Abstract
Objective:
The incidence of skin squamous cell carcinoma (SSCC) has recently been increasing, with diverse clinical manifestations.SSCC could metastasize to lymph nodes or other organs, posing a great threat to life. The present study was designed to investigate the function and underlying mechanism of muscleblind-like protein 1 (MBNL1) in skin squamous cell carcinoma.
Methods:
SCL-1 cell was used for vitro model and transfected with MBNL1 or siMBNL1 plasmids. MTT Assays, LDH activity ELISA, and Transwell chamber migration experiment were used to confirm the effects of MBNL1 on cell growth of SCL-1 cell. Western blot analysis was used to analyze the mechanism of MBNL1 in SCL-1 cell.
Results:
Down-regulation of MBNL1 promoted cell metastasis of SSCC, while up-regulation of MBNL1 reduced cell metastasis of SSCC in vitro. Down-regulation of MBNL1 suppressed the protein expression of T cell intracellular antigen (TIAL1), myogenic determinant 1 (MyoD1) and Caspase-3 in vitro. Consistent with these observations, inhibition of TIAL1 or MYOD1 expression attenuated the effects of MBNL1 in SSCC.
Conclusion:
The present study revealed that MBNL1 suppressed thecancer metastatic capacity of SSCC via by TIAL1/MYOD1/Caspase-3 signaling pathways.
Keywords: MBNL1, TIAL1, MYOD1, skin squamous cell carcinoma, signaling pathways
Introduction
SSCC is a type of malignant tumor that originates from the epidermal or accessory keratinocytes.1 SSCC generally occurs after certain types of skin diseases or precancerous diseases, or is caused by progression from various types of precancerous lesions.1 The incidence rate of non-melanoma skin cancer is second only to basal cell carcinoma (CBCC), which is rapidly increasing at a rate of 3% to 10% annually worldwide.2 The incidence rate of SSCC is second only to CBCC, ranking the second in non-melanoma skin cancer, which is generally characterized by nodules and ulcers.3 SSCC mainly occurs in white population. The occurrence of SSCC is closely associated with sun exposure.2 And the risk of SSCC incidence is also significantly increased in areas with strong UV rays.3
MBNL1 is a member of the MBNL protein family, which is a class of conserved splicing factors, playing important roles in the development of multiple tissues.4 MBNL1 and MBNL2 are lowly expressed in embryonic stem cells and can promote the differentiated-cell like alternative splicing and the transformation from stemness to mature splicing.5 MBNL1 is mainly distributed in adult skeletal muscle, myocardium, brain tissue, intestinal tissue, kidney, liver, lung and placenta.6
T cell intracellular antigen (TIAL1) protein can also shuttle from the nucleus to the cytoplasm, participating in the regulation of various aspects of RNA metabolism.7 TIAL1 protein intracellularly binds with its target mRNA, which generally decreases their stability or inhibits its translation and participates in various cellular functions.7-9
Myogenic determinant 1 (MyoD1) is a class of basic helix-loop-helix, belonging to the subfamily of myogenic regulatory factors, which is composed of MyoD, myogenin (MyoG), Myf5 and MRF4 (Myf6, herculin).8 MyoD1 and myogenin are myogenic regulatory protein that is expressed in the early stage of skeletal muscle differentiation, which is only expressed in embryonic skeletal muscle.8,10 Zhu et al.5 reported that Long non-coding RNA MBNL1-AS1 regulates proliferation, migration, and invasion of cancer stem cells in colon cancer. The present study aimed to investigate the function and mechanism of MBNL1 in skin squamous cell carcinoma.
Microarray Analysis
Microarray experiments were performed at the Genminix Informatics (China). Gene expression was analyzed using the Human Gene Expression 4 x 44 K v2 Microarray Kit (Agilent, Santa Clara, CA). Data were obtained using the Agilent Feature Extraction software.
Cell Culture and Transfection
Human skin squamous cell carcinoma SCL-1 cell was obtained from the Cell Bank (Shanghai Genechem Co., Ltd., Shanghai, China). Cell was cultured in Dulbecco’s modified eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) in an incubator at 37°C with 5% CO2. Cell was transfected using Lipofectamine 2000 (Invitrogen, USA) with negative (Control vectors, 5 ntrol vectorsen, USA) ′), MBNL1 (5′-CTCCCGCTTCTACCGAC-3′ and 5′-TTGGTGCATTTAAGGCGGC-3′) or si-MBNL1 (5′-CTCCCGCTTCTTCTACCGAC-3′) mimics (Sangon Biotech (Shanghai) Co., Ltd.).
MTT Assays and LDH Activity Levels
Cell viability was analyzed using MTT assays (Beyotime) at 0, 24, 48 and 72 h after transfection. 20 µl of MTT solution was added to each well and cells were incubated for 4 h at 37°C. Supernatants were removed and formazan crystals were dissolved in 150 µl dimethylsulfoxide (DMSO). Absorbance was measured using a Multiskan Spectrum Microplate Spectrophotometer (Thermo Scientific™, USA) at a wavelength of 492 nm.
LDH activity levels were measured using LDH activity kits (Beyotime). Absorbance was measured using a Multiskan Spectrum Microplate Spectrophotometer (Thermo Scientific™, USA) at a wavelength of 450 nm.
Transwell Chamber Migration Experiment
100 µl of transfection cells (1 x 104/ml) were resuspended in serum-free medium and were seeded onto each well of the insert. 500 µL of media containing 20% FBS (Gibco) was added outside the transwell culture insert (Thermo Scientific™, USA). Transwells were washed twice with PBS and fixed with 4% formaldehyde for 15 min. Cell was stained with 0.1% of crystal violet for 15 min at room temperature, and then observed using a microscope (x100, Leica, Wetzlar, Germany).
Western Blot Analysis
Total proteins of cell were by using RIPA lysis buffer and protease inhibitor cocktail (PMCF, 1:100, Beyotime) and these protein concentration was determined by using BCA Kit (Beyotime). 50 µg protein samples were loaded to 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transferred onto polyvinyl difluoride (PVDF, Thermo Scientific™, USA) membranes. Membranes was incubated with MBNL1, TIAL1, MYOD1 and GAPDH at 4°C overnight after blocking with 5% non-fat milk in tris-buffered saline with 0.1% tween 20 (TBST). The membrane was washed with TBST and then incubated with anti-rabbit secondary antibody (1:5000) for 2 h at room temperature. Immunoreactive bands were visualized using the ECL kit (Thermo Scientific™, USA) and integrated density of the bands was quantified by Quantity One software 3.0 (Bio-Rad).
Statistical Analysis
Results are expressed as means ± SEM. Student’s t-test was used to analyze the difference between 2 independent groups. One-way analysis of variance (ANOVA) and Tukey’s post test was used to analyze the difference between 2 independent groups. p values < 0.05 were considered to indicate statistical significance.
Results
MBNL1 Suppressed Cancer Metastatic Capacity of SSCC
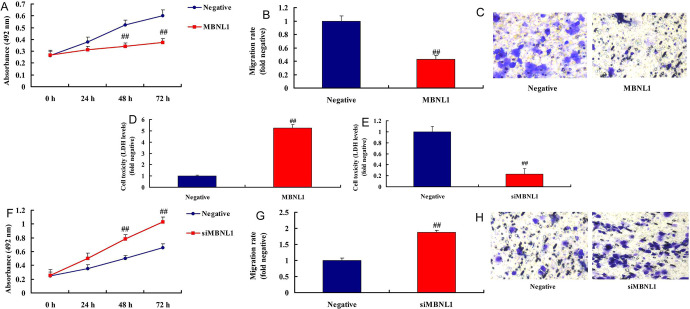
We examined the effects of MBNL1 in SSCC. Over-expression of MBNL1 reduced cell growth and migration rate, and increased LDH activity level in vitro, compared with negative mimics group ( Figure 1A-1D). By contrast, down-regulation of MBNL1 promoted cell growth and migration rate, and reduced LDH activity level in vitro, in comparisons with negative mimics group ( Figure 1E-1 H). In addition, this study revealed that MBNL1 suppressed cell growth in SSCC.
Figure 1.
MBNL1 suppressed cancer metastatic of skin squamous cell carcinoma cell growth, migration rate and LDH activity (A, B, C and D) by over-expression of MBNL1; LDH activity, cell growth, migration rate and (E, F, G, and H) by down-regulation of MBNL1. Negative, negative mimics group; MBNL1, over-expression of MBNL1 group; siMBNL1, down-regulation of MBNL1 group. ##p < 0.01 compared negative group.
TIAL1/MYOD1/Caspase-9/3 Signaling Pathways Was the Target Spot for MBNL1
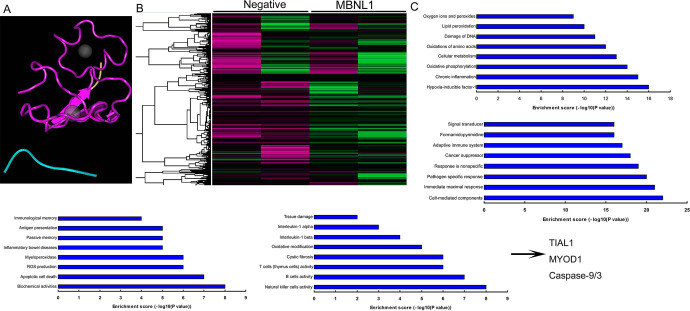
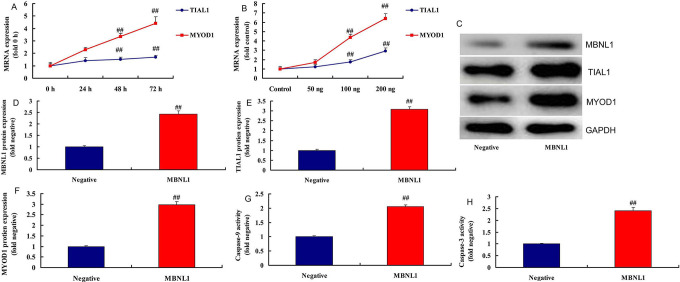
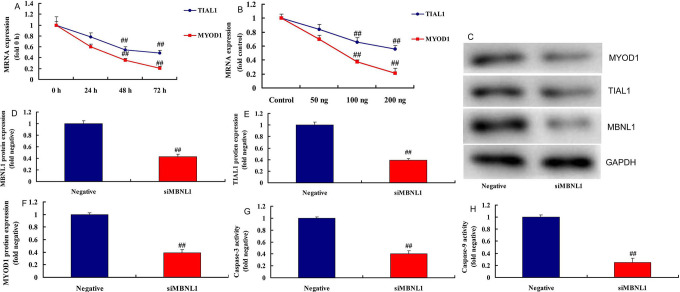
To examine the underlying mechanism of MBNL1 on cell growth in SSCC, we firstly analyzed the destruction of MBNL1 protein (Figure 2A). Gene chip was used to scan gene expression of MBNL1 in SSCC in vitro ( Figure 2B ). As a result, the results showed that TIAL1, MYOD1 and Caspase-9/3 may the target spot for MBNL1 on cell growth in SSCC ( Figure 2C ). In addition, 100 ng MBNL1 plasmid induced the mRNA expression of TIAL1 and MYOD1 in a time-dependent pattern, and 50-200 ng MBNL1 plasmid also induced the mRNA expression of TIAL1 and MYOD1 in a dose-dependent pattern ( Figure 3A-3B ). Western blot analysis showed that MBNL1 plasmid induced the protein expression of MBNL1, TIAL1 and MYOD1 in in vitro model of SSCC, compared with negative group ( Figure 3C-3F ). Over-expression of MBNL1 increased Caspase-9/3 activity levels, in comparison with negative group ( Figure 3G-3 H ). However, 100 ng si-MBNL1 mimics suppressed the mRNA expression of TIAL1 and MYOD1 in a time-dependent pattern ( Figure 4A ). 50-200 ng MBNL1 mimics also decreased the mRNA expression of TIAL1 and MYOD1 in a dose-dependent pattern ( Figure 4B ). Moreover, si-MBNL1 mimics suppressed the protein expression of MBNL1, TIAL1 and MYOD1 in in vitro model of skin squamous cell carcinoma, compared with negative group ( Figure 3C-3F ). Down-regulation of MBNL1 reduced Caspase-9/3 activity levels, in comparison with negative group ( Figure 4G-4 H ). Taken together, this study found that TIAL1/MYOD1/Caspase-9/3 signaling pathway was the target spot of MBNL1 in regulating cell apoptosis of SSCC.
Figure 2.
TIAL1/MYOD1/Caspase-9/3 signaling pathways is target spot for MBNL1 the destruction of MBNL1 protein (A), gene chip (B), analysis result diagram (C). Negative, negative mimics group; MBNL1, over-expression of MBNL1 group.
Figure 3.
MBNL1 regulated TIAL1/MYOD1/Caspase-9/3 signaling pathways TIAL1 and MYOD1 mRNA expression in time-dependence (A), TIAL1 and MYOD1 mRNA expression in does-dependence (B), MBNL1, TIAL1 and MYOD1 protein expression (C, D, E and F), caspase-3/9 activity (G and H). Negative, negative mimics group; MBNL1, over-expression of MBNL1 group. ##p < 0.01 compared negative group.
Figure 4.
SiMBNL1 regulated TIAL1/MYOD1/Caspase-9/3 signaling pathways TIAL1 and MYOD1 mRNA expression in time-dependence (A), TIAL1 and MYOD1 mRNA expression in does-dependence (B), MBNL1, TIAL1 and MYOD1 protein expression (C, D, E and F), caspase-3/9 activity (G and H). Negative, negative mimics group; siMBNL1, down-regulation of MBNL1 group. ##p < 0.01 compared negative group.
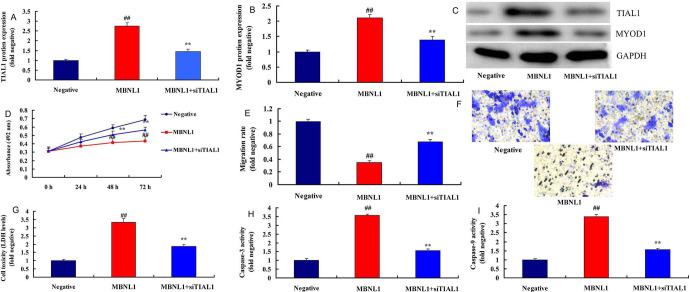
The Inhibition of TIAL1 Attenuated the Effects of MBNL1 on SSCC
Next, we investigated the role of TIAL1 in the effects of MBNL1 on SSCC. si-TIAL1 attenuated the protein expression of TIAL1 and MYOD1 in in vitro model of SSCC by over-expression of MBNL1, in comparison with over-expression of MBNL1 group ( Figure 5A-5C ). The inhibition of TIAL1 attenuated the effects of MBNL1 on the cell growth and migration rate of SSCC, and increased LDH and Caspase-9/3 activity level in vitro, compared with negative mimics group ( Figure 5D-5I ).
Figure 5.
The inhibition of TIAL1 reduced the effects of MBNL1 on skin squamous cell carcinoma TIAL1 and MYOD1 protein expression (A, B and C), cell growth (D), migration rate (E and F), LDH (G) and caspase-3/9 activity (H and I). Negative, negative group; MBNL1, over-expression of MBNL1 group; MBNL1+siTIAL1, over-expression of MBNL1 and si TIAL1 group. ##p < 0.01 compared negative group, **p < 0.01 compared over-expression of MBNL1 group.
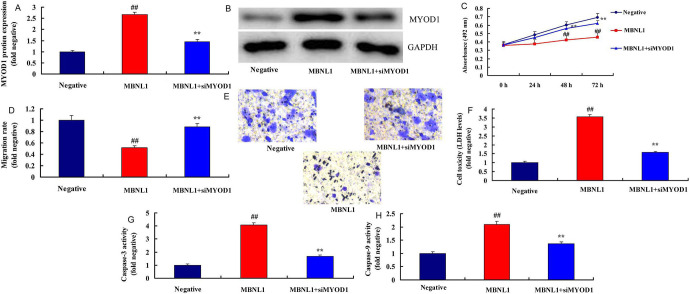
The Inhibition of MYOD1 Attenuated the Effects of MBNL1 on SSCC
To identify the function of MYOD1 in the effects of MBNL1 on SSCC, si-MYOD1 mimics could suppress the protein expression MYOD1 protein expression in in vitro model of SSCC, compared with negative group ( Figure 6A-6B ). Down-regulation of MYOD1 reduced Caspase-9/3 activity levels, in comparison with negative group ( Figure 6G-6 H ). Our results suggested that MBNL1 suppressed the cancer metastatic capacity of SSCC via by the TIAL1/MYOD1/Caspase-9/3 signaling pathway.
Figure 6.
The inhibition of MYOD1 reduced the effects of MBNL1 on skin squamous cell carcinoma MYOD1 protein expression (A and B), cell growth (C), migration rate (D and E), LDH (F) and caspase-3/9 activity (G and H). Negative, negative group; MBNL1, over-expression of MBNL1 group; MBNL1+siMYOD1, over-expression of MBNL1 and si MYOD1 group. ##p < 0.01 compared negative group, **p < 0.01 compared over-expression of MBNL1 group.
Discussion
SSCC is a malignant tumor that occurs in epithelial cells of the epidermis.3 The carcinogenesis and progression of SSCC is a consequence of multi-step progressive and comprehensive effects of both internal and external factors.11 In recent years, studies on the dysregulation of cell proliferation and division, the inactivation of tumor suppressor genes, the activation of oncogenes and the apoptosis of cancer cells have all become research hotspots.11 Our results present showed that MBNL1 suppressed SSCC cell growth and metastatic. Zhu et al.5 reported that Long non-coding RNA MBNL1-AS1 regulates proliferation, migration, and invasion of cancer stem cells in colon cancer.
The TIAL1 protein family is another important RNA-binding protein that has been paid great attention to.12,13 TIAL1 protein can also shuttle between the nucleus and cytoplasm, which is involved in the regulation of various aspects of metabolism. TIAL1 protein binds to their target mRNA within the cells, generally by decreasing their stability or inhibiting their translation.14
MyoD1 gene is the decisive gene for the differentiation of skeletal muscle cell.10,14 MyoD1 is the initiator of cell differentiation, but it cannot directly activate downstream genes. Instead, MyoD1 first activates the downstream related genes to regulate cell proliferation, apoptosis, inflammation and etc.12,15 Our results show that TIAL1/MYOD1/Caspase-9/3 signaling pathways is target spot for MBNL1 in SSCC. Liu et al.16 showed that TIAL1 is as a tumor suppressor by i modulate the p53 pathway.17
The Caspase family is closely associated with the apoptotic mechanism of eukaryotic cells and is involved in the regulation of cell growth and differentiation.18 Caspase-9/3 are important apoptotic executors, and are widely distributed in various types of cells in the form of inactive zymogen, with potent pro-apoptotic ability.19 In this study, we found that Over-expression of MBNL1 increased Caspase-9/3 activity levels in SSCC. Zhao et al.20 suggested that MeCP2 promoted gastric cancer progression through regulating MYOD1/Caspase-3 signaling pathways.
In conclusion, MBNL1 suppressed cancer metastatic of skin squamous cell carcinoma, and TIAL1/MYOD1/Caspase-9/3 signaling pathways is target spot for MBNL1 in skin squamous cell carcinoma. The inhibition of TIAL1 or MYOD1 reduced the effects of MBNL1 on skin squamous cell carcinoma. The results of this study provide evidence that MBNL1 may be a potent therapeutic agent for treatment of skin squamous cell carcinoma by TIAL1/MYOD1/Caspase-9/3 signaling pathways.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article This study was funded by the Natural Science Foundation of Hubei Province (Grant No. 2015CFB379) and Natural Science Foundation of Hubei Province (Grant No. 2017CFB384).
ORCID iD: Jiaorong Chen  https://orcid.org/0000-0003-0933-3939
https://orcid.org/0000-0003-0933-3939
Jiaqi Wang  https://orcid.org/0000-0003-3949-0206
https://orcid.org/0000-0003-3949-0206
Jingyi Qian  https://orcid.org/0000-0002-0220-677X
https://orcid.org/0000-0002-0220-677X
Mengying Bao  https://orcid.org/0000-0003-3274-9940
https://orcid.org/0000-0003-3274-9940
Xin Zhang  https://orcid.org/0000-0003-2957-9784
https://orcid.org/0000-0003-2957-9784
Zheng Huang  https://orcid.org/0000-0001-5143-5192
https://orcid.org/0000-0001-5143-5192
Informed consent: For this type of study informed consent is not required.
References
- 1. Wang X, Liu X, Li D, Wang X, Huang W, Li B. Concurrent selective lymph node radiotherapy and s-1 plus cisplatin for esophageal squamous cell carcinoma: a phase ii study. Ann Surg Oncol. 2019;26(6):1886–1892. [DOI] [PubMed] [Google Scholar]
- 2. Wu F, Zhang S, Xiong A. et al. A phase ii clinical trial of apatinib in pretreated advanced non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e831–e842. [DOI] [PubMed] [Google Scholar]
- 3. Gold KA, Kies MS, William WN, Jr, Johnson FM, Lee JJ, Glisson BS. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase 2 clinical trial. Cancer. 2018;124(10):2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welte T, Tuck AC, Papasaikas P. et al. The RNA hairpin binder TRIM71 modulates alternative splicing by repressing MBNL1. Genes Dev. 2019;33(17-18):1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu K, Wang Y, Liu L, Li S, Yu W. Long non-coding RNA MBNL1-AS1 regulates proliferation, migration, and invasion of cancer stem cells in colon cancer by interacting with MYL9 via sponging microRNA-412-3p. Clin Res Hepatol Gastroenterol. 2019;44(1):101–114. [DOI] [PubMed] [Google Scholar]
- 6. Li P, Xing W, Xu J. et al. microRNA-301b-3p downregulation underlies a novel inhibitory role of long non-coding RNA MBNL1-AS1 in non-small cell lung cancer. Stem Cell Res Ther. 2019;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawakami A, Tian Q, Duan X, Streuli M, Schlossman SF, Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc Natl Acad Sci U S A. 1992;89(18):8681–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai JW, ChangChien YC, Lee JC. et al. The expanding morphological and genetic spectrum of MYOD1-mutant spindle cell/sclerosing rhabdomyosarcomas: a clinicopathological and molecular comparison of mutated and non-mutated cases. Histopathology. 2019;74(6):933–943. [DOI] [PubMed] [Google Scholar]
- 9. Li W, Li Y, Kedersha N. et al. Cell proteins TIA-1 and TIAR interact with the 3’ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol. 2002;76(23):11989–12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shukla A, Narayanan DL, Asher U, Girisha KM. A novel bi-allelic loss-of-function variant in MYOD1: further evidence for gene-disease association and phenotypic variability in MYOD1-related myopathy. Clin Genet. 2019;96(3):276–277. [DOI] [PubMed] [Google Scholar]
- 11. William WN, Feng L, Ferrarotto R. et al. Gefitinib for patients with incurable cutaneous squamous cell carcinoma: a single-arm phase II clinical trial. J Am Acad Dermatol. 2017;77(6):1110–1113. e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Q, Yu H, Yu H, Ma M, Ma Y, Li R. miR2233p/TIAL1 interaction is involved in the mechanisms associated with the neuroprotective effects of dexmedetomidine on hippocampal neuronal cells in vitro. Mol Med Rep. 2019;19(2):805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang T, Delestienne N, Huez G, Kruys V, Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J Cell Sci. 2005;118(Pt 23):5453–5463. [DOI] [PubMed] [Google Scholar]
- 14. Zhao W, Zhao J, Hou M. et al. HuR and TIA1/TIAL1 are involved in regulation of alternative splicing of SIRT1 pre-mRNA. Int J Mol Sci. 2014;15(2):2946–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen W, Huang B, He Y, Shi L, Yang J. Long non-coding RNA RP11-820 promotes extracellular matrix production via regulating miR-3178/MYOD1 in human trabecular meshwork cells. FEBS J. 2019;287(5):978–990. [DOI] [PubMed] [Google Scholar]
- 16. Liu L, Yue H, Liu Q. et al. LncRNA MT1JP functions as a tumor suppressor by interacting with TIAR to modulate the p53 pathway. Oncotarget. 2016;7(13):15787–15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Jiang Y, Lu X. et al. Genomic characterization of cervical cancer based on human papillomavirus status. Gynecol Oncol. 2019;152(3):629–637. [DOI] [PubMed] [Google Scholar]
- 18. Ahagh MH, Dehghan G, Mehdipour M. et al. Synthesis, characterization, anti-proliferative properties and DNA binding of benzochromene derivatives: increased bax/Bcl-2 ratio and caspase-dependent apoptosis in colorectal cancer cell line. Bioorg Chem. 2019;93:103329. [DOI] [PubMed] [Google Scholar]
- 19. Jiao C, Chen W, Tan X. et al. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J Ethnopharmacol. 2020;247:112256. Epub 2019 Oct 3. [DOI] [PubMed] [Google Scholar]
- 20. Zhao L, Liu Y, Tong D. et al. MeCP2 promotes gastric cancer progression through regulating FOXF1/Wnt5a/beta-catenin and MYOD1/caspase-3 signaling pathways. EBioMed 2017;16:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]