Abstract
Genome-wide association study (GWAS) provides a robust and potent tool to retrieve complex phenotypic traits back to their underlying genetics. Maize is an excellent crop for performing GWAS due to diverse genetic variability, rapid decay of linkage disequilibrium, availability of distinct sub-populations and abundant SNP information. The application of GWAS in maize has resulted in successful identification of thousands of genomic regions associated with many abiotic and biotic stresses. Many agronomic and quality traits of maize are severely affected by such stresses and, significantly affecting its growth and productivity. To improve productivity of maize crop in countries like India which contribute only 2% to the world’s total production in 2019–2020, it is essential to understand genetic complexity of underlying traits. Various DNA markers and trait associations have been revealed using conventional linkage mapping methods. However, it has achieved limited success in improving polygenic complex traits due to lower resolution of trait mapping. The present review explores the prospects of GWAS in improving yield, quality and stress tolerance in maize besides, strengths and challenges of using GWAS for molecular breeding and genomic selection. The information gathered will facilitate elucidation of genetic mechanisms of complex traits and improve efficiency of marker-assisted selection in maize breeding.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02799-4.
Keywords: GWAS, Maize, Stress, Productivity, QTLs
Introduction
Maize (Zea mays L.) is an important cereal crop which has a huge demand as food, feed and fodder across the world. In India maize is the third important cereal crop after wheat and rice in terms of production. It has wider adaptability and can be grown in all seasons, i.e., rainy, winter and summer. Apart from serving as basic raw material for production of starch, protein, oil, alcoholic beverages, food sweeteners and fuels, it provides nutrients to maintain human and animal health. Being a C4 plant, it may be a future cereal crop owing to its potential to perform better under conditions of global warming. About 193.7 Mha is under maize cultivation worldwide, producing 1147.7 Mt with an average productivity of 5.75 t/ha in 2019–2020 (FAOSTAT, 2020). The United States has been the major producers of maize contributing ~ 34% of world’s maize production (FAOSTAT, 2020). In India, maize occupied an area of 9.3 Mha with production of 29 Mt; the average productivity was 3.1 t/ha that contributed only ~ 2% to the world’s total production in 2019–2020 (USDA, 2020). Such lower yield level of maize in India provides ample opportunities to enhance its productivity through the development of new and improved technologies and subsequent popularization and adoption.
In plants systems, there are two major approaches to study genotype/trait associations using mapping populations; (i) linkage or QTL mapping, and (ii) association or linkage disequilibrium (LD) mapping (Xu et al. 2017). Association mapping is further classified into two groups (a) candidate gene association mapping and (b) whole genome or genome- wide association study (GWAS) (Ibrahim et al. 2020). The key differences between GWAS and QTL mapping has been explained by a number of researchers (Korte and Farlow, 2013; Alqudah et al. 2020). QTL mapping though resulting in identification and mapping of several QTLs have been failed to conclusively map some traits that are governed by many minor-effect QTLs, e.g., Fusarium ear rot resistance in maize (Wu et al. 2020). Complexity of large maize genome containing > 85% repetitive sequences also hinders fine mapping via QTL mapping (Xiao et al. 2017). QTL mapping has lower resolution power, as well as low speed and allele frequencies (Xu et al. 2017). In contrast, GWAS is an advancement of association mapping which relies upon historical recombination events, accumulated over hundreds of generations thereby, providing higher resolution power and greater allele frequency (Carlson et al. 2019; Alqudah et al. 2020).
Maize is considered as an ideal crop for GWAS (Xiao et al. 2017), and its suitability is mainly due to its genetic variability, distinct sub-populations (Cooper et al. 2019), availability of SNP information and rapid LD decay (Mazaheri et al. 2019). Germplasm screening through GWAS resulted in identification of stress tolerant maize inbred lines (Rodriguez et al. 2010; Farooqi et al. 2016). Adding such improved maize breeding lines into the association panel would be useful to intensify genetic variability and detect more genes of interest. Linkage analysis of stress tolerance traits has resulted in discovery of hundreds of QTLs by utilizing accessions showing extreme phenotypes. Polygenic complex traits such as yield and component traits, plant architecture and stress adaptation are often influenced by more than one gene and genotype × environment interactions. Conventional breeding therefore, has attained limited success in improving maize complex traits. GWAS on the other hand, enables us to reveal natural variation underlying some complex attributes, viz., yield, stress tolerance and biochemical and physiological traits. Researchers are now able to screen out several associated single nucleotide polymorphisms (SNPs), QTLs/ candidate genes for such complex traits through GWAS approach. Advancement in next-generation sequencing (NGS) technologies has led GWAS to become an extensively accepted tool for interpreting marker-trait associations (MTAs) in many plant species (Korte and Farlow 2013). However, it has also been stated that GWAS are likely to result in false implications of markers-trait association (Koning 2011).
Factors affecting production and productivity of maize crop
Maize crop is often exposed to various biotic and abiotic stresses in the field (Li et al. 2017). A large number of insect pests (earworm, corn borer, armyworm, fall armyworm, corn leaf aphid, grasshopper, weevil, thrips) and various pathogens attack maize crop at different growth stages (Singh and Singh 2018). Insect infestation severely damage kernel yield and quality. Similarly, abiotic stress results in stunted growth, elongated leaf (Sowinski et al. 2005), extended stem, proliferated root and elevated production of reactive oxygen species (Foyer et al. 2002).
Water, soil salinity/ alkalinity and temperature (> 35 °C and < 10 °C) stresses have been known to affect uptake of mineral and water (Sabagh et al. 2020), leaf carbon exchange (Tollenaar 1989), chloroplast function (Tao et al. 2016), RuBisCo activity (Perdomo et al. 2017) and conductivity of stomata (Hussain et al. 2018). These stresses additionally, reduce photosynthetic ability (Hussain et al. 2018; Sabagh et al. 2020), lower quantum efficiency of electron transfer at PSII (Li et al. 2020) and also cause abortion of the tassel branches (Bechoux et al. 2000) Excessive male inflorescence size and insufficient pollen may lead to significant yield reduction (Wu et al. 2016). It may cause a change in the composition of leaf pigment (Wijewardana et al. 2016), photo-inhibition (Sabagh et al. 2020) and alteration of the composition of protein present in thylakoid membranes (Sabagh et al. 2020). Further, major abiotic stresses such as drought (Barutcular et al. 2016), heat (Sabagh et al. 2020), salinity, combination of heat and drought (Sehgal et al. 2018) etc. not only reduce growth and development of maize crop but also severely affect nutrient composition (protein, grain starch, ash, crude oil and yield), thus affecting the quality of maize grain. Heat stress during seed filling stage, result in reduced number of endosperm, storage granules and proteins leading to reduced starch and protein accumulation in maize kernels (Monjardino et al. 2005). Mahmood et al. (2010) also reported a significant decrease in forage quality of maize in response to heat stress.
Elucidation and estimation of biotic and abiotic stress tolerance are critical for improving maize productivity (Wang et al. 2016a). Through appropriate screening procedures genotypic variability for stress tolerance in maize can be assessed apart from identifying traits related to stress tolerance (Zaidi et al. 2010). Screening for stress tolerance has been performed on the basis of potential traits including primary traits (grain yield) under stress), and secondary traits (anthesis–silking interval, plant height, ear height, grain yield components). Some other traits such as tassel sterility, silk balling and tassel blast related with thermal stress, stay green traits and brace roots related with water stress have been reported to be highly correlated with stress tolerance and also exhibit high heritability (Nardino et al. 2016; Musvosvi et al. 2018). These traits should also be considered for selection against stress tolerance in maize breeding to identify tolerant lines. Moreover, appraisal of stress tolerance in the field is highly influenced by the environmental conditions and it also becomes difficult to identify causal relationship between specific stress and final yield due to appearance of multiple stresses. GWAS have successfully revealed the genetic etiology by identifying a number of susceptibility loci for common abiotic and biotic stresses in maize.
Genome-wide association study (GWAS)
GWAS is one of the advances of association mapping (AM) which is utilized to identify markers located much closer to the gene of interest. The GWAS approach initially applied for studying human genetics by Klein et al. (2005), scans complete set of DNA or genome using a large number of markers to detect genetic variations correlated with a particular trait. Presently, it has become a routine method in the study of human disease and other complex attributes in many large cohort analyses (Visscher et al. 2012). GWAS have rapidly gained popularity as a standard method though, with some modifications, for discovering genotype–phenotype associations in plants as well. In 2008, scientists performed the first GWAS in maize to reveal loci with prime effect on oleic acid content in seeds by utilizing 8590 loci in 553 elite inbred lines (Belo et al. 2008). Since then, the application of GWAS for various traits has progressed rapidly leading to the release of ‘B73′ reference maize genome (Schnable et al. 2009).
Achievements in maize breeding resulting from GWAS have been reviewed by Xiao et al. (2017) citing several examples of QTL identification with significant effects on complex attributes such as biotic and abiotic stress tolerance. GWAS enables fine mapping of QTL using diverse populations as it faces, large number of historical recombination events which result in the rapid decay of linkage disequilibrium (LD) (Flint Garcia et al. 2003). LD refers to the non-random association of alleles at different loci which is estimated as Lewontin’s (‘D’) and coefficient of correlation (r2) (Hindu et al. 2018). Whether two SNPs are strong or weak LD can be determined using r2 estimate. If r2 value is > 0.2, co-inheritance and presence on same QTL of the two SNPs is revealed (Alqudah et al. 2020). Compared to other species, LD decay is rapid in maize on account of its diverse phenotypic and genotypic nature (Xu et al. 2017). Low LD enables high resolution mapping by depicting only those loci which are tightly linked to the gene of interest having significant association with the trait in question. However, such resolution requires large number of molecular markers as compared to high LD. Since, in case of high LD probability of identification of markers linked to causal variant is also high (Flint Garcia et al. 2003).
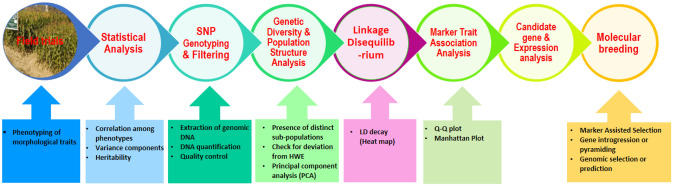
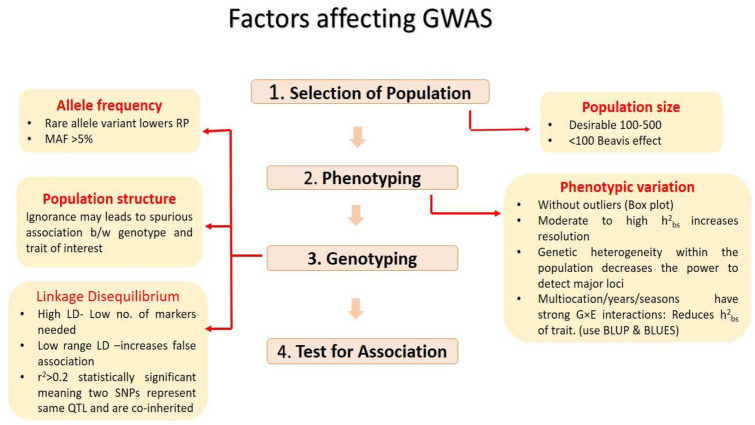
GWAS have been effectively utilized to reveal genomic regions and QTL governing tolerance to diseases like Fusarium ear rot (Zila et al. 2013), sugarcane mosaic virus (Tao et al. 2013), grey leaf spot (Shi et al. 2014), southern corn rust (Zhou et al. 2018), gibberella ear rot (Han et al. 2018) and maize leaf necrosis resistance (Nyaga et al. 2020). GWAS thus, fosters rapid estimation of the genetic construction of complex attributes (McCarthy et al. 2008). It possess great potential to revealing desirable genetic loci governing trait of interest with higher resolution, at economical cost as compared to the traditional linkage mapping (Yu and Buckler 2006). General procedure of conducting GWAS and the factors affecting its accuracy and resolution power are summarised in Figs. 1 and 2, respectively. GWAS have resulted in genetic dissection of many agriculturally relevant traits in maize (Supplementary Table 1).
Fig. 1.
A generalized procedure of genome-wide association study
Fig. 2.
Factors affecting GWAS accuracy and resolution at successive stages.
GWAS on agronomic and quality traits in maize
GWAS have been proved to be an effective tool in complementing significant association between SNP and quality trait and to understand its genetic basis (Wang et al. 2007; Tang et al. 2015). Zhao et al. (2018a) have provided information upon genetic influence of cadmium (Cd) accumulation, enabling quick formation of maize lines or genotypes comprising low Cd content through engineering of positive alleles, utilizing GWAS and QTL mapping approaches. On similar lines, Zhao et al. (2018b) after deciphering the inheritance pattern of lead (Pb) accumulation in grains and leaves, developed low Pb accumulating maize cultivars. Similarly, Hindu et al. (2018) discovered 46 SNPs significantly related with iron and zinc concentrations in kernel, while, Wang et al. (2016b) reported 73, 41 and 82 significant SNPs associated with acid and neutral detergent fiber and in vitro dry matter digestibility, respectively in mature stalk of maize influencing its forage quality. Such SNPs could be very useful in developing nutrition rich maize. Even mechanisms involved in hybrid performance have been elicited by performing multiple hybrid populations based GWAS using, 55 K SNP array for flowering time traits such as days to tassel initiation, silking, anthesis and anthesis–silking interval (Wang et al. 2017). GWAS have also been conducted for seedling traits improvement. Hu et al. (2017a) explored the genetic regulation of seedling traits by brassinosteroid and gibberellin, and correlated seedling stage hormone inhibitor response with field attributes using GWAS approaches. Genetic mechanism of cell wall components in maize stalk has been studied using AM panel of 368 inbred lines of maize in seven different environments (Li et al. 2016a). The scientists observed various loci, e.g., 22, 18 and 24 to be significantly correlated with lignin, cellulose and hemicellulose, respectively. The candidate genes identified by GWAS are known to encode enzymes which are mainly involved in metabolism of cell wall, various transcription factors, protein kinase enzyme and proteins related to various biological pathways in maize (Li et al. 2016a).
GWAS on biotic stress tolerance in maize
GWAS is generating molecular markers that have been broadly utilized for the indirect selection of traits like resistance to corn earworm, which is otherwise not accessible to traditional breeding programmes (Warburton et al. 2017). Such studies pursued by pathway analysis based on a gene-set enrichment method, enable a better insight of the genetic basis and physiological mechanisms of resistance against different insect pests and diseases in addition toelucidating biological route and correlated genes that provide resistance. The gene-set enrichment based GWAS has been used to detect cumulative effect of genes majorly contributing resistance (Tang et al. 2015; Warburton et al. 2017). GWAS have successfully detected the genes and corresponding alleles that govern resistance to maize lethal necrosis (Gowda et al. 2015) and resistance to Gibberella ear rot in dent and flint lines of maize (Han et al. 2018). Mahuku et al. (2016) identified a major QTL qRtsc8-1 responsible for 18–43% of the observed phenotypic variation for tar spot resistance using GWAS. These findings reveal candidate genes encoding protein or enzymes which are mainly involved in signal transduction, stress resistance, various transcriptional and post-transcriptional regulation of cell component synthesis. Thus, in depth analysis is required using ‘Omics’ approach so as to understand the complex molecular behaviour of biotic stress tolerance (Deshmukh et al. 2014).
GWAS on abiotic stress tolerance in maize
Tolerance of maize plants to abiotic stresses such as drought, water submergence, salt and heavy metals have been studied using GWAS. ‘Omics’ approach (Gong et al. 2014) revealed a series of genes involved in cold stress response, e.g., ZmDREB2A, ZmCOI6.1, ZmACA1, ZmERF3 (Nguyen et al. 2009), ZmPP2C2 (Hu et al. 2010), ZmMKK4 (Kong et al. 2011), ZmFKBP (Yu et al. 2012) and ZmMKK1 (Cai et al. 2014). Several candidate genes, viz., ZmVPP1 encoding drought tolerance (Wang et al. 2016c), GRMZM2G013726 encoding anthocyanin synthesis under low phosphorous condition (Xu et al. 2018a) and GRMZM2G377194, GRMZM2G026892, GRMZM2G060349 and GRMZM2G122199 associated with thermo-tolerance (Gao et al. 2019) have been identified. Further, due to the complex architecture of abiotic thermo-tolerance-traits, classical marker-assisted selection approaches fail to meet the need for stable yield under low or high temperature. Therefore, whole genome prediction study has been proposed to know about the genetic factors controlling traits. Similarly, most of the QTLs governing resistance to abiotic stresses at the germination and seedling stages have been identified in maize by conducting experiments either in growth chamber or under field conditions (Huang et al. 2013; Hu et al. 2017b). Occurrence of multiple stresses and unsteady environment, however, complicate assessment of stress tolerance in the field and revilement of causal association between stress and final yield (Riva-Roveda et al. 2016). It is very difficult to simultaneously access yield on account of abiotic stress tolerance and to locate candidate regions of the genome for positional cloning. Few studies have identified QTLs/genes associated with specific components of abiotic stress in different maize germplasm, e.g., Strigens et al. (2013) evaluated 375 maize inbred lines under both field and controlled conditions and obtained 19 highly significant associations with chlorophyll fluorescence and primary growth attributes under chilling stress.
A number of candidate genes and associated SNPs have been identified for various agronomic, quality traits as well as for biotic and abiotic stress tolerance. These can be further utilized in developing climate resilient maize varieties in future breeding programs. Thus, GWAS lead to genetic information on favourable alleles contributing towards the traits and explore new breeding alternatives rather than merely scanning genes associated with a trait of interest.
Identification and cloning of major candidate genes through GWAS in maize
SNPs and QTLs governing various important traits in maize have been identified by GWAS (Supplementary Table 1). Recently, some progress has been made in functional validation of detected allelic variants, e.g., fatty acid desaturase 2 (fad2) is the first candidate gene in maize studied by GWAS (Belo et al. 2008). The study indicated that the difference in oleic acid content of maize kernels is due to the allelic differences between the two fad2 alleles in the 5′ UTR region. Subsequently, Li et al. (2013) identified three new candidate genes significantly associated with oil content (ACP, LACS and COPII), in addition to previously identified four genes (fad2, DGAT1-2, FATB and WRI1a). Plant architectural traits including tassel branch number and main axis length, plant height, ear height, leaf length and width are important factors affecting grain yield in maize. More recently, candidate genes associated with plant height encoding gibberellin 2-oxidase 12 (Zm00001d018617) and auxin response factor 2 (Zm00001d02365) have been reported by Zhang et al. (2019). Optimizing maize stalk trait is desirable to enhance biofuel source. Mazaheri et al. (2019) detected gene Zmm22 associated with plant height and stalk diameter. Expression of Zmm22 gene is reported to modulate reproductive transition in maize (Kaeppler et al. 2020). Similarly, C3H gene is another example which isinvolved in lignin pathway and a NAC domain transcription factor related with secondary cell wall modification (Li et al. 2016a).
Candidate genes Zm00001d034319 and Zm00001d039219 associated with cold tolerance have been identified by integrating GWAS and RNA-seq data in maize (Zhang et al. 2020). In response to cold stress, plants accumulate various antioxidants mediated by Mitogen-activated Protein Kinase Phosphatases (MAPK) pathways (Sinha et al. 2011). The identified candidate genes were found to be associated with fatty acid hydroxylase activity and MAPK signalling pathways. Zm00001d034319 encoding inositol phosphorylceramide-B C-26 hydroxylase shares sequence homology with the fatty acid hydroxylase in rice (LOC_Os03g56820.1) and in Arabidopsis (AT2G34770). Likewise, another gene Zm00001d039219 (encoding a pleckstrin homology domain containing protein) shares homology with AT4G23895 in Arabidopsis and LOC_Os05g51710.1 in rice. Integrating GWAS and co-expression analysis, gene Zm00001d002266 (encoding lysine-specific histone demethylase) and Zm00001d049584 (encoding a wall-associated receptor kinase) governing the expression of seedling root length in response to drought stress has been reported by Guo et al. (2020).
ZmVTE4 governing α-tocopherol levels in maize kernels (Li et al. 2012) has been cloned into yeast and plant transient expression vectors (Zhu et al. 2019); the overexpression of ZmVTE4 resulted in enhanced cadmium tolerance in yeast (Zhu et al. 2019). Similarly, gene ZmMKK1 was amplified and cloned by co-transforming yeast utilizing lithium acetate transformation process (Cai et al. 2014). Introgression of ZmMKK1 gene in transgenic Arabidopsis was reported to enhance drought and salt tolerance. Wang et al. (2016c) cloned salt tolerance gene ZmVPP1 and made a construct in plasmid pSB II for Agrobacterium-mediated transformation. Yang et al. (2013) constructed bacterial artificial chromosome (BAC) clones for ZmCCT gene, providing photoperiod sensitivity in maize. Further, such BAC clones of gene ZmCCT were utilized by Wang et al. (2017) for affirming the role of this candidate gene against Gibberella stalk rot. Thus, GWAS facilitates the characterization of genomic regions utilizing various gene cloning approaches.
Role of GWAS in validation and vice versa
The use of GWAS can obviously allow validation of earlier findings or results of linkage mapping and identification of new MTAs. Hindu et al. (2018) validated SNPs, viz., S3_40522792, S9_151265550 and S2_1926586 (Qin et al., 2012) and S4_161165956, S3_186200393 and S4_167189737 (Jin et al., 2013) governing kernel iron and zinc in maize. There are several studies in maize where significant MTAs were validated found to be associated with the trait again through GWAS. Moreover, findings of GWAS from one study can be validated by same or other study. For instance, Gowda et al. (2015) identified SNPs significantly associated with maize lethal necrosis virus were validated by Nyaga et al. (2020) using GWAS approach. Besides, GWAS-identified genomic regions associated with Fusarium ear rot resistance (Chen et al. 2016) and cadmium accumulation in leaf (Zhao et al. 2018a) were validated through QTL mapping using different populations. Additionally, findings of QTL mapping can also be validated through GWAS indicating its crucial role in validation of earlier results and identification of MTAs.
Konig (2011) explained the importance of validation in GWAS since it results in identification and documentation of false positive candidate genes/QTLs. Misleading associations arise due to several factors such as heterogeneous genetic backgrounds, stratified and related populations etc. These bottlenecks can be circumvented by developing suitable statistical models, incorporating corrections for population stratification and kinship (Hindu et al. 2018). In the case of maize, Galli et al. (2020) reported that GWAS results on inbred lines associated with low heritable traits could not be validated in their hybrid population.
Validation of GWAS results in different populations and environments ensures association between causal variant and the trait to be on account of genetics, and not due to confounding factors. In the case of maize, Sitonik et al. (2019) revealed two major effect QTLs (qMCMV6-17/qMLN6-17 and qMCMV3-108/qMLN3-108) conferring resistance to lethal necrosis and chlorotic mottle viruses. They applied linkage mapping of double haploid populations and a GWAS on tropical and subtropical maize germplasm to validate those genomic regions. However, a previous work had identified three SNPs (S3_90976758, S3_90976749 and S3_114355785) related with only maize lethal necrosis virus. The identified SNPs correspond to the same confidence interval as the QTL qMCMV3-108 (Gowda et al. 2015; Sitonik et al. 2019). Validation thus, enables identification of potential genomic regions for improving germplasm and such populations could be utilized as a source of training population in GS Most of the researchers have used biparental (Hindu et al. 2018) or multiparent (Yang et al. 2018) mapping populations, transgenics or mutants (Mazaheri et al. 2019) for validation of GWAS estimates in many plants species including maize. Other than breeding, functional validation is another approach to verify biological significance of candidate genes (Curtin et al. 2017). GWAS may result in identification of many (tens to hundreds SNPs) loci associated with a particular trait. Olukolu et al. (2014) have reported 44 SNPs significantly associated with hypersensitive defence reactions in maize. In such case, it will be very difficult to reveal candidate genes and biological functions of identified genetic variants. Schaefer et al. (2018) suggested, GWAS coupled with co-expression analysis as an effective tool to categorize single causal candidate gene rather than centralizing on specifying several SNPs or loci associated with target traits.
Strengths and challenges of trait analysis using GWAS
GWAS is an integral and potent tool for linking the genotype–phenotype map. Additionally, GWAS offers several advantages over QTL mapping. It fosters higher resolution power for localizing a QTL and overcoming the two main limitations of QTL analysis, i.e., estimation of allelic diversity arising within a bi-parental mapping population (Borevitz and Nordborg 2003) and lower resolution power. GWAS also, have the potential to reveal causative/predictive factors for a given trait. It can be performed on a breeding or with natural populations (Viana et al. 2016). Implementation of multivariate GWAS approaches for dissecting pleiotropic genes underlying maize inflorescence has been provided by Rice et al. (2020). Similarly, Kaler et al. (2020) reported the immense effects of population structure and family relatedness or cryptic ancestry in providing false positive associations in maize with high LD decay as compared to low LD decay in soya bean It is pertinent to determine the population structure or the pedigree before searching for the MTAs. Application of mixed models are recommended in GWAS for successful reduction in number of false positive MTAs (Zhao et al. 2007) however over fitting of the model may lead to false negatives. Kaler et al. (2020) compared eight different statistical models and suggested that the FarmCPU model as appropriate and effective for GWAS analysis since it controlled both false positive and false negative associations.
Presence of rare allelic variants with small effect, limit the power of GWAS due to increased false positives thereby posing a major challenge (Asimit and Zeggini 2010). It thus becomes difficult to map minor QTLs using conventional GWAS approaches. Several other approaches nonetheless have been used to identify small effect QTLs with less phenotypic effects. Identification and mapping of minor effect QTLs governing plant architecture related traits using a large RIL mapping population and super high-density genotyping in maize has been done by Wang et al. (2018a). More recently, an improved method combining GWAS and machine learning has resulted in significant identification of minor effect QTLs (Zhou and Bellis 2019). Presence of genetic heterogeneity reduces the power of GWAS for recovering variants resulting in weakening of the correlation between phenotype and specific variant. Manchia et al. (2013) described the effect of genetic heterogeneity on GWAS estimates associated with complex diseases. GWAS requires large population size, extensive marker coverage and accurate phenotyping over different environments. Similarly, power of GWAS gradually decreases if a number of polymorphisms responsible for the phenotype remain undetected. Estimation of population structure or pedigree therefore, becomes necessary before searching for the marker-trait association. Sample size involving 400 individuals and traits with moderate to high heritability (70–80%) should be considered for GWAS analysis (Viana et al. 2016).
Prospects of GWAS for molecular breeding and genomic selection in maize
Markers or allelic variants identified by GWAS are readily available for MAS and genomic selection (Galli et al. 2020). Feng et al. (2015) introgressed ZmVTE4 gene in to four weet corn lines through marker-assisted backcrossing. For this purpose, two functional markers InDel7 and InDel118 as detected by Li et al. (2012) were utilized in GWAS for foreground selection of ZmVTE4. Similarly, Goswami et al. (2019) introgressed crtRB1 gene encoding β‐carotene hydroxylase in to elite QPM inbred to combine high provitamin A, tryptophan and lysine in maize. Despite numerous GWAS outputs, limited application in maize molecular breeding has occurred. This is mainly due to, high false discovery rate from GWAS and a lack of validation of estimates from GWAS (Verdeprado et al. 2018). In view of the already available abundant genomic resources for desirable traits, it is suggested to validate GWAS results and deploy molecular markers associated with target traits to enhance the efficacy of breeding programs. Subsequently, introgression of desirable QTLs or genes should be initiated in existing elite maize breeding core set.
Usually, pyramiding of small effect minor QTLs is restricted to an elite core of advanced breeding genotypes through MAS. Minor QTLs are known to be highly determined by genetic background of the population, phenotyping environments and G × E interaction (Liu et al. 2020). Under such scenario, genomic selection or prediction would be more advantageous, compared to MAS. The GWAS estimates are used to predict genomic-estimated breeding values (GEBV) of individuals by capturing both minor and major gene effects in GS. Several researches have been conducted to investigate effectiveness of GS for improving traits in maize using GWAS estimates, e.g., nitrogen use efficiency (Ertiro et al. 2020), Fusarium ear rot resistance (Wu et al. 2020), stalk length (Liu et al. 2020), etc. Such results show medium to high selection accuracies by encompassing MTAs identified by GWAS, in to the prediction model. Thus, GS in combination with GWAS is considered as a versatile genomic tool for ameliorating undecipherable conditions of complex quantitative attributes.
Conclusions
Maize is an excellent cereal crop with wider adaptation to varied environments. In the last few years, whole-genome sequencing and resequencing of the maize genome has resulted in identification of millions of genome-wide SNPs/QTLs/candidate genes. These findings have assisted in mapping of genes governing several yield and component traits, abiotic and biotic stress tolerance and quality traits. Meanwhile, it is important to validate identified loci and candidate genes associated with desirable traits. Generation of such genomic resources will prove to be of much utility in forming whole-genome prediction models.
GWAS can accelerate sustainable improvement in maize production by providing a thorough understanding of the complex genetic mechanisms underlying biotic and abiotic stress tolerance. GWAS are able to facilitate prediction of allele functions representing mutations and candidate genes conferring desirable agronomic traits. Its application in various breeding strategies such as genetic mapping, gene cloning, gene editing and gene pyramiding is likely to lead to fruitful results. It has some constrains though, regarding more complex nature of certain traits, small effects size, genetic heterogeneity, missing genotypes, low allele frequency and unexpected linkage disequilibrium. The possible solutions includes possessing appropriate phenotypic data, updated statistical models and effective validation of candidate loci. If possible, an integration of multi-layer data to GWAS would be helpful in identifying candidate genes and disclosing functional track, underlying desirable traits beyond the genomic clues. There is also need to identify many more physiological, metabolic and other biological causes underlying phenotypic variations. These could be achieved by integration of ‘omics’ data with specific genetic designs and relevant analytical methods.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
KS, JPS and AKS conceived the outline of the article, KS, MTV and PHZ helped in writing the manuscript, KS prepared the table and figures, AKS and BS finalised the manuscript and all authors reviewed and approved the manuscript.
Declarations
Conflict of interest
The authors declare that they have no competing interest in the publication.
References
- Alqudah AM, Sallam A, Baenziger PS, Borner A. GWAS: Fast-forwarding gene identification and characterization in temperate Cereals: lessons from Barley–a review. J Adv Res. 2020;22:119–135. doi: 10.1016/j.jare.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimit J, Zeggini E. Rare variant association analysis methods for complex traits. Ann Rev Genet. 2010;44:293–308. doi: 10.1146/annurev-genet-102209-163421. [DOI] [PubMed] [Google Scholar]
- Barutcular C, Dizlek H, El-Sabagh A, Sahin T, EL-Sabagh, M, Islam MS, Nutritional quality of maize in response to drought stress during grain-filling stages in Mediterranean climate condition. J Exp Biol Agric Sci. 2016;4:644–652. [Google Scholar]
- Bechoux N, Bernier G, Lejeune P. Environmental effects on the early stages of tassel morphogenesis in maize (Zea mays L.) Plant Cell Environ. 2000;23:91–98. [Google Scholar]
- Belo A, Zheng P, Luck S, Shen B, Meyer DJ, Li B, Rafalski A. Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol Genet Genomics. 2008;279:1–10. doi: 10.1007/s00438-007-0289-y. [DOI] [PubMed] [Google Scholar]
- Borevitz JO, Nordborg M. The impact of genomics on the study of natural variation in Arabidopsis. Plant Physiol. 2003;132:718–725. doi: 10.1104/pp.103.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Wang G, Wang L, Pan J, Liu Y, Li D. ZmMKK1, a novel group a mitogen-activated protein kinase kinase gene in maize, conferred chilling stress tolerance and was involved in pathogen defense in transgenic tobacco. Plant Sci. 2014;214:57–73. doi: 10.1016/j.plantsci.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Carlson CH, Gouker FE, Crowell CR, Evans L, DiFazio SP, Smart CD, Smart LB. Joint linkage and association mapping of complex traits in shrub willow (Salix purpurea L) Ann Bot. 2019;124:701–715. doi: 10.1093/aob/mcz047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shrestha R, Ding J, Zheng H, Mu C, Wu J, Mahuku G. Genome-wide association study and QTL mapping reveal genomic loci associated with Fusarium ear rot resistance in tropical maize germplasm. G3. 2016;6:3803–3815. doi: 10.1534/g3.116.034561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JS, Rice BR, Shenstone EM, Lipka AE, Jamann TM. Genome- wide analysis and prediction of resistance to Goss's wilt in maize. Plant Genome. 2019;12:1–10. doi: 10.3835/plantgenome2018.06.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin SJ, Tiffin P, Guhlin J, Trujillo DI, Burghardt LT, Atkins P, Young ND. Validating genome-wide association candidates controlling quantitative variation in nodulation. Plant Physiol. 2017;173:921–931. doi: 10.1104/pp.16.01923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh R, Sonah H, Patil G, Chen W, Prince S, Mutava R, Nguyen HT. Integrating omic approaches for abiotic stress tolerance in soybean. Frontiers Plant Sci. 2014;5:244. doi: 10.3389/fpls.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertiro BT, Labuschagne M, Olsen M, Das B, Prasanna BM, Gowda M. Genetic dissection of nitrogen use efficiency in tropical maize through genome-wide association and genomic prediction. Front Plant Sci. 2020;11:474. doi: 10.3389/fpls.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faostat FAO, Production AC (2020) Food and agriculture organization of the United Nations, Rome, Italy. http://www.fao.org
- Farooqi MQU, Lee JK. Cold stress evaluation among maize (Zea mays L.) inbred lines in different temperature conditions. Plant Breed Biotech. 2016;4:352–361. [Google Scholar]
- Feng F, Wang Q, Liang C, Yang R, Li X. Enhancement of tocopherols in sweet corn by marker-assisted backcrossing of ZmVTE4. Euphytica. 2015;206:513–521. [Google Scholar]
- Flint-Garcia SA, Thornsberry JM, Buckler VES. Structure of linkage disequilibrium in plants. Ann Rev Plant Biol. 2003;54:357–374. doi: 10.1146/annurev.arplant.54.031902.134907. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Vanacker H, Gomez LD, Harbinson J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures. Plant Physiol Biochem. 2002;40:659–668. [Google Scholar]
- Galli G, Alves FC, Morosini JS, Fritsche-Neto R. On the usefulness of parental lines GWAS for predicting low heritability traits in tropical maize hybrids. PLoS ONE. 2020;15:e0228724. doi: 10.1371/journal.pone.0228724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang S, Zhou Z, Wang S, Dong C, Mu C, He K. Linkage mapping and genome-wide association reveal candidate genes conferring thermo-tolerance of seed-set in maize. J Exp Bot. 2019;70:4849–4864. doi: 10.1093/jxb/erz171. [DOI] [PubMed] [Google Scholar]
- Gong F, Yang L, Tai F, Hu X, Wang W. “Omics” of maize stress response for sustainable food production: opportunities and challenges. OMICS. 2014;18:714–732. doi: 10.1089/omi.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Zunjare RU, Khan S, Baveja A, Muthusamy V, Hossain F. Marker-assisted introgression of rare allele of β-carotene hydroxylase (crtRB1) gene into elite quality protein maize inbred for combining high lysine, tryptophan and provitamin A in maize. Plant Breed. 2019;138:174–183. [Google Scholar]
- Gowda M, Das B, Makumbi D, Babu R, Semagn K, Mahuku G, Prasanna BM. Genome-wide association and genomic prediction of resistance to maize lethal necrosis disease in tropical maize germplasm. Theor Appl Genet. 2015;128:1957–1968. doi: 10.1007/s00122-015-2559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Li C, Zhang X, Li Y, Zhang D, Shi Y, Wang T. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2020;292:10380. doi: 10.1016/j.plantsci.2019.110380. [DOI] [PubMed] [Google Scholar]
- Han S, Miedaner T, Utz HF, Schipprack W, Schrag TA, Melchinger AE. Genomic prediction and GWAS of Gibberella ear rot resistance traits in dent and flint lines of a public maize breeding program. Euphytica. 2018;214:6–11. [Google Scholar]
- Hindu V, Palacios-Rojas N, Babu R, Suwarno WB, Rashid Z, Usha R, Nair SK. Identification and validation of genomic regions influencing kernel zinc and iron in maize. Theor Appl Genet. 2018;131:1443–1457. doi: 10.1007/s00122-018-3089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Liu L, Xiao B, Li D, Xing X, Kong X, Li D. Enhanced tolerance to low temperature in tobacco by over-expression of a new maize protein phosphatase 2C, ZmPP2C2. J Plant Physiol. 2010;167:1307–1315. doi: 10.1016/j.jplph.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Hu G, Li Z, Lu Y, Li C, Gong S, Yan S, Zhang Z. Genome-wide association study identified multiple genetic loci on chilling resistance during germination in maize. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-11318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Sanchez DL, Wang C, Lipka AE, Yin Y, Gardner CA, Lubberstedt T. Brassinosteroid and gibberellin control of seedling traits in maize (Zea mays L.) Plant Sci. 2017;263:132–141. doi: 10.1016/j.plantsci.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang J, Li W, Hu W, Duan L, Feng Y, Yue B. Genome-wide association analysis of ten chilling tolerance indices at the germination and seedling stages in maize. J Integ Plant Bio. 2013;55:735–744. doi: 10.1111/jipb.12051. [DOI] [PubMed] [Google Scholar]
- Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L. Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Frontiers Plant Sci. 2018;9:393. doi: 10.3389/fpls.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AK, Zhang L, Niyitanga S, Afzal MZ, Xu Y, Zhang L, Qi J. Principles and approaches of association mapping in plant breeding. Trop Plant Biol. 2020;13:212–224. [Google Scholar]
- Jin T, Zhou J, Chen J, Zhu L, Zhao Y, Huang Y. The genetic architecture of zinc and iron content in maize grains as revealed by QTL mapping and meta-analysis. Breed Sci. 2013;63:317–324. doi: 10.1270/jsbbs.63.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppler S, De Leon N, Foerster JM, Muttoni G (2020). U.S. Patent No. 10,858,664. Washington DC: US Patent and Trademark Office.
- Kaler AS, Gillman JD, Beissinger T, Purcell LC. Comparing different statistical models and multiple testing corrections for association mapping in soybean and maize. Frontiers Plant Sci. 2020;10:1794. doi: 10.3389/fpls.2019.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Bracken MB. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Pan J, Zhang M, Xing XIN, Zhou YAN, Liu Y, Li D. ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ. 2011;34:1291–1303. doi: 10.1111/j.1365-3040.2011.02329.x. [DOI] [PubMed] [Google Scholar]
- Konig IR. Validation in genetic association studies. Brief Bioinfo. 2011;12:253–258. doi: 10.1093/bib/bbq074. [DOI] [PubMed] [Google Scholar]
- Korte A, Farlow A. The advantages and limitations of trait analysis with GWAS: a review. Plant Methods. 2013;9:29. doi: 10.1186/1746-4811-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yang X, Xu S, Cai Y, Zhang D, Han Y, Yan J. Genome-wide association studies identified three independent polymorphisms associated with α-tocopherol content in maize kernels. PLoS ONE. 2012;7:e36807. doi: 10.1371/journal.pone.0036807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Peng Z, Yang X, Wang W, Fu J, Wang J, Liu J. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat Genet. 2013;45:43–50. doi: 10.1038/ng.2484. [DOI] [PubMed] [Google Scholar]
- Li K, Wang H, Hu X, Liu Z, Wu Y, Huang C. Genome-wide association study reveals the genetic basis of stalk cell wall components in maize. PLoS ONE. 2016;11:e0158906. doi: 10.1371/journal.pone.0158906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Cao W, Fang H, Xu S, Yin S, Zhang Y, Yang Z. Transcriptomic profiling of the maize (Zea mays L.) leaf response to abiotic stresses at the seedling stage. Front Plant Sci. 2017;8:290. doi: 10.3389/fpls.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YT, Xu WW, Ren BZ, Zhao B, Zhang J, Liu P, Zhang ZS. High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J Agron Crop Sci. 2020;206:548–564. [Google Scholar]
- Liu X, Hu X, Li K, Liu Z, Wu Y, Wang H, Huang C. Genetic mapping and genomic selection for maize stalk strength. BMC Plant Biol. 2020;20:1–16. doi: 10.1186/s12870-020-2270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S, Wahid A, Javed F, Basra SM. Heat stress effects on forage quality characteristics of maize (Zea mays) cultivars. Int J Agri Biol. 2010;12:701–706. [Google Scholar]
- Mahuku G, Chen J, Shrestha R, Narro LA, Guerrero KVO, Arcos AL, Xu Y. Combined linkage and association mapping identifies a major QTL (qRtsc8-1), conferring tar spot complex resistance in maize. Theor Appl Genet. 2016;129:1217–1229. doi: 10.1007/s00122-016-2698-y. [DOI] [PubMed] [Google Scholar]
- Manchia M, Cullis J, Turecki G, Rouleau GA, Uher R, Alda M. The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PLoS ONE. 2013;8(10):e76295. doi: 10.1371/journal.pone.0076295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri M, Heckwolf M, Vaillancourt B, Gage JL, Burdo B, Heckwolf S, Kaeppler HF. Genome-wide association analysis of stalk biomass and anatomical traits in maize. BMC Plant Biol. 2019;19:45. doi: 10.1186/s12870-019-1653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nature Rev Genet. 2008;9:356. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Monjardino P, Smith AG, Jones RJ. Heat stress effects on protein accumulation of maize endosperm. Crop Sci. 2005;45:1203–1210. [Google Scholar]
- Musvosvi C, Setimela PS, Wali MC, Gasura E, Channappagoudar BB, Patil SS. Contribution of secondary traits for high grain yield and stability of tropical maize germplasm across drought stress and non-stress conditions. Agron J. 2018;110:819–832. [Google Scholar]
- Nardino M, Souza VQD, Baretta D, Konflanz VA, Carvalho IR, Follmann DN, Caron BO. Association of secondary traits with yield in maize F 1's. Cienc Rural. 2016;46:776–782. [Google Scholar]
- Nguyen HT, Leipner J, Stamp P, Guerra-Peraza O. Low temperature stress in maize (Zea mays L.) induces genes involved in photosynthesis and signal transduction as studied by suppression subtractive hybridization. Plant Physiol Biochem. 2009;47:116–122. doi: 10.1016/j.plaphy.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Nyaga C, Gowda M, Beyene Y, Muriithi WT, Makumbi D, Olsen MS, Prasanna BM. Genome-wide analyses and prediction of resistance to MLN in large tropical maize germplasm. Gene. 2020;11:16. doi: 10.3390/genes11010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukolu BA, Wang GF, Vontimitta V, Venkata BP, Marla S. A Genome-wide association study of the maize hypersensitive defense response. PLoS Genet. 2014;10:e1004562. doi: 10.1371/journal.pgen.1004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo JA, Capo-Bauça S, Carmo-Silva E, Galmes J. Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front Plant Sci. 2017;8:490. doi: 10.3389/fpls.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Cai Y, Liu Z, Wang G, Wang J, Guo Y, Wang H. Identification of QTL for zinc and iron concentration in maize kernel and cob. Euphytica. 2012;187:345–358. [Google Scholar]
- Rice BR, Lipka FSB, AE, Multi-Trait Genome-wide Association Studies Reveal Loci Associated with Maize Inflorescence and Leaf Architecture. Plant Cell Physiol. 2020;8(61):1427–1437. doi: 10.1093/pcp/pcaa039. [DOI] [PubMed] [Google Scholar]
- Riva-Roveda L, Escale B, Giauffret C, Pderilleux C. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biol. 2016;16:212. doi: 10.1186/s12870-016-0909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez VM, Romay MC, Ordas A, Revilla P. Evaluation of European maize (Zea mays L.) germplasm under cold conditions. Genet Resour Crop Evol. 2010;57:329–335. [Google Scholar]
- Sabagh AE, Hossain A, Iqbal MA, Barutcular C, Islam MS, Cig F, Jabeen T (2020) Maize Adaptability to Heat Stress under Changing Climate. In: Plant Stress Physiology. Intech Open.
- Schaefer RJ, Michno JM, Jeffers J, Hoekenga O, Dilkes B, Baxter I, Myers CL. Integrating coexpression networks with GWAS to prioritize causal genes in maize. Plant Cell. 2018;30(12):2922–2942. doi: 10.1105/tpc.18.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Minx P. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Sita K, Siddique KH, Kumar R, Bhogireddy S, Varshney RK, Nayyar H. Drought or/and heat-stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front Plant Sci. 2018;9:1705. doi: 10.3389/fpls.2018.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Lv X, Weng J, Zhu H, Liu C, Hao Z, Li X. Genetic characterization and linkage disequilibrium mapping of resistance to gray leaf spot in maize (Zea mays L.) Crop J. 2014;2:132–143. [Google Scholar]
- Singh G, Jaglan MS. Seasonal incidence of different insect-pests in Kharif maize. J Pharmaco Phytochem. 2018;7:3666–3669. [Google Scholar]
- Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitonik C, Suresh LM, Beyene Y, Olsen MS, Makumbi D, Oliver K, Das B, Prasanna BM. Genetic architecture of maize chlorotic mottle virus and maize lethal necrosis through GWAS, linkage analysis and genomic prediction in tropical maize germplasm. Theor Appl Genet. 2019;132:2381–2399. doi: 10.1007/s00122-019-03360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski P, Rudzinska-Langwald A, Adamczyk J, Kubica I, Fronk J. Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. J Plant Physiol. 2005;162:67–80. doi: 10.1016/j.jplph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Strigens A, Freitag NM, Gilbert X, Grieder C, Riedelsheimer C, Schrag TA, Melchinger AE. Association mapping for chilling tolerance in elite flint and dent maize inbred lines evaluated in growth chamber and field experiments. Plant Cell Environ. 2013;36:1871–1887. doi: 10.1111/pce.12096. [DOI] [PubMed] [Google Scholar]
- Tang JD, Perkins A, Williams WP, Warburton ML. Using genome-wide associations to identify metabolic pathways involved in maize aflatoxin accumulation resistance. BMC Genomics. 2015;16:673. doi: 10.1186/s12864-015-1874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Jiang L, Liu Q, Zhang Y, Zhang R, Ingvardsen CR, Xu M. Combined linkage and association mapping reveals candidates for Scmv1, a major locus involved in resistance to sugarcane mosaic virus (SCMV) in maize. BMC Plant Biol. 2013;13:162. doi: 10.1186/1471-2229-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao ZQ, Chen YQ, Chao LI, Zou JX, Peng YAN, Yuan SF, Peng SUI. The causes and impacts for heat stress in spring maize during grain filling in the North China Plain—a review. J Integ Agric. 2016;15:2677–2687. [Google Scholar]
- Tollenaar M. Response of dry matter accumulation in maize to temperature: II. Leaf photosynthesis Crop Sci. 1989;29:1275–1279. [Google Scholar]
- USDA, United States Department of Agriculture. https://www.usda.gov. Accessed Aug 2020
- Verdeprado H, Kretzschmar T, Begum H, Raghavan C, Joyce P, Lakshmanan P, Collard BC. Association mapping in rice: basic concepts and perspectives for molecular breeding. Plant Prod Sci. 2018;21:159–176. [Google Scholar]
- Viana JMS, Mundim GB, e Silva FF, Garcia AAF (2016). Efficiency of genome-wide association study in open-pollinated populations. BioR 050955.
- Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Human Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Bucan M. Pathway-based approaches for analysis of genome wide association studies. Am J Human Genet. 2007;81:1278–1283. doi: 10.1086/522374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yan J, Zhao J, Song W, Zhang X, Xiao Y, Zheng Y. Genome-wide association study (GWAS) of resistance to head smut in maize. Plant Sci. 2012;196:125–131. doi: 10.1016/j.plantsci.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Liu S, Ferjani A, Li J, Yan J, Qin F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat Genet. 2016;48:1233–1241. doi: 10.1038/ng.3636. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang ZP, Liang XL, Weng JF, Lv XL, Zhang DG, Jiang LY. Identification of loci contributing to maize drought tolerance in a genome-wide association study. Euphytica. 2016;210:165–179. [Google Scholar]
- Wang H, Li K, Hu X, Liu Z, Wu Y, Huang C (2016b) Genome-wide association analysis of forage quality in maize mature stalk. BMC Plant Bio1 6:227. [DOI] [PMC free article] [PubMed]
- Wang H, Xu C, Liu X, Guo Z, Xu X, Wang S, Xu Y. Development of a multiple-hybrid population for genome-wide association studies: theoretical consideration and genetic mapping of flowering traits in maize. Sci Rep. 2017;7:40239. doi: 10.1038/srep40239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu S, Fan Y, Liu N, Zhan W, Liu H, Deng M. Beyond pathways: genetic dissection of tocopherol content in maize kernels by combining linkage and association analyses. Plant Biotech J. 2018;16:1464–1475. doi: 10.1111/pbi.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Liu H, Liu Z, Dong X, Guo J, Li W, Chen Z. Identification of minor effect QTLs for plant architecture related traits using super high density genotyping and large recombinant inbred population in maize (Zea mays) BMC Plant Biol. 2018;18:17. doi: 10.1186/s12870-018-1233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton ML, Womack ED, Tang JD, Thrash A, Smith JS, Xu W, Williams WP. Genome- wide association and metabolic pathway analysis of corn earworm resistance in maize. Plant Genome. 2018;11:1–8. doi: 10.3835/plantgenome2017.08.0069. [DOI] [PubMed] [Google Scholar]
- Wijewardana C, Henry WB, Hock MW, Reddy KR. Growth and physiological trait variation among corn hybrids for cold tolerance. Canadian J Plant Sci. 2016;96:639–656. [Google Scholar]
- Wu X, Li Y, Shi Y, Song Y, Zhang D, Li C, Wang T. Joint-linkage mapping and GWAS reveal extensive genetic loci that regulate male inflorescence size in maize. Plant Biotechnol J. 2016;14:1551–1562. doi: 10.1111/pbi.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou Z, Dong C, Chen J, Ding J, Han ZX, Y, Linkage mapping and genome-wide association study reveals conservative QTL and candidate genes for Fusarium rot resistance in maize. BMC Genomics. 2020;21:1–11. doi: 10.1186/s12864-020-6733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liu H, Wu L, Warburton M, Yan J. Genome-wide association studies in maize: Praise and Stargaze. Mol Plant. 2017;10:359–374. doi: 10.1016/j.molp.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li P, Yang Z, Xu C. Genetic mapping of quantitative trait loci in crops. Crop J. 2017;5:175–184. [Google Scholar]
- Xu C, Zhang H, Sun J, Guo Z, Zou C, Li WX, Wang J. Genome-wide association study dissects yield components associated with low-phosphorus stress tolerance in maize. Theor Appl Genet. 2018;1:16. doi: 10.1007/s00122-018-3108-4. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li Z, Li W, Ku L, Wang C, Ye J, Xu M. CACTA-like transposable element in ZmCCT attenuated photoperiod sensitivity and accelerated the post-domestication spread of maize. Proc Natl Acad Sci USA. 2013;110:16969–16974. doi: 10.1073/pnas.1310949110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ramamurthy RK, Qi X, Fernando RL, Dekkers JC, Garrick DJ, Schnable PS. Empirical comparisons of different statistical models to identify and validate kernel row number-associated variants from structured multi-parent mapping populations of maize. G3. 2018;8:3567–3575. doi: 10.1534/g3.118.200636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Buckler ES. Genetic association mapping and genome organization of maize. Curr Opin Biotechnol. 2006;17:155–160. doi: 10.1016/j.copbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang H, Li W, Mu C, Zhang F, Wang L, Meng Z. Genome-wide analysis and environmental response profiling of the FK506-binding protein gene family in maize (Zea mays L.) Gene. 2012;498:212–222. doi: 10.1016/j.gene.2012.01.094. [DOI] [PubMed] [Google Scholar]
- Zaidi PH, Yadav M, Maniselvan P, Khan R, Shadakshari TV, Singh RP, Pal D. Morpho-physiological traits associated with cold stress tolerance in tropical maize (Zea mays L.) Maydica. 2010;55:201–208. [Google Scholar]
- Zhang Y, Wan J, He L, Lan H, Li L. Genome-wide association analysis of plant height using the maize f1 population. Plants. 2019;8:432. doi: 10.3390/plants8100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang J, Xu Q, Wang D, Di H, Huang J, Wang Z. Identification of candidate tolerance genes to low temperature during maize germination by GWAS and RNA-seq approaches. BMC Plant Biol. 2020;20:1–17. doi: 10.1186/s12870-020-02543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Aranzana MJ, Kim S, Lister C, Shindo C, Tang C, Nordborg M. An Arabidopsis example of association mapping in structured samples. PLoS Genet. 2007;3:e4. doi: 10.1371/journal.pgen.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Luo L, Cao Y, Liu Y, Li Y, Wu W, Shen Y. Genome-wide association analysis and QTL mapping reveal the genetic control of cadmium accumulation in maize leaf. BMC Genomics. 2018;19:91. doi: 10.1186/s12864-017-4395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu Y, Wu W, Li Y, Luo L, Lan Y, Liu L. Genome-wide association analysis of lead accumulation in maize. Mol Genet Genomics. 2018;293:615–622. doi: 10.1007/s00438-017-1411-4. [DOI] [PubMed] [Google Scholar]
- Zhou W, Bellis ES (2019) Minor QTLs mining through the combination of GWAS and machine learning feature selection. BioRxiv.
- Zhou G, Hao D, Mao Y, Zhu Q, Chen G, Lu H, Xue L. Identification of genetic loci conferring partial resistance to southern corn rust through a genome-wide association study. Eur J Plant Pathol. 2018;150:1083–1090. [Google Scholar]
- Zhu Q, Zhang J, Yu H, Li L, Chen X, Jiang M, Tan M. Maize cd-tolerant ZmVTE4 encoding γ-tocopherol-methyl-transferase alleviated cd-toxicity through its product α-tocopherol. Environ Exp Bot. 2019;58:171–179. [Google Scholar]
- Zila CT, Samayoa LF, Santiago R, Butron A, Holland JB. A genome-wide association study reveals genes associated with Fusarium ear rot resistance in a maize core diversity panel. G3. 2013;3:2095–2104. doi: 10.1534/g3.113.007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.