Abstract
Objectives
More and more encouraging evidence revealed that immunotherapy could improve clinical outcomes in patients with previously treated non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) variations. However, immunotherapy is still a controversy for NSCLC patients with EGFR mutation.
Method
In this retrospective analysis, we compared the clinical efficacy of pembrolizumab monotherapy (PM), pembrolizumab combined with chemotherapy (P+C) and pembrolizumab combined with anlotinib (P+A) in NSCLC patients with EGFR mutation who had failed on EGFR-TKI and platinum-based chemotherapy.
Result
Eighty-six patients were included in this study. The overall median progression free survival (PFS) was 3.24 months. Multivariate analysis suggested that EGFRL858R and combined therapy were positive prognostic factors of PFS. The overall median OS was 12.28 months. Multivariate analysis found that high PD-L1 expression (≥50%) and combined therapy seemed to be positive prognostic factors of OS. Among the population, 32 patients received PM, 26 patients received P+C and 28 patients received P+A. Up to Jan 30, 2021, the median progression-free survival was 1.5 months in the PM group, 4.30 months in the P+C group and 3.24 months in the P+A group. The median OS were 7.41, 14.92 and 15.97 months, respectively. The ORR were 3.1%, 23.1% and 21.4%.
Conclusion
The addition of chemotherapy or antiangiogenic therapy to pembrolizumab resulted in significantly longer PFS, OS and ORR than pembrolizumab alone in our study. EGFRL858R might be a positive prognostic factor of PFS and high PD-L1 expression might be a positive prognostic factor of OS.
Keywords: non-small cell lung cancer, pembrolizumab, epidermal growth factor receptor, antiangiogenic agent, chemotherapy
Introduction
Targeted therapy has revolutionized the treatment landscape for patients with non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations. Treatment with EGFR-TKIs, which have been developed to the third generation, provides better disease control and longer survival for patients with EGFR mutations (1). At the same time, screening for PD-L1 expression has become standard practice with the rise of immunotherapy. Of interest, the presence of EGFR mutations has been reported to upregulate the expression of PD-L1 (2–7). However, several studies have revealed that high PD-L1 expression predicted poor response to EGFR-TKIs in patients with EGFR-mutant NSCLC and correlated with primary resistance to EGFR-TKIs (8–10). Nevertheless, EGFR-TKIs have shown overwhelming advantages over standard chemotherapy in patients with EGFR-mutant NSCLC. EGFR-TKIs are recommended as the first-line treatment in this population according to the NCCN guidelines. However, treatment options after the development of TKI resistance need to be further explored. Given the growing emphasis on molecular profiling and detection of PD-L1, more detailed treatment guidance is needed for the critical population of patients with advanced NSCLC with both high PD-L1 and EGFR mutations.
Recently, the use of immune checkpoint inhibitors (ICIs) has greatly altered the standard of care for patients with advanced NSCLC without targetable EGFR or ALK genetic aberrations depending on the patient’s PD-L1 expression level. However, immunotherapy is still a controversial for patients with EGFR mutations because several clinical studies, including Checkmate057, Keynote010, POPLAR and OAK, have revealed that immunotherapy failed to improve clinical outcomes in patients with advanced NSCLC with EGFR mutations (11–15). However, the final OS data of the ATLANTIC trial showed that durvalumab improved clinical activity across all cohorts in patients with previously treated advanced NSCLC, including those with EGFR mutations (16). In addition, JAMA oncol reported that the combination of pembrolizumab plus docetaxel improved clinical outcomes in patients with previously treated NSCLC with EGFR variations (17). Furthermore, the ABCP group in the IMPOWER150 trial also prolonged the OS of patients with sensitive EGFR mutations (18). These encouraging results underline the necessity for further investigation into immunotherapy in patients with NSCLC with EGFR mutations. In this study, we collected the clinical records of patients with NSCLC with EGFR mutations who received pembrolizumab at our institution, including pembrolizumab monotherapy (PM), pembrolizumab combined with chemotherapy (P+C) and pembrolizumab combined with anlotinib (P+A). Anlotinib is an antiangiogenic agent that inhibiting VEGFR, FGFR, and PDGFR and has been approved by China National Medical Products Administration (NMPA) (19). In addition, it has been proved to be effective in NSCLC patients with EGFR mutations (20). In this study, we explored the efficacy of PD-1 in previously treated NSCLC patients with EGFR mutation.
Material and Methods
Patients
The medical records of patients with advanced NSCLC with EGFR mutations received pembrolizumab treatment at the Shanghai Chest Hospital between Dec 1, 2017 and Oct 30, 2020 were screened. Eighty-six patients met the following inclusion criteria: (1) stage IV NSCLC (2) positive EGFR mutation [exon 19 deletion mutation (EGFRD19), exon 21 L858R mutation (EGFRL858R), secondary exon 20 T790M mutation and other uncommon sensitive mutation such as G719X, L861R] (3) patients had disease progressed with at least 1 approved EGFR-TKI (patients with 20T790M mutation must had failed on osimertinib) and platinum-based chemotherapy following standard treatment guideline; (4) patients received PM, P+C or P+A (5) Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1. Therapeutic schedule was decided by physician under the principle that patients at high risk of bleeding should not be treated with P+A, patients with severe adverse effects to previous chemotherapy should not chose P+C as priority. This study was approved by the Institutional Review Board of Shanghai Chest Hospital and performed following the declaration of Helsinki.
Treatment and Clinical Response Evaluation
Among the three groups (PM, P+C and P+A), pembrolizumab was administered 200mg intravenously every 3 weeks. Chemotherapy was administrated following the standard NCCN guidelines. Chemotherapy regimens included docetaxel combined with carboplatin (DC) and nab-paclitaxel combined with carboplatin (TC). Antiangiogenic agent was anlotinib (given orally, 8mg once daily on days 1–14 of a 21-day cycle). Disease stage was decided on the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification. Enhanced chest computed tomography (CT) scan and abdominal ultrasound scan were examined every 4 weeks for therapeutic response evaluation. Enhanced brain magnetic resonance imaging (MRI) was examined every 4-6 months if no lesion at baseline and no symptoms thereafter. The response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Detection of Gene and Programmed Death Ligand 1 (PD-L1) Tumor Proportion Score (TPS)
The tissue sample was biopsied at the time of disease diagnosis and disease progression. EGFR detection was performed by the amplification refractory mutation system (ARMS) or by next generation sequencing (NGS). PD-L1 expression was assessed at the time of disease progression, right before the initiation of immunotherapy. TPS was detected by the PD-L1 IHC 22C3 pharmDx assay and was classified into TPS<0, 1-49% and ≥50%.
Statistical Analysis
The χ2 test was used for comparison of categorical variables. The primary endpoints were PFS (from immunotherapy initiation to disease progression or the last follow-up); OS (from immunotherapy initiation to death or the last follow-up) and ORR (the ratio of complete and partial response). The median PFS and OS was estimated using the Kaplan-Meier method and compared by the log-rank test. Hazard ratios (HR) and 95% confidence intervals were estimated by a stratified Cox proportional-hazards model. To avoid the influence of confounding factors, factors with p values less than 0.1 in univariate analysis were included in multivariate analysis. All statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Clinical Features
Eighty-six patients who met the eligibility criteria were included in this study. Of these, most patients were male (55.8%) and non-smoker (52.3%) and had received third or more lines of therapy ( Table 1 ). 17 patients (19.8%) had brain metastasis. The most common EGFR mutation type was EGFRL858R (54.6%), followed by EGFRD19 (25.6%), uncommon sensitive mutation (10.5%) and T790M (9.3%). 75 patients were screened for PD-L1 expression levels immediately before immunotherapy, 17 (19.8%) of whom had a TPS of 0%, 32 (37.2%) of whom had a TPS of 1-49% and 26 (30.2%) of whom had a TPS of 50% or greater.
Table 1.
Clinicopathological characteristics of 86 EGFR-mutated patients treated with pembrolizumab.
| Characteristics | Number | Percent (%) |
|---|---|---|
| Age(median age, Range) | 62(39-80) | – |
| Sex | ||
| Male | 48 | 55.8% |
| Female | 38 | 44.2% |
| Smoking history | ||
| Yes | 41 | 47.7% |
| No | 45 | 52.3% |
| Recurrence after surgery | ||
| Yes | 39 | 45.3% |
| No | 47 | 54.7% |
| Treatment line of PD-1 Inhibitors | ||
| Second line | 4 | 4.7% |
| Third or after line | 82 | 95.3% |
| Brain metastasis | ||
| Yes | 17 | 19.8% |
| No | 69 | 80.2% |
| PD-L1 TPS | ||
| <1% | 17 | 19.8% |
| 1~49% | 32 | 37.2% |
| ≥50% | 26 | 30.2% |
| Unknown | 11 | 12.8% |
| EGFR mutation subtype | ||
| 19del | 22 | 25.6% |
| 21L858R | 47 | 54.6% |
| T790M | 8 | 9.3% |
| Other | 9 | 10.5% |
| Treatment | ||
| PM | 32 | 37.2% |
| P+C | 26 | 30.2% |
| P+A | 28 | 32.6% |
Progression−Free Survival
PD occurred in 64 (74.4%) patients in the overall population, including 26 (81.3%) patients in PM group, 12 (46.2%) patients in P+C group and 26 (92.9%) patients in P+A group. The overall median PFS was 3.24 months (95% CI: 2.46–4.02) ( Figure 1A ). Univariate analysis found that brain metastasis (p = 0.024), PD-L1 expression [p (1-49% vs 0) = 0.027, p (≥50% vs 0) =0.004)] and therapy [p (P+C vs PM) <0.001, p (P+A vs PM) = 0.002)] were associated with PFS ( Table 2 ). Multivariate analysis found that patients with EGFRL858R had longer PFS than those with EGFRD19 (p=0.024), patients in P+C group (p<0.001) and P+A group (p<0.001) had longer PFS than those in PM group ( Table 2 ). These results suggested that EGFR mutation type and treatments were independent prognostic factors of PFS.
Figure 1.
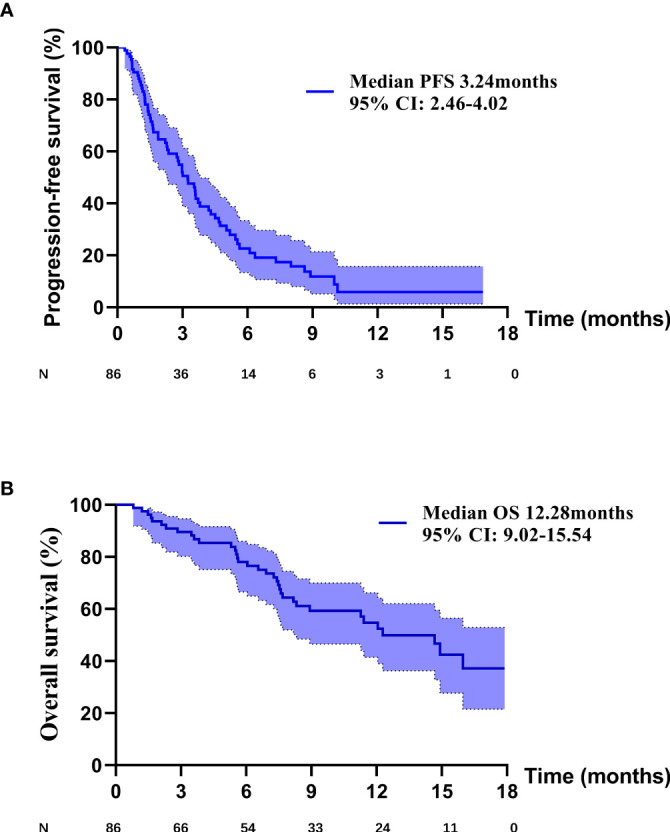
(A) The PFS curve of overall patients. (B) The OS curve of overall patients.
Table 2.
Univariate and multivariable analyses for covariables associated with progression free survival.
| Characteristics | Category | Univariate analysis HR (95 %CI) | p | Multivariate analysis HR (95 %CI) | p |
|---|---|---|---|---|---|
| Age | ≤65 vs >65 years | 0.71 (0.43-1.19) | 0.194 | ||
| Sex | Male vs female | 0.88 (0.53-1.46) | 0.630 | ||
| Smoking history | Yes vs no | 1.43 (0.88-2.35) | 0.153 | ||
| Treatment line | Second line vs posterior line | 1.15 (0.36-3.68) | 0.814 | ||
| Brain | Yes vs No | 1.40 (1.05-1.87) | 0.024 | 0.74 (0.39-1.41) | 0.361 |
| PD-L1 expression | 1-49% vs 0 | 0.46 (0.23-0.92) | 0.027 | 0.47 (0.22-1.03) | 0.058 |
| ≥50% vs 0 | 0.33 (0.16-0.70) | 0.004 | 0.46 (0.21-1.02) | 0.057 | |
| Unknown vs 0 | 0.71 (0.32-1.61) | 0.414 | 0.60 (0.25-1.42) | 0.246 | |
| EGFR mutation | 21L858R vs 19del | 0.60 (0.33-1.09) | 0.092 | 0.41 (0.19-0.90) | 0.024 |
| T790M vs 19del | 0.83 (0.33-2.11) | 0.691 | 0.47 (0.17-1.26) | 0.133 | |
| Others vs 19del | 0.47 (0.19-1.14) | 0.095 | 0.38 (0.14-1.04) | 0.059 | |
| Therapy | I+C vs IM | 0.22 (0.11-0.45) | <0.001 | 0.16 (0.07-0.37) | <0.001 |
| I+A vs IM | 0.41 (0.23-0.73) | 0.002 | 0.31 (0.16-0.57) | <0.001 | |
| I+C vs I+A | 0.53 (0.27-1.06) | 0.071 | 0.55 (0.25-1.19) | 0.126 |
Overall Survival
Death occurred in 35 (40.7%) patients in the overall population, including 18 (56.3%) patients in PM group, 6 (23.1%) patients in P+C group and 11 (39.3%) patients in P+A group. The overall median OS was 12.28 months (95% CI: 9.02–15.54) ( Figure 1B ). Univariate analysis found that PD-L1 expression [p (≥50% vs 0) =0.007)] and therapy [p (P+C vs PM) =0.021, p (P+A vs PM) =0.020)] were associated with OS ( Table 3 ). Multivariate analysis, including brain metastasis, PD-L1 expression and therapy, found that patients with high PD-L1 expression (≥50%) had longer OS than those with negative expression (p=0.039), patients in P+C group (p=0.035) and P+A group (p=0.019) had longer OS than those in PM group ( Table 3 ). Hence, high PD-L1 expression and combined therapy seemed to be positive prognostic factors of OS.
Table 3.
Univariate and multivariable analyses for covariables associated with overall survival.
| Characteristics | Category | Univariate analysis HR (95 %CI) | p | Multivariate analysis HR (95 %CI) | p |
|---|---|---|---|---|---|
| Age | ≤65 vs >65 years | 0.65 (0.32-1.22) | 0.168 | ||
| Sex | Male vs female | 0.67 (0.33-1.35) | 0.264 | ||
| Smoking history | Yes vs No | 1.50 (0.76-2.96) | 0.242 | ||
| Treatment line | Second line vs posterior line | 0.47 (0.14-1.54) | 0.211 | ||
| Brain | No vs Yes | 0.51 (0.24-1.09) | 0.082 | 1.31 (0.88-1.93) | 0.180 |
| PD-L1 expression | 1-49% vs 0 | 0.64 (0.30-1.59) | 0.339 | 0.94 (0.35-2.48) | 0.895 |
| ≥50% vs 0 | 0.22 (0.07-0.67) | 0.007 | 0.30 (0.10-0.94) | 0.039 | |
| Unknown vs 0 | 1.74 (0.67-4.55) | 0.257 | 2.27 (0.84-6.15) | 0.107 | |
| EGFR mutation | 21L858R vs 19del | 0.79 (0.36-1.72) | 0.557 | ||
| T790M vs 19del | 0.83 (0.32-4.32) | 0.802 | |||
| Others vs 19del | 0.58 (0.18-1.86) | 0.361 | |||
| Therapy | I+C vs IM | 0.34 (0.13-0.85) | 0.021 | 0.35 (0.13-0.93) | 0.035 |
| I+A vs IM | 0.41 (0.19-0.87) | 0.020 | 0.40 (0.19-0.86) | 0.019 | |
| I+C vs I+A | 0.82 (0.30-2.23) | 0.700 | 0.88 (0.31-2.46) | 0.807 |
Survival Analysis of Patients in Different Therapy Group
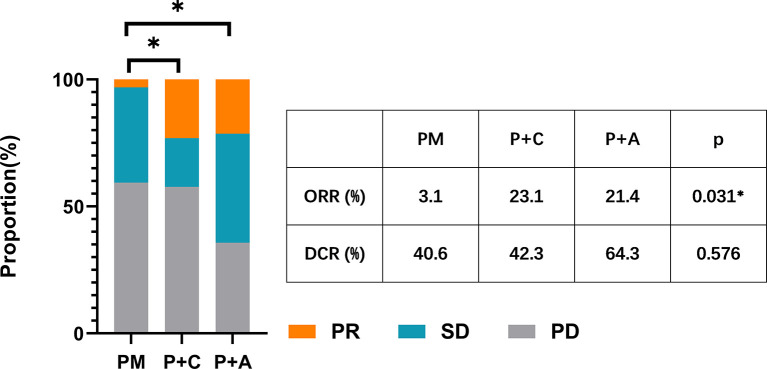
We divided the patients into three groups according to the therapy they received (32 patients in PM group, 26 patients in P+C group and 28 patients in P+A group). The baseline characteristics among the three groups were shown in Table 4 . There were no statistically significant differences in characteristics among the three groups, indicating that no large selection bias existed. The median PFS was 1.5 months (95% CI: 1.19-1.81) in the PM group, 4.30 months (95% CI: 3.21-5.39) in the P+C group and 3.24 (95% CI: 0.96-5.52) months in the P+A group ( Figure 2A ). The median OS of PM, P+A and P+C were 7.41 (95% CI: 4.30-10.52), 14.92 (95% CI: 9.75-20.09) and 15.97 (9.57-22.37) months, respectively ( Figure 2B ). P+C group showed a significant PFS and OS benefit over PM group (p<0.001 and p=0.021). P+A group also revealed a significant PFS and OS benefit over PM group (p=0.002 and p=0.020). The ORR of PM, P+C and P+A group were 3.1%, 23.1% and 21.4% ( Figure 3 ). The difference of objective tumor response showed us the superiority of combined therapy over monotherapy [P+C vs PM (p=0.038), P+A vs PM (p=0.041)]. The DCR were 40.6%, 42.3% and 64.3%.
Table 4.
Clinicopathological characteristics of all patients with different treatments.
| Characteristics | Monotherapy (N=17) (%) | With chemotherapy (N=15) (%) | With anlotinib (N=14) (%) | p value |
|---|---|---|---|---|
| Age | ||||
| Median, range | 61(39-80) | 66(54-78) | 59 (41-78) | 0.081 |
| Sex | 0.763 | |||
| Male | 19 (59.4) | 13 (50.0) | 16 (57.1) | |
| Female | 13 (40.6) | 13 (50.0) | 12 (42.9) | |
| Smoking history | 0.471 | |||
| Yes | 18 (56.2) | 11 (42.3) | 12 (42.9) | |
| No | 14 (43.8) | 15 (57.7) | 16 (57.1) | |
| Recurrence after surgery | 0.800 | |||
| Yes | 16 (50.0) | 11 (42.3) | 12 (42.9) | |
| No | 16 (50.0) | 15 (57.7) | 16 (57.1) | |
| Treatment line of PD-1 Inhibitors | 0.862 | |||
| Second line | 2 (6.3) | 1 (3.8) | 1 (3.6) | |
| Third/after line | 30 (93.7) | 25 (96.2) | 27 (96.4) | |
| Brain metastasis | 0.700 | |||
| Yes | 6 (18.8) | 5 (19.2) | 6 (21.4) | |
| No | 26 (81.2) | 21 (80.8) | 22 (78.6) | |
| PD-L1 TPS | 0.131 | |||
| <1% | 8 (25.0) | 1 (3.8) | 8 (28.6) | |
| 1~49% | 13 (40.6) | 13 (50.0) | 6 (21.4) | |
| ≥50% | 9 (28.1) | 8 (30.8) | 9 (32.1) | |
| Unknown | 2 (6.3) | 4 (15.4) | 5 (17.9) | |
| EGFR mutation subtype | 0.152 | |||
| 19del | 5 (1.6) | 6 (23.1) | 11 (39.3) | |
| 21L858R | 21 (65.6) | 17 (65.4) | 9 (32.1) | |
| T790M | 3 (9.4) | 1 (3.8) | 4 (14.3) | |
| Others | 3 (9.4) | 2 (7.7) | 4 (14.3) |
Figure 2.
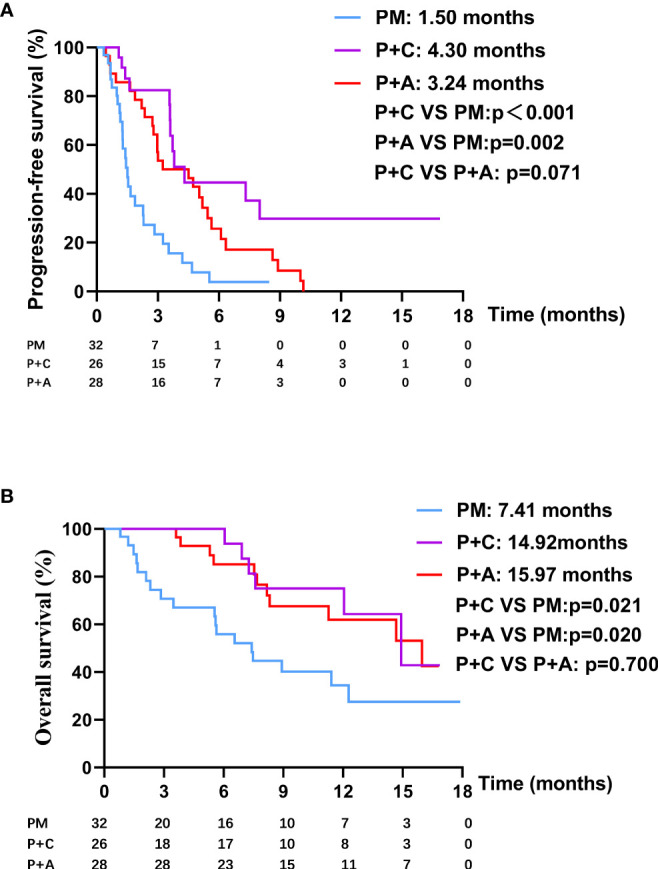
(A) The PFS curve of patients in PM, P+C and P+A groups. (B) The OS curve of patients in PM, P+C and P+A groups.
Figure 3.
The ORR and DCR of patients in PM, P+C and P+A groups. * represents a statistically significant difference.
Subgroup Analysis of Patients in P+C and P+A Group
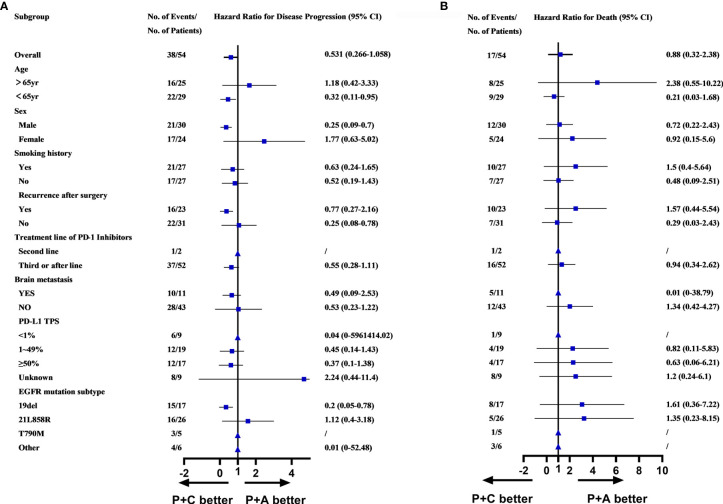
Survival analysis, including PFS, OS and ORR had demonstrated the superiority of combination therapy (P+C and P+A) over monotherapy (PM). However, no significant difference was found between P+C and P+A. We conducted a subgroup analysis of the patients in the P+C and P+A groups to determine the specific characteristics of each treatment. The subgroup analysis of the PFS showed that patients of <65years old (HR=0.32, 95%CI: 0.11-0.95), male patients (HR=0.25, 95%CI: 0.09-0.70), patients that relapsed after surgery (HR=0.25, 95%CI: 0.08-0.78) and patients with EGFRD19 (HR=0.20, 95%CI: 0.05-0.78) preferred P+C to P+A ( Figure 4A ). However, no difference was found in OS subgroup analysis ( Figure 4B ).
Figure 4.
(A) Subgroups analysis of PFS in P+C and P+A groups. (B) Subgroups analysis of OS in P+C and P+A groups. (▲Represented HR cannot be calculated due to the sample.
Discussion
To our knowledge, this is the first study to directly compare the efficacy of PD-1 inhibitor monotherapy, PD-1 inhibitor plus chemotherapy and PD-1 inhibitor plus antiangiogenic agents in patients with previously treated advanced NSCLC with EGFR mutations. In this retrospective study, the addition of chemotherapy or anlotinib to pembrolizumab resulted in significantly prolonger PFS, OS and ORR compared with pembrolizumab alone. The median PFS and OS of the whole population in the present study were 3.24 and 12.28 months, respectively. Of interest, the univariate analysis and multivariate analysis found that combined therapies (P+C and P+A) were positive prognostic factors for both the PFS and OS.
The feasibility of immunotherapy for patients with EGFR mutation has long been controversial. A phase II study (NCT02879994) of pembrolizumab in TKI naive patients with EGFR mutations, advanced NSCLC and PD-L1-positive tumors was suspended due to lack of efficacy, which indicating that pembrolizumab was not suitable as a first-line treatment in this population (21). Besides, Checkmate057, Keynote010, POPLAR and OAK trials showed us the poor efficacy of PD-1/PD-L1 monotherapy in patients with EGFR mutations who had progressed after platinum-based doublet chemotherapy and treatment with EGFR-TKIs (12–15). However, the final overall survival update of the ATLANTIC trial demonstrated a promising OS benefit across all cohorts, especially in patients with EGFR mutations (16). The median OS of patients with NSCLC with EGFR mutations (TPS ≥ 25%) was 16.1 months, which was longer than that observed in patients with TPS ≥ 25% EGFR−/ALK− tumors (median OS of 10.9 months) (16). Of note, the median PFS and OS following PM treatment in our cohort were 1.50 and 7.41 months, respectively, which was consistent with the Keynote010 trial and failed to copy the success of ATLANTIC. This might be due to the difference between PD-1 inhibitors and PD-L1 inhibitors (12).
Recently, Christian et al. reported the results of a phase II clinical trial evaluating the effect of ICI (pembrolizumab) plus chemotherapy (docetaxel) vs chemotherapy (docetaxel) alone in previously treated patients with advanced NSCLC, including patients with sensitizing EGFR mutations who had experienced disease progression after platinum-based chemotherapy. For patients with EGFR variations, the PFS (6.8 vs 3.5 months) and ORR (58.3% vs 23.1%) were statistically significantly different in favor of the combination arm, which highlighted the efficacy of the combination of immunotherapy and chemotherapy. Similarly, another phase II study of immunotherapy (toripalimab) plus chemotherapy in patients with EGFR-mutant advanced NSCLC patients found that the combined treatment yielded encouraging PFS (7.0 months) and ORR (50%) (22). In our cohort, the median PFS and ORR of P+C treatment were 4.30 months and 23.1%, respectively. Our cohort’s PFS rate and ORR were lower than those reported in the aforementioned, which probably because our patients were more heavily treated. Nevertheless, our study verified the benefit of the combination treatment.
The IMPOWER150 trial revealed encouraging PFS and OS following immunotherapy, albeit in a combination therapy pattern, in patients with NSCLC with EGFR mutations (18, 23). The OS was greater in the ABCP arm (29.4 months) than in the BCP arm (18.1 months) but the difference was not statistically significant (HR, 0.60; 95% CI, 0.31-1.14), which might be due to the study’s small sample size, which might due to the small sample (24). In contrast, the IMPOWER130 trial revealed no significant PFS or OS benefit for patients with EGFR and ALK alterations. This difference in results highlighted the necessity of antiangiogenic agents and supported the hypothesis that antiangiogenic agents could enhance immune efficacy, which might be due to the remarkable improvement of antigen-specific T-cell migration, in patients with NSCLC with EGFR mutations in response to antiangiogenic treatment (25). Similarly, the combination of ICIs and antiangiogenic agents in our cohort also yielded greater PFS (HR, 0.41; 95% CI, 0.23-0.73) and OS (HR, 0.41; 95% CI, 0.19-0.87) than ICIs alone. Meanwhile, Zhai et al. found that anlotinib combined with PD-1 inhibitors showed promising efficacy as a third- or further-line treatment for NSCLC (26). The combination treatment achieved a median OS of 17.3 months, which was similar to the OS of 15.97 months in the P+A group in our cohort. This encouraging result suggested that the combination of immunotherapy and antiangiogenic agents might overcome the barriers associated with immunotherapy for patients with EGFR- mutant NSCLC patients.
Our study demonstrated the superiority of combination therapy (pembrolizumab plus anlotinib or pembrolizumab plus chemotherapy) intuitively. However, was found in the survival analysis between the group that received P+A and the group that received P+C. Future research studies with a larger sample size are needed to define the subgroups of patients to determine precise treatment strategies.
Hastings, K. et al. found that different EGFR mutation subtypes responded to ICIs differently (27). EGFRL858R resulted in longer PFS and OS than EGFRD19, which might be due to the higher tumor mutation burden (TMB) in the EGFRL858R group. Consistent with the previous findings, our multivariate analysis in our study also found that EGFRL858R was associated with a longer PFS than EGFRD19 (HR:0.41, p=0.024). However, no OS benefit of EGFRL858R was found, which might be due to the small sample size.
There is no doubt that the expression level of PD-L1 is correlated with the efficacy of pembrolizumab in patients with NSCLC without EGFR mutations (28). Meanwhile, several studies have found that patients with EGFR mutations and PD-L1+ were more likely to respond to ICI monotherapy or ICI plus chemotherapy than those who were PD-L1 negative (22, 29, 30). However, some studies did not address the problem that chemotherapy and EGFR-TKIs might affect the expression of PD-L1. Hence, we utilized the tumor samples that were re-biopsied immediately before immunotherapy to detect PD-L1 expression to reduce bias (31, 32). We assessed the relationship between the efficacy of ICIs and PD-L1 expression and revealed that patients with PD-L1≥50% had longer OS than the OS of the PD-L1 negative group (HR:0.30, p=0.039).
Our study is limited by its retrospective nature. First, the data was collected from one center and the sample size was relatively small. Also, selection bias existed inevitable due to unavoidable missing data. However, the baseline clinical characteristics of patients in the PM, P+C and P+A group were balanced well, indicating that no large selection bias existed. Additionally, the heterogeneity of PD-L1 expression within tumors was inevitably existed though all detection were performed under guideline.
In summary, our analysis revealed that pembrolizumab plus chemotherapy or antiangiogenic agents could significantly prolong the PFS, OS and ORR compared with those observed following treatment with pembrolizumab alone in previously treated patients with advanced NSCLC with EGFR mutations. Our findings highlight the efficacy of the combination strategy of immunotherapy in this specific population. We also found that immunotherapy might be a more promising therapeutic agent for patients with EGFRL858R and patients with PD-L1≥50%. Based on the current findings, we hold the opinion that relevant clinical trials are urgently needed. The efficacy and safety of immunotherapy plus chemotherapy or antiangiogenic therapy in patients with EGFR-mutant NSCLC, especially those with high PD-L1 expression, should be further explored in clinical trials that provide strong evidence-based medicine data.
Data Availability Statement
The datasets presented in this article are not readily available because of the following: ethical requirements for Shanghai chest hospital. Requests to access the datasets should be directed to 18930858216@163.com.
Author Contributions
YC, ZY, and YW have substantial contributions to the conception or design of the work, the collection and analysis of data, and the writing and editing of the article. The rest authors have given substantial contributions to the work by providing editing and writing assistance. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Shanghai Xuhui District municipal health commission [grant number XHLHGG201806] and Shanghai Shenkang three-year project[grant number SHDC 2020CR4017].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Acknowledgments
The authors thank all the patients and their families for their contributions to this study.
References
- 1. Remon J, Ahn MJ, Girard N, Johnson M, Kim DW, Lopes G, et al. Advanced-Stage Non-Small Cell Lung Cancer: Advances in Thoracic Oncology 2018. J Thorac Oncol (2019) 14(7):1134–55. 10.1016/j.jtho.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 2. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol (2015) 10(6):910–23. 10.1097/JTO.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Liu Y. Molecular diagnostic characteristics based on the next generation sequencing in lung cancer and its relationship with the expression of PD-L1. Pathol Res Pract (2020) 216(2):152797. 10.1016/j.prp.2019.152797 [DOI] [PubMed] [Google Scholar]
- 4. Soo RA, Lim SM, Syn NL, Teng R, Soong R, Mok TSK, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer (2018) 115:12–20. 10.1016/j.lungcan.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 5. Tung JN, Lin PL, Wang YC, Wu DW, Chen CY, Lee H. PD-L1 confers resistance to EGFR mutation-independent tyrosine kinase inhibitors in non-small cell lung cancer via upregulation of YAP1 expression. Oncotarget (2018) 9(4):4637–46. 10.18632/oncotarget.23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoneshima Y, Ijichi K, Anai S, Ota K, Otsubo K, Iwama E, et al. PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer (2018) 118:36–40. 10.1016/j.lungcan.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 7. Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer (2019) 134:174–9. 10.1016/j.lungcan.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 8. Hsu KH, Huang YH, Tseng JS, Chen KC, Ku WH, Su KY, et al. High PD-L1 expression correlates with primary resistance to EGFR-TKIs in treatment naive advanced EGFR-mutant lung adenocarcinoma patients. Lung Cancer (2019) 127:37–43. 10.1016/j.lungcan.2018.11.021 [DOI] [PubMed] [Google Scholar]
- 9. Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J, et al. Strong Programmed Death Ligand 1 Expression Predicts Poor Response and De Novo Resistance to EGFR Tyrosine Kinase Inhibitors Among NSCLC Patients With EGFR Mutation. J Thorac Oncol (2018) 13(11):1668–75. 10.1016/j.jtho.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 10. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology (2017) 6(11):e1356145. 10.1080/2162402x.2017.1356145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsumoto Y, Koh Y. Tumor immune microenvironment of EGFR-mutant non-small-cell lung cancer and its impact on therapeutic efficacy. Immunotherapy (2020) 12(7):431–7. 10.2217/imt-2019-0213 [DOI] [PubMed] [Google Scholar]
- 12. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 16. Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. Final overall survival and safety update for durvalumab in third- or later-line advanced NSCLC: The phase II ATLANTIC study. Lung Cancer (2020) 147:137–42. 10.1016/j.lungcan.2020.06.032 [DOI] [PubMed] [Google Scholar]
- 17. Arrieta O, Barron F, Ramirez-Tirado LA, Zatarain-Barron ZL, Cardona AF, Diaz-Garcia D, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol (2020) 6(6):856–64. 10.1001/jamaoncol.2020.0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med (2019) 7(5):387–401. 10.1016/s2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 19. Zhou M, Chen X, Zhang H, Xia L, Tong X, Zou L, et al. China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (London England) (2019) 39(1):36. 10.1186/s40880-019-0383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol (2018) 4(11):1569–75. 10.1001/jamaoncol.2018.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J Thorac Oncol (2018) 13(8):1138–45. 10.1016/j.jtho.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou C. A multi-center phase II study of toripalimab with chemotherapy in patients with EGFR mutant advanced NSCLC patients resistant to EGFR TKIs: Efficacy and biomarker analysis. J Clin Oncol (2020) 38(suppl; abstr e21618). 10.1200/JCO.2020.38.15_suppl.e21618 [DOI] [Google Scholar]
- 23. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 24. Socinski MA, Mok TS, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Abstract CT216: IMpower150 final analysis: Efficacy of atezolizumab (atezo) + bevacizumab (bev) and chemotherapy in first-line (1L) metastatic nonsquamous (nsq) non-small cell lung cancer (NSCLC) across key subgroups. Cancer Res (2020) 80(16 Supplement):CT216–CT. 10.1158/1538-7445.Am2020-ct216 [DOI] [Google Scholar]
- 25. Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun (2016) 7:12624. 10.1038/ncomms12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhai C, Zhang X, Ren L, You L, Pan Q, Pan H, et al. The Efficacy and Safety of Anlotinib Combined With PD-1 Antibody for Third-Line or Further-Line Treatment of Patients With Advanced Non-Small-Cell Lung Cancer. Front Oncol (2021) 10:619010. 10.3389/fonc.2020.619010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol (2019) 30(8):1311–20. 10.1093/annonc/mdz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol (2019) 30(10):1653–9. 10.1093/annonc/mdz288 [DOI] [PubMed] [Google Scholar]
- 29. Liu SY, Dong ZY, Wu SP, Xie Z, Yan LX, Li YF, et al. Clinical relevance of PD-L1 expression and CD8+ T cells infiltration in patients with EGFR-mutated and ALK-rearranged lung cancer. Lung Cancer (2018) 125:86–92. 10.1016/j.lungcan.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 30. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol (2017) 28(7):1532–9. 10.1093/annonc/mdx183 [DOI] [PubMed] [Google Scholar]
- 31. Lacour M, Hiltbrunner S, Lee SY, Soltermann A, Rushing EJ, Soldini D, et al. Adjuvant Chemotherapy Increases Programmed Death-Ligand 1 (PD-L1) Expression in Non-small Cell Lung Cancer Recurrence. Clin Lung Cancer (2019) 20(5):391–6. 10.1016/j.cllc.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 32. Kim TJ, Hong SA, Kim O, Kim SJ, Yang JH, Joung EK, et al. Changes in PD-L1 expression according to tumor infiltrating lymphocytes of acquired EGFR-TKI resistant EGFR-mutant non-small-cell lung cancer. Oncotarget (2017) 8(64):107630–9. 10.18632/oncotarget.22582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available because of the following: ethical requirements for Shanghai chest hospital. Requests to access the datasets should be directed to 18930858216@163.com.