietz GL, Chervin RD, O'Brien LM. Sleep-disordered breathing during pregnancy: future implications for cardiovascular health. Obstet Gynecol Surv. 2014;69(3):164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien LM, Bullough AS, Owusu JT, et al. Snoring during pregnancy and delivery outcomes: a cohort study. Sleep. 2013;36(11):1625–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pamidi S, Marc I, Simoneau G, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71(8):719–725. [DOI] [PubMed] [Google Scholar]
- 19.Kneitel AW, Treadwell MC, O'Brien LM. Effects of maternal obstructive sleep apnea on fetal growth: a case-control study. J Perinatol. 2018;38(8):982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bin YS, Cistulli PA, Ford JB. Population-Based Study of Sleep Apnea in Pregnancy and Maternal and Infant Outcomes. J Clin Sleep Med. 2016;12(6):871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunietz GL, Shedden K, Schisterman EF, Lisabeth LD, Treadwell MC, O'Brien LM. Associations of snoring frequency and intensity in pregnancy with time-to-delivery. Paediatr Perinat Epidemiol. 2018;32(6):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telerant A, Dunietz GL, Many A, Tauman R. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci Rep. 2018;8(1):10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien LM, Owusu JT, Swanson LM. Habitual snoring and depressive symptoms during pregnancy. BMC Pregnancy Childbirth. 2013;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCall WV, Harding D, O'Donovan C. Correlates of depressive symptoms in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2(4):424–426. [PubMed] [Google Scholar]
- 25.Chen YH, Keller JK, Kang JH, Hsieh HJ, Lin HC. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med. 2013;9(5):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005-2008. Sleep. 2012;35(4):461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redhead K, Walsh J, Galbally M, Newnham JP, Watson SJ, Eastwood P. Obstructive sleep apnea is associated with depressive symptoms in pregnancy. Sleep. 2019. [DOI] [PubMed] [Google Scholar]
- 28.Murray D, Cox JL. Screening for depression during pregnancy with the edinburgh depression scale (EDDS). Journal of Reproductive and Infant Psychology. 1990;8(2):99–107. [Google Scholar]
- 29.Lockhart EM, Ben Abdallah A, Tuuli MG, Leighton BL. Obstructive Sleep Apnea in Pregnancy: Assessment of Current Screening Tools. Obstet Gynecol. 2015;126(1):93–102. [DOI] [PubMed] [Google Scholar]
- 30.Cox JL, Holden JM, Sagovsky R. Detection of Postnatal Depression. British Journal of Psychiatry. 1987;150(06):782–786. [DOI] [PubMed] [Google Scholar]
- 31.van Ginkel JR, Linting M, Rippe RCA, van der Voort A. Rebutting Existing Misconceptions About Multiple Imputation as a Method for Handling Missing Data. J Pers Assess. 2020;102(3):297–308. [DOI] [PubMed] [Google Scholar]
- 32.Kamysheva E, Skouteris H, Wertheim EH, Paxton SJ, Milgrom J. A prospective investigation of the relationships among sleep quality, physical symptoms, and depressive symptoms during pregnancy. J Affect Disord. 2010;123(1-3):317–320. [DOI] [PubMed] [Google Scholar]
- 33.Tomfohr LM, Buliga E, Letourneau NL, Campbell TS, Giesbrecht GF. Trajectories of Sleep Quality and Associations with Mood during the Perinatal Period. Sleep. 2015;38(8):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Street LM, Aschenbrenner CA, Houle TT, Pinyan CW, Eisenach JC. Gestational Obstructive Sleep Apnea: Biomarker Screening Models and Lack of Postpartum Resolution. J Clin Sleep Med. 2018;14(4):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clatworthy J The effectiveness of antenatal interventions to prevent postnatal depression in high-risk women. J Affect Disord. 2012;137(1-3):25–34. [DOI] [PubMed] [Google Scholar]
- 37.Muzik M, Marcus SM, Heringhausen JE, Flynn H. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771–788, ix-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McFarland J, Salisbury AL, Battle CL, Hawes K, Halloran K, Lester BM. Major depressive disorder during pregnancy and emotional attachment to the fetus. Arch Womens Ment Health. 2011;14(5):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295(12):1389–1398. [DOI] [PubMed] [Google Scholar]
- 41.Tantrakul V, Numthavaj P, Guilleminault C, et al. Performance of screening questionnaires for obstructive sleep apnea during pregnancy: A systematic review and meta-analysis. Sleep Med Rev. 2017;36:96–106. [DOI] [PubMed] [Google Scholar]
- 42.Abdullah HR, Nagappa M, Siddiqui N, Chung F. Diagnosis and treatment of obstructive sleep apnea during pregnancy. Curr Opin Anaesthesiol. 2016;29(3):317–324. [DOI] [PubMed] [Google Scholar]
- 43.Zaremba S, Mueller N, Heisig AM, et al. Elevated upper body position improves pregnancy-related OSA without impairing sleep quality or sleep architecture early after delivery. Chest. 2015;148(4):936–944. [DOI] [PubMed] [Google Scholar]
- 44.Povitz M, Bolo CE, Heitman SJ, Tsai WH, Wang J, James MT. Effect of treatment of obstructive sleep apnea on depressive symptoms: systematic review and meta-analysis. PLoS Med. 2014;11(11):e1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta MA, Simpson FC, Lyons DC. The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: A systematic review and meta-analysis. Sleep Med Rev. 2016;28:55–68. [DOI] [PubMed] [Google Scholar]
- 46.Zheng D, Xu Y, You S, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular Endpoint randomised trial and meta-analysis. EClinicalMedicine. 2019;11:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bat-Pitault F, Deruelle C, Flori S, et al. Sleep Pattern during Pregnancy and Maternal Depression: Study of Aube Cohort. Journal of Sleep Disorders and Management. 2015;1(1):7. [Google Scholar]
- 48.Sarberg M, Bladh M, Svanborg E, Josefsson A. Postpartum depressive symptoms and its association to daytime sleepiness and restless legs during pregnancy. BMC Pregnancy Childbirth. 2016;16(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gay CL, Richoux SE, Beebe KR, Lee KA. Sleep disruption and duration in late pregnancy is associated with excess gestational weight gain among overweight and obese women. Birth. 2017;44(2):173–180. [DOI] [PubMed] [Google Scholar]