Abstract
Background:
Periodontal disease (PD) is known to be associated with endothelial dysfunction in patients with coronary artery and/or cardiovascular disease. In our study, we sought to explore the virulence of P. gingivalis (Pg) affecting glycogen synthase kinase 3 beta (GSK-3β)/nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/tetrahydrobiopterin (BH4)/ nitric oxide synthase (NOS) expression in primary human aortic endothelial cells (pHAECs).
Methods:
pHAECs were infected for 48 hours with Pg in vitro using the Human oxygen-Bacteria anaerobic coculture technique. Cell viability was determined, and target gene expression changes were evaluated by quantitative real-time polymerase chain reaction at the end of each incubation period.
Results:
Pg impaired pHAEC viability 24 hours post-infection. Pg infection reduced mRNA expression levels of endothelial NOS (eNOS), Nrf2, and Phase II enzymes (heme oxygenase-1, catalase, superoxide dismutase-1) in a time-dependent manner. Significant (P <0.05) increase in the inflammatory markers (interleukin [IL]-1β, IL-6, and tumor necrosis factor-α) were observed in the medium as well as in the infected cells. Interestingly, inducible NOS mRNA levels showed a significant (P <0.05) increase at 12 hours and 24 hours and were reduced at later time points. BH4 (cofactor of eNOS) biosynthesis enzyme dihydrofolate reductase (DHFR, salvage pathway) mRNA levels showed a significant (P <0.05) decrease, while mRNA levels of GSK-3β were elevated.
Conclusions:
These results suggest that periodontal bacterial infection may cause significant changes in the endothelial GSK-3β/BH4/eNOS/Nrf2 pathways, which may lead to impaired vascular relaxation. Greater understanding of the factors that adversely affect endothelial cell function could contribute to the development of new therapeutic compounds to treat PD-induced vascular diseases.
Keywords: GSK-3β, NOS, Nrf2, P. gingivalis, periodontal disease, primary human aortic endothelial cells
1 |. INTRODUCTION
Periodontal disease (PD) is a chronic inflammatory disease characterized by alveolar bone resorption. This chronic inflammation is caused by red complex bacteria, such as Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Treponema denticola (Td), and a bridge complex-forming bacterium, Fusobacterium nucleatum (Fn), in the subgingival sulcus.1 A previous study has depicted the potential ability of Pg invasion into gingival epithelial cells and spreading into the connective tissue.2 P. gingivalis is capable of gaining access to vascular circulation during routine oral activities, such as chewing, brushing, and flossing teeth, and during dental cleanings thus invasion of Pg may contribute to inflammation at sites distant from the oral cavity.3 In vivo results from orally infected mice have further confirmed the systemic dissemination of Pg within aortic wall endothelial cells.4 P. gingivalis has been shown to induce an acute inflammatory response marked by significantly higher Pg-specific immunoglobulin G, a feature linked with atherosclerosis in humans.4 Moreover, endothelial dysfunction and chronic vascular injury has been documented in mice infected with Pg.1,4 Endothelial dysfunction is an independent predictor of cardiovascular events and precedes the development of atherosclerosis and other cardiovascular diseases.5 The overall pathological process is the result of reduced bioavailability of nitric oxide (NO) inhibiting platelet aggregation, expressing adhesion molecules, and promoting arterial smooth muscle cell relaxation.6 Periodontitis-induced endothelial dysfunction occurs in association with substantial reduction in NO bioavailability.7 In addition, a potential correlation between salivary NO concentration and endothelial dysfunction in patients with PD has been reported.8,9 Pre-clinical rodent models of periodontitis further have confirmed that reduced NO levels are correlated with the onset of endothelial dysfunction.10,11
Normal vascular endothelial functions are regulated by endogenous NO synthesis that occurs as a result of enzymatic coupling of endothelial nitric oxide synthase (eNOS) using tetrahydrobiopterin (BH4) as a cofactor.12 Thus, NO is an important molecule that promotes vasodilatation and antisclerotic activity.13 We have reported that apolipoprotein E-null mice had diminished vascular and colon NO synthase (NOS) and serum NO levels with oral polybacterial infection, suggesting an adverse role of PD on NO synthesis.1 Importantly, reduced levels of NO were observed as a consequence of suppression of antioxidant machinery and tended to exacerbate cardiovascular pathologies.1 Further, reactive oxygen species (ROS) were shown to promote inflammation, providing evidence that oxidative stress impairs vascular homeostasis.10,14 A previous study demonstrates that ROS oxidizes BH4 to dihydrobiopterin (BH2), resulting in eNOS uncoupling and O2 as a byproduct rather than NO.15 Our previous work has shown that periodontal bacterial infection in mice results in lowered BH4 availability and reduced respective biosynthetic enzymes, such as guanosine triphosphate cyclohydrolase-1 (GTPCH-1, also known as GCH-1).1
Nrf2 is a transcriptional factor that protects the cells from oxidative stress by regulating the expression of Phase II antioxidant enzymes and in parallel with the NOS pathway, the vascular endothelium regulates homeostasis.1 Phosphorylated Nrf2 inhibits glycogen synthase kinase 3 beta (GSK-3β) activity, a serine-threonine protein kinase.16 Inversely, active GSK-3β phosphorylates Src family kinases, which subsequently phosphorylate the serine residues within the Neh6 domain of Nrf2.12 Our previous study indicates that mice infected with oral pathogens impede vascular Nrf2 activity, leading to reduced Phase II detoxifying enzymes.17 This in turn is postulated to impair the atheroprotective capacity of Nrf2 in endothelial cells.1 Endothelial dysfunction is a key step in the development of atherosclerosis. Although reports indicate that Pg impairs endothelial function, the mechanism involved still remains unclear.18 Here, we investigated whether in vitro infection with Pg using primary human aortic endothelial cells (pHAECs) modulates NOS/BH4/Nrf2/GSK-3β signaling pathways and release of proinflammatory cytokines that are known to be involved in endothelial homeostasis.
2 |. MATERIALS AND METHODS
2.1 |. Pre-culture of Pg
Pg was cultured as described previously.19 Frozen bacterial Pg (strain W83) stocks were grown anaerobically on Trypticase Soy Agar (TSA) blood agar plates (BAP) with 5% sheep blood at 37°C in a BACTRON-300 Anaerobic Chamber for 4 to 7 days. Using sterile loops, Pg was inoculated into 3 mL TSA broth supplemented with hemin and menadione, which allowed bacteria to grow until mid-log phase (OD600 of ≈ 0.8).
2.2 |. Pre-culture of pHAECs
pHAECs were cultured in vascular cell basal medium, supplemented with Endothelial Cell Growth Kit-VEGF, containing recombinant human (RH) vascular endothelial growth factor (VEGF), RH epidermal growth factor (EGF), RH fibroblast growth factor (FGF) basic, RH insulin-like growth factor-1, L-glutamine, heparin sulfate, hydrocortisone hemisuccinate, fetal bovine serum (2%), and ascorbic acid. Cells were cultured in a humidified incubator at 37°C in 5% CO2 and were seeded at ≈ 50% confluency in a 12-well plate containing coverslips. Confluent fourth-to sixth-passage cells were used in all experiments. All reagents were purchased from ATCC or Fisher Scientific.
2.3 |. Protocol for the Human oxygen-Bacteria anaerobic (HoxBan) coculture
The Human oxygen-Bacteria anaerobic (HoxBan) coculture method was performed as described with minor modifications.20 One milliliter of the overnight Pg (W83, 109 colony-forming units) pre-culture was used to inoculate 1,000 mL of freshly autoclaved and cooled-down (≈ 40°C) TSA containing 1% agar. Aliquots of 40 mL of this inoculum were transferred into sterile Falcon 50 mL Conical Centrifuge Tubes and allowed to solidify for 30 minutes. The Pg-inoculated Falcon Tube cultures were transferred to a tissue culture cabinet at ambient air. pHAECs cultured on coverslips (≈ 5,000 cells) were placed upside down on top of the agar and overlaid with 10 mL of pre-warmed (37°C) DMEM medium (without antibiotics). For respective controls (uninfected), pHAECs cultured on coverslips were placed upside down on top of the agar without Pg. Following this process, the coculture tubes were placed in a humidified incubator at 37°C and 5% CO2 and incubated at different time points (0 to 48 hours). The MOI was calculated based on the number of cells per well when seeded. Three separate experiments were performed, each performed in duplicate for each condition.
2.4 |. Trypan blue staining (viability assay)
Trypan blue solution (0.2%) was added for 1 minute to the pHAECs adherent to coverslips. After removing the trypan blue solution, the cells were fixed immediately with 4% paraformaldehyde (10 minutes at 4°C), rinsed four times with PBS, and mounted on glass slides. Trypan blue-negative (viable) cells were quantified microscopically.
2.5 |. Cytokine enzyme-linked immunosorbent assay (ELISA)
Cytokine concentrations in cell culture supernatants were quantified by sandwich ELISA using specific pairs of monoclonal antibodies against tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1α, and IL-1β. The assays were performed according to the manufacturer’s protocols.21 In brief, at the end of the coculture experiment for each time point, pHAECs adherent to coverslips were removed from the HoxBan coculture tubes, and the cell culture supernatants were collected. An aliquot of the supernatant was used for the assay. The concentrations of the cytokines were directly proportional to the color intensity of the samples, respectively. Absorbance was measured spectrophotometrically at 450 nm.
2.6 |. RNA isolation and quantitative real-time polymerase chain reaction analysis
At the end of the co-culture experiment, pHAECs adherent to coverslips were removed from co-culture tubes, and total RNA of cells was extracted with TRIzol. The quality of RNA was determined by NanoDrop. RNA was reverse-transcribed to cDNA using iScript cDNA Synthesis Kit. Quantitative real time-polymerase chain reaction assay was performed using SYBR Green. The sequences of primers for target genes are listed in Table 1. Relative expression levels of target genes were normalized to β-actin, and threshold cycle (Ct) numbers were calculated using 2−ΔΔCT method). All studies were performed in the Meharry Medical College Molecular Biology Core Laboratory.
TABLE 1.
Primers used for quantitative real-time polymerase chain reaction
| Gene | Forward | Reverse |
|---|---|---|
| IL 1α | 5ť-CCAGGCGTAGGTCTGGAGTCTCACTTGTCT-3ť | 5ť-TGTTGCGGCAGGAAGGCTTAGGTATTATTC-3ť |
| IL-1β | 5′-CTTTGAAGCTGATGGCCCTAAA-3′ | 5′-AGTGGTGGTCGGAGATTCGT-3′ |
| IL-6 | 5′-TCAATGAGGAGACTTGCCTG-3′ | 5′- GATGAGTTGTCATGTCCTGC-3′ |
| MCP-1 | 5′-GAAAGTCTCTGCCGCCCTT-3′ | 5′- TTGATTGCATCTGGCTGAGCG-3′ |
| TLR-4 | 5′-GATCTGTCTCATAATGGCTTG-3′ | 5′- GACAGATTCCGAATGCTTGTG-3′ |
| β-actin | 5′-ATCCTCACCCTGAAGTACCC-3′ | 5′-TAGAAGGTGTGGTGCCAGAT-3′ |
| eNOS | 5′-TACGCACCCAGAGCTTTTCT-3′ | 5′-CTTGGTCAACCGAACGAAGT-3′ |
| iNOS | 5ť-CTTCAACCCCAAGGTTGTCTGCAT-3ť | 5ť-ATGTCATGAGCAAAGGCGCAGAAC-3ť |
| nNOS | 5′-GGTGGAGATTAACATTGCTGTCCTA-3′ | 5′-TTCTCCATGTGTTTGATGAAGGACT-3′ |
| GCH-1 | 5′- GAGCATCACCTTGTTCCATTTG -3′ | 5′- GCCAAGTTTACTGAGACCAAGGA -3′ |
| DHFR | 5′-TCGACCATTGAACTGCATCGTCGCC-3′ | 5′-GGAATGGAGAACCAGGTTTTCCTACC-3′ |
| Nrf2 | 5′-TCTCCTCGCTGGAAAAAGAA-3′ | 5′-TAAAGCACAGCCAGCACATT-3′ |
| CAT | 5′-GCGGATTCCTGAGAGAGTGGTAC-3′ | 5′-GCCTGACTCTCCAGCGACTGTGGAG-3′ |
| SOD-1 | 5′-CGGTTGAGATAGACAGG-3′ | 5′-TTAAGTGGTCTTGCACTCG-3′ |
| HO-1 | 5′-CTGTGTAACCTCTGCTGTTCC-3′ | 5′-CCACACTACCTGAGTCTACC-3′ |
2.7 |. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.00 for Windows. Student t-test or one-way ANOVA was used to analyze significant differences between groups. Values of P <0.05 were considered statistically significant.
3 |. RESULTS
3.1 |. Infection with Pg increases pHAEC detachment at longer incubation period
To explore Pg capacity to induce cell death in pHAECs, a series of phase-contrast micrographs were assessed (Fig. 1). Trypan blue staining revealed that >80% of the pHAECs were viable after overnight culture in the HoxBan system in the absence or presence of Pg (Fig. 1). pHAECs co-incubated with Pg exhibited morphologic shrinking, rounding, and detachment beginning at the 24 hours time point compared with uninfected controls at each time point, respectively (Fig. 1G). The pHAEC viability assay showed that cells survived until 24 hours and were reduced significantly (P <0.05) at 36 hours and 48 hours (Fig. 1G).
FIGURE 1.

Infection with Pg compromises cell viability in pHAECs in vitro. A) Uninfected control, B) pHAECs infected with Pg (12 hours), C) pHAECs infected with Pg (18 hours), D) pHAECs infected with Pg (24 hours), E) pHAECs infected with Pg (36 hours), and F) pHAECs infected with Pg (48 hours). Damaged cells (arrows) demonstrated shrinking, rounding, and detachment. Viable cells with an intact plasma membrane exclude trypan blue. Quantification of pHAEC viability by trypan blue staining after co-culture with Pg (G), the values are expressed as a percentage of the control (uninfected with Pg) for each time point, respectively (0 to 48 hours). Microscopic images were at 10× magnification, representative of three independent experiments. Scale bar represents 50 μm. Data were analyzed with one-way ANOVA. The values are mean ± SD (n = 3). *P <0.05 compared with control non-infected cells
3.2 |. Pg infection increases the expression of proinflammatory cytokines
Endothelial cytokine and chemokine secretion is an essential step in the initiation and progression of atherosclerosis.22 Therefore, we measured various cytokines released in the media in response to in vitro infection with Pg and found a time-dependent increase of IL-1β (Fig. 2B). In contrast, IL-1α levels in the infection group were significantly higher only at 48-hour release in the medium (Fig. 2A). Whereas tumor necrosis factor-alpha (TNF-α) and IL-6 in the media secreted were significantly (P <0.05) higher at 24 hours and 36 hours compared with the control group (Figs. 2C and 2D).
FIGURE 2.

Effect of Pg infection on cytokine release by pHAECs in vitro. Results are mean concentrations of IL-1α (A), IL-1β (B), IL-6 (C), and TNF-α (D) at 12 hours to 48 hours after co-culture in cell supernatant (n = 3) for three separate experiments, each performed in duplicate. Bars represent mean values, with error bars representing SD. *P <0.05 compared with control non-infected cells
Pg has been reported to target Toll-like receptor 4 (TLR-4) leading to the secretion of inflammatory cytokines.23 Next, we examined the mRNA expression of IL-1α, IL-1β, IL-6, TNF-α, and TLR-4 in the Pg-exposed cells. Our data depicted in Figures 3A through 3E indicate that infection increases all of the above cytokines and TLR-4 compared with uninfected cells. In addition, our data show that monocyte chemoattractant protein 1 (MCP-1) mRNA expression was reduced significantly (P <0.05) in Pg-exposed cells (Fig. 3F).
FIGURE 3.

In vitro pHAECs co-cultured with Pg stimulates greater cytokine mRNA expressions. Results are mean fold increases in IL-1α (A), IL-1β (B), IL-6 (C), TNF-α (D), MCP-1 (E), and VEGF (F) transcript levels (n = 3, mean ± SD) at 12 hours to 48 hours for three separate experiments, each performed in duplicate. Data were normalized with housekeeping gene (β-actin). *P < 0.05 compared with control non-infected cells
3.3 |. Pg infection differentially influences the expression of eNOS/iNOS
pHAECs co-cultured with Pg revealed that neuronal NOS (nNOS) showed no changes until 36 hours but significantly decreased at 48 hours when co-cultured with Pg (Fig. 4A). eNOS mRNA levels were reduced significantly (P <0.05) beginning at 18 hours until 48 hours compared with the uninfected control group (Fig. 4B). Infection resulted in a significant (P <0.05) increase in the expression levels of inducible NOS (iNOS) at all time points (Fig. 4C). GCH-1 is a rate-limiting enzyme responsible for BH4 biosynthesis via the de novo pathway. Our results show that mRNA expression of GCH-1 (Fig. 4D) remained unchanged throughout the culture period. In contrast, significant (P <0.05) reduction in the expression of dihydrofolate reductase (DHFR), an enzyme responsible for the conversion of oxidized BH2 into reduced BH4 via the salvage pathway (Fig. 4E), was observed in the cells co-cultured with Pg when compared with control non-infected cells.
FIGURE 4.
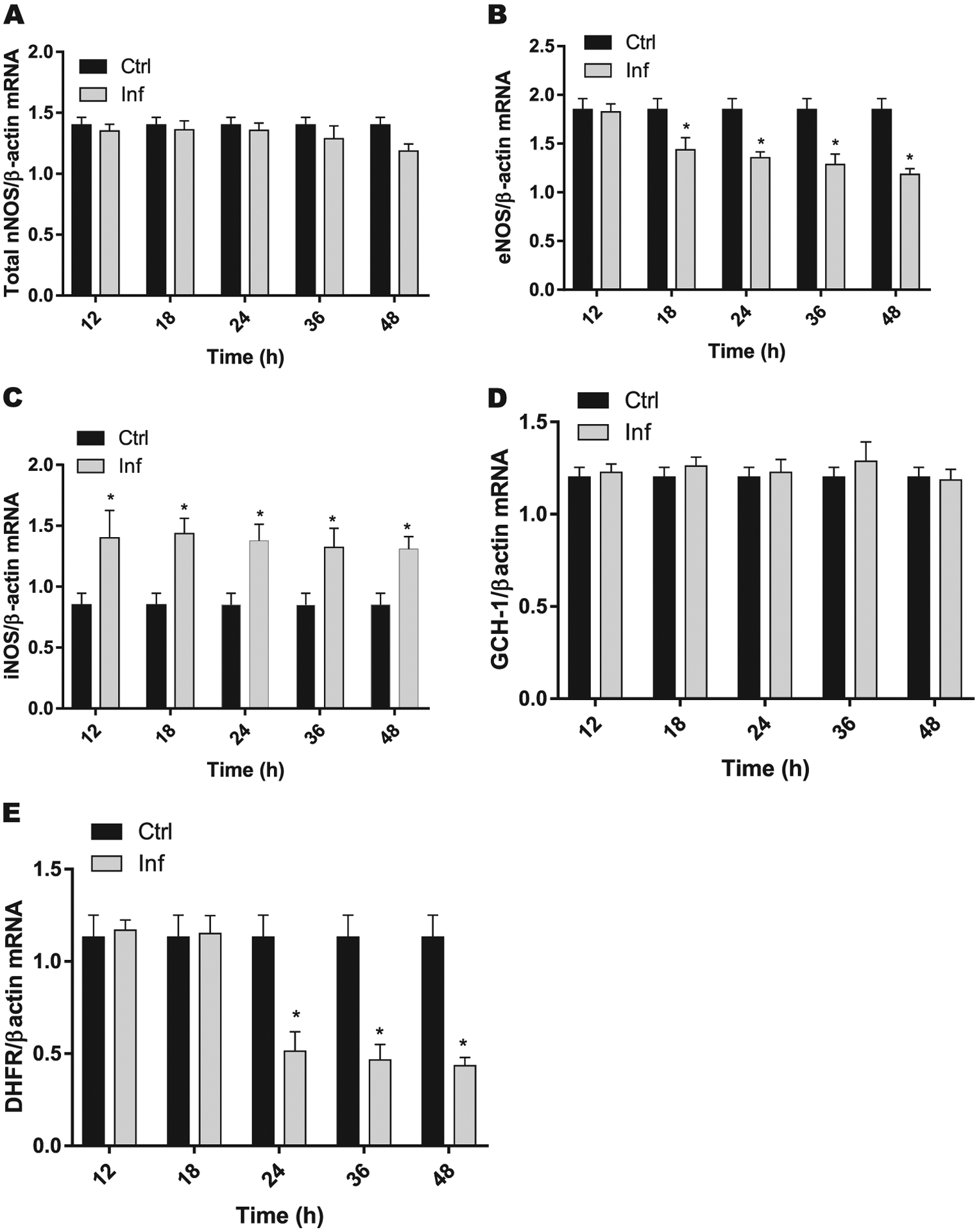
Time-dependent effect of Pg infection on mRNA expressions of total nNOS (A), eNOS (B), iNOS (C), GCH-1 (D), and DHFR (E) in pHAECs (n = 3), for three separate experiments, each performed in duplicate. Data were normalized with housekeeping gene (β-actin). *P < 0.05 compared with control non-infected cells
3.4 |. Pg infection alters GSK-3β/Nrf2/antioxidant enzyme expression
GSK-3β is a fundamental element in the downregulation of the antioxidant cell defense elicited by the transcription factor Nrf2.24 GSK-3β mRNA expressions were elevated significantly (P <0.05) at the 18-hour time point and remained higher until 48 hours when compared with uninfected control cells (Fig. 5A). As seen in Figure 5B, Nrf2 mRNA expression was reduced significantly in infected cells (1.35 ± 0.1 versus 0.34 ± 0.07, P <0.05) when compared with control non-infected cells. The mRNA expressions of catalase (CAT), superoxide dismutase-1 (SOD1), and heme oxygenase-1 (HO-1) are shown in Figures 5C through 5E, respectively. When compared with the uninfected group, the infected groups showed a significant (P <0.05) reduction in the mRNA expressions of CAT (1.28 ± 0.2 versus 0.3 ± 0.1), SOD-1 (1.45 ± 0.2 versus 0.4 ± 0.1), and HO-1 (1.07 ± 0.09 versus 0.27 ± 0.01).
FIGURE 5.

Quantitative real time-polymerase chain reaction analysis of changes in the mRNA levels of selected genes. mRNA expressions of GSK-3β (A), Nrf2 (B), HO-1 (C), CAT (D), and SOD1 (E) in pHAECs co-cultured with Pg at different time periods for three separate experiments, each performed in duplicate. Data were normalized with housekeeping gene (β-actin). *P <0.05 compared with control non-infected cells
4 |. DISCUSSION
Infection with Pg has been proposed as one of the potential bacterial infections for the development of cardiovascular diseases.25 Pg (W83) has been shown to be highly virulent in experimental animal models.26 However, studies are limited on investigating the effect of Pg on endothelial cell survival and various cell signaling mechanisms using in vitro vascular cell culture models. It has been reported that Pg triggers apoptosis in human gingival fibroblasts at later stages of infection.27 Consistent with the above findings, our data also show a deleterious effect of Pg on endothelial cell survival at later stages of infection but not at early time points. Pg produces three cysteine proteases known as gingipains, arginine-gingipains A and B, and lysine-gingipain, which are known for processing/maturation of their own respective cell surface proteins.28,29 Gingipains have been reported to be crucial in apoptosis promotion in human gingival fibroblast and aortic endothelial cells.27,30
Pg has major and minor fimbriae on its cell surface and contributes to persistent infection, leading to periodontitis with elevated levels of IL-1β, IL-6, and TNF-α.18 Gingipains and SerbB from Pg induce IL-6 and IL-8 secretion, thereby influencing the host cytoskeleton and intruding host tissues.31,32 MCP-1, IL-6, and IL-8, stimulated by secretory proteins released by oral commensal bacteria into the blood stream, are known to invade the endothelial layer of the blood vessels.33 Our results from the current study demonstrate that infection with Pg induces the release of IL-1β, IL-6, TNF-α, and TLR-4 in pHAECs. The severity of microbial infection is determined by its inflammatory response, which is known to be regulated by TLR signaling.34 The inflammatory cytokines are enlisted by dendritic cells, which engulf oral bacteria transported and deposited into the vascular sites.35 Taken together, our studies suggest that Pg elevates cytokines and TLR-4 and impairs endothelial cell survival and function.
NO synthases (eNOS, nNOS, and iNOS) catalyze the synthesis of NO and maintain normal functioning of vascular endothelial cells.36 Reduction in NO bioavailability results in NOS uncoupling and elevated levels of ROS, promote cardiovascular disease.1 Both eNOS and nNOS are known to play a beneficial role in maintaining vascular relaxation in various experimental animal models.37 A reduction of NOS enzymes reduces NO synthesis, an impairment in endothelium NO-mediated vascular relaxation.38 Most importantly, endothelium-mediated vascular relaxation is impaired in patients with periodontitis with coronary heart disease.7 However, in vitro data are lacking that show whether NOS and NO expression are altered by oral bacterial infection. Our data herein indicate that both eNOS and nNOS mRNA expression and NO levels were reduced with Pg infection, suggesting that periodontal pathogens play a vital role in endothelium-mediated vascular relaxation. iNOS expression is known to increase with the rate of infection and elevates NO production, thus impairing endothelial cell function.39,40 An earlier study suggests that excess NO production is associated with the invasion of vascular endothelial cells by Pg.41 Elevated levels of NO production cause epithelial cell cytotoxicity and pulmonary epithelial cell damage.40,42 Consistent with previous studies, our study also shows an increase in iNOS expression. In addition, our results further show that NO levels are correlated with the expression of iNOS in pHAECs.
BH4, cofactor for the synthesis of NO by NOS and BH4 bioavailability, maintains the balance between NO and superoxide production.43 BH4 is synthesized from the de novo pathway via a series of reactions involving GCH-1, 6-pyruvoyltetrahydropterin synthase, and sepiapterin reductase. DHFR may regenerate BH4 from BH2 as part of the recycling (salvage) pathway. Both BH4 and BH2 have similar affinity binding to NOS; however, BH4-bound NOS produces NO, whereas BH2-bound NOS promotes uncoupling and NOS-derived superoxide rather than NO. Previous studies demonstrate that cytokine stimulation reduces DHFR activity and BH4 synthesis; sepiapterin treatment restored all of these in adult cardiac myocytes.44 Also in rabbits insufficient activity of DHFR showed impaired vasorelaxation in atherosclerotic vessels.45 Collectively, our data suggest that elevated proinflammatory cytokines observed with infection alters BH4 synthesis, thereby impairing NO-mediated vascular function.
GSK-3β is a key arbitrator for the production of proinflammatory cytokines during bacterial infections, and inhibition of GSK-3β shows an abnormally low sensitivity to oral and any other pathogenic invasions.34 Inhibition of GSK-3β demonstrated a significant reduction of chronic intestinal inflammation in a dextran sodium sulfate-induced colitis model.34 Inflammation is regulated by PI3K-Akt signaling through the TLR module.34 Thus, activation of the PI3K-Akt signaling pathway inhibits TLR-mediated cytokine production, which is regulated differentially by GSK-3β activation.46
In PD tolerance, Nrf2 plays an important role in preserving periodontal tissues against the constant influx of bacterial, neutrophil, and macrophage interactions and reducing oxidative stress in healthy periodontium.47 It has been reported that Nrf2 deficiency in macrophages leads to increased proatherogenic foam cell formation, intensifying atherosclerosis.48 GSK-3β phosphorylates Nrf2, which results in the nuclear exclusion and degradation of Nrf2.49 The in vivo rodent model has shown that inhibition of GSK-3β by lithium activates Nrf2, thus protecting against ventricular arrhythmias.48 The results from our study show that Nrf2 significantly decreases and that GSK-3β increases with Pg infection. This form of Nrf2 regulation switches off the self-protective antioxidant stress response and downregulates the antioxidant defense elicited by Nrf2.50,51 The anti-inflammatory effect of Nrf2 is capable of inhibiting the TNF-α, IL-6, and iNOS expressions.50 Collectively, our data suggest that Nrf2 is an important regulator of pathways leading to toxicity and development of inflammatory diseases.
5 |. CONCLUSIONS
In summary, our findings suggest that the Nrf2 Phase II pathway may play a role in NO-mediated endothelial cell homeostasis. Hence, it is essential to recognize the mechanistic role of endothelial function in pHAEC co-culture models, as such insight could prove beneficial during the development of future therapeutic compounds used to treat the endothelial dysfunction associated with PD.
| 1 | P. gingivalis (W83) | ATCC BAA-308, ATCC, Manassas, VA |
| 2 | Trypticase Soy Agar (TSA) blood agar plates (BAP) with 5% sheep blood | Fisher Scientific, Hampton, NH |
| 3 | BACTRON-300 Anaerobic Chamber | Sheldon Manufacturing, Cornelius, OR |
| 4 | Hemin | Sigma-Aldrich, St. Louis, MO |
| 5 | Menadione | Fisher Scientific, Hampton, NH |
| 6 | Primary human Aortic endothelial cells | ATCC PCS-100–011, Manassas, VA |
| 7 | Vascular cell basal medium | ATCC PCS-100–030, ATCC, Manassas, VA |
| 8 | Endothelial Cell Growth Kit-VEGF | ATCC PCS-100–041, ATCC |
| 9 | Mouse cytokine ELISA kit | Signosis, Santa Clara, CA |
| 10 | TRIzol reagent | Invitrogen, Carlsbad, CA |
| 11 | NanoDrop | Thermo Fisher Scientific, Waltham, MA |
| 12 | iScript cDNA Synthesis Kit | Bio-Rad, Hercules, CA |
| 13 | SYBR Green | Bio-Rad, Hercules, CA |
| 14 | GraphPad Prism, version 8.00 | GraphPad Software, La Jolla, CA |
ACKNOWLEDGMENTS
The National Institute of General Medical Sciences of the National Institutes of Health (NIH), under award number-SC1GM121282 and Health Resources and Services Administration (HRSA) D34HP00002-32-00, supported our research. The Meharry Office of Scientific Editing and Publications (NIH S21MD000104) provided editing services.
Dr. P. R. Gangula filed a patent application for the use of BH4 (tetrahydrobiopterin) in gastroparesis subjects through the University of Texas Medical Branch at Galveston, Texas. All remaining listed authors report no conflicts of interest related to this study.
REFERENCES
- 1.Gangula P, Ravella K, Chukkapalli S, et al. Polybacterial periodontal pathogens alter vascular and gut BH4/nNOS/NRF2-Phase II enzyme expression. PLoS One. 2015;10(6):e0129885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji S, Choi YS, Choi Y. Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis? J Periodontal Res. 2015;50(5):570–585. [DOI] [PubMed] [Google Scholar]
- 3.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–407. [DOI] [PubMed] [Google Scholar]
- 4.Velsko IM, Chukkapalli SS, Rivera MF, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9(5):e97811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoluci MC. Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J Diabetes. 2015;6(5):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liccardo D, Cannavo A, Spagnuolo G, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20(6):1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toda N, Tanabe S, Nakanishi S. Nitric oxide-mediated coronary flow regulation in patients with coronary artery disease: recent advances. Int J Angiol. 2011;20(03):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashi Y, Goto C, Jitsuiki D, et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51(2):446–453. [DOI] [PubMed] [Google Scholar]
- 9.Moura MF, Navarro TP, Silva TA, Cota LOM, Soares Dutra Oliveira AM, Costa FO. Periodontitis and endothelial dysfunction: periodontal clinical parameters and levels of salivary markers interleukin-1β, tumor necrosis factor-α, matrix metalloproteinase-2, tissue inhibitor of metalloproteinases-2 complex, and nitric oxide. J Periodontol. 2017;88(8):778–787. [DOI] [PubMed] [Google Scholar]
- 10.Brito LCW, DalBó S, Striechen TM, et al. Experimental periodontitis promotes transient vascular inflammation and endothelial dysfunction. Arch Oral Biol. 2013;58(9):1187–1198. [DOI] [PubMed] [Google Scholar]
- 11.Campi P, Herrera BS, de Jesus FN, et al. Endothelial dysfunction in rats with ligature-induced periodontitis: participation of nitric oxide and cycloxygenase-2-derived products. Arch Oral Biol. 2016;63:66–74. [DOI] [PubMed] [Google Scholar]
- 12.Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ. The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longev. 2017;2017:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saffi MAL, Furtado MV, Polanczyk CA, et al. Relationship between vascular endothelium and periodontal disease in atherosclerotic lesions: review article. World J Cardiol. 2015;7(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116(3):531–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 2014;20(18):3040–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev. 2019;2019:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangula P, Ravella K, Chukkapalli S, et al. Polybacterial periodontal pathogens alter vascular and gut BH4/nNOS/NRF2-Phase II enzyme expression. PLoS One. 2015;10(6):e0129885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunsch CM, Lewis JP. Porphyromonas gingivalis as a model organism for assessing interaction of anaerobic bacteria with host cells. J Vis Exp. 2015(106):e53408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadaghian Sadabad M, von Martels JZH, Khan MT, et al. A simple coculture system shows mutualism between anaerobic faecalibacteria and epithelial Caco-2 cells. Sci Rep. 2016;5(1):17906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azimzadeh O, Sievert W, Sarioglu H, et al. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res. 2015;14(2):1203–1219. [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nativel B, Couret D, Giraud P, et al. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci Rep. 2017;7(1):15789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuadrado A, Kügler S, Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mougeot J-LC, Stevens CB, Paster BJ, Brennan MT, Lockhart PB, Mougeot FKB. Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries. J Oral Microbiol. 2017;9(1):1281562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333(1):1–9. [DOI] [PubMed] [Google Scholar]
- 27.Urnowey S, Ansai T, Bitko V, Nakayama K, Takehara T, Barik S. Temporal activation of anti- and pro-apoptotic factors in human gingival fibroblasts infected with the periodontal pathogen, Porphyromonas gingivalis: potential role of bacterial proteases in host signalling. BMC Microbiol. 2006;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Murakami Y, Kawata A, Ito S, Katayama T, Fujisawa S. Radical-scavenging and anti-inflammatory activity of quercetin and related compounds and their combinations against RAW264.7 cells stimulated with Porphyromonas gingivalis fimbriae. Relationships between anti-inflammatory activity and quantum chemical par. In Vivo. 2015;29(6):701–710. [PubMed] [Google Scholar]
- 29.Nakayama M, Ohara N. Molecular mechanisms of Porphyromonas gingivalis-host cell interaction on periodontal diseases. Jpn Dent Sci Rev. 2017;53(4):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth GA, Ankersmit HJ, Brown VB, Papapanou PN, Schmidt AM, Lalla E. Porphyromonas gingivalis infection and cell death in human aortic endothelial cells. FEMS Microbiol Lett. 2007;272(1):106–113. [DOI] [PubMed] [Google Scholar]
- 31.Jayaprakash K, Khalaf H, Bengtsson T. Gingipains from Porphyromonas gingivalis play a significant role in induction and regulation of CXCL8 in THP-1 cells. BMC Microbiol. 2014;14: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa Y, Tribble GD, Baker HV, Mans JJ, Handfield M, Lamont RJ. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect Immun. 2008;76(6):2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chhibber-Goel J, Singhal V, Bhowmik D, et al. Linkages between oral commensal bacteria and atherosclerotic plaques in coronary artery disease patients. Biofilms Microbiomes. 2016;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamowicz K, Wang H, Jotwani R, Zeller I, Potempa J, Scott DA. Inhibition of GSK3 abolishes bacterial-induced periodontal bone loss in mice. Mol Med. 2012;18:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Kholy KRJ Genco, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26(6):315–321. [DOI] [PubMed] [Google Scholar]
- 36.Chuaiphichai S, Crabtree MJ, Mcneill E, et al. A key role for tetrahydrobiopterin-dependent endothelial NOS regulation in resistance arteries: studies in endothelial cell tetrahydrobiopterin-deficient mice. Br J Pharmacol. 2017;174(8): 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forte M, Conti V, Damato A, et al. Targeting nitric oxide with natural derived compounds as a therapeutic strategy in vascular diseases. Oxid Med Cell Longev. 2016;2016:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan M, Hou M, Liu J, et al. Regulation of iNOS-Derived ROS generation by HSP90 and Cav-1 in porcine reproductive and respiratory syndrome virus-infected swine lung injury. Inflammation. 2017;40(4):1236–1244. [DOI] [PubMed] [Google Scholar]
- 40.Issaad N, Ait-Lounis A, Laraba-Djebari F. Cytotoxicity and actin cytoskeleton damage induced in human alveolar epithelial cells by Androctonus australis hector venom. Toxin Rev. 2018;37(1):67–74. [Google Scholar]
- 41.Alayan J, Ivanovski S, Gemmell E, Ford P, Hamlet S, Farah CS. Deficiency of iNOS contributes to Porphyromonas gingivalis-induced tissue damage. Oral Microbiol Immunol. 2006;21(6):360–365. [DOI] [PubMed] [Google Scholar]
- 42.Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA, Aldalbahi A. Nanocubes of indium oxide induce cytotoxicity and apoptosis through oxidative stress in human lung epithelial cells. Colloids Surf B Biointerfaces. 2017;156:157–164. [DOI] [PubMed] [Google Scholar]
- 43.Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011;50(11):1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ionova IA, Vásquez-Vivar J, Whitsett J, et al. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;295(5):H2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vásquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol. 2002;22(10):1655–1661. [DOI] [PubMed] [Google Scholar]
- 46.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6(8):777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu AV, Al Saigh M, McCulloch CA, Glogauer M. The role of NrF2 in the regulation of periodontal health and disease. J Dent Res. 2017;96(9):975–983. [DOI] [PubMed] [Google Scholar]
- 48.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50(2):77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Culbreth M, Aschner M. GSK-3β, a double-edged sword in Nrf2 regulation: implications for neurological dysfunction and disease. F1000Res. 2018;7:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vomund S, Schäfer A, Parnham MJ. Brüne B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith RE, Tran K, Smith CC, McDonald M, Shejwalkar P, Hara K. The role of the Nrf2/ARE antioxidant system in preventing cardiovascular diseases. Diseases. 2016;4(4):34. [DOI] [PMC free article] [PubMed] [Google Scholar]


