Abstract
Background:
Sarcopenia is associated with adverse outcomes among older adults with cancer; however, no easily applied sarcopenia measure exists for use in clinical practice. The use of SARC-F, a 5-item self-reported sarcopenia screening questionnaire, among older adults with cancer remains to be investigated.
Methods:
We identified older adults ≥60y with cancer enrolled in the UAB Cancer & Aging Resilience Evaluation (CARE) Registry. Patients completed the SARC-F questionnaire (score of ≥4 positive for sarcopenia). We assessed for differences in GA domain impairments, Health related Quality of Life (HRQoL), and health-care utilization between those with and without sarcopenia using multivariate regression, then assessed the association of sarcopenia with survival using Kaplan-Meier methods and a Cox regression model, adjusting for covariates.
Results:
We identified 256 older adults, median age 69y, 59% male, and 75% whites. The median SARC-F score was 2 (Interquartile range 0–4), and 33% of participants screened positive. Those with sarcopenia had higher odds of having multiple impairments including impaired instrumental activities of daily living (aOR 18.1, 95% CI 7.5–43.8) and frailty (aOR 43.5, 95% CI 17.7–106.8) as well as reduced physical and mental HRQoL (β coefficient −13.6 and −11.5, respectively) and increased ER visits (aOR 2.4, 95% CI 1.3–4.7). Furthermore, sarcopenia was independently associated with inferior overall survival (aHR 2.98; 95% CI 1.1–8.3, p=0.04).
Conclusions:
A third of older adults with cancer in our cohort screened positive for sarcopenia using the SARC-F screening tool that was associated with GA domain impairments, reduced HRQOL, increased ER visits, and inferior overall survival.
Keywords: sarcopenia, SARC-F, geriatric assessment, geriatric oncology, aging, cancer
Precis:
A third of older adults with cancer in our cohort screened positive for sarcopenia using the SARC-F screening tool. Sarcopenia was associated with GA domain impairments, reduced HRQOL, increased ER visits, and inferior overall survival.
Introduction
Age-related loss of muscle mass and strength, known as sarcopenia, is highly prevalent in older adults and is associated with functional impairment, disability, loss of independence, and mortality 1–5. Losses in skeletal muscle mass and strength are apparent as early as the 4th decade of life and progress linearly with increasing age 6. In older adults with cancer, age-related sarcopenia is further complicated by cancer-related malnutrition and inflammation as well as cancer treatment 7,8. The elevated inflammatory response and alterations in metabolism related to cancer cachexia lead to further losses of muscle mass 7,9. Older adults have accelerated losses of appendicular lean mass as compared to non-cancer age-matched controls 10. As sarcopenia in cancer is associated with increased chemotherapy toxicities, disability, and reduced survival, further understanding and recognition of sarcopenia in this population is warranted 10–13.
Among patients with cancer, sarcopenia is typically assessed from routine Computed Tomography (CT) imaging using measurements of muscle from a single cross-sectional slice 14,15. While, low muscle measures obtained using this method have been shown to be associated with an array of adverse outcomes, this information is not yet readily available within the clinic for use in clinical decision-making and only applicable for select patient populations where CT imaging is recommended as part of routine care. Recognizing the challenges of measuring sarcopenia in routine care, the European Working Group on Sarcopenia in Older People (EWGSOP) now recommends use of SARC-F, a five-item self-reported screening questionnaire, for rapid screening of sarcopenia in older adults 16–19. SARC-F has now been validated in several non-cancer cohorts with moderate sensitivity and excellent specificity (94–99%) 17,19,20, yet, no studies have examined the utility of this tool among patients with cancer.
We sought to address this gap in a prospective cohort study of older adults ≥60y with cancer. Our goals of this exploratory study were to a) describe the results of the SARC-F screening measure in this population, 2) examine the prevalence of sarcopenia estimated using SARC-F, 3) evaluate the association between SARC-F-identified sarcopenia and geriatric assessment-identified impairments, health-related quality of life (HRQOL), and health-care utilization, and 4) examine whether SARC-F-identified sarcopenia is associated with mortality.
Methods
Study Population
We used the University of Alabama at Birmingham (UAB) Cancer & Aging Resilience Evaluation (CARE) study to address our study goals. CARE is an ongoing prospective registry enrolling older adults (≥60y) undergoing cancer care at UAB Hospitals and Clinics 21. The age of 60 and older was chosen given the uncertainty of the appropriate age cut-off for employing a geriatric assessment in adults with cancer and prior results have shown similar rates of geriatric assessment identified impairments across older age groups (60–64y, 65–74y, and ≥75y) 22. Since September 2017, over 900 participants have enrolled with both solid tumors and hematologic malignancies. For the current analysis, we included patients enrolled between May 2019 and April 2020, to coincide with the time when SARC-F questionnaire added to the CARE registry. This study was approved by the Institutional Review Board of University of Alabama at Birmingham (IRB-300000092) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
SARC-F Questionnaire
We used the five item SARC-F measure, originally developed in 2013 by Malmstrom and Morley as a screening tool for diagnosing sarcopenia 18. The SARC-F measure consists of the following five components: strength, assistance in walking, rising from a chair, climbing stairs, and falls. Each item is scored between 0 and 2 points yielding a total score from 0 to 10; 0 being the best and 10 being the worst. A score of four or greater is highly sensitive and specific for sarcopenia and predictive of poor outcomes based on prior studies in non-oncology populations 16,18,19.
Geriatric Assessment and Frailty Measures
All participants enrolled in the CARE study undergo a brief patient-reported geriatric assessment using the CARE survey as outlined in the eTable 1 in the Supplement 21. Briefly, the CARE survey is a modified version of the original Cancer and Aging Research Group (CARG) geriatric assessment, augmented to streamline completion and aid in integration into routine oncologic clinical practice. The CARE survey measures activities of daily living (ADL), instrumental ADL (iADL), ability to walk one block, number of falls within 6 months, nutrition, comorbid conditions, cognitive complaints, anxiety, depression, social support, social activities, and self-rated Eastern Cooperative Oncology Group Performance Status (ECOG PS). Domain-specific cut-offs were utilized in accordance with prior literature 21,23–25. Frailty was reported using the CARE Frailty Index based on the principles of deficit accumulation as previously published 26,27. In brief, the CARE Frailty Index includes 44 GA variables identified as health deficits from the CARE survey, each of which are scored as “0” (no deficit) or “1” (deficit present) (Supplement). These values were then combined and divided by the overall number of deficits assessed (range 0–1), and categorized as robust (0–0.2), pre-frail (0.2–0.35), and frail (>0.35) 26. In addition, the CARE survey incorporates the PROMIS 10-item Global Health questionnaire to assess HRQoL, which includes physical and mental Health subscales 28,29.
Additional Variables:
Information on vital status was obtained by linking the data to Accurint database30 and additionally supplemented by review of patient medical records. Vital status information was updated to 6/12/2020. We measured prior healthcare utilization based on participant’s self-report of emergency room (ER) visit or hospitalization in the past year 31. Race, ethnicity, education level, marital status, and employment were obtained by self-report as part of the CARE survey, while cancer stage, cancer type, date of diagnosis, and treatment phase (i.e. pre- or during chemotherapy) were abstracted from review of the electronic medical record.
Statistical Analyses
We used descriptive statistics to examine the demographic and clinical characteristics of participants. We measured the proportion of patients screening positive for sarcopenia and computed its 95% confidence interval using exact binomial (Clopper-Pearson) method 32. Group differences in demographic, clinical, geriatric assessment domain impairments, frailty, HRQOL, and healthcare utilization were examined using appropriate bivariate statistical tests, i.e., Analysis of Variance/Kruskal Wallis test for continuous variables and Chi-squared test/Fisher’s exact test for categorical variables depending on their distribution. Univariate and multivariable logistic regression models were used to examine the association of sarcopenia with geriatric assessment impairments, frailty, and healthcare utilization, reporting odds ratios (ORs) and 95% confidence intervals (CIs). Multiple linear regression was used to study the impact of sarcopenia on physical and mental domains of HRQoL using normalized PROMIS T-scores as the outcome variable. We used Kaplan Meier methods to compare the overall survival distributions between those with and without sarcopenia based on the SARC-F questionnaire and a multivariable Cox regression model, adjusting for age, sex, race, cancer type, cancer stage, and treatment phase. All statistical tests were 2-sided with an alpha < .05 considered statistically significant. We conducted statistical analyses using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC) and STATA 16.0 (StataCorp LLC, College Station, TX).
Results
Between May 2019 and April 2020, 256 were enrolled into the CARE registry and completed the SARC-F questionnaire. The median age of this cohort was 69y (range 60–96y) with 59% male and 75% non-Hispanic white. Common cancers included pancreatic (18%), colorectal (16%), and hepatobiliary (11%); most of the cancers were stage III (29%) or IV (54%) at diagnosis. The median time interval between cancer diagnosis and study participation was 35 days (interquartile range [IQR], 19–92 days) (Table 1).
Table 1.
Demographic and Cancer Characteristics, Overall and by Sarcopenia Status
| Variable | All patients | Sarcopenic | P-value* | |
|---|---|---|---|---|
| No | Yes | |||
| Total Patients | N= 256 | N= 171 | N= 85 | |
| Age, median (IQR) | 69 (64, 74) | 70 (63, 73) | .72 | |
| Sex, n (%) | ||||
| Male | 152 (59.4) | 102 (59.6) | 50 (58.8) | .90 |
| Race, n (%) | ||||
| White | 193 (75.4 ) | 131 (76.6) | 62 (72.9) | .58 |
| Black | 58 (22.7 ) | 36 (21.1) | 22 (25.9) | |
| Other | 5 (2.0 ) | 4 (2.3) | 1 (1.2) | |
| Ethnicity, n (%) | ||||
| Hispanic | 4 (1.6) | 4 (2.3) | 0 (0.0) | .16 |
| Educational Level, n (%) | ||||
| Less than high school | 40 (15.6) | 21 (12.3) | 19 (22.4) | |
| High school graduate | 73 (28.5) | 45 (26.3) | 28 (32.9) | |
| Some college | 47 (18.4) | 32 (18.7) | 15 (17.6) | |
| Associate/Bachelors | 65 (25.4) | 46 (26.9) | 19 (22.4) | |
| Advanced Degree | 31 (12.1) | 27 (15.8) | 4 (4.7) | |
| Employment, n (%) | .04 | |||
| Retired | 157 (61.3) | 107 (62.6) | 50 (58.8) | |
| Disabled | 30 (11.7) | 14 (8.2) | 16 (18.8) | |
| Part-time (<32hr/wk) | 6 (2.3) | 5 (2.9) | 1 (1.2) | |
| Full-time (>32hr/wk) | 32 (12.5) | 26 (15.2) | 6 (7.1) | |
| Other | 31 (12.1) | 19 (11.1) | 12 (14.1) | |
| Marital Status, n (%) | .31 | |||
| Single | 15 (5.9) | 8 (4.7) | 7 (8.2) | |
| Widowed/Divorced | 73 (28.5) | 46 (26.9) | 27 (31.8) | |
| Married | 168 (65.6) | 117 (68.4) | 51 (60.0) | |
| Cancer Type, n (%) | .41 | |||
| Colorectal | 42 (16.4) | 31 (18.1) | 11 (12.9) | |
| Pancreatic | 45 (17.6) | 28 (16.4) | 17 (20.0) | |
| Hepatobiliary | 27 (10.5) | 19 (11.1) | 8 (9.4) | |
| Gastroesophageal | 13 (5.1) | 11 (6.4) | 2 (2.4) | |
| Other# | 129 (50.4) | 82 (48.0) | 47 (55.3) | |
| Cancer Stage, n (%) | .33 | |||
| I-II | 44 (17.2) | 33 (19.3) | 11 (12.9) | |
| III | 73 (28.5) | 50 (29.2) | 23 (27.1) | |
| IV | 139 (54.3) | 88 (51.5) | 51 (60.0) | |
based on comparison between sarcopenia and non-sarcopenic participants
others include neuroendocrine carcinoma (n=17), prostate (n=22), lung (n=29), head & neck, (n=21), bladder (n=6), kidney (n=6), breast (n=4) and others (n=24)
The median SARC-F score was 2 (IQR, 0–4), and 33.2% of the participants screened positive for sarcopenia using a score cutoff of ≥4. Difficulty in climbing a flight of 10 stairs (57.4%) and lifting and carrying 10 pounds (49.2%) were the most commonly impaired components in the SARC-F questionnaire (eTable 1). As compared to those without sarcopenia, patients with sarcopenia more likely to be disabled (18.8% vs 8.2%, P=.04) and less likely to have an advanced degree (15.8% vs 4.7%, P=0.03). Meanwhile, there was no significant differences in the two groups in terms of sex, race/ethnicity, marital status, cancer type, or cancer stage (Table 1).
Next, we compared the prevalence of GA domain impairments between patients with and without sarcopenia. As compared to those without, patients with sarcopenia were more likely to have impaired performance status (aOR 18.2; 95% CI 8.7–38.1; p <0.001), impairments in iADL (aOR 18.1; 95% CI 7.5–43.8 p <0.001) or ADL (aOR 18.8; 95% CI 7.4–47.7; p <0.001), moderate to severe cognitive complaints (aOR 23.2; 95% CI 4.6–116.4; p <0.001), limitations in social activities (aOR 12.3; 95% CI 5.6–27.1, p <0.001), moderate/severe anxiety (aOR 5.2; 95% CI 2.5–11.1, p <0.001), moderate/severe depression (aOR 28.2; 95% CI 7.5–106.5, p <0.001), moderate/severe fatigue (aOR 22.2; 95% CI 8.7–56.9, p <0.001), frailty (aOR 43.5; 95% CI 17.7–106.8, p <0.001), and financial distress (aOR 28.7; 95% CI 13–63.5, p =0.003). However, there were no significant differences in the proportion of patients with vision or hearing impairment, multimorbidity (≥3 comorbidities) and polypharmacy (≥9 medications) between those with and without sarcopenia. Further, those with sarcopenia had lower HRQOL in the physical domain (β coefficient= −13.45, P <.001) as well as the mental domain (β coefficient= −11.39, P <.001), and a higher rates of prior healthcare utilization with ER visits (64.6 vs. 44.9 %, P =0.003), but not hospitalizations (Table 2).
Table 2:
Comparison of geriatric assessment identified impairments, health-related quality of life, and healthcare utilization by Sarcopenia status.
| Geriatric Assessment | Sarcopenia | P-value unadjusted | Adjusted Odds*, 95% CI | P-value adjusted | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Geriatric Assessment Identified Impairments | |||||
| Impaired (≥2) performance status, n (%) | 24 (14.5) | 61 (72.6) | <.001 | 18.15 (8.65–38.07) | <.001 |
| Limitations in walking one block, n (%) | 58 (35.4) | 79 (95.2) | <.001 | 39.03 (12.81–118.97) | <.001 |
| Any IADL dependence, n (%) | 58 (36.0) | 71 (89.9) | <.001 | 18.07 (7.45–43.84) | <.001 |
| Any ADL dependence, n (%) | 7 (4.2) | 37 (45.1) | <.001 | 18.75 (7.36–47.74) | <.001 |
| Mod/Severe Cognitive Complaints, n (%) | 3 (1.8) | 19 (24.7) | <.001 | 23.20 (4.62–116.39) | <.001 |
| ≥3 comorbidities, n (%) | 66 (40.0) | 41 (52.6) | .07 | 1.66 (0.91–3.02) | .10 |
| ≥9 medications daily, n (%) | 33 (20.1) | 25 (31.3) | .06 | 1.74 (0.88–3.39) | .11 |
| Limitations in social activities, n (%) | 19 (11.4) | 43 (55.1) | <.001 | 12.34 (5.61–27.12) | <.001 |
| Moderate/Severe Anxiety, n (%) | 21 (13.0) | 29 (37.2) | <.001 | 5.20 (2.45–11.06) | <.001 |
| Moderate/Severe Depression, n (%) | 5 (3.0) | 23 (29.5) | <.001 | 28.18 (7.46–106.52) | <.001 |
| Vision impairment, n (%) | 40 (24.0) | 32 (40.0) | .009 | 1.41 (0.73–2.74) | .31 |
| Hearing impairment, n (%) | 45 (27.3) | 29 (36.7) | .13 | 1.69 (0.87–3.28) | .12 |
| Mod/severe Fatigue, n (%) | 73 (42.9) | 76 (90.5) | <.001 | 22.24 (8.69–56.91) | <.001 |
| Frail, n (%) | 24 (14.2) | 68 (81.9) | <.001 | 43.49 (17.72–106.75) | <.001 |
| Health-Related Quality of Life | |||||
| Physical Health T-Score, mean (SD) | 47.5 (8.5) | 33.2 (6.5) | <.001 | −13.57# (−15.83 to −11.31) | <.001 |
| Mental Health T-Score, mean (SD) | 50.8 (7.5) | 38.9 (8.1) | <.001 | −11.48# (−13.59 to −9.36) | <.001 |
| Healthcare Utilization | |||||
| Emergency Room visit, n (%) | 75 (44.9) | 53 (64.6) | .003 | 2.50 (1.34–4.67) | .004 |
| Hospitalized at least one night, n (%) | 104 (62.7) | 57 (69.5) | .29 | 1.31(0.71–2.45) | .03 |
Abbreviations: IADL, Instrumental Activities of Daily Living; ADL, Activities of Daily Living. SD, standard deviation; Mod, moderate.
adjusted for age, sex, race, education, cancer type, cancer stage, and treatment phase. Each model only includes one GA domain or health care utilization measure of interest that serves as the dependent variable.
represents the β-coefficient and its 95% Confidence Interval computed using a linear regression model adjusting for the aforementioned covariates.
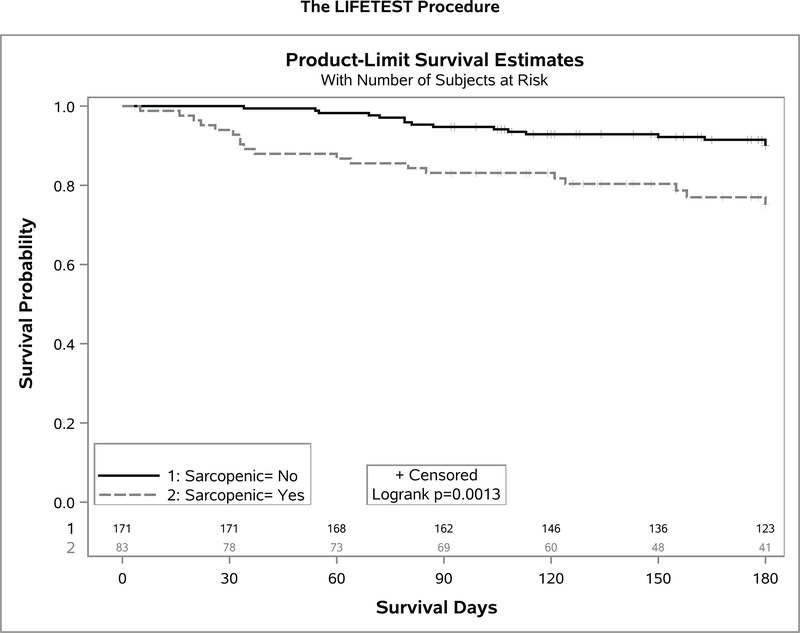
Over a median follow up of 8.6 months (range 1–20 months), 16 (6.3%) patients died. Patients with sarcopenia had a worse overall survival (1y OS 88% vs 95%; log-rank P=.04) (Figure 1). Sarcopenia independently predicted for worse survival (adjusted Hazards Ratio, 2.98; 95% CI 1.07–8.28, p=.04) after adjusting for age, sex, race, cancer type, cancer stage, and treatment phase (Table 3).
Figure 1.
Kaplan Meier plots between those with and without sarcopenia.
Table 3:
Cox Proportional Hazards Regression Model showing the impact of Sarcopenia as measured by SARC-F questionnaire on the overall survival in the study cohort*.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Sarcopenia | 2.98 | 1.07–8.28 | .04 |
| Age (continuous, yr) | 1.01 | 0.94–1.09 | .74 |
| Male Sex | 1.87 | 0.66–5.34 | .24 |
| Race | |||
| - White | Ref | ||
| - Others | 2.24 | 0.71–7.01 | .17 |
| Cancer Type£ | |||
| - Colorectal | Ref | ||
| - Pancreatic/Hepatobiliary | 1.47 | 0.39–5.49 | .57 |
| - Gastroesophageal | 1.85 | 0.18–18.96 | .60 |
| - Others | 0.29 | 0.07–1.20 | .09 |
| Cancer Stage | |||
| - Stage I/II | Ref | ||
| - Stage III | 0.76 | 0.05–12.42 | .85 |
| - Stage IV | 7.41 | 0.93–59.13 | .93 |
HR, Hazards Ratio; CI, confidence interval.
Treatment phase i.e. pre-treatment vs post-treatment was used as a stratifying variable in the model
Index date of follow up for the survival analysis was the time of the GA/SARC-F assessment.
Cancer type was recategorized into colorectal, pancreatic/hepatobiliary, gastroesphageal and others due to small sample size in the original cohort to allow stable model estimates.
Discussion
Sarcopenia has been widely recognized as a risk factor for chemotherapy toxicities, surgical complications, and mortality in cancer patients; however, sarcopenia is determined primarily using CT imaging, making it difficult to identify the at risk population in a busy clinical practice 12,13,33. Using the SARC-F screening measure in a population of older adults with cancer, we identified one third of older adults as sarcopenic. Furthermore, sarcopenia identified using this questionnaire was associated with higher odds of most geriatric assessment domain impairments and frailty, clinically meaningful reduction in physical and mental HRQOL, as well as increased ER utilization. Lastly, sarcopenic patients had inferior overall survival compared to non-sarcopenic.
The prevalence of sarcopenia varies greatly by the study population included and the definition employed. A meta-analysis of the association between CT-based sarcopenia and mortality in cancer patients identified 11 different definitions of sarcopenia employed in the literature 11. The prevalence of sarcopenia varied greatly across the 35 studies ranging from 11% to 74%. Of note, within oncology sarcopenia is commonly defined by muscle mass alone (based on CT imaging), however, in the broader field of geriatrics, the definition of sarcopenia also includes reduced muscle strength and performance 8. The SARC-F screening measure is comprised of 5 questions that directly relate to reduced muscle strength and performance. The correlation of SARC-F with CT-based measures of sarcopenia as commonly employed in oncology remains unknown. Previous studies have demonstrated low to no correlation between CT-based measures of muscle mass and physical performance, making it very likely that sarcopenic patients defined by SARC-F and by CT-based imaging may very well be different groups, albeit both at increased risk of adverse events 34. Further work is necessary to examine the correlation of SARC-F with standard CT-based muscle measures, including a comparison of both with important clinical outcomes. In addition, future prospective work is necessary to evaluate whether the associations of sarcopenia with cancer outcomes is a risk factor distinct from GA variables or not.
Other methods are available to identify sarcopenia each with their own strengths and limitations 35. Body mass index (BMI) is one of the oldest tools used to evaluate obesity and nutritional status, but is unable to account for variability in body composition and particularly limited in its ability to detect sarcopenia 36. Bioelectrical Impedance Analysis (BIA) is an accurate method for detecting sarcopenia in adults with cancer, but requires specific equipment that is not used in routine clinical care and can be influenced by hydration status and is less accurate in patients with morbid obesity (BMI ≥35) 37. Within oncology, the opportunistic use of CT imaging (for clinical care) to assess body composition and identify sarcopenia is the most common method utilized; however, not all adults with cancer undergo CT imaging as part of routine cancer care and to our knowledge real-time body composition analyses from CT imaging is not yet available within the clinic 33. Within the general geriatrics literature, dual-energy x-ray absorptiometry (DEXA) is increasingly used for the assessment of lean body mass, but is rarely employed within oncology except in the evaluation of concurrent osteoporosis 38. In addition, objective measures of gait speed assessment and handgrip strength have been recommended as screening methods for sarcopenia and are potentially scalable in practice, but remain a challenge to implement widely in oncology clinics given the required training, equipment (hand dynamometer), and limited space and time 20. Newer methods of assessing skeletal muscle mass, such as D3-creatine dilution, hold great promise as highly accurate tools for providing direct and accurate measurements of muscle mass, but are still in the investigative phase and not yet employed within oncology 39. Thus, SARC-F has great potential as one of the simplest and most widely scalable forms of screening for sarcopenia within oncology to help identify who may benefit from further evaluation with these other methods.
This study is not without limitations. Given the cross-sectional nature of the association between sarcopenia and GA identified impairments, no causal inferences or directionality can be drawn. No objective measurement of sarcopenia was included in this study as a confirmatory test, and as mentioned above, more work comparing the SARC-F tool to CT-based imaging evaluation is warranted. Furthermore, our participants were required from a single site in the southeastern US and compromised mostly of gastrointestinal malignancies, thus our results may not be generalizable to other populations. Our survival analysis was also limited by a short follow up time. Lastly, our sample size was limited, thus this study serves more as an initial exploration of this tool in older adults with cancer with further work needed in larger more diverse samples and with longer follow up time. Notably, a low sample size also resulted in lack of statistical significance for traditional risk factors of mortality. Our study also has many strengths. Our study is one of the first to examine the use of SARC-F within oncology and contains a comprehensive geriatric assessment that provides a wealth of details usually not included in clinical studies.
While it is widely known that sarcopenia is an important risk factor for many adverse events in oncology, no sarcopenia measure is currently available for use in the oncology clinical practice today. SARC-F is a simple and brief 5-item questionnaire that holds great promise in identifying at-risk older patients with cancer and may assist in targeting interventions to improve outcomes in this growing and vulnerable population. Future work is needed to examine the association with CT-based muscle measures and further examine the association of SARC-F with other cancer outcomes prospectively, including chemotherapy toxicities, maintaining functional independence, and HRQOL.
Supplementary Material
Acknowledgements:
Supported in part by the National Cancer Institute of the National Institutes of Health (K08CA234225). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement
Declaration of Competing Interest the authors have no conflict to disclose.
References:
- 1.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36(1):228–235. [DOI] [PubMed] [Google Scholar]
- 2.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35(4):409–415. [DOI] [PubMed] [Google Scholar]
- 3.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60(3):324–333. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50(5):889–896. [DOI] [PubMed] [Google Scholar]
- 5.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. The journal of nutrition, health & aging. 2008;12(7):433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. The journals of gerontology Series A, Biological sciences and medical sciences. 1997;52(5):B267–276. [DOI] [PubMed] [Google Scholar]
- 7.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. The American journal of clinical nutrition. 2010;91(4):1123S–1127S. [DOI] [PubMed] [Google Scholar]
- 8.Williams GR, Rier HN, McDonald A, Shachar SS. Sarcopenia & aging in cancer. J Geriatr Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. [DOI] [PubMed] [Google Scholar]
- 10.Williams GR, Chen Y, Kenzik KM, et al. Assessment of Sarcopenia Measures, Survival, and Disability in Older Adults Before and After Diagnosis With Cancer. JAMA Netw Open. 2020;3(5):e204783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 12.Rier HN, Jager A, Sleijfer S, Maier AB, Levin MD. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. The oncologist. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Seminars in cell & developmental biology. 2015. [DOI] [PubMed] [Google Scholar]
- 14.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Me. 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 15.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of applied physiology. 2004;97(6):2333–2338. [DOI] [PubMed] [Google Scholar]
- 16.Ida S, Kaneko R, Murata K. SARC-F for Screening of Sarcopenia Among Older Adults: A Meta-analysis of Screening Test Accuracy. Journal of the American Medical Directors Association. 2018;19(8):685–689. [DOI] [PubMed] [Google Scholar]
- 17.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. Journal of the American Medical Directors Association. 2013;14(8):531–532. [DOI] [PubMed] [Google Scholar]
- 19.Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? Journal of the American Medical Directors Association. 2014;15(9):630–634. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams GR, Kenzik KM, Parman M, et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). Journal of geriatric oncology. 2020;11(2):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giri S, Al-Obaidi M, Weaver A, et al. Association Between Chronological Age and Geriatric Assessment Identified Impairments: findings from the CARE registry. J Natl Compr Canc Ne. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. Journal of geriatric oncology. 2014;5(3):245–251. [DOI] [PubMed] [Google Scholar]
- 24.Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams GR, Al-Obaidi M, Dai C, et al. Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies. Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2017;15(7):894–902. [DOI] [PubMed] [Google Scholar]
- 27.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC geriatrics. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2009;18(7):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pergolotti M, Deal AM, Williams GR, et al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer. J Geriatr Oncol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Accurint. L. http://www.accurint.com. Accessed October 30, 2019.
- 31.Knight TG, Deal AM, Dusetzina SB, et al. Financial Toxicity in Adults With Cancer: Adverse Outcomes and Noncompliance. Journal of oncology practice / American Society of Clinical Oncology. 2018:JOP1800120. [DOI] [PubMed] [Google Scholar]
- 32.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. [Google Scholar]
- 33.Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9(7):1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams GR, Deal AM, Muss HB, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget. 2017;8(20):33658–33665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu SC, Khow KS, Jadczak AD, Visvanathan R. Clinical Screening Tools for Sarcopenia and Its Management. Curr Gerontol Geriatr Res. 2016;2016:5978523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shachar SS, Williams GR. The Obesity Paradox in Cancer-Moving beyond BMI-Response. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(6):981. [DOI] [PubMed] [Google Scholar]
- 37.Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Battaglini CL, Williams GR. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. The oncologist. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38(8):940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.