Abstract
Aim:
To evaluate the similarities and differences in barrier function of a peri-implant epithelium (PIE) versus a native junctional epithelium (JE).
Materials and methods:
A mouse model was used wherein titanium implants were placed sub-occlusally in healed extraction sites. The PIE was examined at multiple timepoints after implant placement, to capture and understand the temporal nature of its assembly and homeostatic status. Mitotic activity, hemidesmosomal attachment apparatus, and inflammatory responses in the PIE were compared against a JE. Additionally, we evaluated whether the PIE developed a Wnt-responsive stem cell niche like a JE.
Results:
The PIE developed from oral epithelium (OE) that had, by the time of implant placement, lost all characteristics of a JE. Compared with a JE, an established PIE had more proliferating cells, exhibited lower expression of attachment proteins, and had significantly more inflammatory cells in the underlying connective tissue. Wnt-responsive cells in the OE contributed to an initial PIE, but Wnt-responsive cells and their descendants were lost as the PIE matured.
Conclusions:
Although histologically similar, the PIE lacked a Wnt-responsive stem cell niche and exhibited characteristics of a chronically inflamed tissue, which contributed to its suboptimal barrier functions compared with a native JE.
Keywords: gingiva, oral epithelium, dental implants, Wnt-responsive, inflammation
Introduction
Osseointegration is essential for implant success but equally important is mucointegration e.g., the attachment of soft tissues to the transmucosal portion of an implant (Klinge, Meyle, & Working, 2006). A disruption in mucointegration can manifest as peri-implant mucositis and, if not resolved, can progress to peri-implantitis (Berglundh et al., 2018; O’Neal, Sauk, & Somerman, 1992; Ramanauskaite, Becker, & Schwarz, 2018) Peri-implantitis is universally agreed to begin with a breakdown in this soft tissue attachment (Esposito, Hirsch, Lekholm, & Thomsen, 1998; Lindhe, Meyle, & Group, 2008; Rosen & Froum, 2016; Schwarz, Derks, Monje, & Wang, 2018). Consequently, methods to strength and maintain this soft tissue attachment are critical for the success of an implant.
Comparisons have been made between the peri-implant epithelium (PIE) and the junctional epithelium (JE) around teeth. The JE is a continuously regenerating, non-keratinized epithelial barrier that is permeable, proliferative, and adherent, and JE cells are routinely shed to eliminate microbial invasion (reviewed in (Schroeder & Listgarten, 2003)). Histological analyses (Berglundh et al., 1991; Donley & Gillette, 1991; Lindhe & Berglundh, 1998) have emphasized the resemblance between a PIE and a JE, in that both are continuous with their adjacent keratinized oral epithelia, and that both exhibit an interfacial tissue containing hemidesmosomes and a basal lamina. Below the PIE and JE is a zone of collagen-rich connective tissue, with well-organized marginal bone and small blood vessels. These and other comparative analyses, however, have left open the question of whether the barrier functions of the two tissues are equivalent. Some molecular markers expressed by cells in the JE are also expressed by cells in the PIE (Fujiseki, Matsuzaka, Yoshinari, Shimono, & Inoue, 2003) but it is unclear whether their levels of expression- and by extension, their functions- are equivalent.
We focused on characterizing in both a developing and in a stable PIE, those features known to contribute to epithelial barrier functions. One example is the continual production, migration and shedding of epithelial cells that constitutes an efficient dynamic barrier to microbial invasion. In the JE, we recently demonstrated that epithelial cells are continually produced by a stem cell niche at the base of the JE (Yuan, Chen, Gauer, et al., 2020). We demonstrated that daughter cells arising from this stem cell population differentiate and contribute to the attachment apparatus that anchors the JE to the tooth surface via Laminin5-expressing hemidesmosomes (Yuan, Chen, Van Brunt, et al., 2020). Once epithelial cells reach their adult morphology, mitotic activity must be balanced by cell death and/or extrusion (Guillot & Lecuit, 2013) and this sequence of events is also observed in the JE. Cells at the tip of the JE express Caspase3 and TUNEL (Yajima-Himuro et al., 2014; Yuan, Chen, Gauer, et al., 2020), guaranteeing cell turnover in the JE.
When a tooth is extracted, the JE is gradually replaced by oral epithelium (OE). The OE differs from the JE in many respects: it is keratinized whereas the JE is not; its rate of proliferation is significantly lower than the JE; and it does not have an equivalent attachment apparatus (Yuan, Chen, Van Brunt, et al., 2020). Although the OE contains stem cells, their turnover rates are vastly different than the JE (Yuan et al., 2019).
What happens when an implant is placed into this healed OE? An epithelial interface clearly forms around the dental implant, but whether it is functionally equivalent to a JE- or is more similar to OE- is not known; this knowledge gap served as the impetus for our study. Using an established mouse model of oral implant osseointegration (Coyac et al., 2020; Mouraret et al., 2014; Pei et al., 2017), we undertook a multiscale analysis of the developing and established PIE and compared it against a JE. Using histology, imaging, molecular, and cellular analyses we gained insights into biological signals that have a role in shaping and maintaining the barrier functions of these two epithelial attachments.
Methods and Materials
Experimental groups and animals
A detailed information including all experimental groups is shown in Supplemental Fig. 1 and table 1. All experimental protocols followed ARRIVE guidelines and were approved by the Stanford Committee on Animal Research (#13146). Axin2CreERT2/+, and R26RmTmG/+ mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were housed in a temperature-controlled environment with 12-hour light/dark cycles.
Surgeries
Twenty-one days after tooth extraction (see Supplementary information), an implant was placed in every healed site. A soft tissue flap was raised, a pilot hole was produced using a 0.38 mm diameter drill (Drill Bit City, USA) run at 1000 rpm with saline irrigation. The osteotomy was expanded with a 0.50 mm diameter drill after which a screw-shaped titanium alloy implant (Ti-6AI-4V) with a diameter of 0.62 mm (Retopin, NTI Kahla GmbH, Germany) was manually placed. The implant was positioned ~0.6 mm above the alveolar ridge and gingiva, in a sub-occlusal position. Healing of the soft tissue was examined on post-implant days (PID) 1, 3, 7, 14, and 21. There was no evidence of infection or prolonged inflammation at surgical sites, nor around implants. No antibiotics were given to operated animals.
Statistical analyses
Results are presented as the mean ± standard deviation of independent replicates. GraphPad Prism was used for statistical analyses. Student’s t-test or one-way ANOVA was used. P≤0.05 was considered significant.
Details on lineage tracing, μCT image acquisition, tissue sample preparation, histology and staining, immunohistochemistry, EdU injections and detection, and quantification protocols can be found in supporting Supplementary information.
Results
After tooth extraction, the JE is replaced by an OE
The soft tissue attachment apparatus surrounding the molars was evaluated before and after tooth extraction. One distinguishing characteristic of a JE is its non-keratinized status (Fig. 1A) whereas the OE contains terminally differentiated keratinocytes identified by Filaggrin immunostaining (Fig. 1B). Only JE cells facing the tooth surface expressed the hemidesmosomal markers Laminin5 (Fig. 1C) and integrin ß4 (Fig. 1D); OE cells did not. EdU uptake demonstrated that cells in both the OE and JE are mitotically active (Fig. 1E) but JE cells had a significantly higher rate of cell division (Supplemental Fig. 2).
Figure 1. After tooth extraction, JE barrier characteristics are replaced with OE barrier functions.
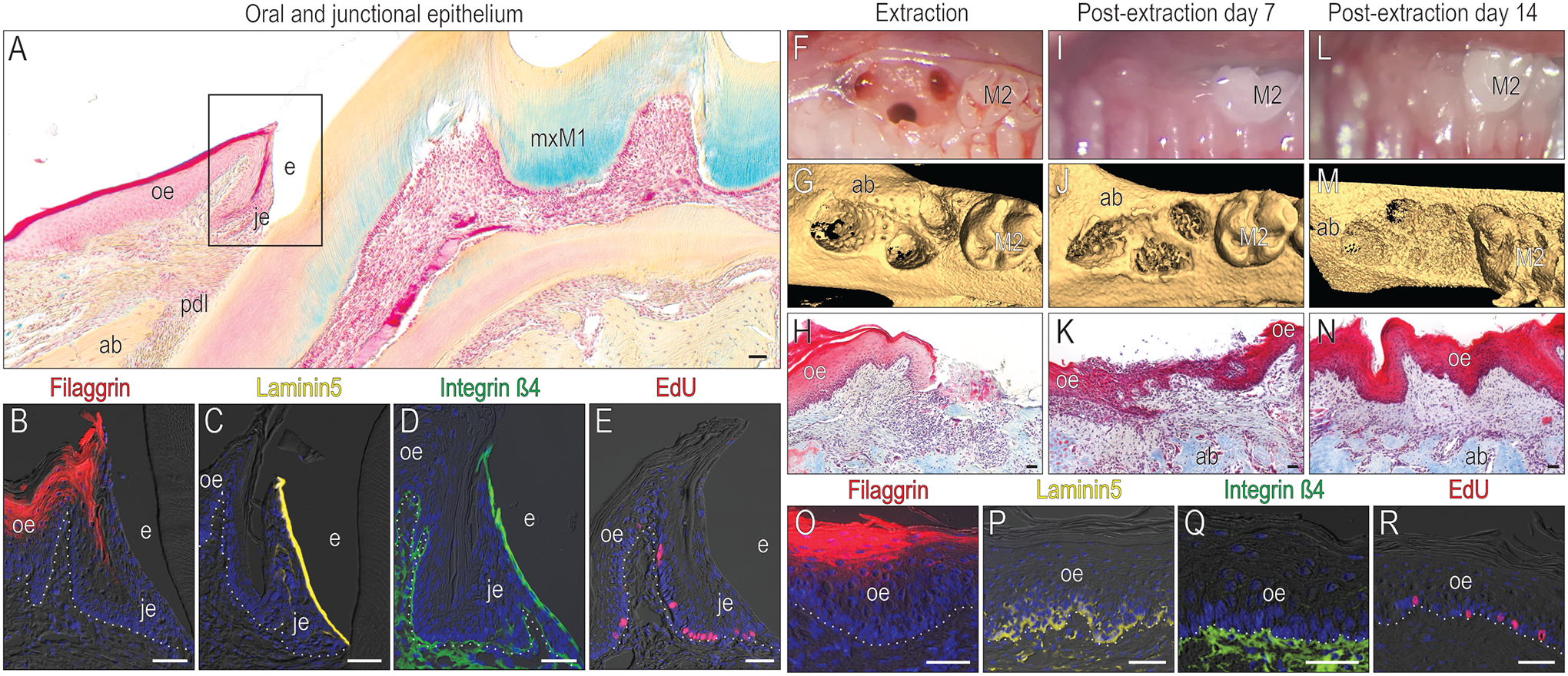
(A) A representative tissue section stained with pentachrome to illustrate keratinized OE (red color), non-keratinized JE (pink), alveolar bone (yellow gold) and connective tissue/PDL. In the OE and JE (the black box area in A), immunohistochemical localization of (B) the terminal keratinocyte marker Filaggrin, (C) the hemidesmosomal markers Laminin5 and (D) Integrin β4; and (E) mitotically active cells that have incorporated EdU. (F) The mxM1 extraction socket viewed clinically and (G) using μCT imaging. (H) Representative tissue section stained with Masson’s trichrome from the post-extraction day (PED) 0 extraction socket. (I) Clinical, (J) μCT, and (K) histological assessment of the PED7 site. (L) Clinical, (M) μCT, and (N) histological assessment of the PED14 site. In this OE, examined 14 days after tooth extraction e.g., PED14, immunohistochemical localization of (O) Filaggrin, (P) Laminin5, and (Q) Integrin β4. (R) Mitotically active cells are identified by EdU. Dotted white lines indicate the demarcation between the epithelium and connective tissue. Abbreviations: ab, alveolar bone; e, enamel; je, junctional epithelium; oe, oral epithelium; pdl, periodontal ligament; mxM1, maxillary first molar; M2, maxillary second molar. Scale bars: 50 μm.
Molars were extracted, leaving the surrounding soft tissues relatively intact (Fig. 1F). Hard tissue repair was monitored by μCT (Fig. 1G) and soft tissue healing was monitored by histology (Fig. 1H). By PED 7, clinical photographs showed minimal inflammation (Fig. 1I), μCT confirmed that bone repair was underway (Fig. 1J), and histological analyses demonstrated re-epithelialization was complete (Fig. 1K). On PED14, healing sites showed no signs of redness or swelling (Fig. 1L), bone repair was still ongoing (Fig. 1M), and a keratinized OE now occupied the space where a JE had once existed (Fig. 1N).
Using the same molecular markers e.g., Filaggrin, Laminin5, Integrin ß4, and EdU, the epithelium over the healed extraction site was re-assessed on PED14. The JE-specific expression patterns had been replaced with OE expression patterns (Fig. 1O–R): terminally differentiated keratinocytes occupied the upper layers of the OE (Fig. 1O). Laminin5 expression was now restricted to basal lamina cells, indicating their hemidesmosomal attachment to the underlying integrin ß4-expressing connective tissue (Fig. 1P,Q). Mitotic activity at the healed site had returned to baseline levels e.g., those observed in an intact OE (Fig. 1R, Supplemental Fig. 2). Thus, the JE disappears following tooth removal and is replaced by OE.
Implant mucointegration is accomplished via a PIE whose structure resembles a JE
To evaluate a developing PIE, we positioned titanium implants in healed maxillary extraction sites (Fig. 2A,B). Compared to its expression in a native JE, Laminin5 was low in the healed OE (Fig. 2C). Into this healed OE, implants were placed at a sub-occlusal, supra-gingival position (Fig. 2D). Implant placement triggered a robust epithelial repair response: Between PID1 and 3, cells at the wound edge proliferated to produce a thickened epithelium adjacent to the implant (Fig. 2E,F).
Figure 2. Implant mucointegration is accomplished via a PIE whose structure resembles a JE.
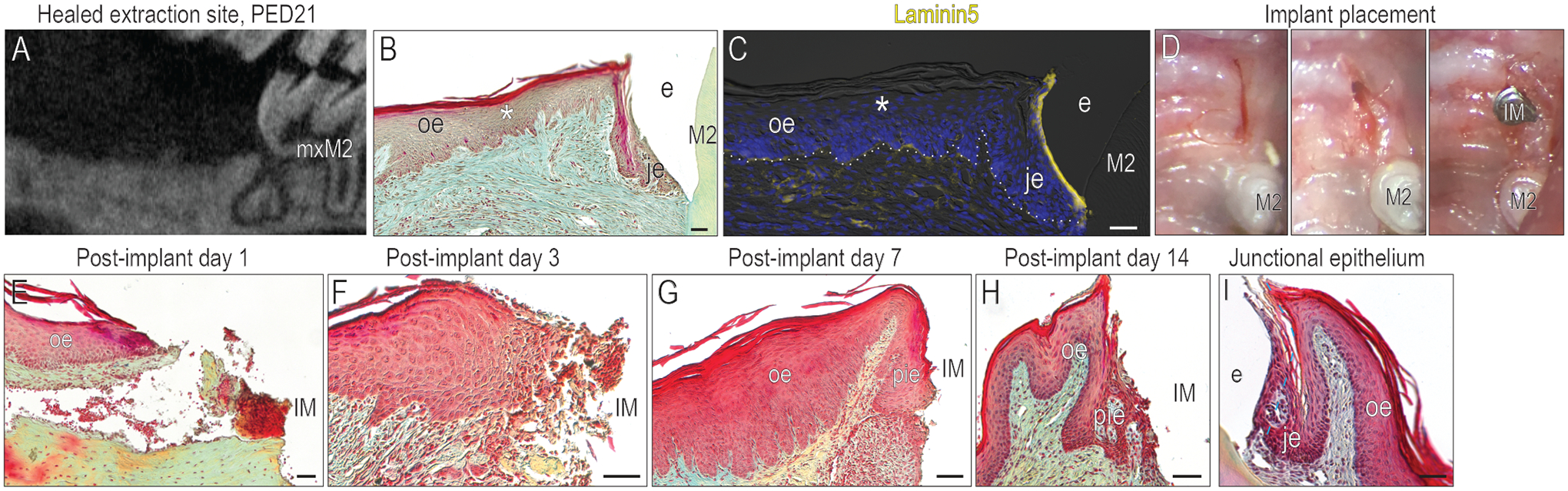
(A) A μCT imaging showing the healed extraction socket on PED21. (B) A representative tissue section stained with pentachrome showing that keratinized OE covered the healed mxM1 extraction site by PED21. (C) Compared to the JE around mxM2, healed OE exhibited low levels of Laminin5. (D) Site preparation for implant placement. The healing of the soft tissue around implant were examined by pentachrome staining on (E) PID1, (F) PID3, (G) PID7, and (H) PID14. (I) A representative tissue section stained with pentachrome showing the intact JE around tooth. Dotted white lines indicate the demarcation between the epithelium and connective tissue. Abbreviations: as in Fig. 1 and IM, implant; PIE, peri-implant epithelium. Scale bars: 50 μm.
By PID7, interfacial tissues had organized themselves into a PIE (Fig. 2G). Analyses on PID14 showed little change in PIE morphology from PID7 (Fig. 2H). Histological similarities were obvious between this PIE and a JE (compare Fig. 2H with 2I), prompting a deeper comparative analysis of the interfacial tissues.
Although structurally similar, the PIE lacks essential barrier features of a JE
We began the comparative analysis by determining how the PIE formed from OE. The OE wound edge was identified by Keratin5 (Fig. 3A); at this edge, robust cell proliferation was evident (Fig. 3B). Laminin5 was not expressed in the leading edge of the wound epithelium (Fig. 3C). By PID3, keratinocytes reached the implant surface (Fig. 3); these keratinocytes retained their exuberant mitotic activity (Fig. 3E); Laminin5 was minimally expressed in these keratinocytes, but strongly expressed in the basal lamina (Fig. 3F). By PID7, keratinocytes approximated the implant surface (Fig. 3G). Mitotic activity remained high (Fig. 3H), and Laminin5 expression was still restricted to the basal lamina (Fig. 3I), indicating that a hemidesmosomal attachment to the implant surface had not yet formed. By PID14, neither Keratin5 nor PCNA expression patterns were significantly altered (Fig. 3J,K) but some interfacial cells now expressed Laminin5 (Fig. 3L). Collectively, these data indicated that a PIE was established by PID14.
Figure 3. Although structurally similar, the PIE lacks essential barrier features of a JE.
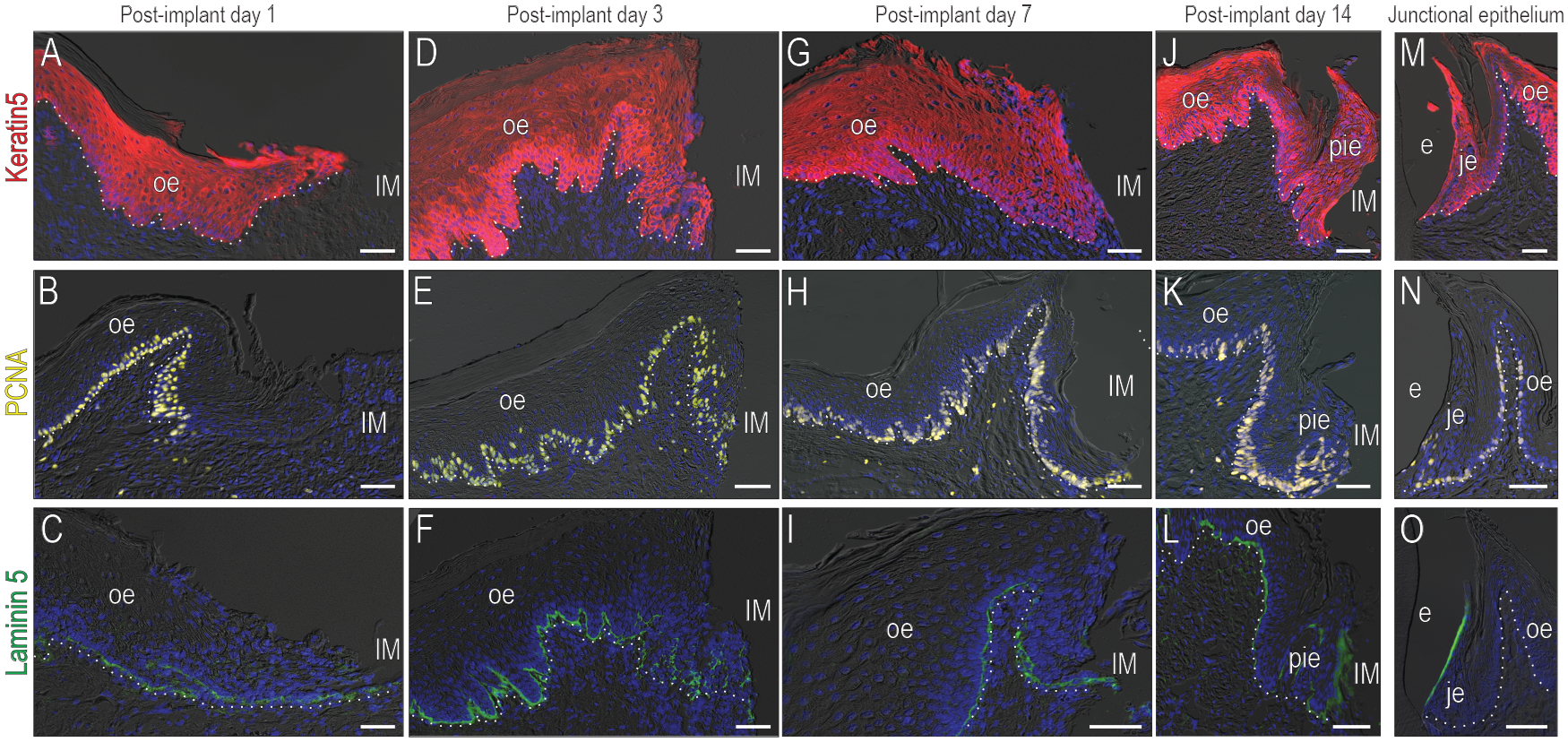
Healing of soft tissues around implants was examined using immunostaining for (A) the keratinocyte marker Keratin5 (B) the proliferating cell marker PCNA and (C) the hemidesmosomal marker Laminin5 on PID1; (D,E,F) on PID3; (G,H,I) on PID7 and (J,K,L) on PID14. The formation of the PIE was compared to (M) Keratin5, (N) PCNA, and (O) Laminin5 expression in a JE.
This established PIE was compared to a native JE. In both sites, keratinocytes contributed to the interfacial tissue (Fig. 3M) but PCNA expression was more widespread in cells of the established PIE (Fig. 3K) than in a JE (Fig. 3N). Laminin5 was weakly expressed by interfacial cells in the PIE compared to a JE (Fig. 3O).
The PIE lacks a Wnt-responsive stem cell niche
A JE harbors a stem cell niche that supports the soft tissue attachment and the mitotic activity of the tissue (Yuan, Chen, Gauer, et al., 2020; Yuan, Chen, Van Brunt, et al., 2020). To determine whether the PIE had a Wnt-responsive stem cell niche similar to the JE’s niche, the lineage tracing strain Axin2CreErt2/+;R26RmTmG/+ was employed. A single dose of tamoxifen was delivered to mice and the JE was evaluated (Fig. 4A). One day after tamoxifen, a subset of basal lamina cells was labeled with GFP (Fig. 4B), demonstrating their Wnt-responsive status. Seven days later, another subset of mice was examined; at this point the entire JE was occupied with GFP+ve cells (Fig. 4C). Since mice had only received one dose of tamoxifen, these additional GFP+ve cells were descended from the original Wnt-responsive population. Three hundred days later, GFP+ve cells still filled the entire JE (Fig. 4D), demonstrating that the original population of Wnt-responsive stem cells was still producing descendants that maintained the JE barrier functions.
Figure 4. The PIE lacks a stem cell niche.
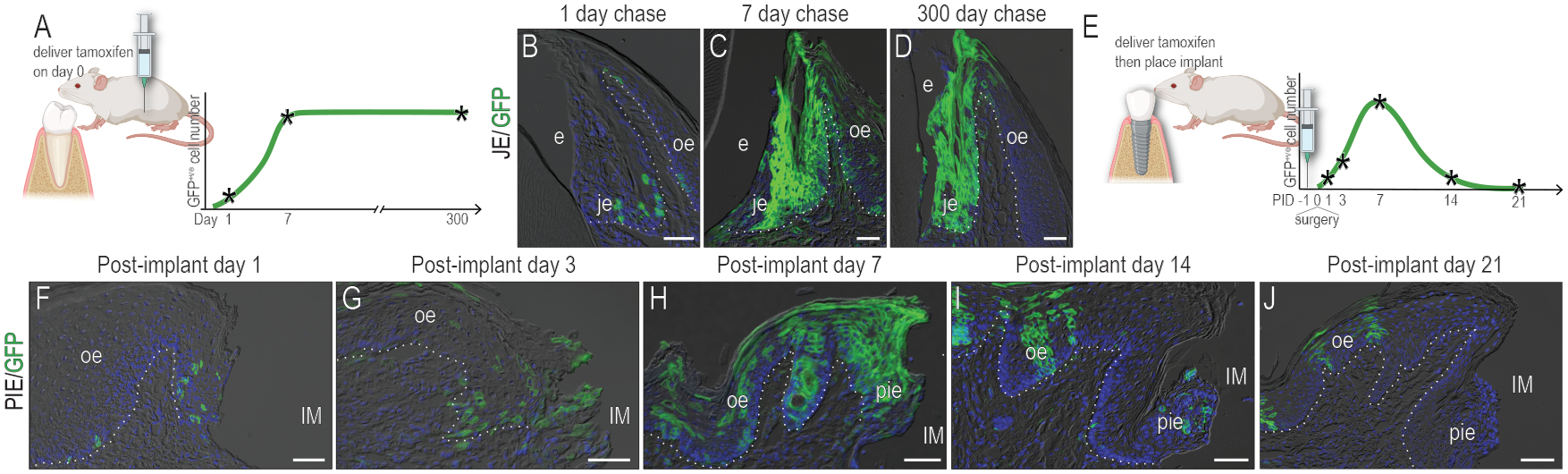
(A) Schematic of lineage tracing. 3M old Axin2CreERT2/+ ;R26RmTmG/+ mice were given a single dose of tamoxifen and GFP+ve cells were analyzed (B) 1 day, (C) 7 days, and (D) 300 days later. (E) Schematic of lineage tracing in the mice with implants. Axin2CreERT2/+ ;R26RmTmG/+ mice were given a single dose of tamoxifen one day before implant placement. GFP+ve cells were analyzed on (F) PID1, (G) PID3, (H) PID7, (I) PID14, and (J) PID21. Dotted white lines indicate the demarcation between the epithelium and connective tissue. Abbreviations: as in Fig. 2. Scale bars: 50 μm.
An equivalent analysis was performed for the PIE (Fig. 4E). Mice received a single dose of tamoxifen one day preceding implant placement; one day after implant placement, a few Wnt-responsive, GFP+ve cells were identified in the basal lamina and in the leading edge of the OE wound (Fig. 4F). On PID3, descendants of the original Wnt-responsive population had expanded near the implant (Fig. 4G). On PID7, the PIE was filled with progeny from the original Wnt-responsive population (Fig. 4H) but thereafter, the number of GFP+ve progeny diminished. By PID14, only a few GFP+ve cells were left in the PIE (Fig. 4I). By PID21, the PIE was devoid of GFP+ve cells (Fig. 4J). GFP+ve clones, however, were still evident in the adjacent OE (Fig. 4J). Collectively, these data demonstrate that a Wnt-responsive stem cell niche like the one that exists in the JE did not form around implants.
The PIE exhibits signs of chronic inflammation
Wnt-responsive stem cells initially contributed to a PIE but when cell turnover exhausted this GFP+ve population a persistent Wnt-responsive stem cell niche failed to take its place. Why did the PIE fail to support the establishment of a stem cell niche? We considered this question by first examining the distribution of mitotically active cells that are produced by a stem cell niche, in the PIE vs. in a JE.
Previous analyses (Fig. 3) indicated more proliferating cells occupied the PIE versus a JE. Here a complementary strategy was used to label a subset of proliferating cells via EdU incorporation. EdU was delivered to mice then 2h later, tissues were harvested and analyzed: an established PIE had significantly more proliferating cells than a JE and moreover, these mitotically active cells were not always restricted to the basal lamina like they were in a JE (compare Fig. 5A,B, supplemental Fig. 3). The pattern of widely disseminated mitotically active cells in the PIE was reminiscent of the elevated cell proliferation seen in chronically inflamed tissues (Kiraly, Gong, Olipitz, Muthupalani, & Engelward, 2015).
Figure 5. The PIE exhibits signs of chronic inflammation.
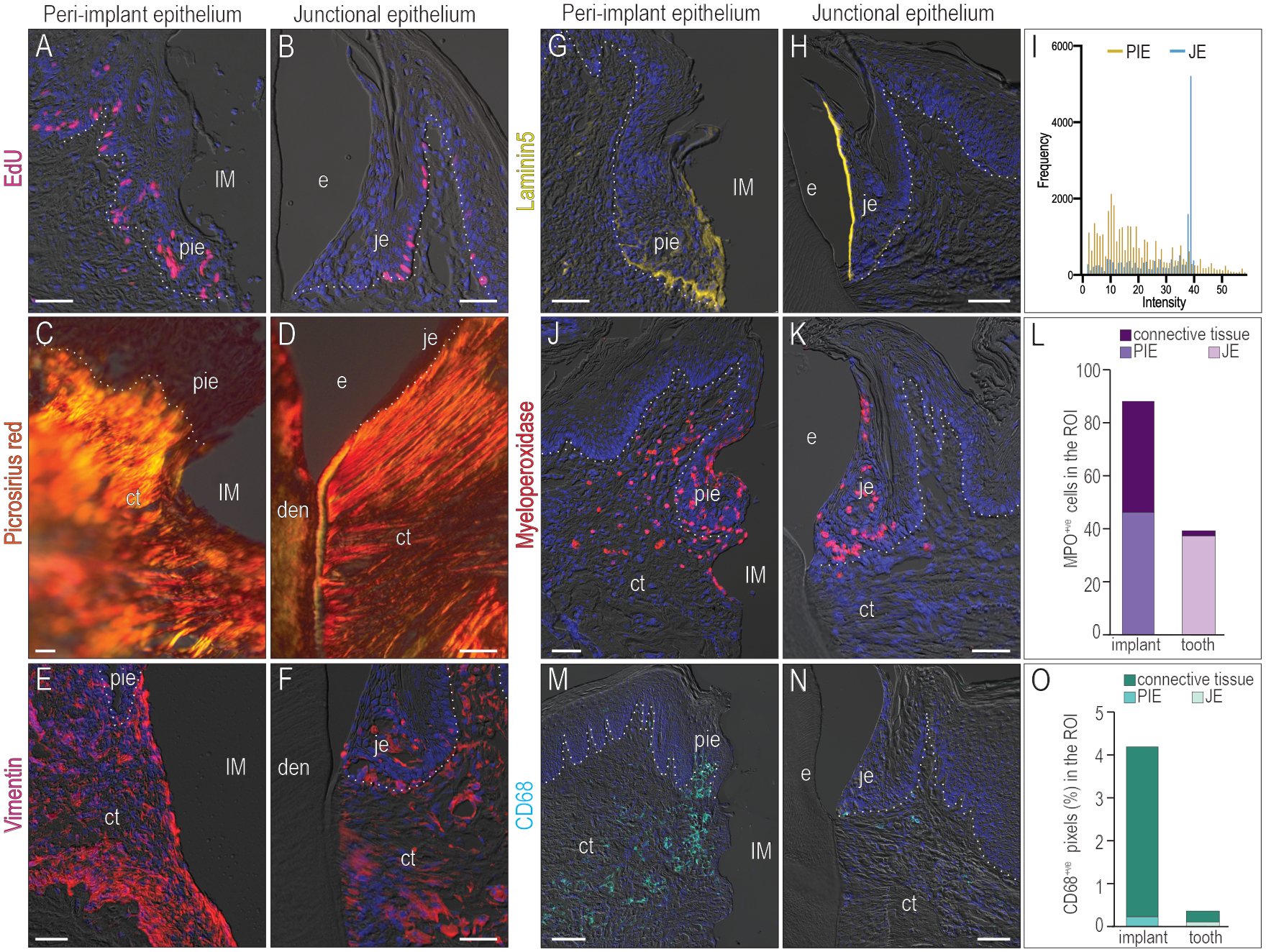
Mitotically active cells were labeled by EdU in (A) the PID14 PIE and (B) the JE. The fibrosis of the connective tissue was examined by (C,D) Picrosirius red staining and (E,F) Vimentin staining. The attachment to (G) the implant and (H) the tooth was evaluated by Laminin5 staining. (I) Histogram of Laminin5 expression. Immunostaining for Myeloperoxidase (MPO), a marker for neutrophils, in the (J) PIE and (K) JE. (L) Quantification of MPO+ve cells in the JE, PIE and connective tissue beneath them. Immunostaining for CD68, a marker for macrophages and monocytes, in the (M) PIE and (N) JE. (O) Quantification of CD68 expression in the JE, PIE and connective tissue beneath them. Dotted white lines indicate the demarcation between the epithelium and connective tissue. Abbreviations: as in Fig. 2 and den, dentin; ct, connective tissue.
Persistent inflammation is typically associated with fibrosis, prompting us to evaluate collagen organization in the PIE. Picrosirius red staining highlighted the thickened collagen fibers in the connective tissue underlying the PIE (Fig. 5C) vs. the thinner, more aligned collagen fibers in the connective tissue underlying a JE (Fig. 5D). The intermediate filament protein Vimentin is a marker of fibrotic, inflamed tissues (dos Santos et al., 2015) and its expression was markedly higher in connective tissue facing the implant surface (Fig. 5E) as opposed to connective tissue under a JE (Fig. 5F).
The expression pattern of Laminin5 was different in the PIE (Fig. 5G) compared with that in the JE (Fig. 5H): in a JE, Laminin5 expression was highest in cells facing the tooth surface (Fig. 5H), corresponding to a signal intensity peak at ~40 in the histogram (blue lines, Fig. 5I). In the PIE, no such peak was observed; instead, the IHC signal was diffusely distributed throughout the basal lamina and in cells facing the implant surface (gold lines, Fig. 5I).
Using myeloperoxidase (Fig. 5J–L) and CD68 (Fig. 5M–O) as markers of neutrophils and monocytes/macrophages, we confirmed that the number of inflammatory cells was significantly higher in the PIE and adjacent connective tissue than in a JE and its associated connective tissue (Fig. 5J,M; quantified in L,O). Collectively, these data supported the conclusion that the PIE exhibited characteristics of a chronically inflamed tissue whose barrier functions were compromised in comparison to a native JE.
Discussion
Across species, soft tissue-implant interfaces share common features
The connection between soft tissues and an implant e.g., mucointegration has been a subject of considerable interest and for obvious reasons: this interface serves as the first biological defense against the ingression of oral pathogens (reviewed in (Sculean, Gruber, & Bosshardt, 2014)). In this preclinical study, sub-occlusal titanium implants were placed into healed extraction sites and the process of mucointegration was followed over time. Imaging and histology coupled with molecular/cellular assays were used to characterize the tissues (Fig. 1). The PIE was examined around sub-occlusal implants while osseointegration was ongoing (Fig. 2–4) and after osseointegration was complete (Fig. 5). At all timepoints, comparisons were made with an intact JE.
Berglundh and colleagues conducted a similar study in beagle dogs but only one timepoint e.g., 3 months post implant, was analyzed (Berglundh et al., 1991). Size differences aside, our findings in a rodent model are remarkably consistent with these data from a dog model. In both species the peri-implant mucosa was keratinized and collagen fibers in the underlying connective tissue were oriented parallel with the implant surface (Figs. 2,5). Clinically, neither implants in dogs nor mice showed overt clinical signs of inflammation (Fig. 2).
There were some noteworthy differences. Although Berglundh reported that peri-implant and gingival tissues were free of inflammatory cell infiltrate, the analysis was limited to histologic inspection (Berglundh et al., 1991). Using molecular markers for neutrophils and macrophages, we found inflammatory cells infiltrated in both the PIE and underlying connective tissue (Fig. 5). These data from a mouse model are consistent with analyses of human peri-implant tissues, where investigators noted inflammatory cell infiltration and robust epithelial cell proliferation in the biopsies (Degidi et al., 2012). We observed the same two characteristics in the murine PIE (Figs. 3,5), leading us to conclude that our mouse model is sufficiently representative of other mammals, including humans, which supports the conclusion that the barrier functions of a JE are superior to those of the PIE.
The mechanoresponsiveness of the PIE and JE
Implants are osseointegrated whereas teeth exhibit a degree of physiological mobility in bone made possible by the fibrous periodontal ligament; as a consequence, the soft tissues attached to implants and teeth will also move differently when then implant/tooth is subjected to loading. In experiments shown here, implants were not functionally loaded but that does not exclude a role for mechanical influences on the form/function of a PIE. Mastication has a measurable impact on the barrier function of the gingiva, as has recently been demonstrated by Moutsopoulos and colleagues (Dutzan et al., 2017). They showed that within the gingiva proper, some T helper cells functions were instigated in response to masticatory forces (Dutzan et al., 2017). We speculate that masticatory forces also play a role in JE homeostasis and likely influence PIE function as well. In that regard, it may be informative to compare the PIE to an ankylosed tooth’s JE because both transmucosal structures would be associated with an “osseointegrated” type of anchorage in bone. In such a situation, masticatory-induced stress/strain distributions in peri-implant versus periodontal soft tissues would be similar.
Surface matters
One of the most obvious differences between the PIE and a JE is the surface to which the epithelia attach. Most studies evaluating the effects of implant surface composition on mucointegration, however, are carried out in tissue culture dishes (Kantarci, Hasturk, & Van Dyke, 2015; Sugawara et al., 2016); consequently, it is impossible to extrapolate from these studies whether one surface supports a better attachment, or is better at preventing peri-implant disease progression than another. Ultrastructural studies comparing implant surfaces have identified hemidesmosomes in the PIE, leading investigators to conclude that epithelial cells form a “firm attachment” to the implant surface (Gould, Westbury, & Brunette, 1984). It should be emphasized, however, that a direct measurement of attachment strength was not performed.
Another point of direct clinical relevance is that fact that in this study, the titanium alloy implants used did not undergo any surface modifications. It is reasonable to expect that different surfaces will elicit different responses from interfacial cells, and such studies could be especially informative because they may allow for ‘ranking’ of various surfaces with regards to their ability to support and maintain more JE-like barrier functions.
Limitations of this study
In order to support claims that one surface is better at promoting/supporting an epithelial attachment over another, there must be tools that are capable of actually measuring such presumptive differences. In the absence of such mechanical strength-testing, a rigorous, quantitative comparison of PIEs formed around implants with different surfaces will have to rely on ultrastructural/molecular/cellular characterizations of the interfaces. In this regard, a JE can serve as the “gold standard”: it constitutes a continuously regenerating defensive barrier that is simultaneously adherent to a hard substrate and yet permeable, and that is regularly shed to eliminate pathogen entry into the underlying connective tissues.
This study did not make use of human tissues but rather employed rodents as a model. Previous studies evaluating the PIE have used dogs and primates, but both species are now largely avoided for preclinical studies. This prompted us to ask, are there notable species-specific differences in a PIE? To address this question, we used the JE from various species as a comparator and found that the tissues from mice (Yuan, Chen, Gauer, et al., 2020; Yuan, Chen, Van Brunt, et al., 2020), rats (Takamori et al., 2017), dogs (Berglundh et al., 1991), primates (Braga & Squier, 1980) and humans (Overman & Salonen, 1994) are remarkably similar from a histologic perspective. While interspecies differences are likely negligible, what will invariably differ is the health status of the attachment apparatus from different species. In humans, the peri-implant tissues available for analysis are typically diseased whereas in rodents, they are typically healthy. Therefore, future studies will take into account how mechanical disruptions and bacterial accumulation adversely affect a JE- and a PIE.
Conclusions
Unlike periodontitis, there are no established treatments for peri-implantitis (Albrektsson & Wennerberg, 2019); therefore primary prevention of the disease is of critical importance (Renvert & Quirynen, 2015). Prevention depends upon a clear understanding of disease etiology; our approach is to understand as much as possible about the tissue responsible for mucointegration e.g., the PIE, and then build on these data to devise methods that enhance its barrier function. Using a mouse model, we demonstrated that mucointegration of an implant is accomplished via a PIE that histologically resembled a JE but whose barrier functions, as shown by three lines of evidence, were inferior. First, the PIE lacks a Wnt-responsive stem cell niche. Since daughter cells arising from this niche form the JE attachment to the tooth (Yuan, Chen, Gauer, et al., 2020; Yuan, Chen, Van Brunt, et al., 2020), loss of this niche is significant, as shown by the spotty hemidesmosomal attachment apparatus in the compared to that in a JE. Third, inflammatory cells populated the PIE connective tissues whereas in a JE, inflammatory cells were restricted to the epithelium. Together, these lines of evidence support the conclusion that the PIE is a sub-optimal barrier compared to a JE.
Supplementary Material
Clinical Relevance.
Scientific rationale for this study:
Mucointegration is critical for implant success but how the peri-implant epithelium (PIE) forms and how its functions compare to a junctional epithelium (JE) are not known.
Principal findings:
Although histologically similar to a JE, the PIE established around titanium alloy implants used here was less effective as a barrier. Around teeth, the barrier functions of a JE depend upon a stem cell population; the PIE lacked this stem cell population.
Practical implications:
Improving the defensive characteristics of a peri-implant epithelium, to more closely mimic the barrier functions of a native JE, are key to dental implant success.
Acknowledgements
We thank Dr. Ye Tian for contributing tissue samples, and Dr. Masaki Arioka and Mr. Isaiah Dawid for contributing μCT imaging data in this study.
Sources of funding
The study was supported by a gift from the Bredt family to J.A.H. and U.S. Department of Health and Human Services National Institutes of Health National Institute of Dental and Craniofacial Research K99DE028585–02 to X.Y.
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflict of Interest Statement
All authors declare that no conflicts of interest exist.
References
- Albrektsson T, & Wennerberg A (2019). On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res, 21 Suppl 1, 4–7. doi: 10.1111/cid.12742 [DOI] [PubMed] [Google Scholar]
- Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, … Zitzmann N (2018). Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol, 89 Suppl 1, S313–S318. doi: 10.1002/JPER.17-0739 [DOI] [PubMed] [Google Scholar]
- Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, & Thomsen P (1991). The soft tissue barrier at implants and teeth. Clin Oral Implants Res, 2(2), 81–90. doi: 10.1034/j.1600-0501.1991.020206.x [DOI] [PubMed] [Google Scholar]
- Braga AM, & Squier CA (1980). Ultrastructure of regenerating junctional epithelium in the monkey. J Periodontol, 51(7), 386–392. doi: 10.1902/jop.1980.51.7.386 [DOI] [PubMed] [Google Scholar]
- Coyac BR, Salvi G, Leahy B, Li Z, Salmon B, Hoffmann W, & Helms JA (2020). A novel system exploits bone debris for implant osseointegration. J Periodontol. doi: 10.1002/JPER.20-0099 [DOI] [PubMed] [Google Scholar]
- Degidi M, Artese L, Piattelli A, Scarano A, Shibli JA, Piccirilli M, … Iezzi G (2012). Histological and immunohistochemical evaluation of the peri-implant soft tissues around machined and acid-etched titanium healing abutments: a prospective randomised study. Clin Oral Investig, 16(3), 857–866. doi: 10.1007/s00784-011-0574-3 [DOI] [PubMed] [Google Scholar]
- Donley TG, & Gillette WB (1991). Titanium endosseous implant-soft tissue interface: a literature review. J Periodontol, 62(2), 153–160. doi: 10.1902/jop.1991.62.2.153 [DOI] [PubMed] [Google Scholar]
- dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, … Ridge KM (2015). Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun, 6, 6574. doi: 10.1038/ncomms7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, … Moutsopoulos NM (2017). On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity, 46(1), 133–147. doi: 10.1016/j.immuni.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Hirsch JM, Lekholm U, & Thomsen P (1998). Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci, 106(3), 721–764. [DOI] [PubMed] [Google Scholar]
- Fujiseki M, Matsuzaka K, Yoshinari M, Shimono M, & Inoue T (2003). An experimental study on the features of peri-implant epithelium: immunohistochemical and electron-microscopic observations. Bull Tokyo Dent Coll, 44(4), 185–199. doi: 10.2209/tdcpublication.44.185 [DOI] [PubMed] [Google Scholar]
- Gould TR, Westbury L, & Brunette DM (1984). Ultrastructural study of the attachment of human gingiva to titanium in vivo. J Prosthet Dent, 52(3), 418–420. doi: 10.1016/0022-3913(84)90459-1 [DOI] [PubMed] [Google Scholar]
- Guillot C, & Lecuit T (2013). Mechanics of epithelial tissue homeostasis and morphogenesis. Science, 340(6137), 1185–1189. doi: 10.1126/science.1235249 [DOI] [PubMed] [Google Scholar]
- Kantarci A, Hasturk H, & Van Dyke TE (2015). Animal models for periodontal regeneration and peri-implant responses. Periodontol 2000, 68(1), 66–82. doi: 10.1111/prd.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly O, Gong G, Olipitz W, Muthupalani S, & Engelward BP (2015). Inflammation-induced cell proliferation potentiates DNA damage-induced mutations in vivo. PLoS Genet, 11(2), e1004901. doi: 10.1371/journal.pgen.1004901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge B, Meyle J, & Working G (2006). Soft-tissue integration of implants. Consensus report of Working Group 2. Clin Oral Implants Res, 17 Suppl 2, 93–96. doi: 10.1111/j.1600-0501.2006.001366.x [DOI] [PubMed] [Google Scholar]
- Lindhe J, & Berglundh T (1998). The interface between the mucosa and the implant. Periodontol 2000, 17, 47–54. doi: 10.1111/j.1600-0757.1998.tb00122.x [DOI] [PubMed] [Google Scholar]
- Lindhe J, Meyle J, & Group, D. o. E. W. o. P. (2008). Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol, 35(8 Suppl), 282–285. doi: 10.1111/j.1600-051X.2008.01283.x [DOI] [PubMed] [Google Scholar]
- Mouraret S, Hunter DJ, Bardet C, Brunski JB, Bouchard P, & Helms JA (2014). A pre-clinical murine model of oral implant osseointegration. Bone, 58, 177–184. doi: 10.1016/j.bone.2013.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal RB, Sauk JJ, & Somerman MJ (1992). Biological requirements for material integration. J Oral Implantol, 18(3), 243–255. [PubMed] [Google Scholar]
- Overman DO, & Salonen JI (1994). Characterization of the human junctional epithelial cells directly attached to the tooth (DAT cells) in periodontal disease. J Dent Res, 73(12), 1818–1823. doi: 10.1177/00220345940730120501 [DOI] [PubMed] [Google Scholar]
- Pei X, Wang L, Chen C, Yuan X, Wan Q, & Helms JA (2017). Contribution of the PDL to Osteotomy Repair and Implant Osseointegration. J Dent Res, 96(8), 909–916. doi: 10.1177/0022034517707513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanauskaite A, Becker K, & Schwarz F (2018). Clinical characteristics of peri-implant mucositis and peri-implantitis. Clin Oral Implants Res, 29(6), 551–556. doi: 10.1111/clr.13152 [DOI] [PubMed] [Google Scholar]
- Renvert S, & Quirynen M (2015). Risk indicators for peri-implantitis. A narrative review. Clin Oral Implants Res, 26 Suppl 11, 15–44. doi: 10.1111/clr.12636 [DOI] [PubMed] [Google Scholar]
- Rosen PS, & Froum SJ (2016). Emerging Issues Associated With Peri-implant Disease. Compend Contin Educ Dent, 37(7), 440–447;quiz448. [PubMed] [Google Scholar]
- Schroeder HE, & Listgarten MA (2003). The junctional epithelium: from strength to defense. J Dent Res, 82(3), 158–161. doi: 10.1177/154405910308200302 [DOI] [PubMed] [Google Scholar]
- Schwarz F, Derks J, Monje A, & Wang HL (2018). Peri-implantitis. J Periodontol, 89 Suppl 1, S267–S290. doi: 10.1002/JPER.16-0350 [DOI] [PubMed] [Google Scholar]
- Sculean A, Gruber R, & Bosshardt DD (2014). Soft tissue wound healing around teeth and dental implants. J Clin Periodontol, 41 Suppl 15, S6–22. doi: 10.1111/jcpe.12206 [DOI] [PubMed] [Google Scholar]
- Sugawara S, Maeno M, Lee C, Nagai S, Kim DM, Da Silva J, … Kondo H (2016). Establishment of Epithelial Attachment on Titanium Surface Coated with Platelet Activating Peptide. PLoS ONE, 11(10), e0164693. doi: 10.1371/journal.pone.0164693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori Y, Atsuta I, Nakamura H, Sawase T, Koyano K, & Hara Y (2017). Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin Oral Implants Res, 28(2), 163–170. doi: 10.1111/clr.12777 [DOI] [PubMed] [Google Scholar]
- Yajima-Himuro S, Oshima M, Yamamoto G, Ogawa M, Furuya M, Tanaka J, … Yamamoto M (2014). The junctional epithelium originates from the odontogenic epithelium of an erupted tooth. Sci Rep, 4, 4867. doi: 10.1038/srep04867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chen J, Gauer J, Xu Q, Van Brunt LA, & Helms JA (2020). The Junctional Epithelium Is Maintained by a Stem Cell Population. J Dent Res, 22034520960125. doi: 10.1177/0022034520960125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Chen J, Van Brunt LA, Grauer J, Xu Q, Pei X, … Helms JA (2020). Formation and regeneration of a Wnt-responsive junctional epithelium. J Clin Periodontol. doi: 10.1111/jcpe.13371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Xu Q, Zhang X, Van Brunt LA, Ticha P, & Helms JA (2019). Wnt-Responsive Stem Cell Fates in the Oral Mucosa. iScience, 21, 84–94. doi: 10.1016/j.isci.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


