Abstract
The painted urchin Lytechinus pictus is a sea urchin in the family Toxopneustidae and one of several sea urchin species that are routinely used as an experimental research organism. Recently, L. pictus has emerged as a tractable model system for establishing transgenic sea urchin lines due to its amenability to long term laboratory culture. We present the first published genome of L. pictus. This chromosomal-level assembly was generated using Illumina sequencing in conjunction with Oxford Nanopore Technologies long read sequencing and HiC chromatin conformation capture sequencing. The 998.9-Mb assembly exhibits high contiguity and has a scaffold length N50 of 46.0 Mb with 97% of the sequence assembled into 19 chromosomal-length scaffolds. These 19 scaffolds exhibit a high degree of synteny compared with the 19 chromosomes of a related species Lytechinus variegatus. Ab initio and transcript evidence gene modeling, combined with sequence homology, identified 28,631 gene models that capture 92% of BUSCO orthologs. This annotation strategy was validated by manual curation of gene models for the ABC transporter superfamily, which confirmed the completeness and accuracy of the annotations. Thus, this genome assembly, in conjunction with recent high contiguity assemblies of related species, positions L. pictus as an exceptional model system for comparative functional genomics and it will be a key resource for the developmental, toxicological, and ecological biology scientific communities.
Keywords: Lytechinus pictus, sea urchin, echinoderm, genome
Significance
The sea urchin Lytechinus pictus has long served as a model system for developmental, toxicological, and ecological studies. Here, we present a chromosomal-level genome assembly as a resource for these communities and other researchers using this important model system.
Introduction
For over a century, biologists have used sea urchin eggs and embryos to make important contributions to the fields of development, cell biology, and gene regulation (Driesch 1891; Evans et al. 1983; Foltz and Hamdoun 2019; Hamdoun and Foltz 2019; Oliveri et al. 2008). The attributes that make sea urchins excellent material for developmental and cell biology research include large quantities of sperm and oocytes, rapid development into pelagic larva with a relatively simple and well-defined set of cell types, and optically clear embryos and larvae that facilitate fluorescence microscopy and in vivo imaging (Foltz and Hamdoun 2019; Hamdoun and Foltz 2019).
Unlike similar research carried out in other model systems, the communities using sea urchins have not converged on a singular species to work with, as researchers typically favor working with local species. For example, Paracentrotus lividus is used in Europe (Campos et al. 2020), Heliocidaris tuberculata is used in Australia (Morris et al. 2019), Hemicentrotus pulcherrimus is used in Asia (Liu et al. 2019), Arbacia spp. and Lytechinus variegatus are used on the east coast of North America (Hogan et al. 2020; Queiroz et al. 2021), and Stronglyocentrotus purpuratus and Lytechinus pictus are used on the west coast of North America (Nesbit and Hamdoun 2020; Peter 2019). The investment in many sea urchin species, instead of just one, has paid out in numerous studies comparing sea urchin (and echinoderm) development, gene regulatory networks, and genomes (Cary and Hinman 2017; Erkenbrack and Thompson 2019; Wang et al. 2020).
Although most sea urchin experimental material is collected from wild populations, a few sea urchin species are regularly bred in captivity including Temnopleurus reevesii (Yaguchi 2019; Yaguchi et al. 2020) and L. variegatus (Heflin and Watts 2016). Long before those studies, Ralph Hinegardner and colleagues (Cameron and Hinegardner 1974; Coffaro and Hinegardner 1977; Hinegardner 1969) established inbred lines of L. pictus. This species is well-suited for rearing in the lab because it is small as an adult (2–4 cm in diameter), has a short generation time (∼4–6 months), and is resistant to infection in captivity (Hinegardner 1975). Lytechinus pictus is used for studying fertilization (Hussain et al. 2017), gastrulation (Aleksanyan et al. 2016), toxicology (Li et al. 2020), and response to ocean acidification (Smith et al. 2019), among other areas. Given the long history of research with L. pictus, our groups previously developed updated culturing methods, obtained developmental transcriptomes, and established a detailed staging system for this species (Nesbit et al. 2019; Nesbit and Hamdoun 2020), with the ultimate goal of establishing inbred and germline transgenic lines.
A key impediment to functional and comparative genomics in L. pictus is the availability of a high contiguity genome. To remove this impediment, we have obtained and annotated a high-quality whole genome sequence for L. pictus. The L. pictus genome follows a recently published genome sequence in a sister species: L. variegatus (Davidson et al. 2020). The L. pictus genome is comparable to that of L. variegatus in size (998 Mb), chromosome number (likely 19), and chromosomal organization, as we show here. The completion of the L. pictus genome thus establishes the sea urchin as an excellent model system for comparative genomic studies as high contiguity assemblies are now available in several species representing a range of taxa (Davidson et al. 2020; Kinjo et al. 2018; Sodergren et al. 2006). Furthermore, the expanding genomics toolkit for L. pictus will maximize its usefulness for a wide range of research fields and support work on establishing stable transgenic sea urchin lines.
Materials and Methods
Tissue Collection
Adult L. pictus were collected near the Scripps Institution of Oceanography, in La Jolla, CA and held in free-flowing sea water aquaria. Spawning was induced by injection of 0.5 M potassium chloride. From one of the males, several hundred microliters of sperm were “dry” collected using a pipette, centrifuged to remove excess seawater and flash frozen in liquid nitrogen and stored at –80 °C. DNA extraction was carried out by Dovetail Genomics (Scott’s Valley, CA).
Dovetail Sequencing and Assembly
A Chicago library was prepared as described previously (Putnam et al. 2016). Briefly, approximately 500 ng of HMW gDNA (mean fragment length = 75 kb) was reconstituted into chromatin in vitro and fixed with formaldehyde. Fixed chromatin was digested with DpnII, the 5′ overhangs filled in with biotinylated nucleotides, and free blunt ends ligated. After ligation, crosslinks were reversed and the DNA purified from protein. Purified DNA was treated to remove biotin that was not internal to ligated fragments. The DNA was then sheared to approximately 350 bp mean fragment size and sequencing libraries were generated using NEBNext Ultra enzymes and Illumina-compatible adapters. Biotin-containing fragments were isolated using streptavidin beads before PCR enrichment of each library. The libraries were sequenced on an Illumina HiSeq X platform to produce 387 million 2 × 150 bp paired end reads, which provided 42.50 × physical coverage of the genome (1–100 kb pairs).
Two Dovetail HiC libraries were prepared in a similar manner as described previously (Lieberman-Aiden et al. 2009). Briefly, for each library, chromatin was fixed in place with formaldehyde in the nucleus and then extracted. Fixed chromatin was digested with DpnII, the 5′ overhangs filled in with biotinylated nucleotides, and free blunt ends ligated. After ligation, crosslinks were reversed and the DNA purified from protein. Purified DNA was treated to remove biotin that was not internal to ligated fragments. The DNA was then sheared to approximately 350 bp mean fragment size and sequencing libraries were generated using NEBNext Ultra enzymes and Illumina-compatible adapters. Biotin-containing fragments were isolated using streptavidin beads before PCR enrichment of each library. The libraries were sequenced on an Illumina HiSeq X platform. The number and length of read pairs produced for each library was: 247 million, 2 × 150 bp for library 1 and 170 million, 2 × 150 bp for library 2. Together, these Dovetail HiC library reads provided 2,771.85 × physical coverage of the genome (10–10,000 kb pairs, supplementary fig. 1, Supplementary Material online).
Five runs of ONT MinION sequencing generated 15 Gb of data representing 19× coverage. The ONT data were assembled with the wtdbg2 assembler, followed by consensus polishing with ONT reads with racon and Illumina polishing with pilon. The input de novo assembly, ONT reads, Chicago library reads, and Dovetail HiC library reads were used as input data for HiRise, a software pipeline designed specifically for using proximity ligation data to scaffold genome assemblies (Putnam et al. 2016). An iterative analysis was conducted. First, Shotgun and Chicago library sequences were aligned to the draft input assembly using a modified SNAP read mapper (https://www.microsoft.com/en-us/research/project/snap/, last accessed March 23, 2021). The separations of Chicago read pairs mapped within draft scaffolds were analyzed by HiRise to produce a likelihood model for genomic distance between read pairs, and the model was used to identify and break putative misjoins, to score prospective joins, and make joins above a threshold. After aligning and scaffolding Chicago data, Dovetail HiC library sequences were aligned and scaffolded following the same method. After scaffolding, shotgun sequences were used to close gaps between contigs.
Synteny Analysis
The 19 largest scaffolds were repeat masked using RepeatMasker v4.0.9 (Smit et al. 2010) and aligned to the 19 chromosomes identified in the L. variegatus genome using D-Genies (Cabanettes and Klopp 2018) with minimap as the aligner to identify homologous chromosomes. The L. pictus scaffolds were renamed for the homology to L. variegatus (e.g., “chr1”), with the remaining chromosomes enumerated and named with the prefix “unplaced_scaffold.”
Annotation
Genome annotation was performed using the MAKER pipeline (Cantarel et al. 2008). A repeat library was generated with RepeatModeler v2.01 (Smit and Hubley 2010) and repeats were identified and masked prior to using RepeatMasker v4.0.9 (Smit et al. 2010). MAKER was run using protein models from S. purpuratus (available on echinobase: http://legacy.echinobase.org/Echinobase/download/downloadfile.php?file=SPU_peptide.fasta.zip; last accessed May 3, 2020) and L. variegatus (available on echinobase: http://legacy.echinobase.org/Echinobase/download/downloadfile.php?file=Lvar2.2-protein.fa.zip; last accessed May 3, 2020) along with a previously assembled transcriptome from L. pictus (Nesbit et al. 2019) for a first round of homology-based and RNA-evidenced gene prediction. The resultant gene models were used for two subsequent rounds of training and ab initio gene prediction using SNAP and Augustus as part of the MAKER pipeline. The gene models were annotated using the notation LPI_XXXXXX and analyzed for “completeness” with BUSCO version 4 using the metazoan gene set (Simão et al. 2015). Functional annotation was performed using BLASTp to compare our gene models to the S. purpuratus protein database, RefSeq database, and Uniprot (Swissprot and Trembl) databases (hits defined as having an e value < 5e-5). The top hit to the Uniprot database was used to source putative GO terms via programmatic retrieval from uniprot.org.
ABC Transporter Annotation
To identify putative ABC transporters, known sequences from human, mouse, and S. purpuratus were used as input for BLAST searches of the L. pictus genome and transcriptome. Redundant sequences and low-quality hits were eliminated. To help confirm the identity of the L. pictus ABC candidates we analyzed the amino acid sequences of the deduced protein. This included manual notation of transmembrane domains, and the Walker A/B/C domains, which are characteristic of ABCs, using Geneious (v11.1.5). Candidate L. pictus ABC transporters that had strong support from our initial BLAST search and contained the predicted structural motifs were further validated with a reciprocal BLAST approach. Each of our candidate sequences served as the query in a BLAST search against annotated genes and proteins in the NCBI, SwisProt, and Echinobase databases. We excluded hypothetical and uncharacterized proteins. Hits were deemed true ABC transporter orthologs if supported with a combination of high (>85%) sequence identity to known ABC transporters, structural motif analysis, and reciprocal BLAST search. To annotate beyond subfamily resolution, we focused on the ABCC candidates from L. pictus and performed phylogenetic analysis to verify individual genes. Sequences were aligned in Geneious (v11.1.5) using the ClustalW program with default settings (gap open = 10.0, gap extension = 0.1, word size = 1, window length = 5, topdiag = 5, pairgap = 3). The resultant alignment was run through ProtTest (v3.4.2) to predict the best fit model for tree construction. A maximum likelihood tree (RaxML-HPC2 on XSEDE) with 1,000 bootstraps was then produced using the LG+G model, with yeast selected as an outgroup, through the CIPRES Science Gateway (v3.3) (Miller et al. 2011). Remaining parameters were used on default settings. The resulting tree was exported and formatted using FigTree (V1.4.4).
Results and Discussion
Lytechinus pictus Genome Assembly and Synteny Analysis
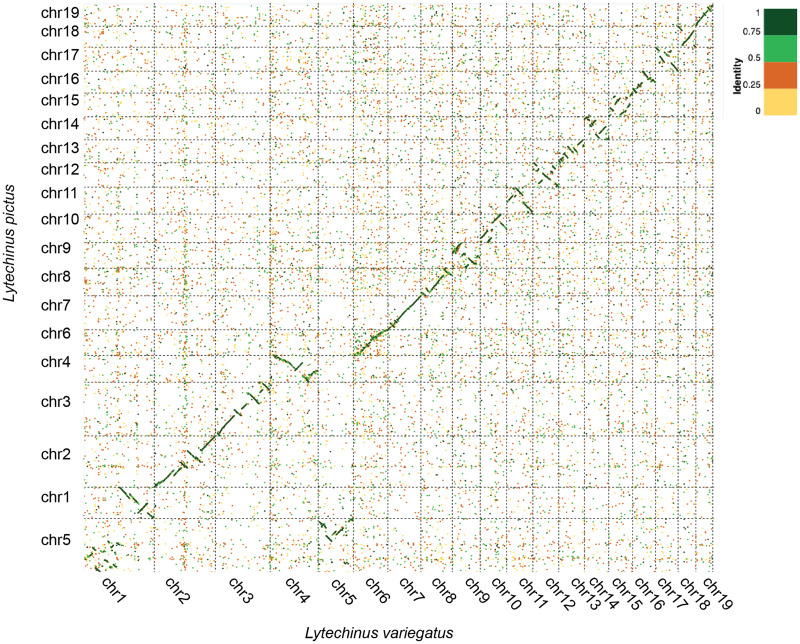
The L. pictus genome assembly, which we term Lpic_2.0 (Lpic_1.0 refers to an unpublished assembly), totals 998.85 Mb of sequences (0.10% gaps) assembled into 1,307 scaffolds with half of the sequence (N50) contained in eight scaffolds longer than 46 Mb (table 1). 97% of the sequence is assembled into nineteen scaffolds longer than 10 Mb, corresponding to putative chromosomes. This result is in agreement with a recent genome sequencing effort in L. variegatus, which also identified 19 chromosome-length scaffolds (Davidson et al. 2020). To identify homologous chromosomes between the two assemblies, we performed a nucleotide alignment between the 19 longest scaffolds in both species (fig. 1). Lytechinus variegatus and L. pictus diverged approximately 5–10 Ma (Zigler and Lessios 2004) and this is reflected in the genome alignment that shows a high overall level of synteny between L. variegatus and L. pictus, albeit with a large number of inversions and translocations, particularly in regard to chromosomes 1 and 5 (fig. 1).
Table 1.
Lytechinus pictus Genome Assembly Statistics
| Assembly | |
|---|---|
| Size | 998.85 Mb |
| Scaffolds | 1,307 |
| N50 scaffold length | 46.003 Mb |
| Longest scaffold | 91.80 Mb |
| Scaffolds > 10 Mb | 19 |
| Sequence in 19 largest scaffolds | 97% |
| N50 contig length | 219.60 kb |
| N (%) | 0.10 |
| GC (%) | 36 |
| BUSCO | |
| Complete | 251 |
| Duplicated | 13 |
| Fragmented | 14 |
| Missing | 25 |
| Annotations | |
| No. of protein coding genes | 28,631 |
| No. of gene models with Sp hit | 25,483 |
| No. of gene models with RefSeq hit | 26,179 |
Fig. 1.
Dot plot alignment of Lytechinus variegatus chromosomal sequences to the 19 largest Lytechinus pictus scaffolds (putative chromosomes). Dot plot was generated using D-Genies (Cabanettes and Klopp 2018) with minimap as the alignment engine. The top 100,000 matches are displayed.
Gene Annotation
To predict protein coding genes, we used the MAKER pipeline (Cantarel et al. 2008). We used available protein models from S. purpuratus and L. variegatus in conjunction with a previously assembled transcriptome for homology and evidence-based gene modeling. We then used the resultant gene models to train Augustus and Snap for two additional rounds of de novo gene prediction. This resulted in 28,631 protein coding gene annotations, including 17,865 with 5′UTRs and 17,760 with 3′UTR sequences (table 1, supplementary table 1, Supplementary Material online). These protein coding gene models contained 92% of the Benchmarking Universal Single-Copy Orthologs (BUSCO) of metazoan genes (Simão et al. 2015). We also functionally annotated the gene models using protein alignments against the S. purpuratus protein, RefSeq protein, and Uniprot (Swissprot and Trembl) databases. 91% (26,179) of the gene models yielded a significant hit to the RefSeq database (blastp, e value < 5e-5) and 89% (25,483) yielded a significant hit to the S. purpuratus protein database. Finally, we used the top hit from Uniprot to associate GO terms to our gene models. All of the annotations can be found in supplementary files 1 and 2, Supplementary Material online.
Manual Curation of ABC Transporter Annotations
To further assess the completeness and quality of the L. pictus genome, we manually examined the annotations of the ATP-Binding Cassette (ABC) transporter superfamily. This group of genes has a number of features which make it attractive for assembly assessment: The ABC transporters are a large multigene family (Dean and Annilo 2005), they include large genes which can span several hundred kilobases (Luciani et al. 1994), and they are well characterized in a related echinoderm, S. purpuratus. The proteins are essential for ion regulation (Bryan et al. 2007), developmental signaling (Petrášek and Friml 2009), cell migration (Kassmer et al. 2015), antigen presentation (Schumacher et al. 1994), and in humans, mutations in these genes are associated with disease (supplementary table 2, Supplementary Material online) (Borst and Elferink 2002; Dean 2002; Gottesman and Ambudkar 2001; Silverton et al. 2011; Tarling et al. 2013). There were 54 L. pictus ABC gene candidates in our assembly, spanning ABCs found in sea urchins (A–H)(Goldstone et al. 2006; Shipp and Hamdoun 2012). These include putative orthologs of disease-relevant genes, such as ABCA1, ABCB1, ABCC1, ABCC9, ABCD1, and ABCG5 (supplementary table 2, Supplementary Material online). To assess the quality of individual annotations we focused on the ABCC subfamily (supplementary fig. 2, Supplementary Material online). A feature of ABC families in the sea urchins is the relative expansion of the ABCC family as compared with humans (Gokirmak et al. 2016). Consistent with this, L. pictus have 20 putative C-subfamily candidates compared with the 12 ABCC members in human (supplementary file 4, Supplementary Material online). Of these C-subfamily members in urchins, the S. purpuratusABCC1 contains 29 exons and spans approximately 45.5 kb. We identified a L. pictusABCC1 candidate of 31 exons and 59.9 kb, encoding a 1,471 aa open reading frame. Similarly, the S. purpuratusABCC4a gene is encoded by 27 exons spanning approximately 43.8 kb in length. A corresponding annotation for this isoform of ABCC4a in L. pictus is 24 exons and approximately 31.7 kb long, encoding a 1,334 aa open reading frame. Collectively these results support the outputs of the automated pipeline annotations, and the significant hits of L. pictus gene models against RefSeq and S. purpuratus protein databases, supporting the “completeness” of our assembly and annotation.
This chromosomal-level genome assembly will be a valuable resource for the design of transgenic lines in this genetically enabled model system that is also well suited to laboratory culture through multiple generations (Hinegardner 1969; Nesbit et al. 2019). Our annotation identifies homologues for the large majority of developmental regulatory genes described in the well-established sea urchin model S. purpuratus, thus enabling evolutionary comparisons between these two model systems. Furthermore, this genome assembly, in conjunction with the recent genome assembly of the sister species L. variegatus represent two of the most complete assemblies in any echinoderm model system. These chromosome-level assemblies will allow for robust synteny analyses to identify conserved cis-regulatory regions between the two species. Together, these two high-contiguity genomes firmly establish the sea urchin as an excellent model system for comparative genomics and represent a major advancement in the field for sea urchin developmental biology.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (ES 027921 and 030318), National Science FOundation (1840844) to A.H., by a Scripps Institution of Oceanography SEED Grant to D.C.L. and A.H., and by a U.C. San Diego Academic Senate Grant to D.C.L. This work used the Extreme Science and Engineering Discovery Environment (XSEDE) Jetstream resource at Indiana University through allocation TG-MCB190132 to J.F.W.
Data Availability
The sequence assembly and annotations can be found at the National Center for Biotechnology Information (NCBI) under Bioproject PRJNA647794. Additionally, annotation files including a GFF3 and functional annotation file can be found in supplementary files 1 and 2, Supplementary Material online.
Literature Cited
- Aleksanyan H, Liang J, Metzenberg S, Oppenheimer SB.. 2016. Terminal alpha-d-mannosides are critical during sea urchin gastrulation. Zygote 24(5):775–782. [DOI] [PubMed] [Google Scholar]
- Borst P, Elferink RO.. 2002. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 71:537–592. [DOI] [PubMed] [Google Scholar]
- Bryan J, et al. 2007. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 453(5):703–718. [DOI] [PubMed] [Google Scholar]
- Cabanettes F, Klopp C.. 2018. D-GENIES: dot plot large genomes in an interactive, efficient and simple way. PeerJ 6:e4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron RA, Hinegardner RT.. 1974. Initiation of metamorphosis in laboratory cultured sea urchins. Biol Bull. 146(3):335–342. [DOI] [PubMed] [Google Scholar]
- Campos S, Troncoso J, Paredes E.. 2020. Major challenges in cryopreservation of sea urchin eggs. Cryobiology 97:295. [DOI] [PubMed] [Google Scholar]
- Cantarel BL, et al. 2008. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18(1):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary GA, Hinman VF.. 2017. Echinoderm development and evolution in the post-genomic era. Dev Biol. 427(2):203–211. [DOI] [PubMed] [Google Scholar]
- Coffaro KA, Hinegardner RT.. 1977. Immune response in the sea urchin Lytechinus pictus. Science 197(4311):1389–1390. [DOI] [PubMed] [Google Scholar]
- Davidson PL, et al. 2020. Chromosomal-level genome assembly of the sea urchin Lytechinus variegatus substantially improves functional genomic analyses. Genome Biol Evol. 12(7):1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. 2002. The human ATP-binding cassette (ABC) transporter superfamily. Bethesda (MD: ): National Center for Biotechnology Information (US; ). [Google Scholar]
- Dean M, Annilo T.. 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 6:123–142. [DOI] [PubMed] [Google Scholar]
- Driesch H. 1891. Entwicklungsmechanische Studien: I. Der Werthe der beiden ersten Furchungszellen in der Echinogdermenentwicklung. Experimentelle Erzeugung von Theil- und Doppelbildungen. II. Über die Beziehungen des Lichtez zur ersten Etappe der thierischen Form-bildung. Zeitschrift Für Wissenschaftliche Zoologie. 53:160–184. Translated as “The potency of the first two cleavage cells in echinoderm development. experimental production of partial and double formations.” In Willier BH and Oppenheimer JM, editors. Foundations of Experimental Embryology. New York: Hafner Press. p. 38–50. [Google Scholar]
- Erkenbrack EM, Thompson JR.. 2019. Cell type phylogenetics informs the evolutionary origin of echinoderm larval skeletogenic cell identity. Commun Biol. 2:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T.. 1983. Cyclin: A protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33(2):389–396. [DOI] [PubMed] [Google Scholar]
- Foltz KR, Hamdoun A.. 2019. Methods in cell biology: echinoderms, Part A, First ed. Cambridge: Academic Press. [Google Scholar]
- Gokirmak T, et al. 2016. Functional diversification of sea urchin ABCC1 (MRP1) by alternative splicing. Am J Physiol-Cell Physiol. 310:C911–C920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone JV, et al. 2006. The chemical defensome: environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev Biol. 300(1):366–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Ambudkar SV.. 2001. Overview: ABC transporters and human disease. J Bioenerget Biomembr. 33(6):453–458. [DOI] [PubMed] [Google Scholar]
- Hamdoun A, Foltz KR.. 2019. Methods in cell biology: echinoderms, Part B, First ed. Cambridge: Academic Press. [Google Scholar]
- Heflin LE, Watts SA.. 2016. Feeding time, frequency and ration affect growth and energy allocation in young adults of the sea urchin Lytechinus variegatus. Aquacult Nutr. 22(5):1055–1064. [Google Scholar]
- Hinegardner RT. 1969. Growth and development of the laboratory cultured sea urchin. Biol Bull. 137(3):465–475. [DOI] [PubMed] [Google Scholar]
- Hinegardner RT. 1975. Morphology and genetics of sea urchin development. Am Zool. 15(3):679–689. [Google Scholar]
- Hogan JD, et al. 2020. The developmental transcriptome for Lytechinus variegatus exhibits temporally punctuated gene expression changes. Dev Biol. 460(2):139–154. [DOI] [PubMed] [Google Scholar]
- Hussain YH, Sadilek M, Salad S, Zimmer RK, Riffell JA.. 2017. Individual female differences in chemoattractant production change the scale of sea urchin gamete interactions. Dev Biol. 422(2):186–197. [DOI] [PubMed] [Google Scholar]
- Kassmer SH, Rodriguez D, Langenbacher AD, Bui C, De Tomaso AW.. 2015. Migration of germline progenitor cells is directed by sphingosine-1-phosphate signalling in a basal chordate. Nat Commun. 6(1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo S, Kiyomoto M, Yamamoto T, Ikeo K, Yaguchi S.. 2018. HpBase: a genome database of a sea urchin, Hemicentrotus pulcherrimus. Dev Growth Differ. 60(3):174–182. [DOI] [PubMed] [Google Scholar]
- Li A, Espinoza J, Hamdoun A.. 2020. Inhibitory effects of neurotoxin β-N-methylamino-l-alanine on fertilization and early development of the sea urchin Lytechinus pictus. Aquat Toxicol. 221:105425. [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E, et al. 2009. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326(5950):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DM, Awazu A, Sakuma T, Yamamoto T, Sakamoto N.. 2019. Establishment of knockout adult sea urchins by using a CRISPR-Cas9 system. Dev Growth Differ. 61(6):378–388. [DOI] [PubMed] [Google Scholar]
- Luciani MF, Denizot F, Savary S, Mattei MG, Chimini G.. 1994. Cloning of 2 novel ABC transporters mapping on human chromosome 9. Genomics 21(1):150–159. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T.. 2011. The CIPRES science gateway: a community resource for phylogenetic analyses. Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery. p. 1–8. doi:10.1145/2016741.2016785
- Morris VB, Kable E, Koop D, Cisternas P, Byrne M.. 2019. Early development of the feeding larva of the sea urchin Heliocidaris tuberculata: role of the small micromeres. Dev Genes Evol. 229(1):1–12. [DOI] [PubMed] [Google Scholar]
- Nesbit KT, et al. 2019. The painted sea urchin, Lytechinus pictus, as a genetically-enabled developmental model. Methods Cell Biol. 150:105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbit KT, Hamdoun A.. 2020. Embryo, larval, and juvenile staging of Lytechinus pictus from fertilization through sexual maturation. Dev Dyn. 249(11):1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri P, Tu Q, Davidson EH.. 2008. Global regulatory logic for specification of an embryonic cell lineage. Proc Natl Acad Sci USA. 105(16):5955–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS. 2019. Chapter 2 – Methods for the experimental and computational analysis of gene regulatory networks in sea urchins. In: Hamdoun A, Foltz KR, editors. Methods in cell biology. Cambridge: Academic Press. p. 89–113. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Friml J.. 2009. Auxin transport routes in plant development. Development 136(16):2675–2688. [DOI] [PubMed] [Google Scholar]
- Putnam NH, et al. 2016. Chromosome-scale shotgun assembly using an in vitro method for long-range linkage. Genome Res. 26(3):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz V, Muxel SM, Inguglia L, Chiaramonte M, Custódio MR.. 2021. Comparative study of coelomocytes from Arbacia lixula and Lythechinus variegatus: cell characterization and in vivo evidence of the physiological function of vibratile cells. Fish Shellfish Immunol. 110:1–9. [DOI] [PubMed] [Google Scholar]
- Schumacher TNM, et al. 1994. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J Exp Med. 179(2):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp LE, Hamdoun A.. 2012. ATP-binding cassette (ABC) transporter expression and localization in sea urchin development. Dev Dyn. 241(6):1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverton L, Dean M, Moitra K.. 2011. Variation and evolution of the ABC transporter genes ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8: implication for pharmacogenetics and disease. Drug Metabol Drug Interact. 26(4):169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM.. 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31(19):3210–3212. [DOI] [PubMed] [Google Scholar]
- Smit AFA, Hubley R.. 2008. –2015. RepeatModeler Open-1.0 [accessed 2020 Feb 6]. Available from: http://www.repeatmasker.org.
- Smit AFA, Hubley R, Green P.. 1996. –2010. RepeatMasker Open-3.0 [accessed 2020 Feb 6]. Available from: http://www.repeatmasker.org.
- Smith KE, et al. 2019. Sea urchin reproductive performance in a changing ocean: poor males improve while good males worsen in response to ocean acidification. Proc Biol Sci. 286(1907):20190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergren E, et al. 2006. The genome of the sea urchin Strongylocentrotus purpuratus. Science 314(5801):941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarling EJ, Vallim TQD, Edwards PA.. 2013. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol Metab. 24(7):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. 2020. Genetic basis for divergence in developmental gene expression in two closely related sea urchins. Nat Ecol Evol. 4(6):831–840. [DOI] [PubMed] [Google Scholar]
- Yaguchi S. 2019. Temnopleurus as an emerging echinoderm model. Methods Cell Biol. 150:71–79. [DOI] [PubMed] [Google Scholar]
- Yaguchi S, et al. 2020. Establishment of homozygous knock-out sea urchins. Curr Biol. 30(10):R427–r429. [DOI] [PubMed] [Google Scholar]
- Zigler KS, Lessios HA.. 2004. Speciation on the coasts of the new world: phylogeography and the evolution of bindin in the sea urchin genus Lytechinus. Evolution 58(6):1225–1241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence assembly and annotations can be found at the National Center for Biotechnology Information (NCBI) under Bioproject PRJNA647794. Additionally, annotation files including a GFF3 and functional annotation file can be found in supplementary files 1 and 2, Supplementary Material online.