Abstract
CD36 is a multifunctional transmembrane glycoprotein abundantly expressed in several cell types. Recent studies have identified CD36 in circulation (cCD36) in several chronic inflammatory diseases, including type 2 diabetes and chronic kidney disease, and proposed cCD36 to be a biomarker of disease activity. Whether cCD36 is present in hyperlipidemia, a condition characterized by oxidative stress and low-grade inflammation, is not known. In addition, the cellular origin of cCD36 and triggers of CD36 release have not been elucidated. We now demonstrate that plasma cCD36 level is increased in hyperlipidemic ApoE−/− and Ldlr−/− mice. Using several cell-specific CD36 knockout mice, we showed that multiple cell types contribute to cCD36 generation in hyperlipidemic conditions, with a particularly strong contribution from endothelial cells. In vitro studies have demonstrated that oxidized phospholipids, ligands for CD36 (oxPCCD36), which are known to accumulate in circulation in hyperlipidemia, induce a robust release of CD36 from several cell types. In vivo studies have demonstrated CD36 release into the circulation of WT mice in response to tail-vein injection of oxPCCD36. These findings document the presence of cCD36 in hyperlipidemia and identify a link between cCD36 and oxidized phospholipids generated under oxidative stress and low-grade inflammation associated with hyperlipidemia.
Keywords: Dyslipidemia, Scavenger receptor, Lipid oxidation, Oxidized phospholipids (oxPC), Circulating CD36, Endothelial cells, Macrophages, Adipocytes, Platelets
Graphical Abstract

Introduction
CD36 is a transmembrane glycoprotein and a member of the class B scavenger receptor family (1). CD36 is highly expressed on microvascular endothelial cells, platelets, macrophages, adipocytes, striated myocytes, mammary epithelial cells, and a lesser expression on several other cells, such as erythrocytes and hepatocytes. CD36 plays a role in lipid and lipoprotein metabolism, atherosclerosis, angiogenesis, thrombosis, and inflammation (2). Recent studies have identified a circulatory form of CD36 (cCD36) (3). cCD36 was first detected in the plasma of diabetes type 2 patients (3), but is also increased in patients with stage 5 chronic kidney disease and several others (3–6). Several studies have suggested that cCD36 might serve as a biomarker of disease activity in chronic inflammatory diseases, including diabetes, non-alcoholic steatohepatitis, and cardiovascular disease (7–11). While these findings suggest the potential importance of cCD36 in these disease conditions, the cellular sources, and triggers of release of cCD36 are still not established. Whether cCD36 is increased in hyperlipidemia, the major cause of cardiovascular disease, is also not known. We have previously demonstrated that hyperlipidemia and oxidative stress lead to accumulation in the circulation of oxidized phospholipids (oxPCCD36), which are specific ligands for scavenger receptor CD36 (12). Oxidized phospholipids have multiple biological activities to a significant extent, mediated by CD36 (13). In this work, we tested whether oxPCCD36 can trigger CD36 release from cells, leading to the generation of cCD36 under hyperlipidemic conditions.
In this study, we demonstrated that plasma cCD36 levels are increased in hyperlipidemic ApoE−/− and Ldlr−/− mice as compared to relatively normolipidemic control mice. Using several cell-specific CD36 knockout mice, we showed that multiple cell types produce cCD36 in hyperlipidemia with a particularly strong contribution from endothelial cells. In vitro studies demonstrated that specific groups of oxPCCD36, γ-hydroxyl or γ-oxo alkenal phospholipids, induce a robust release of CD36 from several cell types expressing high levels of CD36. Furthermore, tail-vein injection of oxPCCD36 into wild type (WT) mice induced CD36 release into the circulation.
Material and methods
Materials
Anti-hCD36 (AF1955) and anti-mCD36 (AF2519) antibodies, and Mouse CD36/SR-B3 DuoSet ELISA kit (DY2519) are from R&D systems (Minneapolis, MN). Amicon Ultra Centrifugal filters were purchase from MilliporeSigma (Burlington, MA). Different members of oxPCCD36, 9-keto-12-oxo-10-dodecenoic acid ester of 2-lyso-phosphocholine (KODA-PC), 9-hydroxy-12-oxo-10-dodecenoic acid esters of 2-lyso-PC (HODA-PC), and 5-keto-8-oxo-6-octenoic acid esters of 2-lyso-PC (KOOA-PC) were synthesized, purified, and the structures were confirmed by multinuclear NMR and high resolution mass spectrometry as previously described (12,14,15). The purity of synthetic lipids was routinely analyzed by high-performance liquid chromatography (HPLC) with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS). If lipids were found to be less than 98% pure, they were re-isolated prior to use. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PLPC) were from Avanti Polar Lipids (Alabaster, AL). All other reagents were purchased from Sigma-Aldrich if not mentioned otherwise.
Experimental animals
Wild type (WT) C57BL/6, ApoE−/−, Pf4-Cre+, Adipoq-Cre+, LysM-Cre+, and Ldlr−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). Tie2eCre mice were generated as mentioned earlier (16). CD36−/−, ApoE−/−-CD36−/−, and CD36fl/fl mice were generated as described earlier (17–19). To generate lines with cell type-specific deficiency of CD36 on ApoE−/− background we initially crossed ApoE−/− mice to CD36fl/fl and several Cre+ mice including macrophage-specific (LysM-Cre+), adipocyte-specific (Adipoq-Cre+), and platelet-specific (Pf4-Cre+) mice. Cell-type-specific CD36 knockout mice were then generated by crossing ApoE−/−-CD36fl/fl to the corresponding ApoE−/−-Cre+ mice line. Endothelial specific CD36 knockout mice on Ldlr−/− background were generated using a similar scheme and Ldlr−/−, Tie2eCre+, and CD36fl/fl mice. We used sex-, age-, and genetic background-matched mice between 8 and 16 weeks of age in our experiments. We used the Cre− littermates as controls for all genotypes. All strains were on the C57BL/6 background. Animals were housed in ventilated cages with ad libitum access to food and water, on a 14:10 light-dark cycle. To induce hyperlipidemia, ApoE−/− mice were fed a Western diet (TD.88137, Envigo-Teklad) and Ldlr−/− mice with high-fat high cholesterol diet (TD.96121, Envigo-Teklad) for 6–8 weeks. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Bone marrow transplantation
Bone marrow transplantation was performed using recipient ApoE−/− mice and bone marrow donor ApoE−/− and ApoE−/−-CD36−/− mice as described (17). Eight-week-old male or female recipient ApoE−/− mice were lethally irradiated with a single dose of whole-body irradiation (900 rads) on the day of transplantation. Bone marrow cells from the donor 7-week- old male or female ApoE−/− and ApoE−/−/CD36−/− mice were isolated and intravenously injected into each recipient mouse. Four weeks later, the mice were fed with a Western diet for 8 to 10 weeks and used for experiments.
Cells
Murine peritoneal macrophages (MPM) were isolated 48–72 hours after thioglycolate injection as described earlier (20). Platelets were isolated by gel filtration as described (21). Primary Human Dermal Microvascular Endothelial Cells; Normal, Neonatal (HDMVECn) cells were purchased from ATCC and cultured as recommended.
Cell differentiation
3T3L1 pre-adipocyte cells were purchased from ATCC and were cultured as recommended. Confluent cells were cultured in differentiation media containing 1μM dexamethasone (Cayman Chemical), 0.5mM 3-isobutyl-1-methylxanthine (IBMX, Cayman Chemical), and 10μg/ml insulin (Sigma-Aldrich) for 3 days. Then cells were cultured in maintenance media containing 10μg/ml insulin for 5–7 days and then used for experiments.
Platelet isolation
Platelet rich plasma (PRP) were separated and platelets were isolated by gel filtration from blood drawn from healthy human donors or mice as described earlier (17).
Tissue lysate preparation
Mice were perfused with 1X PBS and tissues were collected. Lysate were prepared with 1X RIPA buffer in the presence of protease inhibitors.
Blood collection and plasma separation
Blood was collected into anticoagulant EDTA for endothelial specific CD36 deficient mice and corresponding control mice, (Ldlr−/−-Tie2eCre-CD36fl/fl) or rest of the mice in to 3.8% citrate. Plasma was separated by centrifugation at 2000 rpm for 20 minutes.
Sample preparation
To remove cell debris and microparticles, cell culture media, platelet supernatant, and plasma samples were subjected to differential centrifugation using ultra centrifuge as described (22). Samples were centrifuged at 2000 g for 10 minutes at 4°C to remove the cells and cell debris, followed by 10,000 g for 30 minutes at 4°C to remove larger vesicles. Then the samples were centrifuged at 100,000 g for 90 min at 4°C to deplete all the small vesicles including exosomes. For electrophoresis and Western blot analysis the cell culture media and platelet supernatants were further concentrated using a 50kDa centrifugal filter and centrifuging at 10,000 rpm for 20 minutes at 4°C.
Immunoblotting
Immunoblotting was performed as described earlier (17). Platelets and cells were treated with oxPCCD36. Platelets supernatant and cell culture media were collected and processed for sample preparation following above-mentioned method. Platelet and cell pellets were lysed on ice with RIPA buffer containing protease and phosphatase inhibitors. Lysates were centrifuged at 10000 rpm for 10 minutes at 4°C, and supernatants were collected. Protein concentrations were estimated using BioRad protein assay dye reagent and BSA as standard. Proteins were resolved on SDS-PAGE, transferred to PVDF membrane, and probed with protein-specific primary antibodies, followed by horseradish peroxidase (HRP)-conjugated specific secondary antibodies. Immunodetection was performed by enhanced chemiluminescence (ECL, GE Healthcare/Amersham, Buckinghamshire, UK) according to the manufacturer’s protocol.
ELISA
To measure the concentration of circulating CD36 in hyperlipidemic mice; microparticles free plasma was used to detect CD36 using ELISA kit for mCD36 (R&D systems, Minneapolis, MN) following manufacturer’s protocol. A 96 well plate was coated with capture antibody overnight at room temperature. Next day the wells were washed and blocked with blocking reagent for 1h at room temperature. Then the wells were washed and incubated with samples and different concentrations of standard mCD36 for 2h at room temperature. After that the wells were washed and incubated with detection antibody for 1h at room temperature followed by Streptavidin-HRP for 20 min at room temperature. After washing the wells substrate solution was added and incubated for 20 min at room temperature in dark and added the stop solution and mixed well. Optical density was measured at 450nm using a plate reader. The concentration of CD36 in the samples were determined comparing with the known concentrations of standard mCD36.
In vivo oxPCCD36 injection
oxPCCD36 (HODA-PC and KODA-PC) were mixed with PLPC in 1:1 molar ratio or PLPC alone in PBS and 0.1μm vesicles were prepared as described (23). Vesicles were injected into WT mice via tail vein injection to the final concentration of 6μM. After 6 hours the plasma of these mice was collected and analyzed for cCD36.
Statistical analysis
Data are presented as mean ±SD. The statistical significance of differences was evaluated using Student’s t-test. The p values less than 0.05 were considered to be statistically significant.
Results
Circulating CD36 is increased in two models of hyperlipidemic mice
To test whether circulating CD36 (cCD36) is increased in hyperlipidemic conditions, we used the two most commonly used mouse models for hyperlipidemia ApoE−/− and Ldlr−/− mice. ApoE−/− mice were fed either a chow diet or a Western diet, and cCD36 levels in the plasma were assessed by Western blot analysis. cCD36 protein levels were significantly increased in the plasma of hyperlipidemic ApoE−/− mice fed a Western diet as compared to relatively normolipidemic mice that received a chow diet (Fig 1A). A similar result of increased plasma cCD36 was observed in hyperlipidemic Ldlr−/− mice fed a proinflammatory high-fat high cholesterol diet as compared to a chow diet-fed normolipidemic mice (Fig 1A). cCD36 band intensity was increased 4–5 folds in hyperlipidemic mice as compared to corresponding normolipidemic mice (Fig 1B). We also confirmed the elevated levels of cCD36 in hyperlipidemic mice using ELISA (Fig 1C) and demonstrated that the absolute levels of cCD36 in hyperlipidemic murine plasma reached 0.1μg/ml.
Figure 1. Hyperlipidemia induced plasma levels of circulating CD36.
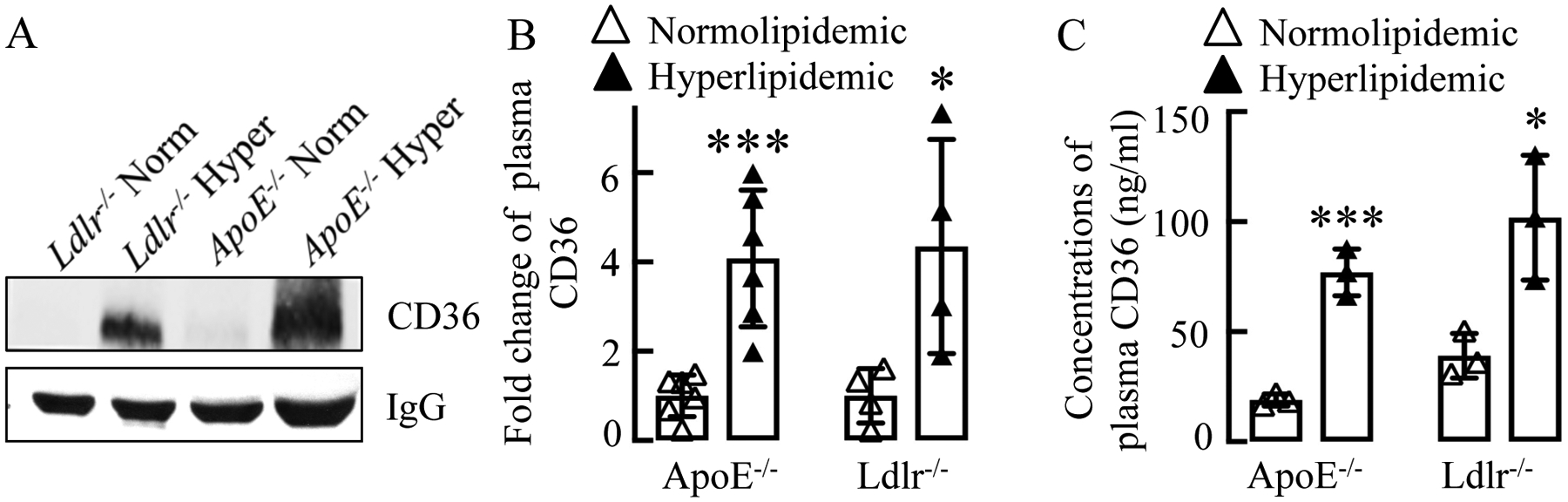
(A, B) Blood was collected from Ldlr−/− and ApoE−/− mice fed with either a Western diet or high fat diet (hyperlipidemic/hyper) or a chow diet (normolipidemic/norm), plasma was isolated, cells and cell debris were removed by centrifugation as described in experimental procedures and circulating CD36 was detected using Western Blotting (A) or ELISA (n=3) (B). (A) Quantification (mean ± SD) of bands intensity in Western blots is shown in the right side panel (n≥4).
oxPCCD36 trigger CD36 release from the cells
The triggers of CD36 release into the circulation from cells are not known, and the cellular mechanisms leading to the generation of cCD36 are poorly understood. Biologically active oxidized phospholipids, specific ligands for CD36 (oxPCCD36), are known to accumulate in tissues and in the circulation of hyperlipidemic mice (21). We next tested whether oxPCCD36 can induce CD36 release from CD36 expressing murine and human cells in vitro. A representative member of oxPCCD36, HODA-PC, but not control unoxidized phospholipid (POPC) induced release of CD36 from murine peritoneal macrophages (MPM), cells that interact with oxPCCD36 in vivo in atherogenesis (Fig 2A). oxPCCD36 induced CD36 release from MPM in a dose-dependent manner at pathophysiologically relevant concentrations of oxPCCD36 (Fig 2B) (21). CD36 release was time-dependent, with a significant increase within 1h and a robust increase at 6h of treatment (Fig 2C). The release of CD36 from cells was accompanied by the loss of cellular CD36 (Fig 2A–C). HODA-PC is a γ-hydroxy alkenal phospholipid and representative of oxPCCD36. We showed that other γ-oxo alkenal phospholipids, members of oxPCCD36 induced CD36 release from MPM (Fig 2D, data for KODA-PC and KOOA-PC are shown).
Figure 2. Oxidized phospholipids, ligands for CD36 induce CD36 release from cells.


(A-D) Murine Peritoneal Macrophages (MPM) were isolated from wild type mice (WT) and incubated with (A) 20μM of oxPCCD36 (HODA-PC) or control non-oxidized phospholipid, POPC for 6 hours (h), (B) indicated concentrations (0–20μM) of oxPCCD36 (HODA-PC) for 6h, (C) 20μM of oxPCCD36 (HODA-PC) for indicated time (0–6h), and (D) 20μM of different members of the specific subgroup of oxPCCD36, γ-hydroxyl alkenal phospholipids (HODA-PC) or γ-oxo alkenal phospholipids (KODA-PC and KOOA-PC). Media was depleted of particles by centrifugation as described in experimental procedures. CD36 was detected in the media and cell lysate by Western blot analysis. Actin is used as a loading control for cellular CD36. The quantification data of band intensity shown in bar graph (n≥3). * p<0.05, ** p<0.01, *** p<0.001, and ns p>0.05.
We then tested whether CD36 release in response to oxPCCD36 is macrophage specific. We found that other major CD36 expressing cells, including primary human dermal microvascular endothelial cells (HDMVECn), platelets, and adipocytes (3T3L1), released CD36 in response to oxPCCD36 whereas unoxidized phospholipid, POPC had no effect (Fig 3A–C).
Figure 3. Oxidized phospholipids induce CD36 release from various CD36 expressing cells.

(A) Primary human dermal microvascular endothelial cells (HDMVECn) were incubated with 20μM oxPCCD36 (HODA-PC) or control POPC for 6h and CD36 was detected in the media and cell lysate by Western blot analysis. (B) Human platelets were isolated from platelet rich plasma by gel filtration and incubated with oxPCCD36 (HODA-PC) and control POPC. Supernatant and lysate were analyzed for CD36 by Western blot analysis. (C) 3T3L1 preadipocytes were differentiated to adipocytes and then incubated with 20μM oxPCCD36 (HODA-PC) or control POPC for 6h and CD36 was detected in the media and cell lysate by Western blot analysis. Actin is used as a loading control for cellular CD36. Right side panels show the quantification data of band intensity (mean ± SD of at least 3 independent experiments). The quantification data of band intensity shown in bar graph (n≥5). * p<0.05, ** p<0.01, *** p<0.001, and ns p>0.05.
Multiple CD36 expressing cells are involved in cCD36 generation in vivo
To identify the source of the cCD36 in hyperlipidemic mice, we assessed the contribution of several types of CD36 expressing cells. Microvascular endothelium, macrophages, adipocytes, and platelets belong to a small group of cells with high expression of CD36. Therefore, we generated macrophage-specific, adipocyte-specific, and platelet-specific CD36 knockout mice using CD36fl/fl mice (19) and corresponding Cre+/− mice (all on ApoE−/− background). Contribution of endothelial CD36 was assessed using endothelial-specific knockout of CD36 on Ldlr−/− background generated by crossing Ldlr−/−-Tie2eCre+/− mice to Ldlr−/−-CD36fl/fl mice. We have validated the cell-type-specific CD36 deficiency by genotyping (data not shown) and Western blot analysis (Fig 4A–D). No changes in the CD36 levels in non-targeted cells were detected (Supplementary Fig S1–S4). Mice were fed hyperlipidemic diet as described in experimental procedures, and the cCD36 levels in the plasma were assessed. We observed a significant reduction in plasma CD36 levels in endothelial-cell specific CD36 deficient mice (Ldlr−/−-Tie2eCre+-CD36fl/fl) as compared to its littermate control Ldlr−/−-Tie2eCre−-CD36fl/fl mice (Fig 4E). We also observed a noticeable reduction in plasma CD36 levels in macrophage-specific (ApoE−/−-LysMCre+-CD36fl/fl), and adipocyte-specific (ApoE−/−-AdipoqCre+-CD36fl/fl) CD36 deficient mice as compared to their corresponding control Cre-negative mice (Fig 4F, G). While all three groups showed a reduction in cCD36, the contribution of endothelial CD36 was particularly pronounced (~50% decrease) in cCD36 levels in Cre positive mice as compared to Cre negative mice (Fig 4E), whereas macrophages and adipocytes showed ~25% decrease (Fig 4F, G). Noticeable contribution of bone marrow (presumably macrophages) derived CD36 to cCD36 was confirmed using hyperlipidemic ApoE−/− chimeras with either ApoE−/−-CD36−/− or ApoE−/− bone marrow (Fig 4I). Interestingly, we detected little changes in cCD36 in platelet specific knockout of CD36 (ApoE−/−-Pf4-Cre+-CD36fl/fl mice, Fig 4H). These data indicate that multiple types of CD36 expressing cells are involved in the production of cCD36 in hyperlipidemic conditions with endothelial cells being the highest contributor.
Figure 4. oxPCCD36 induce cCD36 generation in vivo involving multiple CD36 expressing cells.

(A-D) Cells and tissues were collected from different tissue-specific-CD36 deficient mice as indicated genotypes and lysates were prepared as mentioned in the experimental procedures and CD36 expression was detected by Western blot analysis showing the validation of cell type specific CD36 deficiencies. Ldlr−/−/Tie2e-Cre+/CD36fl/fl, ApoE−/−/LysM-Cre+/CD36fl/fl, ApoE−/−/Adipoq-Cre+/CD36fl/fl, and ApoE−/−/Pf4-Cre+/CD36fl/fl mice are labeled as Cre+ and Ldlr−/−/Tie2e-Cre−/CD36fl/fl, ApoE−/−/LysM-Cre−/CD36fl/fl, ApoE−/−/Adipoq-Cre−/CD36fl/fl, and ApoE−/−/Pf4-Cre−/CD36fl/fl mice are labeled as Cre-, protein ladder (M). (E-H) Plasma from the cell-type-specific mice (Tie2e-Cre+, endothelial cells specific; LysM-Cre+, macrophage specific; Adipoq-Cre+, adipocytes specific; Pf4-Cre+, platelet specific) as mentioned above was collected and assessed for circulatory CD36 by Western blot analysis. (I) ApoE−/− bone marrow chimeric mice with ApoE−/− (ApoE−/−) or ApoE−/−/CD36−/− (ApoE−/−/CD36−/−) bone marrow fed with a Western diet. Plasma was collected as mentioned earlier and and assessed for circulatory CD36 by Western blot analysis. (J) Wild type (WT) mice were injected with non-oxidized control phospholipid, PLPC or oxPCCD36 via tail vein injection. After 6 hours we collected the plasma from those mice and analyzed for circulating CD36 by Western blot analysis as mentioned earlier. The quantification data (mean ± SD) of band intensity of Western blots (n ≥ 4). * p<0.05, ** p<0.01, *** p<0.001, and ns p>0.05.
oxPCCD36 triggers cCD36 generation in vivo
We then tested whether oxPCCD36 can induce CD36 release in circulation in vivo. Wild type mice received intravenous injections of oxPCCD36 (6μM) or control, unoxidized phospholipid (PLPC), plasma was collected 6h later, and the cCD36 levels were assessed by Western blot analysis. Injection of oxPCCD36 induced the accumulation of cCD36 in the plasma, whereas control unoxidized phospholipid PLPC had no effect (Fig 4J). This result strongly suggests the direct role of oxPCCD36 in the generation of cCD36.
Discussion
In this study, we demonstrated that hyperlipidemia in mice is associated with the accumulation of cCD36 that derives from several cellular sources. Furthermore, we have established that oxidized phospholipids, a lipid peroxidation product present in plasma in hyperlipidemia, triggers cCD36 release from various CD36 expressing cells in vitro and in vivo.
Phospholipids represent a major target for lipid peroxidation, and significant levels of oxidized phospholipids were detected in human atherosclerotic lesions, in the circulation of hyperlipidemic mice and patients with low HDL (12,21). More recently, oxidized phospholipids were also detected in several conditions with chronic inflammation (24–28). Oxidized phospholipids have multiple biological activities and can interact with several receptors on the cellular surface, but they are highly specific ligands for scavenger receptors class B, including CD36 (13,29,30). We hypothesized that oxidized phospholipids could be a link between hyperlipidemia, chronic inflammation, and cCD36. Our in vitro experiments demonstrated that oxPCCD36 induce CD36 release from various cells, while native non-oxidized phospholipids had no effect. These results were confirmed in vivo using pathophysiologically relevant concentrations of oxidized phospholipids. Thus, our work identifies a novel biological activity of oxidized phospholipids - induction of the release of cellular CD36. We observed that various representatives of γ-hydroxy/oxo alkenal oxidized phospholipids induced cellular release of CD36. Interestingly, γ-hydroxy/oxo alkenal phospholipids can covalently modify CD36 (31). It remains to be established though, whether a covalent modification is required for the induction of CD36 release from cells. Taken together, our results strongly suggest that oxidized phospholipids represent a link between chronic inflammation and cCD36. They further suggest that increase in cCD36 is likely to be found in other cases of chronic inflammation.
Hyperlipidemia is associated with increased oxidative stress, lipid peroxidation, and accumulation of oxidized phospholipids in circulation and in tissues (24,32–35). To test a hypothesis that hyperlipidemic conditions are associated with the increase in CD36 in the circulation, we used ApoE−/− and Ldlr−/− mice, two most extensively studied mouse models of hyperlipidemia. Both of these models are characterized by chronic pro-oxidant stress and by high levels of individual oxidized phospholipids in circulation in hyperlipidemia (21,36). We detected increased cCD36 in both models. Western-type diet (ApoE−/−) or proinflammatory high fat/high cholesterol diet (Ldlr−/−) induced 4–5 folds increase in cCD36. The connection between hyperlipidemia and increased cCD36 was further confirmed in a limited amount of human samples.
Previous report demonstrated that circulating CD36 is not proteolytically cleaved and associated with a specific subfamily of microparticles instead of being soluble (37). At the same time, other studies showed that CD36 could undergo proteolytic cleavage and be released as a free form of CD36 (38,39). Our findings of cCD36 in microparticles depleted plasma and culture media suggest that oxPCCD36 induced cCD36 might not be microparticles bound. Further mechanistic studies are warranted to characterize the cCD36, generated in response to oxPCCD36.
A variety of cell types express CD36. Using several cell lines and primary cells, we have demonstrated that cCD36 release is not cell-specific, oxPCCD36 induced cCD36 release in vitro from all cells tested. Macrophages, microvascular endothelial cells, adipocytes, and human platelets are known to express CD36 at very high levels and, correspondingly, are potential contributors to cCD36 in vivo. To identify the cellular sources of cCD36 in hyperlipidemia, we generated several conditional knockouts of CD36; we also used bone marrow chimeras to assess the combined role of bone marrow-derived cells. Our data indicate that in vivo endothelial cells likely contribute the most to cCD36, likely due to chronic exposure to circulating oxPC. We observed a more dramatic effect than expected in the bone marrow transplant experiment. While the exact reason for this is not clear, it can not be excluded that other bone marrow-derived CD36 expressing cells besides platelets and macrophages contribute to CD36 generation in hyperlipidemia. The significant contribution of bone marrow-derived cells, and specifically macrophages, as well as adipocytes was also detected indicating that cells outside of circulation still contribute to cCD36. Interestingly, while in vitro experiments showed that human platelets release CD36 in response to oxidized phospholipids, platelet specific CD36 deficient mice showed no significant drop in cCD36 accumulation in hyperlipidemic conditions. This is probably explained by a relatively low expression of CD36 in murine platelets compared to endothelial cells, macrophages, and adipocytes. It should be noted that human platelets express very high levels of CD36; thus, we can not exclude human platelet contribution to the generation of cCD36. cCD36 release from cells in vitro is correlated with a significant decrease in cellular CD36 levels in vitro. While the exact consequences of CD36 loss for the cell function are not clear, it is likely that such loss would alter the lipid metabolism, fatty acids and oxidized lipoproteins uptake, and inflammatory responses to lipid ligands by cells. It should be noted though that in vivo chronic loss of CD36 may be compensated by upregulation of CD36 synthesis stimulated by PPARγ ligands present in oxidized LDL (40). CD36 is critically involved in the sensing and metabolism of native and oxidatively modified lipids and lipoproteins and participates in a number of pathophysiological processes, including inflammatory and immune responses, atherosclerosis, thrombosis, diabetes, and others (2,12,21,41–45). While the role of CD36 as a marker of chronic inflammation was suggested (42,46–49), its role in inflammation is insufficiently understood. The CD36 ectodomain contains binding sites for several ligands, including oxidized phospholipids, and plays a critical role in CD36 function (50–53). Upon ligand binding, CD36 interacts with several co-receptors including toll-like receptors to induce intracellular signaling (17,26,45,54). A recent study showed that free, non-cell-associated CD36 ectodomain in complex with a bacterial ligand, interacted with cell surface TLR and induced pro-inflammatory responses (55). This finding suggests that cCD36 released by cells such as microvascular endothelium in response to oxidized phospholipids may promote an inflammatory response via TLR2 on cells that express no or low levels of CD36 (e.g. circulating immune cells, large vessel endothelial cells) and would otherwise be oblivious to oxidized phospholipids. Such interaction may enhance the pro-inflammatory responses in hyperlipidemia induced by endogenous as well as bacterial stimuli. Future studies are needed to test this hypothesis.
Conclusions
In conclusion, our studies revealed that in hyperlipidemic conditions in mice circulating CD36 in the plasma is highly elevated due to release from multiple cell types with a particularly strong contribution from endothelial cells. We further demonstrated that oxPCCD36, a family of biologically active oxidized phospholipids ligands for CD36 (17), trigger the CD36 release from cells in vitro and in vivo. Taken together, these findings suggest that oxidized phospholipids serve as a mechanistic link between chronic inflammation and cCD36.
Supplementary Material
Highlights.
The circulating CD36 in the plasma is highly elevated during hyperlipidemia.
OxPCCD36, ligands for CD36, trigger the CD36 release from cells in vivo and in vitro.
Multiple cell types contribute to increased plasma levels of CD36 in hyperlipidemia.
OxPCCD36 serve as a mechanistic link between chronic inflammation & cCD36.
Acknowledgements:
Authors want to thank BRU services for their help with mice maintenance/housing.
Funding and additional information.
This work was supported in part by the National Institutes of Health grants HL077213, HL073311, and HL126738 (E.A.P.); HL142772 (T.B.); and also AHA postdoctoral fellowship grant 14POST20230027 (S.B.), Heart & Stroke Foundation Grant in Aid (M.F.), and Motyl Graduate Studentship in Cardiac Sciences (U.R.).
Abbreviations and nomenclature.
- oxPC
Oxidized phospholipids
- oxPCCD36
Oxidized phospholipids, ligands for CD36
- cCD36
Circulating CD36
- HODA-PC
9-hydroxy-10-dodecenedioic acid esters of 2-lysoPC
- KODA-PC
9-keto-12-oxo-10-dodecenoic acid ester of 2-lyso-phosphocholine
- KOOA-PC
5-keto-8-oxo-6-octenoic acid ester of 2-lyso-phosphocholine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PLPC
1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine
- ELISA
Enzyme-linked immunosorbent assay
- HDMVECn
Primary Human Dermal Microvascular Endothelial Cells; Normal, Neonatal
- MPM
Murine peritoneal macrophages
- PBS
Phosphate buffer saline
- PRP
Platelet rich plasma
- RIPA buffer
Radioimmunoprecipitation assay buffer
- ATCC
American Type Culture Collection
- LDL
Low Density Lipoprotein
- HDL
High Density Lipoprotein
- PPARγ
Peroxisome proliferator-activated receptor γ
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors have no conflict of interest to disclose.
References
- 1.Rigotti A, Acton SL, and Krieger M (1995) The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem 270, 16221–16224 [DOI] [PubMed] [Google Scholar]
- 2.Silverstein RL, and Febbraio M (2009) CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handberg A, Levin K, Hojlund K, and Beck-Nielsen H (2006) Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation 114, 1169–1176 [DOI] [PubMed] [Google Scholar]
- 4.Chmielewski M, Bragfors-Helin AC, Stenvinkel P, Lindholm B, and Anderstam B (2010) Serum soluble CD36, assessed by a novel monoclonal antibody-based sandwich ELISA, predicts cardiovascular mortality in dialysis patients. Clin Chim Acta 411, 2079–2082 [DOI] [PubMed] [Google Scholar]
- 5.Glintborg D, Hojlund K, Andersen M, Henriksen JE, Beck-Nielsen H, and Handberg A (2008) Soluble CD36 and risk markers of insulin resistance and atherosclerosis are elevated in polycystic ovary syndrome and significantly reduced during pioglitazone treatment. Diabetes Care 31, 328–334 [DOI] [PubMed] [Google Scholar]
- 6.Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, and Eriksson JW (2010) Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab 95, 1939–1946 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Monzon C, Lo Iacono O, Crespo J, Romero-Gomez M, Garcia-Samaniego J, Fernandez-Bermejo M, Dominguez-Diez A, Rodriguez de Cia J, Saez A, Porrero JL, Vargas-Castrillon J, Chavez-Jimenez E, Soto-Fernandez S, Diaz A, Gallego-Duran R, Madejon A, and Miquilena-Colina ME (2014) Increased soluble CD36 is linked to advanced steatosis in nonalcoholic fatty liver disease. Eur J Clin Invest 44, 65–73 [DOI] [PubMed] [Google Scholar]
- 8.Handberg A, Hojlund K, Gastaldelli A, Flyvbjerg A, Dekker JM, Petrie J, Piatti P, Beck-Nielsen H, and Investigators R (2012) Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med 271, 294–304 [DOI] [PubMed] [Google Scholar]
- 9.Handberg A, Skjelland M, Michelsen AE, Sagen EL, Krohg-Sorensen K, Russell D, Dahl A, Ueland T, Oie E, Aukrust P, and Halvorsen B (2008) Soluble CD36 in plasma is increased in patients with symptomatic atherosclerotic carotid plaques and is related to plaque instability. Stroke 39, 3092–3095 [DOI] [PubMed] [Google Scholar]
- 10.Liani R, Halvorsen B, Sestili S, Handberg A, Santilli F, Vazzana N, Formoso G, Aukrust P, and Davi G (2012) Plasma levels of soluble CD36, platelet activation, inflammation, and oxidative stress are increased in type 2 diabetic patients. Free Radic Biol Med 52, 1318–1324 [DOI] [PubMed] [Google Scholar]
- 11.Shiju TM, Mohan V, Balasubramanyam M, and Viswanathan P (2015) Soluble CD36 in plasma and urine: a plausible prognostic marker for diabetic nephropathy. J Diabetes Complications 29, 400–406 [DOI] [PubMed] [Google Scholar]
- 12.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, and Hazen SL (2002) A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem 277, 38517–38523 [DOI] [PubMed] [Google Scholar]
- 13.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, and Stockl J (2010) Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal 12, 1009–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun M, Deng Y, Batyreva E, Sha W, and Salomon RG (2002) Novel bioactive phospholipids: practical total syntheses of products from the oxidation of arachidonic and linoleic esters of 2-lysophosphatidylcholine(1). The Journal of organic chemistry 67, 3575–3584 [DOI] [PubMed] [Google Scholar]
- 15.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, and Berliner JA (1997) Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 272, 13597–13607 [DOI] [PubMed] [Google Scholar]
- 16.Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, Pober JS, Flavell RA, and Fu XY (2003) Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med 198, 1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswas S, Zimman A, Gao D, Byzova TV, and Podrez EA (2017) TLR2 Plays a Key Role in Platelet Hyperreactivity and Accelerated Thrombosis Associated With Hyperlipidemia. Circ Res 121, 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, and Silverstein RL (1999) A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274, 19055–19062 [DOI] [PubMed] [Google Scholar]
- 19.Nagendran J, Pulinilkunnil T, Kienesberger PC, Sung MM, Fung D, Febbraio M, and Dyck JR (2013) Cardiomyocyte-specific ablation of CD36 improves post-ischemic functional recovery. J Mol Cell Cardiol 63, 180–188 [DOI] [PubMed] [Google Scholar]
- 20.Ding L, Biswas S, Morton RE, Smith JD, Hay N, Byzova TV, Febbraio M, and Podrez EA (2012) Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab 15, 861–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, and Hazen SL (2007) Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med 13, 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, and Govorun VM (2015) Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep 5, 17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, and Hazen SL (2000) Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest 105, 1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaziri ND (2014) Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clin Exp Nephrol 18, 265–268 [DOI] [PubMed] [Google Scholar]
- 25.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, Kawada N, Arakawa T, and Ueda M (2006) Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology 43, 506–514 [DOI] [PubMed] [Google Scholar]
- 26.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, and Tabas I (2010) Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 12, 467–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, and Penninger JM (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, and Fogelman AM (2004) D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation 110, 3252–3258 [DOI] [PubMed] [Google Scholar]
- 29.Zimman A, and Podrez EA (2010) Regulation of platelet function by class B scavenger receptors in hyperlipidemia. Arterioscler Thromb Vasc Biol 30, 2350–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao D, Ashraf MZ, Kar NS, Lin D, Sayre LM, and Podrez EA (2010) Structural basis for the recognition of oxidized phospholipids in oxidized low density lipoproteins by class B scavenger receptors CD36 and SR-BI. J Biol Chem 285, 4447–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao D, Willard B, and Podrez EA (2014) Analysis of covalent modifications of proteins by oxidized phospholipids using a novel method of peptide enrichment. Anal Chem 86, 1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo FB, Barbosa DS, Hsin CY, Maranhao RC, and Abdalla DS (1995) Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis 117, 61–71 [DOI] [PubMed] [Google Scholar]
- 33.Moriel P, Plavnik FL, Zanella MT, Bertolami MC, and Abdalla DS (2000) Lipid peroxidation and antioxidants in hyperlipidemia and hypertension. Biol Res 33, 105–112 [DOI] [PubMed] [Google Scholar]
- 34.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, and Witztum JL (1994) ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb 14, 605–616 [DOI] [PubMed] [Google Scholar]
- 35.Stubiger G, Aldover-Macasaet E, Bicker W, Sobal G, Willfort-Ehringer A, Pock K, Bochkov V, Widhalm K, and Belgacem O (2012) Targeted profiling of atherogenic phospholipids in human plasma and lipoproteins of hyperlipidemic patients using MALDI-QIT-TOF-MS/MS. Atherosclerosis 224, 177–186 [DOI] [PubMed] [Google Scholar]
- 36.Kennedy DJ, Kuchibhotla SD, Guy E, Park YM, Nimako G, Vanegas D, Morton RE, and Febbraio M (2009) Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arterioscler Thromb Vasc Biol 29, 1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkhatatbeh MJ, Mhaidat NM, Enjeti AK, Lincz LF, and Thorne RF (2011) The putative diabetic plasma marker, soluble CD36, is non-cleaved, non-soluble and entirely associated with microparticles. J Thromb Haemost 9, 844–851 [DOI] [PubMed] [Google Scholar]
- 38.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, Flynn E, Zhang Z, Jin YF, Zhang H, and Lindsey ML (2016) CD36 Is a Matrix Metalloproteinase-9 Substrate That Stimulates Neutrophil Apoptosis and Removal During Cardiac Remodeling. Circ Cardiovasc Genet 9, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driscoll WS, Vaisar T, Tang J, Wilson CL, and Raines EW (2013) Macrophage ADAM17 deficiency augments CD36-dependent apoptotic cell uptake and the linked anti-inflammatory phenotype. Circ Res 113, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tontonoz P, and Nagy L (1999) Regulation of macrophage gene expression by peroxisome-proliferator-activated receptor gamma: implications for cardiovascular disease. Curr Opin Lipidol 10, 485–490 [DOI] [PubMed] [Google Scholar]
- 41.Abumrad NA, and Goldberg IJ (2016) CD36 actions in the heart: Lipids, calcium, inflammation, repair and more? Biochim Biophys Acta 1861, 1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Okamura DM, Lu X, Chen Y, Moorhead J, Varghese Z, and Ruan XZ (2017) CD36 in chronic kidney disease: novel insights and therapeutic opportunities. Nat Rev Nephrol 13, 769–781 [DOI] [PubMed] [Google Scholar]
- 43.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, and Moore KJ (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14, 812–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy DJ, and Kashyap SR (2011) Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metab Syndr Relat Disord 9, 239–245 [DOI] [PubMed] [Google Scholar]
- 45.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, and Moore KJ (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himoto T, Tani J, Miyoshi H, Morishita A, Yoneyama H, Kurokohchi K, Inukai M, Masugata H, Goda F, Senda S, Haba R, Ueno M, Yamaoka G, and Masaki T (2013) Investigation of the factors associated with circulating soluble CD36 levels in patients with HCV-related chronic liver disease. Diabetol Metab Syndr 5, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos-Arellano LE, Munoz-Valle JF, De la Cruz-Mosso U, Salgado-Bernabe AB, Castro-Alarcon N, and Parra-Rojas I (2014) Circulating CD36 and oxLDL levels are associated with cardiovascular risk factors in young subjects. BMC Cardiovasc Disord 14, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L, Varghese Z, Moorhead JF, Chen Y, and Ruan XZ (2018) CD36 and lipid metabolism in the evolution of atherosclerosis. Br Med Bull 126, 101–112 [DOI] [PubMed] [Google Scholar]
- 49.Park YM (2014) CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med 46, e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Diaz C, Bargeton B, Abuin L, Bukar N, Reina JH, Bartoi T, Graf M, Ong H, Ulbrich MH, Masson JF, and Benton R (2016) A CD36 ectodomain mediates insect pheromone detection via a putative tunnelling mechanism. Nat Commun 7, 11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kar NS, Ashraf MZ, Valiyaveettil M, and Podrez EA (2008) Mapping and characterization of the binding site for specific oxidized phospholipids and oxidized low density lipoprotein of scavenger receptor CD36. J Biol Chem 283, 8765–8771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glatz JFC, and Luiken J (2018) Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res 59, 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Son NH, Basu D, Samovski D, Pietka TA, Peche VS, Willecke F, Fang X, Yu SQ, Scerbo D, Chang HR, Sun F, Bagdasarov S, Drosatos K, Yeh ST, Mullick AE, Shoghi KI, Gumaste N, Kim K, Huggins LA, Lhakhang T, Abumrad NA, and Goldberg IJ (2018) Endothelial cell CD36 optimizes tissue fatty acid uptake. J Clin Invest 128, 4329–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canton J, Neculai D, and Grinstein S (2013) Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 13, 621–634 [DOI] [PubMed] [Google Scholar]
- 55.Jimenez-Dalmaroni MJ, Xiao N, Corper AL, Verdino P, Ainge GD, Larsen DS, Painter GF, Rudd PM, Dwek RA, Hoebe K, Beutler B, and Wilson IA (2009) Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS One 4, e7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


