Abstract
Background and Purpose–
Brain tissue-resident microglia and monocyte-derived macrophages (MDMs) are innate immune cells that contribute to the inflammatory response, phagocytosis of debris, and tissue repair after injury. We have previously reported that both microglia and MDMs transition from proinflammatory to reparative phenotypes over days after an intracerebral hemorrhage (ICH). However, their individual functional properties in the brain remain largely unknown. Here we characterized the differences between microglia and MDMs and further elucidate their distinct activation states and functional contributions to the pathophysiology and recovery after ICH.
Methods–
Autologous blood injection was used to model ICH in mice. Longitudinal transcriptomic analyses on isolated microglia and MDMs from mice at days 1, 3, 7 and 10 after ICH and naïve controls identified core transcriptional programs that distinguish these cells. Imaging flow cytometry and in vivo phagocytosis assays were used to study phagocytic ability of microglia and MDMs. Antigen presentation was evaluated by ovalbumin-OTII CD4 T-cell proliferation assays with bone marrow-derived macrophages and primary microglia cultures.
Results–
MDMs had higher phagocytic activity and higher erythrophagocytosis in the ICH brain. Differential gene expression revealed distinct transcriptional signatures in the MDMs and microglia after ICH. MDMs had higher expression of major histocompatibility complex class II genes than microglia at all time points and greater ability to induce antigen-specific T-cell proliferation.
Conclusions–
The different ontogeny of microglia and MDMs lead to divergent responses and functions in the inflamed brain as these two cell populations differ in phagocytic functions and antigen-presenting capabilities in the brain after ICH.
Keywords: intracerebral hemorrhage, macrophages, microglia, phagocytosis, innate immunity
Introduction
Macrophages are critical innate immune cells that perform diverse functions in inflammation and homeostasis. They exhibit extensive phenotypic heterogeneity and remarkable functional plasticity.1 As professional phagocytes, macrophages execute host defense and reparative tasks through the phagocytosis of pathogens, tissue debris, and senescent cells.2 In addition, macrophages maintain tissue homeostasis and provide immunoregulatory functions by flexibly transitioning across a spectrum of phenotypes induced by local microenvironmental cues.3
Macrophage populations can be broadly categorized as either tissue-resident macrophages, which are highly specialized cells that perform specific functions for their tissue of residence, or blood monocyte-derived macrophages (MDMs), which traffic into tissues in response to inflammatory and chemokine cues. Microglia, the major tissue-resident macrophages within brain parenchyma, derive from the yolk sac and self-maintain throughout life under physiological conditions.4, 5 In contrast, MDMs do not enter the central nervous system (CNS) in the absence of brain pathology.6, 7 Because they share cell markers and morphologies, microglia and MDMs have been frequently studied as the same population and considered to serve similar functions in the inflamed brain. Recent studies have identified unique markers of microglia (e.g. Tmem119, Sall1, and Fcrls),8, 9 and revealed that even under experimental conditions when MDMs enter the brain, they maintain a highly distinct transcriptional state from that of microglia.10 However, our knowledge regarding whether MDMs and microglia perform similar functions in the setting of inflammation after acute brain injury remains scant.
Neuroinflammation contributes to secondary injury after intracerebral hemorrhage (ICH) and has been a major focus of translational cerebrovascular research.11 ICH is initiated by a rupture of a parenchymal blood vessel resulting in blood leakage into the brain. This mass of blood cells, serum components, injured resident CNS cells, and disrupted extracellular matrix induce a dynamic immune response in the damaged brain. We, and others, have previously shown robust activation of microglia and MDMs in the hemorrhagic brain.12–15 Both populations highly express proinflammatory transcripts and contribute to acute disability after ICH, and they both gradually switch toward reparative phenotypes to aid in ICH brain repair 3–10 days post-hemorrhage.16, 17 Interestingly, we found MDM phenotype is modulated by the engulfment of eryptotic erythrocytes and microglia by TGF-beta signaling in the hemorrhagic brain.16, 17 These findings led to the hypothesis that microglia and MDMs may work together in respond to acute brain injury through performing disparate functions. The ICH model provides an ideal platform where the activation and function of the two myeloid cousins can be studied side-by-side over time. A comprehensive comparison of microglia and MDMs in ICH entails characterization of transcriptional responses, phagocytosis substrates, and functional differences and will advance our knowledge of macrophage function in acute brain injury.
Both microglia and MDMs contribute to the clearance of debris after ICH. We first quantified phagocytosis by microglia and MDMs in a mouse model of ICH and found that MDMs have higher phagocytic ability in the perihematomal region. We then compared the gene expression profiles between the two populations at various time points after ICH. While microglia expressed transcripts that are relevant to synapse remodeling and phospholipid uptake, MDMs increased levels of antigen presentation molecules. We further developed an in vitro culture system that preserves microglia-specific gene expression and tested these transcriptional differences identified in vivo with functional assays. High expression of MHCII on macrophages lead to more efficient antigen presentation and T-cell proliferation when compared to microglia. Together our data reveal distinct responses and functions of microglia and MDMs after ICH and demonstrate how these two populations may work jointly to restore tissue homeostasis in the brain.
Methods
Please see the Data Supplement for detailed experimental description of Materials and Methods.8, 16–20 The data that support the findings are available from the corresponding author upon reasonable request. All animal studies followed the guidelines outlined in Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments) and were approved by the Institutional Animal Care and Use Committees where experiments were performed. A total of 93 mice were used in this study. C57BL/6 male mice and OT-II transgenic mice designated B6.Cg-Tg(TcraTcrb)425Cbn/J (weight, 24–28 g) were purchased from The Jackson Laboratory. The mice were housed under specific-pathogen free (SPF) condition with 12-hour light/dark cycle in a temperature-controlled environment with free accessed to water and food pallets. Mice were randomly allocated to experimental groups by coin flip. ICH was induced by autologous blood injection. Group size calculation is based on our experience in ICH models with the variability in these experiments, accounting for a resulting effect size f (by analysis of variance [ANOVA]) of 0.6 with α < 0.05 and power 0.80.
Statistical Analysis
Analyses were performed with GraphPad Prism (v8), and all values are present as mean ± standard deviation or individual mouse data with a line indicating the mean of the group. Mean values were compared using Student’s t-test for comparison between 2 groups, and 2-way ANOVA with post-hoc Tukey test for multiple-group comparisons. A value of P < 0.05 was considered statistically significant. Transcriptional analyses of data generated from custom nanoString panel were performed in R (v 3.3.3) with further details in Data Supplement.
Results
MDMs Display Higher Phagocytic Activity in the ICH Brain
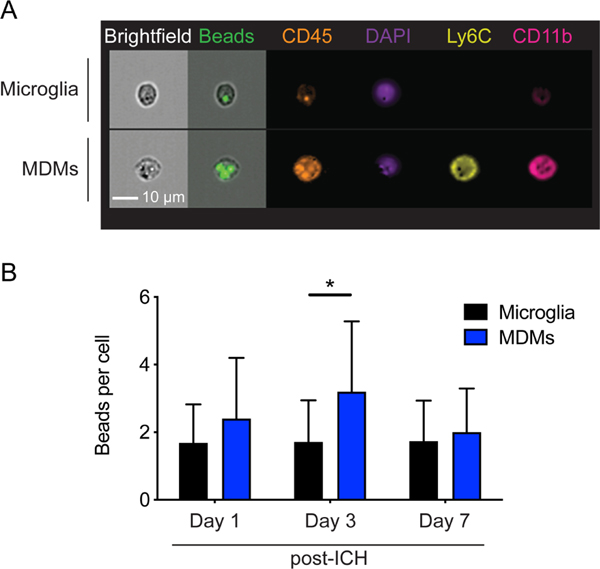
Macrophages clear debris through phagocytosis, thereby restoring tissue homeostasis after injury. Both microglia and MDMs have been shown to play a key role in ICH brain recovery;15–17 however, their phagocytic potential in this context has yet to be fully investigated. We first quantified the phagocytic ability of microglia and MDMs (Gating strategy in Supplemental Figure IA) by injecting a mixture of fluorescent beads and autologous blood to induce ICH in the mouse brain. Our previous work indicated that microglia do not appreciably upregulate CD45 when activated after ICH,21 we therefore defined CD11b+CD45int population as microglia and CD11b+CD45hi population as MDM in our experiments hereafter. A mean 4674 ± 303 microglia and 551 ± 139 macrophages were analyzed per brain across the time points. Engulfment of microspheres was visible in both microglia and MDMs as measured by imaging flow cytometry (Figure 1A). Phagocytosis capacity was quantified in the myeloid cells that had engulfed at least one bead, in order to directly compare the cells that had encountered the phagocytosis substrate and eliminate a bias against microglia which may be remote from the ICH. We found that MDMs had a significantly higher number of intracellular fluorescent beads at day 3 after ICH (Figure 1B), showing that the two phagocytes have different phagocytic capacities in the context of ICH.
Figure 1. MDMs have higher phagocytic capacity in the ICH brain.
A, Representative images show MDMs and microglia from ICH day 3 brains. MDMs are CD11b+CD45hiLy6C+ and microglia are CD11b+CD45int. Fluorescent beads are visible within these cells. B, MDMs phagocytose higher numbers of fluorescent beads in the perihematomal brain tissues on day 3. Data shows mean ± SD. *p < 0.05 by ANOVA with post-hoc Tukey test, n=10.
MDMs Phagocytose More Erythrocytes than Microglia during ICH.
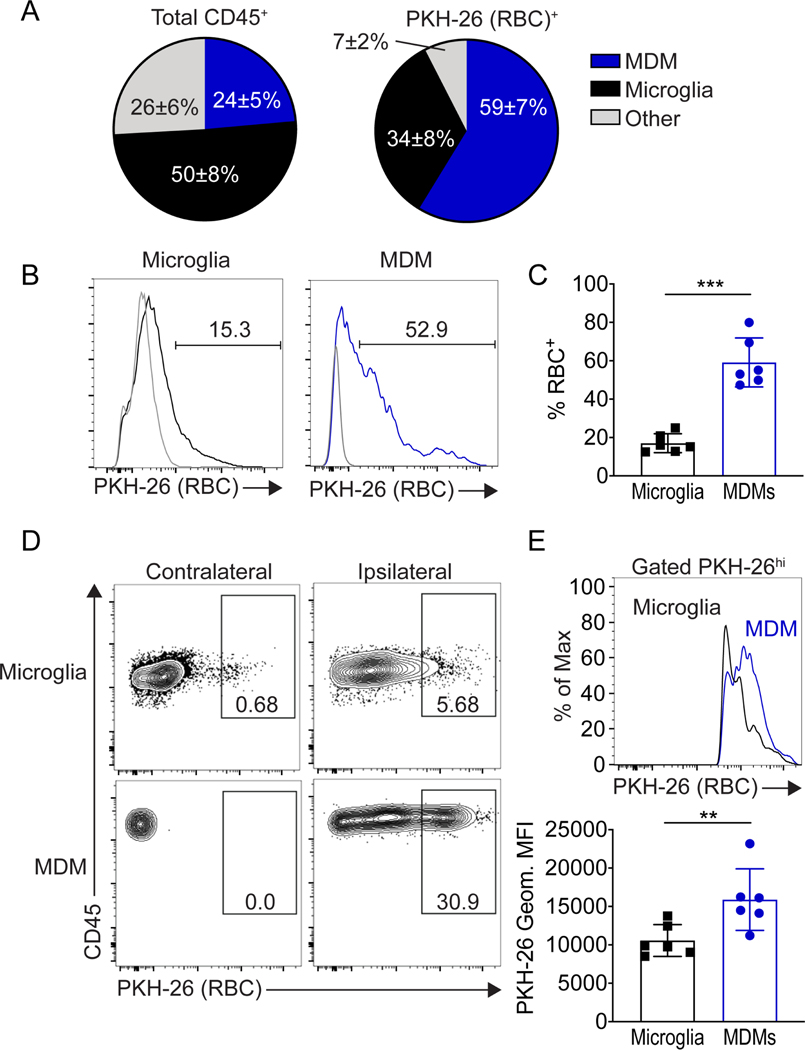
Microglia and macrophages accumulate around the hematoma at day 1 and are found within the hematoma from days 3 to 10 after ICH.12, 21 Additionally, in Ccr2−/− bone marrow chimeras that lack MDM infiltration, the hematoma clearance was delayed.16 These findings, coupled with the differential phagocytic capabilities of MDMs and microglia, led us to speculate that MDMs may be the major cell population that enters and clears the hematoma. To test this in a biologically relevant manner, we injected PKH-26-labeled erythrocytes to induce ICH. We chose day 3 post-ICH, a time point when ICH clearance is ongoing in the murine model21 to measure the erythrocyte fluorescence signal in perihematomal microglia and MDMs by flow cytometry. Of the CD45+ cells in the region, there were twice as many microglia as MDMs. However, when examining all the erythrocyte-positive cells, almost two-thirds of cells that had engulfed an erythrocyte were MDMs (Figure 2A). Additionally, when gating on MDMs and microglia separately, the percentage of MDMs that were positive for PKH-26-erythrocytes was higher than microglia (Figure 2B). We then limited the quantification to cells at the site of injury and therefore to phagocytes exposed to the labeled cells in order to directly compare the efficiency of phagocytosis by MDMs and microglia. The mean fluorescence intensity (MFI) of the erythrocyte-positive MDMs was higher than the erythrocyte-positive microglia (Figure 2C). Regardless of the quantification approach, results were consistent that MDMs have a greater capacity to phagocytose erythrocytes than microglia in the ICH brain.
Figure 2. Erythrophagocytosis of MDMs and microglia in the ICH brain.
A, Pie charts of proportions of MDMs, microglia, and other cells as a percentage of total CD45+ cells (left) and proportions of these cells that had engulfed an erythrocyte as a percentage of the total erythrocyte-positive cells in the perihematomal brain (right). B, Left, representative histogram shows PKH-26-erythrocyte signal from microglia (black line) and MDMs (blue line) at day 3 after ICH compared to the cells from control brains (gray lines). Right, quantification of percentage of PKH-26- erythrocyte positive microglia (17.0±4.9%) and MDMs (59.1±12.8%). Each dot represents an individual animal, mean ± SD, *p < 0.05 by Student’s t-test, n=6. C, Left, gating of PKH-26-positive cells to gate on cells that had phagocytosed erythrocytes. Right, representative histogram shows PKH-26-(erythrocyte) signal from microglia (black line) and MDMs (blue line) with quantification of mean fluorescent intensity (below; each dot represents individual animal), **p < 0.01 by Student’s t-test, n=6. *p < 0.05 by Student’s t-test
Transcriptional Profiling Demonstrates Divergent Microglia and MDM Responses in the Brain after ICH
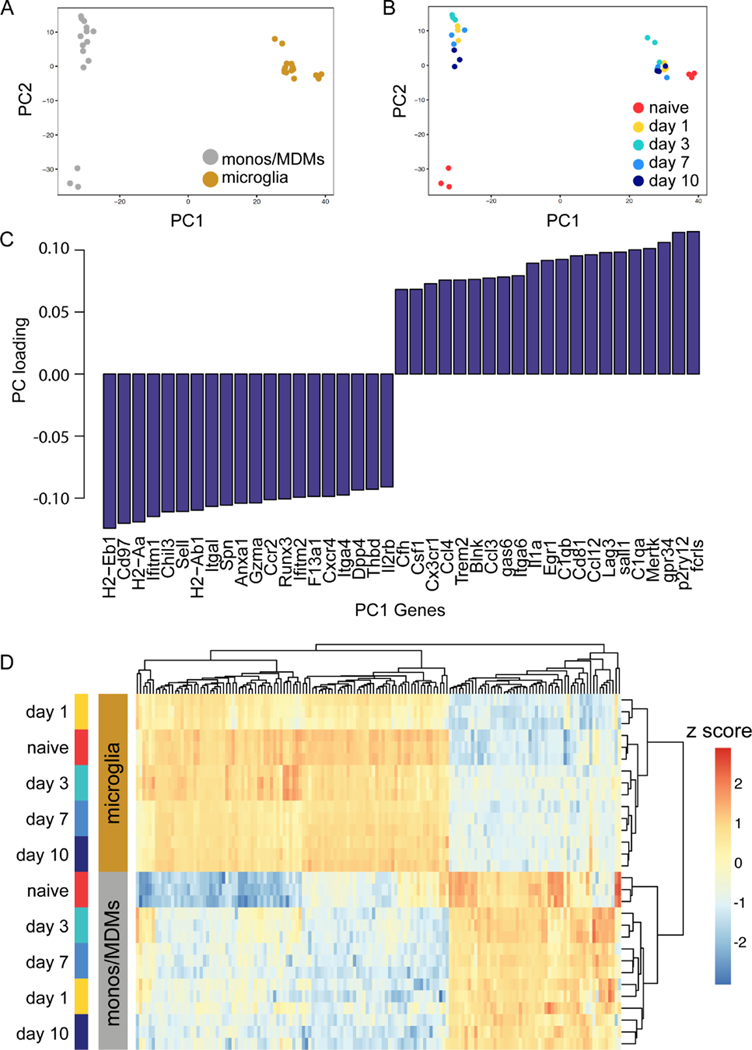
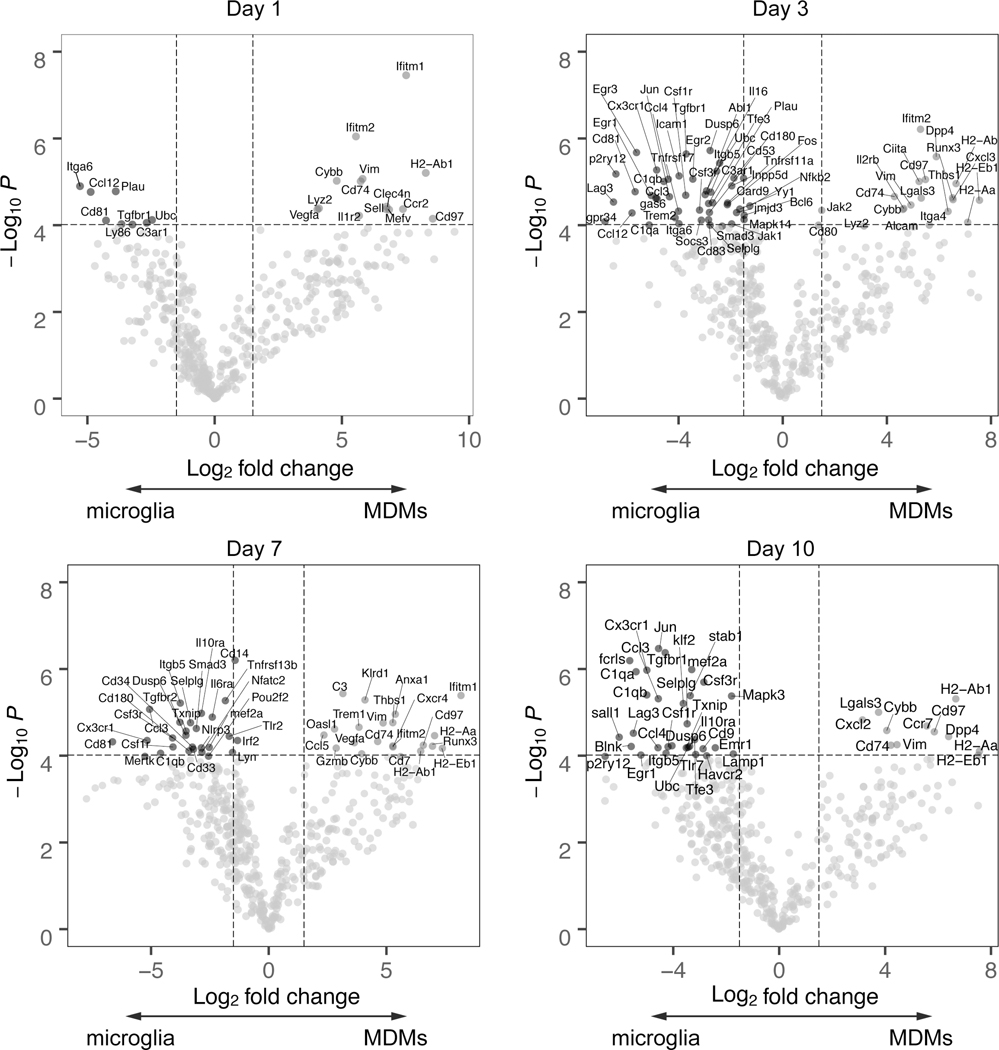
The functional phagocytosis assays suggested that microglia and MDMs may respond to the microenvironment of the post-ICH brain differently based on differential ontogeny or long-lived tissue residence. Alternatively, the damage-associated molecular patterns and other stimuli in the post-ICH brain may overcome these and drive both myeloid populations to produce robust, classically-defined proinflammatory responses followed by repair programs. To better understand the molecular and transcriptional programs that might drive microglia and MDM functional differences, we leveraged transcriptional data sets generated from our lab16, 17. This dataset consists of 780 genes from microglia and MDMs sorted from mouse brains at days 1, 3, 7, and 10 after ICH induction as well as naive microglia and blood monocytes. Microglia signature genes18 such as P2ry12, Fcrls and Mertk were highly expressed in the microglia population whereas Ccr2 was highly expressed in the MDMs (Supplemental Figure IB), providing support for the FACS sorting strategy. Pearson correlation analysis of all samples across all detected genes in both microglia and MDMs followed by clustering shows that cell types cluster closely, and that naïve MDMs are globally different from ICH MDMs at all time points (Supplemental Figure II). We performed principal components analysis (PCA) and found that >80% of the data variance was accounted for by principal components 1 and 2 (Figure 3A). Cell type (monocyte/MDMs versus microglia) strongly drove separation in our data along PC1. The main source of variance along PC2 was the difference between circulating monocytes in the blood of naïve mice and MDMs in the brain (Figure 3B). At no time point did MDMs and microglia in the injured brain cluster together, indicating highly cell-type specific responses. Variable loading plots show top genes that drive this cell-type separation along PC1 (Figure 3C). Notably, Trem2, C1qb, and C1qa, which contribute to synapse pruning and lipoprotein clearance, were highly expressed by microglia in the ICH brain.22, 23 In contrast, multiple major histocompatibility complex (MHC) class II molecules H2-Eb1, H2-Aa, H2-Ab1 were highly expressed by MDMs. Hierarchical clustering of all the differentially expressed genes across samples (adjusted p < 0.05, n=260) demonstrated marked differences between microglia and MDMs (Figure 3D). Notably, the blood monocytes maintained closer transcriptional similarity with the MDMs after ICH than the MDMs and microglia in the brain after ICH, underscoring the persistent effects of ontogeny even in the setting of an inflamed tissue.
Figure 3. Microglia and MDMs are transcriptionally distinct cell populations in ICH.
A-B, PCA plot of ICH microglia and MDMs transcriptome. Each replicate represents pooled samples from three brains collected from mice 1, 3, 7, and 10 days after ICH surgery or the naïve animals. The scatter plots show each sample projected on the first 2 principal components and are color coded according to cell type (A) and time point (B) of each sample. Replicates cluster closely at each cell type. C, Bar plot of the top 20 genes contributing to separation along PC1, the major PC separating microglia and MDMs. D, Heatmap of the Z score of the 469 genes above level of detection across both microglia and MDMs data set. Data were clustered hierarchically in GENE-E using pairwise comparisons at each time point. Data are colored according to row minimum and maximum.
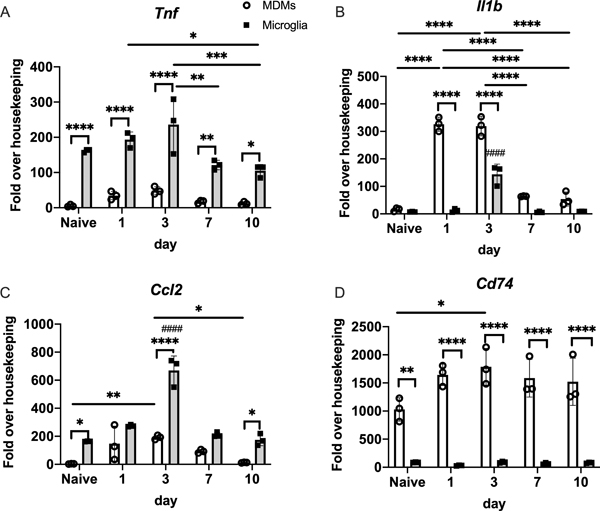
At all time points, microglia had markedly higher expression of the classic inflammatory mediator Tnf (Figure 4A). MDMs showed an early and robust expression of Il1b at days 1 and 3, although microglia did upregulate Il1b at day 3 (Figure 4B). Microglia and MDMs upregulate Ccl2, although the peak expression at day 3 was higher in microglia (Figure 4C). In contrast, MDMs had higher expression of the MHCII invariant chain Cd74 at all time points (Figure 4D). To better understand the gene programs responsible for divergent behaviors in MDMs and microglia over the course of ICH, we visualized the pairwise differential expression analysis between MDMs and microglia at each time point (Figure 5 and Supplemental Figure III–VI). This revealed distinct patterns of gene expression across time and allowed identification of core genes that temporally differentiate microglia and MDMs. Genes associated with microglia identity (e.g. Sall1, P2ry12, Fcrls) and complement genes (C1qa and C1qb) were lost at day 1 after ICH but reemerged at later time points. Consistent with the importance of TGF-beta in the restoration of homeostatic microglia,17 Smad3 was upregulated at days 3 and 7 and Tgfbr1 was upregulated at days 3 and 10 in microglia. Interestingly, Lag3 (CD223), a negative costimulatory molecule on CD4+ T cells24 was increased in microglia at days 3 and 10 after ICH. We also found antigen presentation genes H2aa, H2ab1, H2eb1, and Cd74 consistently upregulated in MDMs from days 3 to 7 after ICH. Additionally, we found that Thbs1 (Thrombospondin 1), a gene relevant to phagocytosis, was increased in MDMs at days 3 and 7.
Figure 4. Distinct gene expression in microglia and MDMs from the ICH brain.
Expression of Tnf, Ccl2, Il1b, and Cd74 in the days 1, 3, 7, and 10 ICH brain-sorted microglia and MDMs. (mean ± SD, n=3, with each replicate pools cells from 3 mice). * p<0.05, ** p<0.01, *** p<0.005, **** p<0.001 for each comparison shown, #### p<0.001 compared to same population at every other time point, by two-way ANOVA with post-hoc Tukey test. Gene expression is reported as fold over housekeeping to normalize for differing cell counts across time points and cell population.
Figure 5. Differential gene expression in microglia and MDMs over time in the ICH brain.
Differential gene expression at each time point. Genes with Log2 fold change > 1.5 and p < 10−4 are labeled on the volcano plots.
To determine what functions might be driven by altered gene expression programs, we performed functional enrichment and gene set enrichment analysis on differentially expressed genes at each time point (Supplemental Tables I and II). Overall, we found that MDMs and microglia were enriched for different gene sets at each time point post ICH. MDMs were enriched for GO functions related to T-cell activation and antigen presentation. In contrast, microglia were enriched for functions related to leukocyte cell migration and cytokine production. These transcriptomic results supported our functional findings and suggested that MDMs and microglia may have important differences in the regulation of antigen-specific CD4 function in the ICH brain.
MDMs Are More Effective at Inducing Antigen-Driven CD4+ T-Cell Proliferation
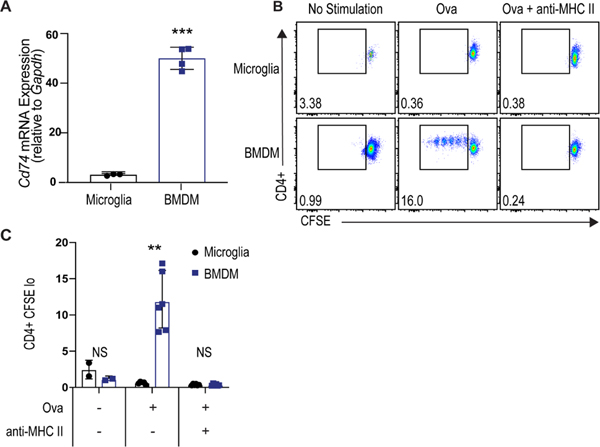
Since the transcriptional analysis showed that microglia upregulated Lag3 and MDMs highly expressed MHC-related antigen presentation genes, we hypothesized that MDMs would be more effective at antigen presentation. First, we developed a microglia culture system building on our work and others8, 18 that improved upon previous culture conditions in preserving the microglial gene signature (Supplemental Figure VII). Microglia were cultured using this system and gene expression was assessed by qPCR. Consistent with in vivo findings during ICH, the expression of Cd74 was higher in bone marrow-derived macrophages (BMDMs) than microglia (Figure 6A). Primary microglia and BMDMs were then assessed for their ability to present antigen and induce proliferation of CD4+ T cells. BMDM and microglia were incubated with ovalbumin and then co-cultured with CD4+ T cells from OTII mice, in which the T-cell receptor is specific for chicken ovalbumin 323–339 peptide presented by MHCII. As predicted, macrophages were more effective at inducing T-cell proliferation to antigen (Figure 6B). This response was MHCII-dependent, as MHCII blocking antibody abrogated the macrophage-induced T-cell proliferation (Figures 6B and C).
Figure 6. BMDMs induce greater antigen-dependent CD4+ T-cell proliferation than microglia.
A, Gene expression of Cd74 in BMDMs and primary microglia by qRT-PCR (***p < 0.001 by Student’s t-test, n=3, each independent experiment includes 3 technical replicates). B, Representative gating of proliferating CD4+ T cells co-cultured with ovalbumin-stimulated microglia or BMDMs with or without anti-MHC II antibody. C, Quantification of proliferating CD4+ cells (Ki67+CFSElow CD4+ cells) from co-culture experiments (** p<0.01 by two-way ANOVA with post-hoc Tukey test, n=6, each independent experiment includes 3 technical replicates).
Discussion
Previous studies have shown that microglia and MDMs play distinct roles in multiple sclerosis,25 Alzheimer’s disease,26 and cerebral ischemia,27 but the functions of these two cell populations in response to ICH have not been extensively studied. Studies in cerebrovascular diseases are critical as the stimuli for acute inflammation and the programs necessary for repair are likely context dependent. Here, we report a comparative analysis of microglia and MDMs with direct quantifications of their phagocytic capacity, transcriptomes and immunostimulatory properties in response to ICH. We establish that 1) MDMs have greater phagocytic capacity than microglia in the context of brain hemorrhage; 2) MDMs are the dominant erythrophagocytic population in the perihematomal region; 3) MDMs and microglia express distinct transcriptional profiles after ICH; 4) BMDMs have greater ability than microglia to induce antigen-specific CD4+ T-cell activation.
As professional phagocytes, macrophages perform host defense through phagocytosis of infectious organisms, aid in tissue debris clearance, and maintenance and restoration of tissue homeostasis. We conducted a bead-based assay to evaluate the phagocytic capacity of microglia and MDM in the ICH brain. In agreement with a previous study using an ex vivo system to compare the phagocytic ability of CD11b+CD45hi and CD11b+CD45low cells,26 we found the phagocytic activity of MDMs to be higher than microglia in the hemorrhagic brain. A previous study from our group showed that efferocytosis of eryptotic erythrocytes induces phenotypic modulation of MDMs,16 causing us to ask whether MDMs and microglia differed in their capacity for erythrophagocytosis in the perihematomal brain. Using in vivo phagocytosis assays, we demonstrated that MDMs rather than microglia are mainly in charge of erythrophagocytosis. We compared percentages of all cells that had phagocytosed erythrocytes as well as the MFI of only those that had taken up fluorescently-labeled erythrocytes. This second analysis was critical because several studies have shown limited microglia migration after injury.10, 28, 29 By gating first on cells that had taken up at least some erythrocyte material, we could ascertain we were comparing microglia to macrophages that had encountered erythrocytes at the injury site. The difference did not appear to be correlated with activation state or pro-inflammatory phenotype, as both cell populations were activated-- although with somewhat different phenotypes (e.g. the MDMs with greater Il1b upregulation while the microglia with greater Tnf upregulation at day 3). Much more nuanced studies of phenotype and phagocytosis of specific substrates are needed in future work. To our knowledge, this presents the first comparison of erythrophagocytosis between tissue-resident and monocyte-derived macrophages in an in vivo model of ICH.
Synapse pruning is a crucial function of microglia in the developmental brain and in regulation of neurogenesis in the adult brain22, 23 suggesting that microglia may preferentially clear endogenous substances such as axonal debris in ICH. On the contrary, MDMs are often in charge of phagocytosing exogenous pathogens in diverse diseases. In our work, while both microglia and MDMs perform erythrophagocytosis, it appears the MDMs do more bulk clearance of the hematoma. Furthermore, our transcriptomic results show that microglia highly express complement genes (C1qb and C1qa) and Trem2, which are essential for engulfing lipoprotein and maintaining synaptic structure.30 Although we were not able to compare the phagocytic activity of microglia and MDMs of neuronal components, previous findings from our group and others suggest a model in which microglia and MDMs can reduce CNS burden through distinct phagocytic functions in the injured brain.16, 17, 31 This divergent phagocytic activity may inform future development of cell specific therapeutics by targeting their “culinary habit” to manipulate the functions of these two cells for treating hemorrhagic stroke.
Macrophages arise from at least three different sources: the embryonic yolk sac, the fetal liver, and the bone marrow.32 Microglia specifically in the developmental CNS are seeded by erythromyeloid progenitor cells from the yolk sac at embryonic day 8.5.33 Their numbers are independently maintained throughout the life and they are not replenished from the circulation.5 Since macrophages can flexibly switch their phenotypes and perform diverse functions through sensing the tissue microenvironment, it has been speculated that MDMs could be influenced by the CNS environment and gain characteristics of microglia in the brain. Studies have revealed that invading MDMs are able to spatially replace microglia while persistently maintain distinct identities from microglia.10 Using ICH as a CNS inflammatory model, our transcriptomic studies support the notion that MDMs and microglia are transcriptionally distinct even when they co-exist in the inflamed microenvironment. Our sorting strategy utilized CD45 expression to differentiate MDMs from microglia, which effectively discriminates between the cells in our model21 and is consistent with other work.9, 34–36 However, it is possible that a small percentage of microglia upregulate CD45 after injury, and future work including single cell transcriptomics or lineage tracing would be needed to further elucidate this possibility. Furthermore, a panel of 780 immune-related genes were sequenced, which while likely adequate to capture many inflammatory genes activated in the cells, may not be able to fully determine the functional heterogeneity between microglia and MDMs. Future work utilizing lineage tracing and/or single cell sequencing techniques will be helpful to further understand the phenotypic spectrum and unexplored subpopulations of these two cell types. However, the functions of the two populations could be reflected by the high expression of complement proteins (C1qa, C1qb) and lipoprotein receptor (Trem2) by microglia, and efferocytosis factor (Thbs1) by MDMs. Notably, in each population, the transcriptional signatures between naïve cells and the cells in the brain after ICH are more similar than the two populations are to each other at any time point. This further supports the idea that a developmental imprint sculpts the properties of microglia and MDMs and regulates their responses in the diseased brain.
In addition to phagocytosis, macrophages also serve as antigen presenting cells (APC) that present fragments of protein antigens in MHC molecules classes I and II to T cells. We found that infiltrating MDMs in the ICH brain expressed high levels of MHC II molecules (H2aa, H2ab1, H2eb1, and Cd74), which distinguished these cells from the tissue-resident microglia that express immunosuppressive molecule Lag3. The MDMs induced antigen-specific CD4+ T-cell proliferation, suggesting they may play a role in promoting T-cell responses in the post-ICH brain. The difference of Cd74 expression in naïve primary BMDM and microglia culture systems as well as higher antigen-presenting capacity in ovalbumin-stimulated BMDM further support the functional and ontological divergence between microglia and MDMs. Although we found that BMDM had lower level of microglia specific genes (Tmem119, P2ry12, Sall1, Fcrls) and higher Cd74 expression, one should note that the ex vivo global transcriptional state is likely different than in vivo.
In acute ischemic stroke models, infarct volumes are reduced in mice lacking T cells37 and when T-cell migration to the brain was inhibited.38, 39 Although it is well established that CD4+ and CD8+ T cells traffic to the perihematomal region in high numbers,11, 40, 41 few studies have focused on delineating T-cell functions after ICH. These CD4+ T cells have been shown to promote blood-brain barrier disruption, brain edema, and functional disability.41–43 In contrast, adoptive transfer of Treg cells within 24 hours of ICH induction reduces brain edema and neurological deficits.44, 45 Expansion of Treg cells before ICH induction by injecting CD28 super-agonist antibody alleviates injury and inflammatory milieu in the perihematomal region.46 While these studies have shed light on the different roles for T cells in ICH, the mechanism through which antigen presentation by macrophages may stimulate harmful or beneficial T cell responses remains unclear. Additional work is needed to understand the interaction between innate and adaptive immune cells in the ICH brain in order to develop pharmaceutical strategies for ICH across the time course of injury and recovery.
In sum, we employed transcriptomic analyses and functional assays to demonstrate the heterogeneity between tissue-resident microglia and recruited MDMs in the hemorrhagic brain. Macrophages are emerging as key mediators of several CNS pathologies. While it remains unclear whether immunotherapeutics promoting tissue repair after ICH need to target MDMs and microglia separately, our findings provide novel insights into the diverse functions of macrophage populations in the brain after injury.
Supplementary Material
Acknowledgments
Sources of Funding
The work was supported by NIH R01NS095993 (Sansing), the Ministry of Science and Technology of Taiwan (MOST 107-2320-B-002-063-MY2, Chang).
Nonstandard Abbreviations:
- BMDM
bone marrow-derived macrophage
- CNS
central nervous system
- ICH
intracerebral hemorrhage
- MDMs
monocyte-derived macrophages
- MHCII
major histocompatibility complex class II
Footnotes
Disclosures
none
References
- 1.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science. 2012;336:86–90 [DOI] [PubMed] [Google Scholar]
- 2.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566 [DOI] [PubMed] [Google Scholar]
- 3.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng J, Ruedl C, Karjalainen K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity. 2015;43:382–393 [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, Wang J, Zhao L, Liang YX, Wu T, et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci. 2018;21:530–540 [DOI] [PubMed] [Google Scholar]
- 6.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger eae progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149 [DOI] [PubMed] [Google Scholar]
- 7.Prinz M, Priller J. The role of peripheral immune cells in the cns in steady state and disease. Nat Neurosci. 2017;20:136–144 [DOI] [PubMed] [Google Scholar]
- 8.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique tgf-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, et al. New tools for studying microglia in the mouse and human cns. Proc Natl Acad Sci U S A. 2016;113:E1738–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronk JC, Filiano AJ, Louveau A, Marin I, Marsh R, Ji E, Goldman DH, Smirnov I, Geraci N, Acton S, et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med. 2018;215:1627–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CF, Wan J, Li Q, Renfroe SC, Heller NM, Wang J. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol Dis. 2017;103:54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung JE, Sun G, Bautista Garrido J, Obertas L, Mobley AS, Ting SM, Zhao X, Aronowski J. The mitochondria-derived peptide humanin improves recovery from intracerebral hemorrhage: Implication of mitochondria transfer and microglia phenotype change. J Neurosci. 2020;40:2154–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Sun G, Ting SM, Song S, Zhang J, Edwards NJ, Aronowski J. Cleaning up after ich: The role of nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2015;133:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362 [DOI] [PubMed] [Google Scholar]
- 16.Chang CF, Goods BA, Askenase MH, Hammond MD, Renfroe SC, Steinschneider AF, Landreneau MJ, Ai Y, Beatty HE, da Costa LHA, et al. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest. 2018;128:607–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor RA, Chang CF, Goods BA, Hammond MD, Mac Grory B, Ai Y, Steinschneider AF, Renfroe SC, Askenase MH, McCullough LD, et al. Tgf-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest. 2017;127:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron. 2017;94:759–773 e758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronstein R, Torres L, Nissen JC, Tsirka SE. Culturing microglia from the neonatal and adult central nervous system. J Vis Exp. 2013:50647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, Kasner SE, McCullough LD, Sansing LH. Ccr2+ ly6c(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, et al. The trem2-apoe pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581 e569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, Shang Y, Oldham MC, Martens LH, Gao F, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class ii antigens. J Exp Med. 1992;176:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangaraju S, Raza SA, Li NX, Betarbet R, Dammer EB, Duong D, Lah JJ, Seyfried NT, Levey AI. Differential phagocytic properties of cd45(low) microglia and cd45(high) brain mononuclear phagocytes-activation and age-related effects. Front Immunol. 2018;9:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation. 2015;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill RA, Damisah EC, Chen F, Kwan AC, Grutzendler J. Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat Commun. 2017;8:15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn SJ, Anrather J, Nishimura N, Schaffer CB. Diverse inflammatory response after cerebral microbleeds includes coordinated microglial migration and proliferation. Stroke. 2018;49:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond TR, Marsh SE, Stevens B. Immune signaling in neurodegeneration. Immunity. 2019;50:955–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L, Zhang B, He X, et al. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via plexin-b2. Nat Neurosci. 2020;23:337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449 [DOI] [PubMed] [Google Scholar]
- 33.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct rna sequencing. Nat Neurosci. 2013;16:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592 [DOI] [PubMed] [Google Scholar]
- 36.Cardona AE, Huang D, Sasse ME, Ransohoff RM. Isolation of murine microglial cells for rna analysis or flow cytometry. Nat Protoc. 2006;1:1947–1951 [DOI] [PubMed] [Google Scholar]
- 37.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and b-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211 [DOI] [PubMed] [Google Scholar]
- 39.Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205 [DOI] [PubMed] [Google Scholar]
- 40.Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, Ostrowski R, Manaenko A, Tang J, Zhang JH. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mracsko E, Javidi E, Na SY, Kahn A, Liesz A, Veltkamp R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45:2107–2114 [DOI] [PubMed] [Google Scholar]
- 42.Zhong Q, Zhou K, Liang QL, Lin S, Wang YC, Xiong XY, Meng ZY, Zhao T, Zhu WY, Yang YR, et al. Interleukin-23 secreted by activated macrophages drives gammadeltat cell production of interleukin-17 to aggravate secondary injury after intracerebral hemorrhage. J Am Heart Assoc. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Liu W, Yuan J, Zhu H, Yang Y, Wen Z, Chen Y, Li L, Lin J, Feng H. T lymphocytes infiltration promotes blood-brain barrier injury after experimental intracerebral hemorrhage. Brain Res. 2017;1670:96–105 [DOI] [PubMed] [Google Scholar]
- 44.Mao LL, Yuan H, Wang WW, Wang YJ, Yang MF, Sun BL, Zhang ZY, Yang XY. Adoptive regulatory t-cell therapy attenuates perihematomal inflammation in a mouse model of experimental intracerebral hemorrhage. Cell Mol Neurobiol. 2017;37:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Z, Yu A, Liu Y, Shen H, Lin C, Lin L, Wang S, Yuan B. Regulatory t cells inhibit microglia activation and protect against inflammatory injury in intracerebral hemorrhage. Int Immunopharmacol. 2014;22:522–525 [DOI] [PubMed] [Google Scholar]
- 46.Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, Zhu WY, Liao MF, Wu LR, Yang YR, et al. Regulatory t cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the il-10/gsk3beta/pten axis. J Cereb Blood Flow Metab. 2017;37:967–979 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.