Abstract
Background:
Opioid use and public insurance have been correlated with worse outcomes in a number of orthopaedic surgeries. These factors have not been investigated with anterior cruciate ligament reconstruction (ACLR).
Purpose/Hypothesis:
To evaluate if narcotic use, physical therapy location, and insurance type are predictors of patient-reported outcomes after ACLR. It was hypothesized that at 1 year postsurgically, increased postoperative narcotic use would be associated with worse outcomes, physical therapy obtained within the authors’ integrated health care system would lead to better outcomes, and public insurance would lead to worse outcomes and athletic activity.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
All patients undergoing unilateral, primary ACLR between January 2015 and February 2016 at a large health system were enrolled in a standard-of-care prospective cohort. Knee injury and Osteoarthritis Score (KOOS) and the Hospital for Special Surgery Pediatric–Functional Activity Brief Scale (HSS Pedi-FABS) were collected before surgery and at 1 year postoperatively. Concomitant knee pathology was assessed arthroscopically and electronically captured. Patient records were analyzed to determine physical therapy location, insurance status, and narcotic use. Multivariable regression analyses were used to identify significant predictors of the KOOS and HSS Pedi-FABS score.
Results:
A total of 258 patients were included in the analysis (mean age, 25.8; 51.2% women). In multivariable regression analysis, narcotic use, physical therapy location, and insurance type were not independent predictors of any KOOS subscales. Public insurance was associated with a lower HSS Pedi-FABS score (–4.551, P = .047) in multivariable analysis. Narcotic use or physical therapy location was not associated with the HSS Pedi-FABS score.
Conclusion:
Increased narcotic use surrounding surgery, physical therapy location within the authors’ health care system, and public versus private insurance were not associated with disease-specific KOOS subscale scores. Patients with public insurance had worse HSS Pedi-FABS activity scores compared with patients with private insurance, but neither narcotic use nor physical therapy location was associated with activity scores. Physical therapy location did not influence outcomes, suggesting that patients be given a choice in the location they received physical therapy (as long as a standardized protocol is followed) to maximize compliance.
Keywords: anterior cruciate ligament reconstruction, opioid use, payer status, KOOS, HSS Pedi-FABS
Anterior cruciate ligament (ACL) injury is one of the most common debilitating injuries among athletes, affecting an estimated 98,000 to 250,000 people each year in the United States. Good short- and long-term success of ACL reconstruction (ACLR) is well-established in the literature.3,6,32,50 Prior studies have identified numerous pre- and intraoperative factors, such as age, body mass index (BMI), and coincident lateral collateral ligament injury, that predict patient outcomes.8,12,13,26,49,53 Preoperative narcotic use has been previously identified as a factor increasing postoperative narcotic use in orthopaedic surgery.4 Preoperative narcotic use has similarly been linked to worse patient-reported outcome measures in spine surgeries.31 A retrospective database study of patients who underwent ACLR identified that 4.71% of patients were still using opioids at 12 months postsurgically, with preoperative opioid use being associated with prolonged postoperative use.1 However, no prospectively enrolled cohort study has examined the relationship of patient factors, including insurance type, physical therapy (PT) location, and narcotic use, with patient-reported outcomes after ACLR.
Postoperative pain control is critical in orthopaedic outcomes given the importance of early and sustained range of motion and activity. However, narcotic use is rising in the United States, with concomitant increases in opioid-related deaths.10,55 In 2016, there were 42,249 deaths due to opioid overdose, with 17,087 deaths due to prescription opioid overdoses.22 More than 80% of heroin users were exposed to prescription opioids before heroin initiation.25 Thus, legal prescriptions for opioids represent many people’s first exposure to them, and as a result, there has been increased emphasis on physician stewardship of these medications.
Orthopaedic surgeons were among the top 3 prescribers of opioid analgesics in 2018, calculated by the mean number of opioid prescriptions by prescriber, writing 5.8% of all prescriptions.19 Preoperative opioid use has been associated with increased postoperative opioid use4 and worse clinical outcomes in an array of orthopaedic procedures, including joint arthroplasty40,41,61 and patellofemoral stabilization surgery.28 Within the context of ACLR, an analysis of a retrospective insurance database showed that preoperative narcotic use was predictive of postoperative narcotic use.2 Another study in which 100 adolescents were interviewed after ACLR found that 99% of the patients filled their opioid prescriptions, with a mean of 60 pills (5 mg each) per patient and 15.5% of patients continuing to use opioids 1 week after surgery.51 Factors predicting postoperative opioid abuse after orthopaedic surgery include lower socioeconomic status, prior pain-medication use, high health-seeking behavior, mental health disorders, insomnia, and substance abuse.43 However, no prospective cohort study has yet determined whether either pre- or postoperative narcotic use is a significant predictor of patient-reported outcomes in ACLR.
PT is a cornerstone of orthopaedic rehabilitation, especially after ACLR.18,27,58 The effects of variation in PT regimens, however, are difficult to quantify and remain the subject of continued research.30 Specific components of PT regimens have also been associated with improvement in outcome measures.59 Variations in PT regimens and delivery systems have prompted orthopaedic surgeons and physical therapists to develop standardized rehabilitation guidelines.58 Because physical therapists within a health system receive training in standardized, monitored guidelines, it is possible that patients who undergo PT at affiliated sites have improved outcomes compared with those whose PT is conducted at other external PT locations.
Compared with private payer insurance, public payer insurance in the United States has been associated with more severe initial presentation in ACL injury and reduced access to postoperative PT services after ACLR.44,57 Public insurance has also been associated with differences in patient-reported outcomes and complications in joint arthroplasty.16,21,36,47 However, the relationship between insurance type and postoperative outcome has not yet been defined in ACLR.
The purpose of this investigation was to determine whether the following are significant predictors of patient-reported outcomes after ACLR: (1) preoperative narcotic use and/or quantity of postoperative narcotic pills, (2) location of PT appointments, and (3) payer type (public vs private).
Methods
Participants
Patients undergoing primary ACLR at a large academic hospital system between January 2015 and February 2016 were selected from the Orthopaedic Minimal Data Set Episode of Care prospective cohort.9 This is a standard-of-care prospective cohort in which all surgical patients are enrolled and where their characteristics and patient-reported outcomes are obtained serially. The study was approved by our hospital’s institutional review board.
Of the 378 patients initially eligible for the study, 3 were excluded at enrollment because of incomplete enrollment and/or technical failure of the data capture system, and 3 more were excluded because they had bilateral reconstructions. Of the 372 patients who had unilateral ACLR and completed the registration questionnaire at enrollment, 260 completed the 1-year postoperative questionnaire (follow-up, 70%) (Figure 1). Of these patients, 2 were removed as there was missing data during retrospective chart review. In total, 258 patients were included in this study.
Figure 1.
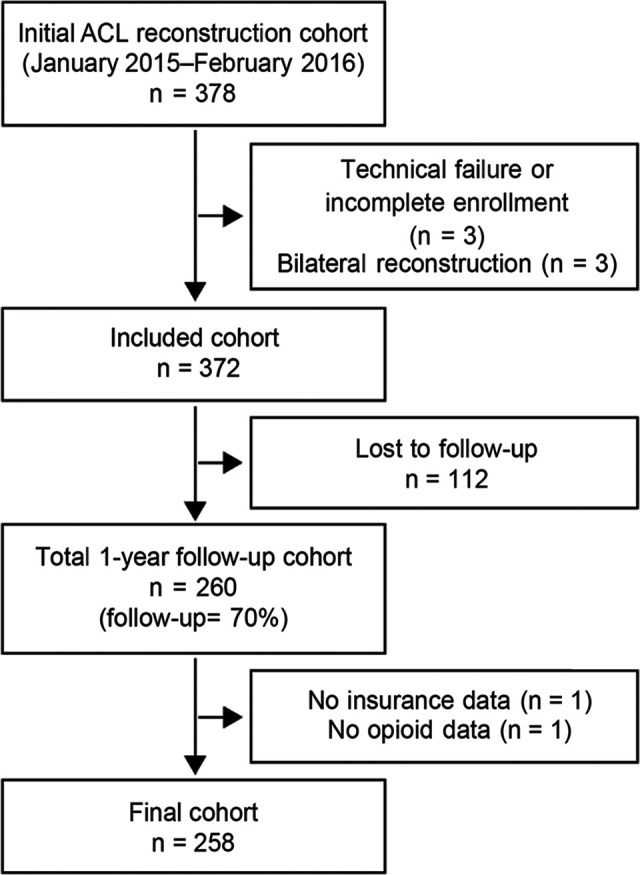
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram for cohort recruitment and 1-year follow-up methodology. ACL, anterior cruciate ligament.
Data Collection
Data were collected from patients before surgery (baseline) and at 1 year after surgery. The 1-year time point was chosen because of the equivalence of Knee injury and Osteoarthritis Outcome Score (KOOS) at 12 and 24 months in a large cohort of patients who had ACLR.48 Preoperatively, each patient was asked to complete questionnaires that collected their data and mental health status (Veterans RAND 12-Item Health Survey Mental Component Summary [VR-12 MCS]), and they were asked to complete patient-reported outcomes preoperatively and at 1-year follow-up. The following patient-reported outcomes were used: KOOS--Pain, KOOS--Physical Function Short Form (PS), KOOS--Quality of Life (QoL), and Hospital for Special Surgery Pediatric–Functional Activity Brief Scale (HSS Pedi-FABS). Only patients who self-identified as athletes (218 patients at baseline, 192 patients at 1 year) were asked to complete the HSS Pedi-FABS questionnaire.
Patient-Reported Outcome Measures
KOOS measures knee function, with KOOS--Pain measuring pain aspects, KOOS-PS measuring physical functioning of the knee, and KOOS-QoL measuring quality of life, including mental and social aspects of knee function.45,46 The KOOS is normalized to a 0 to 100 scale, with 0 indicating extreme functional impairment and 100 indicating no impairment.46 The KOOS has been reported in many studies, such as those performed by the Multicenter Orthopaedic Outcomes Network (MOON) Group,39 Barenius et al,5 Möller et al,38 and Ingelsrud et al.24 The KOOS was chosen over the International Knee Documentation Committee score because of its decreased need for long-term recall and reporting in separate psychometric domains.7 HSS Pedi-FABS measures activity on a 0- to 30-point scale, with 0 meaning activity is extremely impaired and 30 meaning activity is not impaired.14,15
The HSS Pedi-FABS is considered a superset of the Marx activity rating scale, as HSS Pedi-FABS contains the 4 analogous sections in the Marx scale. HSS Pedi-FABS was chosen for all self-identified athlete patients regardless of age, instead of the Marx scale, which has been reported in several large retrospective trials, including those by the MOON Group,33,39,56 to mitigate the latter’s observed ceiling effect.37 Additionally, the time periods differ between HSS Pedi-FABS (measuring activity level over the past month) and the Marx activity rating scale (measuring activity level over the past year). Thus, HSS Pedi-FABS is considered more useful for identifying short-term changes in activity level, such as those seen after ACLR.35 While the HSS Pedi-FABS scale has not been rigorously validated for the adult population, a recent study suggests that it provides similar comparative results with the Marx activity rating scale.35
Surgeon Involvement
Directly after each surgery, the operating surgeon was asked to complete a detailed form that documented intraoperative findings. Electronically captured surgeon input data included physical examination and arthroscopic findings, surgical history, type and source of ACL graft, surgical technique, and concomitant knee pathologies and their associated grades. All 8 surgeons (P.S., L.D.F., K.P.S., J.T.R., M.H.J., A.A.M., R.D.P., J.S.W.) in this study were board-certified orthopaedic surgeons who were staff physicians at our institution. Patient care and surgical planning were directed by the operating surgeon. After each index surgery, patients were provided with an information packet that outlined a specific rehabilitation program to provide to their physical therapist at internal and external PT offices.
Review of the Electronic Medical Record
Narcotic data were collected from the electronic medical record (Epic Systems) based on prescription orders during both the pre- and postoperative periods. The preoperative period was defined as the time of diagnosis to the time of surgery, with a maximum duration of 3 months. The postoperative period was defined from the time of surgery to 6 months after. Narcotics prescribed for knee pain related to the patient’s ACL pathology were included. All refills of narcotics were collected to estimate the number of narcotic pills actually consumed. Insurance information and PT location were similarly collected from the electronic medical record.
Statistical Methods
Continuous variables were summarized using means ± SDs, while categorical factors were summarized with frequencies and percentages. Initially, univariate associations between key predictors (sex, smoking status, graft source, prior contralateral ACLR, maximum osteoarthritis [OA] grade, if preoperative narcotics were prescribed, PT location, insurance payer, BMI, number of postoperative pills prescribed, years of education, age, baseline outcome scores, and VR-12 MCS) and the 4 outcome variables (KOOS--Pain, KOOS-PS, KOOS-QoL, and HSS Pedi-FABS) were assessed using analysis of variance models for categorical factors and Pearson correlations for continuous measures.
Multicollinearity among predictors was assessed using variance inflation factors and condition indices; variance inflation factors >10 and condition indices >30 are generally used to identify strong multicollinearity.29 Because no strong multicollinearity was observed, all predictors were included in the multivariable models. Multicollinearity was also assessed by removing factors associated with the outcome in the multivariable models 1 at a time and refitting the multivariable model. Refitting the models did not lead to any variables changing in significance.
Our primary multivariable models were based on linear regression. These models were used to evaluate whether narcotic use, PT location, and insurance type evaluated with known risk factors for patient-reported outcomes were predictors. Residual patterns were assessed and potential ceiling effects were tested. Initial models included restricted cubic splines for continuous measures to allow for nonlinear effects. No significant nonlinear effects were observed, so the splines were removed to maintain an events per variable ratio of 10:1. Two sensitivity analyses were performed. The first used a censored regression (Tobit) model to account for possible censoring of responses at the extremes of the distribution, while the second used proportional odds logistic regression models to categorically evaluate the endpoints. Both sensitivity analyses agreed with the multivariable models. The chi-square test was used for 2 × 2 contingency table analyses.
A power analysis was performed using a beta value of 0.8 and a minimal clinically important difference (MCID) of 8 for KOOS outcomes and a clinically derived minimal change of 2 for HSS Pedi-FABS.45 The study was adequately powered to detect meaningful differences for all KOOS measures and HSS Pedi-FABS at the predetermined MCID values. For linear and censored regression models, mean effects with 95% CIs and P values are presented. Data management and initial summaries were performed using SAS software (Version 9.4; SAS Institute). Multivariate models were fit using R software (Version 3.3). A significance level of .05 was assumed for all tests.
Results
Study Population
Table 1 lists the predictor variables for the patients used in our study, along with the baseline patient-reported outcome scores. The mean (± SD) age of patients was 25.8 ± 11.8 years, and 51.2% were women. An autograft reconstruction was used in 90.3% of patients, with the rest receiving an allograft tendon. A total of 6.2% of patients had a previous contralateral ACLR, and 14.7% had grade 3 or 4 articular cartilage knee pathologies. Patients were prescribed a mean of 78.2 ± 42.2 narcotic pills during the postoperative period. The majority of the cohort carried private insurance (92.6% of patients), lacked preoperative PT (65.1% of patients), and utilized our health system for postoperative PT (74.8% of patients). Of the patients who were covered by publicly funded insurance, all but 3 were covered under Medicaid.
Table 1.
Predictor Variables (N = 258 Patients)a
| Variable | Value | Variable | Value |
|---|---|---|---|
| Age, y | 25.8 ± 11.8 | Prior contralateral ACLR | |
| Sex | No | 242 (93.8) | |
| Female | 132 (51.2) | Yes | 16 (6.2) |
| Male | 126 (48.8) | Maximum extent of knee OA | |
| BMI | 26.4 ± 5.6 | Normal/grade 1 or 2 | 220 (85.3) |
| Years of education | 13.1 ± 3.7 | Grade 3 or 4 | 38 (14.7) |
| Smoking status | Preoperative narcotic use | ||
| Never smoker | 218 (84.5) | No | 236 (91.5) |
| Former smoker | 22 (8.5) | Yes | 22 (8.5) |
| Current smoker | 18 (7.0) | No. of postoperative narcotic pills prescribed | 78.2 ± 42.2 |
| VR-12 MCS | 54.7 ± 9.8 | Were all preoperative PT visits internal? | |
| Baseline outcome scores | No/None | 168 (65.1) | |
| KOOS-QoL | 38.3 ± 21.0 | Yes | 90 (34.9) |
| KOOS--Pain | 71.1 ± 18.6 | Were all postoperative PT visits internal? | |
| KOOS-PS | 30.3 ± 14.5 | No | 40 (15.5) |
| HSS Pedi-FABSb | 14.0 ± 11.1 | Yes | 193 (74.8) |
| Graft source (surgical limb) | None | 25 (9.7) | |
| Autograft | 233 (90.3) | Insurance category | |
| Allograft | 25 (9.7) | Private | 239 (92.6) |
| Public | 19 (7.4) |
aData are presented as mean ± SD or number of patients (%). ACLR, anterior cruciate ligament reconstruction; BMI, body mass index; HSS Pedi-FABS, Hospital for Special Surgery Pediatric–Functional Activity Brief Scale; KOOS, Knee injury and Osteoarthritis Outcome Score; MCS, Mental Component Summary; OA, osteoarthritis; PS, Physical Function Short Form; PT, physical therapy; QoL, Quality of Life; VR-12, Veterans RAND 12-Item Health Survey.
bn = 218 patients.
Table 2 shows the patient-reported outcome questionnaires at initial enrollment and the 1-year follow-up. Of the 218 patients in the initial cohort who self-identified as athletes and subsequently answered questions pertaining to HSS Pedi-FABS, 26 did not self-identify as an athlete at the 1-year follow-up, resulting in 192 patients with completed HSS Pedi-FABS measures at 1 year. A 2 × 2 contingency table analysis demonstrated no difference in athlete status between those on public versus private insurance (P = .83).
Table 2.
Patient-Reported Outcome Measures at Baseline and 1-Year Follow-Upa
| Outcome Measure | Baseline | 1-Year Follow-Up | ||
|---|---|---|---|---|
| n | Score (Mean ± SD) | n | Score (Mean ± SD) | |
| KOOS-QoL | 258 | 38.3 ± 21.0 | 258 | 71.8 ± 21.4 |
| KOOS--Pain | 258 | 71.1 ± 18.6 | 258 | 89.2 ± 13.1 |
| KOOS-PS | 258 | 68.6 ± 14.6 | 258 | 86.9 ± 12.1 |
| HSS Pedi-FABS | 218 | 14.0 ± 11.1 | 192 | 17.3 ± 9.3 |
aHSS Pedi-FABS, Hospital for Special Surgery Pediatric–Functional Activity Brief Scale; KOOS, Knee injury and Osteoarthritis Outcome Score; PS, Physical Function Short Form; QoL, Quality of Life.
KOOS-QoL
In the multivariable model, only VR-12 MCS (P = .009) and baseline KOOS-QoL (P < .001) remained statistically significant. Every incremental increase in VR-12 MCS led to an increase of 0.346 points for the 1-year KOOS-QoL, and every point increase in the baseline KOOS-QoL measure led to an increase of 0.266 points for the 1-year KOOS-QoL. Narcotic use, PT location, and insurance type were not independent predictors of 1-year KOOS-QoL.
KOOS--Pain
In multivariable analysis, age (β = –0.335; P < .001), VR-12 MCS (β = 0.218; P = .005), baseline KOOS--Pain (β = 0.249; P < .001), and age × education interaction (β = 0.060; P = .001) were all statistically significant for 1-year KOOS--Pain. Specifically, age was negatively associated with 1-year KOOS--Pain at follow-up, indicating that older patients had lower scores, but this effect was attenuated among those with greater education. Narcotic use, PT location, and insurance type were not independent predictors of 1-year KOOS--Pain.
KOOS-PS
In the multivariable analysis, age (β = 0.256; P = .004), baseline KOOS-PS (β = 0.309; P < .001), VR-12 MCS (β = –0.259; P < .001), and age × education interactions (β = –0.069; P < .001) remained significant. As above, older age was associated with worse (higher) scores, but this impact was weakened among those with higher education levels. When performing backward elimination to determine multicollinearity between postoperative narcotic use and other variables included in the multivariable model, we noticed that removing age and the age × education interaction has a large impact on the estimate of postoperative narcotic use (after age and age × education were removed, the multivariable estimate for postoperative narcotics was β = 0.032 with a P value of .058). Narcotic use, PT location, and insurance type were not independent predictors of 1-year KOOS-PS.
HSS Pedi-FABS
In the multivariable analysis, insurance type (public compared with private, –4.551; P = .047), BMI (β = –0.421; P = .009), education (β = –0.722; P < .001), and baseline HSS Pedi-FABS scores (β = 0.140; P = .011) were statistically significant predictors of 1-year HSS Pedi-FABS scores (Table 3). Importantly, those with public insurance, after controlling for other factors, had a lower HSS Pedi-FABS score (a mean of 4.551 points lower) at the 1-year follow-up. Narcotic use, PT location, and insurance type were not independent predictors of 1-year HSS Pedi-FABS. There was no significant difference between patients with no postoperative PT location recorded, patients with all internal PT appointments, and patients with all external PT appointments (the reference level for the multivariable testing of PT location). Subsequent analysis utilizing the Marx activity score–specific questions of HSS Pedi-FABS demonstrated similar significance values to the outcomes for HSS Pedi-FABS, although public insurance was no longer below the predefined significance threshold, with P = .077.
Table 3.
Multivariable Linear Model for HSS Pedi-FABSa
| Factor | Estimate (95% CI) | P Value |
|---|---|---|
| Intercept | 38.969 (28.831 to 49.108) | <.001 |
| Male sex | 0.332 (–2.235 to 2.898) | .8 |
| Smoker: former | –2.811 (–7.613 to 1.991) | .25 |
| Smoker: current | 0.679 (–5.575 to 6.933) | .83 |
| Graft source: allograft | –4.777 (–10.525 to 0.971) | .10 |
| Prior contralateral ACLR | 3.112 (–1.959 to 8.182) | .23 |
| Maximum extent of OA: grade 3 or 4 | –0.662 (–5.024 to 3.701) | .76 |
| Preoperative narcotic use | –2.188 (–6.022 to 1.646) | .26 |
| All preop PT visits, internal | 1.186 (–1.384 to 3.756) | .36 |
| All postoperative PT visits, internal | –2.339 (–5.686 to 1.009) | .17 |
| No postoperative PT visits recorded | –1.823 (–6.598 to 2.951) | .45 |
| Insurance: public | –4.551 (–9.039 to –0.062) | .047 |
| Age | –0.114 (–0.291 to 0.063) | .20 |
| BMI | –0.421 (–0.735 to –0.107) | .009 |
| Years of education | –0.722 (–1.124 to –0.320) | <.001 |
| VR-12 MCS | 0.024 (–0.095 to 0.143) | .69 |
| Baseline HSS Pedi-FABS | 0.140 (0.032 to 0.248) | .011 |
| Postop narcotics, quantity | –0.011 (–0.040 to 0.019) | .47 |
| Age × education | 0.023 (–0.006 to 0.053) | .12 |
aBolded P values indicate statistical significance (P < .05). ACLR, anterior cruciate ligament reconstruction; BMI, body mass index; HSS Pedi-FABS, Hospital for Special Surgery Pediatric –Functional Activity Brief Scale; MCS, Mental Component Summary; OA, osteoarthritis; postop, postoperative; PT, physical therapy; VR-12, Veterans RAND 12-Item Health Survey.
Discussion
The most important findings of our study were that, according to multivariable modeling, narcotic use, PT location, and insurance type were not independent risk factors for KOOS subscales. However, for athletic activity, public insurance type was a significant risk factor for worse HSS Pedi-FABS scores. A summary of all risk factors that were found to be statistically significant predictors of KOOS and HSS Pedi-FABS scores is shown in Table 4.
Table 4.
Summary of Significant Predictors for Each Multivariable Model Outcomea
| Outcome | ||||
|---|---|---|---|---|
| KOOS-QoL | KOOS–Pain | KOOS-PS | HSS Pedi-FABS | |
| Predictor | VR-12 MCS | Age | Age | Insurance type |
| Baseline KOOS-QoL | VR-12 MCS | VR-12 MCS | BMI | |
| Baseline KOOS–Pain | Baseline KOOS-PS | Education | ||
| Age × education | Age × education | Baseline HSS Pedi-FABS | ||
aBMI, body mass index; HSS Pedi-FABS, Hospital for Special Surgery Pediatric–Functional Activity Brief Scale; KOOS, Knee injury and Osteoarthritis Outcome Score; MCS, Mental Component Summary; PS, Physical Function Short Form; QoL, Quality of Life; VR-12, Veterans RAND 12-Item Health Survey.
As narcotic use was not associated with KOOS subscale scores or HSS Pedi-FABS scores, patients should be switched to an analgesic with less addictive potential as soon as their pain levels permit. Previous studies have identified ways to decrease opioid use after orthopaedic surgery, such as through acetominophen52,60 or parecoxib11,23,34 administration. With opioid-related deaths increasing17 and documented negative effects of opioids after surgery,20 providers should attempt to use a multimodal pain-management strategy to reduce opioid addiction burden on patients.
PT location, coded as all within our institution’s network or all outside our network, was not significantly associated with a difference in patient-reported outcomes. Our study was powered to detect a change at least as great as the MCID for the KOOS outcome measures, suggesting that PT location is not a variable that orthopaedic surgeons should focus on when determining treatment plans for patients who have undergone ACLR. All patients who underwent ACLR at our institution receive an informational packet on proper ACL rehabilitation, which includes instructions on exercises similar to those performed in PT.58 As such, patients whose PT was completed outside of our institution’s network received the same protocol as those within our network. PT location is not significantly associated with outcomes, so patients should receive care where it is most convenient for them as long as a standardized protocol is given. Having a standardized ACL therapy program is likely more important than location of therapy.
Insurance payer is only significantly associated with athletic activity as measured by the HSS Pedi-FABS score. Multivariable analysis of HSS Pedi-FABS showed that those with publicly funded insurance scored 4.5 points lower than patients with private insurance after adjustment for all other factors. Insurance type is highly correlated with socioeconomic status as Medicaid is offered as an insurance to those who cannot afford private coverage. It is interesting that this study found a significant effect of insurance on HSS Pedi-FABS after correcting for other known indicators of socioeconomic status. Privately insured patients have previously been shown to have fewer complications54 and decreased resource utilization42 after arthroplasty. Patients with public insurance have more severe presentations as compared with those with private insurance.57 We show that even after controlling for severity and socioeconomic status, patients with public insurance have worse patient-reported outcomes.
We acknowledge several limitations of this study. The 70% follow-up we had in our study could introduce confounders based on selection bias. However, our response rate was still high compared with other orthopaedic cohort studies, and we used multivariable methodology to decrease this bias. Our study duration of 1 year demonstrates only short-term outcomes. Narcotic use was indicated by the number of narcotic pills prescribed to patients, not the number of pills the patient had actually taken. Finally, we are unable to account for differences in unsupervised PT; we did not track whether or how frequently patients performed exercises as instructed. One of our outcome measures, HSS Pedi-FABS, does not have a validated MCID. Thus, conclusions drawn using this outcome measure should be tempered. Of the outcome measures included, HSS Pedi-FABS and KOOS-PS have the most relevant construct to assess outcomes after ACLR; other outcome measures introduce the possibility of bias from miscalculation of outcome. Finally, 26 patients who self-identified as athletes at the time of the index surgery no longer self-identified at the 1-year mark, introducing an important source of bias.
Conclusion
In a prospectively enrolled cohort of patients who had ACLR, we identified that preoperative narcotic usage, postoperative narcotic usage, and PT location did not significantly affect outcome measures at 1 year after surgery in a multivariable analysis. Insurance type was a significant predictor in a multivariate model for HSS Pedi-FABS, with patients receiving public funding for insurance having lower athletic activity. While opioids do not influence outcomes, surgeons should be aware of the national stigma against opioid use and approach pain in a multimodal fashion to maximize patient comfort while minimizing addiction potential. Surgeons should be aware that PT location does not influence outcomes and should thus prescribe PT to be most convenient for the patient. Last, surgeons should be more cognizant of the insurance status of their patient to ensure that those with public funding are functionally improving at an appropriate rate.
Acknowledgment
The authors thank the Cleveland Clinic orthopaedic patients, staff, and research personnel, whose efforts related to regulation, data collection, participant follow-up, data quality control, analyses, and manuscript preparation have made this consortium successful. The authors also thank Thomas E. Anderson, MD, Kim L. Stearns, MD, and Alan W. Davis, MD, for contributing cases. Thanks also to Brittany Stojsavljevic, editorial assistant, Cleveland Clinic Foundation, for editorial management.
Footnotes
Final revision submitted September 10, 2020; accepted October 21, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (award K23AR066133), which supported a portion of M.H.J.’s professional effort. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. L.D.F. has received consulting fees from Zimmer Biomet and hospitality payments from Musculoskeletal Transplant Foundation. M.H.J. is on the scientific advisory board for Samumed. A.A.M. has received educational support from Rock Medical; consulting fees from Arthrosurface, Amniox Medical, Linvatec, Stryker, and Trice; speaking fees from Trice; royalties from Arthrosurface, Zimmer Biomet, and Wolters Kluwer; and hospitality payments from Arthrex, DJO, and Smith & Nephew; and has stock/stock options in Arthrosurface and Trice. R.D.P. has received royalties from Zimmer Biomet and hospitality payments from Smith & Nephew and Musculoskeletal Transplant Foundation. J.T.R. has received consulting fees from Smith & Nephew. P.S. has received educational support from Rock Medical; consulting fees from Arthrex, DJO, and DePuy; speaking fees from Arthrex; and hospitality payments from Musculoskeletal Transplant Foundation. G.S. has received royalties from Oberd. K.P.S. has received research support from Smith & Nephew and DJO; consulting fees from NFL, Cytori, Mitek, Samumed, and Flexion Therapeutics; hospitality payments from DePuy and Biosense Webster; and royalties from Oberd. J.S.W. has received educational support from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Cleveland Clinic Institutional Review Board (study No. 17-554).
Authors: Cleveland Clinic Sports Health; Jaret M. Karnuta, MS (Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio); Sarah Dalton, MD (Case Western Reserve University School of Medicine, Cleveland, Ohio); James Bena, MS (Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, Ohio); Lutul D. Farrow, MD (Cleveland Clinic Orthopaedic Sports Health, Cleveland, Ohio); Joseph Featherall, BS (Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio); Morgan H. Jones, MD, MPH, Anthony A. Miniaci, MD, Richard D. Parker, MD, James T. Rosneck, MD, Paul Saluan, MD, Greg Strnad, MS (Cleveland Clinic Orthopaedic Sports Health, Cleveland, Ohio); Kurt P. Spindler, MD (Cleveland Clinic Lerner College of Medicine and Cleveland Clinic Orthopaedic Sports Health, Cleveland, Ohio); and James S. Williams, MD, Sameer R. Oak, MD (Cleveland Clinic Orthopaedic Sports Health, Cleveland, Ohio).
References
- 1. Anthony C, Westermann RW, Bedard N, et al. Opioid demand after anterior cruciate ligament reconstruction. Orthop J Sports Med. 2017;5(7)(suppl 6):2325967117S00278. [DOI] [PubMed] [Google Scholar]
- 2. Anthony CA, Westermann RW, Bedard N, et al. Opioid demand before and after anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(13):3098–3103. [DOI] [PubMed] [Google Scholar]
- 3. Ardern CL, Sonesson S, Forssblad M, Kvist J. Comparison of patient-reported outcomes among those who chose ACL reconstruction or non-surgical treatment. Scand J Med Sci Sports. 2017;27(5):535–544. [DOI] [PubMed] [Google Scholar]
- 4. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine. 2014;39(25):e1524. [DOI] [PubMed] [Google Scholar]
- 5. Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42(5):1049–1057. [DOI] [PubMed] [Google Scholar]
- 6. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history? A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292–300. [DOI] [PubMed] [Google Scholar]
- 7. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function. Arthritis Care Res. 2011;63(11)(suppl):S208–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox CL, Huston LJ, Dunn WR, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42(5):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curtis GL, Tariq MB, Brigati DP, Faour M, Higuera CA; Cleveland Clinic Orthopaedic Arthroplasty Group. Validation of a novel surgical data capturing system following total hip arthroplasty. J Arthroplasty. 2018;33(11):3479–3483. [DOI] [PubMed] [Google Scholar]
- 10. Dhalla IA, Persaud N, Juurlink DN. Facing up to the prescription opioid crisis. BMJ. 2011;343:D5142. [DOI] [PubMed] [Google Scholar]
- 11. Diaz-Borjon E, Torres-Gomez A, Essex MN, et al. Parecoxib provides analgesic and opioid-sparing effects following major orthopedic surgery: a subset analysis of a randomized, placebo-controlled clinical trial. Pain Ther. 2017;6(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn WR, Spindler KP, Amendola A, et al. Which preoperative factors, including bone bruise, are associated with knee pain/symptoms at index anterior cruciate ligament reconstruction (ACLR)? A Multicenter Orthopaedic Outcomes Network (MOON) ACLR Cohort Study. Am J Sports Med. 2010;38(9):1778–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunn WR, Spindler KP; MOON Consortium. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med. 2010;38(10):2040–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fabricant PD, Robles A, Downey-Zayas T, et al. Development and validation of a pediatric sports activity rating scale: the Hospital for Special Surgery Pediatric Functional Activity Brief Scale (HSS Pedi-FABS). Am J Sports Med. 2013;41(10):2421–2429. [DOI] [PubMed] [Google Scholar]
- 15. Fabricant PD, Suryavanshi JR, Calcei JG, Marx RG, Widmann RF, Green DW. The Hospital for Special Surgery Pediatric Functional Activity Brief Scale (HSS Pedi-FABS): normative data. Am J Sports Med. 2018;46(5):1228–1234. [DOI] [PubMed] [Google Scholar]
- 16. Featherall J, Brigati DP, Faour M, Messner W, Higuera CA. Implementation of a total hip arthroplasty care pathway at a high-volume health system: effect on length of stay, discharge disposition, and 90-day complications. J Arthroplasty. 2018;33(6):1675–1680. [DOI] [PubMed] [Google Scholar]
- 17. Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grant JA, Mohtadi NGH. Two- to 4-year follow-up to a comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction. Am J Sports Med. 2010;38(7):1389–1394. [DOI] [PubMed] [Google Scholar]
- 19. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125(5):1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6)(suppl 1):9–14. [DOI] [PubMed] [Google Scholar]
- 22. Hoots B, Xu L, Kariisa M, et al. 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2018. Accessed March 3, 2021. https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf [Google Scholar]
- 23. Hubbard RC, Naumann TM, Traylor L, Dhadda S. Parecoxib sodium has opioid-sparing effects in patients undergoing total knee arthroplasty under spinal anaesthesia. Br J Anaesth. 2003;90(2):166–172. [DOI] [PubMed] [Google Scholar]
- 24. Ingelsrud LH, Terwee CB, Terluin B, et al. Meaningful change scores in the Knee injury and Osteoarthritis Outcome Score in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46(5):1120–1128. [DOI] [PubMed] [Google Scholar]
- 25. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers—United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95–100. [DOI] [PubMed] [Google Scholar]
- 26. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368(18):1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khazi ZM, Shamrock AG, Hajewski C, et al. Preoperative opioid use is associated with inferior outcomes after patellofemoral stabilization surgery. Knee Surg Sports Traumatol Arthrosc. 2020;28(2):599–605. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72(6):558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kruse LM, Gray B, Wright RW. Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(19):1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89. [DOI] [PubMed] [Google Scholar]
- 32. Magnussen RA, Carey JL, Spindler KP. Does autograft choice determine intermediate-term outcome of ACL reconstruction? Knee Surg Sports Traumatol Arthrosc. 2011;19(3):462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magnussen RA, Reinke EK, Huston LJ, et al. Effect of high-grade preoperative knee laxity on 6-year anterior cruciate ligament reconstruction outcomes. Am J Sports Med. 2018;46(12):2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malan TP, Marsh G, Hakki SI, Grossman E, Traylor L, Hubbard RC. Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty. Anesthesiology. 2003;98(4):950–956. [DOI] [PubMed] [Google Scholar]
- 35. Marom N, Xiang W, Heath M, Boyle C, Fabricant PD, Marx RG. Time interval affects physical activity scores: a comparison of the Marx Activity Rating Scale and the Hospital for Special Surgery Pediatric Functional Activity Brief Scale. Knee Surg Sports Traumatol Arthrosc. 2020;28:2619–2625. [DOI] [PubMed] [Google Scholar]
- 36. Martin CT, Callaghan JJ, Liu SS, Gao Y, Warth LC, Johnston RC. Disparity in total joint arthroplasty patient comorbidities, demographics, and postoperative outcomes based on insurance payer type. J Arthroplasty. 2012;27(10):1761–1765.e1. [DOI] [PubMed] [Google Scholar]
- 37. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. [DOI] [PubMed] [Google Scholar]
- 38. Möller E, Weidenhielm L, Werner S. Outcome and knee-related quality of life after anterior cruciate ligament reconstruction: a long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):786–794. [DOI] [PubMed] [Google Scholar]
- 39. MOON Knee Group; Spindler KP, Huston LJ, et al. Ten-year outcomes and risk factors after anterior cruciate ligament reconstruction: a MOON longitudinal prospective cohort study. Am J Sports Med. 2018;46(4):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morris BJ, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. Preoperative opioid use and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(1):11–16. [DOI] [PubMed] [Google Scholar]
- 41. Pivec R, Issa K, Naziri Q, Kapadia BH, Bonutti PM, Mont MA. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop. 2014;38(6):1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Plate JF, Ryan SP, Goltz DE, Howell CB, Bolognesi MP, Seyler TM. Medicaid insurance correlates with increased resource utilization following total hip arthroplasty. J Arthroplasty. 2019;34(2):255–259. [DOI] [PubMed] [Google Scholar]
- 43. Rhon DI, Snodgrass SJ, Cleland JA, Sissel CD, Cook CE. Predictors of chronic prescription opioid use after orthopedic surgery: derivation of a clinical prediction rule. Perioper Med. 2018;7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogers MJ, Penvose I, Curry EJ, DeGiacomo A, Li X. Medicaid health insurance status limits patient accessibility to rehabilitation services following ACL reconstruction surgery. Orthop J Sports Med. 2018;6(4):2325967118763353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 47. Rosenthal BD, Hulst JB, Moric M, Levine BR, Sporer SM. The effect of payer type on clinical outcomes in total knee arthroplasty. J Arthroplasty. 2014;29(2):295–298. [DOI] [PubMed] [Google Scholar]
- 48. Samuelsson K, Magnussen RA, Alentorn-Geli E, et al. Equivalent Knee injury and Osteoarthritis Outcome Scores 12 and 24 months after anterior cruciate ligament reconstruction: results from the Swedish National Knee Ligament Register. Am J Sports Med. 2017;45(9):2085–2091. [DOI] [PubMed] [Google Scholar]
- 49. Spindler KP, Warren TA, Callison JC, Secic M, Fleisch SB, Wright RW. Clinical outcome at a minimum of five years after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 2005;87(8):1673–1679. [DOI] [PubMed] [Google Scholar]
- 50. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320–2326. [DOI] [PubMed] [Google Scholar]
- 51. Taylor N, Frick S, Killilea S, Dugan-Frost T, Solodiuk J. Opioid use in children and adolescents after anterior cruciate ligament repair. J Healthc Qual. 2018;40(2):97–102. [DOI] [PubMed] [Google Scholar]
- 52. Tsang KS, Page J, Mackenney P. Can intravenous paracetamol reduce opioid use in preoperative hip fracture patients? Orthopedics. 2013;36(2)(suppl):20–24. [DOI] [PubMed] [Google Scholar]
- 53. Ulstein S, Årøen A, Engebretsen L, Forssblad M, Lygre SHL, Røtterud JH. Effect of concomitant cartilage lesions on patient-reported outcomes after anterior cruciate ligament reconstruction: a nationwide cohort study from Norway and Sweden of 8470 patients with 5-year follow-up. Orthop J Sports Med. 2018;6(7):2325967118786219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veltre DR, Sing DC, Yi PH, et al. Insurance status affects complication rates after total hip arthroplasty. J Am Acad Orthop Surg. 2019;27(13):e606–e611. [DOI] [PubMed] [Google Scholar]
- 55. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Westermann R, Spindler KP, Huston LJ, Wolf BR. Outcomes of grade III MCL injuries treated concurrently with ACL reconstruction: a multicenter study. Arthroscopy. 2019;35(5):1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams AA, Mancini NS, Solomito MJ, Nissen CW, Milewski MD. Chondral injuries and irreparable meniscal tears among adolescents with anterior cruciate ligament or meniscal tears are more common in patients with public insurance. Am J Sports Med. 2017;45(9):2111–2115. [DOI] [PubMed] [Google Scholar]
- 58. Wright RW, Haas AK, Anderson J, et al. Anterior cruciate ligament reconstruction rehabilitation: MOON guidelines. Sports Health. 2015;7(3):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wright RW, Preston E, Fleming BC, et al. A systematic review of anterior cruciate ligament reconstruction rehabilitation: part I: continuous passive motion, early weight bearing, postoperative bracing, and home-based rehabilitation. J Knee Surg. 2008;21(3):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang L, Du S, Sun Y. Intravenous acetaminophen as an adjunct to multimodal analgesia after total knee and hip arthroplasty: a systematic review and meta-analysis. Int J Surg. 2017;47:135–146. [DOI] [PubMed] [Google Scholar]
- 61. Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93(21):1988. [DOI] [PubMed] [Google Scholar]


