Abstract
Elucidating the molecular mechanism of the microRNAs in skin fibrosis is critical for identifying a novel therapeutic strategy for hypertrophic scar (HS). In this study, it was shown that miR-210-5p is induced by TGFβ, and that overexpression of miR-210-5p promoted the differentiation of human dermal fibroblasts (HDFs) into myofibroblasts. STAT5A is required for TGFβ-induced STAT3 activity. Here, we show that miR-210-5p attenuated TGFβ-induced STAT3 signaling pathway by suppressing the expression of STAT5A. Taken together, the present study suggests that TGFβ-induced miR-210-5p reduced STAT5A expression, leading to aberrant activation of STAT3, and facilitate skin fibrosis in HDFs.
Keywords: MiR-210-5p, Human dermal fibroblasts, Differentiation, STAT5A, STAT3
Introduction
After the injury, if the deep dermal wounds are disturbed in the initial stage of repair, it may lead to the formation of newly generated tissue, which may evolve into hypertrophic scar (HS) tissue (Niessen et al. 1999). The hypertrophic scar is characterized by the deposition of extracellular matrix (ECM), the angiogenesis of fibroblasts, and their trans-differentiation into myofibroblasts (Park et al. 2019). The adverse physiological and psychological effects of hypertrophic scar formation after wound healing are still the patients’ main medical problems (Martin 1997). Although various treatments can be used to prevent scar formation in the process of skin wound healing, the effect is still unsatisfactory (Berman et al. 2007). Therefore, it is essential to understand the mechanism of scar formation for the development of novel therapies.
Myofibroblasts are known to play critical roles in diverse biological processes, such as fibrosis, wound healing, and immunological reaction (Adegboyega et al. 2002; Owens and Simmons 2013). One of the major mediators in the fibrogenic processes, transforming growth factor beta (TGFβ), has been reported to play a critical role in the differentiation of fibroblasts into myofibroblasts (Varga and Jimenez 1986). In response to TGFβ, several different types of cells, including the dermal fibroblasts, could transdifferentiate into myofibroblasts, which causes excessive contraction and extracellular matrix (ECM) deposition. (Fan et al. 2015; Piersma et al. 2018). Accumulating evidence revealed that TGFβ stimulation induced the expression of αSMA in fibroblasts, leading to the acquisition of myofibroblasts-like properties. Therefore, it can be inferred that myofibroblasts can be defined as TGFβ activated fibroblasts (Desmouliere et al. 1993).
Dysregulation of the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) pathway has been associated with several human diseases, including, autoimmune disease, allergy, and cancer (Hou et al. 2002; O'Shea and Plenge 2012). Recent studies have emphasized the role of STAT3 in skin fibrosis and TGFβ signaling (Pedroza et al. 2018). JAK/STAT1 signaling is involved in the regulating of ultraviolet-induced MMP-1 expression in human dermal fibroblasts. The protein inhibitor of activated STAT 4 (PIAS4) contributes to cirrhosis through regulating SIRI1-dependent Smad3 deacetylation (Kim et al. 2008; Sun et al. 2016), suggesting that JAK/STAT signaling have a widespread effect on the regulation of fibrosis. STAT5A has been shown to play a vital role in immune cells and absence of lymphoid STAT5A lead to loss of CD8+ and regulatory T cells (Treg) (Hoelbl et al. 2006). STAT5 was also identified as a cytosolic signaling molecule involved in proliferation, differentiation, and apoptosis in various cell types (Cumaraswamy and Gunning 2012). However, little is known about the function of STAT5A in dermal fibroblasts differentiation.
MicroRNAs (miRNAs), a class of 17–23 nucleotide non-coding RNAs, have been shown to be involved in diverse biological and pathological processes by binding to 3′-UTR (3′-untranslated region) of the target mRNAs, thus inducing the translational inhibition and/or degradation of mRNAs. Currently, the regulatory effect of microRNAs on skin wound healing is gradually prominent (Li et al. 2016; Zhou et al. 2015). MiR-210-5p is a potent factor of angiogenesis in the hypoxic tumor microenvironment (Fasanaro et al. 2008, 2009). It has been reported that miR-210 was highly expressed in skin lesions, and the upregulation of miR-210 can promote tissue healing in vivo (Feng et al. 2020; Hu et al. 2010; Shoji et al. 2012). Furthermore, miR-210 is involved in liver cirrhosis and fibroblast proliferation (Bodempudi et al. 2014; Watany et al. 2018). However, the role of miR-210-5p in HS remains unclear. In this study, we investigated the function of STAT5A in fibroblast. We also examined the molecular mechanism underlying the relationship between miR-210 and HDFs differentiation. The results showed that down-regulation of STAT5A facilitated the phosphorylation of STAT3 and that miR-210-5p regulated TGFβ-induced HDFs differentiation by targeting STAT5A.
Materials and methods
Cell culture and transfection
Human dermal fibroblasts (HDFs) were obtained from American-type culture collection (ATCC) and cultured at 37 °C in DMEM media containing 10% FBS (Thermo Fisher Scientific, Inc, Waltham, MA, USA). All transfection experiments were carried out with Effectene Transfection Reagent (QIAGEN, DUs, GER) according to the manufacturer’s instructions. The miR-210-5p mimic (mimic), miR-210-5p inhibitor (inhibitor) and scramble control (control) Oligonucleotides were purchased from Sangon Biotech Shanghai (Shanghai, China).
RNA analysis
Total RNA was extracted from HDFs using RNAisoPlus (Takara, Japan) according to the manufacturer’s instructions. 800 ng of total RNA was reversely transcribed into cDNA using PrimeScripTMRT Master Mix cDNA synthesis system (Takara, Japan). TB GreenTM Premix EX TaqTM II (Takara, Japan) was used for quantification. The expression of the gene of interest was normalized to GAPDH RNA level. The following primers were used for qPCR: GAPDH (forward 5′-GGAGCGAGATCCCTCCAAAAT-3′; reverse 5′-GGCTGTTGTCATACTTCTCATGG-3′), αSMA (forward 5′-TTCAATGTCCCAGCCATGTA-3′; reverse 5′-GAAGGAATAGCCACGCTCAG-3′), Col-1 (forward 5′-CGGACGACCTGGTGAGAGA-3′; reverse 5′-CATTGTGTCCCCTAATGCCTT-3′), Col-3 (forward 5′-CCCTGAGGAACGGGAGAGTA-3′; reverse 5′-CTTTCCAACGATCCTCGCCT-3′), STAT5A (forward 5′-GCAGAGTCCGTGACAGAGG-3′; reverse 5′-CCACAGGTAGGGACAGAGTCT-3′).
Western blot analysis
Total proteins were extracted from cells using the RIPA buffer (Solarbio, Beijing, China) in the presence of Protease Inhibitor Cocktail and Protein Phosphatase Inhibitor (Solarbio, Beijing, China). Western blots were performed according to standard procedures.
Reagents and antibodies
The concentration for recombinant human TGFβ (R&D, USA) stimulation was 20 ng/ml. For STAT3 inhibition, cells were treated with 10 μM STAT3 inhibitor C188-9 (Selleck Technology, Inc., Shanghai, China) for 12 h. Primary antibodies: Actin antibody (1:3000, Cell Signaling Technology, Inc., USA); Col-1 antibody (1:2000, Abcam, USA); Col-3 antibody (1:2000, Abcam, USA); αSMA antibody (1:1500, Cell Signaling Technology, Inc., USA); STAT3 antibody (1:2000, Cell Signaling Technology, Inc., USA); p-STAT3 antibody (1:1000, Cell Signaling Technology, Inc., USA); STAT5A antibody (1:2500, Cell Signaling Technology, Inc., USA); p-STAT5A antibody (1:1500, Abcam, USA); JAK2 antibody (1:2000, Cell Signaling Technology, Inc., USA); p-JAK2 antibody (1:1000, Cell Signaling Technology, Inc., USA); Smad2 antibody (1:3000, Cell Signaling Technology, Inc., USA); p-Smad2 antibody (1:1500, Cell Signaling Technology, Inc., USA); Smad3 antibody (1:3000, Abcam, USA); p-Smad3 antibody (1:2000, Abcam, USA).
Luciferase reporter assay and construction
The cDNA of human STAT3 was amplified by PCR and cloned into the pcDNA6.0 empty vector. For a generation of pmirGLO-STAT5A-wt and pmirGLO-STAT5A-mu, the wide-type (WT, 5′-GTAGGACTCGCAGTCAGGGGCA-3′), and mutant (MU, 5′-GTAGGACTCGCAGTCTCCCCGA-3′) 3′-UTR of STAT5A were cloned into the pmirGLO vector (Promega, WI, USA). HDFs were transfected with luciferase constructs and miR-210-5p mimic. After 48 h, a dual-luciferase reporter assay system (Promega, WI, USA) was used to measure the luciferase activity.
Cell immunostaining
Following transfection, HDFs were transferred to 24 well plates containing coverslips, and then cells were washed in 0.5 ml PBS and fixed with 4% formaldehyde for 15 min. Cells were washed twice in 0.5 ml PBT (PBS containing 0.1% Tween-20) and then blocked with 5% BSA for 1 h. Cells were then incubated with indicated primary antibody overnight at 4 ℃ before 2 × 5 min TBST washes. Secondary antibody was incubated for 4 h at room temperature. Immunostaining samples were photographed with an inverted fluorescence microscope (Olympus).
Cell motility analysis
For wound healing assay, 2 × 105 transfected cells were plated into 12-well plates and incubated at 37 °C. After reaching 100% of confluence, cells were wounded by scraping with a 200 µl tip, followed by the washing for 3 times in serum-free medium to remove the detached cells. Subsequently, cells were photographed with an inverted microscope (Olympus). Cell migration was assessed using transwell chambers with a Matrigel (BD Bioscience, USA). Briefly, 2 × 104 transfected cells in serum-free medium were placed into the upper compartment of the chamber. The bottom chambers were filled with a completed medium (added 10% FBS). After incubation for 48 h in a humidified atmosphere of 5% CO2 at 37 °C, the cells on the upper surface of filters were removed from the top well with a cotton swab, while the cells migrated into the lower surface of filters were fixed with 70% methanol for 30 min and stained with 0.2% crystal violet.
Statistical analysis
Data are presented the mean ± SEM of at least three independent experiments. The differences between the two means were analyzed with the Student's t test using IBM SPSS Statistics 22.0. Comparisons among multiple groups were analyzed using one-way ANOVA followed by LSD post hoc test. P-values below 0.05 were considered significantly different.
Results
Downregulation of miR-210-5p inhibits TGFβ-induced αSMA
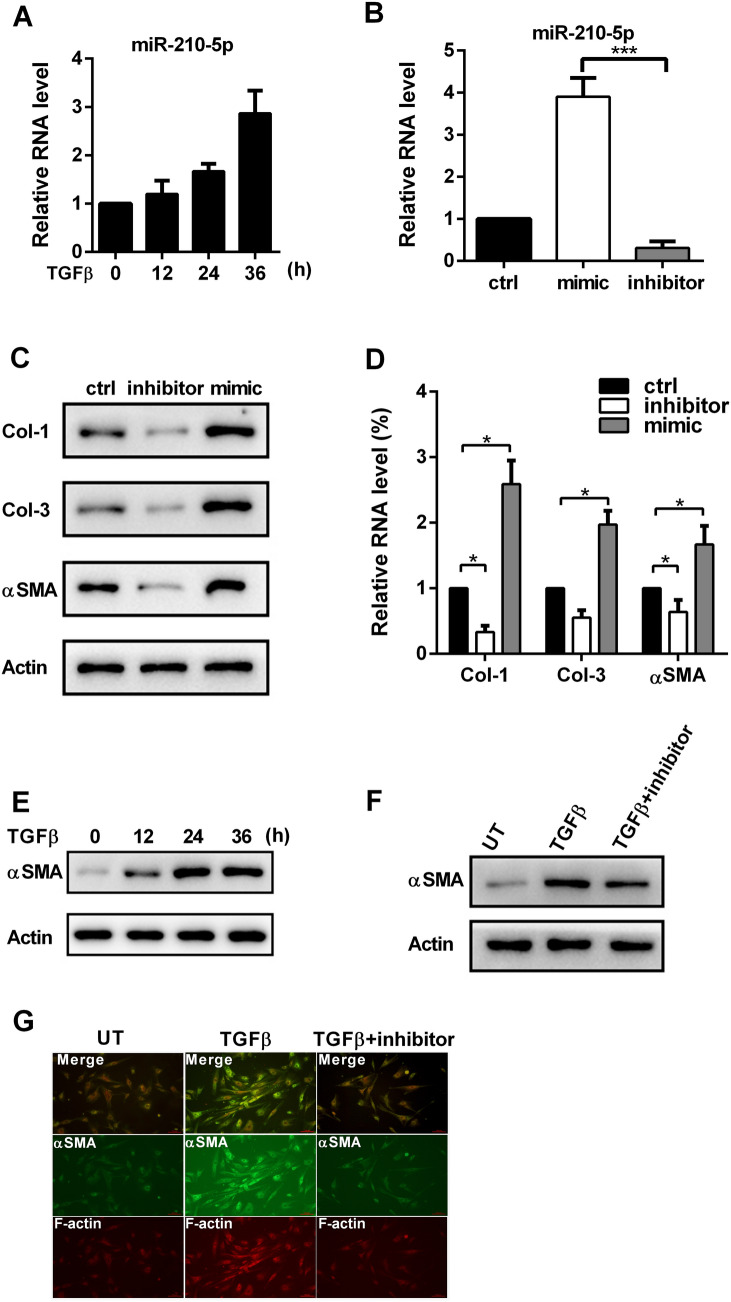
To identify the role of miR-210-5p in the differentiation of skin fibroblasts, we first tested whether miR-210-5p is induced by TGFβ treatment. As shown in Fig. 1a, the expression of miR-210-5p was elevated upon TGFβ stimulation in a time-dependent manner. We performed gain- and loss-function experiments through transfection with inhibitor or mimic of miR-210-5p (Fig. 1b). It was observed that miR-210-5p knockdown decreased the expressions of collagen I (Col-1), collagen III (Col-3), and αSMA in both RNA and protein levels (Fig. 1c and d), whereas miR-210-5p overexpression did the opposite. As expected, TGFβ treatment elevated the expression of αSMA in HDFs (Fig. 1e). Remarkably, the downregulation of miR-210-5p reduced TGFβ-induced αSMA expression (Fig. 1f). This result was further confirmed by immunofluorescence staining, inhibition of miR-210-5p decreased αSMA and F-actin expression upon TGFβ stimulation (Fig. 1g). Together, these results indicated that miR-210-5p is essential for TGFβ-induced differentiation of HDFs into myofibroblasts.
Fig. 1.
MiR-210-5p is required for TGF-induced αSMA. a The expression of miR-210-5p in HDFs was measured by qPCR upon TGFβ treatment. b The expression of miR-210-5p in HDFs was measured by qPCR after transfection with miR-210-5p mimic or inhibitor. c Protein levels of Col-1, Col-3, and αSMA in HDFs were was measured by western blot after transfection with miR-210-5p mimic or inhibitor. d RNA levels of Col-1, Col-3, and αSMA in HDFs were was measured by qPCR after transfection with miR-210-5p mimic or inhibitor. e The expression of αSMA in HDFs was measured by western blot upon TGFβ treatment. f HDFs were treated with or without TGFβ, and the combination of TGFβ and miR-210-5p inhibitor, western blot measured the expression of αSMA. UT, untreated. g F-actin and αSMA staining of HDFs treated with or without TGFβ, and the combination of TGFβ and miR-210-5p inhibitor. Data are represented as the mean ± SEM. Student's t test was performed to analyze the differences (*P < 0.05; ***P < 0.001 in comparison with the indicated group)
MiR-210-5p facilitates human dermal fibroblast migration
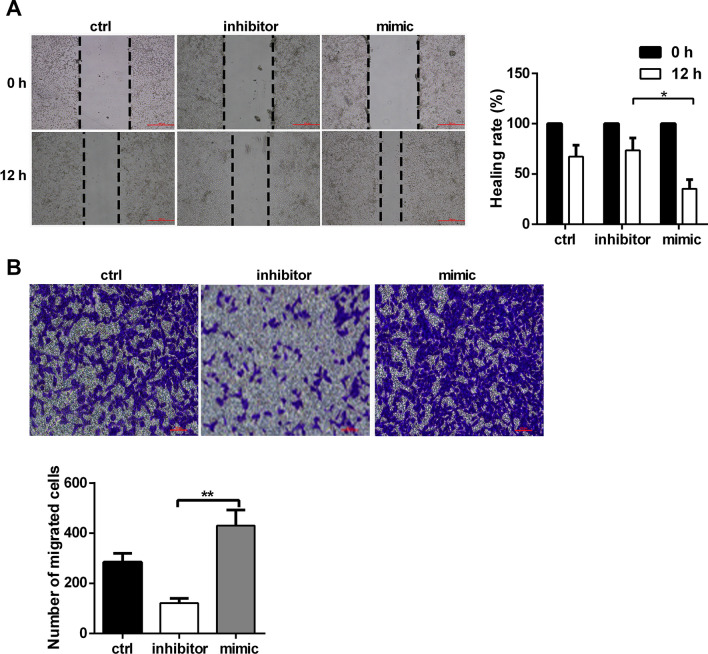
Next, we examined the role of miR-210-5p in fibroblast migration. The results of the wound healing assay indicated that miR-210-5p overexpression significantly enhanced the migratory capacity of HDFs (Fig. 2a). Further, it was observed that miR-210-5p overexpression promoted, whereas miR-210-5p downregulation inhibited migration of HDFs towards fetal bovine serum (Fig. 2b). Collectively, these results indicated that miR-210-5p promoted the motility of HDFs.
Fig. 2.
Overexpression of miR-210-5p promotes the motility of HDFs. a The migration of HDFs was detected by wound healing assay in the presence of miR-210-5p mimic or inhibitor. b After TGFβ treatment, transwell assay of HDFs invasion in the presence of miR-210-5p mimic or inhibitor. Data are represented as the mean ± SEM. Student's t test was performed to analyze the differences (*P < 0.05; **P < 0.01 in comparison with the indicated group)
MiR-210-5p is involved in the regulation of STAT3 signaling in HDFs
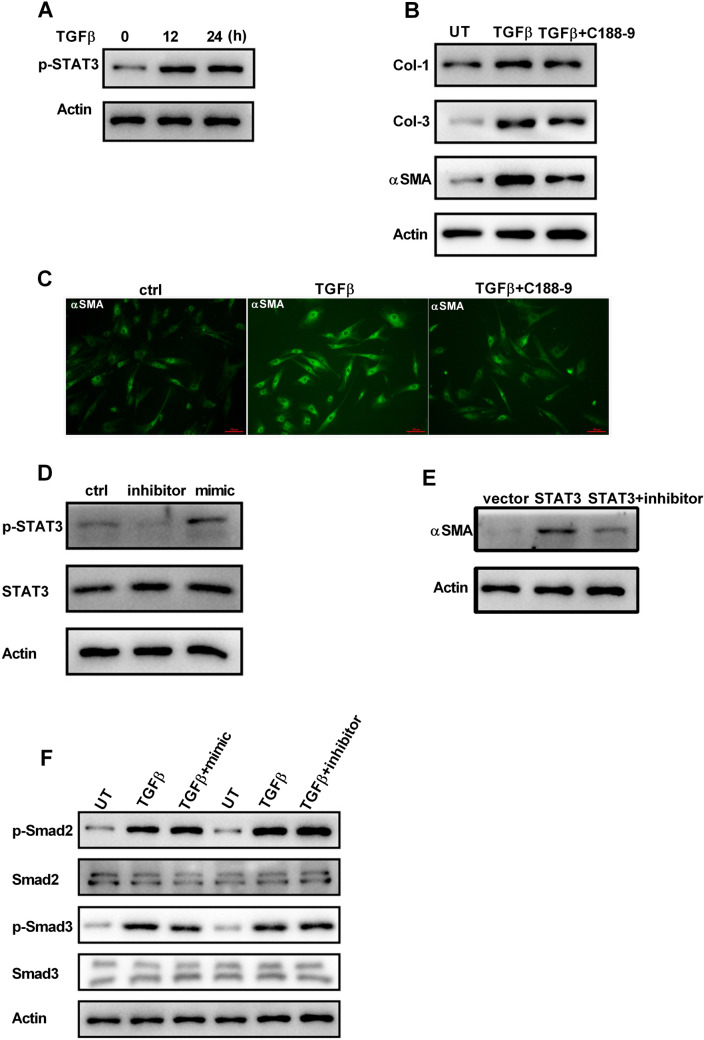
It has been reported that JAK/STAT3 signaling regulated dermal fibroblast function. Therefore, it was hypothesized that STAT3 plays a role in the phenotypical switch from fibroblasts to myofibroblasts. The results indicated that TGFβ stimulation increased the phosphorylation of STAT3 in HDFs (Fig. 3a). Inhibition of STAT3 by C188-9 resulted in a reduction in TGFβ-induced expression of Col-1, Col-3 and αSMA, as well as the amount of αSMA-positive fibroblasts (Fig. 3b and c), suggesting that STAT3 modulates fibrosis signaling in HDFs. Downregulation of miR-210-5p decreased phosphorylated STAT3, whereas miR-210-5p overexpression did the opposite, indicating that miR-210-5p is involved in STAT3-related signaling (Fig. 3d). Overexpression of STAT3 in HDFs results in an increase in αSMA expression, which is reduced by miR-210-5p inhibitor transfection (Fig. 3e). Smad signaling had been reported to play a critical role in fibroblast activation. However, alteration of miR-210-5p had no effect on the activation of Smad2 and Smad3 (Fig. 3f). Together, these results suggested that miR-210-5p regulated STAT3 signaling in response to TGFβ stimulation.
Fig. 3.
MiR-210-5p is required for TGF-induced STAT3 activity. a After TGFβ treatment, the phosphorylated STAT3 was detected by western blot. b HDFs were treated with or without TGFβ, and the combination of TGFβ and STAT3 inhibitor C188-9 (20 µM), western blot measured the expression of Col-1, Col-3, and αSMA. c The αSMA staining of HDFs treated with or without TGFβ, and the combination of TGFβ and STAT3 inhibitor C188-9 (20 µM). d Total and phosphorylated forms of STAT3 were detected by western blot after transfection with miR-210-5p mimic or inhibitor. e The protein level of αSMA in HDFs was detected by western blot after transfection with STAT3 or co-transfection with STAT3 and miR-210-5p inhibitor. f Total and phosphorylated forms of Smad2 and Smad3 were detected by western blot in HDFs treated with or without TGFβ, and the combination of TGFβ and miR-210-5p inhibitor. UT, untreated. Data are represented as the mean ± SEM. Student's t test was performed to analyze the differences (*P < 0.05; **P < 0.01 in comparison with the indicated group)
Inhibition of STAT5A elevated STAT3 activity in HDFs
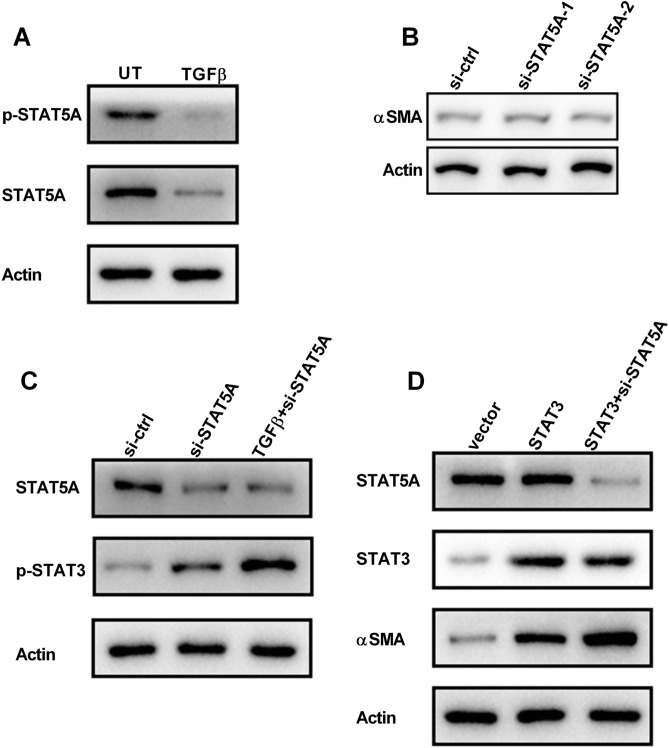
To verify the function of STAT5A in skin fibrosis, HDFs were treated with TGFβ, and STAT5A expression was examined by western blot. It was found that the protein level of STAT5A and phosphorylated STAT5A (p-STAT5A) were decreased upon TGFβ treatment in HDFs (Fig. 4a). However, RNAi-mediated downregulation of STAT5A does not change the basal expression of αSMA in HDFs (Fig. 4b). Earlier studies have shown that in the absence of STAT5, STAT3 can be recruited into the receptor associated with STAT5, resulting in its aberrant activation (Cui et al. 2007; Hosui and Hennighausen 2008). As shown in Fig. 4c, knockdown of STAT5A elevated the phosphorylation of STAT3 in HDFs upon TGFβ treatment (Fig. 4c). Additionally, overexpression of STAT3 increased αSMA expression, which was markedly enhanced by STAT5A silencing (Fig. 4d). These data suggested that the downregulation of STAT5A enhanced the activation of STAT3 in HDFs.
Fig. 4.
Downregulation of STAT5A impedes TGF-induced STAT3 activity. a Total and phosphorylated forms of STAT5A were detected by western blot in HDFs treated with or without TGFβ. UT, untreated. b HDFs were transfected with ctrl-siRNA or STAT5A-siRNA. The protein level of αSMA was detected by western blot. c HDFs were treated with or without TGFβ, and the combination of TGFβ and STAT5A-siRNA, western blot measured the expression of STAT5A and the phosphorylated of STAT3. d HDFs were transfected with STAT3 or the combination of STAT3 and STAT5A-siRNA, western blot measured the expression of STAT5A, STAT3, and αSMA. Data are represented as the mean ± SEM. Student's t test was performed to analyze the differences (*P < 0.05; **P < 0.01 in comparison with the indicated group)
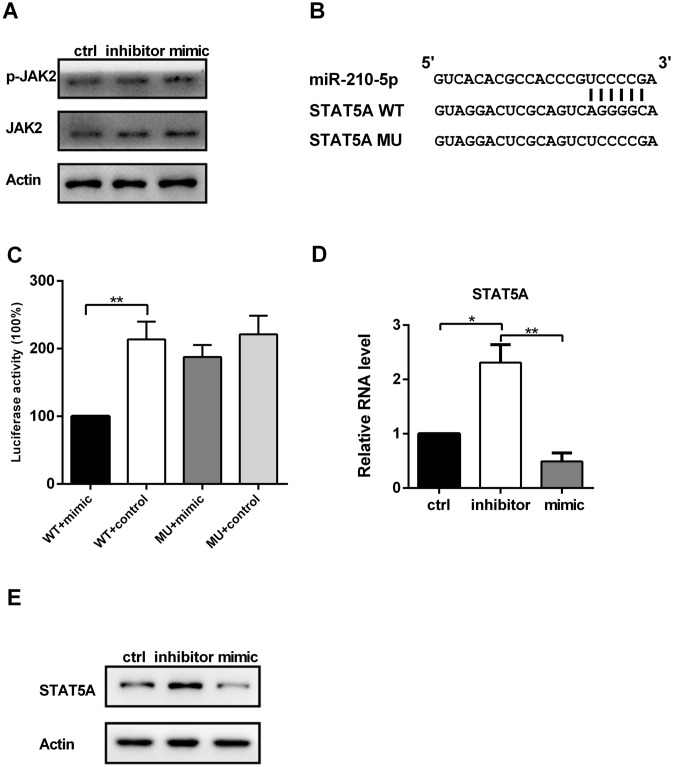
MiR-210-5p regulates JAK/STAT3 signaling by targeting STAT5A
As described thus far, it was shown that miR-210-5p is involved in the differentiation of fibroblasts and the regulation of STAT3 signaling. To further assess the relationship between miR-210-5p and JAK/STAT3 pathway, we examined whether knockdown of miR-210-5p affects JAK2 phosphorylation. Western blot analysis showed that knockdown of miR-210-5p did not affect JAK2 phosphorylation (Fig. 5a). Next, we performed Bioinformatics analysis by databases of TargetScan and MiRanda. The results predicted that miR-210-5p might target STAT5A (Fig. 5b). Therefore, we assessed the direct reaction between miR-210-5p and STAT5A using dual-luciferase reporter assay. Compared with the control groups, the function of luciferase was inhibited by transfecting with miR-210-5p mimic fused to 3′-UTR of STAT5A (Fig. 5c). Consistently, miR-210-5p overexpression decreased, whereas miR-210-5p downregulation increased the expression of STAT5A on both mRNA and protein levels (Fig. 5d and e). Taken together, these results indicated that miR-210-5p directly binds to the 3′-UTR of STAT5A.
Fig. 5.
a Total and phosphorylated forms of JAK2 in HDFs were was measured by western blot after transfection with miR-210-5p mimic or inhibitor. b Graphical representation of the miR-210-5p binding motif at the 3′-UTR of STAT5A. c The luciferase activity displayed by the luciferase reporter constructs which contained either the wild type (WT) or mutation (MU) of STAT5A 3′-UTR after miR-210-5p mimic transfection. d, e) The expression of STAT5A in HDFs was measured by western blot or qPCR after transfection with miR-210-5p mimic or inhibitor. Data are represented as the mean ± SEM. Student's t test was performed to analyze the differences (*P < 0.05; **P < 0.01 in comparison with the indicated group)
Discussion
The long-term inflammation and infection caused by skin injury lead to the failure of normal clearance of myofibroblasts and continuous increment and activation of myofibroblasts. Excessive synthesis and secretion of Col-1 and Col-3, resulting in scar thickening and hardening. HS affects the appearance and limb function; at the same time, it brings great suffering to the patient's body and mind (Bloemen et al. 2009). However, the cause of HS is still not clearly understood.
Skin fibrosis is a remarkable feature of hypertrophic scars, and it mainly occurs in the dermis, the connective tissue layer under the basement membrane and epidermis. Skin fibrosis involves multiple cellular signaling, such as Notch, TGFβ-Smad, and JAK/STAT pathway (Desmouliere et al. 1993; Hu and Phan 2016; Pedroza et al. 2018). Our data showed that STAT3 participates in dermal fibrosis, and it is required for the expressions of αSMA, Col-1 and Col-3 (Fig. 3b). These results were consistent with a previous report showing that inhibition of STAT3 suppressed dermal fibrosis in the bleomycin (BLM) mouse model (Pedroza et al. 2018). In the immune system, STAT5 proteins, including STAT5A and STAT5B, have been shown to play a critical role in the regulation of IL-2-signaling (Imada et al. 1998; Nakajima et al. 1997). However, the role of STAT5 in dermal fibrosis and the underlying molecular mechanism remains largely unexplored. In the present study, it was found that stimulation of HDFs with TGFβ decreased the expression of STAT5A (Fig. 4a). Knockdown of STAT5A facilitated the activation of STAT3 upon TGFβ stimulation, as well as αSMA expression (Fig. 4b and c), indicating that TGFβ-induced STAT3 activation is dependent on STAT5A in HDFs. The association between TGFβ and STAT5 signaling has been previously suggested in different cell lines. For example, TGFβ stimulation reduces the expression of STAT5 in human mast cell (Fernando et al. 2013). In regulatory T cells (Treg), the expression of Foxp3 gene is elevated by TGFβ stimulation by enhancing STAT5 activation following downregulation of SOCS3 expression (Ogawa et al. 2014). The N terminus of STAT5 interacts with TGFβ, and abrogation of STAT5 led to an increase in TGFβ expression as a result of elevated stability (Hosui et al. 2009). Additionally, STAT5 expression is negatively correlated with TGFβ in hepatic fibrosis (Abu El Makarem et al. 2018). It was suggested that inactivated mutations in STAT5 led to dwarfism and immunological disorders (Bernasconi et al. 2006; Kofoed et al. 2003). Unscheduled activation of STAT3 was observed in the primary cells isolated from these patients, and some of the immune disorders observed in these patients may be related to the over-activation of STAT3 rather than the loss of STAT5. For dermal fibrosis, it is likely that under our experimental conditions, stimulation of HDFs with TGFβ suppresses the expression of STAT5A resulted in aberrant activation of STAT3 (Fig. 4).
Mounting evidence has suggested that microRNAs play a critical role in pathological wound healing and the development of skin fibrosis (Li et al. 2017a, 2017b). MiR-210 considered as oncogenes (so-called oncomiRs) due to its high levels of expression in many solid tumors, including breast, cervical, and pancreatic (Grosso et al. 2013; Qin et al. 2014). In this study, it was observed the upregulation of miR-210-5p in HDFs upon TGFβ stimulation. Inhibition of miR-210-5p decreased the expressions of Col-1, Col-3, and αSMA (Fig. 1), suggesting that miR-210-5p is involved in the regulation of skin fibrosis. These results were similar to findings from other studies, for instance, overexpression of miR-210 promotes fibroblast proliferation via suppressing the MNT, a c-myc inhibitor (Bodempudi et al. 2014). Upregulation of miR-210 facilitates epithelial-mesenchymal transition (EMT) via activating JAK/STAT3 signaling in lung cancer cells (Zhang et al. 2019). It was also found that the downregulation of miR-210-5p impeded the migratory capacity of HDFs, suggesting a unique role of miR-210-5p in the EMT process (Fig. 2). Further, miR-210-5p knockdown reduced the activation of STAT3 signaling, indicating that miR-210-5p participates in the regulation of STAT3 (Fig. 3d and e). Stimulation of HDFs with TGFβ increased miR-210-5p and decreased STAT5A expression. This promotes us to explore the relationship between miR-210-5p and STAT5A. Bioinformatics analysis demonstrated that STAT5A is a direct target of miR-210-5p, and this prediction is further confirmed by luciferase reporter assay (Fig. 5). Consistently, miR-210-5p mimic transfection decreased the expression of STAT5A. In addition to the JAK/STAT signaling, miR-210 has been shown to regulate paraquat-induced EMT by suppressing RUNX3 and maintaining HIF-1α signaling (Zhu et al. 2017). Alteration of miR-210-5p does not affect the activity of Smad, indicating that miR-210-5p regulates skin fibrosis via JAK/STAT signaling specifically.
Conclusion
In summary, the results provided evidence that TGF-β treatment increased miR-210-5p in HDFs. MiR-210-5p reduced the expression of STAT5A expression, and activated STAT3 signaling, leading to the differentiation of HDFs into myofibroblasts. Our data revealed an essential role of miR-210-5p in skin fibrosis and suggest a therapeutic potential of miR-210-5p in the hypertrophic scar.
Acknowledgements
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Abu El Makarem MA, El-Sagheer GM, Abu El-Ella MA. The role of signal transducer and activator of transcription 5 and transforming growth factor-β1 in hepatic fibrosis induced by chronic hepatitis c virus infection in egyptian patients. Med Princ Pract Int J Kuwait Univ Health Sci Cent. 2018;27:115–121. doi: 10.1159/000487308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–836. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- Berman B, Perez OA, Konda S, Kohut BE, Viera MH, Delgado S, Zell D, Li Q. A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol Surg off Publ Am Soc Dermatol Surg. 2007;33:1291–1302. doi: 10.1111/j.1524-4725.2007.33280.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Marino R, Ribas A, Rossi J, Ciaccio M, Oleastro M, Ornani A, Paz R, Rivarola MA, Zelazko M, et al. Characterization of immunodeficiency in a patient with growth hormone insensitivity secondary to a novel STAT5b gene mutation. Pediatrics. 2006;118:e1584–1592. doi: 10.1542/peds.2005-2882. [DOI] [PubMed] [Google Scholar]
- Bloemen MC, van der Veer WM, Ulrich MM, van Zuijlen PP, Niessen FB, Middelkoop E. Prevention and curative management of hypertrophic scar formation. Burns J Int Soc Burn Inj. 2009;35:463–475. doi: 10.1016/j.burns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Bodempudi V, Hergert P, Smith K, Xia H, Herrera J, Peterson M, Khalil W, Kahm J, Bitterman PB, Henke CA. miR-210 promotes IPF fibroblast proliferation in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L283–294. doi: 10.1152/ajplung.00069.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46:504–513. doi: 10.1002/hep.21713. [DOI] [PubMed] [Google Scholar]
- Cumaraswamy AA, Gunning PT. Progress towards direct inhibitors of Stat5 protein. Horm Mol Biol Clin Invest. 2012;10:281–286. doi: 10.1515/hmbci-2012-0009. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Hao C, Li M, Dai X, Qin H, Li J, Xu H, Wu X, Zhang L, Fang M, et al. MKL1 is an epigenetic modulator of TGF-beta induced fibrogenesis. Biochem Biophys Acta. 2015;1849:1219–1228. doi: 10.1016/j.bbagrm.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M, et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J Biol Chem. 2009;284:35134–35143. doi: 10.1074/jbc.M109.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Wu R, Zhang S, Kong Y, Liu Z, Wu H, Wang H, Su Y, Zhao M, Lu Q. Topical administration of nanocarrier miRNA-210 antisense ameliorates imiquimod-induced psoriasis-like dermatitis in mice. J Dermatol. 2020;47:147–154. doi: 10.1111/1346-8138.15149. [DOI] [PubMed] [Google Scholar]
- Fernando J, Faber TW, Pullen NA, Falanga YT, Kolawole EM, Oskeritzian CA, Barnstein BO, Bandara G, Li G, Schwartz LB, et al. Genotype-dependent effects of TGF-β1 on mast cell function: targeting the Stat5 pathway. J Immunol (baltimore, Md: 1950) 2013;191:4505–4513. doi: 10.4049/jimmunol.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, Gounon P, Lacas-Gervais S, Noël A, Pouysségur J, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelbl A, Kovacic B, Kerenyi MA, Simma O, Warsch W, Cui Y, Beug H, Hennighausen L, Moriggl R, Sexl V. Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood. 2006;107:4898–4906. doi: 10.1182/blood-2005-09-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosui A, Hennighausen L. Genomic dissection of the cytokine-controlled STAT5 signaling network in liver. Physiol Genomics. 2008;34:135–143. doi: 10.1152/physiolgenomics.00048.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J Exp Med. 2009;206:819–831. doi: 10.1084/jem.20080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SX, Zheng Z, Chen X, Perrimon N. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell. 2002;3:765–778. doi: 10.1016/s1534-5807(02)00376-3. [DOI] [PubMed] [Google Scholar]
- Hu B, Phan SH. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res. 2016;108:57–64. doi: 10.1016/j.phrs.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada K, Bloom ET, Nakajima H, Horvath-Arcidiacono JA, Udy GB, Davey HW, Leonard WJ. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim Y, Lee Y, Chung JH. Ceramide accelerates ultraviolet-induced MMP-1 expression through JAK1/STAT-1 pathway in cultured human dermal fibroblasts. J Lipid Res. 2008;49:2571–2581. doi: 10.1194/jlr.M800112-JLR200. [DOI] [PubMed] [Google Scholar]
- Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, et al. Growth hormone insensitivity associated with a STAT5b mutation. Nengl J Med. 2003;349:1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- Li G, Zhou R, Zhang Q, Jiang B, Wu Q, Wang C. Fibroproliferative effect of microRNA-21 in hypertrophic scar derived fibroblasts. Exp Cell Res. 2016;345:93–99. doi: 10.1016/j.yexcr.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang J, Lei Y, Lyu L, Zuo R, Chen T. MicroRNA-21 in skin fibrosis: potential for diagnosis and treatment. Mol Diagn Ther. 2017;21:633–642. doi: 10.1007/s40291-017-0294-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang J, Zhang W, Liu Y, Li Y, Wang K, Zhang Y, Yang C, Li X, Shi J, et al. MicroRNA-192 regulates hypertrophic scar fibrosis by targeting SIP1. J Mol Histol. 2017;48:357–366. doi: 10.1007/s10735-017-9734-3. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Liu XW, Wynshaw-Boris A, Rosenthal LA, Imada K, Finbloom DS, Hennighausen L, Leonard WJ. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997;7:691–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- Niessen FB, Spauwen PH, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- Ogawa C, Tone Y, Tsuda M, Peter C, Waldmann H, Tone M. TGF-β-mediated Foxp3 gene expression is cooperatively regulated by Stat5, Creb, and AP-1 through CNS2. J Immunol (baltimore, Md: 1950) 2014;192:475–483. doi: 10.4049/jimmunol.1301892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BM, Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6:224–234. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- Park GS, An MK, Yoon JH, Park SS, Koh SH, Mauro TM, Cho EB, Park EJ, Kim KH, Kim KJ. Botulinum toxin type a suppresses pro-fibrotic effects via the JNK signaling pathway in hypertrophic scar fibroblasts. Arch Dermatol Res. 2019;311:807–814. doi: 10.1007/s00403-019-01975-0. [DOI] [PubMed] [Google Scholar]
- Pedroza M, To S, Assassi S, Wu M, Tweardy D, Agarwal SK. Role of STAT3 in skin fibrosis and transforming growth factor beta signalling. Rheumatology. 2018;57:1838–1850. doi: 10.1093/rheumatology/kex347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma B, Wouters OY, Bank RA. alphaII-spectrin and betaII-spectrin do not affect TGFbeta1-induced myofibroblast differentiation. Cell Tissue Res. 2018;374:165–175. doi: 10.1007/s00441-018-2842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Furong W, Baosheng L. Multiple functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer Res CR. 2014;33:50. doi: 10.1186/1756-9966-33-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Nakasa T, Yamasaki K, Kodama A, Miyaki S, Niimoto T, Okuhara A, Kamei N, Adachi N, Ochi M. The effect of intra-articular injection of microRNA-210 on ligament healing in a rat model. Am J Sports Med. 2012;40:2470–2478. doi: 10.1177/0363546512458894. [DOI] [PubMed] [Google Scholar]
- Sun L, Fan Z, Chen J, Tian W, Li M, Xu H, Wu X, Shao J, Bian Y, Fang M, et al. Transcriptional repression of SIRT1 by protein inhibitor of activated STAT 4 (PIAS4) in hepatic stellate cells contributes to liver fibrosis. Sci Rep. 2016;6:28432. doi: 10.1038/srep28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Jimenez SA. Stimulation of normal human fibroblast collagen production and processing by transforming growth factor-beta. Biochem Biophys Res Commun. 1986;138:974–980. doi: 10.1016/s0006-291x(86)80591-5. [DOI] [PubMed] [Google Scholar]
- Watany MM, Hagag RY, Okda HI. Circulating miR-21, miR-210 and miR-146a as potential biomarkers to differentiate acute tubular necrosis from hepatorenal syndrome in patients with liver cirrhosis: a pilot study. Clin Chem Lab Med. 2018;56:739–747. doi: 10.1515/cclm-2017-0483. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, Li G, Tang J, Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18:40. doi: 10.1186/s12943-019-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Zhang Q, Zhang Y, Fu S, Wang C. Aberrant miR-21 and miR-200b expression and its pro-fibrotic potential in hypertrophic scars. Exp Cell Res. 2015;339:360–366. doi: 10.1016/j.yexcr.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang J, Meng X, Xie H, Tan J, Guo X, Han P, Wang R. A positive feedback loop promotes HIF-1alpha stability through miR-210-mediated suppression of RUNX3 in paraquat-induced EMT. J Cell Mol Med. 2017;21:3529–3539. doi: 10.1111/jcmm.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]