Abstract
Rotator cuff tears involving the musculotendinous junction with a significant amount of tendon still attached to the footprint laterally represent a challenging scenario for shoulder arthroscopists. Because of these challenges, adjunctive techniques to bridge tissue gaps may be required, and biologic augmentation may be considered to improve the healing environment. The following technique presents a stepwise approach to accomplishing the dual goals of a stable anatomic repair and biologic augmentation of this difficult pattern of rotator cuff pathology.
Technique Video
Video 1 demonstrates the technique for arthroscopic rotator cuff repair with bone marrow concentrate dermal allograft augmentation.
The most common pattern of rotator cuff tear involves an avulsion of the supraspinatus and/or infraspinatus and teres minor from the greater tuberosity. Less commonly, the full thickness tears also can occur at, or close to, the musculotendinous junction. A recent study by Walcott et al.1 reports these types of tears occurred in only 9/502 (1.79%) patients over a 3-year span. This pattern of tear often leaves a significant amount of tendon still attached to the lateral footprint. This presents a more challenging clinical entity because debridement of the lateral tendon stump may leave inadequate tissue for primary repair or create a nonanatomic repair with increased tension on a primary repair of the medial tendon directly to the greater tuberosity. Conversely, retention of the tendon stump laterally requires tendon-tendon healing which is biologically less favorable.
Options for arthroscopic management include medialization of the footprint, side-to-side convergence stitches, tendon-tendon repair, or some combination of these.1, 2, 3, 4, 5 The technique we describe is a combination of a tendon-tendon repair with augmentation and acellular dermal allograft infused with bone marrow aspirate concentrate (BMAC). It accomplishes our goals of stable, anatomic repair with biologic augmentation of this difficult pattern of rotator cuff pathology.
Surgical Technique
Patient Positioning and Diagnostic Arthroscopy
The surgical technique is demonstrated in Video 1. A schematic of graft placement is demonstrated in Figure 1. The patient receives an upper extremity interscalene block before administration of general anesthesia, and examination is performed with the patient under anesthesia while supine with comparison to contralateral shoulder. The patient is then positioned in the beach chair position, and standard anatomic landmarks are identified.
Fig 1.
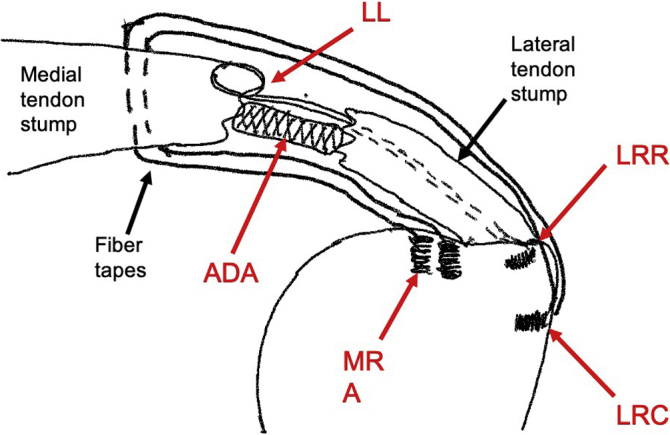
Left shoulder, coronal plane. Schematic demonstrates two medial row anchors (MRA) with FiberTape sutures passed through the tendon proximal to the musculotendinous junction. A single looped locking suture (LL) is shown for simplicity with its distal ends passed through the tendon stump exiting laterally where it is reduced to a lateral reduction anchor (LRR). After provisional reduction to the LRR anchor, the FiberTape suture tails are fixed to a lateral row compression anchor (LRC) (only one is demonstrated for simplicity) as in standard SpeedBridge technique. Together the construct creates a “suture cage” where the acellular dermal allograft (ADA) is secured between the tendon edges to promote a healing response.
A standard posterior portal is used to enter the glenohumeral joint, and diagnostic arthroscopy is performed. If associated pathology of the superior labrum, long head of the bicep tendon, or chondromalacia of the glenoid and humeral head are identified, these are treated concomitantly as indicated. In the case of significant preoperative stiffness, capsular releases may be performed anteriorly and posteriorly to allow for better mobilization of the glenohumeral joint. The subscapularis tendon is examined and repaired as necessary.
BMAC Harvest
One option for harvest of BMAC is from the proximal humerus (Fig 2). The bone marrow aspirate is prepared according to manufacturer instructions using the Angel system (Arthrex Inc., Naples, FL). After preparation of the BMAC, the final product is used to infuse the acellular dermal allograft, ArthroFLEX (Arthrex Inc.), in a small sterile basin on the back table. The graft is covered with a sterile blue towel until implantation.
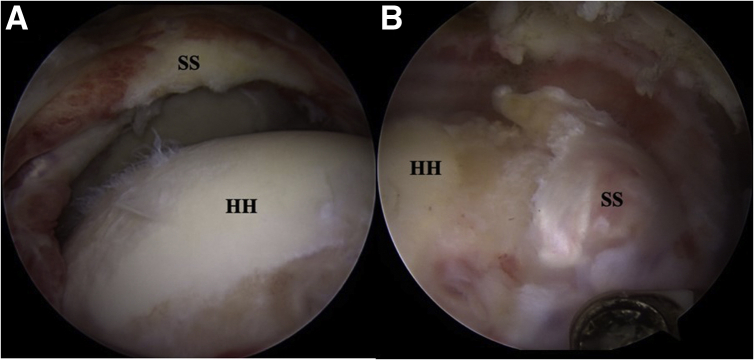
Fig 2.
A, Right shoulder posterior view, demonstrating the exposed humeral head and the exposed medial supraspinatus stump. B, Same shoulder posterior view, demonstrating the right lateral tendon supraspinatus stump. SS, Supraspinatus tendon; HH, humeral head.
Confirmation of Tear Pattern
When indicated, a standard bursectomy and subacromial decompression is then performed with a standard midlateral portal. The camera is then placed through the lateral portal to properly examine the tear pattern, perform releases, and mobilize the tissue, as well as to confirm the transtendinous pattern including the remnants of the supraspinatus or infraspinatus still attached to the greater tuberosity (Fig 3). Posterolateral and anterolateral portals are created for visualization and suture management during the repair.
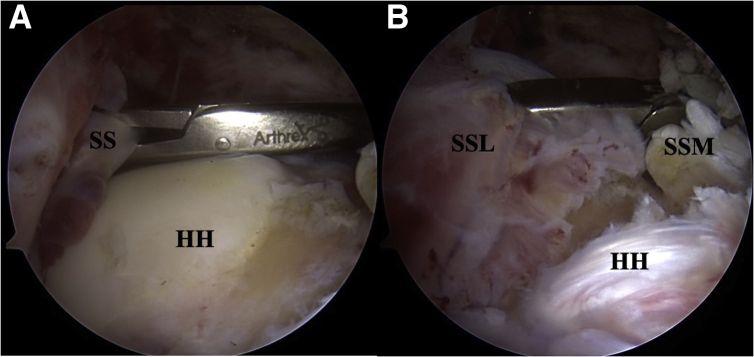
Fig 3.
A, Right shoulder posterior view. An arthroscopic cuff grasper is introduced in from the lateral portal. B, A trial reduction is performed reducing the medial supraspinatus (SSM) to the lateral tendon stump (SSL). SS, Supraspinatus tendon; HH, humeral head.
Placement of Medial Row Anchors
Next, 3 medial row knotless suture anchors, 4.75 mm Bio-composite SwiveLock (Arthrex Inc.), loaded with SutureTape (Arthrex Inc.) are placed just lateral to the articular margin. These three anchors serve as the medial row just medial to the rotator cuff tissue remnant laterally (Fig 4). The FiberTape (Arthrex Inc.) is then passed through the rotator cuff tissue anteriorly, centrally, and posteriorly using a suture passage device being attentive to including any delaminated portions of the superior capsular tissue. This is consistent with a standard SpeedBridge construct technique.
Fig 4.
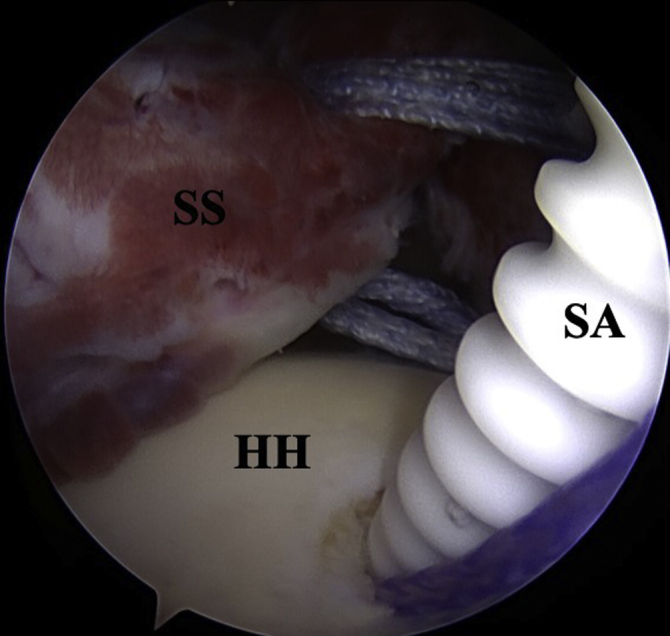
Right shoulder posterior view, the footprint of the greater tuberosity is medialized and the suture anchor is placed just medial to the lateral tendon stump. SA, Suture anchor; SS, supraspinatus tendon; HH, humeral head.
Placement of Locking Reduction Anchors
Locking looped reduction sutures (FiberLink; Arthrex Inc.) are then placed with a suture passing device into the leading edge of the remaining medial tendon. These sutures are cinched over the medial tendon stump as has been previously described.6 Next, the free ends of the locked reduction sutures are shuttled through the lateral portion of the cuff remaining on the greater tuberosity utilizing a SutureLasso (Arthrex Inc.) (Fig 5). Attention is taken to optimize reduction of the intratendinous tear just lateral to the musculotendinous junction. The sutures should pass through the midportion of the lateral tendon stump rather than exiting inferiorly or superiorly, which could result in malreduction of the medial tissue during final tensioning.
Fig 5.
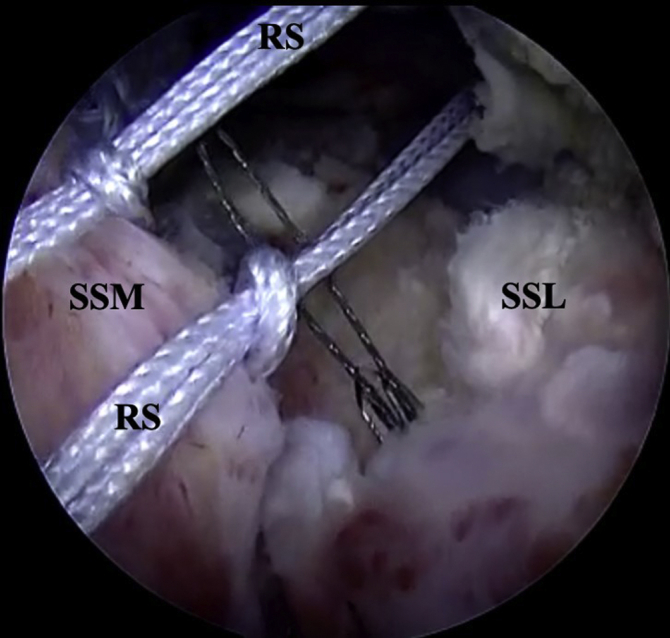
Right shoulder posterior view, previously passed locked reduction sutures are shuttled through the lateral tendon stump with a SutureLasso. RS, Reduction suture; SSM, medial supraspinatus tendon; SSL, lateral supraspinatus tendon.
Placement of Dermal Allograft
A 30° PowerPick (Arthrex Inc.) is then used to microfracture the greater tuberosity to stimulate a healing response (Fig 6). The BMAC infused acellular dermal allograft (ArthroFLEX; Arthrex Inc.) is then carefully introduced into the subacromial space and placed between the medial tendon stump medially, the lateral tendon stump laterally, the greater tuberosity and medial row (FiberTape; Arthrex Inc.) inferiorly, and the locking reduction loops superiorly. This creates a “sandwich” effect of the biologic augmentation at the site of the side to side rotator cuff repair (Fig 7).
Fig 6.
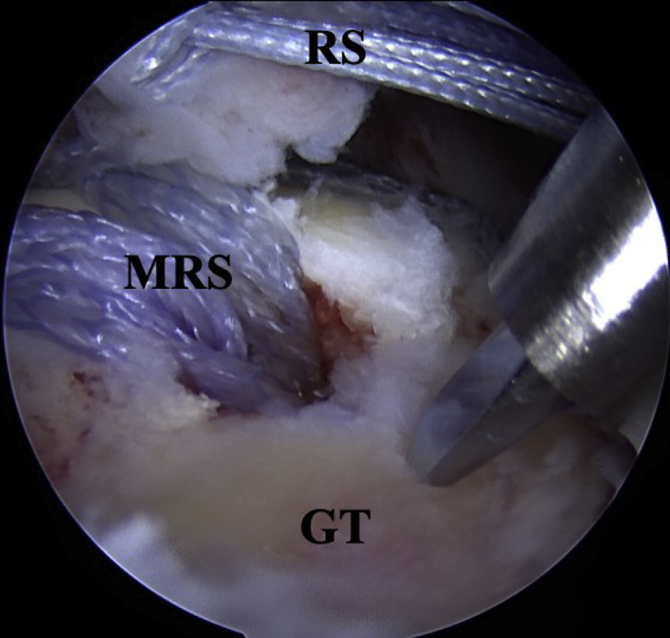
Any remaining exposed footprint is microfractured to stimulate a healing response. RS, Reduction suture; MRS, medial row suture; GT, greater tuberosity.
Fig 7.
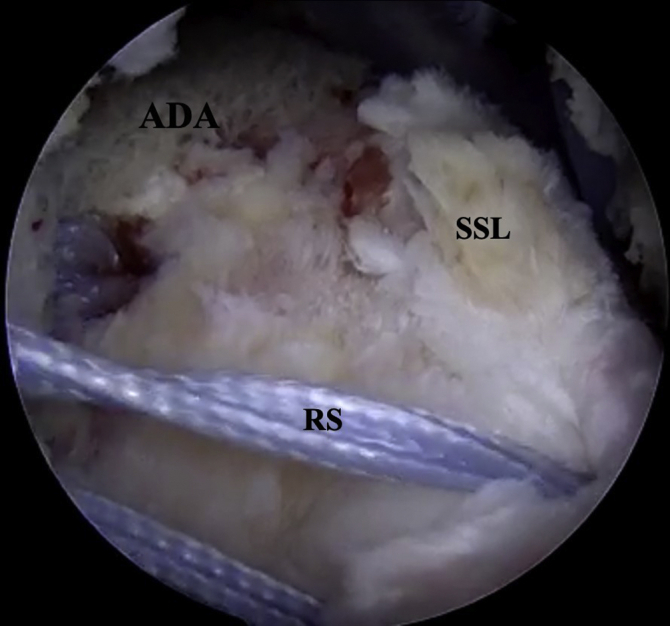
Right shoulder posterior view, the acellular dermal allograft is placed at the anatomic footprint between the medial reduction suture and the lateral reduction suture. ADA, Acellular dermal allograft; RS, reduction suture; SSL, lateral supraspinatus tendon.
Final Reduction and Fixation
The locking looped reduction sutures are attached laterally to a central reduction to reduce the rotator cuff and graft to the greater tuberosity as has been previously described.6 The remaining FiberTapes are then placed in a crisscrossed fashion to lateral knotless anchors, SwiveLock (Arthrex Inc.), completing a double-row SpeedBridge construct to add compression to the repair (Fig 8).
Fig 8.
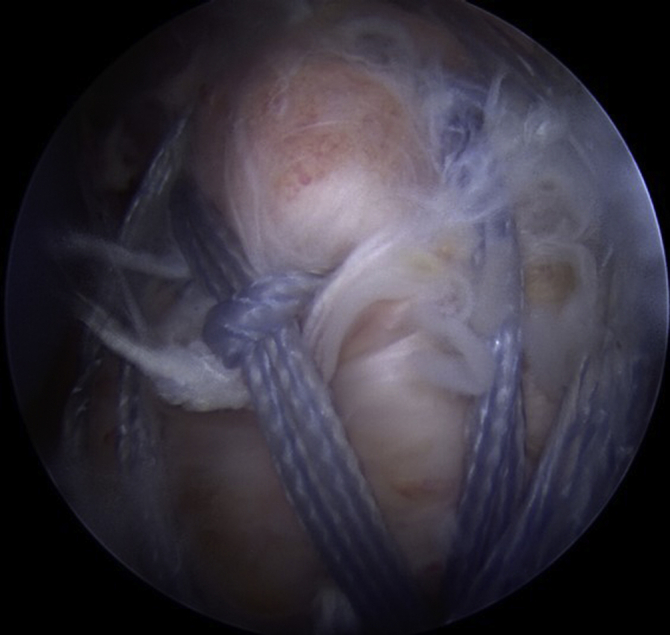
Right shoulder, lateral view. The final fixation construct is pictured.
Postoperative Rehabilitation
After surgery, the patient is placed in a brace in neutral position with a small abduction pillow to protect the repair. Full active range of motion to the elbow, wrist and hand are allowed immediately. The patient wears the sling at all times, except while showering and during formal physical therapy, for the first 6 weeks. The patient is not allowed to perform any active range of motion nor passive abduction for 6 weeks, consistent with a conservative massive rotator cuff repair protocol. The patient is instructed to perform closed chain passive table slides and scapular stabilization exercises for 1 month. After 6 weeks the patient will wean from the sling and begin active and passive range of motion exercises and introducing strengthening around 3 months after surgery.
Discussion
Transtendon rotator cuff tears are a rare and challenging clinical entity. When recognized, the next challenge is to preserve and incorporate the lateral tendon still attached to the footprint. Failure to incorporate the lateral tendon can result in overtensioning of the medial tendon to the anatomic footprint. The described technique combines a side-to-side repair of the medial and lateral tendons with a modified double row transosseous rotator cuff repair. Finally, the addition of BMAC and acellular dermal allografts improve the biomechanical and biologic environment for definitive healing.
Walcott et al.1 reported a case series of transtendinous rotator cuff tears with an average remaining stump tendon of at least 1 cm who underwent an arthroscopic repair with side-to-side (tendon-tendon) technique. They reported acceptable clinical outcome scores, with their technique preserving the lateral tendon stump.1 Other authors have suggested variations on the tendon-tendon repair technique with some modifications.2,3 In contrast to our presented technique, these authors do not discuss augmentation in their surgical techniques. Given the biologic challenges of tendon-tendon healing, it is reasonable to consider supplementing the primary repair.
Because of the inherent challenges and poor outcomes with primary repair of large to massive rotator cuff tears, the use of dermal allografts has emerged as an increasingly common treatment adjunct. Hall et al.7 presented a retrospective series of patients with rotator cuff tears at the musculotendinous junction who underwent arthroscopic repair with dermal allograft. At 2 years after operation, the repairs all remain intact.7 Others have presented improvement in clinical outcomes scores with dermal allograft through a mini-open approach.8 Our technique builds on these previous techniques by combining an arthroscopic approach to transtendinous tears with an all-arthroscopic augmentation with dermal allograft.
Literature regarding the use of BMAC in rotator cuff repair is limited. Hernigou et al.,9 in a small case-controlled series, suggested that BMAC harvested from the iliac crest may increase the rate healing of repairs, as well as prevent further ruptures at 10-year follow-up. Similarly, Muench et al.10 demonstrated improvements in clinical outcomes with BMAC and platelet-rich plasma in the setting of massive rotator cuff tears. BMAC from the proximal humerus can be aspirated effectively and with no substantial rises in postoperative complications and has demonstrated the presence of osteogenic progenitor cells.11 Harvesting BMAC during subpectoral biceps tenodesis allows the surgeon to exploit the same drill hole in the bicipital groove. There is a reasonable rationale for obtaining BMAC at the surgical site because of the limited morbidity to the patient and convenience for the treating surgeon.
The primary advantage to the described technique is that, by combining the procedures, they can successfully be performed in one arthroscopic setting. This is technically challenging and requires significant arthroscopic skill in suture management and advanced arthroscopic techniques. Other potential drawbacks to this procedure are the costs associated with allograft tissue and BMAC. Finally, arthroscopic passage of the allograft can be challenging and may require to a mini-open approach.
In conclusion, transtendon rotator cuff tears represent a unique injury pattern that can be challenging to recognize and treat. We present a reproducible technique for arthroscopic repair and biologic augmentation.
Table 1.
Pearls and Pitfalls
| Pearls |
| Consider BMAC harvest from the proximal humerus, the subpectoral tenodesis site provides a convenient harvest location with minimal added morbidity. |
| Utilize knotless suture anchors for augmenting the rotator cuff repair. This can be done at the anterior and posterior peripheral edges of the repair. |
| The use of a central lateral reduction anchor facilitates reduction and allows for even compression when passing the lateral row of knotless anchors for completion of the SpeedBridge construct. |
| By obtaining the BMAC early in the procedure, this allows for more time to soak the acellular dermal allograft during other portions of the procedure. |
| The presoaked dermal allograft is placed in between the layers of suture in the repair construct in a secure location, providing a “sandwich” effect of biologic augmentation. |
| Pitfalls |
| Placement of acellular dermal allograft can be challenging. Consider using additional sutures or holding the graft in place with a grasper while the lateral row is tensioned. |
| Reduce the tear based on tear pattern, as anatomically as possible, malreduction increases the risk of overtensioning the repair and may compromise outcomes. |
BMAC, Bone marrow aspirate concentrate.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.B.G. is a consultant for Arthrex, has stock in ROM3, stock options in Doximity, is on a clinical advisory board for Smart Medical Devices, and received institutional support from Synthes/DePuy, Stryker, Pacific Medical, Smith and Nephew, outside the submitted work. D.G. reports other from Arthrex, other from Medacta, other from Smith & Nephew, other from Stryker, other from DJO, other from Breg, other from FX, other from Zimmer, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Video 1 demonstrates the technique for arthroscopic rotator cuff repair with bone marrow concentrate dermal allograft augmentation.
References
- 1.Walcott M.E., Daniels S.D., Sinz N.J., Field L.D., Higgins L.D. Traumatic full-thickness transtendinous rotator cuff tears: a case series. J Shoulder Elbow Surg. 2017;26:62–67. doi: 10.1016/j.jse.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Kelly B.J., Field L.D. Arthroscopic repair of medial transtendinous rotator cuff tears. Arthrosc Tech. 2017;6:e2217–e2221. doi: 10.1016/j.eats.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S., Trenhaile S. Arthroscopic repair of rare transtendinous rotator cuff tear: utilizing established portals and a posterior superior accessory portal. Arthrosc Tech. 2019;8:e419–e422. doi: 10.1016/j.eats.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clavert P., Le Coniat Y., Kempf J.F., Walch G. Intratendinous rupture of the supraspinatus: anatomical and functional results of 24 operative cases. Eur J Orthop Surg Traumatol. 2016;26:133–138. doi: 10.1007/s00590-015-1716-0. [DOI] [PubMed] [Google Scholar]
- 5.Park S.E., Panchal K., Jeong J.J., Kim Y.Y., Kim J.H., Lee J.Y., Ji J.H. Intratendinous rotator cuff tears: prevalence and clinical and radiological outcomes of arthroscopically confirmed intratendinous tears at midterm follow-up. Am J Sports Med. 2015;43:415–422. doi: 10.1177/0363546514556741. [DOI] [PubMed] [Google Scholar]
- 6.Lee B.D., Gilmer B.B., Lang S.D., Guttmann D. A modified SpeedBridge technique for retracted or delaminated rotator cuff repairs. Arthrosc Tech. 2019;8:e1373–e1378. doi: 10.1016/j.eats.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall T., Danielson K., Brandenburg S., Matelic T. A case series of recurrent musculotendinous rotator cuff tears repaired and augmented with dermal allograft: clinical outcomes at two years. J Shoulder Elbow Surg. 2020;29:2264–2271. doi: 10.1016/j.jse.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A.K., Hug K., Berkoff D.J. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40:141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 9.Hernigou P., Lachaniette C.H., Delambre J. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 10.Muench L.N., Kia C., Jerliu A. Clinical outcomes following biologically enhanced patch augmentation repair as a salvage procedure for revision massive rotator cuff tears. Arthroscopy. 2020;36:1542–1551. doi: 10.1016/j.arthro.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Mazzocca A.D., McCarthy M.B., Chowaniec D.M., Cote M.P., Arciero R.A., Drissi H. Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. Am J Sports Med. 2010;38:1438–1447. doi: 10.1177/0363546509360924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 demonstrates the technique for arthroscopic rotator cuff repair with bone marrow concentrate dermal allograft augmentation.
Video 1 demonstrates the technique for arthroscopic rotator cuff repair with bone marrow concentrate dermal allograft augmentation.