Abstract
A recent randomized, multicenter trial did not show benefit of a CXCR1/2 receptor inhibitor (Reparixin) when analysis included marginal islet mass (>3,000 IEQ/kg) for allotransplantation and when immunosuppression regimens were not standardized among participating centers. We present a post-hoc analysis of trial patients from our center at the University of Chicago who received an islet mass of over 5,000 IEQ/kg and a standardized immunosuppression regimen of anti-thymocyte globulin (ATG) for induction. Twelve islet allotransplantation (ITx) recipients were randomized (2:1) to receive Reparixin (N = 8) or placebo (N = 4) in accordance with the multicenter trial protocol. Pancreas and donor characteristics did not differ between Reparixin and placebo groups. Five (62.5%) patients who received Reparixin, compared to none in the placebo group, achieved insulin independence after only one islet infusion and remained insulin-free for over 2 years (P = 0.08). Following the first ITx with ATG induction, distinct cytokine, chemokine, and miR-375 release profiles were observed for both the Reparixin and placebo groups. After excluding procedures with complications, islet engraftment on post-operative day 75 after a single transplant was higher in the Reparixin group (n = 7) than in the placebo (n = 3) group (P = 0.03) when islet graft function was measured by the ratio of the area under the curve (AUC) for c-peptide to glucose in mixed meal tolerance test (MMTT). Additionally, the rate of engraftment was higher when determined via BETA-2 score instead of MMTT (P = 0.01). Our analysis suggests that Reparixin may have improved outcomes compared to placebo when sufficient islet mass is transplanted and when standardized immunosuppression with ATG is used for induction. However, further studies are warranted. Investigation of Reparixin and other novel agents under more standardized and optimized conditions would help exclude confounding factors and allow for a more definitive evaluation of their role in improving outcomes in islet transplantation. Clinical trial reg. no. NCT01817959, clinicaltrials.gov
Keywords: Islet transplantation, immune modulation, immunosuppressive regimens, induction, chemokines, chemokine receptors
Introduction
Instant blood-mediated inflammatory reaction (IBMIR) is a major factor impacting islet engraftment and transplantation outcomes, causing a substantial (>50%) loss of islet mass after intraportal infusion1,2. Reparixin is a potent CXCR1/2 inhibitor and thus it was hypothesized that it could limit IBMIR and improve islet engraftment. Despite success in preclinical and single center clinical studies3–5, a recent phase 3 multicenter trial did not confirm this beneficial effect of Reparixin. However, the trial was performed without the standardization of donor selection, islet processing, and immunosuppression regimens among the participating centers5. We hypothesize that, under optimal conditions, Reparixin may indeed improve islet engraftment. These conditions include the transplantation of a standard islet mass (i.e., higher than marginal) and induction of immunosuppression with potent anti-thymocyte globulin (ATG). Herein, we present a post-hoc analysis limited to patients from our center only in order to exclude the effect of donor selection, variation in islet isolation technique, and the technical aspects of islet transplantation, which may have contributed to the negative results of the multi-center trial.
Materials and Methods
Study Design and Procedures
Out of the total 46 subjects with type 1 diabetes mellitus (T1DM) and life-threatening hypoglycemia participating in the phase 3 multicenter trial, we analyzed results from 12 (26%) patients enrolled at our center at the University of Chicago. Our cohort was composed of 8 patients assigned to the Reparixin group and 4 in the placebo group. Chicago was the only participating center from the United States in the trial, and thus followed additional exclusion criteria required by the Food and Drug Administration (FDA) which are presented in Supplemental Table S1. Pancreas donor selection at out center was based on the North American Islet Donor Score (NAIDS) previously developed and subsequently validated at our center6. Pancreata qualified for islet isolation only from donors with a NAIDS above 50 and with a cold ischemia time of less than 12 hours. Islet isolation was performed according to the Collaborative Islet Transplantation (CIT) Consortium protocol utilizing Liberase (Roche, Indianapolis, IN) for enzymatic digestion7. The islet isolation product was preserved in an incubator at a temperature of 22°C for up to 72 hours, including up to 6 hours of storage in the infusion bag prior to transplantation. Ultrasound-guided percutaneous transhepatic portal venous access was obtained by an interventional radiologist under local anesthesia and intravenous moderate sedation. A 4 Fr or 5 Fr end-hole catheter (Kumpe, Cook Medical, Bloomington, IN) was positioned in the main portal vein using fluoroscopy. Islets suspended in Transplant Media (CMRL 1066 Transplant Media) with 1 M HEPES (Corning Cell Sciences, Tewksbury, MA) supplemented 10% Human Serum Albumin were infused under gravity. Portal pressure was measured before, mid- and after islet infusion. For hemostasis, the catheter was retracted into the hepatic parenchymal track, which was then embolized with gelatin sponge (Surgifoam, Ethicon, Somerville, NJ) and detachable coils (Interlock, Boston Scientific, Marlborough, MA). For thromboembolic prophylaxis, heparin at a dose of 70 U/kg of recipient body weight was added to the islet infusion in the bag(s), followed by an intravenous drip for 48 hours titrated to a goal PTT of 50–60 seconds, and then subcutaneous fractionated heparin for 2 weeks.
For induction of immunosuppression, anti-thymocyte globulin (ATG) (Thymoglobulin, Genzyme, Cambridge, MA) was infused in divided doses for a total of 6 mg/kg of patient body weight, starting one day before islet transplantation. One dose of methylprednisolone (1 mg/kg) was given as premedication during the first ATG infusion only. No other steroids were administered in order to avoid confounding the interpretation of the anti-inflammatory effects of Reparixin. Basiliximab (Simulect, Novartis Pharmaceuticals, East Hanover, NJ) at a standard dose of 20 mg was infused intravenously immediately before and 3 days after a second islet transplant. Maintenance immunosuppression included oral mycophenolate mofetil at the target dose of 1000 mg twice a day or mycophenolic acid 720 mg twice a day. The goal for tacrolimus serum level was in the range of 8–10 ng/ml during first 3 months and 7–9 ng/ml afterwards. The dose was adjusted, if medically necessary, when adverse events occurred.
Study procedures performed according to the common multicenter trial protocol5
Based on the randomization number, patients received Reparixin at a dose of 2.772 mg/kg/hour or placebo in continuous infusion through a central line over 7 days starting 12 hours before islet transplantation as previously described5. If insulin independence was not achieved by day 75, each subject received a second islet transplant with repeat infusion of the previously assigned Reparixin or placebo, as performed during the first islet transplant. Islet product release criteria included: viability over 70%, purity over 20%, pellet volume less than 10 mL, islet mass transplanted above 3,000 islet equivalent (IEQ) per recipient body weight in kilograms (IEQ/kg) per transplant. Islet engraftment and function was assessed on day 75 ± 5 after islet infusion using the mixed meal tolerance test (MMTT)8. Patients were followed for 1 year after the last islet infusion5.
Post Hoc Islet Engraftment Analysis
In our analysis, we aimed to standardize conditions and measurements of islet engraftment and limit factors, which may have confounded the evaluation of the effect of Reparixin vs. placebo on islet engraftment during the multi-center trial.
Islet engraftment indices
Measurement of engraftment based only on islet mass engrafted would be biased by the differences in islet mass infused in each patient. Therefore, we evaluated islet engraftment based on the ratio of islet mass engrafted to total islet mass infused in IEQ. Engrafted islet mass was determined by measuring islet graft function as assessed by different indices.
In the multi-center trial, islet mass engrafted was determined by measurement of islet graft insulin secretion (using c-peptide as a proxy) which was calculated from the area under the curve (AUC) for serum c-peptide in 120 minute MMTT. Therefore, islet engraftment was determined as the AUC of c-peptide standardized by total IEQ infused but it was also additionally corrected for patient body weight in order to account for differences in insulin requirements/insulin sensitivity between individual patients (AUC c-peptide/IEQ/kg) (Fig. 1).
Figure 1.
Different indices for the calculation of islet engraftment.
In our sub-analysis, we chose to account for the difference in insulin requirements and insulin sensitivity between individual patients in a different, arguably more accurate way. We determined the islet mass engrafted based on an islet graft function calculated from insulin secretion (AUC for serum c-peptide as proxy) in relation to serum glucose changes (AUC for serum glucose) during MMTT (Fig. 1). Therefore, islet engraftment was then calculated as the AUC c-peptide /AUC glucose/IEQ.
We also assessed islet engraftment based on islet function by utilizing the BETA-2 index which takes into account a single value of fasting blood glucose and c-peptide, HbA1c, and daily dose of insulin per body weight9–12. After standardizing for the islet mass infused, we calculated islet engraftment as BETA-2/IEQ.
For comparison, we also determined islet engraftment based on more crude approximations of islet function with limited standardization: MMTT AUC c-peptide, MMTT AUC c-peptide/IEQ, BETA-2.
Assessment of islet engraftment on day 75
We focused our analysis on islet engraftment measured on post-operative day 75, when post-transplant recovery and islet graft revascularization were largely complete12. We did not measure engraftment at 1 year follow-up as many different factors could affect islet graft function between day 75 and 365 (e.g., rejection, autoimmune recurrence, drug toxicity) and thus would confound the assessment of engraftment.
Assessment of islet engraftment after single infusion in patients with undetected stimulated serum c-peptide
We decided to assess islet engraftment after a single transplant and only in patients with undetectable stimulated serum c-peptide prior to islet infusion in order to avoid measurement of endogenous islet function.
Exclusion of transplants where islet engraftment was compromised by factors independent from Reparixin
In order to exclude the influence of external factors on islet engraftment which were independent from Reparixin, we excluded cases with early rejection, severe cytokine release syndrome, and primary nonfunction as the rate of these complications did not differ between the Reparixin and placebo groups. Three cases in the Reparixin group and 2 cases in the placebo group were thus excluded. Similarly, we assessed islet engraftment after second islet transplant in 3 patients with negative c-peptide prior to transplantation after primary nonfunction of the first islet transplant.
Metabolic Outcomes
Metabolic outcomes based on change in HbA1c, incidence of severe hypoglycemic episodes (SHE), daily requirements of insulin, and insulin dependence were included as secondary endpoints similarly as in the common trial protocol5. We additionally assessed 1 year islet graft function using the BETA-2 score and Igls classification9–13.
Plasma (Serum) Cytotoxin and MicroRNA-375 (miR-375) Profile after Intraportal Islet Transplantation
Serum samples were drawn before and 6, 12, 24, 72, 120, and 168 hours after each islet transplant. Concentrations of CXCL8, CXCL-9, CXCL-10, IL-6, IL-10, CCl-2 (MCP-1), CCL-3, CCL-4, miR-375, and miR-375/IEQ were assessed at each time point to analyze the profile of chemokine, cytokine, and miR-375 secretion during the first 7 days after each ITx in both the Reparixin and placebo groups.
Statistical Analysis
Descriptive statistics are expressed as median, range, and percentages as appropriate. The variables were tested for normal distribution and analyzed with Student’s t-test. In case of non-normally distributed data we used the Mann-Whitney U test. To compare categorical variables, we used Fisher’s exact test. We used the t-test to compare dependent variables of log10-transformed AUCs for the analysis of cytokine, chemokine, and miR-375 serum levels. For all analysis, a P-value <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient and Donor Characteristics
The characteristics of patients in the intention to treat cohort were comparable between the Reparixin and placebo groups (NS): median recipient weight was 75.5 kg and median BMI 25.5 kg/m2 (Table 1). Pancreas donor characteristics were also similar in both groups and did not differ significantly: median donor age was 41 years; median weight was approximately 100 kg and BMI 33 kg/m2 with HbA1c <5.9% (41 mmol/mol) in both groups (Table 1). Islet quality assessed in vitro with the glucose stimulated insulin release (GSIR) assay and in vivo by islet transplantation in nude mice was highly comparable in both groups (Table 1) (NS).
Table 1.
Baseline Donor, Recipient, and Islet Characteristics. Baseline characteristics of islet transplant recipients, pancreas donors, and islets. Quality of islets was assessed by in vitro and in vivo assays. Islet mass infused did not differ statistically between the Reparixin and placebo groups (P > 0.05). Values presented are medians with minimum and maximum ranges in parentheses.
| Recipient characteristics | Reparixin N = 8 | Placebo N = 4 | P |
|---|---|---|---|
| Age at 1st ITx (years) | 46 (28–56) | 40 (30–48) | 0.57 |
| Sex (M/F) | 4M/ 4F | 1M/ 3F | 0.57 |
| Weight at 1st ITx (kg) | 77 (42.6–93.8) | 74 (58.0–82.4) | 0.51 |
| BMI at 1st ITx (kg/m2) | 26 (18.9–29.9) | 25 (21.8–29.9) | 0.84 |
| Pre ITx HbA1c (%) Pre ITx HbA1c (mmol/mol) |
7.4 (6.8–8.1) 57 (51–65) |
7.0 (6.5–8.6) 53 (48–70) |
0.51 |
| Pre ITx daily insulin (U) | 34 (29–67) | 36 (29–48) | 0.99 |
| Duration of T1DM (years) | 32 (16–44) | 31 (15–47) | 0.19 |
| Donors characteristics | 16 donors (12 ITx) | 10 donors (8 ITx) | 0.83 |
| Age (years) | 42.5 (17–63) | 41.5 (20–57) | 0.71 |
| Sex (M/F) | 12 M/4F | 7 M/3F | 0.90 |
| Weight (kg) | 98.3 (59–151) | 103 (93–179) | 0.24 |
| Donor Scoring (NAIDS) | 73 (30–100) | 76 (47–88) | 0.87 |
| BMI (kg/m2) | 33.1 (23.8–42.7) | 32.9 (29.5–50.8) | 0.33 |
| Islet quality assays | |||
| Pre culture GSIR | 1.5 (1.01–3.41) | 1.8 (0.46–11) | 0.90 |
| Pre ITx GSIR | 1.71 (0.78–10.95) | 1.43 (0.46–5) | 0.27 |
| Viability (%) | 93. (82.1–97.5) | 94.18 (87–97.5) | 0.82 |
| Bioassay (human ITx in nude mice) reversal of hyperglycemia* (%) |
5/5 (100%) |
4/4 (100%) |
|
| Islet mass infused | |||
| Islet mass first ITx (IEQ/kg) | 6,074 (5,494–9,993) |
7,328 (5,662–8,716) |
0.8 |
| Islet mass second ITx (IEQ/kg) | 7,066 (5,772–9,197) |
7,419 (5,511–8,419) |
0.88 |
| Total mass infused per patient (IEQ/kg) | 10,863 (5,494–16,209) |
13,737 (13,192–17,135) |
0.15 |
ITx: islet transplantation; NAIDS: North American Islet Donor Score; GSIR: glucose-stimulated insulin release; *hyperglycemia (blood glucose >200 mg/ml) was induced with streptozocin injection, reversal of hyperglycemia (blood glucose <200 mg/ml) by transplanted islets was confirmed by measurement of hyperglycemia after excision of the islet graft.
Comparison of Islet Mass Transplanted and Intra-Operative Portal Vein Pressures
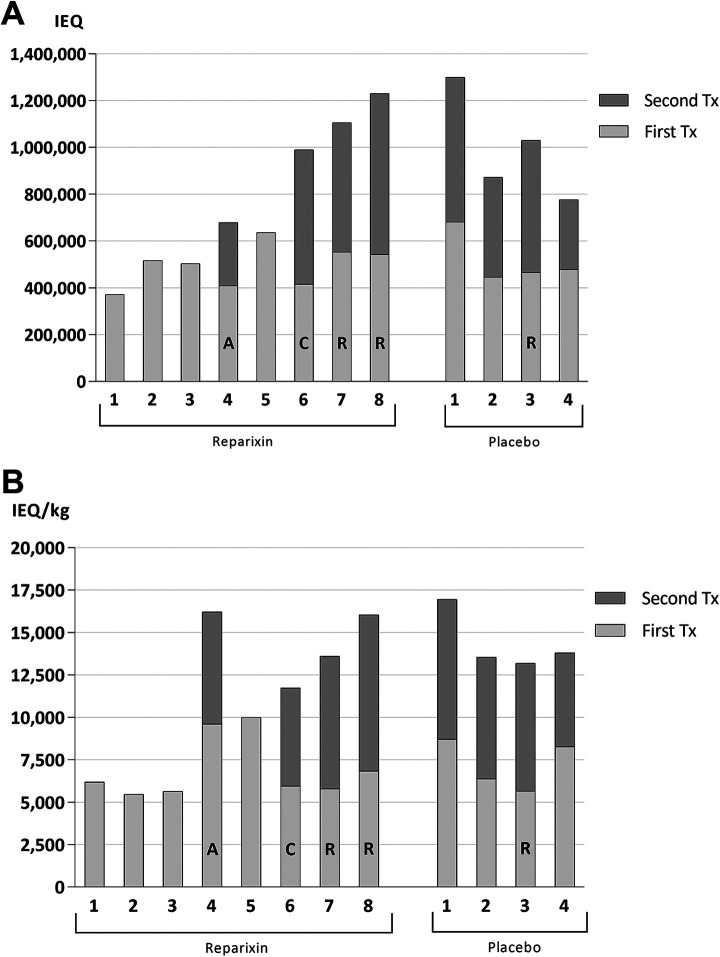
Patients in both the Reparixin and placebo groups were transplanted similar islet masses during the first ITx: median islet mass was 510,155 IEQ (371,821–635,554) in the Reparixin group vs. 473,013 IEQ (445,833–681,565) in the placebo group and median IEQ/kg was 6,074 (5,464–9,993) vs 7,328 (5,655–8,716), respectively (NS) (Table 1). All patients in the placebo group, and only patients with complications in the Reparixin group, underwent a second ITx and all these patients received a similar islet mass (NS) (Table 1 and Fig. 2A, B).
Figure 2.
Transplanted islet mass Patients in both groups were transplanted a similar islet mass expressed in IEQ (A) and IEQ/kg (B) during the first ITx (light gray bars). All patients from the placebo group and only patients with complications in the Reparixin group required a second ITx (dark gray bars). (A: moderate DSA; R: antibody mediated rejection; C: severe cytokine release syndrome). IEQ: islet equivalent.
In order to reach the required islet mass (>3,000 IEQ/kg), islets were combined from two donors in single islet transfusions for 4 (33%) patients in the Reparixin group and in 2 (25%) patients in the placebo group. Altogether, 16 donor pancreata were utilized for 12 islet transplants in the Reparixin group and 10 donor pancreata for 8 islet transplants in the placebo group.
Median pellet volume of the final islet product infused was similar in both the Reparixin and placebo groups: 5 ml (3–10) and 6 ml (3–7.5), respectively (NS). Portal pressure (PP) during the islet infusion fluctuated similarly in both groups; peak PP never exceeded 20 mmHg in either group and all islet infusions were successfully completed. For all the above listed parameters, there was no difference between groups.
Complications
Our first patient in the Reparixin group developed severe headache, fever, and hypotension consistent with a grade 2 CTCAE severe cytokine release syndrome (sCRS)14,15 but was responsive to intravenous fluids. This reaction was triggered by a standard second dose of 1.5 mg/kg ATG infused intravenously without steroids over 6 hours post-transplant. For the following patients, the ATG dose per infusion and the rate were lowered to achieve delivery over 18 hours and spread over 7 days of admission, maintaining a goal total dose of 6 mg/kg of body weight. Acetaminophen and diphenhydramine were given prior to infusion and re-dosed every 6 hours. The remaining patients experienced mild transient headache, nausea, and malaise related to the infusion of ATG, but these symptoms subsided with intravenous hydration and additional doses of acetaminophen, diphenhydramine, and antiemetics.
The incidence rate of other complications did not differ significantly between the Reparixin and placebo groups. Intraperitoneal bleeding or liver hematoma after intraportal infusion developed after 2/12 (16.6%) transplant procedures in the Reparixin and in 2/8 (25%) in the placebo group. Patients in both groups responded to blood transfusion without the need for surgical intervention. None of the patients developed portal vein thrombosis or any other thromboembolic complications.
Two patients (25%) in the Reparixin group and one patient (25%) in the placebo group developed high levels of donor specific HLA antibody (DSA) with PRA >90% within first 75 days of initial islet transplant. In combination with signs of islet dysfunction, this suggests the diagnosis of antibody mediated rejection (AMR). Another patient from the Reparixin group developed a low/medium level of DSA with some loss of islet function and required a second islet transplant before obtaining long-term insulin independence. One patient in the placebo group developed primary non function by 8 weeks post-transplant without any DSA detected.
All patients received standard antibiotic prophylaxis during the islet infusion—cefazolin intravenously for 24 hours as well as fluconazole for 3 months, valganciclovir for 6 months in patients at high risk for cytomegalovirus (CMV) or acyclovir in medium and low risk patients. One patient at high risk developed CMV pneumonia 2 months after completion of a 6-month course of valganciclovir prophylaxis. No unexpected adverse events were observed related or possibly related to the studied medication or to the islet transplantation procedure. The adverse event profile did not differ between the Reparixin and placebo groups in our cohort and the profile was similar to that described in the multicenter trial as a whole5.
Patient Enrolment, Follow up, and Compliance
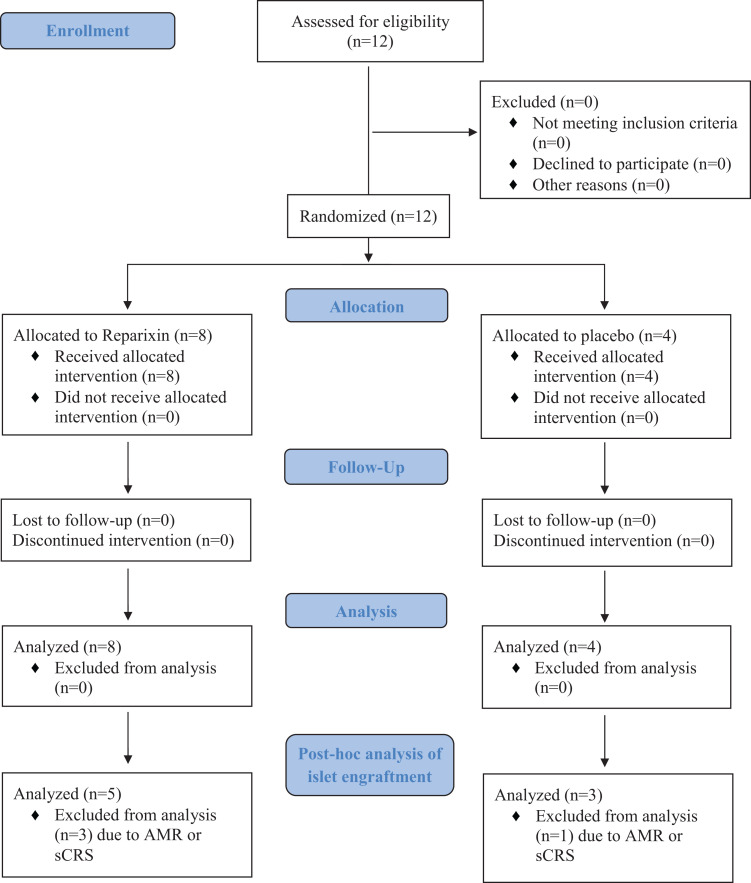
All patients at our site complied with the trial protocol and received the study agent Reparixin or placebo as randomized (Fig. 3). No patients discontinued the intervention, withdrew consent, nor were any patients lost to follow-up. All patients in our cohort were followed until the end of the multicenter trial, 1 year after the last transplant (e.g., 1 year after the first ITx if patient remained insulin independent, otherwise 1 year after second ITx). At that time point the insulin independence rate after a maximum of two islet infusions was 6/8 (75%) in the Reparixin group and 2/4 (50%) in the placebo group.
Figure 3.
CONSORT 2010 flow diagram.
Islet Engraftment After Single Islet Transplant
Islet engraftment after single islet transplant was measured after the first islet infusion for patients except for those who experienced primary non-function with undetectable serum c-peptide and underwent a second islet infusion. For these patients with a second islet infusion, islet engraftment was measured after the second transplant. One patient in each group was excluded due to partial islet graft function after the first transplant. Altogether, we assessed islet engraftment in 7 patients from the Reparixin group (in 5 after 1st transplant and in 2 after their 2nd transplant) and in 3 patients from the placebo group (in 2 after 1st transplant and in 1 after the 2nd transplant). Patient and transplant characteristics in the post-hoc islet engraftment analysis did not differ between the Reparixin and placebo groups (Table 2).
Table 2.
Patient and Transplant Characteristics in Post-hoc Islet Engraftment Analysis. There were no statistically significant differences in patient and islet transplant characteristics between the Reparixin and placebo groups in the post-hoc islet engraftment analysis.
|
Subject
# |
Number of ITx procedures |
Weight
(kg) |
BMI
(kg/m2) |
Pre-Tx
daily insulin requirement (units/day) |
Transfused Mass
(IEQ) |
Transfused Mass
(IEQ/kg) |
Pallet
volume (mL) |
Opening portal
pressure (mmHg) |
Closing portal
pressure (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| Reparixin (N = 7) | |||||||||
| 1 | 1st | 60.1 | 24.4 | 26 | 371,821 | 6,187 | 3.8 | 12 | 14 |
| 2 | 1st | 91.5 | 28.2 | 41 | 516,542 | 5,676 | 7 | 14 | 14 |
| 3 | 1st | 92.2 | 27.8 | 35 | 503,767 | 5,494 | 2.9 | 12 | 13 |
| 4 | 1st | 42.6 | 18.9 | 20 | 409,389 | 9,610 | 9.5 | 16 | 17 |
| 5 | 1st | 63.6 | 23.9 | 34 | 635,554 | 9,993 | 5 | 8 | 8 |
| 6 | 2nd | 62.4 | 23.5 | 37 | 573,884 | 9,197 | 5 | 4 | 4 |
| 7 | 2nd | 95.8 | 30.2 | 47 | 553,002 | 5,772 | 6.5 | 15 | 14 |
| Median (range) | 63.6 (42.6–95.8) | 24.4 (18.9–30.2) |
35
(20–47) |
516,542 (371,821–635,554) | 6,187 (5,494–9,993) | 5 (2.9–9.5) | 12 (4–16) | 14 (4–17) | |
| Placebo (N = 3) | |||||||||
| 1 | 1st | 78.2 | 23.6 | 60 | 681,565 | 8,716 | 6.5 | 8 | 8 |
| 2 | 1st | 70 | 27.3 | 36 | 445,833 | 6,378 | 3 | 11 | 18 |
| 3 | 2nd | 74.9 | 27.2 | 43 | 564,627 | 7,530 | 7.6 | 14 | 17 |
| Median (range) | 74.9 (70–78.2) | 27.2 (23.6–27.3) | 43 (36–60) | 564,627 (445,833–681,565) | 7,530 (6,378–8,716) | 6.5 (3–7.6) | 11 (8–14) | 17 (8–18) | |
| P-value | 0.83 | >0.99 | 0.18 | 0.51 | 0.83 | 0.91 | 0.65 | 0.33 | |
Islet engraftment based on the MMTT AUC c-peptide-to-glucose ratio, standardized for IEQ per islet infusion, on day 75 after the first ITx was significantly higher for patients who received Reparixin compared to placebo. Median AUC c-peptide [ng/mL] / AUC glucose [mg/dL]/IEQ × 108 on day 75 was 6.02 (5.01–11.99) and statistically higher in the Reparixin group than 4.56 (1.66–5.02) in the placebo group (P = 0.03) (Fig. 4A). Engraftment measured by BETA-2 score standardized by IEQ × 106 in the first transplant was significantly higher in the Reparixin group than in placebo, median 41.33 × 106 (32.24–63.36) vs 27.7 × 106 (20.8–30), respectively (P = 0.01) (Fig. 4B). Islet engraftment was above 30 (BETA-2 score) and higher in every patient in the Reparixin group compared to any of patients in the placebo group. Islet engraftment as determined by c-peptide secretion only or not standardized by islet mass transplanted showed no statistical difference between the two groups (Supplemental Fig. S1A-E). Islet engraftment after first islet transplant was not statistically different in the Reparixin and placebo groups.
Figure 4.
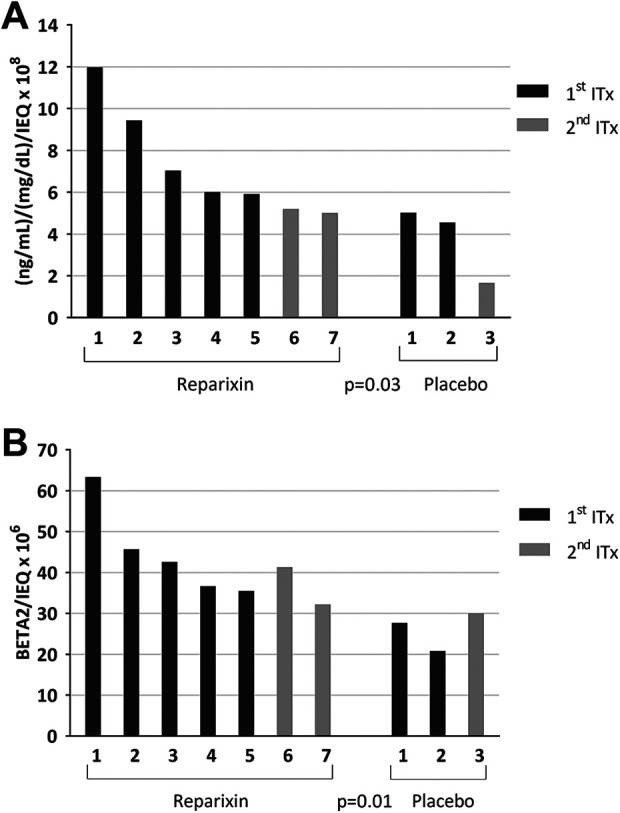
Islet engraftment on day 75 after single islet transplant in post-hoc islet engraftment analysis cohort. (A) Islet engraftment assessed using the AUC c-peptide/AUC glucose/IEQ index was significantly higher in the Reparixin group compared to placebo (P = 0.03). (B) A similar result was found when islet engraftment was determined based on islet function as measured by BETA-2 score standardized with IEQ: (BETA-2/IEQ) (P = 0.01). IEQ: islet equivalent.
Secondary Endpoints
Secondary endpoints included insulin independence, HbA1c, and incidence of severe hypoglycemic episodes (SHE). There was no statistically significant difference between the treatment and placebo groups at our center (Table 3).
Table 3.
Secondary Endpoints in Patients in the Reparixin vs Placebo Group in Our Cohort. There was no statistically significant difference in the secondary endpoints between the Reparixin and placebo groups in our cohort (P > 0.05). A column presenting data from the previously published multicenter trial cohort is added for context.
| Secondary endpoints |
Reparixin
N = 8 |
Placebo N = 4 | Multicenter trial cohort 5 |
|---|---|---|---|
|
Cumulative SHE
between day 75 and 1 year |
0 | 0 | 0 |
| HbA1c (%), median | 7.4---5.8---5.7 | 7.0---5.9---5.9 | 8.1---6.3---6.0 |
| HbA1c (mmol/mol), median | |||
| preTx---day 75---1 year after last ITx | 57---40---39 | 53---41---41 | 65---45---42 |
|
HbA1C ≤ 6% (≤42 mmol/mol)
and no SHE at 1 year |
75% (6/8) | 75% (3/4) | N/A |
|
HbA1C ≤ 6.5% (≤ 48 mmol/mol)
and no SHE at 1 year |
75% (6/8) | 75% (3/4) | N/A |
|
HbA1C≤ 7% (≤ 53 mmol/mol)
and no SHE at 1 year |
100% | 100% | 71% |
| Change in HbA1c day 75 after 1st ITx %, (value) |
–24% (–1.85) | –19% (–1.30) | N/A |
|
Change in HbA1c 1 year after last ITx %, (value) |
–22% (–1.6) | –14% (–0.95) | N/A |
| Insulin independence | |||
| At any time point | 87.5% (7/8) | 100% (4/4) | 21.2% (7/33)* |
| At day 75 after 1st ITx | 50% (4/8) | 25% (1/4) | 3% (1/33)* |
| At 1 year after one ITx (1st or 2nd) | 62.5% (5/8) | 0 | 0* |
| At 1 year after last ITx | 75% (6/8) | 50% (2/4) | 17.2% (5/29)* |
|
Functioning islet graft at 1 year after last ITx (serum c-peptide >0.3ng/ml) |
100% (8/8) | 100% (4/4) | 82.7% (24/29*) |
| Insulin requirements | |||
|
Change at day 75 after 1st ITx %, median value |
–100% (–0.41) | –67.65% (–0.35) | –44% (–0.23) |
|
Change at 1 year after last ITx %, median value |
–100% (–0.44) | –93% (–0.48) | –44% (–0.33) |
| Clinical outcomes based on islet graft function at 1 year after last ITx | |||
| beta score 16 | |||
| Success rate, [beta score ≥ 6] (%) | 75% | 75% | 38% |
| Median beta score | 7.5 | 6.5 | N/A |
| Igls classification 13 | |||
| Success rate [optimal and good function] | 8/8 100% | 4/4 100% | ∼50% |
| BETA-2, (median)9 | 19.4 | 14.7 | N/A |
| TEF/IEQ/kg (median)17 | 68 | 42 | N/A |
* Rates after excluding patients from our cohort (rates only for the remaining patients in the trial)
Tx: islet transplantation; SHE: severe hypoglycemic episodes; TEF: transplant estimated function; IEQ: islet equivalent dose.
Severe Hypoglycemic Episodes and Glucose Control
Six months prior to ITx, all patients in both groups reported severe hypoglycemic episodes (SHE). Despite insulin regimens optimized by endocriologists, patients in the Reparixin group experienced 11 (7–96) SHEs and in placebo group 12 (3–75). Comparatively, no patients in either group experienced SHE during the follow-up after last transplant (between 3 and 12 months). 75% of patients in both groups maintained HbA1c at 6% (42 mmol/mol) or below, while the remaining 25% of patients maintained HbA1c at 7% (53 mmol/mol) or below (Table 3).
Insulin Independence after one ITx
Five out of eight patients (62.5%) from the Reparixin group achieved and maintained insulin independence for 1 year and even for 2 years after only a single successful ITx; of these, four patients achieved independence after the first ITx and one patient after her second ITx (due to complete graft loss after the first ITx) (Fig. 5A). No patients from the placebo group were able to maintain insulin independence at 1 year after only a single successful ITx (P = 0.08).
Figure 5.
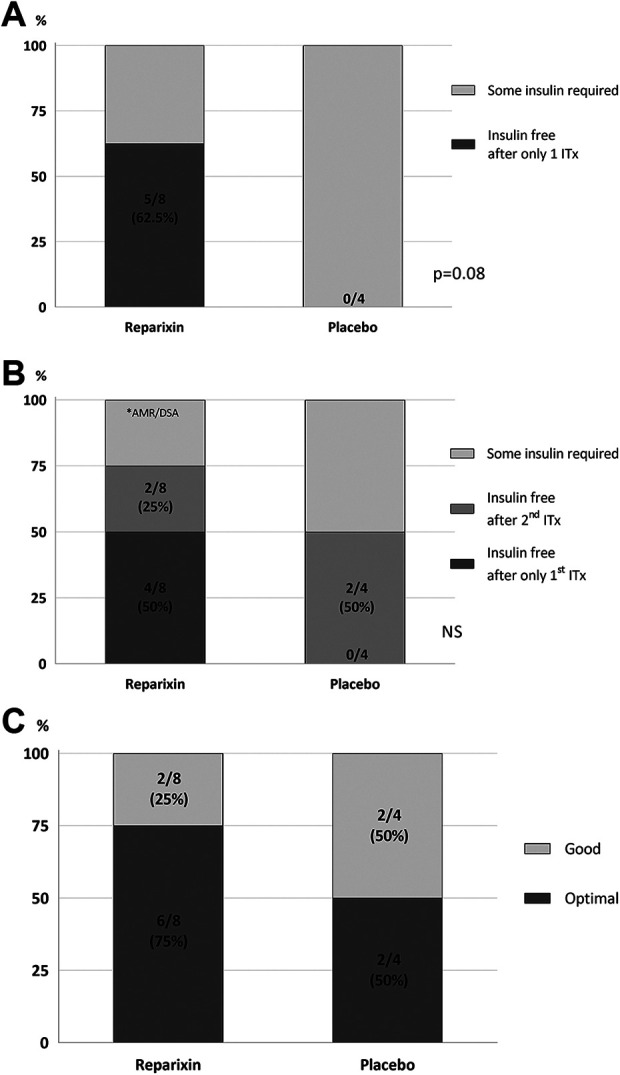
Clinical outcomes. (A) Insulin independence at 1 and 2 years after single ITx; five out of eight patients (62.5%) from the Reparixin group achieved and maintained insulin independence over 1 and even 2 years of follow-up after only one islet Tx, whereas not one patient from the placebo group was able to maintain insuling independence for even 1 year (P = 0.08). (B) Insulin independence at 1 year after last ITx; At the completion of the study 1 year after last ITx, 6/8 patients (75%) were still insulin independent in the Reparixin group vs. 2/4 patients (50%) in the placebo group. (C) Successful (optimal and good) clinical outcome as defined by the Igls classification was 100% at 1 year after last ITx in both the Reparixin and placebo groups, with a higher rate of optimal outcome in the Reparixin group.
Overall Insulin Independence
All patients except one [11/12 (91%)] became insulin independent during the duration of the study. At one and 2 year follow-up after the last ITx, 6/8 patients (75%) were still insulin independent in the Reparixin group vs. 2/4 patients (50%) in the placebo group (Fig. 5B). The only patients in the Reparixin group who did not achieve or maintain insulin independence for at least 1 year were patients who experienced AMR (n = 2) or sCRS (n = 1) early after the transplant.
Clinical Outcomes Based on the Igls Classification13, Beta Score16, BETA-2 Score9, and TEF/IEQ17
All patients in our cohort had successful (i.e., optimal or good) outcomes at 1 year after the last islet transplant as defined by the Igls classification (Fig. 5C). 75% of patients in the Reparixin group and 50% of patients in the placebo group achieved optimal outcomes, while the rest had good outcomes. Islet function based on beta score, BETA-2 score and TEF/IEQ was not statistically different for the two groups (Table 3).
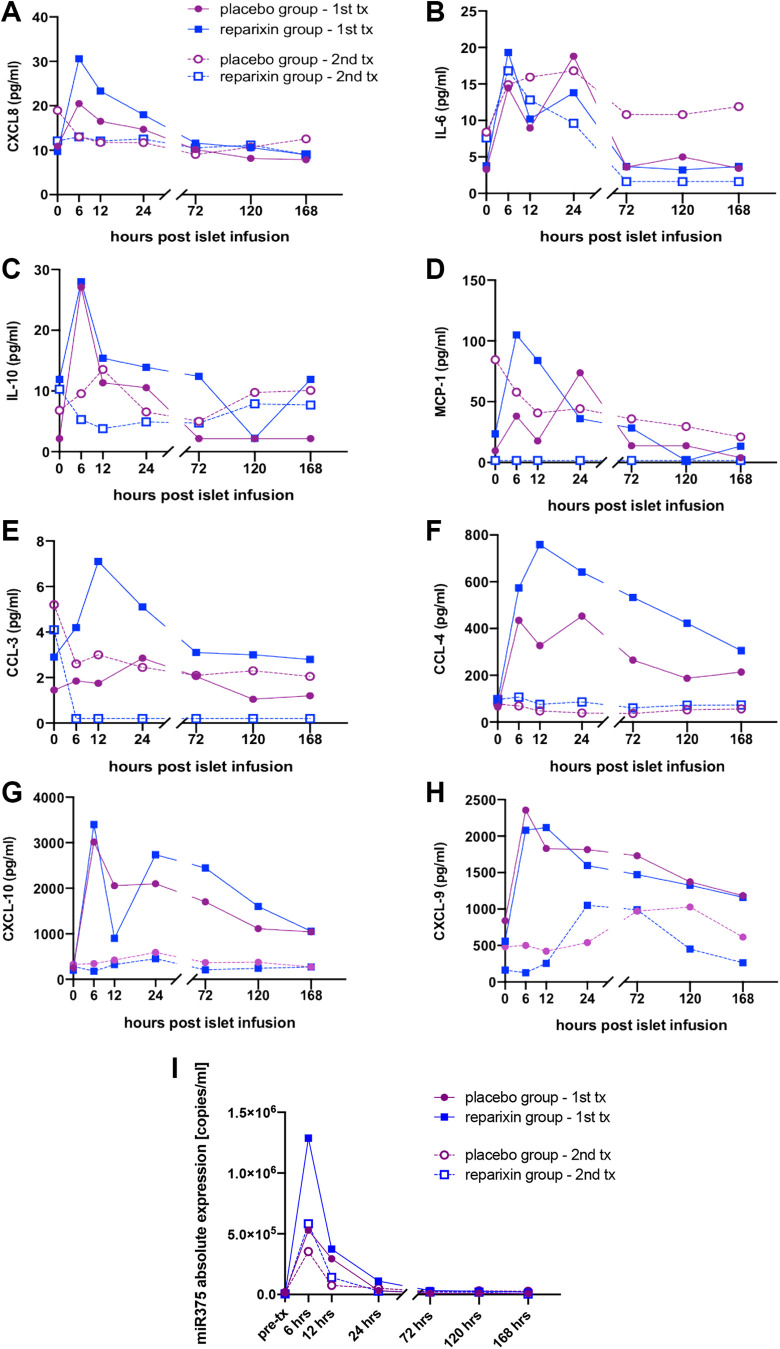
Profiles of Cytokines, Chemokines, and miR-375 Released after ITx
There was a significant increase of CCL4 and IL-6 secretion during the first 6–12 hours aftet ITx with subseqent decrease over time in the placebo group after the 1st ITx (P < 0.05); this was not observed for the remaining chemokines nor for cytokines or miR-375 (Fig. 6A-I, Table 4).
Figure 6.
Cytokine, chemokine, and miR-375 release profiles after islet transplantation. Concentration of CXCL8 (a), IL-6 (b), IL-10 (c), MCP-1 (CCL2) (d), CCL-3 (e), CCL-4 (f), CXCL-10 (g), and CXCL-9 (h) miR-375 (i) was measured in patients in the Reparixin (blue square) group and the placebo (purple circle) group before induction of immunosuppression (pre-ITx) and 6, 12, 24, 72, 120, 168 hours after the 1st (single line) and 2nd (dashed line) islet infusion.
Table 4.
Indentification of Cytokine, Chemokine, and miR-375 Characteristic Release Trends Over Time with a Peak 6–12 Hours after ITx and Subsequent Downfall.
Characteristic Trend of Selected Released Cytokines, Chymokines, and miR-375, with a Peak 6–12 Hours After ITx with Subsequent Downfall Was Identified. In placebo group such characteristic trend was observed for CCL4 and IL-6 secretion after the 1st ITx (P < 0.05) but not for the remaining chemokines, cytokines or miR-375 (P > 0.05). In the Reparixin group, the same trend was observed, not only for CCL4 and IL-6, as observed in the placebo group, but also for CXCL8, MCP-1 (CCL2), CCL3, CXCL10, as well as miR-375 and miR-375/IEQ (P < 0.05). For the 2nd ITx, the above described trends were observed only for miR-375 and miR-375/IEQ and only in the Reparixin group (P < 0.05).
| P-values | ||||
|---|---|---|---|---|
| ITx1 | ITx2 | |||
| Reparixin | Placebo | Reparixin | Placebo | |
| CXCL8 | 0.0003 | 0.4475 | 0.7095 | 0.9498 |
| CCL2 | 0.0108 | 0.0547 | 0.8875 | 0.9200 |
| CCL3 | 0.0304 | 0.0978 | 0.8574 | 0.7833 |
| CCL4 | 0.0009 | 0.0186 | 0.6790 | 0.9933 |
| CXCL10 | 0.0002 | 0.1783 | 0.3509 | 0.9433 |
| CXCL9 | 0.1069 | 0.2289 | 0.4005 | 0.1070 |
| IL-6 | 0.0031 | 0.0135 | 0.1273 | 0.8649 |
| IL-10 | 0.0552 | 0.1611 | 0.9215 | 0.9490 |
| miR-375 | <0.0001 | 0.1571 | 0.0252 | 0.1210 |
| miR-375/IEQ | 0.0001 | 0.0730 | 0.0030 | 0.1200 |
In the Reparixin group, the same trend over time was observed not only for CCL4 and IL-6, as in the placebo group, but also for CXCL8, MCP-1 (CCL2), CCL3, CXCL10, as well as for miR-375 and miR-375/IEQ (P < 0.05). Of note, ATG infusion took place during the same time after the 1st ITx in both groups. For the 2nd ITx, the trends described above were observed only for miR-375 and miR-375/IEQ and only in the Reparixin group (P < 0.05) (Table 4). Of note, Basiliximab was used instead of ATG for induction of immunosuppression after the 2nd ITx.
Cytokine, Chemokine, and miR-375 Release after Each Transplant in the Reparixin and Placebo Groups
Cytokine release during the first 7 days after 1st ITx (ATG) was significantly higher for CCL3 and CCL4 in the 1st ITx in the Reparixin group compared to placebo (P = 0.0103, 0.0347) when determined by AUC. There was no statistical difference in cytokine AUC after the 2nd ITx between the Reparixin and placebo groups (Table 5).
Table 5.
Comparison of Cytokine, Chemokine, and miR-375 Release between the Reparixin and Placebo Groups in the 1st and 2nd ITx.
Comparison of cytokine, chemokine, and miR-375 release based on AUC from selected time point measurements over the first 7 days for the Reparixin and placebo groups undergoing 1st and 2nd ITx. T-test for independent samples was used to compare log10-transformed AUC values. Cytokine release during the first 7 days after 1st ITx (ATG) was determined by AUC and was significantly higher for CCL3 and CCL4 in the 1st Tx in the Reparixin group compared to placebo (P = 0.0103, 0.0347). There was no statistically significant difference in cytokine AUC between Reparixin and placebo after the 2nd ITx.
| 1st Itx | 2nd ITx | |||||
|---|---|---|---|---|---|---|
| Reparixin | Placebo | P-value | Reparixin | Placebo | P-value | |
| CXCL8 [pg/ml] AUC | 2,531 (2,225–2,655) | 1,855 (1,589–2,122) | 0.3506 | 1,724 (1,663–3,111) | 1,793 (1,295–3,062) | 0.8282 |
| MCP1 [pg/ml] AUC | 6,253 (4,185–7,290) | 2,457 (505–4,410) | 0.1261 | 0 (0–15,326) | 5,580 (1,565–22,430) | 0.4500 |
| CCL3 [pg/ml] AUC | 498 (312–600) | 100 (55–145) | 0.0103 | 12 (0–616) | 380 (123–738) | 0.4395 |
| CCL4 [pg/ml] AUC | 82,145 (71,118–114,322) | 43,135 (41,955–44,315) | 0.0347 | 12,066 (7,384–16,558) | 7,853 (6,593–18,126) | 0.8078 |
| CXCL10 [pg/ml] AUC | 259,935 (248,002–312,488) | 259,569 (192,149–326,989) | 0.6507 | 37,829 (32,835–75,387) | 60,938 (43,213–106,459) | 0.4142 |
| CXCL9 [pg/ml] AUC | 259,935 (248,002–312,488) | 259,569 (192,149–326,989) | 0.4646 | 37,829 (32,835–75,387) | 60,938 (43,213–106,459) | 0.1813 |
| IL-6 [pg/ml] AUC | 1071 (819–2,103) | 1000 (815 –1,186) | 0.5628 | 826 (648 –1,310) | 1,909 (763–3,059) | 0.3353 |
| IL-10 [pg/ml] AUC | 1,684 (1,148–2,527) | 512 (85–939) | 0.3201 | 666 (69 –1,574) | 1,273 (132–2,738) | 0.7617 |
| miR-375 [copies/ml] AUC | 22,150,200 (16,210,800–27,911,400) | 7,923,300 (4,916,400–10,930,200) | 0.3077 | 13,484,640 (4,500,660–20,379,213) | 10,389,225 (6,809,736–15,889,425) | 0.8686 |
| miR-375 [copies in 1 ml /infusion IEQ] AUC | 35 (30–75) | 13 (10–16) | 0.2782 | 30 (8–50) | 25 (13–34) | 0.8222 |
AUC of cytokine release during the first 7 days after 1st ITx (ATG) was significantly higher for CXCL10 compared to 2nd ITx (Basiliximab) in both the Reparixin and placebo groups (P < 0.05).
CXCL4 release was higher after 1st ITx compared to after the 2nd ITx only in the Reparixin group but not in the placebo group (P = 0.0154) (Table 6). There was no statistically significant difference for the remaining cytokines, chemokines, or miR-375 released after the 1st ITx nor after the 2nd ITx in both the Reparixin and placebo groups.
Table 6.
Mixed Models for Treatment (Reparixin vs Placebo), and Induction of Immunosupression (1st vs 2nd ITx) in Reparixin vs Placebo. Cytokine released during the first 7 days after the 1st ITx (ATG) based on the calculated AUC was significantly higher for CXCL10 compared to the 2nd ITx (Basiliximab) in both the Reparixin and placebo groups (P < 0.05). CXCL4 release was higher after the 1st ITx compared to after the 2nd ITx only in the Reparixin group but not in the placebo group (P = 0.0154).
| Reparixin vs placebo mixed models | 1st vs 2nd Tx Reparixin (paired t-test) |
1st vs 2nd Tx placebo (paired t-test) |
|
|---|---|---|---|
| P-value | P-value | P-value | |
| CXCL8 [pg/ml] AUC | 0.6381 | 0.4053 | 0.9252 |
| MCP1 [pg/ml] AUC | 0.7814 | 0.2491 | 0.3032 |
| CCL3 [pg/ml] AUC | 0.6708 | 0.2396 | 0.7445 |
| CCL4 [pg/ml] AUC | 0.0522 | 0.0154 | 0.0702 |
| CXCL10 [pg/ml] AUC | 0.9901 | 0.0194 | 0.0315 |
| CXCL9 [pg/ml] AUC | 0.7775 | 0.0516 | 0.4398 |
| IL-6 [pg/ml] AUC | 0.6000 | 0.5814 | 0.5948 |
| IL-10 [pg/ml] AUC | 0.7348 | 0.4307 | 0.6616 |
| miR-375 [copies/ml] AUC | 0.2054 | 0.6976 | 0.4667 |
| miR-375/IEQ [copies in 1 ml /infusion IEQ] AUC | 0.1918 | 0.6659 | 0.4262 |
Overall, when ATG was used for immunosuppressive induction, the use of Reparixin led to a significantly higher release of CCL3 and CCL4 within the first 7 days after the 1st ITx when compared to placebo (P = 0.0103, 0.0347). Additionally, Reparixin led to significantly higher release of CCl4 during the 1st ITx compared to the 2nd ITx. This difference did not exist for the placebo group. There was no other statistically significant difference in the release of the remaining cytokines or chemokines evaluated between the Reparixin and placebo groups (P > 0.05). Only the release of CLCX10 was increased during the 1st ITx compared to the 2nd ITx in both groups.
There was no difference in the amount of miR-375 and miR-375/IEQ released during the first 7 days post-ITx between the Reparixin and placebo groups, between the first and second transplant, as well as between transplants leading to insulin independence, partial function, or failure.
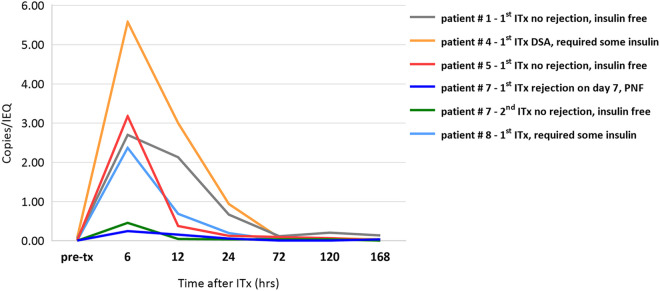
Of note, the patient who developed acute rejection on day 7 after their 1st ITx had miR-375 levels 10-fold lower in the first 7 days when compared to the remaining patients who did not develop complications. Interestingly, this patient’s AUC miR-375 release was in the same low range after uncomplicated 2nd ITx, which resulted in long-term insulin independence while miR-375 release was again 5 fold higher in remaining patients at the same time (Fig. 7).
Figure 7.
Comparison of miR-375/IEQ release profiles in one patient with antibody mediated rejection (AMR) and successful islet transplant with remaining patients after islet transplantation Patient # 7 (1st ITx) who developed AMR on day 7 with complete islet graft loss (primary non-function (PNF)) after his 1st ITx had miR-375/IEQ levels 10-fold lower in the first 7 days when compared to two other patients # 5 (1st ITx) and # 1 (1st ITx) who did not develop complications. Interestingly, AUC miR-375/IEQ levels in the same patient (# 7) were in the same range during his uncomplicated 2nd ITx, which resulted in long-term insulin independence (patient # 7 2nd ITx), and still were over 5 fold lower compared to other patients with insulin independence - # 5 (1st ITx) and # 1 (1st ITx). Two other patients - # 4 (1st ITx) and # 8 (1st ITx), who developed donor specific antibody (DSA) with some islet graft dysfunction and required continuous insulin support, also had similar miR-375/IEQ release profiles when compared to those with excellent islet graft function - # 5 (1st ITx) and # 1 (1st ITx). MiR-375/IEQ in first 7 days post-transplant did not correlate with islet graft function.
Discussion
Optimal islet engraftment is critical in order to achieve long-term insulin independence after islet transplantation12. Sufficient quantity and quality of islet mass must be transplanted in the setting of favorable local conditions at the engraftment site for optimal islet mass engraftment. Results from the Edmonton group indicate that transplantation of an islet mass over 5,000 IEQ/kg per transplant and a total cumulative islet mass of 10,000 IEQ/kg are essential for effective engraftment and subsequent insulin independence18. Despite positive preclinical and phase 1/2 study results, a recent multicenter trial did not demonstrate any benefit of Reparixin, a CXCL8 receptor inhibitor which modulates the inherently inflammatory intraportal environment, on engraftment. Islet engraftment and long-term insulin independence did not differ in the Reparixin and the placebo groups, however, the multi-center trial was limited by suboptimal study conditions including a lower- marginal islet mass transplanted (at least 3,000 IEQ/kg per transplant and a total over 6,000 IEQ/kg for two transplants) and the absence of a standardized immunosuppression regimen5.
Post-hoc analysis of our small cohort suggests that Reparixin may improve engraftment if optimized conditions are ensured including the use of a standard rather than marginal islet mass for transplant and the use of a potent anti-thymocyte globulin for induction of immunosuppression. Each of the patients in our cohort received over 5,000 IEQ/kg of islet mass during each transplant whereas 49% of the remaining transplants in the multicenter trial contained a lower islet mass5. A higher islet mass enhanced islet engraftment and graft function in our cohort12. Additionally, previous reports corroborate our observation that ATG in combination with anti-inflammatory (anti-TNF) therapy during initial ITx optimized conditions for islet engraftment and led to superior outcomes as compared to basiliximab19.
In terms of complications, our post-hoc analysis demonstrates a comparable rate of major and minor complications as experienced in other trials5,20,21. The overall rate of bleeding in our cohort was 20% (4/20 procedures) which was higher than the 9% reported in another multicenter study but lower than in other centers which decided to continue to perform islet transplantation via a mini-laparotomy20,22. The rate of hemorrhage in our series did not differ statistically between the two groups and the observed events are unlikely to be related to Reparixin. The most common source of hemorrhage was at the puncture site on the surface of the liver after withdrawal of the infusion catheter despite gelatin sponge embolization23. Patient heparinization, which was used to prevent portal vein thrombosis, also increased the risk of hemorrhage. Hemorrhagic complications such as subcapsular and intrahepatic hematomas manifest as pain in right upper quadrant of the abdomen, or in the case of intra-abdominal hemorrhage, as pain in the lower abdomen. In our cohort, we noted hemorrhage initially but this complication decreased as more experience helped us avoid puncture of the intrahepatic arterial branches when advancing the catheter through liver parenchyma en route to cannulating braches of the portal vein. In some 25%–33% of cases, the islet mass was derived from two donors for one infusion in order to reach the required islet mass for transplantation; however, no thromboembolic events were noted.
In regards to immunosuppression, we experienced a relatively high AMR and de novo DSA rate during the first transplant. However, the rate was the same in both groups. In our opinion, this complication was related to inadequate immunosuppression in the initial post-transplant period in our cohort. Furthermore, a complete lack of steroids, even for premedication prior to ATG infusion, led to frequent episodes of malaise, nausea, vomiting, and headaches. We later decreased the dose and rate of ATG infusion and also eliminateted delay in the introduction of the therapeutic doses of tacrolimus and mycophenolate. Although none of the patients enrolled in the study had any HLA antibodies detected (PRA was zero) prior to transplant, 3 of 4 patients who developed de novo DSA had a sensitization event prior to islet transplantation (i.e., miscarriage or blood transfusion), which may have additionally triggered DSA production.
All but one of our patients [11/12 (92%)], became insulin independent during the study period, in contrast to only 7/34 (20%) at the other trial centers suggesting that our center employed optimized conditions.5 Our protocol enabled a more controlled examination of Reparixin and enabled us to demonstrate that, contrary to the multicenter trial, it may improve islet engraftment and insulin independence. In fact, in our study the Reparixin group achieved 62.5% insulin independence at 1 year after a single islet infusion in contrast to none in the placebo group with a comparable islet mass infused. This outcome after a single islet infusion is superior to the majority of other reports and comparable with the three best series for islet allotransplantation24–27. Of note, 2/5 patients (40%) in our study had a body weight over 91 kg with a BMI of approximately 28 kg/m2 and received only a minimal total islet dose (below 5,700 IEQ/kg) and yet were still able to maintain insulin independence over 2 years after a single islet infusion. This is similar to the experience reported by Rickels MR et al.27. In contrast, all patients in the two other available reports, by Hering BJ et al. and Posselt AM et al., weighed less than 67 kg with a BMI of 25 kg/m2 or less, and required roughly 6,000 IEQ/kg or more to obtain comparable rates of insulin independence at 1-year25,26. Thus, it is possible that the multicenter trial did not demonstrate improved outcomes with Reparixin because too few islets were transplanted.
The combination of tacrolimus and mycophenolate was as effective as rapamycin-based regimens in our cohort with 50% and higher rates of insulin independence27,28. The remaining 37.5% of patients in the Reparixin group had severe adverse events including rejection or severe cytokine release syndrome. While these rates are comparable to the placebo group, they nevertheless compromise islet engraftment, and thus preclude accurate assessment of the effect of Reparixin on engraftment. Cytokine release syndrome after ATG infusion, as observed in our very first patient, has been well described in almost all patients participating in trials testing ATG in new onset T1DM even with low doses of steroids19. We addressed this problem in subsequent patients by reducing the infusion rate of ATG, dividing the same total dose into lower individual doses, and spreading the dosing over a longer period of time. Steroid avoidance, slow introduction of ATG, as well as maintenance immunosuppression led to a higher than expected acute AMR and de novo DSA rate. This complication could be possibly prevented in future studies by using methylprednisolone during the first few days after transplanting and with earlier introduction of therapeutic immunosuppression.
Although we did not find a statistically significant difference in the AUC for c-peptide/IEQ/kg, which was used to assess islet engraftment in the trial, our post-hoc analysis of AUC c-peptide/AUC glucose standardized by transplanted islet mass expressed in IEQ was statistically significant. The ratio of serum c-peptide and glucose concentration directly measures islet function and normalizes it better than body weight by accounting for differences in insulin resistance between individual. Additionally, we determined a beneficial effect of Reparixin compared to placebo on islet engraftment by day 75 based on BETA-2 score normalized by islet mass of the islet graft. We have previously validated the utility of the BETA-2 score in assessing islet graft function9–12. A detectable difference in the serum cytokine profile (including ligand CXCL8) was not expected, as Reparixin is an allosteric receptor inhibitor that selectively blocks CXCR1 and CXCR2 without hindering ligand internalization or measurably impacting serum proinflammatory cytokines levels29. Accordingly, we did not observe any difference in the serum CLCX8 profile in Reparixin vs placebo group in our cohort (Fig. 6). We hypothesized that ATG improves conditions for islet engraftment over basiliximab based on previous studies showing improved long-term insulin independence with ATG utilized along with TNF inhibitor etanercept/infliximab30. A lower rate and daily dose of ATG which we applied, led to no observed difference in the release of most cytokines in the placebo group after the 1st ITx with ATG compared to the 2nd ITx with Basiliximab. As in previous reports pertaining to islet transplantation, we found the same profile of miR-375 release from islets after transplantation with a peak around 6–12 hours after ITx. Although rising levels of miR-375 has been correlated with islet destruction and worse clinical outcomes we did not note this correlation31,32.
Based on the multicenter trial results, Reparixin appears to have no advantage. However, despite the advantages of a multicenter trial design, the heterogeneity of the study may have led to an inability to appreciate an effect (i.e., a type II error). In the trial, different transplant centers employed different strategies for donor selection, islet isolation, immunosuppression, and a variable transplanted islet mass. Herein, we suggest that a separate analysis of a cohort of patients who received Reparixin under more standardized settings may help elucidate the effect of Reparixin and suggest criteria for further evaluation. Selective analysis based on our cohort potentially uncovered confounding factors which could have affected the clinical outcome, and identified specific conditions in which Reparixin might be beneficial for islet engraftment. We did not find a significantly higher overall cytokine /chemokine release in the placebo group during the first ITx when ATG was used compared to the second ITx when Basiliximab was utilized. However, Reparixin significantly increased the release of CCL4 and CCL3 when compared to placebo during the 1st ITx, while no difference was observed during the 2nd ITx. Taken together, in the setting of the more favorable islet engraftment and clinical outcomes in our cohort, this pattern may represent a signature cytokine release profile related to the beneficial synergistic effect of Reparixin and ATG on islet engraftment. The observation of a significantly different release profile of several cytokines, chemokines, and miR-375 in the Reparixin group compared to placebo supports this hypothesis.
We suggest that Reparixin could be further evaluated under the more optimized and standardized conditions described herein (i.e., using ATG for induction and utilizing islet mass of at least 5,000 IEQ/kg per transplant). A direct comparison of Reparixin with etanercept, the most commonly used anti-inflammatory agent, could be be considered21,25,28,30. Our analysis confirmed that islet allotransplantation is a safe and effective therapy for patients with T1DM and life-threatening hypoglycemia. All patients had positive outcomes based on Igls classification criteria including substantially improved HbA1c, and more importantly, no severe hypoglycemic events. None of the patients experienced SHE, which in combination with no unexpected and mostly mild expected adverse events, likely led to a substantially improved quality of life. Insulin independent patients maintained long-term stable islet graft function.
There are a number of limitations to our study. Due to the low number of patients in our post-hoc analysis and the exclusion of cases where engraftment was affected by other factors unrelated to the new drug/placebo, we cannot draw valid statistical conclusions regarding the benefit of the new agent. However, this analysis suggests that the lack of standardization of the critical conditions for optimal islet engraftment may have led to an inability of the trial to detect the effect of Reparixin. This should be taken into consideration in the design of future trials involving islet transplantation.
Furthermore, our report is limited by the small number of patients, the presence of complications, and the use of post-hoc analysis. Nonetheless, the results are encouraging and suggest the need for further investigations. Our analysis highlights the need for continuous optimization of the procedure, defining critical modifiable factors for success, and additional standardizations of technical and clinical protocols. High variability in islet processing protocols between European centers was highlighted in a recent report33. Introduction of islet transplantation as a standard-of-care procedure in the USA, as it currently is practiced in Canada, Australia, and many European countries would help increase the number of patients who could benefit from islet transplantation and would also help stimulate research and funding34–36.
In summary, despite the negative outcomes of a multicenter trial, our post-hoc analysis of a subgroup of patients suggests that Reparixin may positively affect islet engraftment. Our analysis highlights the importance of standardizing clinical protocols and ensuring optimized islet engraftment. Investigation of Reparixin and future novel agents requires standardized and optimized conditions in order to exclude confounding factors and to facilitate a more definitive examination of their role in islet transplantation.
Supplemental Material
Supplemental Material, sj-doc-1-cll-10.1177_09636897211001774 for Post-Hoc Analysis of a Randomized, Double Blind, Prospective Study at the University of Chicago: Additional Standardizations of Trial Protocol are Needed to Evaluate the Effect of a CXCR1/2 Inhibitor in Islet Allotransplantation by Piotr J. Bachul, Karolina Golab, Lindsay Basto, Steven Zangan, Jordan S. Pyda, Angelica Perez-Gutierrez, Peter Borek, Ling-Jia Wang, Martin Tibudan, Dong-Kha Tran, Roi Anteby, Gabriela S. Generette, Jędrzej Chrzanowski, Wojciech Fendler, Laurencia Perea, Kumar Jayant, Aaron Lucander, Celeste Thomas, Louis Philipson, J. Michael Millis, John Fung and Piotr Witkowski in Cell Transplantation
Supplemental Material, sj-docx-1-cll-10.1177_09636897211001774 for Post-Hoc Analysis of a Randomized, Double Blind, Prospective Study at the University of Chicago: Additional Standardizations of Trial Protocol are Needed to Evaluate the Effect of a CXCR1/2 Inhibitor in Islet Allotransplantation by Piotr J. Bachul, Karolina Golab, Lindsay Basto, Steven Zangan, Jordan S. Pyda, Angelica Perez-Gutierrez, Peter Borek, Ling-Jia Wang, Martin Tibudan, Dong-Kha Tran, Roi Anteby, Gabriela S. Generette, Jędrzej Chrzanowski, Wojciech Fendler, Laurencia Perea, Kumar Jayant, Aaron Lucander, Celeste Thomas, Louis Philipson, J. Michael Millis, John Fung and Piotr Witkowski in Cell Transplantation
Acknowledgements
This study was funded by Dompé Farmaceutici S.p.A. as a part of multi-center trial. We would like to thank Dr. Lorenzo Piemonti from University of Milan, and Dr. Luisa Daffonchio, Dr. Pier Adelchi Ruffini, and Marcello Allegretti from Dompé Farmaceutici S.p.A for including our center in the trial and for supporting it in its execution. We would like to thank Dr. Amittha Wickrema and Diane Ostrega, directors of cGMP facility at University of Chicago, and their staff for providing us continuous access to the islet isolation laboratory as well as for quality control oversight of islet production and processing. Special thanks to Dr. Martin Jendrisak and the Gift of Hope team in Chicago for their help and support in providing cadaveric pancreata for the purpose of the study. We acknowledge support from US Public Health Service Grant P30DK020595 and the Kovler Family Fund.
Abbreviations
- AMR
antibody mediated rejection
- ATG
anti-thymocyte globulin
- AUC
area under the curve
- BMI
Body Mass Index
- CITC
Collaborative Islet Transplantation Consortium
- CMV
cytomegalovirus
- CRS
cytokine release syndrome
- CTCAE
Common Terminology Criteria for Adverse Events
- CXCL
CXC Chemokine Ligand
- DSA
donor specific antibody
- FDA
Food and Drug Administration
- GSIR
glucose stimulated insulin release
- HLA
human leukocyte antigen
- IBMIR
instant blood-mediated inflammatory reaction
- IEQ
Islet equivalent dose
- IL
interleukin
- Itx
islet transplantation
- MCP-1
Monocyte Chemoattractant Protein-1
- miR-375
microRNA-375
- MMTT
mixed meal tolerance test
- NAIDS
North American Islet Donor Score
- NS
not significant
- PP
portal pressure
- PTT
partial thromboplastin time
- PRA
panel-reactive antibody
- SHE
severe hypoglycemia event
- T1DM
type 1 diabetes mellitus
- TEF
transplant estimated function
- TNF
tumor necrosis factor
Footnotes
Ethical Approval: Ethical approval for this study was obtained from Institution Review Board (IRB # 12-2046) of University of Chicago.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institution Review Board of University of Chicago approved protocol (IRB # 12-2046).
Statement of Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: P.W. served as a co-PI in another multi-center trial in islet auto-transplantation and as a consultant for Dompé farmaceutici S.p.A. in another study involving liver transplantation. The author(s) declared no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Definitions and Abbreviations: Insulin independence – no exogenous insulin treatment for ≥ 14 consecutive days, with adequate glycemic control defined as fasting glucose < 140 mg/dL more than 3 times per week, HbA1c < 7% (53 mmol/mol), and 2-h postprandial glucose not exceeding 180 mg/dL more than 4 times per week or during a mixed meal tolerance test.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Dompé farmaceutici S.p.A. and University of Chicago Diabetes Research and Training Center US Public Health Service Grant P30DK020595.
ORCID iD: Piotr J. Bachul  https://orcid.org/0000-0002-7694-1793
https://orcid.org/0000-0002-7694-1793
Jordan S. Pyda  https://orcid.org/0000-0002-2833-3953
https://orcid.org/0000-0002-2833-3953
Piotr Witkowski  https://orcid.org/0000-0002-4459-6673
https://orcid.org/0000-0002-4459-6673
Prior Presentation: Parts of this study were presented in abstract form at the American Diabetes Association 79th Scientific Sessions, San Francisco, CA, June 7–11, 2019 and at the 17th World Congress of the International Pancreas & Islet Transplant Association, Lyon, France, 2 - 5 July 2019.
Supporting Information: Additional Supporting Information may be found in the online version of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory response in islet transplantation. Int J Endocrinol. 2014;2014:451035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennet W, Groth C-G, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–133. [DOI] [PubMed] [Google Scholar]
- 3. Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, Dugnani E, Sordi V, Magistretti P, Daffonchio L, Ruffini PA. et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012;122(10):3647–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pawlick RL, Wink J, Pepper AR, Bruni A, Abualhassen N, Rafiei Y, Gala-Lopez B, Bral M, Shapiro AMJ. Reparixin, a CXCR1/2 inhibitor in islet allotransplantation. Islets. 2016;8(5):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maffi P, Lundgren T, Tufveson G, Rafael E, Shaw JAM, Liew A, Saudek F, Witkowski P, Golab K, Bertuzzi F, Gustafsson B. et al. Targeting CXCR1/2 does not improve insulin secretion after pancreatic islet transplantation: a phase 3, double-blind, randomized, placebo-controlled trial in type 1 diabetes. Diabetes Care. 2020;43(4):710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gołębiewska JE, Bachul PJ, Wang L, Matosz S, Basto L, Kijek MR, Fillman N, Gołąb K, Tibudan M, Dębska-Ślizień A, Millis JM. et al. Validation of a new north American islet donor score for donor pancreas selection and successful islet isolation in a medium-volume islet transplant center. Cell Transplant. 2019;28(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, Clarke WR. et al. National institutes of health–sponsored clinical islet transplantation consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes. 2016;65(11):3418–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shankar SS, Vella A, Raymond RH, Staten MA, Calle RA, Bergman RN, Cao C, Chen D, Cobelli C, Dalla Man C, Deeg M. et al. Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of β-cell function: results from the foundation for the national institutes of health biomarkers consortium investigative series. Diabetes Care. 2016;39(9):1602–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forbes S, Oram RA, Smith A, Lam A, Olateju T, Imes S, Malcolm AJ, Shapiro AMJ, Senior PA. Validation of the BETA-2 score: an improved tool to estimate beta cell function after clinical islet transplantation using a single fasting blood sample. Am J Transplant. 2016;16(9):2704–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gołębiewska J, Solomina J, Kijek MR, Kotukhov A, Basto L, Gołąb K, Bachul PJ, Konsur E, Ciepły K, Fillman N, Wang LJ. et al. External validation of the newly developed beta-2 scoring system for pancreatic islet graft function assessment. Transplant Proc. 2017;49(10):2340–2346. [DOI] [PubMed] [Google Scholar]
- 11. Gołębiewska JE, Solomina J, Thomas C, Kijek MR, Bachul PJ, Basto L, Gołąb K, Wang L, Fillman N, Tibudan M, Ciepły K. et al. Comparative evaluation of simple indices using a single fasting blood sample to estimate beta cell function after islet transplantation. Am J Transplant. 2018;18(4):990–997. [DOI] [PubMed] [Google Scholar]
- 12. Bachul PJ, Gołębiewska JE, Basto L, Gołąb K, Anteby R, Wang L, Tibudan M, Thomas C, Fendler W, Lucander A, Grybowski DJ. et al. BETA-2 score is an early predictor of graft decline and loss of insulin independence after pancreatic islet allotransplantation. Am J Transplant. 2020;20(3):844–851. [DOI] [PubMed] [Google Scholar]
- 13. Rickels MR, Stock PG, de Koning EJP, Piemonti L, Pratschke J, Alejandro R, Bellin MD, Berney T, Choudhary P, Johnson PR, Kandaswamy R. et al. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl Int. 2018;31(4):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Institute of Cancer. Common terminology criteria for adverse events (CTCAE). 2010, version 4.0.
- 15. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryan EA, Paty BW, Senior PA, Lakey JRT, Bigam D, Shapiro AMJ. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care. 2005;28(2):343–347. [DOI] [PubMed] [Google Scholar]
- 17. Caumo A, Maffi P, Nano R, Bertuzzi F, Luzi L, Secchi A, Bonifacio E, Piemonti L. Transplant Estimated Function: a simple index to evaluate -cell secretion after islet transplantation. Diabetes Care. 2008;31(2):301–305. [DOI] [PubMed] [Google Scholar]
- 18. Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 19. Gitelman SE, Gottlieb PA, Rigby MR, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L. et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB. et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shapiro AMJ. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012;9(4):385–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caiazzo R, Vantyghem M-C, Raverdi V, Bonner C, Gmyr V, Defrance F, Leroy C, Sergent G, Hubert T, Ernst O, Noel C. et al. Impact of procedure-related complications on long-term islet transplantation outcome. Transplantation. 2015;99(5):979–984. [DOI] [PubMed] [Google Scholar]
- 23. Low G, Hussein N, Owen RJT, Toso C, Patel VH, Bhargava R, Shapiro AMJ. Role of imaging in clinical islet transplantation. RadioGraphics. 2010;30(2):353–366. [DOI] [PubMed] [Google Scholar]
- 24. Al-Adra DP, Gill RS, Imes S, O’Gorman D, Kin T, Axford SJ, Shi X, Senior PA, Shapiro AMJ. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9):1007–1012. [DOI] [PubMed] [Google Scholar]
- 25. Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm S-H, Zhang H-J, Parkey J, Hunter DW. et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830. [DOI] [PubMed] [Google Scholar]
- 26. Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, Kerlan RK, Fong L, Vincenti FG, Hering BJ, Bluestone JA. et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-lfa-1 antibody efalizumab. Am J Transplant. 2010;10(8):1870–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, Fuller C. et al. Improvement in β-Cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes. 2013;62(8):2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shapiro AMJ. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011;11(5):345–354. [DOI] [PubMed] [Google Scholar]
- 29. Goldstein LJ, Perez RP, Yardley D, Han LK, Reuben JM, Gao H, McCanna S, Butler B, Ruffini PA, Liu Y, Rosato RR. et al. A window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res. 2020;22(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DER, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am. J. Transplant. 2012;12(6):1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanak MA, Takita M, Shahbazov R, Lawrence MC, Chung WY, Dennison AR, Levy MF, Naziruddin B. Evaluation of MicroRNA375 as a novel biomarker for graft damage in clinical islet transplantation. Transplantation. 2015;99(8):1568–1573. [DOI] [PubMed] [Google Scholar]
- 32. Roels S, Costa OR, Tersey SA, Stangé G, De Smet D, Balti EV, Gillard P, Keymeulen B, Ling Z, Pipeleers DG, Gorus FK. et al. Combined analysis of GAD65, miR-375, and unmethylated insulin DNA following islet transplantation in patients with T1D. J Clin Endocrinol Metab. 2019;104(2):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nano R, Kerr-Conte JA, Scholz H, Engelse M, Karlsson M, Saudek F, Bosco D, Antonioli B, Bertuzzi F, Johnson PRV, Ludwing B. et al. Heterogeneity of human pancreatic islet isolation around Europe. Transplantation. 2020;104(1):190–196. [DOI] [PubMed] [Google Scholar]
- 34. Ricordi C, Japour A. Transplanting islet cells can fix brittle diabetes. Why isn’t it available in the U.S.? CellR4. 2019;7:e2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dębska-Ślizień A, Wszoła M, Bachul P, Gulczyński J, Żygowska I, Berman A, Gołębiewska J, Komorniczak M, Witkowski P. Islet transplantation – perspective from poland. CellR4. 2019;7:e2786. [PMC free article] [PubMed] [Google Scholar]
- 36. Witkowski P, Philipson L, Kaufman DB, Ratner L, Abouljoud MS, Bellin M, Buse J, Kandeel F, Stock P, Mulligan D, Markmann JF. et al. The demise of islet allotransplantation in the us: a call for an urgent regulatory update the “islets for us” collaborative. Am J Transplant. 2021. 21(4):1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-doc-1-cll-10.1177_09636897211001774 for Post-Hoc Analysis of a Randomized, Double Blind, Prospective Study at the University of Chicago: Additional Standardizations of Trial Protocol are Needed to Evaluate the Effect of a CXCR1/2 Inhibitor in Islet Allotransplantation by Piotr J. Bachul, Karolina Golab, Lindsay Basto, Steven Zangan, Jordan S. Pyda, Angelica Perez-Gutierrez, Peter Borek, Ling-Jia Wang, Martin Tibudan, Dong-Kha Tran, Roi Anteby, Gabriela S. Generette, Jędrzej Chrzanowski, Wojciech Fendler, Laurencia Perea, Kumar Jayant, Aaron Lucander, Celeste Thomas, Louis Philipson, J. Michael Millis, John Fung and Piotr Witkowski in Cell Transplantation
Supplemental Material, sj-docx-1-cll-10.1177_09636897211001774 for Post-Hoc Analysis of a Randomized, Double Blind, Prospective Study at the University of Chicago: Additional Standardizations of Trial Protocol are Needed to Evaluate the Effect of a CXCR1/2 Inhibitor in Islet Allotransplantation by Piotr J. Bachul, Karolina Golab, Lindsay Basto, Steven Zangan, Jordan S. Pyda, Angelica Perez-Gutierrez, Peter Borek, Ling-Jia Wang, Martin Tibudan, Dong-Kha Tran, Roi Anteby, Gabriela S. Generette, Jędrzej Chrzanowski, Wojciech Fendler, Laurencia Perea, Kumar Jayant, Aaron Lucander, Celeste Thomas, Louis Philipson, J. Michael Millis, John Fung and Piotr Witkowski in Cell Transplantation