Abstract
Purpose of review
The emergence of severe acute respiratory syndrome coronavirus 2 virus, which causes coronavirus disease 2019 (COVID-19), led to the declaration of a global pandemic by the World Health Organization on March 11, 2020. As of February 6, 2021, over 105 million persons have been infected in 223 countries and there have been 2,290,488 deaths. As a result, emergency medical services and hospital systems have undergone unprecedented healthcare delivery reconfigurations. Here, we review the effects of the COVID-19 pandemic on out-of-hospital cardiac arrest (OHCA) epidemiology and systems of care.
Recent findings
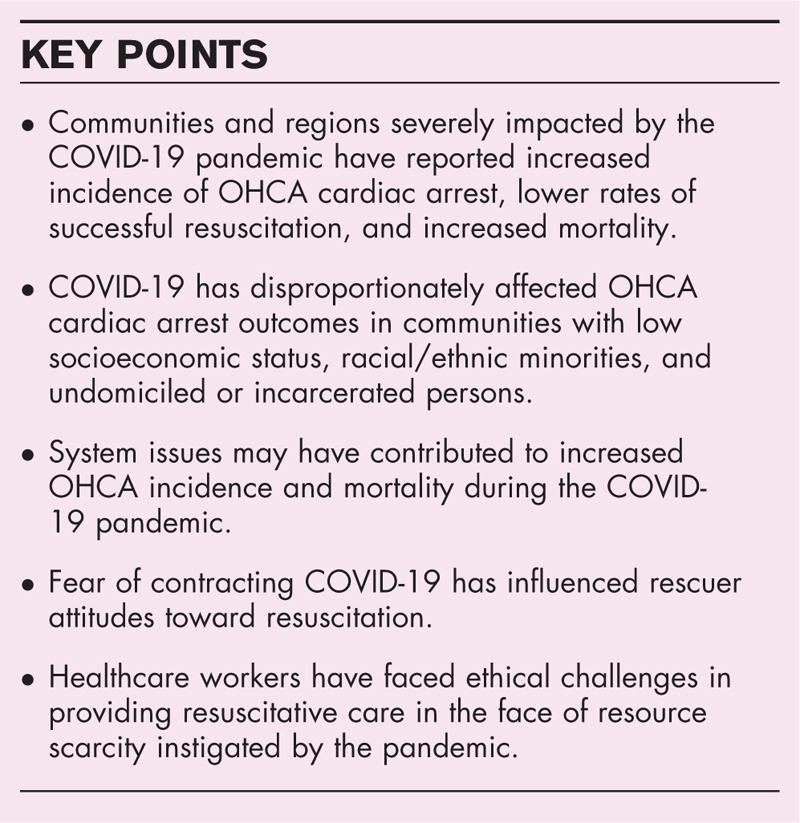
Areas severely affected by the pandemic have reported increased incidence of OHCA, lower rates of successful resuscitation, and increased mortality. COVID-19 has significantly impacted patient outcomes through increased disease severity, decreased access to care, and the reshaping of emergency medical response and hospital-based healthcare systems and policies. The pandemic has negatively influenced attitudes toward resuscitation and challenged providers with novel ethical dilemmas provoked by the scarcity of healthcare resources.
Summary
The COVID-19 pandemic has had direct, indirect, psychosocial, and ethical impacts on the cardiac arrest chain of survival.
Keywords: cardiac arrest, coronavirus disease 2019, management, outcomes, review
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped β-coronavirus with >80% genetic similarity to SARS-CoV-1 and bat coronavirus RaTG13 that is transmitted via respiratory droplets, infects cells through interaction of the viral spike protein with the angiotensin-converting enzyme 2 receptor, and leads to the clinical syndrome of coronavirus disease 2019 (COVID-19) [1]. COVID-19 initially presents with fever, upper respiratory symptoms, myalgias, and other nonspecific symptoms and progresses to severe infection in 14% of cases at a median of 6–8 days from exposure [2–4]. Severe COVID-19 is characterized by progressive pneumonia and a profound hyper-inflammatory state complicated by acute respiratory distress syndrome, cardiovascular complications, shock, and death in 42% of patients admitted to intensive care [5–7]. The World Health Organization (WHO) declared a global pandemic on March 11, 2020 and as of February 6, 2021, over 105 million persons have been infected and 2,290,488have died [8]. Severely affected areas have reported increased incidence of out-of-hospital cardiac arrest (OHCA), lower rates of successful cardiopulmonary resuscitation (CPR), and increased mortality [9–11]. Several small cases series have shown low survival of in-hospital cardiac arrest (IHCA) in patients with COVID-19, but whether IHCA outcomes differ between patients with and without COVID-19 is uncertain [12–14]. Further investigation is currently ongoing to explore outcomes from cardiac arrest given this ongoing pandemic. Here we review the epidemiology and pathogenesis of COVID-19-related OHCA, discuss the impact of healthcare system restructuring on patient outcomes, explore the influence of COVID-19 on attitudes toward resuscitation, and describe novel ethical challenges provoked by the scarcity of healthcare resources during the pandemic.
Box 1.
no caption available
EPIDEMIOLOGY AND PATHOGENESIS OF CARDIAC ARREST DURING THE CORONAVIRUS DISEASE 2019 PANDEMIC
Communities and regions severely impacted during the early stages of the COVID-19 pandemic reported increased incidence of OHCA, lower rates of successful resuscitation, and increased mortality [9–11,15]. Compared to the prior year, return of spontaneous circulation (ROSC) after OHCA fell from 25 to 11% in New York, 31 to 18% in Northern Italy, and 23 to 13% in Paris [9–11]. An international meta-analysis of 10 studies and 35,379 OHCAs found a 2.2-fold increased incidence and significantly decreased survival (OR 0.67, 95% CI 0.49–0.91; P = 0.01) during the pandemic as compared to 2019 [16▪▪]. Lim and colleagues also noted increased frequency of OHCA occurring at home, increased prevalence of nonshockable rhythms, decreased rates of intubation, and decreased survival to both hospital admission and discharge. An analysis from the Cardiac Arrest Registry to Enhance Survival (CARES) registry of 19,303 OHCAs occurring in the United States (US) from March 16 to April 30 in both 2019 and 2020 showed increased incidence of OHCA (88.5 ± 64.1 vs. 69.7 ± 49.8 per million residents; P < 0.01), decreased rates of ROSC (23.0% vs. 29.8%; adjusted rate ratio [ARR], 0.82 [95% CI, 0.78–0.87]; P < 0.01), and decreased survival to hospital discharge (6.6% vs. 9.8%; ARR, 0.83 [95% CI, 0.69–1.00]; P = 0.048), especially in communities that were moderately to severely affected by COVID-19 [17▪▪].
The direct contribution of COVID-19 on excess OHCA incidence and mortality is unknown and has been limited by undertesting for SARS-CoV-2 among patients where resuscitation was not initiated or was terminated prior to hospital presentation [18▪▪,19]. The higher observed incidence and mortality of OHCA during the pandemic may be due to increased illness severity, akin to similar findings during influenza outbreaks [9,20,21]. For some patients with COVID-19, cardiac arrest may be caused by acute hypoxemic respiratory failure, as evidenced by increased prevalence of nonshockable initial rhythms during the pandemic [16▪▪,17▪▪]. Nonetheless, COVID-19 has also been associated with cardiac manifestations including vascular inflammation, myocarditis, and cardiac arrhythmias [22,23]. Myocardial injury in COVID-19 has multiple potential mechanisms and has been associated with negative patient outcomes [24,25]. However, a recent analysis suggested that myocardial injury in severe COVID-19 is a function of patient age and comorbidities and that the adverse prognosis of myocardial injury in COVID-19 relates primarily to critical illness and multiorgan injury, similar to patients with non-COVID acute respiratory distress syndrome [26]. Finally, COVID-19 can cause a hypercoagulable state that has been associated with thrombotic events including acute myocardial infarction (MI), stroke, and pulmonary embolism [27,28]. Further studies are needed to fully understand the pathogenesis of COVID-19 and its implications for cardiac arrest management.
IMPACT OF HEALTHCARE SYSTEM RESTRUCTURING ON CARDIAC ARREST OUTCOMES
System issues may have contributed to increased OHCA incidence and mortality early during the COVID-19 pandemic. Healthcare systems underwent a rapid and unprecedented reconfiguration to limit face-to-face contact and accommodate telemedicine and as a result many nonurgent cardiovascular diagnostic tests and elective procedures were deferred, postponed, or canceled [29–34]. These actions may have unintentionally limited or delayed access to care for patients at high risk of suffering OHCA. Patient fear of contracting COVID-19 may have led to avoidance of care [35]. Indeed, recent studies have demonstrated that more than 25% of victims of OHCA interact with the healthcare system in the preceding 90 days and rates of hospitalization for acute MI, heart failure, and stroke markedly decreased during the pandemic [36–41]. This fall in hospitalizations coincided with a nationwide decrease in emergency medical services (EMS) responses and a doubling of EMS-attended deaths [42]. A single-center study in Denver observed a fall in ambulance activations and a 2.2-fold increased incidence of OHCA following a statewide ‘shelter-in-place’ order that the authors attributed to increased MI-related OHCA and decreased access to care [43]. Moreover, COVID-19 has disproportionately affected communities with low socioeconomic status, racial/ethnic minorities, and undomiciled or incarcerated persons that already suffer poor access to healthcare, lower rates of CPR, and delayed EMS response [44–48]. Lai et al. found that Black, Hispanic, and Asian persons were at increased risk of COVID-19-related OHCA and death even after adjusting for comorbidities that unequally affect minority populations, underscoring the systemic inequalities in the US healthcare system both prior to and during the pandemic [9].
Healthcare system restructuring was not limited to hospitals and policy changes among EMS agencies aimed to protect frontline providers may have negatively impacted OHCA outcomes. These interim recommendations included screening 911 calls for likelihood of COVID-19, limiting the number responding personnel, pausing chest compressions during aerosolizing procedures such as intubation, and not transporting patients without ROSC to hospitals [49]. A meta-analysis of 6 studies of OHCA during the COVID pandemic noted prolonged EMS response times, a reduction in EMS-initiated resuscitations, and lower rates of ROSC and survival to hospital discharge [50▪]. However, this analysis was confounded by missing data and differences in outcome measures and thus subject to substantial heterogeneity across studies. A subsequent analysis of OHCA across the US found no differences in EMS response times or duration of treatment during the pandemic as compared to prior years [17▪▪]. However, the authors did note a substantial decrease in ROSC in all included communities, including those with low COVID-19 mortality rates, suggesting that interim recommendations may have been implemented overly broadly and thus unnecessarily hampered the ability of EMS personnel to effectively respond to OHCA in lower prevalence areas [17▪▪].
Finally, changes in the management strategy for emergent medical conditions, including acute MI, may have contributed to excess cardiac arrest mortality during the pandemic. As the first nation to be struck by COVID-19, China established a fibrinolysis-first strategy for ST-elevation MI (STEMI) to avoid provider exposure [51]. However, subsequent studies have shown that this strategy was associated with lower rates of timely coronary reperfusion and increased rates of recurrent MI, cardiogenic shock, and heart failure [52]. Despite major society guidelines advocating for the ongoing utilization of primary percutaneous coronary intervention (PCI) for STEMI during the pandemic, multiple international studies have shown significant reductions in PCI and prolonged door-to-balloon and total ischemia times that may have contributed to higher mortality [53,54,55▪,56]. Notably, a study of 524 patients with OHCA complicated by acute MI in the United Kingdom observed lower rates of coronary angiography, longer time to reperfusion, and increased mortality during the pandemic [57]. Anecdotal reports have suggested increased incidence of mechanical complications of MI during the pandemic, emphasizing the negative implications of delayed or missed patient presentation and reduced use of PCI [58,59]. Indeed, a hospital system in New York reported a 4.97-fold increase in OHCA with a concomitant 50% reduction in acute coronary syndrome admissions that correlated with a spike in COVID-19-related deaths (correlation coefficient 0.954; P < 0.0001) [60]. The recently announced North American COVID-19 STEMI registry may shed further light on the impact of COVID-19 on STEMI management and its association with OHCA outcomes [61].
ATTITUDES TOWARD RESUSCITATION DURING THE CORONAVIRUS DISEASE 2019 PANDEMIC
Early CPR and defibrillation provide OHCA victims with the highest likelihood of survival. CPR is described as an aerosol-generating procedure by the WHO considering case reports of coronavirus transmission during chest compressions [62]. However, there is a paucity of evidence regarding whether CPR truly generates aerosols or is associated with transmission of COVID-19 [63,64]. Personal protective equipment (PPE) is recommended for healthcare workers performing aerosol-generating procedures during resuscitation of patients with unknown COVID-19 status to reduce risk of exposure [49,65]. Nevertheless, the majority of OHCA occurs in the home where bystanders are unlikely to have access to PPE [66]. In these circumstances, the rescuer is frequently a close personal contact of the victim and thus the provision of CPR is unlikely to significantly change their personal risk of infection [65].
Despite the clear benefits of early CPR for cardiac arrest, the COVID-19 pandemic has inculcated a culture of fear that may negatively influence the willingness of bystanders to perform resuscitative maneuvers due to a perception of increased risk of personal harm [67,68]. Newspapers have reported disturbing instances where bystanders did not perform CPR for fear of contracting COVID-19 [68]. A recent social media study with over 1,300 lay-person respondents from 26 countries showed a decreased willingness to assess an unresponsive stranger, perform chest compressions, or apply an automatic external defibrillator during the pandemic [69▪]. However, determining whether perceptions of risk are influencing bystander CPR on a broader scale has been a challenge. Investigators in Northern Italy and Paris observed dramatically decreased rates of bystander CPR during the COVID-19 pandemic, whereas data from Seattle, New York, and Pittsburgh have shown no significant change in bystander resuscitation [9–11,18▪▪,70] The CARES analysis found no significant difference in the rates of witnessed cardiac arrest (41.1% vs 43.7%; standardized difference [SD] 5.4%) bystander CPR (47.7% vs. 46.8%; SD 1.7%) or bystander defibrillation (5.7% vs 8.1%; SD 9.4%) in the US during the pandemic as compared to the same period in 2019 [17▪▪]. The available data suggests that the rapid identification of pulselessness and prompt initiation of chest compressions by bystanders, with use of immediately available PPE or improvised PPE, should remain the preferred response to witnessed OHCA. Clearly, the willingness of the lay public to perform resuscitation is fragile and strong guidance and support from public health agencies, through CPR education and novel technologies, is needed to maintain a robust community response to OHCA and prevent a potentially disastrous and demoralizing decline in bystander CPR [71,72].
ETHICS OF RESUSCITATION DURING THE PANDEMIC
The COVID-19 pandemic has precipitated significant ethical challenges in the setting of strained and overcrowded hospitals, declining availability of resources, and the need to allocate scarce life-sustaining treatments. During crisis standards of care triggered by the COVID-19 surge, during which healthcare demand outpaced supply and clinical needs were unable to be met, the usual assumption that resuscitation should be provided unless a do-not-resuscitate (DNR) order exists has been challenged by a strain on resources [73▪,74▪▪]. Such concerns are not limited to decisions for initial resuscitation because resource availability for postcardiac arrest care following a successful resuscitation must also be considered. Per Emanual et al., classical ethical principles prevail for assigning scarce-resources during COVID-19 and have resulted in the following six recommendations: maximize benefits; prioritize health workers; do not allocate on a first-come, first-served basis; be responsive to evidence; recognize research participation; and equally apply these principles to both COVID-19 and non-COVID-19 patients [75▪▪]. The American Medical Association has recommended that experienced teams perform triage duties during crisis standards of care to relieve the moral burden upon treating clinicians and minimize conflicts [76,77].
Although triage teams offer a deliberate approach to the ethical determination of inpatient resource allocation, prehospital and emergency services are frequently burdened with the ethical exigencies of making resuscitation decisions unexpectedly with limited consultative support. Physicians are not obligated to offer or provide CPR if medically inappropriate and should enlist a second physician who is uninvolved in the patients care to review their decision-making. Family assent should be sought but is not specifically required to apply a unilateral DNR in the absence of agreement by the surrogate decision-maker [74▪▪]. Conversely, a selective approach has been proposed that would limit resuscitation to <6 min if ROSC is not achieved in cases of unwitnessed, asystolic, or recurrent arrest [78▪]. For other cardiac arrests, the authors endorse the use of the sequential organ failure assessment score to further guide resuscitative efforts [78▪].
Ultimately, the COVID-19 pandemic has underscored the vital importance of advanced care planning in serious illness and aging. Clear delineation of a medical decision-maker, medical durable power of attorney, or healthcare proxy should be encouraged as a component of routine primary care. If feasible, patient wishes should be sought prior to a potential medical decompensation and supported during crisis. The provision of palliative care resources in the emergency setting is one method to ensure that patients and families are supported during the unexpected catastrophe of an OHCA during the COVID-19 pandemic.
CONCLUSIONS
The incidence and mortality of OHCA have increased significantly during the COVID-19 pandemic due to a combination of the direct biological impact of the virus on patients, the indirect effects of healthcare system restructuring and associated changes in preventive and emergency care practice, and psychosocial attitudes toward resuscitation. Healthcare workers have faced ethical challenges in providing resuscitative care in the face of resource scarcity instigated by the pandemic. Despite the rapid pace of investigation, additional studies are needed to explore the effect of COVID-19 on the incidence and outcomes of OHCA among socioeconomically disadvantaged persons, the relationship between decreased access to care and COVID-19-related OHCA, and potential interventions to maintain a robust community response to OHCA. A thorough and complete understanding of the impact of COVID-19 on the cardiac arrest chain of survival may not occur until resolution of the pandemic. At the time of writing, recently approved vaccines raise hope for the future, yet challenges to immunization programs in acquiring and distributing sufficient doses to conclude the global pandemic are significant and their effect will not be immediate [79–81]. Thus, it is imperative to continue investigating and addressing the effects of COVID-19 on cardiac arrest systems of care in order to improve the chances of meaningful survival for as many victims as possible and inform the response to future pandemics.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Cevik M, Kuppalli K, Kindrachuk J, et al. Virology, transmission, and pathogenesis of SARS-CoV-2. Bmj 2020; 371:m3862. [DOI] [PubMed] [Google Scholar]
- 2.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. Morb Mortal Wkly Rep 2020; 69:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a Report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama 2020; 1239–1242. [DOI] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. Jama 2020; 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. Jama 2020; 323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med 2020; 382:2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia 2020; 75:1340–1349. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Coronavirus disease (COVID-19) pandemic.December 19, 2020. [Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 9.Lai PH, Lancet EA, Weiden MD, et al. Characteristics associated with out-of-hospital cardiac arrests and resuscitations during the novel coronavirus disease 2019 pandemic in New York City. JAMA Cardiol 2020; 5:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldi E, Sechi GM, Mare C, et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med 2020; 383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marijon E, Karam N, Jost D, et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health 2020; 5:e437–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thapa SB, Kakar TS, Mayer C, et al. Clinical outcomes of in-hospital cardiac arrest in COVID-19. JAMA Intern Med 2020; 181:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modes ME, Lee RY, Curtis JR. Outcomes of cardiopulmonary resuscitation in patients with COVID-19-limited data, but further reason for action. JAMA Intern Med 2020; 181:281–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayek SS, Brenner SK, Azam TU, et al. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. Bmj 2020; 371:m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Recher M, Baert V, Leteurtre S, Hubert H. Consequences of coronavirus disease outbreak on paediatric out-of-hospital cardiac arrest in France. Resuscitation 2020; 155:100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪▪.Lim ZJ, Ponnapa Reddy M, Afroz A, et al. Incidence and outcome of out-of-hospital cardiac arrests in the COVID-19 era: a systematic review and meta-analysis. Resuscitation 2020; 157:248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]; A systematic review of 10 studies and 35,379 OHCAs demonstrating increased incidence and mortality of OHCA and significant variation in resuscitation practices during the COVID-19 pandemic.
- 17▪▪.Chan PS, Girotra S, Tang Y, et al. Outcomes for out-of-hospital cardiac arrest in the United States during the coronavirus disease 2019 pandemic. JAMA Cardiol 2020; 6:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large study of 19,303 OHCAs occuring between March 16 and April 30 in 2019 and 2020 using data from the Cardiac Arrest Registry to Enhance Survival (CARES) that showed increased incidence of OHCA, decreased rates of sustained ROSC, and decreased survival during the COVID-19 pandemic, even in areas with low burden of COVID-19.
- 18▪▪.Sayre MR, Barnard LM, Counts CR, et al. Prevalence of COVID-19 in out-of-hospital cardiac arrest: implications for bystander cardiopulmonary resuscitation. Circulation 2020; 142:507–509. [DOI] [PubMed] [Google Scholar]; A study of 1,067 OHCAs occuring between January and April 2020 in Seattle and King County, WA, demonstrating a low prevalence of laboratory- confirmed COVID-19 or COVID-19-like illness. The findings reflect the challenge in determining the effect of COVID-19 on excess OHCA incidence and mortality due to undertesting for SARS-CoV-2 and additionally have implications when considering the risk-benefit of changes to resuscitation guidelines.
- 19.Baldi E, Sechi GM, Mare C, et al. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J 2020; 41:3045–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siscovick DS, Raghunathan TE, Lin D, et al. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol 2000; 152:674–677. [DOI] [PubMed] [Google Scholar]
- 21.Madjid M, Miller CC, Zarubaev VV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J 2007; 28:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020; 5:831–840. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020; 41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bavishi C, Bonow RO, Trivedi V, et al. Special article - acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis 2020; 63:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giustino G, Croft LB, Stefanini GG, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020; 76:2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metkus TS, Sokoll LJ, Barth AS, et al. Myocardial injury in severe COVID-19 compared to non-COVID acute respiratory distress syndrome. Circulation 2020; 143:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iba T, Levy JH, Levi M, et al. Coagulopathy of coronavirus disease. Crit Care Med 2020; 48:1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prevention. CfDCa. Healthcare Facilities: Managing Operations During the COVID-19 Pandemic. June 28, 2020. [Google Scholar]
- 30.Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC's Interventional Council and SCAI. J Am Coll Cardiol 2020; 75:2372–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadavath S, Mohan J, Ashraf S, et al. Cardiac catheterization laboratory volume changes during COVID-19-findings from a cardiovascular fellows consortium. Am J Cardiol 2020; 130:168–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi AD, Abbara S, Branch KR, et al. Society of Cardiovascular Computed Tomography guidance for use of cardiac computed tomography amidst the COVID-19 pandemic Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2020; 14:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkpatrick JN, Mitchell C, Taub C, et al. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Soc Echocardiogr 2020; 33:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skali H, Murthy VL, Al-Mallah MH, et al. Guidance and best practices for nuclear cardiology laboratories during the COVID-19 pandemic: an information statement from ASNC and SNMMI. Circ Cardiovasc Imaging 2020; 13:e011761. [DOI] [PubMed] [Google Scholar]
- 35.Dubey S, Biswas P, Ghosh R, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr 2020; 14:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuvy M, Koh M, Qiu F, et al. Healthcare utilization prior to out-of-hospital cardiac arrest: a population-based study. Resuscitation 2019; 141:158–165. [DOI] [PubMed] [Google Scholar]
- 37.De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med 2020; 383:88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall ME, Vaduganathan M, Khan MS, et al. Reductions in heart failure hospitalizations during the COVID-19 pandemic. J Card Fail 2020; 26:462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gluckman TJ, Wilson MA, Chiu ST, et al. Case rates, treatment approaches, and outcomes in acute myocardial infarction during the coronavirus disease 2019 pandemic. JAMA Cardiol 2020; 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jasne AS, Chojecka P, Maran I, et al. Stroke code presentations, interventions, and outcomes before and during the COVID-19 pandemic. Stroke 2020; 51:2664–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baum A, Schwartz MD. Admissions to veterans affairs hospitals for emergency conditions during the COVID-19 pandemic. Jama 2020; 324:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerner EB, Newgard CD, Mann NC. Effect of the coronavirus disease 2019 (COVID-19) pandemic on the U.S. emergency medical services system: a preliminary report. Acad Emerg Med 2020; 27:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holland M, Burke J, Hulac S, et al. Excess cardiac arrest in the community during the COVID-19 pandemic. JACC Cardiovasc Interv 2020; 13:1968–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pareek M, Bangash MN, Pareek N, et al. Ethnicity and COVID-19: an urgent public health research priority. Lancet 2020; 395:1421–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med 2020; 17:e1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. Circulation 2020; doi: 10.1161/CIRCULATIONAHA.120.052278. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akiyama MJ, Spaulding AC, Rich JD. Flattening the curve for incarcerated populations – Covid-19 in Jails and Prisons. N Engl J Med 2020; 382:2075–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelson DP, Sasson C, Chan PS, et al. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19: from the emergency cardiovascular care committee and get with the guidelines-resuscitation adult and pediatric task forces of the American Heart Association. Circulation 2020; 141:e933–e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50▪.Scquizzato T, Landoni G, Paoli A, et al. Effects of COVID-19 pandemic on out-of-hospital cardiac arrests: A systematic review. Resuscitation 2020; 157:241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]; A meta-analysis of 6 studies in the US and Europe that showed decreased ROSC and survival to hospital discharge during the pandemic as compared to a nonpandemic period. Additionally, the authors observed a decrease in bystander-witnessed OHCA and bystander-initiated CPR, increased ambulance response times, and decrease rates of resuscitation attempts by EMS during the pandemic period.
- 51.Han Y, Zeng H, Jiang H, et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID-19 epidemic. Circulation 2020; 141:e810–e816. [DOI] [PubMed] [Google Scholar]
- 52.Leng WX, Yang JG, Li XD, et al. Impact of the shift to a fibrinolysis-first strategy on care and outcomes of patients with ST-segment-elevation myocardial infarction during the COVID-19 pandemic-The experience from the largest cardiovascular-specific centre in China. Int J Cardiol 2020; 329: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahmud E, Dauerman HL, Welt FGP, et al. Management of acute myocardial infarction during the COVID-19 pandemic: A Consensus Statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). Catheter Cardiovasc Interv 2020; 96:336–345. [DOI] [PubMed] [Google Scholar]
- 54.Chieffo A, Stefanini GG, Price S, et al. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur Heart J 2020; 41:1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020; 75:2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study of 9 high-volume cardiac catheterization laboratories in the US that demonstrated a 38% decrease in STEMI activations in March 2020 as compared to the preceding 14 months.
- 56.De Luca G, Verdoia M, Cercek M, et al. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol 2020; 76:2321–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rashid Hons M, Gale Hons CP, Curzen Hons N, et al. Impact of coronavirus disease 2019 pandemic on the incidence and management of out-of-hospital cardiac arrest in patients presenting with acute myocardial infarction in England. J Am Heart Assoc 2020; 9:e018379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pilato E, Pinna GB, Parisi V, et al. Mechanical complications of myocardial infarction during COVID-19 pandemic: an Italian single-centre experience. Heart Lung 2020; 49:779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qureshi WT, Al-Drugh S, Ogunsua A, et al. Post myocardial infarction complications during the COVID-19 pandemic – a case series. Cardiovasc Revasc Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mountantonakis SE, Saleh M, Coleman K, et al. Out-of-hospital cardiac arrest and acute coronary syndrome hospitalizations during the COVID-19 surge. J Am Coll Cardiol 2020; 76:1271–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dehghani P, Davidson LJ, Grines CL, et al. North American COVID-19 ST-segment-elevation myocardial infarction (NACMI) registry: rationale, design, and implications. Am Heart J 2020; 227:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christian MD, Loutfy M, McDonald LC, et al. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis 2004; 10:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown E, Chan LM. Should chest compressions be considered an aerosol-generating procedure? A literature review in response to recent guidelines on personal protective equipment for patients with suspected COVID-19. Clin Med 2020; 20:e154–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Couper K, Taylor-Phillips S, Grove A, et al. COVID-19 in cardiac arrest and infection risk to rescuers: a systematic review. Resuscitation 2020; 151:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perkins GD, Morley PT, Nolan JP, et al. International Liaison Committee on Resuscitation: COVID-19 consensus on science, treatment recommendations and task force insights. Resuscitation 2020; 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiguchi T, Okubo M, Nishiyama C, et al. Out-of-hospital cardiac arrest across the World: first report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020; 152:39–49. [DOI] [PubMed] [Google Scholar]
- 67.Perman SM. Overcoming fears to save lives: COVID-19 and the threat to bystander CPR in out-of-hospital cardiac arrest. Circulation 2020; 142:1233–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scquizzato T, Olasveengen TM, Ristagno G, et al. The other side of novel coronavirus outbreak: Fear of performing cardiopulmonary resuscitation. Resuscitation 2020; 150:92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪.Grunau B, Bal J, Scheuermeyer F, et al. Bystanders are less willing to resuscitate out-of-hospital cardiac arrest victims during the COVID-19 pandemic. Resusc Plus 2020; 4:100034. [DOI] [PMC free article] [PubMed] [Google Scholar]; An international social media study with 1,360 respondents in 26 countries assessing the willingness of bystanders to provide resuscitation for OHCA. Compared to the prepandemic period, there were significant decreases in willingness to check for a pulse, perform chest compressions, provide rescue breaths, or apply an automatic external defibrillator during the COVID-19 pandemic.
- 70.Elmer J, Okubo M, Guyette FX, et al. Indirect effects of COVID-19 on OHCA in a low prevalence region. Resuscitation 2020; 156:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bobrow BJ, Panczyk M, Subido C. Dispatch-assisted cardiopulmonary resuscitation: the anchor link in the chain of survival. Curr Opin Crit Care 2012; 18:228–233. [DOI] [PubMed] [Google Scholar]
- 72.Ringh M, Rosenqvist M, Hollenberg J, et al. Mobile-phone dispatch of laypersons for CPR in out-of-hospital cardiac arrest. N Engl J Med 2015; 372:2316–2325. [DOI] [PubMed] [Google Scholar]
- 73▪.Kramer DB, Lo B, Dickert NW. CPR in the Covid-19 era – an ethical framework. N Engl J Med 2020; 383:e6. [DOI] [PubMed] [Google Scholar]; A perspective on CPR during the COVID-19 pandemic made specific recommendations for crisis standards for CPR including an acknowledgement of resource limitations when discussion code status and goals of care, forgoing CPR in certain circumstances, and altering resuscitation practices to ensure the safety of healthcare personnel.
- 74▪▪.Kirkpatrick JN, Hull SC, Fedson S, et al. Scarce-resource allocation and patient triage during the COVID-19 pandemic: JACC review topic of the week. J Am Coll Cardiol 2020; 76:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of ethical principles and values guiding decisions about triaging and allocating limited resources during crisis standards of care related to the COVID-19 pandemic.
- 75▪▪.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020; 382:2049–2055. [DOI] [PubMed] [Google Scholar]; A perspective discussing the ethical considerations underlying the fair allocation of scarce medical resources during the COVID-19 pandemic. The authors make specific recommendations including maximizing the benefit of available resources, prioriting front-line healthcare workers, avoiding first-come first-served resource allocation, remaining responsive to changes in scientific evidence, participation in research, and maintaining equality in care between patients with and without COVID-19.
- 76.Altevogt BM, Stroud C, Hanson SL, Hanfling D, Gostin LO. Institute of Medicine Committee on Guidance for Establishing Standards of Care for Use in Disaster Situations. National Academies Press (US) Copyright 2009 by the National Academy of Sciences. All rights reserved, Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations: A Letter Report. Washington (DC): 2009. [PubMed] [Google Scholar]
- 77.American Medical Association. Crisis standards of care: Guidance from the AMA Code of Medical Ethics. 2020 [Available from: https://www.ama-assn.org/delivering-care/ethics/crisis-standards-care-guidance-ama-code-medical-ethics. [Google Scholar]
- 78▪.Hsu A, Weber W, Heins A, et al. A proposal for selective resuscitation of adult cardiac arrest patients in a pandemic. J Am Coll Emerg Physicians Open 2020; 1:408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]; A proposal for a literature-based, justice-informed ethical framework for selecting treatment options for CPR during crisis standards of care.
- 79.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med 2020; 383:1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]


