Abstract
Background and Purpose:
Physical exercise offers therapeutic potentials for several central nervous system disorders, including stroke and cardiovascular diseases. However, it is still mostly unknown whether and how exercise preconditioning affects the prognosis of intracerebral hemorrhage (ICH). In this study, we examined the effects of preconditioning on ICH pathology in mature adult mice using treadmill exercise.
Methods:
Male C57BL/6J (25-week old) mice were subjected to 6 weeks of treadmill exercise followed by ICH induction. Outcome measurements included various neurological function tests at multiple time points and the assessment of lesion volume at 8 days after ICH induction. In addition, plasma soluble factors and phagocytotic microglial numbers in the peri-lesion area were also measured to determine the mechanisms underlying the effects of exercise preconditioning.
Results:
The 6-week treadmill exercise preconditioning promoted recovery from ICH-induced neurological deficits in mice. In addition, mice with exercise preconditioning showed smaller lesion volumes and increased numbers of phagocytotic microglia. Furthermore, the levels of several soluble factors, including endostatin, IGFBP-2 and -3, MMP-9, osteopontin and pentraxin-3, were increased in the plasma samples from ICH mice with exercise preconditioning compared to ICH mice without exercise.
Conclusion:
These results suggest that mice with exercise preconditioning may suffer less severe injury from hemorrhagic stroke, and therefore, a habit of physical exercise may improve brain health even in aging people.
Keywords: Intracerebral hemorrhage, treadmill exercise, preconditioning, recovery, phagocytosis
Introduction:
Intracerebral hemorrhage (ICH) is one of the major types of stroke, which is characterized by rupture of arterial blood vessels and formation of hematoma within the brain parenchyma. ICH causes severe neurological deficits and high mortality and is considered a life-threatening and disabling event 1. Recent clinical and pre-clinical studies have demonstrated the efficacy of physical activity in suppressing the progression of ICH 2, 3. However, because ICH patients may suffer from neurological deficits, physical exercise is sometimes difficult after brain injury. In this study, we used a collagenase-induced striatum ICH model, a well-validated mouse model of ICH, to examine how preconditioning with treadmill exercise ameliorates ICH-induced brain injury.
Materials and methods:
Data Transparency -
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Research Design -
The experimental schedule is summarized in Figure 1a. 25-week old mice were randomly separated into 2 groups (sedentary, n=18; treadmill, n=11) at 6 weeks before intracerebral hemorrhage (ICH) induction. Following a week-long habituation period of treadmill exercise, mice in the treadmill group were placed on a treadmill where they ran at a maximum speed of 10 m/min for 60 min/day for 5 weeks on weekdays. The mice in the sedentary group were placed on the treadmill without running for the same duration as the treadmill group and were randomly separated into 2 groups (sham, n=8; ICH, n=10) before starting the ICH operation. ICH was induced by a microinjection of collagenase into the striatum region. Behavioral tests (beam-walking test, adhesive removal test and corner-turning test) were conducted at 1 day before and 1, 3 and 7 days after ICH induction. Body weight was measured daily from 1 day before ICH induction until the day of sacrifice. At 8 days after ICH induction, all mice were sacrificed; brain samples were used for histological examinations, and blood samples were used for an antibody-based protein array experiment. All experimental procedures were reviewed and approved by a Subcommittee for Research Animal Care of the Massachusetts General Hospital IACUC, and we used an institutionally approved animal protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory. Please see our Supplementary Information for detailed procedures of our experiments.
Figure 1. Experimental design, body weight changes and injury volume after ICH induction.
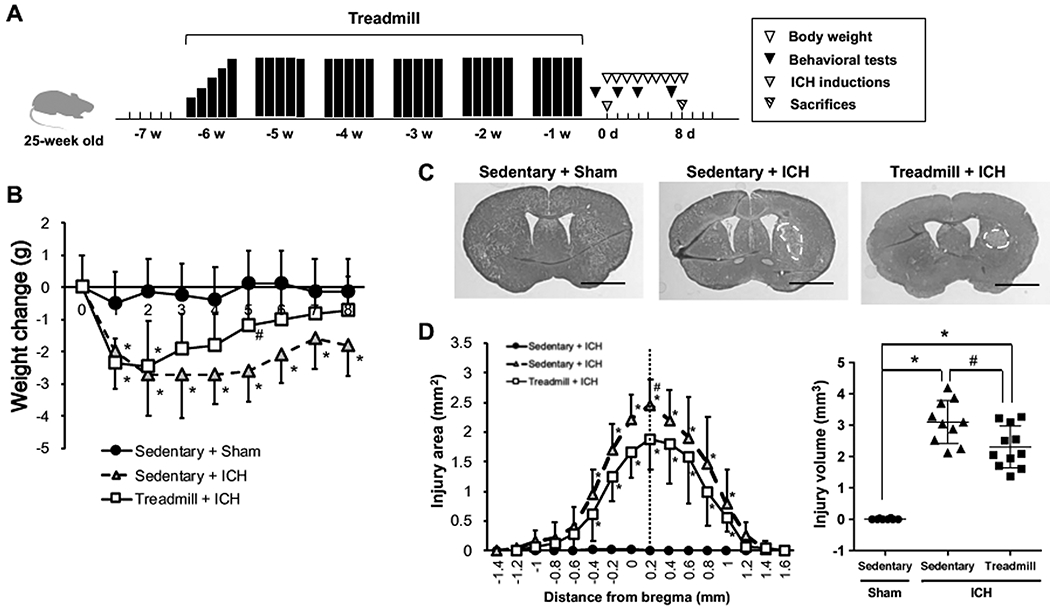
(a) Schematic overview for research design of this study. (b) Body weight changes after ICH induction. (c) Representative images of Nissl-stained coronal sections obtained at 8 days after ICH induction in Sedentary+Sham (left), Sedentary+ICH (middle) and Treadmill+ICH (right). Scale bars = 3 mm. (d) Quantitative results of lesion area (left) and volume (right) at 8 days after ICH induction. Data are expressed as mean ± SD. N=8 for Sedentary+Sham, N=10 for Sedentary+ICH, and N=11 for Treadmill+ICH. *P < 0.05 vs Sedentary+Sham, #P < 0.01 vs Sedentary+ICH.
Statistical Methods -
All measurements/analyses were conducted by operators who were blinded to the group allocations. Statistical analysis was conducted by unpaired t-test, one or two-way repeated-measures analysis of variance followed by post-hoc multiple comparisons test. Differences with P < 0.05 were considered statistically significant, and data were expressed as mean ± SD.
Results:
After ICH induction, mice suffered from body weight loss regardless of exercise preconditioning, but mice with exercise preconditioning showed faster recovery from ICH-induced body weight loss (Figure 1b). In addition, at 8 days after ICH induction, the lesion volume in the exercise group was significantly smaller than that of the mice in the sedentary group (Figure 1c–d). Mice with exercise preconditioning also showed a better outcome in neurological function after ICH in the beam-walking test and the adhesion tape removal test (Figure 2a–c). On the other hand, in the corner-turning test, which is performed to detect sensorimotor dysfunction, there was no difference between the sedentary/ICH and treadmill/ICH groups (Figure 2d), indicating that some neurological deficits caused by ICH may not be ameliorated by exercise preconditioning. To be noted, the control group mice (e.g. sham operation on sedentary mice) did not show any deficits in all three neurological function tests (Figure 2a–d), confirming that the habituation procedure and anesthesia did not affect mouse behavior.
Figure 2. Behavioral outcomes after ICH induction.
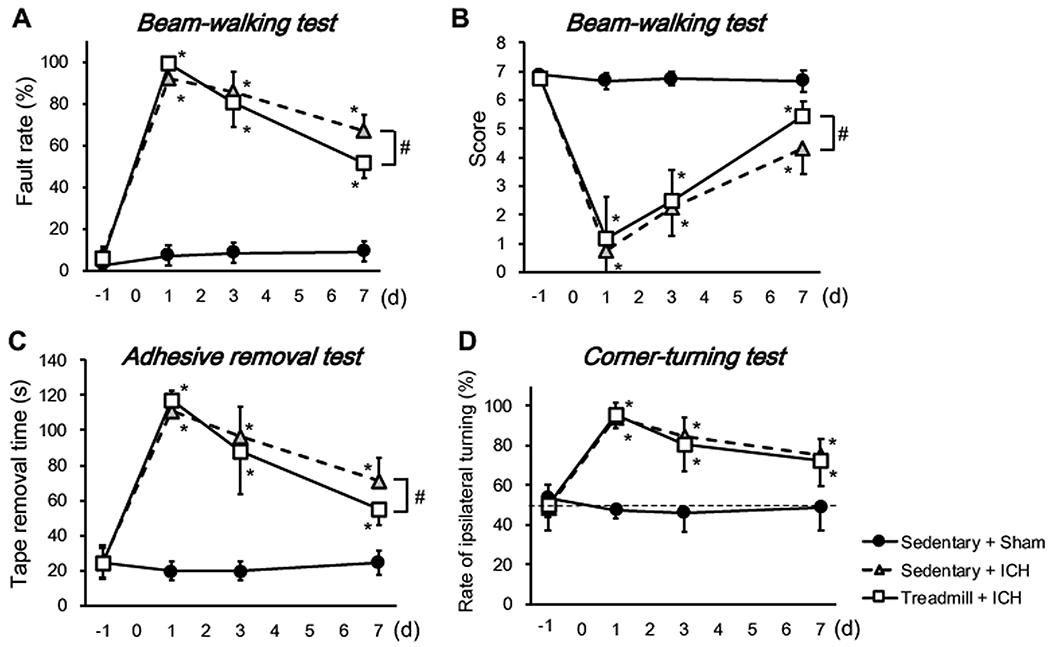
(a) The fault rate of the beam-walking test. (b) The scores of the beam-walking test. (c) The adhesive removal test. (d) The corner-turning test. Data are mean ± SD. N=8 for Sedentary+Sham, N=10 for Sedentary+ICH, and N=11 for Treadmill+ICH. *P < 0.05 vs Sedentary+Sham, #P < 0.01 vs Sedentary+ICH.
After determining that exercise preconditioning has beneficial effects on ICH recovery as evaluated by histological and behavioral assays, we asked whether there are parallel changes in biological responses in circulating blood and endothelial/glial cells. At 8 days after ICH induction, mice with exercise preconditioning had larger circulating amounts of multiple soluble factors compared to ICH mice without exercise preconditioning (Figure 3a and Supplementary Figure I). Immunostaining showed no significant differences in collagen IV (a marker of basement membrane on blood vessels) and GFAP (a marker for reactive astrocytes) in the peri-lesioned area between sedentary/ICH and exercise/ICH groups (Figure 3b–c). However, the number of CD36/Iba1-double positive cells (e.g. phagocytic microglia/macrophage) in the exercise group was significantly larger at day 8 after ICH induction (Figure 3d).
Figure 3. An angiogenesis array analysis in plasma and histological analysis.
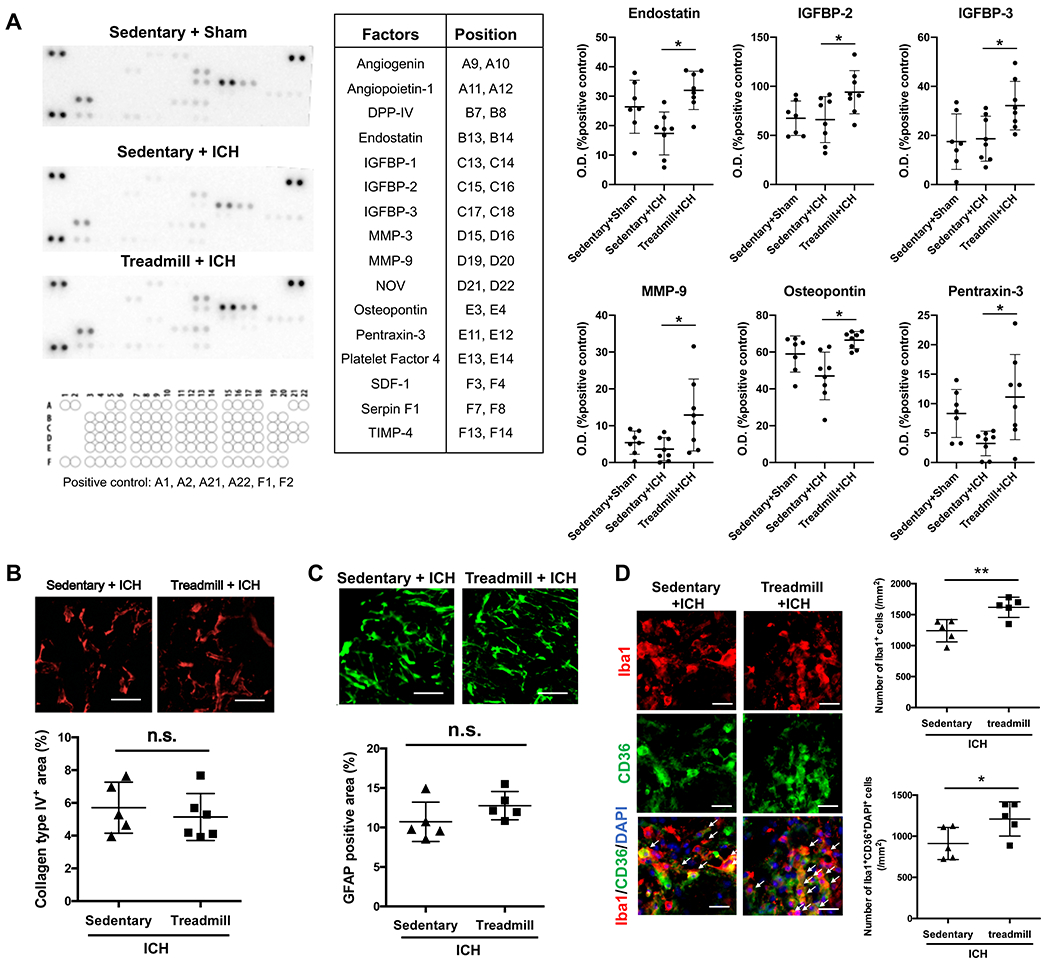
(a) Protein Array results. Representative images of membranes of protein array (left half) and quantitative results for 8 factors that showed significant differences between Sedentary+ICH vs Treadmill+ICH (right half). Data are expressed as mean ± SD. N=7 for Sedentary+Sham, N=8 for Sedentary+ICH and treadmill+ICH. *p < 0.05. Please see Supplementary Figure I for the full set of array data. (b) Collagen type IV staining in peri-hematoma region. Scale bar = 50 μm. Data are expressed as mean ± SD. N=5 for sedentary+ICH group, N=6 for treadmill+ICH group. (c) GFAP staining. Scale bars = 100 μm. Data are expressed as mean ± SD. N=5 for each group. n.s. = not significance. (d) Iba1 (green) and CD36 (red) staining in peri-hematoma region. Arrows indicate representative triple-positive cells. Scale bars = 20 μm. Data are expressed as mean ± SD. N=5 for each group. *p < 0.05.
Discussion:
In this study, we demonstrate that in mature adult mice, preconditioning with treadmill exercise (i) reduces lesion volume, (ii) promotes recovery from neurological deficits and body weight loss, (iii) increases the level of pro-survival factors in plasma, and (iv) induces the phagocytic phenotype in microglia. Because polypharmacy among elderly patients has become a serious social issue 4, our current study provides a proof-of-concept that a habit of physical exercise may offer a new therapeutic intervention to help protect brain health against hemorrhagic insults.
Lesion/hematoma volumes are correlated with functional outcome after ICH 5, 6, and microglial phagocytosis is an important mechanism of hematoma resorption after ICH 7. In our mouse ICH model, hematoma volumes decreased from day 1 to day 8, and the hematoma volume showed a correlation with the lesion volume (Supplementary Figure II). In addition, consistent with the fact that circulating factors regulate microglial responses after brain injury 8, our study confirmed that mice with exercise preconditioning showed increased levels of multiple soluble factors in plasma after ICH, including osteopontin, which is known to regulate microglial motility and phagocytosis 9. Therefore, our findings may provide an additional example of the importance of microglial involvement in ICH pathology. However, our current study is somewhat preliminary in that we lack (i) direct evidence for the effects of exercise preconditioning in hematoma resorption in ICH mice (ii) time-course experiments for the effects of exercise preconditioning in lesion volumes and glial changes after ICH, and (iii) detailed mechanism study regarding the roles of circulating factors in ICH pathology. Future studies are necessary to address these points, because gaining a better understanding of the mechanisms by which these systemic changes regulate lesion/hematoma volume through physical exercise will lead to the development of a non-pharmacological therapy for ICH.
In summary, we demonstrate that preconditioning with treadmill exercise contributes to neuroprotection against hemorrhagic stroke in mice. The findings support the idea that physical exercise may improve brain health even in aging people. To further our understandings of the efficacy of exercise preconditioning, further studies are warranted to dissect the mechanisms of how exercise preconditioning ameliorates detrimental changes in the brain as well as in circulating blood.
Supplementary Material
Acknowledgments
Sources of Funding:
Supported in part by NIH.
Non-standard Abbreviations and Acronyms:
- ICH
intracerebral hemorrhage
- IGFBP
insulin-like growth factor-binding protein
- MMP
matrix metallopeptidase
- GFAP
glial fibrillary acidic protein
Footnotes
DISCLOSURE: None
Contributor Information
Keita Kinoshita, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; Department of Chemico-Pharmacological Sciences, Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, Japan.
Gen Hamanaka, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Ryo Ohtomo, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Hajime Takase, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Kelly K. Chung, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Josephine Lok, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Eng H. Lo, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
Hiroshi Katsuki, Department of Chemico-Pharmacological Sciences, Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, Japan.
Ken Arai, Neuroprotection Research Laboratory, Departments of Radiology and Neurology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA.
References:
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato C, Tanji K, Shimoyama S, Chiba M, Mikami M, Koeda S, Sumigawa K, Akahira K, Yamada J. Effects of voluntary and forced exercises on motor function recovery in intracerebral hemorrhage rats. Neuroreport. 2020;31:189–196 [DOI] [PubMed] [Google Scholar]
- 3.Thrift AG, Donnan GA, McNeil JJ. Reduced risk of intracerebral hemorrhage with dynamic recreational exercise but not with heavy work activity. Stroke. 2002;33:559–564 [DOI] [PubMed] [Google Scholar]
- 4.Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: A systematic review. J Am Geriatr Soc. 2014;62:2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita H, Hijioka M, Hisatsune A, Isohama Y, Iwamoto S, Terasawa H, Katsuki H. Mri-based analysis of intracerebral hemorrhage in mice reveals relationship between hematoma expansion and the severity of symptoms. PLoS One. 2013;8:e67691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The func score. Stroke. 2008;39:2304–2309 [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: The role of microglia/macrophages. Stroke. 2009;40:S92–94 [DOI] [PubMed] [Google Scholar]
- 8.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim ID, Lee H, Jin YC, Lee JK. Osteopontin peptide icosamer containing rgd and slayglr motifs enhances the motility and phagocytic activity of microglia. Exp Neurobiol. 2017;26:339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


