Abstract
Background:
Scapulothoracic bursitis is a significant clinical condition that limits day-to-day function. Arthroscopic scapular debridement and resection have provided satisfactory outcomes; however, techniques, approaches, and recommendations remain varied. Novel bony parameters have also gained increasing interest owing to their value in preoperative planning.
Purpose:
To assess midterm clinical outcomes after the arthroscopic management of scapulothoracic bursitis and to identify and measure novel bony parameters on preoperative magnetic resonance imaging.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 8 patients underwent arthroscopic scapular debridement and bursectomy; 5 of the 8 patients underwent additional medial scapulectomy. There were 5 male (62.5%) and 3 female (37.5%) patients with a mean age of 30.1 ± 12.3 years (range, 19-58 years). Inclusion criteria for surgery were patients with symptomatic scapulothoracic bursitis for whom extensive nonoperative modalities had been utilized for at least 6 months but failed. Outcome measures included the Oxford Shoulder Score (OSS), University of California Los Angeles (UCLA) shoulder rating scale, Constant Shoulder Score (CSS), and visual analog scale (VAS) for pain. The bony parameters included scapular shape, anterior offset, costomedial angle, and medial scapular corpus angle (MSCA).
Results:
The follow-up duration was at least 2 years for all patients (mean follow-up, 25.0 ± 4.1 months [range, 24-35 months]). The majority of patients had a concave-shaped scapula (62.5%). The mean anterior offset was 24.3 ± 3.4 mm, and the mean costomedial angle was 132.3° ± 9.6°. Half the patients had a positive MSCA, while the other half had a negative MSCA. A statistically significant improvement was observed in the OSS, UCLA, CSS, and VAS scores from preoperatively to 2-year follow-up (P < .001 for all). No complications were observed.
Conclusion:
Arthroscopic scapular debridement and resection provided satisfactory midterm clinical outcomes for the treatment of scapulothoracic bursitis.
Keywords: scapulothoracic bursitis, scapulectomy, superomedial angle resection, anterior offset, costomedial angle
Scapulothoracic bursitis is a rare but significant clinical condition that can limit the day-to-day function of patients. Symptoms typically include pain and difficulty with overhead activity and are worse with repetitive motion. A clicking, crunching, grating, or snapping sensation is often felt. Patients may occasionally present with referred pain to the neck or radiculopathy precipitated by activity. Various causes of scapulothoracic bursitis and crepitus have been proposed, and these include overuse syndromes, abnormal scapular morphology, glenohumeral dysfunction, postsurgical or posttraumatic changes (from malunion or surrounding callus formation due to fractures), osseous and soft tissue masses, and scapular dyskinesis.4,19,24 Its early recognition is crucial to reduce unnecessary disability.7
Classically, nonoperative treatment8 such as physical therapy, which involves periscapular strengthening, combined with oral or intramuscular analgesia,3,24 is effective for symptoms due to scapular dyskinesis or benign nonosseous lesions. On the other hand, surgical management should be considered if there are osseous lesions or if patients have failed a prolonged trial of nonoperative therapy. Surgical management can be classified into arthroscopic or open surgery. While an open approach has demonstrated good outcomes, it has lost popularity because of concerns of morbidity from large incisions, poorer postoperative rehabilitation, and the risk of nerve palsies.12
For this condition, magnetic resonance imaging (MRI) is deemed most useful among all other imaging modalities in detecting and characterizing soft lesions and avoiding misdiagnoses.24 Yet, there have been limited clinical studies discussing scapulothoracic bursitis and corresponding imaging. In clinical studies with available imaging, MRI findings appear variable.19 Of late, certain novel bony parameters, including the medial scapular corpus angle (MSCA), costomedial angle, anterior offset, and scapular morphology, have gained traction in the literature1,16,23 for the purposes of preoperative planning to determine the ideal extent of scapular resection.
The aim of this study was to assess the midterm clinical outcomes after the arthroscopic management of symptomatic scapulothoracic bursitis and to measure parameters such as the MSCA, costomedial angle, anterior offset, and scapular morphology on preoperative MRI.18,22,23
Methods
This study was determined to be exempt from ethics review. A retrospective review was conducted, yielding 8 patients with a diagnosis of symptomatic scapulothoracic bursitis who underwent arthroscopic scapulothoracic debridement, bursectomy, and resection by a single surgeon (D.T.T.L.) from 2007 to 2018. Inclusion criteria for surgery were patients with symptoms consistent with scapulothoracic bursitis in whom extensive nonoperative modalities had failed, defined as having undergone at least 6 months of extensive physical therapy, and having trialed both oral and injectable analgesics. The inclusion criteria are in line with what has been proposed by most authors of the existing literature.24 Exclusion criteria included fractures, infections, tumors, and the presence of concomitant shoulder abnormalities that may account for similar symptoms.
All included patients were first diagnosed clinically and further aided by MRI. MRI was performed to identify fibrous bands and rule out other malignant conditions that may mimic scapulothoracic bursitis. MRI also aided preoperative planning regarding the extent of debridement and resection and helped narrow down the location of fibrous bands to be sectioned or excised.
We performed MRI with contrast targeting the scapula, as routine shoulder MRI typically does not include the entire scapula with its medial border. T1- and T2-weighted fat-saturated sequences were performed in the coronal, sagittal, and axial planes. The slice thickness was set at 4 mm and gap thickness at 1 mm, with utilization of a cardiac coil and field of view ranging between 20 and 25 cm. The scan range for axial imaging covered the entire scapula. Sagittal imaging was performed perpendicular to the scapula. Coronal imaging was performed parallel to the scapula.
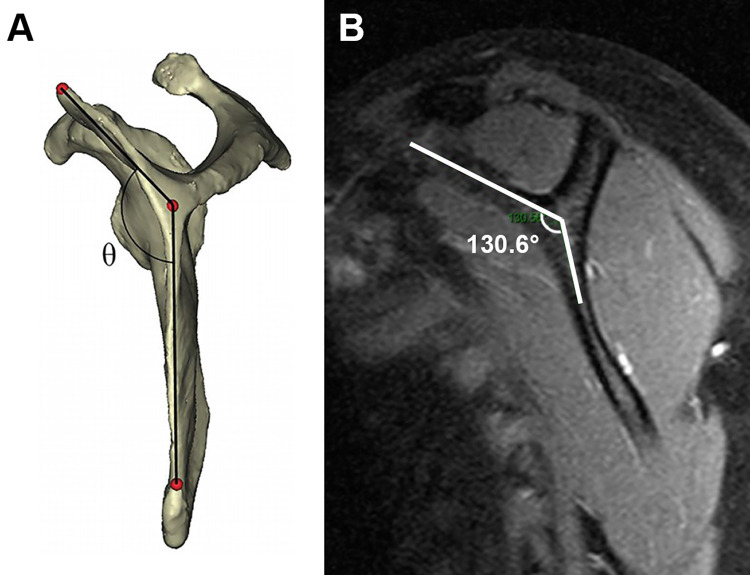
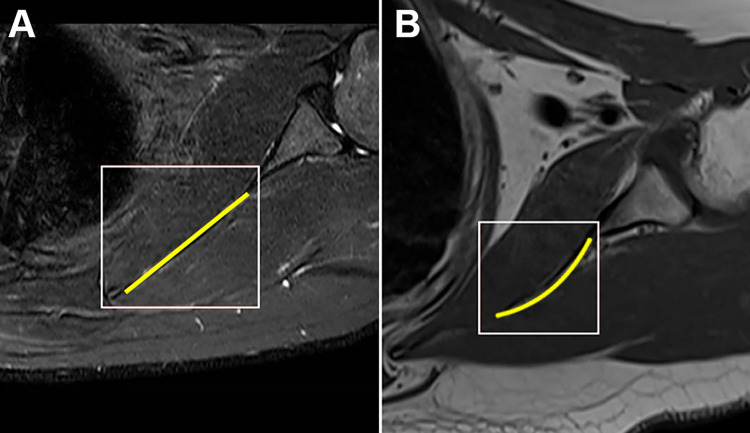
The scapular morphology, anterior offset, MSCA, and costomedial angle were also retrospectively measured on preoperative MRI for all patients (Table 1). All images were assessed by 2 surgeons (D.T.T.L. and K.L.P.). The anterior offset23 was measured on sagittal-plane MRI and was represented by the horizontal distance from an estimated line drawn along the scapular body plane to the superomedial angle of the scapula (Figure 1). The costomedial angle23 was defined as the angle between the superior and inferior scapular wings along the medial border of the scapula (Figure 2). The scapular shape (straight, S-shaped, and concave) was assessed from superior to inferior in the axial plane (Figure 3). The MSCA was measured on axial-plane MRI; a positive MSCA was defined as anterior scapular angulation toward the thorax, while a negative MSCA was defined as posterior scapular angulation away from the thorax. An example of a scapula with a negative MSCA is demonstrated in Figure 4.
Table 1.
Bony Parameters Assessed on Preoperative Magnetic Resonance Imaging
| Patient No. | Scapular Shape | Anterior Offset, mm | Costomedial Angle, deg | Medial Scapular Corpus Angle |
|---|---|---|---|---|
| 1 | Straight | 28.9 | 128 | Positive |
| 2 | Concave | 22.6 | 131 | Negative |
| 3 | Concave | 20.1 | 144 | Positive |
| 4 | Concave | 22.2 | 123 | Positive |
| 5 | Concave | 26.3 | 149 | Negative |
| 6 | Concave | 27.1 | 133 | Negative |
| 7 | Straight | 20.0 | 122 | Negative |
| 8 | S-shaped | 26.8 | 128 | Positive |
Figure 1.
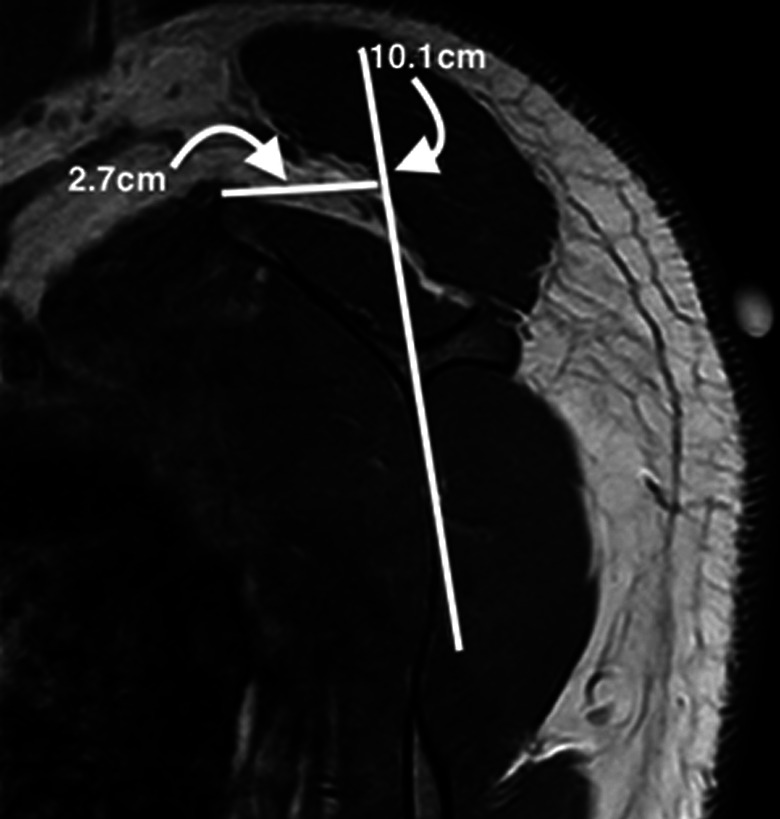
Sagittal magnetic resonance imaging showing the assessment of the anterior offset, measured as the horizontal distance from a line drawn along the plane of the scapula (vertical white line) to the superomedial angle of the scapula. In this example, the offset was 2.7 cm.
Figure 2.
(A) Reference schematic showing the assessment of the costomedial angle,23 defined as the angle between the superior and inferior scapular wings along the medial border of the scapula. (B) The costomedial angle of 130.6° as measured on a sagittal cut of the scapula on magnetic resonance imaging.
Figure 3.
Axial magnetic resonance imaging showing a (A) straight-shaped scapula and (B) concave-shaped scapula.
Figure 4.
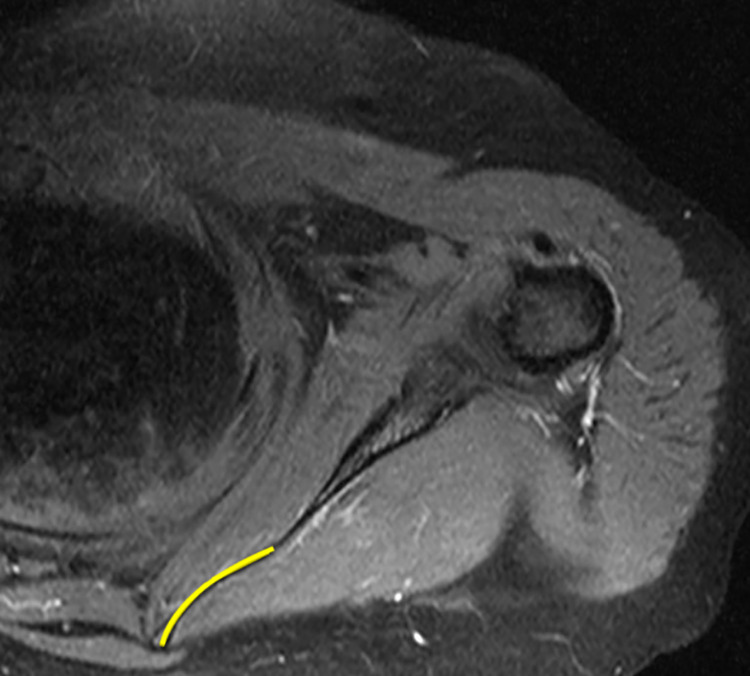
Axial magnetic resonance imaging of the scapular region demonstrating a negative medial scapular corpus angle (posterior scapular angulation away from the thorax; yellow line).
For the operative procedure, the patient was placed in a prone position with both the operative extremity and posterior thorax prepared and draped in a sterile fashion. The arm was secured with tape to ensure a stable position. The patient’s arm was internally rotated in extension (the chicken wing position).13 This allowed artificial winging of the scapula, thereby increasing the amount of space between the scapula and the thorax, to facilitate easier portal placement. Overall, 3 arthroscopic portals were created. The medial portal was first created at the level of the scapular spine, 3 cm medial to the medial border of the scapula, followed by the superior portal (Bell portal),20 identified by the junction of the medial and middle thirds of the scapula, 1 to 2 cm superior to the scapula’s superior border. A 3.5 mm–diameter 30° arthroscope was used. The last inferior portal was placed 4 cm inferior to the medial portal and was optional.
After the creation of the portals, diagnostic arthroscopic surgery was performed to assess the superomedial border. The arthroscopic field was irrigated under pressure. An infusion pump with a mean pressure of 50 mm Hg was utilized to inflate the scapulothoracic space. The bursal layer was identified. It was analogous to the subacromial space. Further debridement was performed using a 4.5-mm arthroscopic shaver blade to clear the scapular borders and improve visualization. A radiofrequency device (VAPR VUE Radiofrequency System; Mitek) was employed to ensure hemostasis and also to clear the bony edges of the scapula. The prominent superior border of the scapula, termed the “tubercle of Luschka,” was identified, burred, shaved, and coplaned until flat. A switching stick was employed to exchange the working and viewing portals, which provided better visualization for more optimal resection.21
Postoperatively, all patients were placed in an arm sling and engaged in passive range of motion exercises with senior physical therapists before discharge. The passive exercises were then escalated to active range of motion exercises at 1 week, followed by strengthening exercises by the end of the first month. Postoperative rehabilitation placed particular focus on restoring scapular kinematics and strengthening relevant muscles such as the levator scapulae and rhomboids, with the ultimate aim of allowing return to sports or overhead activity.
Retrospective analysis of a prospectively collected registry collating preoperative and postoperative scores was performed. Data were collected by an assigned member of the study team (G.J.Z.) in 2019 using a standardized template form. Symptoms and functionality were objectively measured via the Constant Shoulder Score (CSS),5 Oxford Shoulder Score (OSS),6 and University of California Los Angeles (UCLA) shoulder rating scale25 by a trained physical therapist. The CSS5 is utilized to categorize the outcomes after shoulder surgery into unsatisfactory, fair, good, very good, and excellent. It is one of the most commonly used scoring systems because of its easy applicability and is the gold standard in Europe.2 It assesses the following domains: pain, range of movement, activities of daily living, and strength. It is a good measure for the assessment of most shoulder surgical procedures, with the exception of instability surgical procedures. It has been shown to reliably assess improvements in functionality after surgery.6 The OSS,6 which was first established in 1996, is a clinical outcome measure widely used in recent clinical studies. There have since been multiple variations.6 It has undergone rigorous testing for validity, reliability, and sensitivity to change and has been shown to be a robust tool for assessing the outcomes of shoulder surgery.14 It consists of 12 questions, 4 of which are related to pain, and is measured based on the patient’s perspective of his or her own outcome. The UCLA scale25 is measured in 5 sections, which include pain, function, range of movement, strength, and patient satisfaction.
Statistical analysis of pre- and postoperative outcomes was performed by a biostatistician (Y.H.) using the Student t test (2-tailed paired test), with statistical significance defined as P < .05. R software Version 3.5.1 (R Core Team) used for all calculations.
Results
Our study comprised a total of 8 patients, consisting of 5 male (62.5%) and 3 female (37.5%) patients with a mean age of 30.1 ± 12.3 years (range, 19-58 years) and a mean body mass index of 26.3 ± 13.2 kg/m2 (range, 21.2-32.4 kg/m2). All patients underwent arthroscopic scapular debridement and bursectomy; 5 of the 8 patients required additional medial scapulectomy because of a visibly prominent tubercle of Luschka. The mean follow-up duration was 25.0 ± 4.1 months (range, 24-35 months). No patients were lost to follow-up.
Table 1 shows the assessment of bony parameters on preoperative MRI. The majority of patients (62.5%) had a concave-shaped scapula. The mean anterior offset was 24.3 ± 3.4 mm (range, 20.0-28.9 mm), and the mean costomedial angle was 132.3° ± 9.6° (range, 122°-149°). Half of the patients had a positive MSCA, while the other half had a negative MSCA.
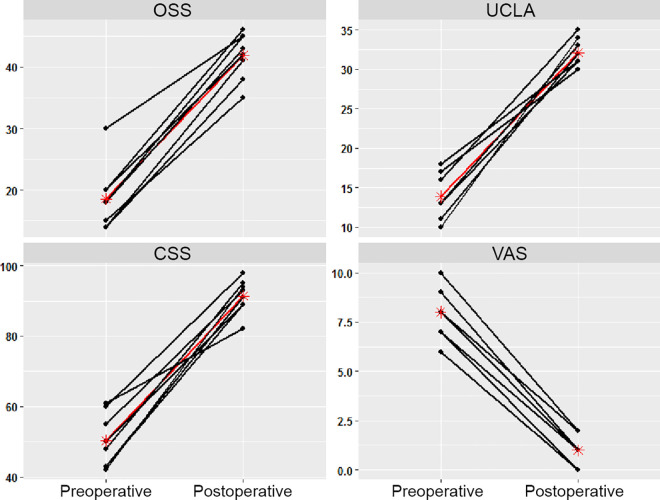
Subjectively, 7 of 8 patients experienced pain-free range of motion and a resolution of crepitus postoperatively at 2 years. One patient had a recurrence of left periscapular pain at 2-month follow-up but still reported a reduction in crepitus and satisfaction with surgery. At 2-year follow-up, a significant improvement was observed on all 4 patient-reported outcome measures compared with preoperatively (P < .001 for all) (Figure 5 and Table 2). The visual analog scale score improved notably from a mean of 8.0 preoperatively to 1.0 postoperatively.
Figure 5.
Preoperative and 2-year postoperative functional outcome scores. The y-axis denotes outcome measure values. Black lines represent the raw data of individual patients, while red lines and asterisks represent mean values. CSS, Constant Shoulder Score; OSS, Oxford Shoulder Score; UCLA, University of California Los Angeles; VAS, visual analog scale.
Table 2.
Preoperative and 2-Year Postoperative Functional Outcome Scoresa
| Preoperative | 2-y Postoperative | P Valueb | |
|---|---|---|---|
| OSS | 18.6 ± 5.2 (14-30) | 41.9 ± 3.8 (14-30) | <.001 |
| UCLA | 13.9 ± 2.9 (10-18) | 32.1 ± 1.7 (30-35) | <.001 |
| CSS | 50.2 ± 7.7 (42-60) | 91.4 ± 4.9 (82-98) | <.001 |
| VAS | 8.0 ± 1.3 (6-10) | 1.0 ± 0.8 (0-3) | <.001 |
aData are shown as mean ± SD (range). A statistically significant improvement was seen in all 4 scores. CSS, Constant Shoulder Score; OSS, Oxford Shoulder Score; UCLA, University of California Los Angeles; VAS, visual analog scale.
bPaired t test.
Discussion
The results from this retrospective case series demonstrate that in patients with scapulothoracic bursitis refractory to nonoperative management, arthroscopic debridement and scapulectomy produced good midterm functional outcomes. Objectively, this was evidenced by a statistically significant improvement in outcome scores, and subjectively, all patients expressed that they would undergo surgery again. Observations of bony parameters also revealed that the majority of our patients had a concave scapula (62.5%), suggesting that it may be a predisposing factor for the development of scapulothoracic bursitis.22 Our study also showed a narrow range of values for the anterior offset and costomedial angle.
Comparative studies are lacking in the current literature, but it is generally suggested that arthroscopic scapular surgery is favored over an open procedure, as it allows accelerated rehabilitation, a quicker return to full function, improved cosmesis, and reduced scar formation.21 It is a fairly safe procedure with a low incidence of complications.12 This is consistent with our patient data set in which no complications were observed.
The feasibility of arthroscopic scapular debridement and resection has been widely established in case series and reviews,9,10,15,17,24 with multiple clinical studies9,21 reporting a significant reduction in pain after resection of the superomedial border of the scapula. Merolla et al14 evaluated 10 patients who were treated with arthroscopic surgery after having failed nonoperative therapy and found that all postoperative scores had significantly improved compared with preoperative scores. Although our study has a small number of patients, the results of our study show consistency with these findings.
In the existing literature, the common predisposing factors for scapulothoracic bursitis described are usually anatomic variations or localized structural problems. There has been no detailed analysis on the demographic risk. Comparing patient demographics across studies, one study by Millett et al15 reported a mean age of 33 years at the time of surgery, strikingly similar to our mean of 30.1 years, which leads us to wonder if certain age groups of patients are more predisposed to scapulothoracic bursitis.
Beyond its feasibility, the techniques, approaches, and recommendations for arthroscopic scapular surgery remain extremely varied. During surgery, while it is widely agreed on that surgical resection of the superomedial angle plus bursectomy can provide much relief in most cases, there are few studies that guide us on the extent of resection that is necessary. It remains contentious if empiric bony resection of the superomedial border should be advocated. While it was demonstrated in Millett et al’s15 study that patients with additional scapuloplasty demonstrated higher satisfaction, Memon et al12 noted that if empiric resection were adopted, it would be unwarranted in a significant proportion of patients. Even if resection were advocated, recommendations for the degree of resection vary from 1 to 7 cm, with some suggesting removal of the entire superomedial corner. The amount of resection needed to achieve adequate scapulothoracic space decompression (SSD) is unknown.23 Currently, the most commonly utilized technique is 2-cm (superior to inferior) by 3-cm (medial to lateral) triangular resection,23 and this technique was utilized in our study for all cases requiring additional scapulectomy. While small-scale studies exist to demonstrate surgical feasibility,13,23 further large-scale studies are required to determine the exact extent of resection required to achieve an optimal outcome.
The general trend of recent studies seems to be moving toward early detection and early intervention with either nonoperative techniques or arthroscopic surgery, as an increased delay in treatment and increased age are associated with poorer outcomes.11,13 Early preoperative imaging may help facilitate this, but the literature on this, especially in conjunction with clinical studies, is sparse. Another unresolved issue in the literature is that no study has described the use of preoperative imaging techniques to assist in surgical decision making.12
A recent controlled laboratory study using cadaveric models by Tahal et al23 suggested that the anterior offset and costomedial angle were the 2 most important bony parameters to evaluate the effectiveness of partial scapulectomy during preoperative planning for patients with scapulothoracic bursitis. The clinical relevance of the anterior offset has been discussed in previous studies. Based on the study by Tahal et al, patients with an anterior offset of less than 20 mm were unlikely to achieve adequate SSD with 3-cm resection. If the anterior offset fell between 20 and 35 mm, there was an estimated 60% success rate for adequate SSD with 3-cm resection. If the anterior offset exceeded 35 mm, 2-cm resection was sufficient for achieving adequate SSD.23 Dimitri et al, in their study of cadaveric specimens with an unknown history of scapulothoracic bursitis, reported a mean anterior offset of 27.26 ± 8.6 mm.23 A separate study by Bell et al1 reported the mean anterior offset of asymptomatic and symptomatic scapulae to be 27.86 ± 9.8 and 28.56 ± 7.9 mm, respectively, with no significant difference. Our patients demonstrated a mean anterior offset of 24.3 ± 3.4 mm. Despite a mild discrepancy in anterior offset values across these studies, operative considerations likely remain similar, as they all fall within the range of 20 to 35 mm, and 3 cm should be recommended. Patients who underwent 2-cm by 3-cm resection in our study attained good clinical relief, thereby supporting this recommendation.
To date, there are no clinical studies that have attempted to measure the costomedial angle while also discussing functional outcomes. However, in the cadaveric study by Tahal et al,23 the mean costomedial angle derived from 20 pairs of shoulder specimens was found to be 144.1° ± 9.6°. The mean costomedial angle in our study was 132.3° ± 9.6°. In Tahal et al’s study, it was noted that maximum SSD with 3-cm resection significantly correlated with the patients’ values of the costomedial angle. Their study noted that no scapula with a costomedial angle greater than 157.9° was able to achieve 5-mm SSD with 3-cm resection, while all scapulae with a costomedial angle less than 126.4° managed at least 5-mm SSD with 2-cm resection. While our study did not measure the extent of SSD, all patients in our study cohort had a costomedial angle that did not exceed the upper limit proposed (157.9°). Patients who underwent additional scapulectomy (2-cm [superior to inferior] by 3-cm [medial to lateral] triangular resection) attained clinical relief as well as objective improvements on outcome measures.
Some studies have shown that scapular morphology may also play a role. In Millett et al’s16 study, all included patients with a concave scapula had snapping scapula syndrome (SSS). Another study showed that patients with a concave-shaped scapula and a positive MSCA have a 12-fold increased risk of SSS.22 In our study, it was noted that the majority of our patients (62.5%) had a concave-shaped scapula, while there was an even divide between a positive and negative MSCA. However, according to the cadaveric study by Tahal et al,23 it was concluded that “MSCA and scapula shape may have a role in the development of SSS but did not appear to be important to consider when planning surgical SSD.” Consolidating both findings, we feel that the presence of a concave-shaped scapula may be a stronger predisposing factor than a positive MSCA and is of greater clinical significance. It may be useful to routinely examine scapular morphology on imaging to supplement a clinical evaluation. Considering the points raised above, it may be worthwhile advocating preoperative MRI1 and recording the extent of resection to further validate the findings of the current literature. Further large-scale studies are required to validate current findings regarding the use of novel bony parameters for guidance in operative planning.
Limitations
We acknowledge several limitations to our study. The small sample size of our study with a lack of a nonoperative control group limits statistical analyses. Although our data were attained through a retrospective review, biases were reduced through the use of objective and patient-centered questionnaires. However, considering the rarity of scapulothoracic bursitis, there is no validated outcome measure specifically targeting scapular disorders; thus, the use of the UCLA scale, CSS, and OSS may not have been the most ideal. Although observation bias was reduced to a minimum through the use of blinded observers for data collection, there might have been selection bias owing to the absence of randomization. We lacked postoperative imaging to objectify our surgical results, but this was to maintain a cost-effective approach to treatment.
Apart from selection bias, there were limited threats to the external validity of the study results. Our sample was composed of a homogeneous population undergoing similar interventions.
Conclusion
Arthroscopic scapular debridement and resection is a feasible treatment method for scapulothoracic bursitis, demonstrating significant improvements in midterm clinical outcomes. Further large-scale prospective studies that assess long-term outcomes are required to better substantiate the validity of this treatment.
Footnotes
Final revision submitted September 13, 2020; accepted October 23, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was waived by the SingHealth Centralised Institutional Review Board (No. 2017-2757).
References
- 1. Bell SN, Troupis JM, Miller D, Alta TD, Coghlan JA, Wijeratna MD. Four-dimensional computed tomography scans facilitate preoperative planning in snapping scapula syndrome. J Shoulder Elbow Surg. 2015;24(4):e83–e90. [DOI] [PubMed] [Google Scholar]
- 2. Blønd L, Rechter S. Arthroscopic treatment for snapping scapula: a prospective case series. Eur J Orthop Surg Traumatol. 2014;24(2):159–164. [DOI] [PubMed] [Google Scholar]
- 3. Chang WH, Kim YW, Choi S, Lee SC. Comparison of the therapeutic effects of intramuscular subscapularis and scapulothoracic bursa injections in patients with scapular pain: a randomized controlled trial. Rheumatol Int. 2014;34(9):1203–1209. [DOI] [PubMed] [Google Scholar]
- 4. Conduah AH, Baker CL, Baker CL. Clinical management of scapulothoracic bursitis and the snapping scapula. Sports Health. 2010;2(2):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 6. Ebrahimzadeh MH, Birjandinejad A, Razi S, Mardani-Kivi M, Reza Kachooei A. Oxford Shoulder Score: a cross-cultural adaptation and validation study of the Persian version in Iran. Iran J Med Sci. 2015;40(5):404–410. [PMC free article] [PubMed] [Google Scholar]
- 7. Gaskill T, Millett PJ. Snapping scapula syndrome: diagnosis and management. J Am Acad Orthop Surg. 2013;21(4):214–224. [DOI] [PubMed] [Google Scholar]
- 8. Groh G, Simoni M, Allen T, Dwyer T, Heckman M, Rockwood C. Treatment of snapping scapula with a periscapular muscle strengthening program. J Shoulder Elbow Surg. 1996;5(2):S6. [Google Scholar]
- 9. Islam SU, Choudhry MN, Akbar S, Waseem M. Outcome of scapulothoracic arthroscopy for painful snapping scapula. Open Orthop J. 2017;11:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kartus J-T. Editorial commentary. Arthroscopy of the scapulothoracic joint for treatment of snapping scapula syndrome: minimum 2-year results in a large series, a long-awaited publication. Arthroscopy. 2017;33(4):733. [DOI] [PubMed] [Google Scholar]
- 11. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251–2262. [DOI] [PubMed] [Google Scholar]
- 12. Memon M, Kay J, Simunovic N, Ayeni OR. Arthroscopic management of snapping scapula syndrome improves pain and functional outcomes, although a high rate of residual symptoms has been reported. Knee Surg Sports Traumatol Arthrosc. 2018;26(1):221–239. [DOI] [PubMed] [Google Scholar]
- 13. Menge TJ, Horan MP, Tahal DS, Mitchell JJ, Katthagen JC, Millett PJ. Arthroscopic treatment of snapping scapula syndrome: outcomes at minimum of 2 years. Arthroscopy. 2017;33(4):726–732. [DOI] [PubMed] [Google Scholar]
- 14. Merolla G, Cerciello S, Paladini P, Porcellini G. Scapulothoracic arthroscopy for symptomatic snapping scapula: a prospective cohort study with two-year mean follow-up. Musculoskelet Surg. 2014;98 Suppl 1:41–47. [DOI] [PubMed] [Google Scholar]
- 15. Millett PJ, Gaskill TR, Horan MP, van der Meijden OA. Technique and outcomes of arthroscopic scapulothoracic bursectomy and partial scapulectomy. Arthroscopy. 2012;28(12):1776–1783. [DOI] [PubMed] [Google Scholar]
- 16. Millett PJ, Smith S, Ho C, Spiegl U. Significant association between snapping scapula syndrome and anterior angulation of the superomedial scapular angle. Orthop J Sports Med. 2014;2(7 Suppl 2):2325967114S0006. [Google Scholar]
- 17. Morse BJ, Ebraheim NA, Jackson WT. Partial scapulectomy for snapping scapula syndrome. Orthop Rev. 1993;22(10):1141–1144. [PubMed] [Google Scholar]
- 18. Mozes G, Bickels J, Ovadia D, Dekel S. The use of three-dimensional computed tomography in evaluating snapping scapula syndrome. Orthopedics. 1999;22(11):1029–1033. [DOI] [PubMed] [Google Scholar]
- 19. Osias W, Matcuk GR, Skalski MR, et al. Scapulothoracic pathology: review of anatomy, pathophysiology, imaging findings, and an approach to management. Skeletal Radiol. 2018;47(2):161–171. [DOI] [PubMed] [Google Scholar]
- 20. Pavlik A, Ang K, Coghlan J, Bell S. Arthroscopic treatment of painful snapping of the scapula by using a new superior portal. Arthroscopy. 2003;19(6):608–612. [DOI] [PubMed] [Google Scholar]
- 21. Saper M, Kasik C, Dietzel D. Arthroscopic scapulothoracic decompression for snapping scapula syndrome. Arthrosc Tech. 2015;4(6):e631–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spiegl UJ, Petri M, Smith SW, Ho CP, Millett PJ. Association between scapula bony morphology and snapping scapula syndrome. J Shoulder Elbow Surg. 2015;24(8):1289–1295. [DOI] [PubMed] [Google Scholar]
- 23. Tahal DS, Katthagen JC, Marchetti DC, et al. A cadaveric model evaluating the influence of bony anatomy and the effectiveness of partial scapulectomy on decompression of the scapulothoracic space in snapping scapula syndrome. Am J Sports Med. 2017;45(6):1276–1282. [DOI] [PubMed] [Google Scholar]
- 24. Warth RJ, Spiegl UJ, Millett PJ. Scapulothoracic bursitis and snapping scapula syndrome: a critical review of current evidence. Am J Sports Med. 2015;43(1):236–245. [DOI] [PubMed] [Google Scholar]
- 25. Wylie JD, Beckmann JT, Granger E, Tashjian RZ. Functional outcomes assessment in shoulder surgery. World J Orthop. 2014;5(5):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]