Abstract
Objective.
Anxiety is common in older adults with cancer (OACs) and their caregivers and is associated with poor outcomes including worse physical symptoms, poor treatment adherence and response, and longer hospitalizations. This study examined the feasibility, acceptability, adherence, and preliminary efficacy of a cognitive-behavioral therapy (CBT) intervention for OACs and their caregivers.
Method.
Patients with active cancer age 65 years and older and their caregivers were randomized to Managing Anxiety from Cancer (MAC), a seven-session CBT-based psychotherapy intervention delivered over the telephone or usual care. Patients and caregivers completed the intervention separately with licensed social workers. Self-report measures of anxiety, depression, and quality of life were administered after randomization and following intervention completion. Analyses were conducted separately for patients and caregivers and at the dyad level. Hierarchical Linear Modeling accounted for the within-dyad intraclass correlation coefficients (ICCs) by random intercepts associated with the dyads.
Results.
Twenty-nine dyads were randomized; 28 (96.6%) patients and 26 (89.7%) caregivers completed all study procedures. Of dyads randomized to MAC, 85.7% (n = 12) of patients and caregivers completed all seven sessions. Most patients (≥50%) and over 80% of caregivers rated the overall intervention and intervention components as “moderately” to “very” helpful. MAC was associated with a greater reduction in anxiety among dyads than usual care, the effect of MAC was greater in caregivers than in patients, and improvement in patient anxiety was associated with the reduction in caregiver anxiety. However, these results did not reach statistical significance.
Significance of results.
This pilot study demonstrates the feasibility of MAC and suggests strategies for improving acceptability, with a focus on adherence. Furthermore, these results indicate that MAC is promising for the reduction of anxiety in OAC–caregiver dyads and may be particularly beneficial for OAC caregivers. Larger randomized controlled trials are needed to evaluate the efficacy of MAC.
Keywords: Anxiety, Cancer, Caregiver, Cognitive-behavioral therapy, Older adult
Introduction
Anxiety is a normal response to a perceived threat and is characterized by worry that is difficult to control, restlessness, difficulty concentrating and sleeping, fatigue, and muscle tension (American Psychiatric Association, 2013). The objective threat posed by cancer makes the high rates of anxiety in cancer patients and survivors unsurprising and understandable (Deimling et al., 2017). For older adults, the threat of cancer is potentially magnified by threats that accompany aging (Holland, 2016; Dura-Ferrandis et al., 2017) such as age-related cognitive (Parpa et al., 2014; Bluethmann et al., 2016) and physical changes (Zhang et al., 2018). Cancer symptoms and treatment effects such as fatigue and nausea can add to and exacerbate these age-related physical and cognitive changes (Derks et al., 2016; Klepin et al., 2016; Dura-Ferrandis et al., 2017; Oh, 2017; van Abbema et al., 2017).
Older adults with cancer (OACs) experience high rates of anxiety, with over 40% reporting clinically significant anxiety (Teunissen et al., 2006; Kasparian et al., 2009; Nelson et al., 2010). OAC anxiety is associated with worse physical symptoms (Bruera et al., 2000; Brown and Kroenke, 2009; Delgado-Guay et al., 2009; Reddy et al., 2009; Salvo et al., 2012); poor quality of life (Smith et al., 2003; Horney et al., 2011) and treatment adherence and response (Fujii et al., 2001; Greer et al., 2008; Pedersen et al., 2010); difficulty communicating with the healthcare team (Schag and Heinrich, 1989); longer hospitalizations (Prieto et al., 2002); and higher risk for mortality (Chan et al., 2015). Thus, effective anxiety treatments for OACs have the potential to reduce distress, improve quality of life, and enhance treatment engagement and response.
OACs often rely heavily on informal or unpaid caregivers. Almost two-thirds (62%) of the 2.8 million U.S. cancer caregivers care for an older adult (National Alliance for Caregiving, 2016). The physical and cognitive impact of cancer and aging and the burden of cancer treatment place notable stress on OACs’ caregivers (National Alliance for Caregiving and AARP Public Policy Institute, 2015). Over one-third of cancer caregivers report clinically significant anxiety (Rossi Ferrario et al., 2003; Gotze et al., 2014; Gotze et al., 2016) that can be more severe than patient anxiety (Rossi Ferrario et al., 2003; Gotze et al., 2014). In addition, patients whose caregivers report elevated anxiety are at increased risk for clinically significant anxiety themselves (Bambauer et al., 2006; Segrin et al., 2007; Gotze et al., 2014). Therefore, treating anxiety in informal caregivers is vital to improving caregiver quality of life and OAC anxiety.
Cognitive-behavioral therapy (CBT) is a time-limited problem-focused (Moorey and Greer, 2002; Osborn et al., 2006) psychological treatment that targets thoughts and behaviors that increase distress (Moorey and Greer, 2002; Freeman, 2004). CBT is efficacious for anxiety in cancer patients (Osborn et al., 2006; Moyer et al., 2009; Greer et al., 2012), including when telephone-delivered (Brenes et al., 2015; Watson et al., 2017). Based on this research, CBT has been designated as first-line treatment for anxiety in cancer patients by the American Society of Clinical Oncology (Andersen et al., 2014). CBT is particularly appropriate for OACs due to the high adverse effect rates of and limited research on psychotropic medications in OACs (Molton and Terrill, 2014; Nightingale et al., 2015) and the prevalence (Lees and Chan, 2011; Prithviraj et al., 2012) and dangers of polypharmacy (Lees and Chan, 2011; Badgwell et al., 2013; Maher et al., 2013). However, effect sizes for CBT in older adults are smaller than in samples of the general population (Westen and Morrison, 2001), suggesting that modifications to CBT interventions that consider the unique needs of older adults are needed (Ayers et al., 2007). Finally, CBT for OACs and their caregivers must address the dual threat of cancer and aging.
The purpose of this pilot study is to evaluate the feasibility and acceptability of and adherence to a CBT intervention for anxiety in OACs and their caregivers. In addition, this study examines the preliminary efficacy of the intervention relative to usual care. We hypothesized that the intervention would be feasible and acceptable to OACs and their caregivers and that OACs and their caregivers would adhere to the intervention. In addition, we hypothesized a medium effect of the intervention relative to usual care.
Methods
Participants and procedures
All study procedures were approved by the Institutional Review Boards of all participating sites. All participants provided informed consent. Participants were recruited from June 2017 to June 2020. Recruitment began at one academic medical institution and was completed at a second institution in the same city due to the principal investigator’s change of employment. Participants were identified through self-referral from fliers posted in participating clinics, referrals from oncology providers, and medical chart reviews by study staff. Patients were recruited from the lymphoma, gynecologic, lung, pancreatic, myeloma, breast, gastrointestinal, and genitourinary outpatient cancer clinics.
Eligible patients were 65 years of age or older with a diagnosis of cancer currently on active treatment or within 6 months of treatment completion. Patients identified a primary unpaid caregiver age 21 years or older and patients and caregivers were enrolled as a dyad. In eligible dyads, the patient and/or caregiver had a score of ≥8 on the Anxiety Subscale of the Hospital Anxiety and Depression Scale and the patient and caregiver were fluent in English and able to communicate over the telephone. Dyads were excluded if one or both members received CBT since the patient’s cancer diagnosis, the patient’s anxiety was restricted to a phobic reaction to a medical procedure (e.g., needle phobia), one dyad member was too weak or cognitively impaired to complete study procedures per the treating oncologist, or one member met diagnostic criteria for schizophrenia, substance use or dependence, and/or bipolar disorder or endorsed active suicidal ideation.
Patients and caregivers completed baseline measures administered by study staff over the telephone. Dyads were then randomly assigned to the anxiety intervention (Managing Anxiety from Cancer; MAC) or usual care using block randomization with block sizes of four and six. Allocation concealment was ensured using the sequentially numbered, opaque sealed envelopes procedure (Doig and Simpson, 2005). Follow-up assessments were administered over the telephone by a study staff member blind to treatment condition. Participants were compensated $20 for completing baseline assessments and $30 for completing follow-up assessments.
Treatment conditions
Managing Anxiety from Cancer
MAC is a seven-session CBT-based psychotherapy intervention delivered over the telephone by licensed social workers. Each weekly session is 45–60 min in length and teaches a new skill for managing anxiety. OACs and caregivers are enrolled as dyads but complete MAC with separate interventionists. Modifications to CBT for older adults were informed by the Contextual, Cohort-based, Maturity, Specific challenge model (CCMSC) which integrates principles from gerontology and psychotherapy to inform approaches with older adults (Knight and McCallum, 1998; Kropf et al., 2017). For example, MAC normalizes anxiety and psychotherapy to address stigma toward psychological services common in older adults. Strategies for coping with cancer-specific stressors were integrated throughout the intervention such as acceptance-based strategies for coping with uncontrollable stressors (e.g., pending scan results). The Stage Model for Psychotherapy Manual Development informed the development of the therapist manuals (Carroll and Nuro, 2002). Additional details about the content and development of MAC have been published elsewhere (Trevino et al., 2020).
Usual care
Dyads randomized to usual care received ongoing oncologic and medical care and were monitored by their treatment teams, consistent with standard clinical practice.
Measures
Sample characteristics
Demographic characteristics were assessed by patient/caregiver self-report and included sex, age, education, race and ethnicity, marital status, patient–caregiver relationship type, whether the patient and caregiver live together, the number of hours spent providing care (caregiver only), and the percentage of informal care provided by the caregiver (caregiver only).
Disease characteristics were obtained by the electronic medical record review by study staff and included cancer type/primary site, current treatment, presence of metastatic disease, and clinical trial enrollment. Performance status was also obtained from the medical record in the form of the Eastern Cooperative Oncology Group Scale of Performance Status scale (ECOG score), a six-point physician rating from “Fully active, able to carry on all pre-disease performance without restriction” (0) to “dead” (5) (Oken et al., 1982).
Feasibility, acceptability, and adherence
Intervention feasibility was assessed with attrition rates, the number of sessions was completed by patients and caregivers, and the number of weeks was required to complete the intervention. Feasibility was defined as ≥70% of dyads randomized to MAC complete at least five sessions and ≥70% of all dyads complete study measures. Interventionist fidelity to MAC was evaluated with a checklist completed by trained raters that assessed the delivery of core intervention components and the use of appropriate therapeutic techniques (e.g., shows positive regard, uses active listening skills, etc.). Fidelity ratings were conducted for 15% of MAC sessions. Fidelity was defined as delivering ≥70% of intervention components using ≥70% of the therapeutic techniques.
Acceptability was assessed post-intervention. Patients and caregivers in the intervention condition rated the perceived helpfulness of the overall intervention and specific intervention components. Each item was rated on a five-point scale from “not at all helpful” (0) to “very helpful” (4). Patients and caregivers also completed multiple choice questions assessing the acceptability of the number of MAC sessions, session frequency, and the amount of information in the intervention. Acceptability was defined as ≥70% of patients and caregivers scoring 2 or greater on the Likert scale items.
Participant adherence to MAC was assessed with clinician ratings of whether patients and caregivers completed between-session MAC exercises. Adherence was defined a priori as ≥70% of patients and caregivers completing at least four of the six between-session MAC exercises.
Outcomes
Anxiety and depression were assessed at baseline and follow-up with the Hospital Anxiety and Depression Scale (HADS), a 14-item self-report measure (Zigmond and Snaith, 1983; Snaith and Zigmond, 1986). The HADS contains a seven-item anxiety subscale and a seven-item subscale assessing depressive symptoms. Each item is rated on a 0–3 scale; item scores are summed with higher scores indicating greater distress. The HADS has been evaluated extensively in cancer patients (Hopwood et al., 1991; Moorey et al., 1991) and caregivers (Grov et al., 2005; Gough and Hudson, 2009; Lambert et al., 2011), is psychometrically sound, and has demonstrated superior performance compared with other measures (Mitchell, 2010).
Quality of life was assessed at baseline and follow-up. Patients completed the Functional Assessment of Cancer Therapy-General (FACT-G), a reliable and valid 27-item self-report scale that assesses health-related quality of life (Cella et al., 1993; Cella et al., 2002; Webster et al., 2003; Luckett et al., 2010). Each item is rated on a Likert scale from 0 to 4. Caregivers completed the Caregiver Quality of Life-Cancer scale, which is a reliable and valid 35-item measure of quality of life in cancer caregivers (Weitzner et al., 1999; Edwards and Ung, 2002). Each item is rated on a Likert scale from 0 to 4. On both measures, item responses are summed and higher scores indicate better quality of life.
Statistical analyses
Descriptive statistics were used to report participant characteristics, feasibility, acceptability, and adherence. Baseline differences by treatment condition were examined with t-test and chi-square analyses. Interventionist fidelity was examined using frequency statistics of the percentage of fidelity items completed by interventionists.
Raw scores for all outcome measures (anxiety, depression, and quality of life) were standardized into z-scores by the means and standard deviations of baseline scores from patients randomized to usual care. Thus, these standardized means will be zero for all outcomes for patients randomized to usual care.
Separate analyses for patients and caregivers were carried out by evaluating the standardized post-treatment mean scores and Cohen’s d for participants randomized to usual care. Dyadic data were analyzed using the Hierarchical Linear Modeling (HLM) approach in Atkins (2005) to test the treatment effect. Three HLM models were fitted, one for each post-treatment outcome (anxiety, depression, and quality of life) adjusting for baseline outcome of the same scale, MAC condition (UC was the referent), and a MAC by baseline score interaction. All HLM analyses accounted for the within-dyad intraclass correlation coefficients (ICCs) by random intercepts associated with the dyads. The unit of analysis in the HLM was based on individuals nested within dyads because psychosocial outcomes are likely to be correlated between the patient and caregiver. Descriptive analyses were conducted using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA) and the HLM was done using R statistical software 3.5.2.
Results
Sample characteristics
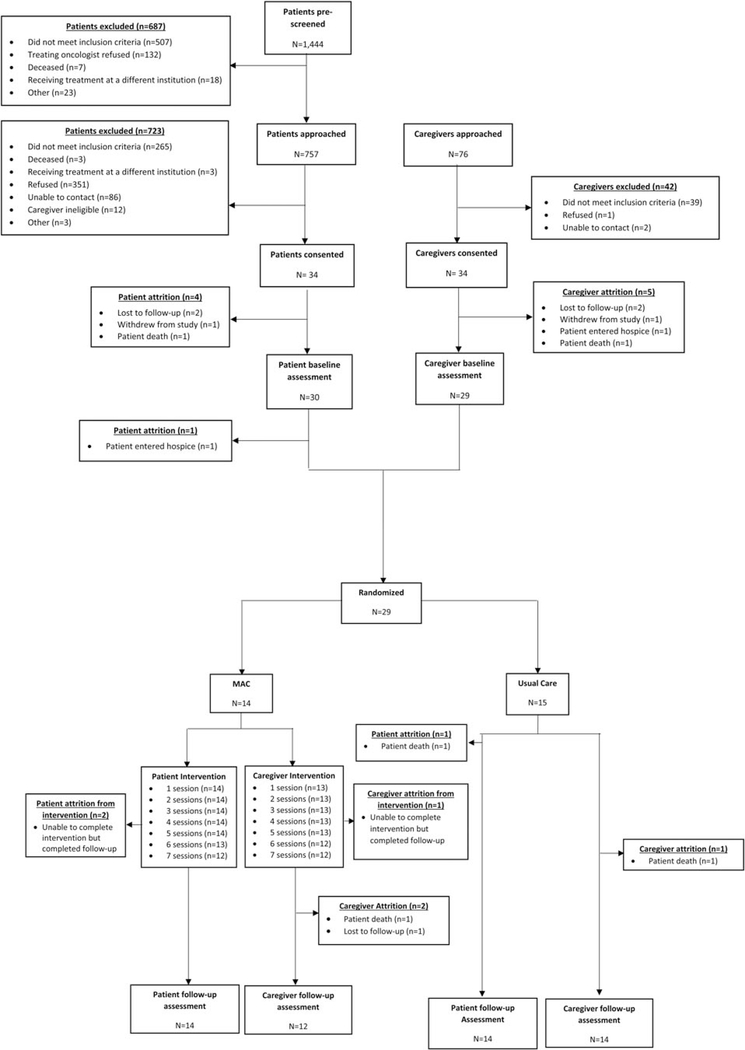
Of 757 patients approached regarding study participation, 351 (46.4%) refused, 265 (35.0%) did not meet inclusion criteria, 86 (11.4%) could not be contacted, 12 (1.6%) had an ineligible caregiver, and 9 (1.2%) were excluded for other reasons. Thirty-four patients (4.5%) were consented to the study. Of the 34 patients and caregivers consented, 30 (88.2%) patients and 29 (85.3%) caregivers completed baseline assessments and 29 dyads were randomized (MAC: n = 14 and usual care: n = 15). Figure 1 contains detailed information regarding reasons for exclusion and attrition over the course of the study.
Fig. 1.
CONSORT diagram.
Patients had a mean age of 71.76 years (SD = 5.73) and were 55.2% female (n = 16). Patients were primarily white (n = 28, 96.6%), non-Latino/a (n = 29, 100.0%), married (n = 21, 72.4%), and highly educated (post-graduate: n = 14, 48.3%). Caregivers had a mean age of 64.24 years (SD = 16.74) and were primarily female (n = 19, 65.5%), white (n = 26, 89.7%), non-Latino/a (n = 27, 93.1%), married (n = 19, 65.5%), and highly educated (post-graduate: n = 12, 41.4%). Over two-thirds (n = 20, 69.0%) of caregivers were the patient’s spouse and most caregivers lived with the patient (n = 25, 86.2%). Patient and caregiver characteristics did not differ across treatment conditions (all p’s > 0.05; Table 1).
Table 1.
Sample characteristics overall and by treatment condition
| Total N (%) | MAC (n = 14) | Usual care (n = 15) | p-value | |
|---|---|---|---|---|
| Patients (n = 29) | ||||
| Age (M, SD) | 71.76 (5.73) | 72.36 (5.67) | 71.20 (5.93) | 0.60 |
| Gender | 0.20 | |||
| Male | 13 (44.8) | 8 (57.1) | 5 (33.3) | |
| Female | 16 (55.2) | 6 (42.9) | 10 (66.7) | |
| Ethnicity | – | |||
| Latino | 0 (0) | 0 (0) | 0 (0) | |
| Non-Latino | 29 (100) | 14 (48.3) | 15 (51.7) | |
| Race | – | |||
| White | 28 (96.6) | 14 (100) | 14 (93.3) | |
| Black | 1 (3.4) | 0 (0) | 1 (6.7) | |
| Education | 0.86 | |||
| College or less | 15 (51.7) | 7 (50.0) | 8 (53.3) | |
| Post-graduate | 14 (48.3) | 7 (50.0) | 7 (46.7) | |
| Partner status | 0.91 | |||
| Married/Partnered | 21 (72.4) | 10 (71.4) | 11 (73.3) | |
| Other | 8 (27.6) | 4 (28.6) | 4 (26.7) | |
| Relationship with caregiver | 0.78 | |||
| Spouse | 20 (69.0) | 10 (71.4) | 10 (66.7) | |
| Other | 9 (31.0) | 4 (28.6) | 5 (33.3) | |
| Live with caregiver | 0.25 | |||
| Yes | 25 (86.2) | 11 (78.6) | 14 (93.3) | |
| No | 4 (13.8) | 3 (21.4) | 1 (6.7) | |
| Cancer type | 0.83 | |||
| Lung | 3 (10.3) | 2 (14.3) | 1 (6.7) | |
| Gynecologic | 6 (20.7) | 2 (14.3) | 4 (26.7) | |
| Breast | 4 (13.8) | 2 (14.3) | 2 (13.3) | |
| Colorectal | 3 (10.3) | 1 (7.1) | 2 (13.3) | |
| Lymphoma | 4 (13.8) | 2 (14.3) | 2 (13.3) | |
| Genitourinary | 8 (27.6) | 5 (35.7) | 3 (20.0) | |
| Pancreatic | 1 (3.4) | 0 (0) | 1 (6.7) | |
| Metastatic disease* | 0.92 | |||
| No | 9 (37.5) | 4 (36.4) | 5 (38.5) | |
| Yes | 15 (62.5) | 7 (63.6) | 8 (61.5) | |
| Receiving chemotherapy* | 0.94 | |||
| No | 4 (14.8) | 2 (15.4) | 2 (14.3) | |
| Yes | 23 (85.2) | 11 (84.6) | 12 (85.7) | |
| Receiving radiation* | 0.96 | |||
| No | 25 (92.6) | 12 (92.3) | 13 (92.9) | |
| Yes | 2 (7.4) | 1 (7.7) | 1 (7.1) | |
| Enrolled in a clinical trial* | 0.16 | |||
| No | 22 (81.5) | 12 (92.3) | 10 (71.4) | |
| Yes | 5 (18.5) | 1 (7.7) | 4 (28.6) | |
| ECOG score* | 0.22 | |||
| Fully active (0) | 7 (30.4) | 5 (41.7) | 2 (18.2) | |
| Light work (1) | 16 (69.6) | 7 (58.3) | 9 (81.8) | |
| Caregivers (n = 29) | ||||
| Age (M, SD) | 64.24 (16.74) | 65.43 (16.55) | 63.13 (17.42) | 0.72 |
| Gender | 0.52 | |||
| Male | 10 (34.5) | 4 (28.6) | 6 (40.0) | |
| Female | 19 (65.5) | 10 (71.4) | 9 (60.0) | |
| Ethnicity | 0.96 | |||
| Latino | 2 (6.9) | 1 (7.1) | 1 (6.7) | |
| Non-Latino | 27 (93.1) | 13 (92.9) | 14 (93.3) | |
| Race | 0.08 | |||
| White | 26 (89.7) | 14 (100) | 12 (80.0) | |
| Other | 3 (10.3) | 0 (0) | 3 (20.0) | |
| Education | 0.18 | |||
| College or less | 17 (58.6) | 10 (71.4) | 7(46.7) | |
| Post-graduate | 12 (41.4) | 4 (28.6) | 8 (53.3) | |
| Partner status | 0.36 | |||
| Married/Partnered | 19 (65.5) | 8 (57.1) | 11 (73.3) | |
| Other | 10 (34.5) | 6 (42.99) | 4 (26.7) | |
| Amount of patient’s care provided* | 0.55 | |||
| 0–39% | 14 (51.9) | 7 (58.3) | 7 (46.7) | |
| 40+% | 13 (48.1) | 5 (41.7) | 8 (53.3) | |
| Length of caregiving (months; M, SD) | 26.25 (29.37) | 36.71 (35.52) | 15.79 (17.15) | 0.06 |
Totals less than 29 are due to missing data.
Feasibility, acceptability, and adherence
Of the dyads randomized, 28 (96.6%) patients and 26 (89.7%) caregivers completed follow-up measures, exceeding the a priori benchmark of 70%. Of the 14 dyads randomized to MAC, all patients (100%) completed at least five intervention sessions, 13 (92.9%) completed six sessions, and 12 (85.7%) completed all seven sessions. For caregivers, 13 (92.9%) completed five sessions and 12 (85.7%) completed six and seven sessions, exceeding the a priori benchmark for feasibility. Patients who completed five sessions did so in an average of 4.95 weeks (SD = 1.47); patients who completed seven sessions did so in an average of 6.96 weeks (SD = 0.82). Caregivers completed five sessions in an average of 4.48 weeks (SD = 0.71) and seven sessions in 6.94 weeks (SD = 1.40).
Interventionists delivered the intervention with fidelity. Interventionists delivered 79.14% of intervention components and used 96.6% of the therapeutic techniques with patients and 78.61% of intervention components using 99.12% of the therapeutic techniques with caregivers.
Most patients (≥50%) rated the overall intervention and intervention components as “moderately” to “very” helpful (Table 2). Patients also reported that MAC had an acceptable number of sessions (n = 9, 75%), that weekly sessions were acceptable (n = 12, 100%), and that MAC had the right amount of information (n = 9, 100%). Patients were split regarding whether to include dyadic sessions in MAC with 41.7% (n = 5) preferring separate interventions, 33.3% (n = 4) preferring some joint sessions, and 25.0% (n = 3) preferring a completely combined intervention.
Table 2.
MAC acceptability ratings
| Patients | Caregivers | |
|---|---|---|
| Overall helpfulness | ||
| Mean, SD | 2.42 (1.51) | 2.36 (1.03) |
| Moderately to very helpful (n, %) | 8 (66.7) | 9 (81.8) |
| Scheduling form | ||
| Mean, SD | 1.67 (1.67) | 1.27 (1.49) |
| Moderately to very helpful (n, %) | 6 (50.0) | 4 (36.4) |
| Values/expertise check-in (patients only) | ||
| Mean, SD | 2.25 (1.36) | – |
| Moderately to very helpful (n, %) | 8 (66.7) | – |
| Self-care check-in (caregiver only) | ||
| Mean, SD | – | 2.90 (1.29) |
| Moderately to very helpful (n, %) | – | 8 (80.0) |
| Case examples | ||
| Mean, SD | 2.08 (1.51) | 2.27 (1.42) |
| Moderately to very helpful (n, %) | 7 (58.3) | 9 (81.8) |
| Practice plan | ||
| Mean, SD | 2.67 (1.61) | 2.18 (1.25) |
| Moderately to very helpful (n, %) | 9 (75.0) | 9 (81.8) |
Over 80% of caregivers rated MAC and MAC components as “moderately” to “very” helpful (Table 2). The exception was the Scheduling Form which was designed to help patients and caregivers record and remember session appointments. Only 36.4% (n = 4) of caregivers found the Scheduling Form helpful. Caregivers also reported that MAC had an acceptable number of sessions (n = 7, 70.0%), that weekly sessions were acceptable (n = 9, 90.0%), and that MAC contained the right amount of information (n = 8, 72.7%). Caregivers were split regarding whether to combine the patient and caregiver interventions with 45.5% (n = 5) expressing a preference for separate interventions, 36.4% (n = 4) preferring some joint sessions, and 18.2% (n = 2) preferring a completely combined intervention.
Adherence to MAC was assessed with clinician ratings of patient and caregiver completion of between-session MAC exercises. Most patients (n = 8, 61.5%) but less than half of caregivers (n = 5, 41.7%) completed at least four of the six exercises.
Anxiety, depression, and quality of life
Table 3 shows the descriptive statistics for all three outcomes, stratified by treatment (MAC vs. UC), role in the dyad (patients vs. caregivers), and time (baseline vs. post-treatment). In the UC and MAC conditions, patients reported Cohen’s d differences in anxiety (d = −0.11; −0.14), depression (d = −0.21; 0), and quality of life (d = 0.29; 0) in the UC and MAC conditions, respectively. Caregivers showed a Cohen’s d difference in anxiety (d = −0.06; −0.41), depression (d = 0.03; −0.04), and quality of life (d = −0.08, 0.26) in the UC and MAC conditions, respectively. Finally, the change in patients’ anxiety (r = 0.14, p = 0.516) was positively associated with the change in caregiver anxiety, while the change in patient and caregiver depression (r = −0.12, p = 0.703) and quality of life (r = −0.5, p = 0.709) were negatively associated but not statistically significant.
Table 3.
Analyses for patients and caregivers (n = 29 dyads: n = 14 MAC, n = 15 usual care)
| Patients |
Caregivers |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Post-treatment |
Baseline |
Post-treatment |
||||||||||||
| M | SD | d* | M | SD | d* | Post-Tx - Baseline (d) | M | SD | d* | M | SD | d* | Post-Tx - Baseline (d) | ||
| Anxiety | Usual Care | 7.53 | 3.02 | 0.00 | 7.21 | 3.45 | −0.11 | −0.11 | 8.40 | 3.54 | 0.29 | 8.21 | 3.26 | 0.23 | −0.06 |
| MAC | 5.64 | 4.01 | −0.63 | 5.21 | 3.62 | −0.77 | −0.14 | 9.45 | 2.93 | 0.64 | 8.21 | 3.07 | 0.23 | −0.41 | |
| Depression | Usual Care | 5.73 | 4.74 | 0.00 | 4.71 | 4.07 | −0.22 | −0.22 | 4.80 | 3.03 | −0.20 | 4.64 | 3.08 | −0.23 | −0.03 |
| MAC | 5.36 | 2.31 | −0.08 | 5.36 | 3.84 | −0.08 | 0.00 | 3.50 | 2.35 | −0.47 | 3.33 | 2.81 | −0.51 | −0.04 | |
| Quality of life | Usual Care | 72.82 | 17.31 | 0.00 | 77.84 | 13.29 | 0.29 | 0.29 | 89.54 | 15.47 | 0.97 | 88.22 | 13.63 | 0.89 | −0.08 |
| MAC | 79.56 | 10.61 | 0.39 | 79.65 | 12.65 | 0.39 | 0.01 | 89.30 | 14.12 | 0.95 | 93.74 | 14.98 | 1.21 | 0.26 | |
M = Mean; SD = Standard Deviation.
Cohen’s d with respect to the mean and SD of baseline scores for participants randomized to usual care.
Table 4 shows the results for the dyadic HLM analyses across anxiety, depression, and quality of life. Dyads randomized to the MAC condition had a greater reduction in anxiety than UC, although not statistically significant at the 0.05 level (b = −0.244, p = 0.298). A nonsignificant difference was also observed in the outcomes of depression (b = −0.091, p = 0.647) and quality of life (b = 0.180, p = 0.733). Baseline scores were strongly associated with the post-treatment outcomes of the same domain, i.e., for anxiety (b = 0.622, p < 0.001), depression (b = 0.803, p < 0.001), and quality of life (b = 0.695, p < 0.001). No significant interaction effects were found between receiving the treatment condition and baseline scores for all outcomes.
Table 4.
Hierarchical linear model estimates for dyadic post-treatment z-scores (anxiety, depression, and quality of life)
| Model 1: Anxiety | Model 2: Depression | Model 3: Quality of life | ||||
|---|---|---|---|---|---|---|
| Fixed effect: Predictors | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value |
| Intercept | 0.015 | 0.924 | −0.050 | 0.714 | 0.172 | 0.253 |
| Treatment Condition | −0.244 | 0.298 | −0.091 | 0.647 | 0.180 | 0.426 |
| Baseline score | 0.622 | <0.001 | 0.803 | <0.001 | 0.695 | <0.001 |
| Treatment Condition × Baseline | 0.180 | 0.317 | −0.308 | 0.280 | −0.073 | 0.733 |
| Random effect | Variance | SD | Variance | SD | Variance | SD |
| Dyad-level | 0.108 | 0.329 | 0.084 | 0.289 | 0.011 | 0.106 |
| Individual-level | 0.495 | 0.704 | 0.298 | 0.546 | 0.404 | 0.635 |
| ICC | 0.179 | 0.219 | 0.027 | |||
Observations: 54 individuals, 28 dyads.
All raw scores of outcome measures (anxiety, depression, and quality of life) were standardized into z-scores by the mean and SD of baseline scores for patients randomized to usual care. Bold values indicate statistically significant p-values.
Discussion
This pilot study examined the feasibility and acceptability of and adherence to an anxiety intervention for OACs and their caregivers. The intervention was feasible and interventionists delivered it with fidelity. Most patients rated the intervention as acceptable although at lower levels than a priori benchmarks. Caregivers, however, exceeded a priori benchmarks for acceptability. Patients and caregivers had low rates of completion of between-session exercises with higher completion rates in patients than caregivers. Preliminary efficacy testing was limited by the small sample size and intervention effects were not statistically significant. For patients, the effect of MAC was small for anxiety with no effect for depression and quality of life. Caregivers who received MAC experienced a small to moderate reduction in anxiety, a small improvement in quality of life, and no change in depression. In dyadic analyses, dyads randomized to MAC experienced a greater reduction in anxiety than dyads in usual care with smaller changes in depression and quality of life. Finally, improvement in patient anxiety was associated with improvements in caregiver anxiety. However, these results did not reach the level of statistical significance.
Feasibility analyses indicated that patients and caregivers who initiated study procedures and the intervention completed at high rates. The use of the telephone may have facilitated this high completion rate by reducing the burden of the intervention and study procedures. In the era of COVID-19, the ability to provide remotely delivered services has become increasingly important. In addition, numerous barriers to in-person participation in mental health services exist in the normal course of cancer care such as travel burden, limited institutional resources (e.g., space), symptom burden, demanding treatment schedules, and multiple responsibilities of caregivers (e.g., parenting, work) (Latte-Naor and Mao, 2019).
While completion rates were high, so were rates of refusal to participate in the study. Dyads who self-select to participate may perceive a greater personal need for the intervention that motivates treatment completion. However, dyads who may have benefited likely refused. Future studies that examine the reasons patients and caregivers refuse to participate will inform modifications to the intervention, study procedures, and clinical care that reduce refusal rates and increase patient and caregiver engagement in mental health services.
While most patients rated the intervention as helpful, the ratings did not meet the a priori acceptability benchmark. The small sample may have contributed to this finding; for most acceptability ratings, a higher rating by one additional patient would have exceeded the benchmark. However, patient ratings of intervention components point to strategies for improving acceptability including removing the Scheduling Form, improving the case examples, and incorporating dyadic sessions. Notably, caregiver ratings of acceptability exceeded a priori benchmarks. Caregivers tend to be overlooked by the healthcare system and fewer interventions have been developed for caregivers than for patients (Sklenarova et al., 2015; Huang et al., 2019). The high burden of cancer caregiving and dearth of available support may have enhanced caregivers’ views of the intervention.
The intervention component that received among the highest acceptability ratings was the Practice Plan, the exercise designed to promote the use of intervention strategies between sessions. Yet, the completion rate for the Practice Plans was low, consistent with prior research on CBT (Hundt et al., 2014). However, research also indicates that homework completion is associated with better outcomes (Kazantzis et al., 2003; Hundt et al., 2014). Therefore, promoting the completion of homework exercises is important to ensuring patients maximally benefit from CBT. Strategies to improve adherence to the Practice Plan may include simplification of the exercise to reduce burden and spending more time during the session to explain the exercise and discuss barriers to completion (Kazantzis et al., 2003).
Analyses of intervention efficacy were limited by a small sample size and lack of adequate statistical power. Furthermore, despite rigorous randomization procedures, patients randomized to MAC had notably lower baseline anxiety levels than patients in the usual care condition which likely attenuated treatment effects. Despite this, dyads randomized to MAC reported greater reductions in anxiety than dyads randomized to usual care, although not statistically significant or conclusive due to the small sample size. In combination with extensive prior research on CBT efficacy (Osborn et al., 2006; Moyer et al., 2009; Greer et al., 2012), this finding supports the potential of MAC as an effective treatment for anxiety in OAC–caregiver dyads. In addition to this dyadic effect, MAC was associated with greater reductions in anxiety in OACs and caregivers, respectively relative to usual care. Notably, the treatment effect was greater in caregivers than in patients. As previously stated, caregivers are typically not provided with mental health services during the course of patients’ care. In addition, attention to caregivers often focuses on improving their ability to care for the patient rather than on their personal needs. Therefore, MAC may have addressed a significant gap in resources available to caregivers. Finally, the positive relationship between improvements in patient anxiety and reductions in caregiver anxiety supports the importance of treating the OAC–caregiver dyad rather than focusing on a single dyad member, consistent with prior research on the interdependence of patient and caregiver distress (Bambauer et al., 2006; Segrin et al., 2007; Gotze et al., 2014).
Intervention effects for depression and quality of life were smaller than for anxiety in dyadic and individual analyses with a minimal change associated with MAC. These findings suggest that, as designed, MAC content targets anxiety specifically and may be particularly appropriate for patients who are suffering from anxiety symptoms.
Strengths of this study include use of a randomized design, treatment of OAC–caregiver dyads, and modifications to CBT to address the dual stressors of cancer and aging. However, this study was conducted in academic medical settings in an urban location with a largely white and highly educated sample, limiting the generalizability to other populations and settings. Dyads were excluded if the patient or caregiver had received CBT since the patient’s cancer diagnosis. However, dyads may have received CBT prior to the diagnosis with an unknown impact on their response to MAC. Finally, data were collected at two institutions consecutively. These institutions are in the same area of a single city, serve similar patient populations, and are academic medical institutions. Furthermore, study staff and interventionists and the research protocol were largely identical across institutions. Yet, this transition may have increased error within study data.
Future research that identifies strategies for improving intervention acceptability, particularly for patients may enhance intervention efficacy. In addition, the examination of the intervention in more diverse patient and caregiver populations will improve intervention generalizability and inform cultural tailoring of the intervention to meet the unique needs of diverse populations. Finally, few patients have access to evidence-based mental health care. Considering implementation strategies early in the process of intervention development will promote the future implementation of MAC with the potential to enhance implementation of other similar psychotherapy interventions.
The results of this study indicate that the MAC intervention is feasible and acceptable, particularly for caregivers. The findings point to strategies for improving patient acceptability and increasing adherence to homework exercises. Preliminary analyses indicate MAC has strong potential to reduce anxiety in OAC–caregiver dyads with particular benefit for caregivers. Future research is needed on strategies for improving OAC and caregiver adherence and to establish intervention efficacy.
Acknowledgments
The authors acknowledge Simon Cohen and Chrystal Marte for their work on recruitment for this study and the Memorial Sloan Kettering Psycho-oncology in Aging and Cancer (PAC MSK) research laboratory for feedback on prior versions of this manuscript.
Funding. This research was funded by the National Institute on Aging and American Federation for Aging Research (K23 AG048632, Trevino).
Footnotes
Conflicts of interest. The authors do not have conflicts to disclose.
References
- American Psychiatric Association, DSM-5 Task Force (2013) Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. American Psychiatric Publishing, Inc. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Andersen BL, DeRubeis RJ, Berman BS, et al. (2014) Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. Journal of Clinical Oncology 32(15), 1605–1619. doi: 10.1200/jco.2013.52.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins DC (2005) Using multilevel models to analyze couple and family treatment data: Basic and advanced issues. Journal of Family Psychology 19(1), 98–110. doi: 10.1037/0893-3200.19.1.98 [DOI] [PubMed] [Google Scholar]
- Ayers CR, Sorrell JT, Thorp SR, et al. (2007) Evidence-based psychological treatments for late-life anxiety. Psychology and Aging 22(1), 8–17. doi: 10.1037/0882-7974.22.1.8 [DOI] [PubMed] [Google Scholar]
- Badgwell B, Stanley J, Chang GJ, et al. (2013) Comprehensive geriatric assessment of risk factors associated with adverse outcomes and resource utilization in cancer patients undergoing abdominal surgery. Journal of Surgical Oncology 108(3), 182–186. doi: 10.1002/jso.23369 [DOI] [PubMed] [Google Scholar]
- Bambauer KZ, Zhang B, Maciejewski PK, et al. (2006) Mutuality and specificity of mental disorders in advanced cancer patients and caregivers. Social Psychiatry and Psychiatric Epidemiology 41(10), 819–824. doi: 10.1007/s00127-006-0103-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluethmann SM, Mariotto AB and Rowland JH (2016) Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology, Biomarkers & Prevention 25(7), 1029–1036. doi: 10.1158/1055-9965.epi-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes GA, Danhauer SC, Lyles MF, et al. (2015) Telephone-delivered cognitive behavioral therapy and telephone-delivered nondirective supportive therapy for rural older adults with generalized anxiety disorder: A randomized clinical trial. JAMA Psychiatry 72(10), 1012–1020. doi: 10.1001/jamapsychiatry.2015.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF and Kroenke K (2009) Cancer-related fatigue and its associations with depression and anxiety: A systematic review. Psychosomatics 50(5), 440–447. doi: 10.1176/appi.psy.50.5.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera E, Schmitz B, Pither J, et al. (2000) The frequency and correlates of dyspnea in patients with advanced cancer. Journal of Pain and Symptom Management 19(5), 357–362. [DOI] [PubMed] [Google Scholar]
- Carroll KM and Nuro KF (2002) One size cannot fit all: A stage model for psychotherapy manual development. Clinical Psychology: Science and Practice 9(4), 396–406. [Google Scholar]
- Cella DF, Tulsky DS, Gray G, et al. (1993) The functional assessment of cancer therapy scale: Development and validation of the general measure. Journal of Clinical Oncology 11(3), 570–579. [DOI] [PubMed] [Google Scholar]
- Cella D, Hahn EA and Dineen K (2002) Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Quality of Life Research 11(3), 207–221. [DOI] [PubMed] [Google Scholar]
- Chan CM, Wan Ahmad WA, Yusof MM, et al. (2015) Effects of depression and anxiety on mortality in a mixed cancer group: A longitudinal approach using standardised diagnostic interviews. Psycho-Oncology 24(6), 718–725. doi: 10.1002/pon.3714 [DOI] [PubMed] [Google Scholar]
- Deimling GT, Brown SP, Albitz C, et al. (2017) The relative importance of cancer-related and general health worries and distress among older adult, long-term cancer survivors. Psycho-Oncology 26(2), 182–190. doi: 10.1002/pon.4015 [DOI] [PubMed] [Google Scholar]
- Delgado-Guay M, Parsons HA, Li Z, et al. (2009) Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Supportive Care in Cancer 17(5), 573–579. doi: 10.1007/s00520-008-0529-7 [DOI] [PubMed] [Google Scholar]
- Derks MG, de Glas NA, Bastiaannet E, et al. (2016) Physical functioning in older patients with breast cancer: A prospective cohort study in the TEAM trial. Oncologist 21(8), 946–953. doi: 10.1634/theoncologist.2016-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig GS and Simpson F (2005) Randomization and allocation concealment: A practical guide for researchers. Journal of Critical Care 20(2), 187–191. doi: 10.1016/j.jcrc.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Dura-Ferrandis E, Mandelblatt JS, Clapp J, et al. (2017) Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (alliance). Psycho-Oncology 26(11), 1914–1921. doi: 10.1002/pon.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards B and Ung L (2002) Quality of life instruments for caregivers of patients with cancer: A review of their psychometric properties. Cancer Nursing 25(5), 342–349. [DOI] [PubMed] [Google Scholar]
- Freeman A (2004) Clinical Applications of Cognitive Therapy. New York: Springer. [Google Scholar]
- Fujii M, Ohno Y, Tokumaru Y, et al. (2001) Manifest anxiety scale for evaluation of effects of granisetron in chemotherapy with CDDP and 5FU for head and neck cancer. Supportive Care in Cancer 9(5), 366–371. [DOI] [PubMed] [Google Scholar]
- Gotze H, Brahler E, Gansera L, et al. (2014) Psychological distress and quality of life of palliative cancer patients and their caring relatives during home care. Supportive Care in Cancer 22(10), 2775–2782. doi: 10.1007/s00520-014-2257-5 [DOI] [PubMed] [Google Scholar]
- Gotze H, Brahler E, Gansera L, et al. (2016) Anxiety, depression and quality of life in family caregivers of palliative cancer patients during home care and after the patient’s death. European Journal of Cancer Care. doi: 10.1111/ecc.12606 [DOI] [PubMed] [Google Scholar]
- Gough K and Hudson P (2009) Psychometric properties of the hospital anxiety and depression scale in family caregivers of palliative care patients. Journal of Pain and Symptom Management 37(5), 797–806. doi: 10.1016/j.jpainsymman.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Greer JA, Pirl WF, Park ER, et al. (2008) Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. Journal of Psychosomatic Research 65(6), 549–552. doi: 10.1016/j.jpsychores.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JA, Traeger L, Bemis H, et al. (2012) A pilot randomized controlled trial of brief cognitive-behavioral therapy for anxiety in patients with terminal cancer. Oncologist. doi: 10.1634/theoncologist.2012-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov EK, Dahl AA, Moum T, et al. (2005) Anxiety, depression, and quality of life in caregivers of patients with cancer in late palliative phase. Annals of Oncology 16(7), 1185–1191. doi: 10.1093/annonc/mdi210 [DOI] [PubMed] [Google Scholar]
- Holland J (2016). Double whammy of cancer and aging. Personal Communication. [Google Scholar]
- Hopwood P, Howell A and Maguire P (1991) Screening for psychiatric morbidity in patients with advanced breast cancer: Validation of two self-report questionnaires. British Journal of Cancer 64(2), 353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horney DJ, Smith HE, McGurk M, et al. (2011) Associations between quality of life, coping styles, optimism, and anxiety and depression in pretreatment patients with head and neck cancer. Head & Neck 33(1), 65–71. doi: 10.1002/hed.21407 [DOI] [PubMed] [Google Scholar]
- Huang LW, Smith AK and Wong ML (2019) Who will care for the caregivers? Increased needs when caring for frail older adults with cancer. Journal of the American Geriatrics Society 67(5), 873–876. doi: 10.1111/jgs.15863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundt NE, Amspoker AB, Kraus-Schuman C, et al. (2014) Predictors of CBT outcome in older adults with GAD. Journal of Anxiety Disorders 28 (8), 845–850. doi: 10.1016/j.janxdis.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparian NA, McLoone JK and Butow PN (2009) Psychological responses and coping strategies among patients with malignant melanoma: A systematic review of the literature. Archives of Dermatology 145(12), 1415–1427. doi: 10.1001/archdermatol.2009.308 [DOI] [PubMed] [Google Scholar]
- Kazantzis N, Pachana NA and Secker DL (2003) Cognitive behavioral therapy for older adults: Practical guidelines for the use of homework assignments. Cognitive and Behavioral Practice 10(4), 324–332. doi:doi: 10.1016/S1077-7229(03)80050-0 [DOI] [Google Scholar]
- Klepin HD, Tooze JA, Pardee TS, et al. (2016) Effect of intensive chemotherapy on physical, cognitive, and emotional health of older adults with acute myeloid leukemia. Journal of the American Geriatrics Society 64(10), 1988–1995. doi: 10.1111/jgs.14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight BG and McCallum TJ (1998) Adapting psychotherapeutic practice for older clients: Implications of the contextual, cohort-based, maturity, specific challenge model. Professional Psychology: Research and Practice 29(1), 15–22. doi: 10.1037/0735-7028.29.1.15 [DOI] [Google Scholar]
- Kropf N, Cummings SM and Cummings S (2017) Evidence-Based Treatment with Older Adults: Theory, Practice, and Research. New York: Oxford University Press. [Google Scholar]
- Lambert S, Pallant JF and Girgis A (2011) Rasch analysis of the hospital anxiety and depression scale among caregivers of cancer survivors: Implications for its use in psycho-oncology. Psycho-Oncology 20(9), 919–925. doi: 10.1002/pon.1803 [DOI] [PubMed] [Google Scholar]
- Latte-Naor S and Mao JJ (2019) Putting integrative oncology into practice: Concepts and approaches. Journal of Oncology Practice 15(1), 7–14. doi: 10.1200/jop.18.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J and Chan A (2011) Polypharmacy in elderly patients with cancer: Clinical implications and management. The Lancet Oncology 12(13), 1249–1257. doi: 10.1016/s1470-2045(11)70040-7 [DOI] [PubMed] [Google Scholar]
- Luckett T, Butow PN, King MT, et al. (2010) A review and recommendations for optimal outcome measures of anxiety, depression and general distress in studies evaluating psychosocial interventions for English-speaking adults with heterogeneous cancer diagnoses. Supportive Care in Cancer 18(10), 1241–1262. doi: 10.1007/s00520-010-0932-8 [DOI] [PubMed] [Google Scholar]
- Maher RL, Hanlon J and Hajjar ER (2013) Clinical consequences of polypharmacy in elderly. Expert Opinion on Drug Safety. doi: 10.1517/14740338.2013.827660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ (2010) Short screening tools for cancer-related distress: A review and diagnostic validity meta-analysis. Journal of the National Comprehensive Cancer Network 8(4), 487–494. [DOI] [PubMed] [Google Scholar]
- Molton IR and Terrill AL (2014) Overview of persistent pain in older adults. American Psychology 69(2), 197–207. doi: 10.1037/a0035794 [DOI] [PubMed] [Google Scholar]
- Moorey S and Greer S (2002) Cognitive Behaviour Therapy for People with Cancer. New York: Oxford University Press. [Google Scholar]
- Moorey S, Greer S, Watson M, et al. (1991) The factor structure and factor stability of the hospital anxiety and depression scale in patients with cancer. British Journal of Psychiatry 158, 255–259. [DOI] [PubMed] [Google Scholar]
- Moyer A, Sohl SJ, Knapp-Oliver SK, et al. (2009) Characteristics and methodological quality of 25 years of research investigating psychosocial interventions for cancer patients. Cancer Treatment Reviews 35(5), 475–484. doi: 10.1016/j.ctrv.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Alliance for Caregiving (2016) Cancer Caregiving in the U.S.: An Intense, Episodic, and Challenging Care Experience. Available at: https://www.caregiving.org/wp-content/uploads/2020/05/CancerCaregivingReport_FINAL_June-17-2016.pdf (accessed October 31, 2020).
- National Alliance for Caregiving, AARP Public Policy Institute (2015) Caregiving in the U.S., 2015. Available at: https://www.aarp.org/content/dam/aarp/ppi/2015/caregiving-in-the-united-states-2015-report-revised.pdf (accessed October 31, 2020).
- Nelson CJ, Balk EM and Roth AJ (2010) Distress, anxiety, depression, and emotional well-being in African-American men with prostate cancer. Psycho-Oncology 19(10), 1052–1060. doi: 10.1002/pon.1659 [DOI] [PubMed] [Google Scholar]
- Nightingale G, Hajjar E, Swartz K, et al. (2015) Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. Journal of Clinical Oncology 33(13), 1453–1459. doi: 10.1200/jco.2014.58.7550 [DOI] [PubMed] [Google Scholar]
- Oh PJ (2017) Predictors of cognitive decline in people with cancer undergoing chemotherapy. European Journal of Oncology Nursing 27, 53–59. doi: 10.1016/j.ejon.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 5(6), 649–655. [PubMed] [Google Scholar]
- Osborn RL, Demoncada AC and Feuerstein M (2006) Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. The International Journal of Psychiatry in Medicine 36(1), 13–34. [DOI] [PubMed] [Google Scholar]
- Parpa E, Tsilika E, Gennimata V, et al. (2014) Elderly cancer patients’ psychopathology: A systematic review: Aging and mental health. Archives of Gerontology and Geriatrics 60(1), 9–15. doi: 10.1016/j.archger.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Pedersen AE, Sawatzky JA and Hack TF (2010) The sequelae of anxiety in breast cancer: A human response to illness model. Oncology Nursing Forum 37(4), 469–475. doi: 10.1188/10.onf.469-475 [DOI] [PubMed] [Google Scholar]
- Prieto JM, Blanch J, Atala J, et al. (2002) Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. Journal of Clinical Oncology 20(7), 1907–1917. [DOI] [PubMed] [Google Scholar]
- Prithviraj GK, Koroukian S, Margevicius S, et al. (2012) Patient characteristics associated with polypharmacy and inappropriate prescribing of medications among older adults with cancer. Journal of Geriatric Oncology 3(3), 228–237. doi: 10.1016/j.jgo.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Parsons HA, Elsayem A, et al. (2009) Characteristics and correlates of dyspnea in patients with advanced cancer. Journal of Palliative Medicine 12(1), 29–36. doi: 10.1089/jpm.2008.0158 [DOI] [PubMed] [Google Scholar]
- Rossi Ferrario S, Zotti AM, Massara G, et al. (2003) A comparative assessment of psychological and psychosocial characteristics of cancer patients and their caregivers. Psycho-Oncology 12(1), 1–7. doi: 10.1002/pon.626 [DOI] [PubMed] [Google Scholar]
- Salvo N, Zeng L, Zhang L, et al. (2012) Frequency of reporting and predictive factors for anxiety and depression in patients with advanced cancer. Clinical Oncology 24(2), 139–148. doi: 10.1016/j.clon.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Schag CA and Heinrich RL (1989) Anxiety in medical situations: Adult cancer patients. Journal of Clinical Psychology 45(1), 20–27. doi: [DOI] [PubMed] [Google Scholar]
- Segrin C, Badger T, Dorros SM, et al. (2007) Interdependent anxiety and psychological distress in women with breast cancer and their partners. Psycho-Oncology 16(7), 634–643. doi: 10.1002/pon.1111 [DOI] [PubMed] [Google Scholar]
- Sklenarova H, Krumpelmann A, Haun MW, et al. (2015) When do we need to care about the caregiver? Supportive care needs, anxiety, and depression among informal caregivers of patients with cancer and cancer survivors. Cancer 121(9), 1513–1519. doi: 10.1002/cncr.29223 [DOI] [PubMed] [Google Scholar]
- Smith EM, Gomm SA and Dickens CM (2003) Assessing the independent contribution to quality of life from anxiety and depression in patients with advanced cancer. Palliative Medicine 17(6), 509–513. [DOI] [PubMed] [Google Scholar]
- Snaith RP and Zigmond AS (1986) The hospital anxiety and depression scale. British Medical Journal 292(6516), 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen SC, de Haes HC, Voest EE, et al. (2006) Does age matter in palliative care? Critical Reviews in Oncology/Hematology 60(2), 152–158. doi: 10.1016/j.critrevonc.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Trevino KM, Stern A and Prigerson HG (2020) Adapting psychosocial interventions for older adults with cancer: A case example of Managing Anxiety from Cancer (MAC). Journal of Geriatric Oncology 11(8), 1319–1323. doi: 10.1016/j.jgo.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Abbema D, van Vuuren A, van den Berkmortel F, et al. (2017) Functional status decline in older patients with breast and colorectal cancer after cancer treatment: A prospective cohort study. Journal of Geriatric Oncology 8(3), 176–184. doi: 10.1016/j.jgo.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Watson M, White C, Lynch A, et al. (2017) Telephone-delivered individual cognitive behavioural therapy for cancer patients: An equivalence randomised trial. Psycho-Oncology 26(3), 301–308. doi: 10.1002/pon.4338 [DOI] [PubMed] [Google Scholar]
- Webster K, Cella D and Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health and Quality of Life Outcomes 1, 79. doi: 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzner MA, Jacobsen PB, Wagner H Jr. et al. (1999) The Caregiver Quality of Life Index-Cancer (CQOLC) scale: Development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Quality of Life Research 8(1–2), 55–63. [DOI] [PubMed] [Google Scholar]
- Westen D and Morrison K (2001) A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: An empirical examination of the status of empirically supported therapies. Journal of Consulting and Clinical Psychology 69(6), 875–899. [PubMed] [Google Scholar]
- Zhang X, Sun M, Liu S, et al. (2018) Risk factors for falls in older patients with cancer. BMJ Supportive & Palliative Care 8(1), 34–37. doi: 10.1136/bmjspcare-2017-001388 [DOI] [PubMed] [Google Scholar]
- Zigmond AS and Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67(6), 361–370. [DOI] [PubMed] [Google Scholar]