Abstract
Geranylgeranyl diphosphate synthase (GGDPS), an enzyme in the isoprenoid biosynthetic pathway (IBP), produces the isoprenoid (geranylgeranyl pyrophosphate, GGPP) used in protein geranylgeranylation reactions. Our prior studies utilizing triazole bisphosphonate-based GGDPS inhibitors (GGSIs) have revealed that these agents represent a novel strategy by which to induce cancer cell death, including multiple myeloma and pancreatic cancer. Statins inhibit the rate-limiting enzyme in the IBP and potentiate the effects of GGSIs in vitro. The in vivo effects of combination therapy with statins and GGSIs have not been determined. Here we evaluated the effects of combining VSW1198, a novel GGSI, with a statin (lovastatin or pravastatin) in CD-1 mice. Twice-weekly dosing with VSW1198 at the previously established maximally tolerated dose in combination with a statin led to hepatotoxicity, while once-weekly VSW1198-based combinations were feasible. No abnormalities in kidney, spleen, brain or skeletal muscle were observed with combination therapy. Combination therapy disrupted protein geranylgeranylation in vivo. Evaluation of hepatic isoprenoid levels revealed decreased GGPP levels in the single drug groups and undetectable GGPP levels in the combination groups. Additional studies with combinations using 50% dose-reductions of either VSW1198 or lovastatin revealed minimal hepatotoxicity with expected on-target effects of diminished GGPP levels and disruption of protein geranylgeranylation. Combination statin/GGSI therapy significantly slowed tumor growth in a myeloma xenograft model. Collectively, these studies are the first to demonstrate that combination IBP inhibitor therapy alters isoprenoid levels and disrupts protein geranylgeranylation in vivo as well as slows tumor growth in a myeloma xenograft model, thus providing the framework for future clinical exploration.
Keywords: isoprenoid biosynthesis, statins, geranylgeranyl diphosphate synthase, inhibitor, myeloma Chemical compounds studied in this article, lovastatin (PubChem CID: 53232), pravastatin (PubChem CID: 54687)
Graphical abstract

1. Introduction
The isoprenoid biosynthetic pathway (IBP) is responsible for the generation of both sterol and non-sterol isoprenoids (Fig. 1). Farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) are two non-sterol isoprenoids produced by the IBP and are of significance because they serve as the isoprenoid donors in protein prenylation reactions. The IBP is one of the most heavily targeted pathways in medicine. Statins are the most widely used IBP inhibitors to date, with approximately 15 million Americans prescribed these agents for the management of hyperlipidemia. Statins inhibit HMG-CoA reductase, which is the rate-limiting enzyme in the IBP pathway. Nitrogenous bisphosphonates (NBPs), inhibit the enzyme farnesyl diphosphate synthase (FDPS), and are used to treat a variety of bone disorders, including osteoporosis, metastatic bone disease and myeloma bone disease. In vitro, both statins and NBPs have proven to be useful tools to study the role of prenylation in modulating the activity of members of the Ras small GTPase super family (e.g., Ras, Rho and Rab family members), by virtue of the ability of these agents to deplete cells of both FPP and GGPP and thereby disrupt both farnesylation and geranylgeranylation [1–3]. However, due to their high affinity for bone uptake and limited systemic distribution, the use of NBPs is limited to the treatment of bone diseases. There was significant interest in the potential anti-tumor effects of statins, particularly as agents that could disrupt oncogenic Ras activity by disrupting Ras prenylation [4]. In general, the in vitro studies that evaluated the activity of various statins in cancer cell lines utilized drug concentrations in the micromolar range [5]. As standard doses of statins result in submicromolar concentrations [6], it has been assumed that these doses are insufficient to disrupt prenylation in vivo. Several phase I studies demonstrated that higher doses of statins could be administered, but this was associated with off-target effects and no significant anti-tumor activity [7, 8].
Fig. 1. Overview of the Isoprenoid biosynthetic pathway.
Key enzymes are outlined in black boxes and key inhibitors are in red.
Rab GTPases are a family of geranylgeranylated proteins that mediate intracellular trafficking events. Disruption of Rab geranylgeranylation results in mislocalization of Rabs and interruption of intracellular trafficking processes [9]. In the IBP, geranylgeranyl diphosphate synthase (GGDPS) catalyzes the synthesis of the isoprenoid donor (GGPP) which is used in protein geranylgeranylation reactions mediated by geranylgeranyl transferase (GGTase) I and I. Thus, inhibition of GGDPS results in disruption of protein geranylgeranylation, including Rab geranylgeranylation. We have previously characterized a novel GGDPS inhibitor (GGSI) termed VSW1198 [10–12]. VSW1198 potently inhibits GGDPS with an IC50 of 45 nM in in vitro enzyme studies and disrupts protein geranylgeranylation at concentrations as low as 30 nM in cell culture studies [10]. Preclinical studies with VSW1198 have demonstrated achievement of systemic distribution, feasibility of repeat dosing, a half-life permitting once- or twice-weekly dosing schedules, in vivo disruption of protein geranylgeranylation and metabolic stability [12].
The therapeutic potential of GGSIs has been established through our studies demonstrating that these agents induce apoptosis of myeloma cells and pancreatic cancer cells through disruption of Rab geranylgeranylation, leading to induction of ER stress in vitro [12–14]. These GGSIs have anti-tumor activity in vivo in myeloma and pancreatic cancer xenograft models [12–14]. We have also shown that submicromolar concentrations of lovastatin potentiate GGSI-induced intracellular light chain accumulation and disruption of Rap1a prenylation in myeloma cell lines [11]. Whether statins potentiate the effects of GGDPS inhibition in vivo has not previously been established. Furthermore, it is unknown whether standard doses of statins alter FPP and GGPP levels in vivo. To this end, we have evaluated the effects of combination GGSI and statin therapy in CD-1 mice. These studies reveal that statin therapy, when used in conjunction with twice-weekly dosing of VSW1198, results in hepatic injury. However, dose modifications of VSW1198 allowed for safe co-administration with statins. These studies demonstrated that combination IBP inhibitor therapy alters isoprenoid levels and disrupts protein prenylation in vivo as well as slows tumor growth in a myeloma xenograft model, thus providing the framework for future clinical exploration of this combination therapy.
2. Material and Methods.
2.1. Chemicals.
VSW1198 [10] was kindly provided by Prof. David Wiemer (University of Iowa). Lovastatin and pravastatin were obtained from LKT Laboratories (St. Paul, MN, USA).
2.2. Animals.
Female CD-1 and NOD-SCID mice between the ages of 6–8 weeks were purchased from Charles River. All animals were kept in the University of Nebraska Medical Center (UNMC) animal facility at a temperature of 23–25 °C, relative humidity of 50–70% and 12/12 hour light/dark cycles. The UNMC Institutional Animal Care and Use Committee approved all studies (protocol no. 16–132-11-FC). Lovastatin was first dissolved in 1 mL of ethanol. NaOH (1.5 mL, 0.1N) was added and the solution was heated to 50 °C for 2 hours. The pH was adjusted to between 6–8 and the solution was further diluted in 0.5% w/v methyl cellulose. Pravastatin was dissolved directly in 0.5% w/v methyl cellulose. Lovastatin and pravastatin were administered to mice daily via oral gavage. VSW1198 was prepared in sterile phosphate buffered saline (PBS) and administered via the tail vein either once- or twice-weekly. Details regarding dose and schedule are shown in Table 1.
Table 1.
Dose levels of VSW1198 and statin (lovastatin or pravastatin)
| Dose Level | VSW1198 (mg/kg) | Statin (mg/kg) |
|---|---|---|
| 0 | 0.1 (days 2, 5, 9) | 10 (days 1–11) |
| −1 | 0.1 (days 2, 9) | 10 (days 1–11) |
| −2a | 0.05 (days 2, 9) | 10 (days 1–11) |
| −2b | 0.1 (days 2, 9) | 5 (days 1–11) |
2.3. Blood analysis.
Blood samples were obtained either by cheek bleeds or at time of euthanasia. Samples were analyzed using an Abaxis Vetscan2 instrument and the preventative profile plus rotor (Patterson Veterinary, Greeley, CO, USA). Creatine kinase (CK) levels were measured using a colorimetric assay (BioAssay Systems, Hayward, CA, USA).
2.4. qRT-PCR.
Tissue samples were homogenized in TRIzol and RNA was isolated using alcohol precipitation according to manufacturer’s directions (Invitrogen, Carlsbad, CA, USA). RNA (1 μg) was reverse transcribed to cDNA using the i-Script cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). iTaq Universal SYBR Green Supermix was mixed with cDNA and gene specific primers and qRT-PCR was performed using a CFX96 real time machine. Data analysis, including statistical calculations, was completed using the Bio-Rad CFX manager 3.1 software. Gene expression was normalized to that of PP1A.
2.5. Immunoblot.
Tissue samples were placed in T-Per (Invitrogen, Waltham, MA, USA) supplemented with protease and phosphatase inhibitors and homogenized using a handheld tissue homogenizer. The BCA method was used to quantify protein concentration. Equal amounts of protein were run on SDSPAGE gels and transferred to PVDF membranes. Blots were incubated overnight at 4 °C in primary antibody (Rap1a, Santa Cruz sc-373968 and GAPDH, Cell Signaling Technology 5174S) and then for 2 hours at room temperature in secondary antibody. Protein was visualized using an electrochemiluminescence detection kit and a Bio-Rad Chemidoc imaging system. Uncropped western blot images are presented in supplemental figures 12 and 13.
2.6. Histopathological analysis.
Mouse organs were fixed in formalin for 96 hours and stored in 70% ethanol. Tissues were embedded in paraffin, sectioned and stained (H&E) using standard methods at the UNMC Tissue Science Facility.
2.7. FPP/GGPP analysis.
Hepatic concentrations of FPP and GGPP were quantitated using a validated LC-MS/MS method as described previously [15]. Each sample was accurately weighed and homogenized with triple distilled de-ionized water at a 7- fold dilution factor using a TissueLyserII (Qiagen, Germantown, MD, USA). Controls and liver homogenates (100 μL) sample were spiked with internal standard (10 μL) and analytes extracted by solid phase extraction using Oasis® HLB 1cc/30mg SPE cartridges (Waters Corporation, Milford, MA, USA) as previously described [15]. Concentrations for each isoprenoid were normalized to tissue weight. The method was linear from 0.04 to 20 ng/ml for FPP and GGPP in hepatic tissue.
2.8. Myeloma xenograft studies.
NOD-SCID mice were subcutaneously inoculated in the flank with MM.1S cells (10 million cells in 0.1 mL of sterile saline mixed 1:1 with Matrigel). When tumors became palpable (11 days), mice were randomly divided into treatment and control groups (n=8 per group). Mice were administered either PBS (once weekly by IV injection), lovastatin (10 mg/kg, daily by oral gavage), VSW1198 (0.05 mg/kg, once weekly by IV injection), or both lovastatin and VSW1198. Treatment was administered for up to three weeks followed by one week off. Tumor volume was recorded three times per week using a caliper. Mice were euthanized either when tumors reached 2000 mm3 or on day 29 (post the start of treatment). Two mice, one from the lovastatin group and one from the VSW1198 group, were excluded from tumor growth analysis due to intramuscular tumor growth. The following equation was used to calculate tumor volume: 4π/3 × (width/2)2 × (length/2).
2.9. Statistical Analysis.
T-tests (two-tailed) were used to calculate statistical significance between control and treated groups. The Shapiro-Wilk normality test was used to test the normality assumption of the t-test. If the normality assumption was not met a Mann-Whitney nonparametric test was used instead. Linear mixed models (LMM) were used to look at changes in tumor burden over time. Tumor burden was modeled on the log10 scale. The model included fixed effects for group, day and the group × day interaction and day is modeled as a continuous variable. A random slope and intercept was fit for each mouse. Distributional assumptions of the LMM were assessed using residual plots. Kaplan-Meyer method was used to estimate overall survival distributions by treatment group. Overall survival was defined as time from start of treatment to death. GraphPad Prism version 7.04 (San Diego, CA, USA) and SAS software version 9.4 (Cary, NC, USA) were used for data analysis.
3. Results.
3.1. Twice-weekly dosing of VSW1198 in combination with statin therapy causes hepatotoxicity.
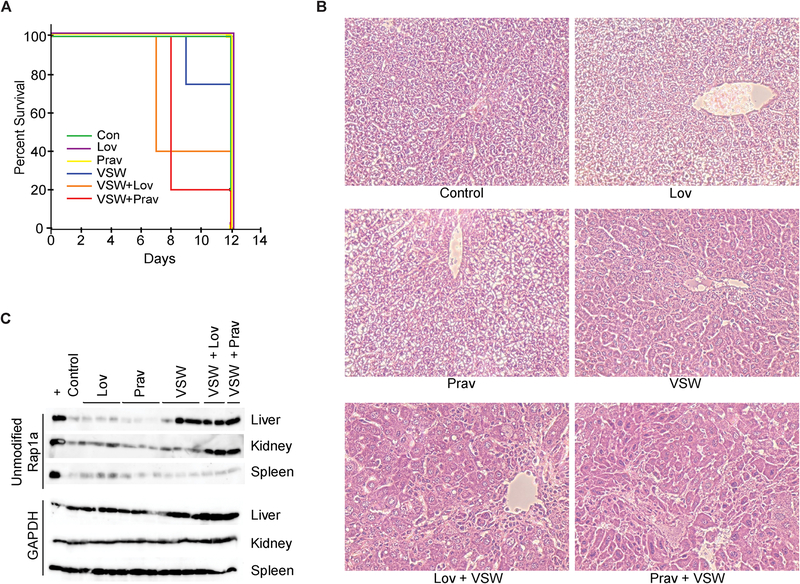
Previous studies had established the feasibility of twice-weekly administration of VSW1198 (0.1 mg/kg) [12]. To determine whether this dosing schedule could be combined with daily statin treatment, cohorts of CD-1 mice were administered PBS (control), lovastatin, pravastatin, VSW1198, or a combination of lovastatin/VSW1198 or pravastatin/VSW1198 for a period of 11 days before being sacrificed on day 12 (Table 1, dose level 0). Statins were administered at a standard dose of 10 mg/kg daily via oral gavage. The combination cohorts experienced early mortality in 60% of VSW1198/lovastatin-dosed mice and 80% of the VSW1198/pravastatin-dosed mice (Fig. 2A). Examination of the surviving animals revealed that the livers from the combination cohorts were characterized by centrilobular hepatocyte injury (cellular swelling and nuclear enlargement/pleomorphism) and death with associated infiltration by inflammatory cells (Fig. 2B). Histopathological analysis of kidney, spleen, brain and skeletal muscle did not reveal any significant abnormalities in any of the treatment groups, however (Fig. S1). Immunoblot analysis using an antibody that only recognizes unmodified Rap1a revealed accumulation of unmodified Rap1a in the livers of mice administered VSW1198 with or without statins and in the kidneys of mice given the combination treatment (Fig. 2C). To better understand the mechanisms underlying the observed toxicity, a second experiment utilizing twice-weekly VSW1198 dosing in combination with lovastatin or pravastatin was performed with animals sacrificed on day 7. Analysis of blood samples at time of sacrifice revealed significantly elevated hepatic transaminase levels with more modest elevations in alkaline phosphatase and total bilirubin levels (Table 2). A decrease in glucose levels was also observed. Examination of liver tissues by H&E staining again confirmed hepatocyte injury as the primary pathological finding (Fig. S2). Immunoblot analysis revealed marked accumulation of unmodified Rap1a in the hepatic samples, consistent with disruption protein geranylgeranylation (Fig. S3).
Fig. 2. Twice-weekly dosing of VSW1198 in combination with statin therapy causes hepatotoxicity.
(A) Kaplan-Meyer survival curve showing time to death for mice administered twice-weekly VSW1198 (VSW, 0.1 mg/kg), daily lovastatin (Lov, 10 mg/kg), daily pravastatin (Prav, 10 mg/kg) or a combination of VSW and statin (n=5 per group). Mice were dosed for 11 days before being sacrificed on day 12. (B) H&E sections of livers collected on day 12. 400X magnification. (C) Immunoblot showing unmodified Rap1a in liver, kidney and spleen tissue. The “+” denotes lysate from RPMI-8226 cells incubated with 10 μM lovastatin for 48 hours. Control denotes tissues collected from an untreated CD-1 mouse. GAPDH is shown as a loading control.
Table 2.
Blood analysis from mice administered twice-weekly dosing of VSW1198 in combination with statin therapy (dose level 0).
| Normal range | Lov+VSW | Prav+VSW | |
|---|---|---|---|
| BUN | 9 to 33 | 32.3 ± 18.1 | 25.8 ± 12.2 |
| CRE | 0.2 to 0.9 | 0.4 ± 0.2 | 0.3 ± 0.1 |
| ALT | 17 to 77 | 1608.8 ± 744.3 | 1920.4 ± 178 |
| ALP | 35 to 222 | 306.8 ± 178.2 | 392.6 ± 117 |
| AST | 54 to 298 | 1816.3 ± 367.5 | > 2000 |
| TBIL | 0 to 0.9 | 1.0 ± 1.5 | 1.1 ± 1 |
| GLU | 140 to 263 | 98.5 ± 27.9 | 87.4 ± 27.8 |
| CA | 6 to 13 | 10.8 ± 0.9 | 11.1 ± 0.3 |
| TP | 3.9 to 6.4 | 5.5 ± 0.5 | 5.0 ± 0.5 |
| ALB | 2.5 to 4.6 | 4.8 ± 0.4 | 4.5 ± 0.5 |
| GLOB | 1.2 to 2.2 | 0.8 ± 0.2 | 0.6 ± 0.2 |
| Na | 110 to 195 | 151.0 ± 4.7 | 150.4 ± 3 |
| K | 4 to 10.5 | 8.5 ± 0.1 | 8.5 ± 0 |
| Cl | NR | 106.8 ± 6.5 | 107.2 ± 3.8 |
| tCO2 | NR | 23.8 ± 6.1 | 24.6 ± 6.9 |
Blood was collected on day 7. N=5 mice per group. Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CA, calcium; Cl, chloride; CRE, creatinine; GLOB, globulin; GLU, glucose; K, potassium; LDH, lactate dehydrogenase; Lov, lovastatin; Na, sodium; NR, not reported; Prav, pravastatin; TBIL, total bilirubin; tCO2, total carbon dioxide; TP, total protein; VSW, VSW1198.
3.2. Once-weekly dosing of VSW1198 in combination with statin therapy is better tolerated.
Given the hepatotoxicity observed with the twice-weekly VSW1198/statin combinations, we subsequently investigated combination therapy using once-weekly VSW1198 administration. CD-1 mice were administered PBS (control), lovastatin (10 mg/kg daily), pravastatin (10 mg/kg daily), VSW1198 (0.1 mg/kg IV on days 2 and 9), or a combination of lovastatin plus VSW1198 or pravastatin plus VSW1198 for a period of 11 days before being sacrificed on day 12 (n=5 per group, dose level −1 (Table 1)). All mice in the VSW1198/lovastatin cohort survived until the experimental endpoint, however 20% of the VSW1198/pravastatin cohort experienced early mortality (Fig. 3A). Analysis of blood collected at day 12 showed mild elevation in hepatic transaminases in a portion of the statin + VSW1198 dosed mice, with no elevations in alkaline phosphatase or total bilirubin levels (Fig. 3B, Table 3). Histopathological examination of liver tissues showed only mild hepatocyte swelling and nuclear enlargement in mice administered VSW1198 in conjunction with either lovastatin or pravastatin (Fig. 3C). No histopathological abnormalities were observed in the kidney or spleen (Fig. S4). Immunoblot analysis revealed accumulation of unmodified Rap1a in tissue samples from mice administered VSW1198 with or without statins, demonstrating that once-weekly VSW1198 treatment is sufficient to disrupt protein prenylation in vivo (Fig. 3D).
Fig. 3. Evaluation of once-weekly VSW1198 in combination with statins.
(A) Kaplan-Meyer survival curve showing time to death for mice administered once weekly VSW1198 (VSW, 0.1 mg/kg, day 2 and 9), daily lovastatin (Lov, 10 mg/kg), daily pravastatin (Prav, 10 mg/kg) or a combination of VSW and statin (n=5 per group). Mice were dosed for 11 days and sacrificed on day 12. (B) Blood levels of ALT and AST at time of sacrifice. (C) H&E sections of livers collected at time of sacrifice. (D) Immunoblot analysis of unmodified Rap1a in liver, kidney and spleen tissues. GAPDH is shown as a loading control. Lysates generated from RPMI-8226 myeloma cells treated with lovastatin were used as a positive control (“+”)for unmodified Rap1a protein, while tissue from untreated CD-1 mice served as negative control (“−“).
Table 3.
Blood analysis from mice administered once-weekly dosing of VSW1198 in combination with statin therapy (dose level -1).
| Normal range | VSW | Lov | Prav | Prav+VSW | Lov+VSW | |
|---|---|---|---|---|---|---|
| BUN | 9 to 33 | 19 ± 2.7 | 16.3 ± 1 | 16 ± 2.9 | 19.5 ± 3.1 | 14 ± 2.2 |
| CRE | 0.2 to 0.9 | 0.2 ± 0 | 0.3 ± 0.1 | <0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| ALT | 17 to 77 | 78.8 ± 17.9 | 53 ± 10.6 | 51.0 ± 11.3 | 322.8 ± 232.2 | 150 ± 38.8 |
| ALP | 35 to 222 | 107.8 ± 12 | 44 ± 11.2 | 55.3 ± 15 | 80 ± 40 | 73.3 ± 15.3 |
| AST | 54 to 298 | 167.5 ± 88.1 | 213.5 ± 78.6 | 172.8 ± 5.1 | 210.7 ± 174.8 | 202.3 ± 99.5 |
| TBIL | 0 to 0.9 | 0.3 ± 0 | 0.2 ± 0 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0 |
| GLU | 140 to 263 | 133.8 ± 16.2 | 197.5 ± 31 | 206.8 ± 21.6 | 81.3 ± 21.5 | 96.8 ± 8.8 |
| CA | 6 to 13 | 10.4 ± 0.3 | 10.7 ± 0.2 | 11.2 ± 0.2 | 11.1 ± 0.2 | 11.3 ± 0.2 |
| TP | 3.9 to 6.4 | 5.5 ± 0.3 | 5.3 ± 0.1 | 5.4 ± 0.2 | 5.9 ± 0.4 | 6.15 ± 0.2 |
| ALB | 2.5 to 4.6 | 4.6 ± 0.1 | 4.6 ± 0.2 | 4.6 ± 0.1 | 4.9 ± 0.3 | 5.0 ± 0.2 |
| GLOB | 1.2 to 2.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.3± 0.1 |
| Na | 110 to 195 | 152 ± 2.6 | 153.8 ± 2.1 | 157.5 ± 4.7 | 153.5 ± 3.3 | 148.8 ± 1 |
| K | 4 to 10.5 | 8.2 ± 0.4 | 8.0 ± 0.3 | 8.3 ± 0.2 | 8.0 ± 0.2 | 7.6 ± 0.7 |
| Cl | NR | 110 ± 2.8 | 108.3 ± 1.5 | 109 ± 1.2 | 108.5 ± 1.9 | 106 ± 1.4 |
| tCO2 | NR | 24.8 ± 0.5 | 31.8 ± 1.7 | 31.5 ± 1.7 | 25.8 ± 3.8 | 25.8 ± 1.9 |
| CK | 29.3–810 | 226.5 ± 119 | 385 ± 16.2 | 368.6 ± 17.7 | 18.7 ± 15.7 | 13.2 ± 1.3 |
Blood was collected on day 12. N=4 per group. Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CA, calcium; CK, creatine kinase; Cl, chloride; CRE, creatinine; GLOB, globulin; GLU, glucose; K, potassium; LDH, lactate dehydrogenase; Lov, lovastatin; Na, sodium; NR, not reported; Prav, pravastatin; TBIL, total bilirubin; tCO2, total carbon dioxide; TP, total protein; VSW, VSW1198.
3.3. Statin and GGSI treatment alter hepatic FPP and GGPP levels.
Using our established LC-MS/MS methodology to measure FPP and GGPP levels, [15] we sought to determine whether once-weekly VSW1198 treatment, single agent lovastatin or pravastatin, or combined GGSI and statin therapy altered in vivo isoprenoid levels. Liver tissues were analyzed from mice who were treated on dose level −1. As statins act at the earliest part of the IBP pathway, they should result in a decrease in the cellular levels of both FPP and GGPP, while GGSI mediated inhibition of GGDPS should deplete GGPP levels while increasing FPP levels [16]. As predicted, evaluation of hepatic isoprenoid levels revealed a decrease in both FPP and GGPP levels in mice administered either lovastatin or pravastatin (Fig. 4). We observed a decrease in GGPP and an increase in FPP in the GGSI-dosed mice, while in the combination group the addition of statin to GGSI abrogated the GGSI-induced increase in FPP and further depleted GGPP to undetectable levels (Fig. 4). To evaluate whether the changes in isoprenoid levels were associated with changes in FDPS or GGDPS levels, qRT-PCR analysis was performed. No significant changes in FDPS levels were observed and 2–3 fold increases in GGDPS levels were observed in GGSI- or combination-treated mice (Fig S5).
Fig. 4. tatin and GGSI treatment alters hepatic isoprenoid levels.
FPP and GGPP levels from livers of CD-1 mice treated once weekly with VSW1198 (VSW, 0.1 mg/kg), daily lovastatin (Lov, 10 mg/kg), daily pravastatin (Prav, 10 mg/kg) or a combination of VSW and statin (dose level −1). n=5 mice per group; *p<0.05, **p<0.01, ***p<0.001 two-sided student t-test and/or Mann-Whitney.
3.4. Evaluation of additional dosing regimens.
In an attempt to further abrogate liver toxicity we evaluated alternative dosing regimens in which either VSW1198 or lovastatin were dose-reduced by 50%. Mice were administered 5 mg/kg lovastatin daily, 0.05 mg/kg VSW1198 once-weekly, 10 mg/kg lovastatin + 0.05 mg/kg VSW1198 (Table 1, dose level −2a) or 5 mg/kg lovastatin + 0.1 mg/kg VSW1198 (Table 1, dose level −2b). All mice survived the 11-day dosing period and were sacrificed on day 12 for further analysis. Analysis of blood samples obtained from the combination cohorts revealed only mild elevations of ALT (131 ± 54 for −2a; 209 ± 107 for −2b; normal range 17 to 77) and AST (261 ± 94 for −2a, 299 ± 72 for −2b, normal range 54 to 298) (Table S2). Evaluation of liver sections showed only mild hepatocyte swelling and reactive nuclear changes in the combination groups with no effects on the spleen or kidney across all treatment groups (Fig. S6). Immunoblot analysis showed increased levels of unmodified Rap1a in the combination groups relative to the single agent groups (Fig. S7). Furthermore, hepatic GGPP levels were significantly reduced across all four treatment groups relative to control mice (Fig. S8), although in contrast to dosing level −1, the combination groups did have detectable levels of GGPP. Collectively, both modified dosing regimens showed equivalent benefit in reducing liver toxicity, while maintaining on-target effects.
3.5. Combination treatment with lovastatin and VSW1198 slows myeloma tumor growth in vivo.
Previous studies utilizing an analog of VSW1198 revealed that twice-weekly dosing of the GGSI significantly delayed tumor growth in a myeloma xenograft model [17]. We subsequently established that once-weekly dosing of VSW1198 (0.1 mg/kg) also delayed tumor growth compared to solvent control (p<0.05, linear mixed model, Fig. S9). To determine whether the addition of a statin to GGSI could enhance anti-tumor activity in vivo, cohorts of mice were treated as per dose level −2a (solvent control, lovastatin 10 mg/kg, VSW1198 0.05 mg/kg weekly, or combination therapy), using our established flank xenograft model with MM.1S cells. Once tumors were palpable, treatment was initiated and continued for 3 weeks followed by one week off. Mice were sacrificed when tumors reached 2 cm3 or on day 29 (from start of treatment). We observed that once-weekly dosing of VSW1198 significantly slowed tumor growth relative to controls (p<0.05, Fig. 5A) while lovastatin alone did not exert a statistically significant effect. However, the reduction in tumor growth was most pronounced in those mice who received a combination of VSW1198 and lovastatin (p<0.001, Fig. 5A). The combination of these two drug also produced significantly smaller tumor sizes at the terminal endpoint, whereas neither VSW1198 nor lovastatin when used alone had any effect on final tumor weight (Fig. 5B). Mice who received lovastatin and VSW1198 showed reduction in overall body weight during the treatment period, although this change was not statistically significant (Fig. S10). Analysis of tumor specimens and liver specimens revealed that despite treatment being held for one week prior to sacrifice, several of the tumor samples from mice receiving combination therapy showed evidence of unmodified Rap1a as well as all liver samples (Fig. 5C). In contrast, samples from single agent treatment cohorts did not show significant accumulation of unmodified Rap1a (Fig. S11). Collectively, these results illustrate that lovastatin potentiates the anti-tumor activity of VSW1198 in vivo.
Fig. 5. Combination therapy with lovastatin and VSW1198 slows tumor growth in vivo.
(A) Tumor growth curve for MM.1S flank xenograft. Mice were administered either PBS (once weekly, n=8), lovastatin (10 mg/kg, daily, n=7), VSW1198 (0.05 mg/kg, once weekly, n=7), or both lovastatin and VSW1198 (n=8). Data are shown as average ± standard error (*p<0.05, ***p<0.001, linear mixed model). (B) Final tumor volume for MM.1S flank xenografts at time of sacrifice. Data are shown as average ± stdev. (**p<0.01, two-sided student t-test). (C). Immunoblot analysis of unmodified Rap1a in tumor and liver specimens from a representative control mouse (“Con”) as well as the animals in the combination treatment cohort. GAPDH is shown as a loading control. Lysates generated from RPMI-8226 myeloma cells treated with lovastatin were used as a positive control (“+”) for unmodified Rap1a protein.
4. Discussion.
We have previously reported the results from initial preclinical studies of VSW1198, a triazole bisphosphonate that functions as a potent and specific GGSI [12]. Notably, VSW1198 has therapeutic potential because of the agent’s drug-like properties, including prolonged half-life, metabolic stability, and systemic distribution [12]. The present study is the first to evaluate the in vivo effects of combining GGSI and statin therapy. We show that while administration of twice-weekly dosing of VSW1198 in combination with statins induces hepatotoxicity, once-weekly dosing regimens of VSW1198 with statins are feasible. Furthermore, these studies confirmed that GGSI and statin therapy alters isoprenoid levels and disrupts protein geranylgeranylation in vivo. Notably, this is the first in vivo study to evaluate the change in hepatic isoprenoid levels following statin/GGSI treatment, as previous reports only evaluated baseline levels of FPP and GGPP in control mice or in GGSI-treated mice [18]. In addition, we present the first in vivo investigation showing anti-tumor effects of combination IBP inhibitor therapy in a xenograft tumor model.
There is rationale within the literature to suggest that combining statins with other IBP pathway inhibitors may provide a therapeutic benefit. Particular interest has been paid to agents that target the enzyme farnesyltransferase (FTase), as it is responsible for the prenylation of oncogenic Ras proteins.[4] One group showed that use of an FTase inhibitor (FTI) prodrug in combination with lovastatin caused cell cycle G1 arrest, increased apoptosis and reduction in Ras prenylation in two human NF1 malignant peripheral nerve sheath tumor (MPNST) cell lines [19]. Other in vitro studies have demonstrated that combining statins with an FTI produces synergistic cytotoxic effects in multiple cancer cell types [20–22]. Strategies that target Rab geranylgeranylation but conserve farnesylation include inhibition of either GGDPS or GGTase II. One group demonstrated that a GGTase II inhibitor (designed as a GGPP derivative that competitively inhibits GGPP binding to the enzyme) in combination with lovastatin, promotes G0/G1 cell-cycle arrest, blocks cellular proliferation, and promotes autophagy in MPNST cells [23].
Studies evaluating the effect of combining GGDSP inhibitor therapy with statins are limited. One report found that combinations of lovastatin with a GGSI inhibited K562 cell growth in a synergistic manner [24]. These studies also showed enhanced induction of apoptosis in vitro, following treatment with lovastatin and the GGSI [24]. Our studies showed that incubation of myeloma cells with 0.1 μM lovastatin (a concentration too low to disrupt protein geranylgeranylation) resulted in the potentiation of effects induced by GGSIs consistent with enhanced disruption of protein geranylgeranylation [11]. These findings lead us to hypothesize that statin therapy may enhance the activity of GGSI through further depletion of GGPP levels and subsequent disruption of Rab geranylgeranylation. Notably, evaluation of hepatic isoprenoid levels following a 12-day treatment period with statins and/or GGSI revealed undetectable GGPP levels in the combination groups, while single drug groups displayed reduced but detectable levels of GGPP in the liver (Fig. 4). The work by Tong et al., demonstrated GGSI-induced changes in tissue FPP and GGPP levels but the authors did not demonstrate whether these changes were sufficient to disrupt protein geranylgeranylation in vivo [18]. That we observed greater decrease in GGPP levels compared to FPP levels in statin-treated mice may be a reflection of the complex regulation of flux through the IBP and the myriad biological processes which utilize FPP and GGPP.
The increased toxicity observed with combination GGSI/statin treatment may be the result of the marked GGPP depletion in the liver, particularly as our combination studies exploring further dose reductions revealed minimal hepatic toxicity in conjunction with detectable GGPP levels (Fig. S8). The cytotoxic effects of lovastatin in cancer cells can be prevented by add-back of GGPP [25–30]. Our own studies in myeloma and pancreatic cancer cells reveal that depletion of GGPP and disruption of Rab geranylgeranylation is responsible for the observed pro-apoptotic effects of GGSI therapy [13, 14]. While we speculate that GGPP depletion promotes hepatotoxicity, the question remains if it is the result of disrupting GGTase I substrate prenylation, GGTase II substrate prenylation, or depletion of downstream isoprenoid species that require GGPP. Hepatocyte-specific knockout of GGTase I in mice caused severe hepatocellular disease with elevation in liver transaminases, histopathological changes in hepatocyte morphology and reduced overall survival, suggesting that GGTase I activity is essential for the viability of hepatocytes [31]. There are limited data regarding the in vivo toxicity of GGTase I inhibitors. One report assessed the in vivo activity of a GGTase I inhibitor (P661A6) in a mouse model of pancreatic cancer and reported mild elevation in liver transaminase levels without histopathological changes [32]. A Phase 1 clinical trial using a GGTase I inhibitor (GGTI-2418) in patients with solid tumors reported no dose limiting toxicities [33]. One patient had grade 3 elevation of alkaline phosphatase and grade 4 elevation in bilirubin, but this was in the context of malignant biliary obstruction due to disease progression [33]. Only one preclinical study using a GGTase II inhibitor (3-PEHPC) in the 5T2MM myeloma mouse model has been reported, but did not include toxicity data [34]. It is possible that the GGSI-induced hepatotoxicity could be an off-target effect related to the specific chemical structure, however our previous studies showed that a structurally similar triazole-based compound with negligible activity as a GGSI did not cause liver damage. Thus it is more likely that the hepatotoxicity observed with VSW1198 (as well as a closely related GGSI, RAM2061 [17]), is related to GGDPS inhibition. It is less likely that GGSI-induced cytotoxicity is due to increased FPP levels [24] as the addition of statins abrogates the GGSI-induced increase in FPP levels while increasing hepatic damage. Future pre-clinical studies using additional structural analogs would provide further insight into the relationship between GGSI chemical structure and hepatotoxicity.
A meta-analysis of 135 randomized clinical trials evaluating patients taking a variety of statins showed a higher risk of transaminase elevations (odds ratio 1.51, 95% confidence interval 1.24–1.84) which appeared to be dose-dependent [35]. However, on the basis of the National Lipid Association’s Liver Expert Panel and Statin Safety Task Force panel [36, 37] along with review of post-marketing data, the Federal Drug Association concluded that the currently marketed statins are associated with very low risk of serious liver injury and that therefore routine monitoring of liver enzyme tests was not necessary [38]. Of note, our studies evaluated two statins: lovastatin and the more hydrophilic pravastatin reported to have greater hepatoselectivity [39]. While the addition of GGSI to either statin induced hepatotoxicity at higher doses of the GGSI, there was a signal that the combination therapy was more problematic when pravastatin was used as the statin (Fig 2A, Fig 3A–B). Future studies will be needed to more rigorously evaluate the mechanism by which GGSI/statin combination therapy promotes hepatotoxicity and the degree to which the identity of the statin influences outcomes.
Aside from the liver function tests, the only other abnormality noted in the blood sample analyses was the finding of decreased glucose levels in the combination cohorts (Tables 1–2). This is unlikely to be a result of decreased oral intake due to esophageal irritation from the oral gavage of the statins, as similar findings were not observed in the statin-only groups. However, it is possible that the mice in the combination cohorts did have decreased oral intake due to the underlying hepatotoxicity. Intriguingly, there is literature connecting GGDPS and insulin resistance. Higher expression of GGDPS in the liver, adipose tissue and muscle was noted in mice with obesity and insulin resistance (ob/ob mice) compared to control animals [40]. In addition, studies utilizing muscle-specific GGDPS knock-out mice demonstrated improved systemic insulin sensitivity [41]. Blood glucose levels were lower in fed knockout animals compared to the fed wildtype mice [41]. Ex vivo studies revealed enhanced glucose uptake in skeletal muscle from heterozygote knockout mice compared wildtype controls [41]. Thus, further studies investigating the effects of GGSIs, with or without statins, on insulin sensitivity are warranted.
There has been considerable interest in determining the in vivo anti-cancer activity of inhibitors targeting the IBP pathway [42–44]. Results of phase 1 trials showed that high dose statins can achieve serum levels in the micromolar range but produced unwanted side effects and achieved minimal antitumor efficacy [7, 8]. There continue to be efforts to combine statins with other anti-cancer agents across multiple different tumor types [45]. It has been hypothesized that standard doses of statins used to treat hyperlipidemia are insufficient to disrupt protein prenylation, however this has not been rigorously evaluated. In one study, AML patients were administered single doses of lovastatin (ranging from 40 to 200 mg) followed by a blood draw at 6 hours [46]. A maximum lovastatin bioactivity of 234 nM in plasma was observed in patients administered the highest dose of lovastatin [46]. An alteration in Ras prenylation in peripheral blood mononuclear cells from these patients was not observed, however given the half-life of Ras proteins, it is not likely that significant accumulation of unprenylated Ras would occur within 6 hours [47, 48]. We and others have demonstrated the anti-cancer activity of GGSIs in mouse xenograft models [14, 17, 49, 50], however at present, GGSI therapy has not yet advanced to clinical trial testing.
5. Conclusions.
The present study has determined the feasibility of combination therapy with a potent GGSI and several clinically used statins. Our previous studies found hepatotoxicity as the primary barrier to dose escalation for VSW1198 [12]. In the present study, hepatic injury was more pronounced in mice administered a combination of GGSI and statin than with VSW1198 alone, which we propose is the result of further depletion of hepatic GGPP levels. We did not observe substantial differences in toxicity between the more lipophilic lovastatin and the more hydrophilic pravastatin when used in combination with GGSI. Further studies evaluating other statins with varying degrees of hepatoselectivity [51] are warranted. We conclude that once-weekly low-dose GGSI treatment combined with a statin is feasible and that this dosing regimen significantly slowed myeloma tumor growth in vivo. As development of GGSI therapeutics progress to eventual phase 1 clinical trials, it will be imperative to determine whether concomitant statin use (for hyperlipidemia) can be safely permitted and whether this combination strategy can be used to enhance the anti-tumor efficacy of the GGSIs.
Supplementary Material
Acknowledgments
Funding. This work was supported by the National Institutes of Health (Grants P30 CA036727) and the American Society of Hematology.
Footnotes
Disclosure. The authors declare no relevant conflicts of interest. SAH has received honoraria from Adaptive Biotechnologies, Celgene, Genentech, GSK, Sanofi, Sorrento, Takeda
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations.
- 1.Thurnher M, Nussbaumer O, and Gruenbacher G, Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res, 2012. 18(13): p. 3524–31. [DOI] [PubMed] [Google Scholar]
- 2.Bergstrom JD, et al. , Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys, 2000. 373(1): p. 231–41. [DOI] [PubMed] [Google Scholar]
- 3.van Beek E, et al. , Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun, 1999. 264(1): p. 108–11. [DOI] [PubMed] [Google Scholar]
- 4.Brock EJ, et al. , How to Target Activated Ras Proteins: Direct Inhibition vs. Induced Mislocalization. Mini Rev Med Chem, 2016. 16(5): p. 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinensky M, et al. , Differential inhibitory effects of lovastatin on protein isoprenylation and sterol synthesis. J Biol Chem, 1990. 265(32): p. 19937–41. [PubMed] [Google Scholar]
- 6.Pan HY, et al. , Comparative pharmacokinetics and pharmacodynamics of pravastatin and lovastatin. J Clin Pharmacol, 1990. 30(12): p. 1128–35. [DOI] [PubMed] [Google Scholar]
- 7.Thibault A, et al. , Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Res, 1996. 2(3): p. 483–91. [PubMed] [Google Scholar]
- 8.Holstein SA, et al. , Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol, 2006. 57(2): p. 155–64. [DOI] [PubMed] [Google Scholar]
- 9.Gomes AQ, et al. , Membrane targeting of Rab GTPases is influenced by the prenylation motif. Mol Biol Cell, 2003. 14(5): p. 1882–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills VS, et al. , Potent triazole bisphosphonate inhibitor of geranylgeranyl diphosphate synthase. ACS Med Chem Lett, 2015. 6(12): p. 1195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen C, et al. , Olefin isomers of a triazole bisphosphonate synergistically inhibit geranylgeranyl diphosphate synthase. Mol Pharmacol, 2017. 91(3): p. 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haney SL, et al. , Preclinical investigation of a potent geranylgeranyl diphosphate synthase inhibitor. Invest New Drugs, 2018. 36(5): p. 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holstein SA and Hohl RJ, Isoprenoid biosynthetic pathway inhibition disrupts monoclonal protein secretion and induces the unfolded protein response pathway in multiple myeloma cells. Leuk Res, 2011. 35(4): p. 551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haney SL, et al. , Inhibition of geranylgeranyl diphosphate synthase is a novel therapeutic strategy for pancreatic ductal adenocarcinoma. Oncogene, 2019. 38(26): p. 5308–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhonker YS, et al. , Simultaneous Quantitation of Isoprenoid Pyrophosphates in Plasma and Cancer Cells Using LC-MS/MS. Molecules, 2018. 23(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holstein SA, Tong H, and Hohl RJ, Differential activities of thalidomide and isoprenoid biosynthetic pathway inhibitors in multiple myeloma cells. Leuk Res, 2010. 34(3): p. 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haney SL, et al. , In Vivo Evaluation of Isoprenoid Triazole Bisphosphonate Inhibitors of Geranylgeranyl Diphosphate Synthase: Impact of Olefin Stereochemistry on Toxicity and Biodistribution. J Pharmacol Exp Ther, 2019. 371(2): p. 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong H, et al. , Quantitative determination of farnesyl and geranylgeranyl diphosphate levels in mammalian tissue. Anal Biochem, 2008. 378(2): p. 138–43. [DOI] [PubMed] [Google Scholar]
- 19.Wojtkowiak JW, et al. , Induction of apoptosis in neurofibromatosis type 1 malignant peripheral nerve sheath tumor cell lines by a combination of novel farnesyl transferase inhibitors and lovastatin. J Pharmacol Exp Ther, 2008. 326(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding N, et al. , A triple combination of atorvastatin, celecoxib and tipifarnib strongly inhibits pancreatic cancer cells and xenograft pancreatic tumors. Int J Oncol, 2014. 44(6): p. 2139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan MA, et al. , Combining prenylation inhibitors causes synergistic cytotoxicity, apoptosis and disruption of RAS-to-MAP kinase signalling in multiple myeloma cells. Br J Haematol, 2005. 130(6): p. 912–25. [DOI] [PubMed] [Google Scholar]
- 22.Yonemoto M, et al. , J-104,871, a novel farnesyltransferase inhibitor, blocks Ras farnesylation in vivo in a farnesyl pyrophosphate-competitive manner. Mol Pharmacol, 1998. 54(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 23.Sane KM, et al. , A novel geranylgeranyl transferase inhibitor in combination with lovastatin inhibits proliferation and induces autophagy in STS-26T MPNST cells. J Pharmacol Exp Ther, 2010. 333(1): p. 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudakovic A, et al. , Inhibition of geranylgeranyl diphosphate synthase induces apoptosis through multiple mechanisms and displays synergy with inhibition of other isoprenoid biosynthetic enzymes. J Pharmacol Exp Ther, 2008. 324(3): p. 1028–36. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal B, et al. , Mechanism of lovastatin-induced apoptosis in intestinal epithelial cells. Carcinogenesis, 2002. 23(3): p. 521–8. [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, et al. , Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells. Leukemia, 2001. 15(9): p. 1398–407. [DOI] [PubMed] [Google Scholar]
- 27.Crosbie J, et al. , Statins inhibit proliferation and cytotoxicity of a human leukemic natural killer cell line. Biomarker research, 2013. 1(1): p. 33–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanae M, et al. , Statin-induced apoptosis via the suppression of ERK1/2 and Akt activation by inhibition of the geranylgeranyl-pyrophosphate biosynthesis in glioblastoma. Journal of experimental & clinical cancer research : CR, 2011. 30(1): p. 74–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson JM, et al. , Targeting the Mevalonate Pathway Suppresses VHL-Deficient CC-RCC through an HIF-Dependent Mechanism. Mol Cancer Ther, 2018. 17(8): p. 1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang P, et al. , In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. Br J Cancer, 2014. 111(8): p. 1562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang SH, et al. , Severe hepatocellular disease in mice lacking one or both CaaX prenyltransferases. J Lipid Res, 2012. 53(1): p. 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, et al. , In vivo antitumor effect of a novel inhibitor of protein geranylgeranyltransferase-I. Mol Cancer Ther, 2009. 8(5): p. 1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karasic TB, et al. , A Phase I Study of GGTI-2418 (Geranylgeranyl Transferase I Inhibitor) in Patients with Advanced Solid Tumors. Target Oncol, 2019. 14(5): p. 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson MA, et al. , Geranylgeranyl transferase type II inhibition prevents myeloma bone disease. Biochem Biophys Res Commun, 2008. 377(2): p. 453–457. [DOI] [PubMed] [Google Scholar]
- 35.Naci H, Brugts J, and Ades T, Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes, 2013. 6(4): p. 390–9. [DOI] [PubMed] [Google Scholar]
- 36.Cohen DE, Anania FA, and Chalasani N, An assessment of statin safety by hepatologists. Am J Cardiol, 2006. 97(8a): p. 77c–81c. [DOI] [PubMed] [Google Scholar]
- 37.McKenney JM, et al. , Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol, 2006. 97(8a): p. 89c–94c. [DOI] [PubMed] [Google Scholar]
- 38.FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-important-safety-label-changes-cholesterol-lowering-statin-drugs#data.
- 39.Schachter M, Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundamental & Clinical Pharmacology, 2005. 19(1): p. 117–125. [DOI] [PubMed] [Google Scholar]
- 40.Vicent D, Maratos-Flier E, and Kahn CR, The branch point enzyme of the mevalonate pathway for protein prenylation is overexpressed in the ob/ob mouse and induced by adipogenesis. Molecular and cellular biology, 2000. 20(6): p. 2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao W, et al. , Lipid-induced Muscle Insulin Resistance Is Mediated by GGPPS via Modulation of the RhoA/Rho Kinase Signaling Pathway. The Journal of biological chemistry, 2015. 290(33): p. 20086–20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regulska K, et al. , Beyond the boundaries of cardiology: Still untapped anticancer properties of the cardiovascular system-related drugs. Pharmacol Res, 2019. 147: p. 104326. [DOI] [PubMed] [Google Scholar]
- 43.Longo J, et al. , Statins as Anticancer Agents in the Era of Precision Medicine. Clin Cancer Res, 2020. [DOI] [PubMed] [Google Scholar]
- 44.Göbel A, et al. , Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochim Biophys Acta Rev Cancer, 2020. 1873(2): p. 188351. [DOI] [PubMed] [Google Scholar]
- 45.Matusewicz L, Czogalla A, and Sikorski AF, Attempts to use statins in cancer therapy: An update. Tumour Biol, 2020. 42(7): p. 1010428320941760. [DOI] [PubMed] [Google Scholar]
- 46.Lewis KA, Holstein SA, and Hohl RJ, Lovastatin alters the isoprenoid biosynthetic pathway in acute myelogenous leukemia cells in vivo. Leuk Res, 2005. 29(5): p. 527–33. [DOI] [PubMed] [Google Scholar]
- 47.Ulsh LS and Shih TY, Metabolic turnover of human c-rasH p21 protein of EJ bladder carcinoma and its normal cellular and viral homologs. Mol Cell Biol, 1984. 4(8): p. 1647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holstein SA, Wohlford-Lenane CL, and Hohl RJ, Consequences of mevalonate depletion. Differential transcriptional, translational, and post-translational up-regulation of Ras, Rap1a, RhoA, AND RhoB. J Biol Chem, 2002. 277(12): p. 10678–82. [DOI] [PubMed] [Google Scholar]
- 49.Reilly JE, Neighbors JD, and Hohl RJ, Targeting protein geranylgeranylation slows tumor development in a murine model of prostate cancer metastasis. Cancer Biol Ther, 2017. 18(11): p. 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reilly JE, et al. , Targeting geranylgeranylation reduces adrenal gland tumor burden in a murine model of prostate cancer metastasis. Clin Exp Metastasis, 2015. 32(6): p. 555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmadi Y, et al. , The effects of statins with a high hepatoselectivity rank on the extra-hepatic tissues; New functions for statins. Pharmacol Res, 2020. 152: p. 104621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.