Abstract
Herein, we report a magnetically retrievable mixed-valent Fe3O4@SiO2/Pd0/PdIINP (5) nanocomposite system for tandem Suzuki coupling/transfer hydrogenation reaction. The nanocomposite 5 was prepared first by making a layer of on followed by deposition of and sorption of ions successively onto the surface of Fe3O4@SiO2NP. The nanocomposite was characterized by powder XRD, electron microscopy (SEM-EDS and TEM-EDS) and XPS spectroscopy techniques. The mixed-valent present onto the surface of nanocomposite 5 was confirmed by XPS technique. Interestingly, the mixed-valent nanocomposite Fe3O4@SiO2/Pd0/PdIINP (5) exhibited tandem Suzuki coupling/transfer hydrogenation reaction during the reaction of aryl bromide with aryl boronic acid (90% of C). The nanocomposite 5 displayed much better reactivity as compared to the monovalent Fe3O4@SiO2/Pd0NP (3) (25% of C) and Fe3O4@SiO2/PdIINP (4) (15% of C) nanocomposites. Further, because of the presence of magnetic , the nanocomposite displayed its facile separation from the reaction mixture and reused at least for five catalytic cycles.
Subject terms: Catalysis, Materials chemistry
Introduction
The design and development of environmentally benign efficient catalytic systems which can conduct multiple mechanistically distinct reaction (tandem reaction) in one-pot is a fascinating area of contemporary research1,2. Such catalytic systems offer a greener way by reducing reaction steps, cost of the process, and most importantly reduce the generation of waste during a reaction1,2. The most common and successful strategy to conduct tandem reactions is to combine multiple mechanically distinct catalytic centers in one-pot under a suitable reaction condition. A major disadvantage of this strategy is catalytic incompatibility which reduces tandem efficiency3–5. The alternative is to incorporate different catalytic units into a single molecular framework6–10 or integrate into a suitable solid matrix4,11–13. In both cases, a well-defined ligand-framework is required to achieve desired tandem output. Adding two different metal centers into a single molecular framework/suitable solid matrix is challenging and cumbersome. Moreover, separation of catalysts after completion of the reaction and their reuse is also a concern.
Both Suzuki–Miyaura C–C coupling reaction14 and transfer hydrogenation reaction15–17 have become an indispensable tool in organic synthesis and pharmaceutical chemistry as they offer access of facile C–C bond formation and hydrogenation without use of hazardous hydrogen gas. A large number of efficient catalytic systems (both homogeneous and heterogeneous) have been developed for conducting C–C coupling reaction and transfer hydrogenation reaction. Many of the catalytic systems are used in industrial processes and are commercially available. There are many important biaryl organic motifs, crucial for the preparation of natural products and chiral pharmaceuticals, which requires both C–C coupling as well as transfer hydrogenation reaction steps4,11,12. A catalyst system capable of conducting both mechanistically distinct C–C coupling reaction and transfer hydrogenation reactions in one-pot is highly appealing. Nevertheless, catalyst systems capable conducting tandem Suzuki coupling/transfer hydrogenation reaction are limited4,10–12. In most of the cases, heterobimetallic systems (Pd–Ru) have been used as catalysts for conducting aforesaid tandem reaction6–9,18. Interestingly, recently our group has observed that the tandem Suzuki coupling/transfer hydrogenation reaction can be conducted using bimetallic system efficiently.19 The palladium center in its zero-oxidation state () initiates the C–C coupling reaction, whereas the palladium center in +2 oxidation state () carried out transfer hydrogenation reaction. It is to be noted that in all the cases, a well-defined ligand-framework is used to make the catalysts. It will be highly attractive if such kind of catalyst systems can be made without ligand-framework. Another interesting feature of a great catalytic system is its separability and reusability. This allows the catalyst to be separated and reused after catalytic conversion and thereby enhances the catalytic efficiency. Thus, designing a catalyst system without the use of ligand with facile separation ability is highly appealing.
With their high surface to volume ratio, interesting redox, optical, and catalytic properties, nanoparticles are attractive and become a fascinating tool for the development of tailor-made materials. The nanoparticles extend the easy incorporation of second nanoparticles onto the first one and also allow facile surface modification using different functionalities. This way, different interesting properties can easily be inserted into a single nanoparticle and thereby achieving materials with advanced features and desired properties. Considering this, several multimetallic nanoparticles systems have been developed and studied. However, the study of catalytic tandem reactivity is relatively less20–24.
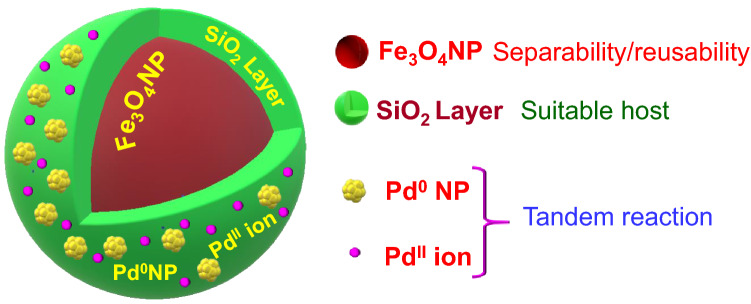
Utilizing the benefits of nanoparticles and advantages of one-pot multistep reactions, in the present contribution, we wish to demonstrate a magnetically retrievable mixed-valent multimetallic Fe3O4@SiO2/Pd0/PdIINP (5) nanocatalytic system (Fig. 1) for efficient tandem Suzuki coupling/transfer hydrogenation reaction. is used to insert magnetic property, and a layer of on helps to host PdNP as well as ions into the nanocomposite without use of any ligands. The nanocatalytic system 5 is characterized by SEM, TEM, powder XRD and XPS techniques. The catalytic reactivity of the synthesized nanocatalytic system is studied by conducting tandem Suzuki coupling/transfer hydrogenation reaction using aryl boronic acid and aryl bromide. Further, to compare the performance of the mixed-valent Fe3O4@SiO2/Pd0/PdIINP (5) system to the monovalent Fe3O4@SiO2/Pd0NP (3) and Fe3O4@SiO2/PdIINP (4) were also synthesized and their tandem reactivity was studied.
Figure 1.
Magnetically retrievable Fe3O4@SiO2/Pd0/PdIINP (5) nanocatalyst system and its advantages.
Results and discussion
The magnetic nanoparticles was synthesized by following the procedure reported elsewhere25. Briefly, a mixture of and (1:1 mole ratio) in alkaline solution (pH = 10) was heated at C for 1 h to yield (1). A layer of was incorporated over to form Fe3O4@SiO2NP (2) by adding tetraethylorthosilicate (TEOS) to it and stirring for 18 h25. Palladium nanoparticle was deposited onto the surface of Fe3O4@SiO2NP in situ by reducing using at low temperature Fe3O4@SiO2/Pd0NP (3)26. Palladium ions () on the surface of Fe3O4@SiO2NP was sorbed by adding solution with stirring to yield NP (4). Mixed-valent Fe3O4@SiO2/Pd0/PdIINP (5) was achieved by mixing Fe3O4@SiO2/Pd0NP and solution. All the nanoparticles were separated magnetically, followed by successive washing with triple distilled water and dried under oven for further use. A layout for the preparation of mixed-valent Fe3O4@SiO2/Pd0/PdIINP (5) is shown in Scheme S1.
The crystalline nature of the nanoparticles was examined by powder X-ray diffraction (XRD) study. Figure S1 represents the powder XRD pattern of the nanoparticles. The powder XRD pattern of (1) displayed peaks centered at 2 values = 30.8, 36.3, 43.9, 54.3 (s), 57.9, 63.5 and 75 (s) which could be indexed as (220), (311), (400), (422) and (511) plane corresponding to cubic lattice of (1)[JCPDS File No: 19-0629]27–30. A broad band centered at along with the bands for was observed while a layer of silica over introduced (2)29. No significant change other than a new but very weak signal at for (3) was observed while was deposited onto 31. No change for Fe3O4@SiO2/Pd0/PdIINP (4) was observed during deposition of ion to . No additional peak was observed for Fe3O4@SiO2/Pd0/PdIINP (5) during the deposition of ion onto .
The surface morphology of the nanoparticles was investigated by Field Emission Scanning Electron Microscopy (FE-SEM) technique. The representative images are presented in Figures S2 and S3. It is observed from the images that the particles sizes are uniform and varying in the range . After deposition of onto (2), the (3) gets aggregated with particle size nm. No significant changes were observed in the morphology while the incorporation of ions (4) (particle size ). The morphology of Fe3O4@SiO2/Pd0/PdIINP (5) (particle size ) remains the same after the introduction of ions onto (3). The observed peaks corresponding to iron, silicon and palladium in the EDS spectra, indicating their presence in the nanocomposites (Figures S2 and S3). The elemental mapping of Fe3O4@SiO2/Pd0/PdIINP (5) further supports the presence of the elements in the nanocomposite (Figure S3).
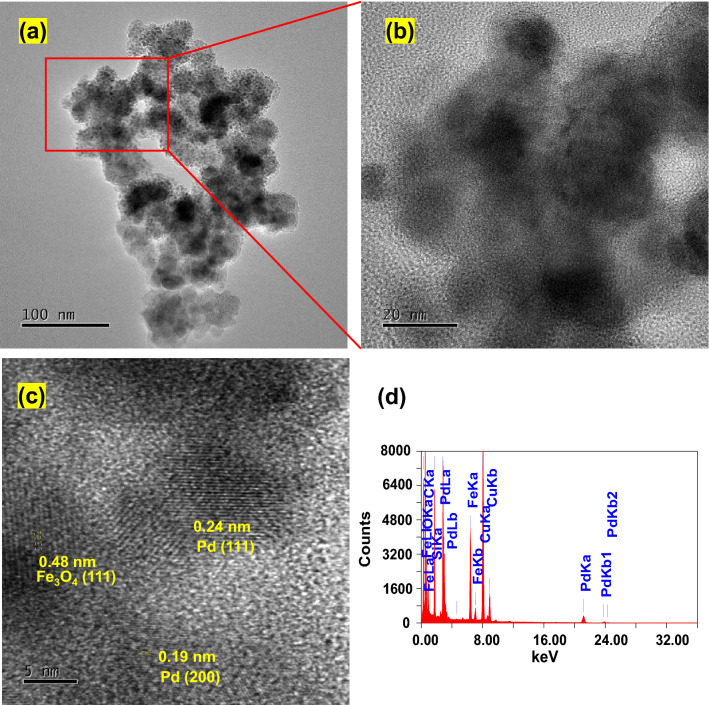
The formation of Fe3O4@SiO2/Pd0/PdIINP (5) was further confirmed by high-resolution transmission images microscopy (HRTEM) (Fig. 2a,b). The EDS analysis of 5 shown the peaks corresponding to iron, palladium and silicon, which are in line with SEM analysis (vide supra) (Fig. 2d). The HRTEM image of the nanocomposite 5 has also shown the lattice fringes 0.48 nm for oxidized iron (020)32, and 0.19 nm for palladium (200)33,34 and 0.224 nm for palladium (111)34,35 which further confirms the presence of the elements (Fig. 2c).
Figure 2.
TEM and HRTEM images of Fe3O4@SiO2/Pd0/PdIINP (5) nanocomposite.
To elucidate the surface composition and oxidation state of the elements present in the nanocomposite 5, X-ray photoelectron spectroscopy (XPS) study was conducted (Fig. 3). The XPS survey scan spectrum of 5 discloses the presence of iron, silicon, and palladium (Fig. 3a). The high resolution XPS spectra of 5 display peaks at 101.8 eV corresponding to (Fig. 3c)36; 710.26 eV and 723.85 eV corresponding to and of and (Fig. 3d)37,38. The peaks at 334.78 eV and 340.16 eV; 337.73 eV and 342.85 eV correspond to and of metallic palladium () and oxidized palladium [: PdO or (Fig. 3b)26,31,33,39–46. This confirms presence of mixed-valent palladium ( and ) in nanocomposite 5.
Figure 3.
(a) XPS survey scan spectrum and high-resolution (b) Pd and , (c) Si 2p and (d) Fe and of Fe3O4@SiO2/Pd0/PdIINP (5).
It is well-known that both and can conduct efficient Suzuki coupling reaction19,40,47–49. Recent literatures also demonstrate that palladium (both and ) can catalyze transfer hydrogenation reaction19,50–57. We, thus, presumed that the nanocatalyst Fe3O4@SiO2/Pd0/PdIINP (5) can conduct mechanically distinct Suzuki coupling and transfer hydrogenation reactions as it comprises palladium in both as well as oxidation state. With this hypothesis, we attempted to conduct tandem Suzuki coupling/transfer hydrogenation reactions. The tandem reaction was carried out using p-bromoacetophenone and phenylboronic acid in as solvent and NaOH as base (Table 1). It was observed that the nanocatalyst 5 resulted in excellent yield of tandem product (entry 1, Table 1). The tandem reaction using nanocatalyst 5 was tested by varying solvents (Table 1). It was observed that the is the best solvent for tandem reaction (entries 1, 5 and 6, Table 1). Further, the tandem reaction was found to be highly dependent on selection of base. The formation of tandem product C (90 %) was best during the use of NaOH as base (entry 1, Table 1). It was 85% under same reaction condition while KOH was used (entry 2, Table 1). The formation of tandem product (C) was significantly reduced (56%) when was used as base (entry 3, Table 1). Surprisingly, no tandem product (C) was formed when the base was used (entry 4, Table 1), however, the yield of coupling product (B) was appreciably high (93%) (entry 4, Table 1). This suggests that the use of bases like KOH and NaOH plays an important role to result in the formation of tandem product (C). Thus, the optimum reaction condition for the nanocatalyst 5 to achieve the best tandem efficiency is mmol/0.14 mmol/0.7 mmol, 1 mL and reaction time 6 h (entry 1, Table 1). The tandem reaction using nanocatalyst 3 and 4 were also conducted. Both the nanocatalyst 3 and 4 under identical reaction condition resulted in much inferior yield of tandem product C (25% and 15%) as compared to the mixed-valent nanocatalyst 5 (entries 7 and 8, Table 1) leaving a major amount of hydrogenated product A (65% and 76%, respectively). This implies the importance of the presence of palladium in both ( and ) oxidation states. It is also observed that pre-attainment of suitable oxidation states of metal atoms and for two mechanistically different reactions leads to much higher catalytic efficiency than that of monovalent or systems27. Both the nanocatalyst (1) and (2) without any were unable to yield the tandem product (A). However, the nanocatalyst 1 was found to act as a good transfer hydrogenation catalyst (Yield of A, 90%), whereas, the nanocatalyst 2 exhibited poor yield of A (30%) using excess of catalyst (5 mg in each cases) and longer reaction time 18 h (entry 11 and 12, Table 1).
Table 1.
Tandem Suzuki coupling/transfer hydrogenation of 4-bromoacetophenone and phenylboronic acid.
|
| ||||||
|---|---|---|---|---|---|---|
| Entry | Cat. | ROH | Base | |||
| A | B | C | ||||
| 1 | 5 | NaOH | 0 | Trace | 90 | |
| 2 | 5 | KOH | Trace | 0 | 85 | |
| 3 | 5 | 10 | ||||
| 4 | 5 | 0 | 93 | 0 | ||
| 5 | 5 | n-PrOH | NaOH | 0 | 45 | 10 |
| 6 | 5 | NaOH | 0 | Trace | 0 | |
| 7 | iPrOH | NaOH | 0 | 0 | 15 | |
| 8b | 3+4 | iPrOH | NaOH | 0 | 0 | 75 |
| 9c | 3 | NaOH | 65 | 0 | ||
| 10c | 4 | NaOH | 76 | 0 | ||
| 11c,d | 2 | NaOH | 30 | 0 | 0 | |
| 12c,d | 1 | NaOH | 90 | 0 | 0 | |
Reaction condition: mmol/0.14 mmol/0.7 mmol, ROH 1.0 mL, reaction temperature , reaction time 6 h.
Isolated yields ( NMR Yield).
Amount of 3 and 4 is 1.0 mg each.
Reaction time 18 h.
Catalyst amount 5.0 mg.
Next, we conducted a time monitored reaction profile study of 4-bromoacetophenone and phenylboronic acid in the presence of NaOH in at 85 using nanocatalyst 5 (Fig. 4). It appears from the reaction profile diagram that the Suzuki coupling reaction and transfer hydrogenation reaction occur simultaneously, and the rate of C–C coupling reaction is faster than the transfer hydrogenation reaction (Fig. 4). In fact, the transfer hydrogenation reaction follows an induction period of 15 min. This suggests that the C–C coupling reaction is more facile than the transfer hydrogenation reaction under this set of reaction condition. The reaction profile study further demonstrates the consecutive nature of mechanistically independent reactions, where reaction initiated with C–C coupling reaction between 4-bromoacetophenone and phenylboronic acid in step I, and subsequently, the coupling product (B) gets hydrogenated to yield the tandem product (C) in step II. Palladium mediated C–C coupling reaction or transfer hydrogenation is known; however, tandem reaction utilizing mixed-valent palladium centers () is rare27,56,58. Thus, the present ligand-free mixed-valent nanocatalyst system 5 is an important addition to this tandem catalysis.
Figure 4.

Reaction profile diagram for the tandem Suzuki coupling/transfer hydrogenation reaction catalyzed nanocomposite 5.
Further, the reversibility of the catalytic tandem Suzuki-Miyaura C–C coupling/transfer hydrogenation reaction was monitored using NMR spectroscopy by conducting the reverse reaction using the tandem product 1-([1,-biphenyl]-4-yl)ethan-1-ol (C) under the identical reaction condition. No trace of the progress of the reaction was observed in NMR spectrum, indicating irreversible nature of the catalytic cycle.
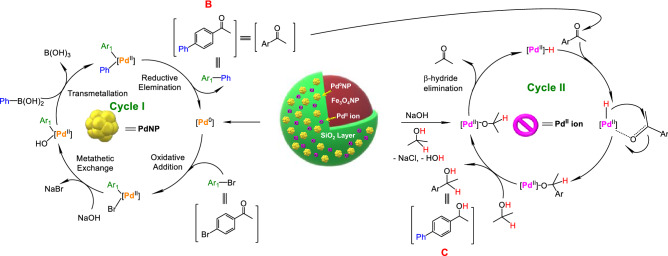
Based on this experimental finding and literature data a plausible tandem reaction mechanism is proposed (Fig. 5)59–63. The tandem reaction is initiated with the formation of Suzuki-Miyaura C–C coupling reaction product (B) between 4-bromoacetophenone and phenylboronic acid at center (catalytic cycle I). The formed coupling product then enters into the catalytic cycle II, where it gets transfer hydrogenated to tandem product (C) catalyzed by center in the presence of as hydrogen donor.
Figure 5.
Plausible mechanism for tandem Suzuki coupling/transfer hydrogenation reaction catalysed by Fe3O4@SiO2/Pd0/PdIINP (5) nanocomposite59–63.
Next, we extended our study to check the generality of the tandem Suzuki coupling/transfer hydrogenation reaction. The tandem reaction was conducted using different boronic acids of varying substituents (Table 2). In all the cases moderate to good yield of tandem product was registered. This suggest that the present nanocatalyst 5 has a good tolerance against various substrates. Further, the nature of substituents and their position in both boronic acid and haloarylketone are found to have a significant influence on the formation of tandem products.
Table 2.
Tandem Suzuki coupling/transfer hydrogenation of 4-bromoacetophenone.
Reaction condition: Catalyst/4-bromoacetophenone/Ar-B(OH)2/NaOH 1 mg/0.1 mmol/0.14 mmol/0.7 mmol, iPrOH 1 mL, reaction temperature 85 °C, reaction time 6 h. aIsolated yields.
Next, we performed mercury drop test to check the heterogeneity of the catalyst 56,64,65. The yield of tandem product C reduced significantly to 15% (entry 7, Table 1) upon addition of metallic mercury during the course of reaction. This suggests the heterogeneous nature of the nanocatalyst 5. Further, to check the heterogeneity and leaching of palladium to the solution, hot filtration study was performed by following the reported procedure66. The filtrate did not show any catalytic activity under the optimized reaction condition. This further suggests that there is no leaching of the catalyst and the catalysis is heterogeneous in nature. Nevertheless, leaching of a small fraction of palladium from the nanocomposite surface to the solution may not be ruled out as there is formation of small amount of tandem product (C) during mercury poisoning test (entry 7, Table 1).
Another interesting feature of the present mixed-valent nanocatalyst Fe3O4@SiO2/Pd0/PdIINP (5) is its separability and reusability from the solution as it consists of magnetic core. The nanocatalyst 5 exhibited its facile separation ability from aqueous solution by using an external magnet during its preparation. To check the separability and reusability, the nanocatalyst 5 was magnetically retrieved from the reaction mixture after completion of the reaction. The magnetically separated nanocatalyst 5 was washed thoroughly with , dried, and reused for the tandem reaction. Interestingly, the tandem reactivity of nanocatalyst 5 did not change significantly, even after 5 cycles (Fig. 6). This demonstrates the efficient recyclability and reusability of nanocatalyst 5, which is a desirable criterion for a good catalyst. Catalyst system exhibiting good tandem efficacy as well as excellent separation ability, and reusability are relatively rare. Thus, the present mixed-valent nanocatalyst 5 is an important example of tandem catalysis.
Figure 6.

Reusability of Fe3O4@SiO2/Pd0/PdIINP (5) nanocatalyst towards tandem Suzuki coupling/transfer hydrogenation reaction.
Conclusions
In conclusion, we have developed a magnetically retrievable efficient mixed-valent nanocatalyst Fe3O4@SiO2/Pd0/PdIINP (5). The nanocatalyst was characterized by FE-SEM-EDS, TEM, powder XRD techniques. The mixed-valent palladium () was confirmed by XPS analysis. Unlike mono-valent (3) and Fe3O4@SiO2/PdIINP (4), the mixed-valent Fe3O4@SiO2/Pd0/PdIINP (5) nanocatalyst exhibits excellent tandem Suzuki coupling/transfer hydrogenation reactivity. The mixed-valent nanocatalyst 5 also offers facile magnetic separation and its repeated use for several cycles. A catalyst exhibiting tandem reactivity along with facile separation ability is rare. Thus, we envision that the present ligand-free mixed-valent nanocatalyst system 5 is an important example that will be useful for the communities working in the area of tandem catalysis. conclusions section should come in this section at the end of the article, before the acknowledgements.
Experimental
Materials and methods
All chemicals were purchased from commercial sources and used as received. All glassware was cleaned using aqua regia, thoroughly washed with double distilled water, and rinsed with copious amount triple distilled water and dried in the oven. All reactions were carried out under an inert atmospheric condition using standard Schlenk techniques under the dinitrogen atmosphere unless and otherwise stated.
Instrumentation
and NMR spectra were recorded using Bruker 400 NMR spectrometer using as solvent. FE-SEM images and EDS analyses were recorded using Zeiss Merlin compact Microscope and Oxford instruments, respectively by drop casting the nanoparticles sample on carbon tape. HRTEM images were acquired using JEOL JEM 2100 electron microscope. Powder XRD analyses were carried out using a Bruker D8 Advance Diffractometer (Bruker AXS) with Cu radiation ( Å) over a range of with a scanning rate of . The samples for powder XRD was prepared by making a thin film of nanocomposites on glass slide.
Synthesis of NP (1)
The nanoparticles was prepared by following the reported procedure25. Briefly, (278 mg, 1 mmol) and (400 mg, 1 mmol) were dissolved in 30 mL of water (3:1). The pH of the solution was adjusted to 10.0 by adding a solution of (25%) with stirring. The reaction mixture was then heated at for 1 h with constant stirring to yield (1). The reaction mixture was cooled to room temperature for further use.
Synthesis of NP (2)
The nanoparticles was also prepared by following the reported procedure25. To the solution of in-situ generated (1) at room temperature, tetraethylorthosilicate (TEOS) (1 mL, 4.48 mmol) was added with vigorous stirring. The stirring was continued for 18 h at room temperature. The formed (2) was magnetically retrieved, washed several times with water and dried for further use (weight 500 mg).
Synthesis of NP (3)
To 30 mL aqueous suspension of (2), an aqueous solution of (50 mg, 0.15 mmol) was added with continuous stirring. The stirring was continued for 6 h and cooled it in ice bath. To this cold solution, an aqueous solution of (50 mg, 1.32 mmol) was added with stirring. The stirring was continued for an additional 12 h. The formed magnetic (3) was then separated using an external magnet and washed several times with distilled water followed by ethanol and dried under vacuum and used for further studies.
Synthesis of Fe3O4@SiO2/PdIINP (4)
To the aqueous solution (pH 10) of (2), an aqueous solution of (50 mg, 0.15 mmol) was added with continuous stirring. The stirring was continued for 18 h. It was then washed several times with distilled water followed by ethanol and dried under vacuum to yield Fe3O4@SiO2/PdIINP (4).
Synthesis of NP (5)
The (3) was suspended in 30 mL distilled water, and 4 mL of ammonia solution was added to it to reach pH 10.0. To this solution, 50 mg (0.15 mmol) of was added and stirred for 18 h. The obtained nanoparticles was washed several times with distilled water followed by ethanol and dried under vacuum to yield (5) nanocomposite.
Study of catalytic activity
In a 10 mL Schlenk tube 0.1 mmol of 4-bromoacetophenon and 0.14 mmol of phenylboronic acid, 0.7 mmol of NaOH and 1 mg of catalyst were taken. The reaction vessel was degassed and filled with dinitrogen by using standard Schlenk techniques. To this reaction mixture, 1 mL dry was added. The dinitrogen environment of the reaction mixture was maintained by using a balloon filled with nitrogen gas. The reaction mixture was then heated at reflux for 6 h with continuous stirring in a preheated () oil bath. The reaction was quenched by adding 4 mL dichloromethane (DCM). The crude products were then charged in a silica gel column. The pure products were isolated either by column chromatography or preparatory thin layered chromatography by using 90% hexane and 10% ethyl acetate as eluent. The compounds were dried under vacuum, and isolated yields were calculated. NMR and NMR spectra of the dried products were recorded.
The identical reaction condition as above was maintained for plotting the reaction profile diagram. The reactions were quenched at different time intervals using DCM. Flash chromatography was carried out to remove the base and catalyst from the reaction mixture. The reaction mixture was then dried at reduced pressure. The composition of products in the reaction mixture at different time intervals was calculated using NMR spectroscopy by comparing the peak intensities of the compounds with the peak intensity of mesitylene (internal standard).
Supplementary Information
Acknowledgements
The authors would like to acknowledge the Council of Scientific and Industrial Research (File No. 01(2932)/18/EMR-II) and the Science and Engineering Research Board (SERB) (File No. CRG/2018/000173 dated 28/02/2019) for financial supports. The authors would like to dedicate this article to Professor Wolfgang Kaim on his 70th birthday.
Author contributions
P.S.: Conducted partial experiments and carried out the partial characterization, data analysis. S.M.: Conducted partial experiments and carried out the partial characterization, data analysis. A.S.: Assist in conducting experiments and conducted some of the characterizations and figure preparation and data analysis. Dr. S.P.: Manuscript writing and figure preparation, data analysis, etc. All the authors reviewed the manuscript
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Parminder Singh and Saumyaranjan Mishra.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88528-6.
References
- 1.Lee JM, Na Y, Han H, Chang S. Cooperative multi-catalyst systems for one-pot organic transformations. Chem. Soc. Rev. 2004;33:302–312. doi: 10.1039/b309033g. [DOI] [PubMed] [Google Scholar]
- 2.Wasilke J-C, Obrey SJ, Tom Baker R, Bazan GC. Concurrent tandem catalysis. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]
- 3.Lohr TL, Marks TJ. Orthogonal tandem catalysis. Nat. Chem. 2015;7:477–482. doi: 10.1038/nchem.2262. [DOI] [PubMed] [Google Scholar]
- 4.Shu X, Jin R, Zhao Z, Cheng T, Liu G. An integrated immobilization strategy manipulates dual active centers to boost enantioselective tandem reactions. Chem. Commun. 2018;54:13244–13247. doi: 10.1039/C8CC07841F. [DOI] [PubMed] [Google Scholar]
- 5.Blaser HU, Federsel H-J. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions. Wiley-VCH; 2010. [Google Scholar]
- 6.Dehury N, Tripathy SK, Sahoo A, Maity N, Patra S. Facile tandem Suzuki coupling/transfer hydrogenation reaction with a bis-heteroscorpionate Pd–Ru complex. Dalton Trans. 2014;43:16597–16600. doi: 10.1039/C4DT02465F. [DOI] [PubMed] [Google Scholar]
- 7.Zanardi A, Mata JA, Peris E. Well-defined Ir/Pd complexes with a triazolyl-diylidene bridge as catalysts for multiple tandem reactions. J. Am. Chem. Soc. 2009;131:14531–14537. doi: 10.1021/ja906028g. [DOI] [PubMed] [Google Scholar]
- 8.Mata JA, Hahn FE, Peris E. Heterometallic complexes, tandem catalysis and catalytic cooperativity. Chem. Sci. 2014;5:1723–1732. doi: 10.1039/C3SC53126K. [DOI] [Google Scholar]
- 9.Pezük LG, Şen B, Hahn FE, Türkmen H. Heterobimetallic complexes bridged by imidazol[4,5- f ][1,10]-phenanthrolin-2-ylidene: Synthesis and catalytic activity in tandem reactions. Organometallics. 2019;38:593–601. doi: 10.1021/acs.organomet.8b00882. [DOI] [Google Scholar]
- 10.Bitzer MJ, Kühn FE, Baratta W. Tandem Suzuki–Miyaura/transfer hydrogenation reaction catalyzed by a Pd–Ru complex bearing an anionic dicarbene. J. Catal. 2016;338:222–226. doi: 10.1016/j.jcat.2016.02.031. [DOI] [Google Scholar]
- 11.Meng J, et al. Switchable catalysts used to control Suzuki cross-coupling and aza-michael addition/asymmetric transfer hydrogenation cascade reactions. ACS Catal. 2019;9:8693–8701. doi: 10.1021/acscatal.9b01593. [DOI] [Google Scholar]
- 12.Zhang G, et al. Multiple functionalized hyperbranched polyethoxysiloxane promotes Suzuki coupling asymmetric transfer hydrogenation one-pot enantioselective organic transformations. ChemCatChem. 2018;10:1882–1888. doi: 10.1002/cctc.201701256. [DOI] [Google Scholar]
- 13.Mahato SK, et al. Fe-polyaniline composite nanofiber catalyst for chemoselective hydrolysis of oxime. J. Colloid Interface Sci. 2018;513:592–601. doi: 10.1016/j.jcis.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Miyaura N, Yamada K, Suzuki A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979;20:3437–3440. doi: 10.1016/S0040-4039(01)95429-2. [DOI] [Google Scholar]
- 15.Knoevenagel E, Bergdolt B. Ueber das Verhalten des -Dihydroterephtalsäuredimethylesters bei höheren Temperaturen und in Gegenwart von Palladiummohr. Berichte der Dtsch. Chem. Gesellschaft. 1903;36:2857–2860. doi: 10.1002/cber.19030360334. [DOI] [Google Scholar]
- 16.Wieland H. Über Hydrierung und Dehydrierung. Berichte der Dtsch. Chem. Gesellschaft. 1912;45:484–493. doi: 10.1002/cber.19120450171. [DOI] [Google Scholar]
- 17.Braude EA, Linstead RP. Hydrogen transfer. Part I. Introductory survey. J. Chem. Soc. 1954 doi: 10.1039/JR9540003544. [DOI] [Google Scholar]
- 18.Majumder A, Naskar R, Roy P, Maity R. Homo- and heterobimetallic complexes bearing NHC ligands: Applications in -arylation of amide, Suzuki–Miyaura coupling reactions, and tandem catalysis. Eur. J. Inorg. Chem. 2019 doi: 10.1002/ejic.201801570. [DOI] [Google Scholar]
- 19.Dehury N, Maity N, Tripathy SK, Basset J-M, Patra S. Dinuclear tetrapyrazolyl palladium complexes exhibiting facile tandem transfer hydrogenation/Suzuki coupling reaction of fluoroarylketone. ACS Catal. 2016;6:5535–5540. doi: 10.1021/acscatal.6b01421. [DOI] [Google Scholar]
- 20.Kaur M, Pramanik S, Kumar M, Bhalla V. Polythiophene-encapsulated bimetallic Au–FeO nano-hybrid materials: A potential tandem photocatalytic system for nondirected C(sp2)-H activation for the synthesis of quinoline carboxylates. ACS Catal. 2017;7:2007–2021. doi: 10.1021/acscatal.6b02681. [DOI] [Google Scholar]
- 21.Woo H, et al. A new hybrid nanocatalyst based on Cu-doped Pd–FeO for tandem synthesis of 2-phenylbenzofurans. J. Mater. Chem. A. 2015;3:20992–20998. doi: 10.1039/C5TA05111H. [DOI] [Google Scholar]
- 22.Jagadeesan D. Multifunctional nanocatalysts for tandem reactions: A leap toward sustainability. Appl. Catal. A. 2016;511:59–77. doi: 10.1016/j.apcata.2015.11.033. [DOI] [Google Scholar]
- 23.Miyamura H, Kobayashi S. Tandem oxidative processes catalyzed by polymer-incarcerated multimetallic nanoclusters with molecular oxygen. Acc. Chem. Res. 2014;47:1054–1066. doi: 10.1021/ar400224f. [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi Y, Fujiwara K, Sugano Y, Ichikawa S, Hirai T. N-monoalkylation of amines with alcohols by tandem photocatalytic and catalytic reactions on TiO2 loaded with Pd nanoparticles. ACS Catal. 2013;3:312–320. doi: 10.1021/cs300756f. [DOI] [Google Scholar]
- 25.Nasir Baig RB, Varma RS. Magnetic silica-supported ruthenium nanoparticles: An efficient catalyst for transfer hydrogenation of carbonyl compounds. ACS Sustain. Chem. Eng. 2013;12:805–809. doi: 10.1021/sc400032k. [DOI] [Google Scholar]
- 26.Bhardwaj M, Sharma H, Paul S, Clark JH. FeO@SiO/EDAC-Pd(0) as a novel and efficient inorganic/organic magnetic composite: Sustainable catalyst for the benzylic C-H bond oxidation and reductive amination under mild conditions. New J. Chem. 2016;40:4952–4961. doi: 10.1039/C5NJ03413B. [DOI] [Google Scholar]
- 27.Arroniz C, Chaubet G, Anderson EA. Dual oxidation state tandem catalysis in the palladium-catalyzed isomerization of alkynyl epoxides to furans. ACS Catal. 2018;8:8290–8295. doi: 10.1021/acscatal.8b02248. [DOI] [Google Scholar]
- 28.Jiang Y, Cai W, Tu W, Zhu M. Facile cross-link method to synthesize magnetic FeO@SiO-chitosan with high adsorption capacity toward hexavalent chromium. J. Chem. Eng. Data. 2019;64:226–233. doi: 10.1021/acs.jced.8b00738. [DOI] [Google Scholar]
- 29.Baby TT, Ramaprabhu S. SiO coated FeO magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta. 2010;80:2016–2022. doi: 10.1016/j.talanta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Prilepskii AY, et al. Urokinase-conjugated magnetite nanoparticles as a promising drug delivery system for targeted thrombolysis: Synthesis and preclinical evaluation. ACS Appl. Mater. Interfaces. 2018;10:36764–36775. doi: 10.1021/acsami.8b14790. [DOI] [PubMed] [Google Scholar]
- 31.Pino N, et al. Structure, activity, and selectivity of bimetallic Pd–Fe/SiO and Pd–Fe/-AlO catalysts for the conversion of furfural. J. Catal. 2017;350:30–40. doi: 10.1016/j.jcat.2017.03.016. [DOI] [Google Scholar]
- 32.Hu H, et al. Unique role of ionic liquid in microwave-assisted synthesis of monodisperse magnetite nanoparticles. Chem. Commun. 2010;46:3866–3868. doi: 10.1039/b927321b. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, et al. A hierarchical heterostructure based on Pd nanoparticles/layered double hydroxide nanowalls for enhanced ethanol electrooxidation. J. Mater. Chem. A. 2013;1:5840–5846. doi: 10.1039/c3ta10588a. [DOI] [Google Scholar]
- 34.Deng QF, Zhang ZF, Cui FJ, Jia LH. Highly dispersed Pd–MnOx nanoparticles supported on graphitic carbon nitride for hydrogen generation from formic acid-formate mixtures. Int. J. Hydrog. Energy. 2017;42:14865–14871. doi: 10.1016/j.ijhydene.2017.04.294. [DOI] [Google Scholar]
- 35.Liu M, et al. The green synthesis of PdO/Pd anchored on hierarchical ZnO microflowers with a synthetic effect for the efficient catalytic reduction of 4-nitrophenol. New J. Chem. 2020;44:7035–7041. doi: 10.1039/D0NJ00001A. [DOI] [Google Scholar]
- 36.Oluwasina OO, Lajide L, Owolabi B. Microcrystalline cellulose from plant wastes through sodium hydroxide-anthraquinone-ethanol pulping. BioResources. 2014;9:3554–3570. doi: 10.15376/biores.9.4.6166-6192. [DOI] [Google Scholar]
- 37.Wang L, et al. Fabrication of hierarchical graphene@FeO@SiO@polyaniline quaternary composite and its improved electrochemical performance. J. Alloys Compd. 2015;634:232–238. doi: 10.1016/j.jallcom.2015.02.062. [DOI] [Google Scholar]
- 38.Lo CK, Xiao D, Choi MMF. Homocysteine-protected gold-coated magnetic nanoparticles: Synthesis and characterisation. J. Mater. Chem. 2007;17:2418–2427. doi: 10.1039/b617500g. [DOI] [Google Scholar]
- 39.Sá S, et al. Magnetically recyclable magnetite–palladium (Nanocat-Fe–Pd) nanocatalyst for the Buchwald–Hartwig reaction. Green Chem. 2014;16:3494–3500. doi: 10.1039/C4GC00558A. [DOI] [Google Scholar]
- 40.Veisi H, Najafi S, Hemmati S. Pd(II)/Pd(0) anchored to magnetic nanoparticles (FeO) modified with biguanidine-chitosan polymer as a novel nanocatalyst for Suzuki–Miyaura coupling reactions. Int. J. Biol. Macromol. 2018;113:186–194. doi: 10.1016/j.ijbiomac.2018.02.120. [DOI] [PubMed] [Google Scholar]
- 41.Tang Y, et al. Effect of Fe state on electrocatalytic activity of Pd–Fe/C catalyst for oxygen reduction. Appl. Surf. Sci. 2010;256:4196–4200. doi: 10.1016/j.apsusc.2010.01.124. [DOI] [Google Scholar]
- 42.Li T, et al. Scalable synthesis of Ag networks with optimized sub-monolayer Au–Pd nanoparticle covering for highly enhanced SERS detection and catalysis. Sci. Rep. 2016;6:1–11. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celebi M, Yurderi M, Bulut A, Kaya M, Zahmakiran M. Palladium nanoparticles supported on amine-functionalized SiO for the catalytic hexavalent chromium reduction. Appl. Catal. B Environ. 2016;180:53–64. doi: 10.1016/j.apcatb.2015.06.020. [DOI] [Google Scholar]
- 44.Wang Q, et al. Hierarchical structure based on Pd(Au) nanoparticles grafted onto magnetite cores and double layered shells: Enhanced activity for catalytic applications. J. Mater. Chem. A. 2013;1:12732–12741. doi: 10.1039/c3ta12814h. [DOI] [Google Scholar]
- 45.Shaikh MN. Pd nanoparticles on green support as dip-catalyst: A facile transfer hydrogenation of olefins and: N-heteroarenes in water. RSC Adv. 2019;9:28199–28206. doi: 10.1039/C9RA06285H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marx S, Baiker A. Beneficial interaction of gold and palladium in bimetallic catalysts for the selective oxidation of benzyl alcohol. J. Phys. Chem. C. 2009;113:6191–6201. doi: 10.1021/jp808362m. [DOI] [Google Scholar]
- 47.Paul S, Islam MM, Islam SM. Suzuki–Miyaura reaction by heterogeneously supported Pd in water: Recent studies. RSC Adv. 2015;5:42193–42221. doi: 10.1039/C4RA17308B. [DOI] [Google Scholar]
- 48.Varadwaj GBB, Rana S, Parida K. Pd(0) nanoparticles supported organofunctionalized clay driving C–C coupling reactions under benign conditions through a Pd(0)/Pd(II) redox interplay. J. Phys. Chem. C. 2014;118:1640–1651. doi: 10.1021/jp410709n. [DOI] [Google Scholar]
- 49.Zhao Y, et al. Fabrication of Te@Pd core–shell hybrids for efficient C–C coupling reactions. J. Phys. Chem. C. 2012;116:7416–7420. doi: 10.1021/jp212197r. [DOI] [Google Scholar]
- 50.Scholz D, Aellig C, Hermans I. Catalytic transfer hydrogenation/hydrogenolysis for reductive upgrading of furfural and 5-(hydroxymethyl)furfural. ChemSusChem. 2014;7:268–275. doi: 10.1002/cssc.201300774. [DOI] [PubMed] [Google Scholar]
- 51.Hauwert P, Boerleider R, Warsink S, Weigand JJ, Elsevier CJ. Mechanism of Pd(NHC)-catalyzed transfer hydrogenation of alkynes. J. Am. Chem. Soc. 2010;132:16900–16910. doi: 10.1021/ja1062407. [DOI] [PubMed] [Google Scholar]
- 52.Németh J, Kiss Á, Hell Z. Palladium-catalyzed transfer hydrogenation of nitrobenzenes: Investigation of the selectivity. React. Kinet. Mech. Catal. 2014;111:115–121. doi: 10.1007/s11144-013-0633-7. [DOI] [Google Scholar]
- 53.Cummings SP, Le TN, Fernandez GE, Quiambao LG, Stokes BJ. Tetrahydroxydiboron-mediated palladium-catalyzed transfer hydrogenation and deuteriation of alkenes and alkynes using water as the stoichiometric h or D atom donor. J. Am. Chem. Soc. 2016;138:6107–6110. doi: 10.1021/jacs.6b02132. [DOI] [PubMed] [Google Scholar]
- 54.Mandal PK, McMurray JS. Pd–C-induced catalytic transfer hydrogenation with triethylsilane. J. Org. Chem. 2007;72:6599–6601. doi: 10.1021/jo0706123. [DOI] [PubMed] [Google Scholar]
- 55.Ciszek B, Fleischer I. Homogeneous palladium-catalyzed transfer hydrogenolysis of benzylic alcohols using formic acid as reductant. Chem. Eur. J. 2018;24:12259–12263. doi: 10.1002/chem.201801466. [DOI] [PubMed] [Google Scholar]
- 56.Nie R, et al. Transfer hydrogenation of bio-fuel with formic acid over biomass-derived N-doped carbon supported acid-resistant Pd catalyst. Catal. Sci. Technol. 2017;7:627–634. doi: 10.1039/C6CY02461K. [DOI] [Google Scholar]
- 57.Mahato SK, Ul Islam R, Acharya C, Witcomb MJ, Mallick K. Polymer-stabilized palladium nanoparticles for the chemoselective transfer hydrogenation of -unsaturated carbonyls: Single-step bottom-up approach. ChemCatChem. 2014;6:1419–1426. [Google Scholar]
- 58.Cui X, et al. Pd-doped Ni nanoparticle-modified N-doped carbon nanocatalyst with high Pd atom utilization for the transfer hydrogenation of nitroarenes. Green Chem. 2018;20:1121–1130. doi: 10.1039/C7GC03710D. [DOI] [Google Scholar]
- 59.Balanta A, Godard C, Claver C. Pd nanoparticles for C–C coupling reactions. Chem. Soc. Rev. 2011;40:4973–4985. doi: 10.1039/c1cs15195a. [DOI] [PubMed] [Google Scholar]
- 60.Zhang B, Yan N. Towards rational design of nanoparticle catalysis in ionic liquids. Catalysts. 2013;3:543–562. doi: 10.3390/catal3020543. [DOI] [Google Scholar]
- 61.Bej A, Ghosh K, Sarkar A, Knight DW. Palladium nanoparticles in the catalysis of coupling reactions. RSC Adv. 2016;6:11446–11453. doi: 10.1039/C5RA26304B. [DOI] [Google Scholar]
- 62.Ellis PJ, Fairlamb IJS, Hackett SFJ, Wilson K, Lee AF. Evidence for the surface-catalyzed Suzuki–Miyaura reaction over palladium nanoparticles: An operando XAS study. Angew. Chem. 2010;122:1864–1868. doi: 10.1002/ange.200906675. [DOI] [PubMed] [Google Scholar]
- 63.Pentsak EO, Ananikov VP. Pseudo-solid-state Suzuki–Miyaura reaction and the role of water formed by dehydration of arylboronic acids. Eur. J. Org. Chem. 2019 doi: 10.1002/ejoc.201900410. [DOI] [Google Scholar]
- 64.Dehury N, Maity N, Tripathy S, Basset J-M, Patra S. Dinuclear tetrapyrazolyl palladium complexes exhibiting facile tandem transfer hydrogenation/Suzuki coupling reaction of fluoroarylketone. ACS Catal. 2016;6:5535–5540. doi: 10.1021/acscatal.6b01421. [DOI] [Google Scholar]
- 65.Depasquale J, Kumar M, Zeller M, Papish ET. Variations on an NHC theme: Which features enhance catalytic transfer hydrogenation with ruthenium complexes? Organometallics. 2013;32:966–979. doi: 10.1021/om300547f. [DOI] [Google Scholar]
- 66.Ji Y, Jain S, Davis RJ. Investigation of Pd leaching from supported Pd catalysts during the heck reaction. J. Phys. Chem. B. 2005;109:17232–17238. doi: 10.1021/jp052527+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.