Abstract
Background:
Recent studies show a mechanistic link between gut microbiota-dependent formation of the atherosclerosis- and thrombosis-promoting metabolite trimethylamine N-oxide (TMAO) and cardiovascular disease (CVD). The clinical utility of TMAO in apparently healthy subjects for predicting incident CVD risks is unclear.
Methods and Results:
In the EPIC-Norfolk community-based study, we examined baseline fasting levels of TMAO and two of its nutrient precursors, choline and betaine, in a case:control design study comparing apparently European healthy middle-aged participants who subsequently develop CVD (Cases, n=908) versus those who did not (Controls, n=1,273) over an ensuing average follow-up period of 8 years. In participants who developed CVD vs controls, higher plasma TMAO (3.70 [IQR 2. 50–6.41]μM vs 3.25 [IQR 2.19–52,1.15]μM; p<0.001) and choline levels (9.09 [IQR 7.87–10.53]μM vs 8.89 [IQR 7.66–10.13]μM; p=0.001) were observed. Following adjustments for traditional risk factors, elevated TMAO (adjusted odds ratio (OR) 1.58 [95% confidence interval (CI) 1.21–2.06], p<0.001) and choline levels (adjusted OR 1.31 [95%CI 1.00–1.72], p<0.05) remained predictive of incident CVD development. The clinical prognostic utility of TMAO remained significant and essentially unchanged regardless of the level of cutoff chosen between 1.5 uM (10%ile) to 10.5 uM (90%ile).
Conclusion:
In apparently healthy participants of the community-based middle-aged EPIC-Norfolk population, elevated plasma levels of the gut microbe-dependent metabolite TMAO, and its nutrient precursor choline, predict incident risk for CVD development independent of traditional risk factors.
Keywords: Trimethylamine N-oxide, choline, betaine, cardiovascular disease
One-Sentence Summary
In apparently healthy people with no history of cardiovascular diseases, elevated trimethylamine N-oxide (TMAO) levels is associated with incident development of coronary artery disease.
INTRODUCTION
Over the past decade, numerous studies have revealed a role of the gut microbiome in both cardiovascular and metabolic health, as well as disease susceptibility.1 Animal model mechanistic studies supported by clinical observational studies, implicate the meta-organismal (i.e. involving both gut microbe and human host) trimethylamine N-oxide (TMAO) pathway as a participant in the development of both atherosclerotic heart disease and its adverse thrombotic events.2–8 The overall pathway begins with gut microbe metabolism of phosphatidylcholine or L-carnitine, nutrients commonly found in a Western diet, and the generation of trimethylamine (TMA), a noxious smelling waste product of gut microbial metabolism. Following absorption into the portal circulation, TMA is rapidly metabolized in the liver to produce TMAO, which is eventually eliminated by excretion into urine via the kidneys. Meanwhile, other related metabolites such as choline and betaine were also identified as being closely associated with TMAO in an iterative series of case-control discovery cohorts.5
The contributory role of the TMAO pathway in the development of coronary artery disease (CAD) in the general population, particularly amongst people who are apparently healthy, has not been established. Herein, we investigate the prognostic value of baseline TMAO levels and the propensity for incident coronary artery disease (CAD) events in apparently healthy middle-aged people from the general population.
METHODS
Study population.
We performed a nested case-control study among participants of the EPIC (European Prospective Investigation Into Cancer and Nutrition)-Norfolk prospective population study, a community-based cohort.9 EPIC is a collaboration of population cohorts in 10 European countries, designed to assess the determinants of cancer and other diseases. The EPIC-Norfolk cohort, which is part of the EPIC study, has been described in detail previously. In brief, between 1993 and 1997, investigators recruited 25,663 apparently healthy men and women between 40 and 79 years old, all residents of Norfolk, United Kingdom, and performed a baseline survey.
Sample acquisition.
Non-fasting blood samples were obtained by venipuncture into plain and citrate bottles. Blood samples for assay were processed at the Department of Clinical Biochemistry, University of Cambridge, and stored at −80°C.
Study endpoints.
All individuals have been flagged for death certification at the U.K. Office of National Statistics, with vital status ascertained for the entire cohort. Participants admitted to hospital were identified using unique National Health Service number by data linkage with the East Norfolk Health Authority database, which identifies all hospital contacts throughout England and Wales for Norfolk residents. Participants were identified as having CAD during follow-up if they had a hospital admission and/or died with CAD as underlying cause. CAD was defined as code 410 to 414 according to the International Classification of Diseases-9th revision. We report results with follow-up to November 2003, an average of 8 years. The study was approved by the Norwich District Health Authority Ethics Committee, and all participants gave informed consent. We considered only individuals who did not report a history of heart attack or stroke at the baseline clinic visit. Cases were 908 individuals in whom fatal or nonfatal CAD developed during follow-up. Controls (n=1273) remained free of CAD during follow-up. Control participants were individually matched to each case subject by gender, age (within 5 years), general practice site of enrollment, and date of visit (within 3 months).
TMAO, choline and betaine measurements.
Plasma samples that has never been utilized before were thawed and re-aliquoted following case/control identification in 2003 (average during from collection to aliquoting was 8 years). Aliquots remained frozen until measurements in 2014. Quantification of fasting plasma TMAO, choline, and betaine levels was performed utilizing stable isotope dilution liquid chromatography with on-line tandem mass spectrometry (LC/MS/MS) as previously described.5, 10 Specifically, TMAO, choline, and betaine were quantified with the use of a stable-isotope-dilution assay and high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry on Shimadzu 8050 triple quadrupole mass spectrometer with ultra-high-performance liquid chromatography interface.5, 10
Statistical analysis.
The Student’s t-test or Wilcoxon rank sum test for continuous variables and chi-square test for categorical variables were used to examine the difference between the groups. Odds ratio (OR) and 95% confidence intervals (95%CI) for CAD were calculated using logistic regression models. Levels of TMAO were then adjusted for traditional cardiac risk factors in a multivariable model including age, gender, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and history of diabetes mellitus. Additional adjustment with creatinine was performed. Because of the nonlinearity of the TMAO-CHD relationship, a restricted cubic spline was used in logistic regression models. P values < 0.05 were considered statistically significant. All statistical analyses were performed using R version 9.2 (Vienna, Austria).
RESULTS
Table 1 illustrates the baseline characteristics of our study population that are age- and sex-matched. In general, cases have greater burden of traditional risk factors. Plasma TMAO (3.70 [IQR 2.50–6.41] μM vs 3.25 [IQR 2.19–5.15] μM; p<0.001) and choline levels (9.09 [IQR 7.87–10.53] μM vs 8.89 [IQR 7.66–10.13] μM; p=0.001) were higher in cases vs controls. In contrast, plasma betaine levels were similar between cases and controls (30.8[IQR 25.0–37.3] μM vs 31.2[IQR 25.6–37.2] μM; p=0.66).
Table 1.
Baseline characteristics
| Characteristic | All (n=2,181) | Control (n=1,273) | Case (n=908) | p |
|---|---|---|---|---|
| Age (years) | 65 ± 8 | 65 ± 8 | 66 ± 8 | 0.002 |
| Male (%) | 65 | 65 | 65 | 0.969 |
| Diabetes (%) | 3.7 | 1.6 | 6.5 | <0.001 |
| Smoking (%) | 63.0 | 58.7 | 69.2 | <0.001 |
| LDL-C (mmol/L) | 4.0 (3.4–4.8) | 4.0 (3.3–4.7) | 4.2 (3.6–4.9) | <0.001 |
| HDL-C (mmol/L) | 1.3 (1.0–1.5) | 1.3 (1.1–1.6) | 1.2 (1.0–1.5) | <0.001 |
| Triglycerides (mmol/L) | 1.8 (1.3–2.5) | 1.6 (1.2–2.3) | 1.9 (1.4–2.7) | <0.001 |
| TMAO (μM) | 3.4 (2.3–5.7) | 3.3 (2.2–5.2) | 3.7 (2.5–6.4) | <0.001 |
| Choline (μM) | 9.0 (7.7–10.3) | 8.9 (7.7–10.1) | 9.1 (7.9–10.5) | 0.001 |
| Betaine (μM) | 31.0 (25.3–37.2) | 31.2 (25.6–37.2) | 30.8 (25–37.3) | 0.659 |
Expressed in mean ± standard deviation or median (interquartile range) Abbreviation: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Table 2 illustrates the odds ratios and 95% confidence intervals (CI) for future CAD events stratified according to increasing quartile (Q) of TMAO, choline, and betaine levels. Elevated baseline TMAO levels (Q4 vs Q1, unadjusted odds ratio 1.86 (95%CI 1.46–2.37); p<0.001) and choline levels (Q4 vs Q1, unadjusted odds ratio 1.51(95% CI 1.18–1.92); p<0.001) were observed to confer increased likelihood of development of CAD over the ensuing follow-up period (average of 8 years). Following adjustments for traditional risk factors, elevated plasma levels of TMAO (adjusted odds ratio 1.58 (95%CI 1.21–2.06); p<0.001) and choline (adjusted odds ratio 1.31(95%CI 1.00–1.72); p<0.05) remained predictive of CAD development. In contrast, betaine did not confer any prognostic value, both in unadjusted and adjusted analyses (Table 2).
Table 2.
Odds Ratio for Future Coronary Artery Disease Events by TMAO, Choline, or Betaine Quartiles
| TMAO | ||||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Range (μM) | <2.32 | 2.32–3.42 | 3.43–5.66 | ≥5.67 |
| Event rate n/N (%) | 188/545(34.5) | 228/545(41.8) | 222/545(40.7) | 270/546(49.5) |
| Unadjusted OR (95%CI) | 1 | 1.37(1.07–1.75)* | 1.31(1.02–1.67)* | 1.86 (1.46–2.37)*** |
| Adjusted OR Model 1 (95%CI) | 1 | 1.31(1.01–1.70)* | 1.16 (0.89–1.52) | 1.58 (1.21–2.06)*** |
| Adjusted Model 2 (95%CI) | 1 | 1.24 (0.95–1.62) | 1.06 (0.81–1.40) | 1.41(1.07–1.85)* |
| Choline | ||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Range (μM) | <7.73 | 7.73–9.00 | 9.01–10.30 | ≥10.31 |
| Event rate n/N (%) | 205/545(37.6) | 227/545(41.7) | 216/545(39.6) | 260/546(47.6) |
| Unadjusted OR (95%CI) | 1 | 1.18 (0.93–1.51) | 1.09 (0.85–1.39) | 1.51 (1.18–1.92)*** |
| Adjusted OR Model 1 (95%CI) | 1 | 1.14 (0.88–1.48) | 1.02 (0.78–1.33) | 1.31 (1.00–1.72)* |
| Adjusted OR Model 2 (95%CI) | 1 | 1.06 (0.81–1.39) | 0.91 (0.69–1.19) | 1.1 (0.83–1.47) |
| Betaine | ||||
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Range (μM) | <25.34 | 25.34–31.04 | 31.05–37.23 | ≥37.24 |
| Event rate n/N (%) | 237/545(43.5) | 225/545(41.3) | 217/545(39.8) | 229/546(41.9) |
| Unadjusted OR (95%CI) | 1 | 0.91 (0.72–1.16) | 0.86 (0.68–1.09) | 0.94 (0.74–1.19) |
| Adjusted OR Model 1 (95%CI) | 1 | 0.86 (0.66–1.12) | 0.81 (0.62–1.07) | 1.03 (0.79–1.36) |
| Adjusted OR Model 2 (95%CI) | 1 | 0.83 (0.63–1.08) | 0.77 (0.59–1.01) | 0.95 (0.72–1.26) |
Abbreviations: OR=odds ratio; TMAO=Trimethylamine N-oxide.
Adjusted model 1 includes age, sex, systolic blood pressure, LDL cholesterol, HDL cholesterol, smoking, and diabetes mellitus.
Adjusted model 2 includes age, sex, systolic blood pressure, LDL cholesterol, HDL cholesterol, smoking, diabetes mellitus, and log(creatinine).
p<0.05;
p<0.01,
p<0.001
To further explore if there is a specific cut-off value for TMAO level that may be of particular clinical prognostic utility, we explored the likelihood of future CAD by different cut-off values, ranging between approximately the 10th and 90th percentile cutoff levels within the EPIC-Norfolk population. Remarkably, we observed largely consistent odds ratios across the range of TMAO cut-off values (1.5–10.5 μM, Figure 1). We observed a dose-dependent increased in CAD risk with increase in circulating TMAO levels by cubic spline analysis (Figure 2). In contrast, the prognostic value of choline within the population was less consistent, especially at lower concentration cutoff values, and betaine demonstrated no cutoff level that revealed clinical prognostic utility (Figure 1). Specifically, 496 out of 2,181 (23%) participants had TMAO levels ≥6 μM (close to the 4th quartile of the originally published CAD cohort of 6.2 μM as previously described4), and it is associated with a 1.27 relative risk compared to those with TMAO < 6 μM.
Figure 1.
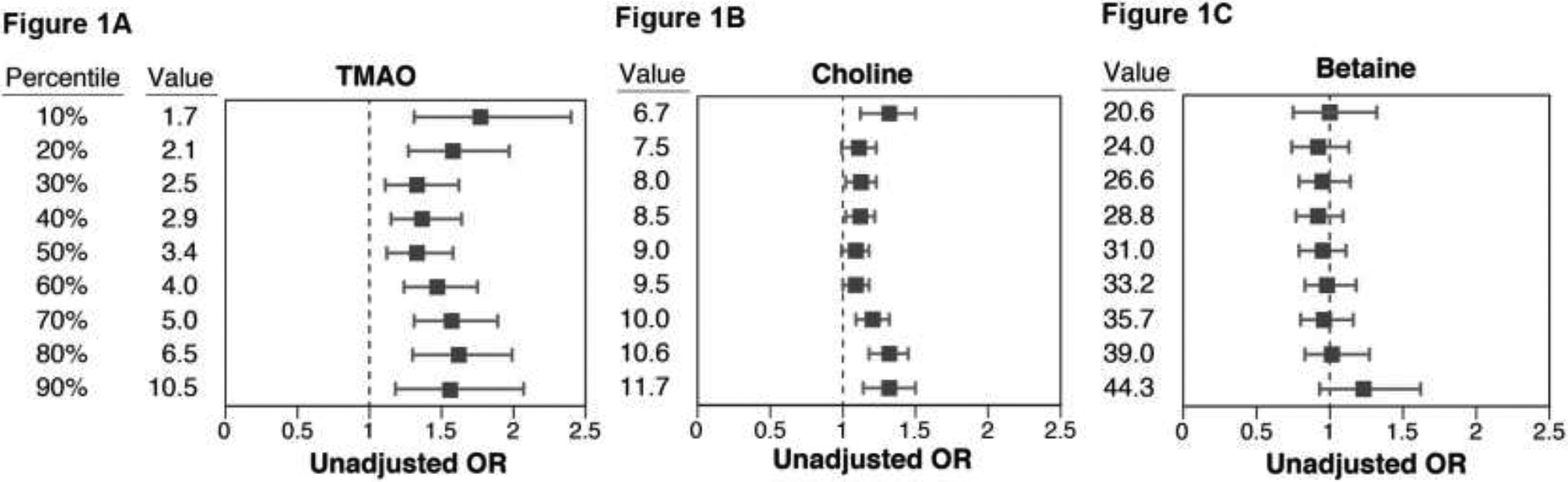
Odds Ratio (OR) for Future Coronary Artery Disease Across the Range of Cut-off Values (in deciles, with 218 cases per decile increment) of 1) Trimethylamine N-oxide (TMAO); 2) choline; and 3) betaine.
Figure 2.
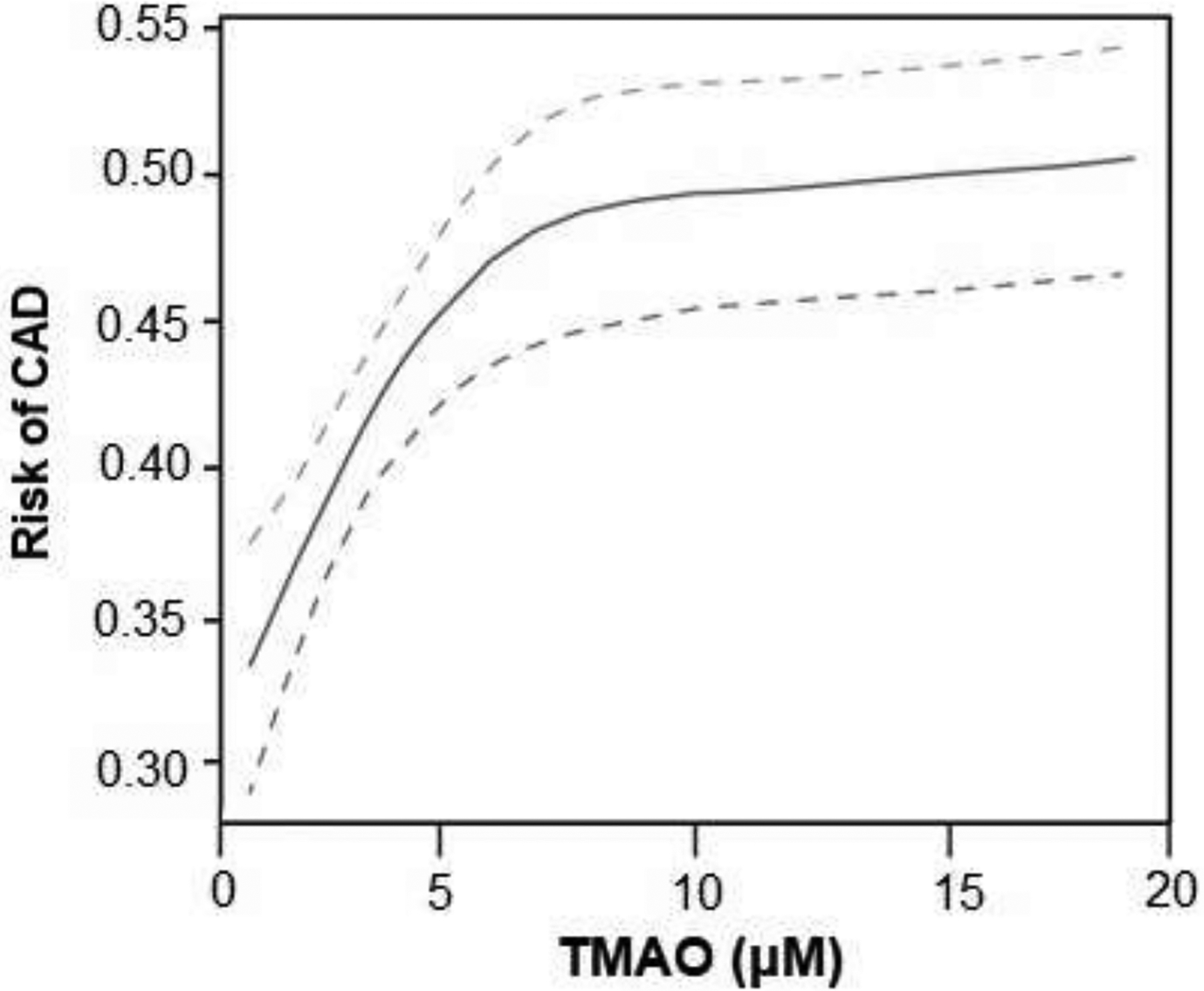
Predicted Risk of Coronary Artery Disease (CAD) across Fasting Plasma Levels of Trimethylamine N-oxide (TMAO)
DISCUSSION
The key finding from our study is that in a Europe-based nested case-control comparison in the EPIC-Norfolk community-based population study, plasma TMAO levels are higher in those who subsequently developed CVD versus those who did not. Plasma TMAO levels were found to provide incremental and independent risk prediction of future development of CVD beyond traditional risk factors. Moreover, in parallel with previous observations from secondary prevention cohorts,4, 11, 12 plasma TMAO levels above 6 μM identify a subset of patients who are vulnerable in developing incident CAD, and should warrant more globally aggressive risk factor modification.
Through both animal model studies and clinical association studies examining patient populations, TMAO has been broadly implicated in participating in the progression of atherosclerosis and thrombosis risk,2–8 as well as both adverse ventricular remodeling and heart failure risks,13–15 and chronic kidney disease progression and adverse CVD outcomes risks.16–19 While there have also been smaller (n<400) cohorts in which systematic TMAO levels did not track with carotid atherosclerosis,20 or with either history of myocardial infarction or incidence of cardiovascular events over 8 years,21 several recent large meta-analyses of up to 17 clinical studies involving 26,167 subjects and CVD risks confirmed an association of cardiovascular mortality and adverse CVD event risk with heightened TMAO levels.11, 12, 22 Moreover, the association of TMAO levels and heightened CVD and mortality risks were observed across multiple geographic regions, including Europe, Asia, North and South America. However, despite the wealth of studies focused on TMAO and cardiometabolic diseases risks, it is notable that virtually all clinical association studies for TMAO have focused on those who have prevalent disease (especially CAD). Three prior studies have examined TMAO levels in a primary prevention cohort – one in a subset of patients in the Coronary Artery Risk Development in Young Adults Study (CARDIA) showed TMAO was not associated with measures of coronary artery calcification as surrogates of atherosclerosis among healthy early-middle-aged adults;23 while another Canadian multiethnic population showed a significant, graded association with prevalent cardiovascular disease.24 Neither of these studies have prospectively examined the development of future CAD events. Recently, a nested case-control study from China identified 86 new diagnosis of CVD over 4.83 years of follow-up. These investigators observed higher TMAO levels in patients with incident CVD cases compared to those without incident CVD, similar to our observations albeit a much smaller cohort.25 Like prior studies, the prognostic value of TMAO, while partially attenuated following adjustments for renal function, remained statistically significant. Thus, accumulation of the atherogenic gut microbial metabolite TMAO in patients with renal insufficiency, a vulnerable population with known heightened cardiovascular risks, may in part be explained by TMAO.
Insights from the community-based EPIC-Norfolk study provide not only confirmed the prognostic value of TMAO in the general population, but allowed us to construct a more reliable normal range that is clinically relevant. As shown in Figures 1 and 2, there exists a “dose-dependent” relationship between TMAO levels and incident CAD risk. For example, regardless of the level of cutoff chosen between the 10th and 90th percentile of the cohort, those with TMAO levels above the cutoff have approximately 1.6-fold increased risk for development of incident CAD versus those whose levels are below that cutoff (Figure 1). The present studies significantly add to the growing data linking the TMAO pathway to heightened CVD risk. In combination with the wealth of other studies on TMAO, they also have important potential clinical implications. Foremost, the present studies suggest that maintaining lower TMAO levels, even amongst apparently healthy middle-aged participants, may help to reduce incident risk for development of CVD (Table 2). Toward that end, several studies have indicated potential TMAO lowering strategies. For example, dietary efforts aimed at reducing red meat and dietary ingestion of the TMAO precursors phosphatidylcholine, and carnitine, such as with a Mediterranean diet, have been associated with lower TMAO levels,26, 27 even though some recent studies have challenged these findings by observing no significant associations between changes in TMAO levels (but improvements in choline levels) with adherence to Mediterranean diet or lifestyle modifications.28, 29 A recent study from the Women’s Health Initiative further demonstrated that long-term increases in TMAO were associated with higher coronary artery disease risk. Further, participants adherent to healthy dietary patterns demonstrated lower increase in TMAO and reduced CVD risk.30 Furthermore, in a small recent clinical intervention study, low dose aspirin both reduced the heightened platelet responsiveness associated with TMAO exposure, and resulted in a modest reduction in TMAO levels.7 Clearly, many factors may affect TMAO levels, and we caution the reliance of a single timepoint measurement to determine risk profiles. Nevertheless the notion that one can identify a cohort of individuals at increased CVD risk due to elevated TMAO, and then potentially impact that risk profile through reduction in TMAO, such as via changes in dietary habits and other potential interventions, warrants further exploration. It is worth noting that non-lethal small molecule inhibitors of microbial choline TMA lyase activity, the presumed major pathway responsible for TMAO generation, have shown efficacy in inhibiting arterial foam cell formation and atherosclerosis progression rate in the apolipoprotein E−/− mouse mode.31 More recently, a family of non-lethal mechanism based suicide substrate inhibitors was shown to serve as a new family of drugs capable of reducing TMAO-dependent platelet responsiveness and thrombosis potential in murine models of arterial injury and thrombus generation.32 In the context of the present studies, since TMAO levels were associated with a dose-dependent risk for incident CAD development in apparently healthy middle aged participants, it remains to be seen whether interventions that reduce TMAO levels will reduce CAD risks, or attenuate thrombosis risks in participants at increased risks for thrombotic events.
There are several limitations of this study that are noted. First, only a single timepoint measurement of TMAO was assessed for risk prediction. Nevertheless, for any given biomarker, the ability to stratify risk at one time point should be similar as it moves to another time point. While there is clear biological variability within individuals as in any known metabolite (e.g. glucose, cholesterol), our previous study in apparently healthy individuals demonstrated that there was heterogeneity across individuals that can be amplified when measuring them in response to different dietary protein intakes. None-the-less, these prior studies also showed consecutive day sampling of circulating TMAO was relatively consistent.27 In addition, while we were able to quantify the atherogenic metabolites previous reported from mechanistic studies, circulating TMA was not investigated since TMA has a high vapor pressure and is well known to be unstable over long periods of storage, even under optimal conditions without specific preparations. As a nested case-control design that leveraged sample availability in the selection of cases and controls, this violates the randomness required for selection, and would have led to biased analyses if using traditional weighted Cox regression analytical methods.
CONCLUSION
In the EPIC-Norfolk community-based study of apparently healthy people with no history of CVD, elevated TMAO levels are associated with incident development of CAD. Further investigations into potentially beneficial approaches to attenuate TMAO levels are warranted.
Take Home Figure.
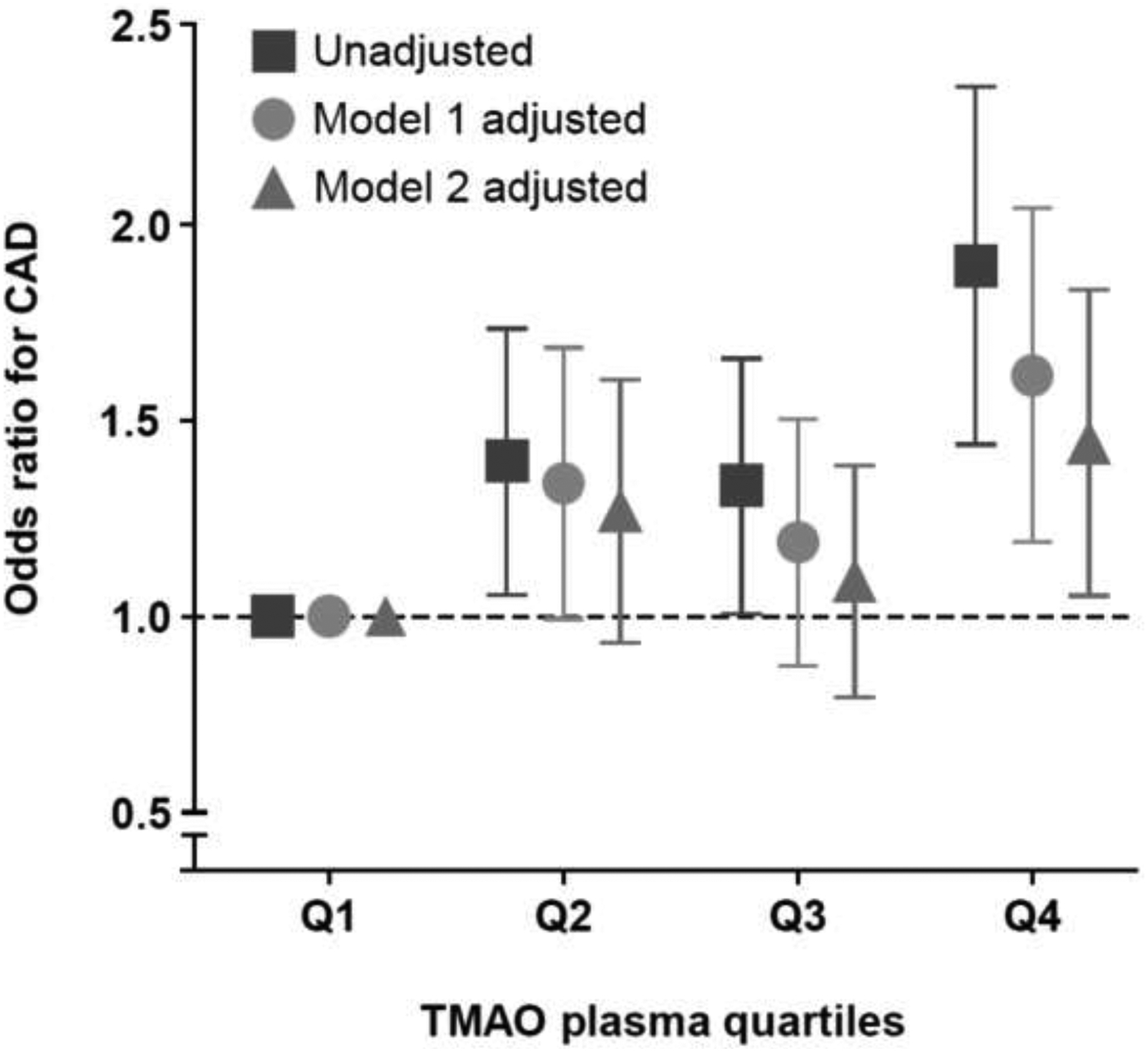
Unadjusted and Adjusted Odds Ratio (OR) for Future Coronary Artery Disease (CAD) Across Quartiles of Trimethylamine N-oxide (TMAO). Model 1 adjusted for age, sex, systolic blood pressure, LDL cholesterol, HDL cholesterol, smoking, and diabetes mellitus. Model 2 adjusted for Model 1 plus log(creatinine).
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the EPIC-Norfolk prospective population study.
Funding Support: The EPIC-Norfolk Study is funded by Cancer Research UK grant number 14136 and the Medical Research Council grant number G1000143. This research was supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, R01DK106000, R01HL126827, WT and SH; P01HL-147823 to SH, R01HL130819 to ZW). MN is supported by a personal ZONMW-VIDI grant 2013 [016.146.327] and a Dutch Heart Foundation CVON IN CONTROL Young Talent Grant 2013. Dr. Hazen was partially supported by a gift from the Leonard Krieger endowment. Mass spectrometry studies were performed on instruments housed in a facility supported in part by the Case Western Reserve University CTSA (UL1TR000439), and a Center of Innovations Award by Shimadzu. The study reported here was additionally supported by Le Ducq consortium grant 17CVD01 (to MN and SH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Drs. Wang and Hazen are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Drs. Wang and Hazen have received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics, and Procter & Gamble. Dr. Hazen is a paid consultant for Proctor & Gamble, and has received research funds from Proctor & Gamble, Pfizer Inc., and Roche Diagnostics. Dr. Tang is a consultant for Sequana Medical A.G., Owkin Inc, CardiolRx Inc, and Relypsa Inc, and has received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation, all unrelated to the contents of this paper. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Tang WHW, Backhed F, Landmesser U and Hazen SL. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WH, DiDonato JA, Lusis AJ and Hazen SL. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ and Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y and Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ and Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ and Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W, Wang Z, Tang WHW and Hazen SL. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation. 2017;135:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL and Luscher TF. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A and Wareham N. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999;80 Suppl 1:95–103. [PubMed] [Google Scholar]
- 10.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM and Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi J, You T, Li J, Pan T, Xiang L, Han Y and Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G and Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Heaney LM, Bhandari SS, Jones DJ and Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102:841–8. [DOI] [PubMed] [Google Scholar]
- 14.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y and Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL and Hazen SL. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafi T, Powe NR, Meyer TW, Hwang S, Hai X, Melamed ML, Banerjee T, Coresh J and Hostetter TH. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J Am Soc Nephrol. 2017;28:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA and Yu AS. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J Am Soc Nephrol. 2016;27:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS and Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto C, Levin A and Can PI. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–52. [DOI] [PubMed] [Google Scholar]
- 20.Skagen K, Troseid M, Ueland T, Holm S, Abbas A, Gregersen I, Kummen M, Bjerkeli V, Reier-Nilsen F, Russell D, Svardal A, Karlsen TH, Aukrust P, Berge RK, Hov JE, Halvorsen B and Skjelland M. The Carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis. 2016;247:64–9. [DOI] [PubMed] [Google Scholar]
- 21.Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H and von Eckardstein A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243:638–44. [DOI] [PubMed] [Google Scholar]
- 22.Heianza Y, Ma W, Manson JE, Rexrode KM and Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc. 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer KA, Benton TZ, Bennett BJ, Jacobs DR Jr., Lloyd-Jones DM, Gross MD, Carr JJ, Gordon-Larsen P and Zeisel SH. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc. 2016;5:e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS and McQueen MJ. The Relationship Between Trimethylamine-N-Oxide and Prevalent Cardiovascular Disease in a Multiethnic Population Living in Canada. Can J Cardiol. 2015;31:1189–94. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L, Zheng J, Xie Y, Li Z, Guo X, Sun G, Sun Z, Xing F and Sun Y. Serum gut microbe-dependent trimethylamine N-oxide improves the prediction of future cardiovascular disease in a community-based general population. Atherosclerosis. 2019;280:126–131. [DOI] [PubMed] [Google Scholar]
- 26.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW and Ercolini D. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM and Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson ML, Malin SK, Wang Z, Brown JM, Hazen SL and Kirwan JP. Effects of Lifestyle Intervention on Plasma Trimethylamine N-Oxide in Obese Adults. Nutrients. 2019;11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guasch-Ferre M, Hu FB, Ruiz-Canela M, Bullo M, Toledo E, Wang DD, Corella D, Gomez-Gracia E, Fiol M, Estruch R, Lapetra J, Fito M, Aros F, Serra-Majem L, Ros E, Dennis C, Liang L, Clish CB, Martinez-Gonzalez MA and Salas-Salvado J. Plasma Metabolites From Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention With Mediterranean Diet) Study. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, Rexrode KM, Manson JE and Qi L. Long-Term Changes in Gut Microbial Metabolite Trimethylamine N-Oxide and Coronary Heart Disease Risk. J Am Coll Cardiol. 2020;75:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ and Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC and Hazen SL. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]


