Abstract
We undertook a prospective matched cohort study of patients with Staphylococcus aureus bacteremia (SAB) and gram-negative bacteremia (GNB) to compare the characteristics, outcomes and chemokine and cytokine response in transplant recipients to immunocompetent, non-transplant recipients. Fifty-five transplant recipients (GNB n=29; SAB n=26) and 225 non-transplant recipients (GNB n=114; SAB n=111) were included for clinical analysis. Transplant GNB had a significantly lower incidence of septic shock than non-transplant GNB (10.3% vs 30.7%, p=0.03). Thirty-day mortality did not differ significantly between transplant and non-transplant recipients with GNB (10.3% vs 15.8%, p=0.57) or SAB (0.0% vs 11.7%, p=0.13). Next, transplant patients were matched 1:1 with non-transplant patients for the chemokine and cytokine analysis. Five cytokines and chemokines were significantly lower in transplant GNB vs non-transplant GNB: IL-2 (median [IQR]: 7.1pg/mL [7.1, 7.1] vs 32.6pg/mL [7.1, 88.0]; p=0.001), MIP-1β (30.7pg/mL [30.7, 30.7] vs 243.3pg/mL [30.7, 344.4]; p=0.001), IL-8 (32.0pg/mL [5.6, 53.1] vs 59.1pg/mL [39.2, 119.4]; p=0.003), IL-15 (12.0pg/mL [12.0, 12.0] vs 12.0pg/mL [12.0, 126.7]; p=0.03), and IFN-α (5.1pg/mL [5.1, 5.1] vs 5.1pg/mL [5.1, 26.3]; p=0.04). Regulated on Activation Normal T Cell Expressed and Secreted (RANTES) was higher in transplant SAB vs non-transplant SAB (mean [SD]: 750.2pg/mL [194.6] vs 656.5pg/mL [147.6]; p=0.046).
1.0. INTRODUCTION
Solid organ transplantation has revolutionized the prognosis of patients with end-stage organ disease. Long term immunosuppression is fundamental to graft survival, yet confers an increased risk of infection.1 Although bacteremia is a leading cause of death in patients who have undergone solid organ transplantation2, the epidemiology and risk factors for adverse outcomes of bacteremia in this population are incompletely understood. The potential impact of long-term therapeutic immunosuppression on host inflammatory response to bacteremia among transplant recipients is also poorly understood. Prior investigators have suggested that immunosuppression in patients with bacteremia should be temporarily reduced out of concern for increased risk for morbidity and mortality from sepsis,2,3 though this remains an area in need of research.
The host immune response to bacteremia involves a cascade of pro-and anti-inflammatory cytokines and chemokines. Specific cytokines are associated with gram-negative vs gram-positive bacterial infections, as well as with adverse outcomes.4–8 However, no study to date has reported cytokine or chemokine levels in solid organ transplant recipients with bacteremia or compared them to cytokine levels seen in bacteremic patients without organ transplantation. In the current investigation, we used a prospective, matched cohort design to define the epidemiology, outcomes and host inflammatory response to Staphylococcus aureus bacteremia (SAB) and gram-negative bacteremia (GNB) in patients with and without solid organ transplantation.
2.0. METHODS
2.1. Study Design and Sample
All eligible inpatients with either monomicrobial SAB or GNB from 2/8/2006 to 4/27/2015 at Duke University Medical Center and Duke Regional Hospital were prospectively enrolled in the Bloodstream Infections Biorepository (BSIB). Clinical data, microbiologic specimens and patient sera were secured for all enrolled patients. Patients from the BSIB were eligible for inclusion into the present study if they were bacteremic with S. aureus, Escherichia coli, or Klebsiella pneumoniae. The GNB population was restricted to patients with bacteremia due to E. coli and K. pneumoniae to minimize inflammatory response heterogeneity potentially introduced by multiple bloodstream pathogens. From this cohort, patients who were recipients of a solid organ transplant (lung, heart, kidney, liver, pancreas, or combination therein) were included for analysis. The control group was composed all immunocompetent non-transplanted patients who were bacteremic with the same organism and had requisite sera available for cytokine and chemokine analysis. Patients not meeting the definition of “immunocompetent” (defined below) were excluded.
For the chemokine and cytokine analysis, transplant BSIB patients were matched 1:1 with non-transplant BSIB patients. Subjects were matched on age, race, gender, bacteria species and methicillin resistance status (if SAB). Matching was performed by an investigator (F.R.) who was blinded to clinical outcome. In the event where there was more than one potential matched control for a given transplant subject, the matched control was selected at random.
This study was IRB approved. Patients (or their legally authorized representative) provided written informed consent. If a patient died prior to notification of blood culture results, the subjects were enrolled using an IRB-approved Notification of Decedent Research.
2.2. Definitions
Immunocompetent was defined as absence of any of the following comorbidities: 1) diabetes mellitus, 2) human immunodeficiency virus (HIV), 3) hemodialysis dependence, 4) active malignancy, and 5) receipt of immunosuppressive medication (i.e., corticosteroids greater than the equivalent of 10mg prednisone daily, anti-proliferative agents, biologics, monoclonal antibodies, calcineurin inhibitors, or mTOR inhibitors). Route of infection was classified as 1) hospital-acquired, 2) community-acquired, healthcare-associated, or 3) community-acquired, non-healthcare-associated, in accordance with prior definitions.9 Source of infection was defined as the primary focus of the bacteremia using the standardized CDC/NHSN criteria10 and was adjudicated independently by two independent reviewers (E.E. and M.D.) with differences resolved by consensus. Bacteremia with no single identifiable source was placed in the “unknown” category. Persistent GNB was defined as positive follow up blood culture with the same organism drawn at least 24 hours after initial culture.11 Persistent SAB was defined as positive follow-up blood culture with the same organism drawn ≥5 days after initial culture.12 Patients were considered to have metastatic infection if they developed any of the following: infective endocarditis, septic emboli, septic thrombophlebitis, vertebral osteomyelitis, septic arthritis, a metastatic abscess or other deep tissue abscess, as defined in Souli et al.12 Duration of symptoms was defined as the patient-reported time from onset of symptoms to date of first positive blood culture. Need for invasive procedure was defined as requiring a surgical intervention to control or treat the infection (i.e., joint washout, incision and drainage of an abscess, cardiac device removal, chest tube placement, or percutaneous nephrostomy tube placement). APACHE-II scores, including Acute Physiology Scores (APS), were calculated on the day of index positive blood culture.13 All-cause mortality was reported at 30 days and 90 days from date of first positive blood culture.
Acute kidney injury (AKI) was defined as serum creatinine reaching 1.5 times the baseline or increase by ≥ 0.3mg/dL within 48 hours of onset of bacteremia in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) guidelines.14 Septic shock was defined as sepsis with hypotension (systolic blood pressure ≤90 mm Hg) and perfusion abnormalities as previously described.15 Transplant recipients were defined as having a reduction in immunosuppression if the patient’s immunosuppressive medication was removed, dose-reduced (if an antimetabolite) or goal calcineurin inhibitor trough was reduced at any time during the hospitalization after the diagnosis of bacteremia was made.
2.3. Laboratory studies
Bacterial isolates were speciated by the Duke Clinical Microbiology Laboratory using standard techniques. Minimum inhibitory concentration (MIC) values were determined using the MicroScan Walkaway system (microbroth dilution method) as described previously. The MIC breakpoint values for each antibiotic were defined according to the most recent Clinical and Laboratory Standards Institute guidelines.16 Multidrug-resistant (MDR) phenotype was defined as non-susceptible to at least 1 antibiotic in ≥3 relevant antimicrobial categories.17
2.4. Cytokine and chemokine profiling
Acute phase serum samples were collected within 72h of index positive blood culture. Serum cytokines and chemokines were quantified using a 25-plex bead array assay (Invitrogen LHC0009M) performed according to the manufacturer’s recommended protocol and read using a Bio-Plex 200 reader (Bio-Rad). The included cytokines and chemokines were eotaxin, granulocyte-macrophage colony-stimulating factor 2 (GM-CSF), interferon alpha (IFN- α), interferon gamma (IFN- γ), interleukin 1 beta (IL-1 β), interleukin 1 receptor antagonist (IL-1RA), interleukin 10 (IL-10), interleukin 12 (IL-12), interleukin 13 (IL-13), interleukin 15 (IL-15), interleukin 17A (IL-17A), interleukin 2 (IL-2), interleukin 2 receptor (IL-2R), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 8 (IL-8), interferon-inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), monokine induced by gamma interferon (MIG), macrophage inflammatory protein 1 alpha (MIP-1α), macrophage inflammatory protein 1 beta (MIP-1 β), Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted (RANTES) and tumor necrosis factor alpha (TNF-α). Data were analyzed using Bio-Plex Manager software (Bio-Rad). Any analyte concentration below the lower limit of quantitation was replaced with one-half of the lower limit of quantitation.
2.5. Statistical methods
Results were summarized using mean with standard deviation (SD), median with interquartile range (IQR), or with frequency with percentage where appropriate. Reviewer agreement for source of infection was assessed by using the Cohen’s kappa test. To determine the effect of transplantation status on clinical characteristics and outcomes for GNB and SAB respectively, Wilcoxon rank sum test was used to analyze continuous variables and chi-square or Fisher’s exact test were used for categorical variables. Chi-square or Fisher’s exact tests were used to assess whether there was a relationship between the type of bacteremia and the induction therapy, and between immunosuppressive medication and related outcomes in the transplanted sample. The effect of transplantation status on each of the 25 cytokines and chemokines for GNB and SAB, respectively, were assessed using either the equal or unequal variance version of the Student’s t test or Wilcoxon rank sum test. For cytokines and chemokines that demonstrated a statistically significant relationship with transplantation status, multiple gamma regression models with a log link were assessed to determine the effect of transplantation status after accounting for the source of infection, immunosuppressive regimen, duration of symptoms and septic shock. To assess the relationship between transplantation status and 30-day mortality, a logistic regression model was postulated for GNB and SAB respectively after adjusting for duration of symptoms and source of infection. The threshold for assessing statistical significance was set at 2-sided alpha=0.05. Analyses were conducted using SAS 9.4 Statistical Software (SAS Institute Inc., Cary, NC).
3.0. RESULTS
3.1. Clinical characteristics of the study sample
A total of 2633 patients were enrolled in the BSIB during the study period. After applying the inclusion and exclusion criteria, 280 patients (10.6%) with bacteremia were identified for analysis [Figure 1]. Fifty-five of the patients were transplant recipients (GNB N=29, SAB N=26).
Figure 1: Study design.
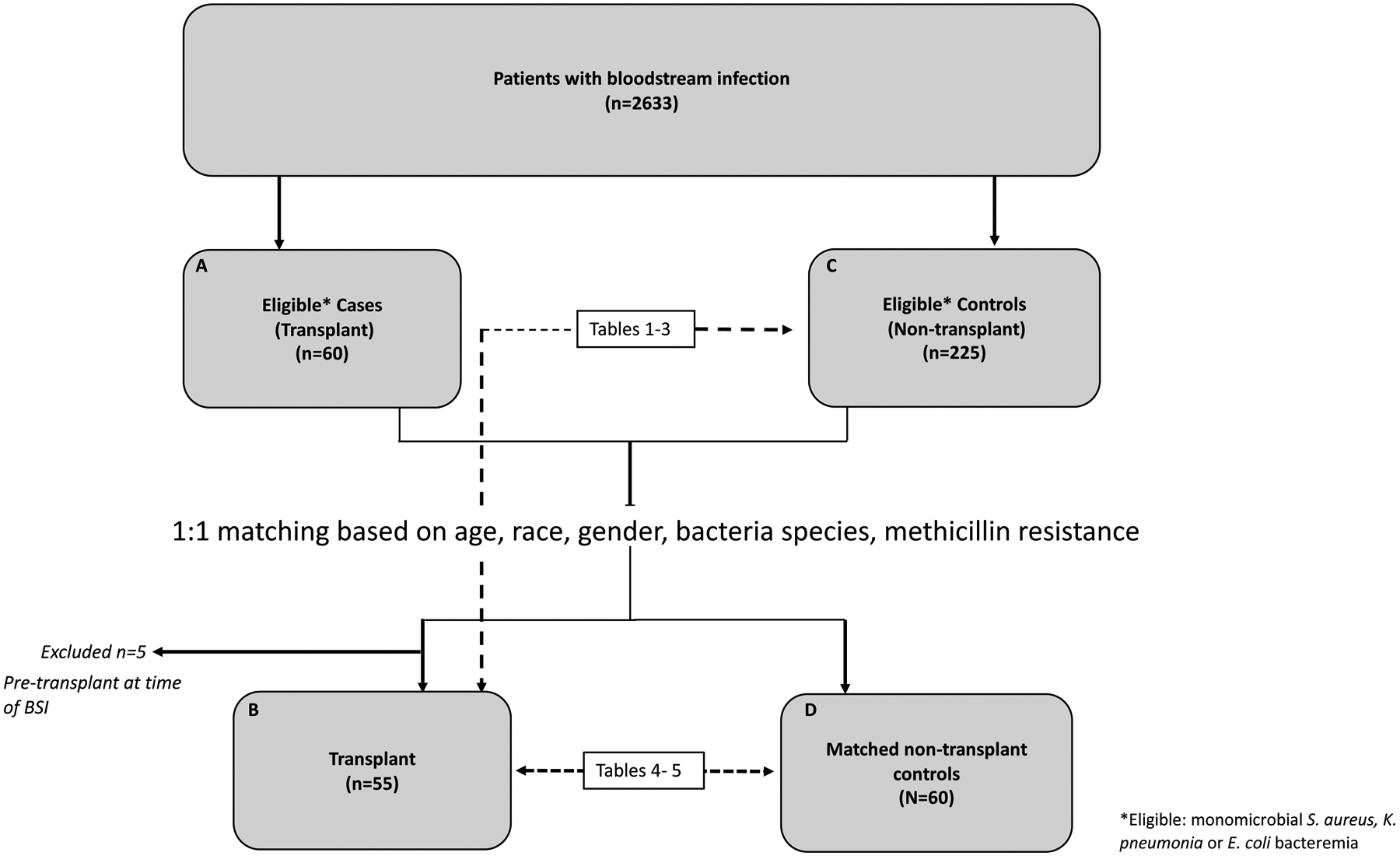
Clinical analysis was performed on the 55 transplant cases and 227 eligible controls (Boxes B and C). Corresponding tables for this comparison are tables 1–3. Chemokine and cytokine analysis was performed on the 55 transplant cases and 60 matched controls (Boxes B and D). Corresponding tables for this comparison are tables 4–5.
SAB, S. aureus bacteremia; GNB, gram-negative bacteremia
The demographic and clinical characteristics are reported in Table 1. Overall, route of infection differed significantly between transplant and non-transplant GNB (p<0.0001) and transplant and non-transplant SAB (p=0.003). None of the transplant recipients developed community-acquired, non-healthcare-associated bacteremia (GNB: 0% transplant vs. 37.7%, SAB: 0% transplant vs. 27.0% non-transplant). Among the GNB patients, GU source was the most common source in the transplant and non-transplant groups (51.7% and 45.6 % respectively), followed by GI (20.7% and 28.9%, respectively). Among SAB patients, source of infection significantly differed between transplant and non-transplant groups (p=0.0005). An endovascular source of SAB was more common in the transplant group as compared to the non-transplant group (transplant: 53.8%, non-transplant: 16.2%) whereas a skin/soft tissue source was the leading identifiable source of infection in the non-transplant group (transplant: 7.7%, non-transplant: 24.3%). Between the two independent reviewers, agreement for the source of infection was high for GNB (κ=0.99, p<0.0001) and for SAB (κ=0.95, P<0.0001). Duration of symptoms was significantly shorter for transplant SAB compared to non-transplant SAB (median [IQR] 1.0 days [1.0, 2.0] vs 4.0 [1.0, 7.0], p=0.0002). The APACHE II score was significantly higher in the bacteremic transplant compared to non-transplant groups: (median [IQR]: transplant GNB 15.0 [13.0, 16.0] vs non-transplant GNB 12.0 [9.0, 16.0], p=0.02; transplant SAB 15.5 [13.0, 20.0] vs non-transplant SAB 12.0 [9.0, 16.0], p=0.004). The Acute Physiology Score was significantly higher in the transplant SAB group compared to non-transplant SAB group (median [IQR]: 8.0 [5.0, 12.0] vs 5.0 [2.0, 9.0]; p=0.01).
Table 1:
Clinical characteristics of Transplant and Non-Transplant Recipients with Bacteremia.
| No. (%) of patients | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Transplant GNB N=29 | Non-Transplant GNB N=114 | P value | Transplant SAB N=26 | Non-Transplant SAB N=111 | P value |
| Age, Median (IQR) | 58.0 (42.0, 67.0) | 61.5 (50.0, 77.0) | 0.101 | 55.0 (45.0, 62.0) | 60.0 (46.0, 70.0) | 0.161 |
| Gender (Female) | 12 (41.4%) | 54 (47.4%) | 0.562 | 10 (38.5%) | 48 (43.2%) | 0.662 |
| Race (Black) | 8 (27.6%) | 30 (26.3%) | 0.892 | 11 (42.3%) | 24 (21.6%) | 0.032 |
| Organ Transplanted | ||||||
| Lung | 5 (17.2%) | -- | 9 (34.6%) | -- | ||
| Heart | 3 (10.3%) | -- | 4 (15.4%) | -- | ||
| Kidney | 15 (51.7%) | -- | 11 (42.3%) | -- | ||
| Liver | 3 (10.3%) | -- | 2 (7.7%) | -- | ||
| Lung & Kidney | 1 (3.4%) | -- | -- | |||
| Kidney & Pancreas | 1 (3.4%) | -- | -- | |||
| Liver & Kidney | 1 (3.4%) | -- | -- | |||
| Months from transplant to bacteremia, Median (IQR) | 62.9 (9.2, 226.3) | -- | -- | 125.9 (17.5, 271.6) | -- | -- |
| Route | <0.00013 | 0.0033 | ||||
| Hospital-acquired | 5 (17.2%) | 21 (18.4%) | 7 (26.9%) | 21 (18.9%) | ||
| Community-acquired, healthcare-associated | 24 (82.8%) | 50 (43.9%) | 19 (73.1%) | 60 (54.1%) | ||
| Community-acquired, non-healthcare-associated | 0 (0.0%) | 43 (37.7%) | 0 (0.0%) | 30 (27.0%) | ||
| Source | 0.813 | 0.00053 | ||||
| Unknown | 2 (6.9%) | 11 (9.6%) | 2 (7.7%) | 32 (28.1%) | ||
| Bone | 0 (0.0%) | 1 (0.9%) | 0 (0.0%) | 6 (5.4%) | ||
| Endovascular infection | 2 (6.9%) | 6 (5.3%) | 14 (53.8%) | 18 (16.2%) | ||
| CNS | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) | ||
| GI Infection | 6 (20.7%) | 33 (28.9%) | 1 (3.8%) | 3 (2.7%) | ||
| GU Infection | 15 (51.7%) | 52 (45.6%) | 0 (0.0%) | 2 (1.8%) | ||
| Respiratory/Lung | 3 (10.3%) | 6 (5.3%) | 7 (26.9%) | 13 (11.7%) | ||
| Skin/soft tissue | 1 (3.4%) | 2 (1.8%) | 2 (7.7%) | 27 (24.3%) | ||
| Joint | 0 (0.0%) | 3 (2.6%) | 0 (0.0%) | 10 (9.0%) | ||
| Duration of Symptoms (days) | 2.0 (1.0, 5.0) | 1.0 (1.0, 4.0) | 0.331 | 1.0 (1.0, 2.0) | 4.0 (1.0, 7.0) | 0.00021 |
| APACHE II, Median (IQR) | 15.0 (13.0, 16.0) | 12.0 (9.0, 16.0) | 0.021 | 15.5 (13.0, 20.0) | 12.0 (9.0, 16.0) | 0.0041 |
| APS, Median (IQR) | 7.0 (4.0, 9.0) | 6.0 (3.0, 10.0) | 0.931 | 8.0 (5.0, 12.0) | 5.0 (2.0, 9.0) | 0.011 |
| MDR Organism | 4 (13.8%) | 16 (14.0%) | 1.003 | |||
GNB, gram-negative bacteremia; SAB, S. aureus bacteremia; IQR, interquartile range; SD, standard deviation; CNS, central nervous system; GI, gastrointestinal; GU, genitourinary; APS, Acute Physiology Score; MDR, multidrug resistant.
Wilcoxon;
Chi-square;
Fisher Exact
3.2. Clinical outcomes of the study sample
Clinical outcomes are reported in Table 2. For GNB, the rate of septic shock was lower in the transplant group compared to the non-transplant group (10.3% vs 30.7%, p=0.03). Hemodialysis-dependent patients were excluded from the non-transplant population, but were eligible for inclusion in the transplant population. Hence, among non-hemodialysis-dependent patients, the rate of AKI in patients with SAB was significantly higher among transplant recipients than non-transplant recipients (transplant SAB 73.3% vs non-transplant SAB 31.5%, p=0.002). The incidence of AKI among bacteremic patients did not significantly differ by type of organ transplanted [Supplemental Table 1]. There were no observed differences in mortality at 30 days or 90 days between transplant and non-transplant recipients with either GNB or SAB respectively. After adjusting for duration of symptoms and source of infection, we did not find evidence of a significant relationship between transplant status and 30-day mortality among patients with GNB [Table 3]. A similar model could not be evaluated for SAB because all 13 of the SAB patients that died within 30 days were non-transplant recipients. All patients were rapidly initiated on adequate empiric antimicrobial therapy due to the institutional practice of commencing vancomycin and a broad-spectrum beta-lactam at the time of suspected bloodstream infection. Additionally, all infected or suspected infected central intravenous catheters were removed in accordance with standard institutional practice. Twelve (41.3%) transplant recipients with GNB and 8 (30.8%) with SAB underwent reduction of immunosuppression after diagnosis of bloodstream infection.
Table 2:
Outcomes of Transplant and Non-Transplant Recipients with Bacteremia.
| No. (%) of Patients | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Transplant GNB N=29 | Non-Transplant GNB N=114 | P value | Transplant SAB N=26 | Non-Transplant SAB N=111 | P value |
| Septic shock | 3 (10.3%) | 35 (30.7%) | 0.031 | 3 (11.5%) | 14 (12.6%) | 0.881 |
| Disseminated intravascular coagulation | 0 (0.0%) | 3 (2.6%) | 1.002 | 1 (3.8%) | 0 (0.0%) | 0.192 |
| Acute lung injury / acute respiratory distress | 1 (3.4%) | 10 (8.8%) | 0.462 | 1 (3.8%) | 9 (8.1%) | 0.692 |
| Acute Kidney Injury | 16 (64.0%) | 58 (50.9%) | 0.231 | 11 (73.3%) | 35 (31.5%) | 0.0021 |
| Metastatic infection | -- | -- | -- | 10 (38.5%) | 60 (54.1%) | 0.192 |
| Infective endocarditis | -- | -- | -- | 5 (19.2%) | 17 (15.3%) | 0.572 |
| Persistent bacteremia | 3 (10.3%) | 10 (8.8%) | 0.732 | 6 (23.1%) | 47 (42.3%) | 0.082 |
| Clostridium difficile colitis within 1-year post bacteremia | 1 (3.4%) | 10 (8.8%) | 0.462 | 4 (15.4%) | 6 (5.4%) | 0.102 |
| Reduction in Immunosuppression | 12 (41.4%) | -- | 8 (30.8%) | -- | ||
| Length of stay (days), median (IQR) | 9.0 (6.0, 12.0) | 7.0 (4.0, 13.0) | 0.123 | 10.5 (7.0, 25.0) | 14.0 (8.0, 25.0) | 0.473 |
| 30-day all-cause mortality | 3 (10.3%) | 18 (15.8%) | 0.572 | 0 (0.0%) | 13 (11.7%) | 0.132 |
| 90-day all-cause mortality | 3 (10.3%) | 24 (21.1%) | 0.292 | 3 (11.5%) | 21 (18.9%) | 0.572 |
GNB, gram-negative bacteremia; SAB, S. aureus bacteremia; IQR, interquartile range
11 of the 16 transplant GNB were kidney transplant recipients.
3 of the 11 transplant SAB were kidney transplant recipients.
Chi-square;
Fisher Exact;
Wilcoxon
Table 3:
Odds Ratios and 95% Confidence Intervals for the relationship between transplantation status and 30-day mortality in GNB after adjusting for relevant confounders
| Logistic regression model showing the relationship between transplantation status and 30-day mortality after adjusting for relevant confounders for GNB (N=141) | ||
|---|---|---|
| Variable | OR (95% CI) | P-Value |
| Transplantation Status (Reference = No Transplant) | 0.61 (0.16, 2.32) | 0.47 |
| Duration of Symptoms (Reference = Less than 2 days) | 0.65 (0.24, 1.75) | 0.39 |
| Source of Infection (Reference = Unknown) | ||
| Source of Infection: GI | 0.28 (0.05, 1.64) | 0.16 |
| Source of Infection: GU | 0.47 (0.10, 2.09) | 0.32 |
| Source of Infection: Other | 1.311 (0.26, 6.60) | 0.74 |
GNB, gram-negative bacteremia, CI, confidence interval; GI, gastrointestinal; GU, genitourinary
3.3. Inflammatory cytokine and chemokine levels in acute phase sera
Next, we compared levels of inflammatory cytokines in the acute phase sera of patients with and without organ transplantation who were bacteremic with the same pathogen (e.g., S. aureus or GNB) [Table 4]. Among patients with GNB, transplant recipients exhibited significantly lower levels of IL-2 (median [IQR]: 7.1 pg/mL [7.1, 7.1] vs 32.6 pg/mL [7.1, 88.0]; p=0.001), MIP-1β (30.7 pg/mL [30.7, 30.7] vs 243.3 pg/mL [30.7, 344.4]; p=0.001, IL-8 (32.0 pg/mL [5.6, 53.1] vs 59.1 pg/mL [39.2, 119.4]; p=0.003), IL-15 (12.0 pg/mL [12.0, 12.0] vs 12.0 pg/mL [12.0, 126.7]; p=0.03), and IFN-α (5.1 pg/mL [5.1, 5.1] vs 5.1 pg/mL [5.1, 26.3]; p=0.04) [Figure 2]. Among patients with SAB, transplant recipients exhibited significantly higher levels of RANTES (mean [SD]: 750.2 pg/mL [194.6] vs 656.5 pg/mL [147.6]; p=0.046). After adjusting for source of infection, immunosuppression class, duration of symptoms and septic shock, IL-2 in GNB was the only cytokine where the statistically significant relationship between transplantation status and cytokine concentration remained significant [Table 5].
Table 4:
Cytokine Concentration by Transplant Status
| Cytokine Concentration (by Transplant Status) | ||||||
|---|---|---|---|---|---|---|
| Transplant GNB (N=29) | Non- Transplant GNB (N=30) | p value | Transplant SAB (N=26) | Non-Transplant GNB (N=30) | p value | |
| Eotaxin, Median (IQR) | 34.9 (23.4, 39.9) | 37.8 (24.5, 44.0) | 0.511 | 52.7 (32.5, 87.4) | 54.9 (39.9, 89.4) | 0.851 |
| IFN-α, Median (IQR) | 5.1 (5.1, 5.1) | 5.1 (5.1, 26.3) | 0.041 | 5.1 (5.1, 36.4) | 5.1 (5.1, 24.0) | 0.381 |
| IL-1RA, Median (IQR) | 331.3 (194.4, 699.8) | 566.1 (365.4, 1826.0) | 0.061 | 842.2 (411.9, 1712.8) | 748.7 (509.9, 1942.2) | 0.791 |
| IL-10, Median (IQR) | 6.0 (6.0, 6.0) | 6.0 (6.0, 6.0) | 0.671 | 6.0 (6.0, 34.2) | 6.0 (6.0, 33.3) | 0.801 |
| IL-12, Median (IQR) | 81.6 (47.3, 134.4) | 68.6 (34.3, 103.2) | 0.401 | 84.8 (40.6, 235.6) | 138.3 (75.0, 333.0) | 0.191 |
| IL-15, Median (IQR) | 12.0 (12.0, 12.0) | 12.0 (12.0, 126.7) | 0.031 | 11.3 (11.3, 92.4) | 11.3 (11.3, 92.4) | 0.911 |
| IL-2, Median (IQR) | 7.1 (7.1, 7.1) | 32.6 (7.1, 88.0) | 0.0011 | 6.9 (6.9, 95.9) | 28.6 (6.9, 98.8) | 0.861 |
| IL-2R, Median (IQR) | 74.6 (28.5, 189.1) | 134.3 (28.5, 298.4) | 0.461 | 266.9 (120.8, 467.4) | 221.1 (28.4, 330.6) | 0.481 |
| IL-6, Median (IQR) | 25.9 (3.5, 86.5) | 38.7 (15.8, 97.2) | 0.211 | 56.2 (15.6, 97.7) | 72.7 (34.0, 102.3) | 0.281 |
| IL-8, Median (IQR) | 32.0 (5.6, 53.1) | 59.1 (39.2, 119.4) | 0.0031 | 84.4 (38.7, 114.8) | 103.0 (62.5, 254.4) | 0.091 |
| IP-10, Median (IQR) | 6.8 (4.2, 12.7) | 6.2 (3.8, 11.9) | 0.741 | 6.9 (4.3, 16.2) | 10.1 (3.9, 17.1) | 0.801 |
| MCP-1, Median (IQR) | 496.5 (291.1, 728.4) | 448.6 (355.8, 807.7) | 0.841 | 736.5 (412.7, 1142.8) | 912.7 (469.2, 1458.9) | 0.511 |
| MIG, Median (IQR) | 82.7 (65.2, 226.0) | 75.9 (13.1, 139.1) | 0.431 | 204.6 (72.0, 357.1) | 149.1 (94.0, 211.7) | 0.361 |
| MIP-1α, Median (IQR) | 8.9 (8.9, 8.9) | 8.9 (8.9, 8.9) | 0.211 | 8.8 (8.8, 49.1) | 8.8 (8.8, 44.8) | 0.861 |
| MIP-1β, Median (IQR) | 30.7 (30.7, 30.7) | 243.3 (30.7, 344.4) | 0.0011 | 193.6 (88.0, 362.7) | 234.3 (131.0, 444.8) | 0.291 |
| RANTES, Mean (SD) | 544.8 (106.5) | 523.4 (137.3) | 0.512 | 750.2 (194.6) | 656.5 (147.6) | 0.0462 |
| GM-CSF, Median (IQR) | 3.5 (3.5, 3.5) | 3.5 (3.5, 3.5) | 0.621 | 3.5 (3.5, 3.5) | 3.5 (3.5, 3.5) | 0.321 |
| IFN-γ, Median (IQR) | 3.0 (3.0, 3.0) | 3.0 (3.0, 3.0) | 0.341 | 3.0 (3.0, 3.0) | 3.0 (3.0, 3.0) | 0.381 |
| IL-1β, Median (IQR) | 6.1 (6.1, 6.1) | 6.1 (6.1, 6.1) | 0.331 | 6.0 (6.0, 6.0) | 6.0 (6.0, 6.0) | 0.291 |
| IL-13, Median (IQR) | 7.2 (7.2, 7.2) | 7.2 (7.2, 7.2) | .2 | 6.9 (6.9, 6.9) | 6.9 (6.9, 6.9) | 0.921 |
| IL-17A, Median (IQR) | 14.9 (14.9, 14.9) | 14.9 (14.9, 14.9) | .2 | 14.9 (14.9, 14.9) | 14.9 (14.9, 14.9) | 0.301 |
| IL-4, Median (IQR) | 31.4 (31.4, 31.4) | 31.4 (31.4, 31.4) | 0.331 | 30.6 (30.6, 30.6) | 30.6 (30.6, 30.6) | 0.731 |
| IL-5, Median (IQR) | 5.1 (5.1, 5.1) | 5.1 (5.1, 5.1) | .2 | 5.0 (5.0, 5.0) | 5.0 (5.0, 5.0) | .2 |
| IL-7, Median (IQR) | 9.4 (9.4, 9.4) | 9.4 (9.4, 9.4) | 0.321 | 9.0 (9.0, 9.0) | 9.0 (9.0, 9.0) | 0.291 |
| TNF-α, Median (IQR) | 2.9 (2.9, 2.9) | 2.9 (2.9, 2.9) | 0.331 | 2.8 (2.8, 2.8) | 2.8 (2.8, 2.8) | 0.921 |
GNB, gram-negative bacteremia; SAB, S. aureus bacteremia;
Wilcoxon
Equal Variance T-Test
Figure 2A-F: Cytokine and chemokine levels differ by transplant status in patients with gram-negative bacteremia and S. aureus bacteremia.
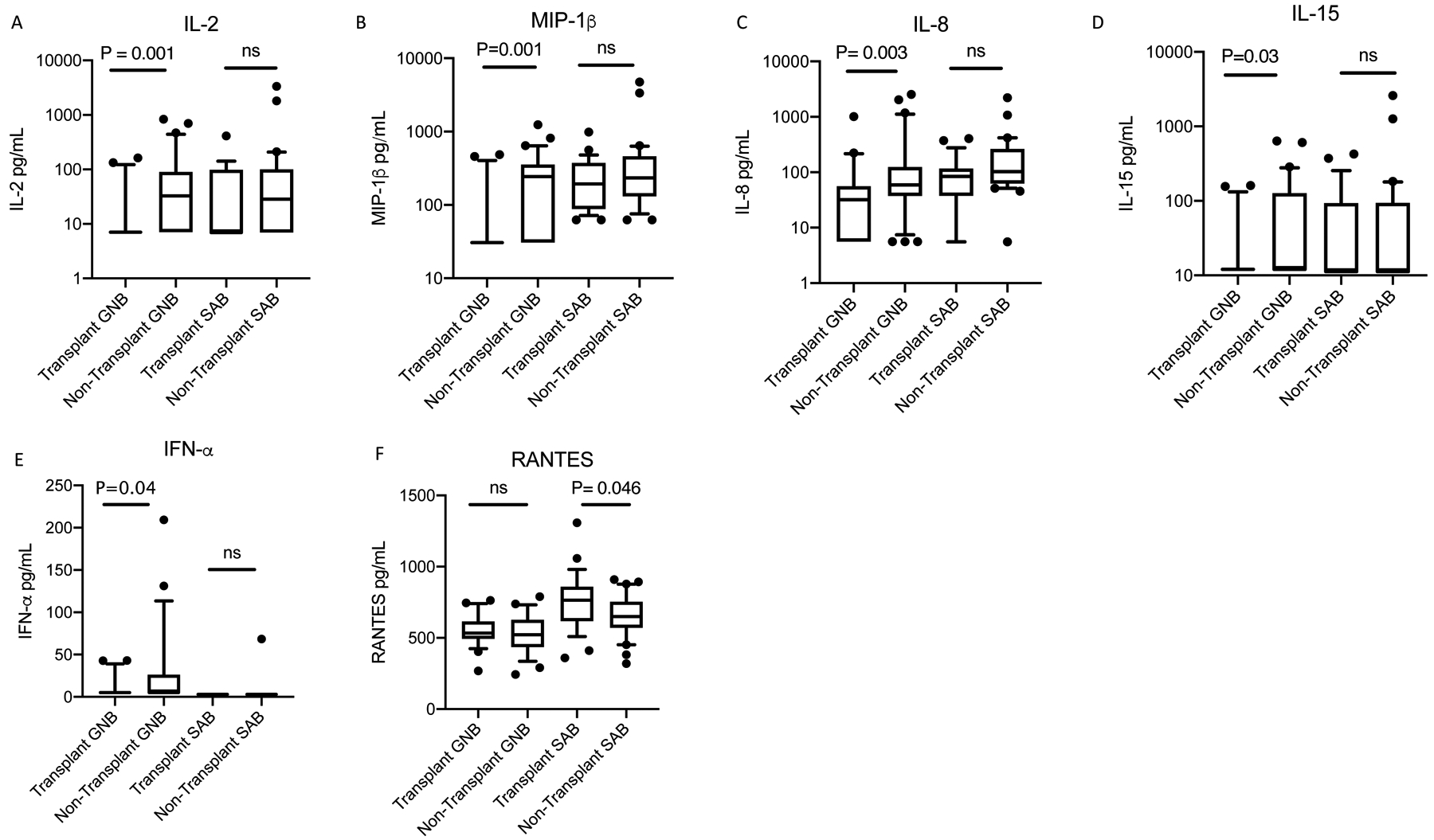
GNB, gram-negative bacteremia; SAB, S. aureus bacteremia; ns, not significant. Box plot extends from 25th to 75th percentile. The line in middle of box denotes median. Whiskers extend to 10th and 90th percentile. P-values were determined using Wilcoxon Rank Sum tests. Analyte concentrations below the lower limit of quantitation were replaced with one-half of the lower limit of quantitation. Thus when multiple patients had cytokine levels below the limit of detection, the 1st and 3rd quartiles were identical to the median.
Table 5:
Gamma Regression (with log link) parameter estimates and 95% confidence intervals when modeling transplantation status on IL-2 GNB cytokine concentration
| Gamma Regression (with log link) parameter estimates and 95% confidence intervals when modeling transplantation status on IL-2 GNB cytokine concentration (N=59) | ||
|---|---|---|
| Covariates | Estimate (95% CI) | P-value |
| Intercept | 4.71 (3.86, 5.56) | <0.0001 |
| Transplantation Status | −3.94 (−6.91, −0.97) | 0.009 |
| Source of Infection (Reference = GU) | Reference | |
| GU | ||
| Endovascular Infection | −1.82 (−3.30, −0.33) | 0.02 |
| GI | 0.39 (−0.38, 1.16) | 0.32 |
| Joint | −2.18 (−4.45, 0.09) | 0.06 |
| Respiratory/Lung | 1.00 (−0.42, 2.41) | 0.17 |
| Skin/soft tissue | −0.56 (−2.85, 1.73) | 0.63 |
| Unknown | 0.22 (−0.87, 1.33) | 0.69 |
| Immunosuppressive Regimen (Reference = None) | Reference | |
| None | ||
| Monotherapy (steroid) | 1.18 (−0.68, 3.05) | 0.21 |
| CNI + steroid or CNI + antimetabolite | 2.74 (−0.76, 6.23) | 0.12 |
| CNI + mTOR inhibitor + steroid | 1.69 (−1.48, 4.87) | 0.30 |
| CNI + antimetabolite + steroid | 2.32 (−0.64, 5.28) | 0.12 |
| Duration of Symptoms (48 hours or more) (Reference: < 48 hours) | −0.57 (−1.28, 0.13) | 0.11 |
| Septic Shock | −0.82 (−1.69, 0.05) | 0.07 |
GNB, gram-negative bacteremia; CI, confidence interval; GU, genitourinary; GI, gastrointestinal; CNI, calcineurin inhibitor.
Cytokine concentrations were also compared between transplant recipients who did and did not undergo reduction of immunosuppression [Supplemental Table 2]. Among transplant recipients with GNB, patients with immunosuppression reduction had significantly higher levels of MIG (median [IQR]: 185.0 pg/mL [86.5, 319.4] vs 71.8 [63.8, 88.0]; p=0.04). Among transplant patients with SAB, patients with immunosuppression reduction had significantly lower levels of Eotaxin (mean [SD]: 47.4 [18.8] vs. 86.8 [64.5]; p=0.04). There were no differences in the levels of measured cytokines or chemokines between patients with bacteremia due to K. pneumoniae vs. E. coli (data not shown).
4.0. DISCUSSION
Our study challenges the long-held assumption that the pharmacologic immunosuppression of transplant recipients contributes to a worse overall outcome in the setting of bacteremia. In a carefully matched prospective, cohort study design that addressed multiple clinical confounders, we found that patients with and without organ transplantation who developed bacteremia did not experience significant differences in the rate of mortality, and that transplant recipients with GNB experienced a significantly lower incidence of septic shock than non-transplant recipients.
We found no significant difference in mortality between bacteremic patients with and without solid organ transplantation for the GNB and SAB groups, respectively. Specifically, after adjusting for relevant confounders, we did not find evidence of a significant change in odds of 30-day mortality based on transplant status. While our sample was underpowered to detect such differences in mortality, our results generally agree with two recent studies of bacteremia in solid organ transplant recipients which found lower rates of 30-day mortality among transplant recipients as compared to non-transplant recipients.18,19 There are two possible explanations for these results. First, it is possible that transplant recipients who develop bacteremia experience shorter time to symptom recognition, medical evaluation and empiric antibiotic therapy due to close contact with their transplant team and the healthcare system. Indeed, the transplant recipients with SAB had a significantly shorter duration of symptoms compared to non-transplant recipients. Second, it is possible that the immunosuppressants taken by transplant recipients may attenuate the paradoxically deleterious effects of exuberant inflammatory responses that can occur during sepsis, a concept proposed by Kalil et al.19 This latter hypothesis is supported by two of our results: (1) that transplant GNB group experienced a significantly lower incidence of septic shock as compared to the non-transplant GNB group, and (2) our preliminary cytokine and chemokine results.
To our knowledge, this is the first report to describe cytokine and chemokine levels in bacteremic solid organ transplant recipients. Importantly, we found that pro-inflammatory cytokines IL-2, IL-8, IL-15, IFN-α and the monocyte chemoattractant MIP-1β were significantly lower in transplant vs non-transplant patients with GNB. We suspect that immunosuppressive medication used in the transplant group is at least in part responsible for the lower marker levels in the transplant GNB group. After adjusting for source of infection, immunosuppressive class, duration of symptoms and presence or absence of septic shock, a statistically significant relationship remained between transplant status and IL-2 concentration. This suggests that during GNB, transplant recipients appear to exhibit a lower pro-inflammatory state than non-transplant recipients.
None of the aforementioned cytokine and chemokine levels differed significantly based on transplant status in patients with SAB, likely reflecting fundamental differences in the immunopathogenesis of SAB and GNB.20 RANTES was the only cytokine that was significantly different between transplant and non-transplant recipients with SAB, and was in fact higher in the transplant recipients than non-transplant recipients. After adjusting for source of infection, immunosuppressive regimen, septic shock and duration of symptoms, this difference was no longer significant. RANTES is a pro-inflammatory chemokine that is known to be up-regulated in response to S. aureus.21 It is induced primarily by platelets, fibroblasts, CD8+ T cells and epithelial cells, and functions as a chemotactic agent to recruit leukocytes to the site of infection.22 Our results suggest that despite immunosuppression, transplant recipients are capable of producing a robust pro-inflammatory response to SAB, akin to immunocompetent, non-transplant recipients.
The role of immunosuppression reduction during bloodstream infection is unknown.23 Some investigators have suggested modification of the patient’s immunosuppressive regimen (e.g., withholding the antimetabolite agent or reducing goal calcineurin inhibitor troughs) on the basis of a theoretical improvement in host immune response.2,23–25 However, this practice may paradoxically increase risk of harm, as reductions in immunosuppressants can increase the risk of allograft rejection and graft failure.26 In the current study, a total of 20 transplant recipients underwent a reduction in immunosuppression due to bacteremia, however we were unable to conclude how this affected outcomes. Further studies need to evaluate whether immunosuppression in transplant recipients carries an increased risk of mortality during bacteremia.
While mortality was not significantly different in transplant and non-transplant recipients with bacteremia, the epidemiology of these infections differed substantially among the two populations. All of the transplant recipients developed healthcare-associated bacteremia while significantly fewer non-transplant recipients developed healthcare-associated bacteremia. This difference likely reflects the ongoing contact that transplant recipients have with the healthcare system. Additionally, the exclusion of hemodialysis-dependent patients from the non-transplant cohort may account for part of this difference. Nevertheless, our findings highlight that transplant patients with indwelling central catheters are a particularly vulnerable population for SAB and underscores the importance of removing intravascular catheters whenever possible.
Transplant recipients with SAB developed AKI significantly more frequently than matched patients without transplantation. This trend was seen among those receiving known nephrotoxic immunosuppressants such as calcineurin inhibitors as well as patients receiving calcineurin inhibitor sparing immunosuppressive regimens. Additionally, AKI did not occur with significantly greater incidence in kidney transplant recipients as compared to non-kidney transplant recipients and patients that did not undergo transplantation. While the reason for the higher rate of AKI among transplant recipients is unknown, a possible mechanism is sepsis-leading to acute tubular necrosis. If our finding is validated, future studies should evaluate the utility of AKI as a biomarker for severity of sepsis as compared to markers such as SIRS criteria which rely on factors that may be impaired in immunocompromised patients, including fever and leukemoid response1,27.
Our study used a large, unique biorepository from clinically well-characterized patients with bacteremia and matched transplant and non-transplant patients by age, race, gender, and bacterial species. Nonetheless, it had limitations. The overall sample size and the number of each type of organ transplant was small, limiting study power. Biological specimens were collected at a single acute phase time point instead of longitudinally over the course of the infection. It is possible that there is variability of cytokine concentrations within this initial 72-hour time interval, and that timing of antimicrobial therapy may influence concentrations. Additionally, we were unable to assess when reduction in immunosuppression occurred relative to biological specimen collection, and thus unable to determine whether reduction in immunosuppression affected chemokine or cytokine levels and patient outcome. The generalizability of the findings may be limited to patients with bacteremia due to S. aureus, E. coli, and K. pneumoniae. Finally, because this was a hypothesis-generating study, we did not apply multiple testing corrections and these inference-based analyses should be completed in a larger, external validation dataset. It is important to note that the large number of analyses performed increases the risk of false positive findings.
5.0. CONCLUSIONS
We present the first study to examine cytokine and chemokine levels in solid organ transplant recipients with bacteremia. Our results challenge the long-held belief that transplant status portends poorer outcomes in SAB and GNB. We provide evidence that mortality may not be linked to immunosuppression, and that transplant recipients experience a lower incidence of septic shock than non-transplant recipients during GNB. Larger studies are needed to validate our findings, to further elucidate the role of immunosuppression on host immune response to bacteremia in transplant recipients, and to determine how we may use that information to further improve outcomes of sepsis in transplant recipients.
Supplementary Material
ACKNOWLEDGEMENTS
Work contained in this manuscript were made possible by the following grants from the National Institutes of Health (K24-AI093969 [VGF]; R01-AIO68804 [VGF]; 1KL2TR002554 [JTT]; 1KL2TR002554 [SAM]) and NIAID (T32 -AI100851 [EME]). Biomarker profiling was performed under the management of Dr. Andrew N. Macintyre and direction of Dr. Gregory D. Sempowski in the Immunology Unit of the Duke Regional Biocontainment Laboratory (RBL), which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607). The Duke BERD Methods Core’s support for this project was made possible (in part) by Grant Number UL1TR002553 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Abbreviations:
- AKI
Acute Kidney Injury
- APS
Acute Physiology Score
- BSIB
Bloodstream Infection Biorepository
- CMV
Cytomegalovirus
- GM-CSF
Granulocyte-Macrophage Colony-Stimulating Factor 2
- IFN-α
Interferon alpha
- IFN- γ
Interferon gamma
- IL-1β
Interleukin 1 beta
- IL-1RA
interleukin 1 receptor antagonist
- IL-2
Interleukin 2
- IL-2R
Interleukin 2 Receptor
- IL-4
Interleukin 4
- IL-5
Interleukin 5
- IL-6
Interleukin 6
- IL-7
Interleukin 7
- IL-8
Interleukin 8
- IL-10
Interleukin 10
- IL-12
Interleukin 12
- IL-13
Interleukin 13
- IL-15
Interleukin 15
- IL-17A
Interleukin 17A
- IQR
Interquartile Range
- IP-10
interferon-inducible protein 10
- KDIGO
Kidney Disease: Improving Global outcomes
- SAB
Staphylococcus aureus bacteremia
- GI
Gastrointestinal
- GNB
Gram-negative bacteremia
- GU
Genitourinary
- HIV
Human Immunodeficiency Virus
- MIP-1 β
Macrophage inflammatory protein 1 beta
- MDR
Multi-Drug Resistant
- MIC
Minimum Inhibitory Concentration
- MIG
Monokine induced by gamma interferon
- MIP-1α
Macrophage inflammatory protein 1 alpha
- MCP-1
monocyte chemoattractant protein 1
- RANTES
Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted
- SD
Standard Deviation
- TNF-α
Tumor necrosis factor alpha
Footnotes
DISCLOSURES
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. VGF reports personal fees from Novartis, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny, Amphliphi Biosciences. Integrated Biotherapeutics; C3J, grants from NIH, MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck; Medical Biosurfaces; Locus; Affinergy; Contrafect; Karius; Genentech, Regeneron, Basilea, Janssen, from Green Cross, Cubist, Cerexa, Durata, Theravance; Debiopharm, Royalties from UpToDate; and a patent sepsis diagnostics pending.
SUPPORTING INFORMATION:
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. [DOI] [PubMed] [Google Scholar]
- 2.Kalil AC, Dakroub H, Freifeld AG. Sepsis and solid organ transplantation. Curr Drug Targets. 2007;8(4):533–541. [DOI] [PubMed] [Google Scholar]
- 3.Guenette A, Husain S. Infectious Complications Following Solid Organ Transplantation. Crit Care Clin. 2019;35(1):151–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe R, Oda S, Sadahiro T, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care. 2010;14(2):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206(10):1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minejima E, Bensman J, She RC, et al. A Dysregulated Balance of Proinflammatory and Anti-Inflammatory Host Cytokine Response Early During Therapy Predicts Persistence and Mortality in Staphylococcus aureus Bacteremia. Crit Care Med. 2016;44(4):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Guimaraes AO, Peck MC, et al. Risk stratification biomarkers for Staphylococcus aureus bacteraemia. Clin Transl Immunology. 2020;9(2):e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guimaraes AO, Cao Y, Hong K, et al. A Prognostic Model of Persistent Bacteremia and Mortality in Complicated Staphylococcus aureus Bloodstream Infection. Clin Infect Dis. 2019;68(9):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman ND, Kaye KS, Stout JE, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. [DOI] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. [DOI] [PubMed] [Google Scholar]
- 11.Maskarinec SA, Park LP, Ruffin F, et al. Positive follow-up blood cultures identify high mortality risk among patients with gram negative bacteremia. Clin Microbiol Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souli M, Ruffin F, Choi SH, et al. Changing Characteristics of Staphylococcus aureus Bacteremia: Results From a 21-Year, Prospective, Longitudinal Study. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 16.CLSI. Performance standards for antimicrobial susceptibility testing. 27th ed. In: Patel JB, Weinstein MP, Eliopoulos GM, Jenkins SG, Lewis SJ, Limbago B, Mathers AJ, Mazzulli T, Patel R, Richter SS, Satlin M, Swenson JM, Traczewski MM, Turnidge JD, Zimmer BL, eds. CLSI Document M100. Wayne, Pennsylvania, USA: Clinical Laboratory Standards Institute; 2017. [Google Scholar]
- 17.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. [DOI] [PubMed] [Google Scholar]
- 18.Malinis MF, Mawhorter SD, Jain A, Shrestha NK, Avery RK, van Duin D. Staphylococcus aureus bacteremia in solid organ transplant recipients: evidence for improved survival when compared with nontransplant patients. Transplantation. 2012;93(10):1045–1050. [DOI] [PubMed] [Google Scholar]
- 19.Kalil AC, Syed A, Rupp ME, et al. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis. 2015;60(2):216–222. [DOI] [PubMed] [Google Scholar]
- 20.Hessle CC, Andersson B, Wold AE. Gram-positive and Gram-negative bacteria elicit different patterns of pro-inflammatory cytokines in human monocytes. Cytokine. 2005;30(6):311–318. [DOI] [PubMed] [Google Scholar]
- 21.Strindhall J, Lindgren PE, Löfgren S, Kihlström E. Clinical isolates of Staphylococcus aureus vary in ability to stimulate cytokine expression in human endothelial cells. Scand J Immunol. 2005;61(1):57–62. [DOI] [PubMed] [Google Scholar]
- 22.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22(2):83–87. [DOI] [PubMed] [Google Scholar]
- 23.Timsit JF, Sonneville R, Kalil AC, et al. Diagnostic and therapeutic approach to infectious diseases in solid organ transplant recipients. Intensive Care Med. 2019;45(5):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu GW, Ju MJ, Zheng YJ, et al. An interdisciplinary approach for renal transplant recipients with severe pneumonia: a single ICU experience. Intensive Care Med. 2014;40(6):914–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmaldienst S, Horl WH. Bacterial infections during immunosuppression - immunosuppressive agents interfere not only with immune response, but also with polymorphonuclear cell function. Nephrol Dial Transplant. 1996;11(7):1243–1245. [PubMed] [Google Scholar]
- 26.Bige N, Zafrani L, Lambert J, et al. Severe infections requiring intensive care unit admission in kidney transplant recipients: impact on graft outcome. Transpl Infect Dis. 2014;16(4):588–596. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer RG, Crabtree TD, Gleason TG, Antevil JL, Pruett TL. Impact of solid organ transplantation and immunosuppression on fever, leukocytosis, and physiologic response during bacterial and fungal infections. Clin Transplant. 1999;13(3):260–265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


