Abstract
Lung cancer, of which non-small lung cancer is the most common subtype, represents the leading cause of cancer related-death worldwide. It is now recognized that a significant proportion of these patients present alterations in certain genes that drive oncogenesis. In recent years, more of these so-called oncogenic drivers have been identified, and a better understanding of their biology has allowed the development new targeted agents. This review aims to provide an update about the current landscape of driver mutation in non-small-cell lung cancer. Alterations in Kirsten rat sarcoma, epidermal growth factor receptor, MET, anaplastic lymphoma kinase, c-ROS oncogene 1, v-raf murine sarcoma viral oncogene homolog B, neurotrophic receptor tyrosine kinase, human epidermal growth factor 2, neuregulin-1 and rearranged during transfection are discussed, as well as agents targeting these alterations. Current standards of treatment as well as promising future strategies are presented. Currently, more than fifteen targeted agents are food and Drug administration-approved for seven oncogenic drivers in non-small-cell lung cancer, highlighting the importance of actively searching for these mutations. Continuous and future efforts made in defining the biology of each of these alterations will help to elucidate their respective resistance mechanisms, and to define the best treatment strategy and therapeutic sequence.
Keywords: Non-small cell lung cancer, Driver mutations, Tyrosine kinase inhibitors, Targeted agents, Oncogenes
Core Tip: We have reviewed the current literature about the impact of detecting oncogenic mutations in non-small cell lung cancer (NSCLC). Over the years, the adoption of next generation sequencing has rendered it easier to determine and detect possible oncogenic driver mutations, leading to the development of several targeted therapies. The clinical impact and benefit for patients is important in terms of quality and quantity of life. These therapies are more effective than standard chemotherapy treatment. We have reviewed the data to explain what has been done, is ongoing and shall be done in the future for patients with oncogene-driven NSCLC.
INTRODUCTION
Lung cancer is the most common malignancy and the leading cause of cancer related deaths worldwide (18.4% of total cancer deaths), with non-small cell lung cancer (NSCLC) being the most common subtype, accounting for approximately 85% of all diagnosed cases[1]. The majority of NSCLC patients display advanced disease when diagnosed and thus have poor prognosis[2]. It is well established that acquired genetic alterations in certain driver genes result in tumor growth and invasiveness, and that patients harbouring certain mutations may benefit from targeted therapies[3] (Figure 1). Indeed, a randomized clinical trial reported that advanced NSCLC patients harbouring activating mutations in the epidermal growth factor receptor (EGFR), one of the major oncogenic drivers of NSCLC, exhibited longer progression-free survival (PFS) when treated with a tyrosine kinase inhibitor (TKI), gefitinib, compared to those treated with standard platinum-based chemotherapy[4]. However, those who were treated with TKI drugs can acquire secondary resistance mutations, in which case a new treatment regimen is needed to maintain therapeutic effects[5,6]. In addition to EGFR, NSCLC patients carrying anaplastic lymphoma kinase (ALK) or c-ROS oncogene 1 (ROS1) rearrangement were shown to respond well to a different TKI drug[7-9], crizotinib, while v-raf murine sarcoma viral oncogene homolog B (BRAF) mutated NSCLC patients can be treated with a combination of BRAF inhibitors, dabrafenib and trametinib[10]. These findings suggest that the identification of mutation profiles of NSCLC is critical in order to prescribe suitable TKI therapy as well as elucidate the molecular basis of drug resistance to provide timely treatment adjustment. Since 2018, the American Society of Clinical Oncology (ASCO) has recommended routine mutation testing for driver genes including EGFR, ALK, ROS1 and BRAF in clinical practice for patients with metastatic NSCLC. Although there are currently no targeted drugs for Kirsten rat sarcoma (KRAS) or neuroblastoma rat sarcoma (NRAS) mutated NSCLCs[11,12], mutation testing for these genes has also been recommended due to their proven impact on clinical outcomes of NSCLC patients[13]. This review aims to provide an update about the impact and importance of detecting mutations in oncogenes in patients with advanced NSCLC.
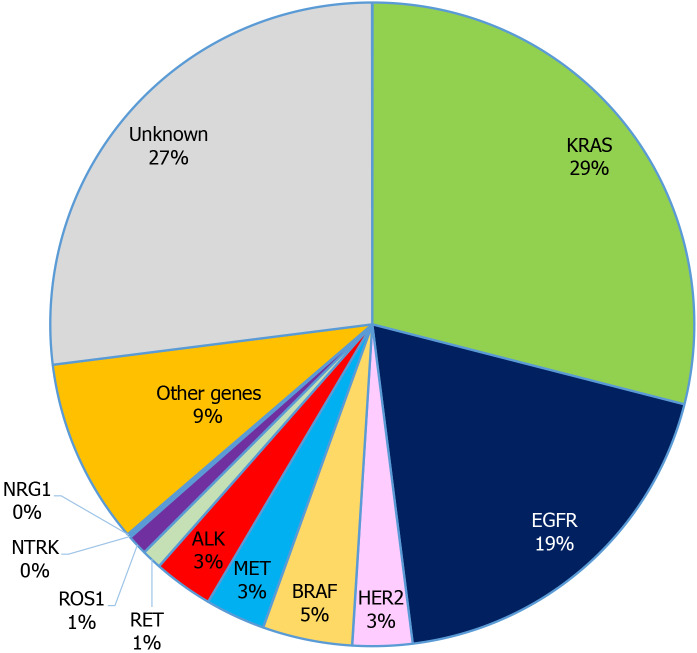
Figure 1.
Incidence of oncogenic drivers in non-small cell lung cancer. KRAS: Kirsten rat sarcoma; EGFR: Epidermal growth factor receptor; ALK: Anaplastic lymphoma kinase; HER2: Human epidermal growth factor 2; ROS1: c-ROS oncogene 1; NTRK: Neurotrophic receptor tyrosine kinase; RET: Rearranged during transfection; NRG1: Neuregulin-1.
KRAS
The rat sarcoma (RAS) genes (KRAS, NRAS, Harvey rat sarcoma viral oncogene homolog) represent the most frequent human oncogenes. Up to 30% of NSCLC harbor a mutation in the KRAS oncogene, making KRAS the most commonly detected oncogenic driver in lung cancer[11]. The KRAS proteins belong to the small guanosine triphosphate (GTP)ase family, involved in intracellular signaling. In response to extracellular signaling, KRAS proteins switch between two states: The GTP-bound “on-state” and general dental practitioner-bound “off-states”. When “on”, KRAS activates downstream signaling pathways, mainly the mitogen activated protein kinase and extracellular signal regulated kinase (MAPK/ERK) and phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) C1 signaling pathways, ultimately promoting cellular division and proliferation.
Some KRAS mutations, such as those in exons 2 and 3, which prevent GTP hydrolysis and prevent switching KRAS signaling off, result in a constitutive activation of KRAS proteins. KRAS mutation is more frequent in adenocarcinoma, and can be detected by next generation sequencing (NGS)[14]. The most common mutations involve a substitution in the codon 12 or 13. The KRAS G12C mutation found in approximately 13% of NSCLC[15], is of particular interest, as it has become a therapeutic target. It is more frequently detected in smokers, while KRAS G12D is more common among non-smokers[11]
Currently, the standard of care for KRAS-mutated NSCLC follows that of non-oncogene-driven NSCLC, consisting of immunotherapy with or without platinum-based chemotherapy.
Two Specific KRAS G12C TKIs have emerged, sotorasib (AMG510) and adagrasib (MRTX849). In the Codebreak100 phase I/II trial, 59 KRAS G12C mutated, previously treated advanced or metastatic NSCLC patients received sotorasib orally. The objective response rate (ORR) was 32.2%, with a median duration of response of 10.9 mo[16].
Sotorasib is currently being tested in the randomized phase III, Codebreak200 trial, vs docetaxel in the second-line setting (NCT04303780). The primary endpoint is PFS, with overall survival (OS) as a secondary endpoint[17].
Adagrasib represents another specific KRAS G12C TKI. In the phase I/II KRYSTAL-1 trial[18], 79 patients with pre-treated NSCLC received adagrasib 600 mg twice daily. Among the 51 patients evaluable for response, an ORR of 45% was observed. The most frequent side effects included nausea, vomiting and diarrhea, mostly grade 1-2.
Others KRAS G12C inhibitors are currently being tested in phase I/II clinical trials: JNJ-74699157[19] and Gadolinia-Doped Ceria-6036[20]. As fewer than 50% of patients initially respond to sotorasib or adagrasib, we can assume that some patients present intrinsic resistance to KRAS G12C inhibition. This hypothesis is supported by preclinical evidence demonstrating resistant cell lines[21]. One explanation is that tumor cells may not exclusively rely on the RAS pathway for survival and proliferation[22]. As an example, RAS-independent activation of the PI3K/AKT/mTORC1 signaling pathway could be associated with resistance to KRAS inhibition[23]. Another mechanism of resistance could be the heterogeneous distribution of KRAS mutations in different tumor sites within the same patient[24]. Adaptive resistance also emerges under the selective pressure of KRAS TKIs. One mechanism of adaptive resistance could consist in the amplification of upstream drivers, such as receptor tyrosine kinases/ Src homology 2 domain-containing phosphatase 2 (RTKs/SHP2), that result from KRAS inhibition. Indeed, the diminution of ERK activity driven by KRAS G12C TKIs has been shown to suppress the ERK-mediated feedback inhibition of RTKs/SHP2, further activating N-Ras, H-Ras, and K-RasG12C, and ultimately restoring the activity of the MAPK/ERK signaling[21,25].
Although clinical data are scarce, it is likely that KRAS G12C inhibitors are not effective in the majority of the patients harboring KRAS G12C mutations. There has been a growing interest to combine the KRAS G12C inhibitors with targeted agents or immune checkpoint inhibitors (ICIs)[26]. Based on preclinical data discussed above, adagrasib is currently being tested in association with the SHP2 inhibitor TNO155 in early clinical phases[27]. Associations with ICIs also represent an interesting approach, as in some preclinical models, KRAS G12C positive tumor cell lines exhibit an immunosuppressive environment that is disrupted by KRAS inhibition[28,29].
Besides those targeting G12C, other KRAS inhibitors are in development, such as MRTX1133, a KRAS G12D inhibitor currently in investigational new drug enabling studies[30], or BI 1701963, a molecule targeting son of sevenless-1, an activator of KRAS, which could allow inhibition of the KRAS pathway regardless of the mutation[31].
Finally, other approaches targeting KRAS include an mRNA vaccine targeting KRAS mutant cells, a strategy that has already entered a phase I clinical trial[32], as preclinical data revealed an immune cell response in animal models[33].
EGFR
EGFR is one of the four members of the human epidermal growth factor (HER) family transmembrane receptors (HER1/EGFR, HER2, HER3, and HER4). Each HER receptor is an inactive monomer that dimerizes with a receptor of the same type or with another member of the HER family in response to ligand binding. The receptor activation triggers a complex downstream signaling network which leads cell replication[34]. The dysregulated receptor function or disruptions in any downstream EGFR processes may result in cell transformation and malignancy.
The prevalence of the mutation in the EGFR oncogenes is 50% among Asian patients with lung adenocarcinoma and 15% among Western patients[35]. Mutations leading to excessive EGFR activity are most common among non-smokers, young, female, Asian lung cancer patients[36]. Exon 19 deletions or L858R point mutations in exon 21 account for 90% percent of the activating mutations in the tyrosine kinase domain of EGFR, resulting in constitutional activation of EGFR without ligand-induced stimulation, thus promoting cell proliferation, survival, and dissemination[37,38].
EGFR mutations can be detected by immunohistochemistry (IHC) or NGS. TKIs are the standard front-line therapy for metastatic EGFR mutant NSCLC for a decade, with three generations of TKIs that demonstrated better outcomes and lower toxicity compared to standard chemotherapy[39], with a median PFS of 11.0 mo (gefitinib or erlotinib) vs 5.6 mo (chemotherapy)[40]. To date, there are five United States Food and Drug Administration (FDA)-approved TKIs as the standard treatment for patients with activating EGFR mutations in NSCLC, including first-generation gefitinib and erlotinib, second-generation afatinib and dacomitinib, and third generation osimertinib[41]. However, it has been shown that resistance systematically develops to those treatments[42,43], mediated by mechanisms such as T790M secondary mutations, activation of other EGFR pathways, development of concurrent mutations or histological transformation. The T790M exon 20 mutation is rarely found in EGFR TKI-naive disease but is the most frequent cause of resistance to first- and second-generation EGFR TKIs (50%-60% of cases)[44] possibly by the presence of a mutated clone before treatment, which would be free to grow into the dominant clone under selective TKI pressure. It is the indication for which osimertinib, an EGFR-TKI that selectively inhibits both EGFR-TKI-sensitizing and EGFR T790M resistance mutations, was first developed. It improves outcomes in second-line after disease progression with T790M mutations[44,45], as well as in the front-line setting (PFS 18.9 mo vs 10.2 mo; OS 38.6 mo vs 31.8 mo) with an improved safety profile[43,46].
In order to improve outcomes of EGFR mutant NSCLC patients, combination therapy of EGFR TKIs with other agents have been tested, with interesting perspectives. A meta-analysis was conducted regarding the efficacy and safety of vascular EGFR inhibitors in combination with chemotherapy for patients with advanced NSCLC showing improved PFS, ORR and disease control rate, but without an impact on OS[47]. Results are pending from the Japanese phase II study comparing osimertinib alone vs osimertinib plus chemotherapy in second-line setting for patients with T790M mutation. Also, the phase III FLAURA2 study started recruiting in 2019, studying osimertinib with or without platinum-pemetrexed chemotherapy as first-line treatment in EGFR mutated advanced patientswith NSCLC (ClinicalTrials.gov Identifier: NCT04035486). Numerous studies are ongoing regarding the best sequence to use. At this time, the combination of chemotherapy and TKIs is not standard practice.
MET
The proto-oncogene MET is located on chromosome 7q21-q31. It encodes for a transmembrane receptor (c-Met or MET) also known as hepatocyte growth factor receptor. This tyrosine kinase receptor activates downstream RAS/ERK/MAPK, PI3K/AKT, Wnt/β-catenin, and signal transducer and activator of transcription (STAT) signaling pathways, that can drive cell proliferation, survival, migration, invasion, angiogenesis, and transition from epithelial to mesenchymal[48]. MET dysregulation encompasses a heterogeneous array of alterations, with two main subgroups: MET amplifications and MET exon 14 mutations, leading to prolonged activation of the cellular MET receptor and downstream proliferation pathways[11]. Regarding exon 14, aberrant splicing and skipping of exon in the messenger RNA transcript can result from somatic missense mutations, insertions, deletions, and concomitant insertions and deletions.
MET alterations are found in fewer than 5% of patients with NSCLC, mainly adenocarcinoma, often with concurrent mutations (i.e., EGFR, ALK). Detection of MET amplification is assessed by fluorescence in situ hybridization (FISH), and exon 14 skipping is most often completed through DNA or RNA NGS.
Due to the various diversity of MET dysregulation, there was a need to identify the oncogenic role of each type of MET alteration and defining the adequate targeted therapy. Over the last 20 years, several agents have been developed to target MET such as multikinase MET inhibitors (crizotinib, cabozantinib, MGCD265, AMG208, altiratinib, golvatinib), selective MET inhibitors (capmatinib, tepotinib, tivantinib) and monoclonal antibody (onartuzumab, emibetuzumab, ficlatuzumab, rilotumumab). A retrospective registry (IMMUNOTARGET) of advanced NSCLC patients who received immunotherapy showed that only 16% of them demonstrated a partial response, with a short median PFS of 3.4 mo[49]. Small cohorts showed that MET TKIs offer a promising treatment option in patients with exon 14 skipping with response rates from 25% to 68%, and a median duration of response of 9 to 16 mo. In the retrospective data analysis of a phase 1 PROFILE 1001 study, data supported that MET exon 14 skipping in NSCLC confers sensitivity to direct MET inhibitors with a median OS of 24.6 mo vs 8.1 mo among patients receiving crizotinib compared with those who did not[50]. In patients with advanced or metastatic NSCLC with a confirmed MET exon 14 skipping mutation, the use of tepotinib, a highly selective oral MET inhibitor, was associated with a partial response in approximately half the patients (ORR 46%, with a median duration of response of 11.1 moin a recent phase 2 study[51]. In advanced MET exon 14 skipping NSCLC, capmatinib showed substantial antitumor activity particularly in previously untreated patients (ORR 68%), with a median duration of response of 12.6 mo[51]. Limited efficacy was observed in previously treated patients with MET amplification (ORR 7 to 12% of patients with capmatinib).
ALK
ALK is a transmembrane receptor tyrosine kinase that can activate multiple signaling cascades such as the PI3K-AKT, Crkl-C3G, MAP kinase kinase kinase 2/3-mitogen-activated protein kinase kinase (MEK)5-ERK5, Janus kinase (JAK)-STAT, and MAPK pathways. Its involvement is known in development, then subsequently silenced in adult tissues. However, several ALK gene alterations have been identified in tumors, including point mutations, deletions, and rearrangements leading to ALK reactivation. Various ALK-fusion proteins have been described that result from numerous chromosomal rearrangements, with formation of dimers by the amino-terminal portion of the ALK fusion proteins resulting in the activation of the ALK protein kinase domain that plays a key role in the tumorigenic process. The consequent ALK expression can activate multiple downstream known cancer signaling pathways [PI3K/AKT, JAK/STAT, and RAS/rapidly accelerated fibrosarcoma (RAF)/ MEK/ERK][52].
ALK rearrangements are detected in approximately 5% of advanced NSCLC[8], 2 to 7% in all, up to 19% for stage IV. ALK alterations are mainly found in adenocarcinomas (97%), while squamous cell carcinomas comprise 3%[7]. ALK positivity is found in a fifth of never to light smokers with lung cancer. Methods of diagnosis can include FISH, IHC, or NGS.
The superiority of the TKI, crizotinib, over chemotherapy in first-line ALK+ advanced NSCLC was proven in 2014, with an ORR of 74% vs 45%, PFS 10.9 mo vs 7 mo, and 1-year survival 84% vs 79% (median OS not reached in the crizotinib group)[53]. However, resistance to treatment invariably develops, often with progression in the brain, motivating the development of new generations of TKI. Second- (alectinib, brigatinib, ceritinib) and third-generation (lorlatinib) TKIs have proven their efficacy, with the third-generation lorlatinib who led to a 72% improvement in PFS compared with crizotinib in first-line treatment[54]. After failure of second-generation ALK TKIs, concurrent administration of platinum/pemetrexed-based chemotherapy with an ALK TKI shows efficacy in a recent retrospective study[55] (PFS 6.8 mo with the combination vs 3.2 mo for chemotherapy alone; suggesting a potential role for continued ALK inhibition. Some studies are currently underway, evaluating the addition of ceritinib to nivolumab (NCT02393625, completion June 2021) or ceritinib with trametinib (NCT03087448, completion June 2022).
Recently, a next-generation ALK inhibitor, ensartinib, demonstrated promising efficacy in the first-line treatment for advanced disease in a preplanned interim analysis (phase III eXalt3 study), with a median PFS of 25.8 mo with ensartinib in the intent-to-treat population vs 12.7 mo with crizotinib) (NCT02767804, study completion March 2021). Another new ALK inhibitor, TQ-B3139, is being tested vs crizotinib in the first line setting (NCT04009317, completion April 2022).
ROS1
ROS1 encodes a tyrosine kinase receptor, belonging to the insulin receptor family, and structurally related to the ALK protein. Its natural ligand remains undefined.
ROS1 rearrangement was described in glioblastoma, before being recognized as a potential oncogenic driver in NSCLC[56]. Various ROS1 alterations have been described in cancers: overexpression, splice variants (usually leading to a truncated protein lacking the intracellular domain), amplification, mutations and finally fusions with another partner gene. In contrast to fusions, the pathogenic significance of other alterations is undetermined[57].
At the moment, ROS1 fusions have identified with 55 different partners genes in different cancer types, including more than 20 fusion partners in NSCLC. The CD74-ROS1, EZR-ROS1, SDC4-ROS1 and SLC34A2-ROS1 fusion represent the most common rearrangements[58]. All resultant fusion proteins retain an intact ROS1 intracellular kinase domain which, as a result of the rearrangement, becomes constitutively activated. The activated kinase triggers intracellular signaling pathways, such as the RAS–RAF-MEK-ERK, PI3K-AKT-mTOR and JAK-STAT3 pathways, ultimately leading to cellular survival and division.
As with other common driver mutations, patients harboring a ROS1 rearrangement tend to be younger and more frequently non-smokers and Asian[56]. ROS1 is almost always found in adenocarcinoma subtypes, where it represents 1%-2% of the cases[59]. ROS1 alterations rarely occur with other driver mutations[60].
ROS1 rearrangement can be detected by FISH or NGS[14]. IHC is sensitive and can be used as a screening test. However, specificity remains poor and a positive result must be confirmed by FISH assays or NGS. A treatment decision should not be based on IHC results alone.
ROS1 rearranged NSCLC seem to have better response to chemotherapy, especially pemetrexed-based, when compared to both tumors with other driver mutations and wild-type tumors[61]. On the other hand, some data, although scarce, show modest response to ICIs[62].
Several tyrosine kinase inhibitors have shown clinical activity in ROS1-positive NSCLC. The phase I PROFILE 1001 trial of crizotinib included 50 patients with ROS1-fusion NSCLC, among which an overall response rate of 72%, and a median PFS of 19.2 mo were reported[63]. Importantly, more than 80% of patients had received at least one previous line of treatment. An updated analysis reported an impressive median OS of 51 mo[64]. Phases 2 trials in Europe and East Asia have consistently reported similar ORRs of 63%-69%[65,66]. In these studies, crizotinib demonstrated a favorable safety profile. However, crizotinib has limited intracranial activity. The central nervous system represents a common site of progression in ROS1-positive NSCLC treated with crizotinib[67]. Crizotinib should be avoided as a first-line agent in case of untreated brain metastasis.
After progression on crizotinib, lorlatinib represents an option. It was evaluated in 69 patients with ROS1-positive NSCLC[68]. In 40 patients that had received prior crizotinib, the ORR was 35%, with a median PFS of 8.5 mo. In 21 crizotinib-naive patients in the same study, lorlatinib demonstrated an ORR of 62%, with a median duration of response comparable to crizotinib of 25.3 mo and a median PFS of 19.3 mo. Lorlatinib also showed intracranial activity, with intracranial response rates of 50% and 64% in crizotinib pretreated and crizotinib-naïve patients respectively.
Ceritinib is a selective ALK inhibitor that also exhibits activity against ROS1 kinase. In a phase phase II trial involving 32 Asian patients with ROS1-rearranged NSCLC the ORR was 67%[69]. Interestingly, two patients that had received prior crizotinib experienced no response.
Entrectinib represents another tyrosine kinase inhibitor with activity against the ROS1 kinase. In a phase II study among 53 patients with ROS1-positive NSCLC, the majority of which had received previous platinum-based chemotherapy, the ORR reached 77%[70]. Responses seem durable, with median duration of response of 24.6 mo. Intracranial activity was also observed, with an intracranial ORR of 55%, with a median duration of response for central nervous system lesions of 12.9 mo.
Repotrectinib represents a next generation TKI targeting ROS1, ALK and tropomyosin receptor kinase (TRK). Its potency for ROS1 exceeds that of crizotinib by more than 90-fold. Reprotrectinib also shown early signs of efficacy against ROS1 resistance mutations[70,71].
Resistance to TKIs ultimately arise in nearly all patients. Intrinsic resistance to ROS1 inhibition implies solvent-front or gate-keeper point mutations in the ROS1 kinase domain, the most common being the ROS1 G2032R, that precludes binding to crizotinib[72]. To date, more than 20 mutations conferring resistance to various TKI have described[57]. Moreover, resistance to ROS1 inhibition may probably arise from the activation of parallel signaling pathways such as KRAS, BRAF or MET. Emergence of BRAF and KRAS activating mutations and MET-amplification in ROS1 positive NSCLC treated with crizotinib or lorlatinib have been described[73,74]. Upon progression, the role of sequencing ROS1 TKIs remains unclear.
BRAF
BRAF is an intracellular protein kinase that plays a relevant role in the MAPK/ERK pathway, including numerous proteins with kinase domains (RAF, MEK, ERK) that carry signal transduction from membrane receptors to DNA in the nucleus of the cell[75]. BRAF is an oncogene located on chromosome 7 involved in several cell functions, including growth, proliferation, survival and differentiation.
BRAF mutations are found in about about 5.5% of cancers, with almost 200 BRAF mutants and many RAF translocations identified in human cancers[76]. Those alterations generate structural modifications of the protein that are responsible for permanent activation of MAPK pathway and resistance to inhibitory feedback signals.
The frequency of BRAF mutations is about 5 to 8% in lung adenocarcinomas[10], with higher incidence in melanoma (50%), thyroid carcinoma (30% to 70%) and colorectal cancer (5% to 20%). Its presence predicts poor outcome for the latter three. While the V600E activating mutation is the most common BRAF variant found in solid tumors (90%), it only accounts for half of BRAF mutations in NSCLC. Non-V600E variants are more common in males, and all BRAF variant are more common among smokers. It is important to note that clinical characteristics of patients with NSCLC harboring BRAF mutation are difficult to clearly identify due to small numbers of patients in trials.
BRAF mutations can be detected by using immunohistochemistry for V600E exclusively or DNA sequencing on the tumor tissue for the two types.
The efficacy of ICIs is uncertain, and is based on conflicting retrospective data and case series, thus not routinely recommended. Therapies targeting BRAF mutations were developed for melanoma with great success, then used for NSCLC. For lung cancer, numerous small studies tested RAF inhibitors (vemurafenib, dabrafenib) alone or in combination with MEK inhibitors (trametinib, cobimetinib). Best responses were observed when treatment was combined, especially in V600E patients in first-line of dabrafenib-trametinib with a mOS of 24.6 mo[77]. Front-line use of double BRAF/MEK inhibition if V600E mutation is found in advanced NSCLC is now suggested in current guidelines. Other agents such as multikinase inhibitors sorafenib and dasatinib showed responses in case reports[78,79].
Combining MEK and BRAF inhibitors has proven to be more effective than single-agents for the treatment of BRAF-mutant advanced tumors, but does not prevent the emergence of resistance[79]. Indeed, studies on melanoma describe intrinsic adaptive (other pathways alteration) or acquired (de novo) resistance in BRAF mutated cancers treated with targeted inhibitors. Thus, further research is warranted to establish clear therapeutic algorithms.
NEUROTROPHIC RECEPTOR TYROSINE KINASE
The three neurotrophic receptor tyrosine kinase (NTRK) genes (NTRK1, NTRK2 and NTRK3) encode tyrosine kinase receptors for neutrophins, involved in neuronal development, survival and proliferation[80,81].
Oncogenic fusions involving the NTRK genes (e.g., apposition of the 3′ region of the NTRK gene with the 5′ sequence of a fusion partner gene) occur across many cancer types[82]. More than 25 gene partners have been described[83]. Yet, all these fusions result in a constitutively activated and overexpressed TRK kinase, that activates downstream pathways involved in cellular proliferation, such as the MAPK and PI3K/AKT[84].
NTRK fusions are very frequent in a few rare cancer types: Secretory carcinoma, mammary analogue secretory carcinoma, infantile fibrosarcoma and cellular mesoblastic nephromas, in which they are detected in more than of 90% of patients[83]. On the other hand, the NTRK fusion occurs at a very low frequency in various common cancers types, such as colorectal, breast, thyroid and lung cancers. In the latter, it represents under 1% of cases[85,86].
NTRK fusion can be detected either by IHC, FISH or DNA-and RNA-based NGS. Pan-TRK IHC has been shown to have high specificity and sensitivity in the detection of fusion protein expression. RNA-based NGS is preferred to DNA-only based technique.
The European Society for Medical Oncology (ESMO) guidelines suggest using FISH testing (or real-time reverse transcription polymerase chain reaction) in tumors with highly prevalent NTRK fusions, as it probably represents the most cost-effective strategy. In situations where such alterations are uncommon, NGS technique should be used upfront if available or as a confirmatory test, after a positive IHC “screening test”. Using NGS upfront has the advantage to allow the detection of other potentially targetable molecular alterations.
Larotrectinib is an oral TRK inhibitor that has shown durable activity in NTRK positive advanced tumors. In an analysis of three phase I/II trials, the ORR among patient with TRK-fusion positive cancer was 79%, with 80% of responses ongoing at 12 mo[15]. Among 12 patients with lung cancer, a similar response rate was reported (75%). The most frequent grade 3-4 adverse events related to larotrectinib were increased alanine aminotransferase, anemia, and decreased neutrophil count. Grade 3-4 adverse events occurred in 46% of patients.
Entrectinib represents another TRK inhibitor, also active for ROS1 and ALK, and specifically designed to cross the blood-brain barrier[87]. Entrectinib demonstrated an ORR of 57% in small non-randomized studies, among 54 patient with TRK-positive advanced tumors, including 10 patients with NSCLC (ORR 70% in the latter)[87]. The median overall duration of response was 10 mo. Of note, an objective intracranial response rate of 54.5% was reported in a cohort of 12 patients with NTRK fusion-positive tumors with brain metastasis[88]. The most common adverse events reported with entrectinib (> 20% of patients) were fatigue, gastro-intestinal disorders, weight gain and cognitive impairment. While the most threatening serious adverse events consisted of congestive heart failure, QT prolongation, central nervous system effects, hepatotoxicity, and vision disorders.
Based on these results, both larotrectinib and entrectinib have been granted accelerated approval by the FDA for metastatic or unresectable NTRK-fusion positive solid tumors, that have progressed following treatment or have no satisfactory standard therapy[89,90].
Despite a prolonged response, resistance to larotrectinib and entrectinib is expected to emerge in most patients[83], via different mechanisms: Solvent-front mutations and xDFG substitution[91]. Larotrectinib (LOXO)-195 and repotrectinib represent second-generation TRK-inhibitors, currently under development capable to overcome resistance to first-generation of TRK-inhibitors. LOXO-195 is a highly selective inhibitor of all 3 TRK kinases. LOXO-195 or selitrectinib was evaluated in a phase I trial and a FDA expanded access single patient protocol, including 31 patients in total, all of whom had received prior treatment with a TRK inhibitor[92]. Of note, 7 patients were pediatric. The most frequent treatment-emergent adverse events were dizziness (65%), ataxia (60%), nausea (50%), vomiting (40%), anemia (30%) and gait disturbance (30%), with 5 double-lumen endobronchial tube (4 ataxia/dizziness) in total. Among the 29 patients evaluable for efficacy, an ORR of 34% was reported.
Repotrectinib[91] is another second-generation NTRK inhibitor, designed to overcome acquired resistance mutations to larotrectinib/entrectinib[93,94] Case reports showed partial response in patient presenting acquired resistance to larotrectinib or entrectinib[87]. Repotrectinib is currently being tested in phase I/II clinical trial (NCT03093116)[95].
HER2
The HER2/neu gene is located on the chromosome 17q12, and encodes the HER2 protein, which belongs to the epidermal growth factor receptor (ERBB) family of tyrosine kinase receptors. Other members of the ERBB family include EGFR (ERBB-1), HER3 (ERBB3), and HER-4 (ERBB4). The ERBB receptors are transmembrane proteins, composed of an extracellular ligand binding domain, an α-helical trans membrane segment and an intracellular tyrosine kinase domain. Ligand binding induces receptor dimerization, and auto-phosphorylation and activation of the intracellular kinase domain[96]. Of note, no natural ligand of HER2 has been identified. Nevertheless, HER2 can undergo dimerization with other ERBB receptors[97], and represents their preferred dimerization partner. The activated kinase domain that results then triggers downstream signaling pathways, such as MAPK, PI3K/AKT, protein kinase C and STAT, promoting cellular proliferation and inhibiting apoptosis[98]. Genetic alterations in HER2 can result in constitutive dimerization and activation of downstream signaling pathways, finally leading to uncontrolled cellular proliferation.
HER2 mutations is found in approximately 1%-3% of NSCLC, primarily in adenocarcinoma, in non-smokers and women[99]. Mutations usually consist of in frame insertions or point mutation in exon 20. On the contrary, HER2 amplification in NSCLC is not associated with benefit of anti-HER2 therapy[100].
Retrospective data indicate that HER2 targeted therapy present some degree of activity in HER2-mutated NSCLC. In a European cohort of 57 pre-treated HER2 mutated NSCLC patients receiving trastuzumab-based regimen, an ORR of 50% was reported, with a PFS of 5.1 mo[101]. In a prospective phase II trial, the antibody-drug conjugate ado-trastuzumab emtansine demonstrated an ORR of 44% in NSCLC patients harboring HER2 mutations, with a median PFS of 5 mo[102]. The most common treatment-related adverse events included grade 1 or 2 infusion reactions, thrombocytopenia, and elevated aspartate aminotransferase or alanine aminotransferase. No grade 4 or 5 adverse events were reported. More recently, the DESTINY-Lung01 trial evaluating the safety and efficacy of trastuzumab-deruxtecan, another antibody-drug conjugate targeting HER2, were presented at the ASCO meeting[103]. The DESTINY-Lung01 trial includes 2 cohorts: HER2 amplified (based on IHC) and HER2 mutated tumors[104]. Interim results concerning only the HER2 mutated population were reported. Of the 42 patients who received trastuzumab-deruxtecan, with a median of 2 prior treatment lines, the confirmed ORR was 61.9%, with a median duration of response not reached at data cut-off, and a median PFS estimation of 14 mo. However, toxicity was not negligible, as 64.3% of patients presented grade 3 or more adverse events (52.4 % drug-related). Of note, 11.9% of developed drug-related interstitial lung disease, all grade 2. Treatment related adverse events led to treatment interruption, dose reduction or treatment cessation in 59.5%, 38.1% and 23.8% of patients respectively. The randomized phase II DESTINY-Lung02 trial (NCT04644237) will compare a lower dose regimen of 5.4 mg/kg every 3-wk (Q3W) to the 6.4 mg/kg Q3W regimen used in DESTINY-Lung01 trial[105].
NEUREGULIN-1
The neuregulin-1 (NRG1) gene is located on the long arm of chromosome 10 (10q23.1 region) and encodes a growth factor belonging to the complex family of proteins called heregulins, structurally related to the stimulation of ERBB receptors tyrosine kinase activity and EGF signals[106]. NRG1-receptor binding activates the ERBB2-ERBB3 heterocomplex and controls proliferation, differentiation, and survival in both normal and tumor cells through the predominant signaling cascades PI3K-AKT and MAP kinase[107].
NRG1 oncogenic gene rearrangements or fusions are rare, with an incidence of 0.2% on a wide retrospective molecular profiling that tested over 21’850 solid tumors[108]. They have been identified across a wide range of tumors including NSCLC (especially mucinous adenocarcinoma subtype), gallbladder cancer, pancreatic cancer, renal cell carcinoma, ovarian cancer and hepatic cholangiocarcinoma[109].
Detection of NRG1 gene fusions in solid tumors are made through RNA NGS[110]. NRG1 aberrations appear to be mutually exclusive with oncogenic alterations in EGFR, KRAS, ALK, ROS1, and rearranged during transfection (RET).
NRG1 was first described in NSCC in 2014[110]. Invasive mucinous adenocarcinoma (IMA), representing approximately 5% of lung adenocarcinomas, are known to be more aggressive than more common types, such as acinar or papillary adenocarcinoma. KRAS mutations had been the only oncogenic driver commonly detected in IMAs (in 50%–80% of cases), but CD74-NRG1 fusions are now detected in 14.7% of KRAS negative IMAs. NRG1 positivity confers worse outcomes in lung adenocarcinoma in retrospective data[111]. Given the potential therapeutic implications of this genetic alteration, the interest in evaluating the prevalence of NRG1 fusions has increased over the last five years.
The use of afatinib (tyrosine kinase inhibitor, targeting ERBB) showed interesting results in case reports for the treatment of NRG1 fusion-positive in cancers of lung (SDC4-, SLC3A2- and CD74-NRG1 gene fusion) and hepatocellular (ATP1B1-NRG1 gene fusion) origin[112]. Further studies are ongoing.
RET
The RET gene, located on the chromosome 10 (10q11.2), encodes a tyrosine kinase receptor located to the cell surface. Its intracellular kinase domain shares 37% homology with the ALK kinase domain[113]. RET is a receptor for Glial Cell Line-Derived neurotrophic factor (GDNF), a family extracellular signaling molecules notably involved in neuronal development[114]. The binding of GDNF to a co-receptor GDRalpha and then to RET, leads to a RET-dimerization and further autophosphorylation of the kinase domains. This activates the intracellular signal transduction process, notably the RAS, MAPK/ERK, PI3K/AKT and JAK/STAT pathways[113]. RET is normally involved in enteric nervous system and urogenital tract development. RET loss of function is associated with Hirschprung disease[115], and activating mutations to MEN-2 syndrome[116]. Rearrangements of the RET gene (e.g., the apposition of the C-terminal region of RET protein with the N-terminal region of another protein) can induce a constitutive activation of the RET kinase.
Rearrangements in RET with various partners have been described, the most common in NSCLC being KIF5B and CCDC6[117]. They have been identified in 1%-2% of lung adenocarcinomas, more often in never smoker and younger patients[113]. IHC is probably unreliable for the detection of RET-rearrangements, and FISH or NGS are preferred[113].
The phase I/II LIBRETTO 001 trial[118] evaluated selpercatinib in NSCLC patients. It showed an ORR of 64% in 105 patients previously treated with platinum-based chemotherapy and 85% in previously untreated patient (39 patients). Median duration of response was 17.5 mo in previously treated patients. Interestingly, among the 11 patients with central nervous system disease, 91% had an intracranial response.
Following these results, selpercatinib was granted accelerated approval by the FDA[119]. Selpercatinib is currently being evaluated in phase III, vs platinum-based chemotherapy +/- pembrolizumab, in the LIBRETTO-431 trial[120].
Pralsetinib (BLU-667), another selective RET inhibitor has also been granted accelerated approval from the FDA based on the results of the ARROW-study (NCT03037385), a basket trial in which patients with RET-fusions positive cancer received pralsetinib 400mg orally once daily. Among 89 patients with RET-fusion positive NSCLC pretreated with platinum-based chemotherapy, ORR was 57%, with 80% of ongoing responses at 6 mo[121]. Among 27 patients with previously untreated NSCLC, the ORR was 70% with 58% of response ongoing at 6 mo. Praseltinib is currently being compared to the standard treatment approach of platinum-based chemotherapy ± pembrolizumab in RET-fusion positive NSCLC in the AcceleRET trial (NCT04222972)[122].
Other non-RET-selective TKIs have shown some activity in RET-fusion positive NSCLC: Cabozantinib for example displayed an ORR of 28% in a single-center, phase II trial, of patients with RET-rearranged NSCLC, of whom 75% had received prior chemotherapy[123]. In an international registry of patients with RET-rearranged NSCLCs, cabozantinib, vandetanib, and sunitinib had rather limited activity, with overall response rates of 37%, 18%, and 22%, respectively[124].
Different mechanisms of RET-inhibition resistance have been described including the emergence of solvent front mutation in the RET-gene (e.g. in the RET G810 residue, in the kinase solvent front)[125] as well as acquired MET or KRAS amplifications[126].
CONCLUSION
Driver mutations have significantly altered the diagnostic work-up and reshaped the oncology treatment paradigm. Recently, several new driver mutations have been identified in metastatic NSCLC, with some leading to therapeutic success and others, failure. We have summarized the current landscape of actionable oncogenic alterations and their therapies in Table 1. A better understanding of the biology of various subtypes of each driver mutation will help not only to match the optimal treatment to each patient, but also elucidate their respective resistance mechanisms, allowing for greater precision medicine. Many trials are ongoing, some of them through serial tumor or plasma biopsies and multiplex molecular testing. The optimal targeted therapy sequence for each driver mutation is yet to be fully determined. Furthermore, it is unclear whether a multi-kinase inhibitor or highly selective therapy is the best choice for some alterations, though the latter tend to have more favourable toxicity profiles.
Table 1.
Selected oncogenic drivers and their treatments in non-small cell lung cancer
|
|
Targeted therapy
|
ORR
|
PFS
|
OS
|
| EGFR | Erlotinib1[40] | 62%-83% | 9.7-13.1 mo | 19.3-26.3 mo |
| Gefitinib1[4] | 73.7% | 9.5 mo | 22 mo | |
| Afatinib1[5] | 56%-67% | 11.1 mo | 25.8 mo | |
| Dacomitinib1[127] | 74.9% | 14.7 mo | 34 mo | |
| Osimertinib1[128] | 80% | 18.9 mo | 38.6 mo | |
| ALK | Crizotinib1[53] | 75.5% | 11 mo | 57 mo |
| Ceritinib1[129] | 72.5% | 16.6 mo | NA | |
| Brigatinib0[130] | 79% | 24 mo | NA | |
| Alectinib1[131] | 82.9% | 35 mo | NA | |
| Lorlatinib1[54] | 76% | NA | NA | |
| ROS1 | Crizotinib1[132] | 63-72% | 15.9-19.2 mo | 51 mo |
| Lorlatinib1[133] | 62% | 19.3 mo | NA | |
| Entrectinib1[134] | 77% | 19 mo | NA | |
| BRAF | Dabrafenib-trametinib1[135] | 64% | 10.9 mo | 24.6 mo |
| MET | Crizotinib[50] | 32% | 7.3 mo | NA |
| Cabozantinib[136] | NA | NA | NA | |
| Capmatinib1[137] | 68% | 9.7 mo | NA | |
| Tepotinib[138] | 46% | NA | NA | |
| NTRK | Entrectinib1[139] | 70% | NA | NA |
| Larotrectinib1[140] | 75% | NA | NA | |
| RET | Selpercatinib1[141] | 85% | NA | NA |
| Pralsetinib1[121] | 70% | NA | NA | |
| KRAS | Sotorasib[142] | 32.2% | 10.2 mo | NA |
| Adagrasib[143] | 45% | NA | NA | |
| HER2 | Trastuzumab-deruxtecan[144] | 62% | 14 mo | NA |
| NRG1 | Afatinib[112] | NA | NA | NA |
Food and Drug Administration approved. ORR: Objective response rate; PFS: Progression-free survival; OS: Overall survival; EGFR: Epidermal growth factor receptor; ALK: Anaplastic lymphoma kinase; ROS1: c-ROS oncogene 1; NTRK: Neurotrophic receptor tyrosine kinase; RET: Rearranged during transfection; KRAS: Kirsten rat sarcoma; HER2: Human epidermal growth factor 2; NRG1: Neuregulin-1; BRAF: V-raf murine sarcoma viral oncogene homolog B. NA: Not available.
Given the portfolio of possible mutations and targeted therapies to offer, multiplex NGS testing should be standard practice. Barring a therapeutic emergency, no patient should be started on systemic therapy before a comprehensive molecular analysis has been completed. In the twentieth century, every gene matters, and it would be unethical to deny patients access to proven targeted therapies given the efficacy and favourable toxicity profile of such drugs.
Footnotes
Conflict-of-interest statement: Mathieu Chevallier and Maxime Borgeaud declare no potential conflicts of interest; Alfredo Addeo has received compensation from Bristol-Myers Squibb, AstraZeneca, Merck Sharpe & Dohme, Takeda, Pfizer, Roche and Boehringer Ingelheim for participating on advisory boards; Alex Friedlaender has received compensation from Roche, Pfizer, Merck Sharpe & Dohme, and Bristol-Myers Squibb for participating in advisory boards.
Manuscript source: Invited manuscript
Peer-review started: January 27, 2021
First decision: March 1, 2021
Article in press: April 7, 2021
Specialty type: Oncology
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen X S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
Contributor Information
Mathieu Chevallier, Department of Oncology, University Hospital Geneva, Geneva 1205, Switzerland.
Maxime Borgeaud, Department of Oncology, University Hospital Geneva, Geneva 1205, Switzerland.
Alfredo Addeo, Department of Oncology, University Hospital Geneva, Geneva 1205, Switzerland.
Alex Friedlaender, Department of Oncology, University Hospital Geneva, Geneva 1205, Switzerland; Department of Oncology, Clinique Générale Beaulieu, Geneva 1206, Switzerland. alex.friedlaender@hcuge.ch.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Birring SS, Peake MD. Symptoms and the early diagnosis of lung cancer. Thorax. 2005;60:268–269. doi: 10.1136/thx.2004.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS, Squire J, Thunnissen E, Ladanyi M. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 6.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedlaender A, Banna G, Patel S, Addeo A. Diagnosis and Treatment of ALK Aberrations in Metastatic NSCLC. Curr Treat Options Oncol. 2019;20:79. doi: 10.1007/s11864-019-0675-9. [DOI] [PubMed] [Google Scholar]
- 8.Addeo A, Tabbò F, Robinson T, Buffoni L, Novello S. Precision medicine in ALK rearranged NSCLC: A rapidly evolving scenario. Crit Rev Oncol Hematol. 2018;122:150–156. doi: 10.1016/j.critrevonc.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Addeo A, Metro G. First-line alectinib for ALK-positive lung cancer: is there room for further improvement? Drugs Context. 2018;7:212537. doi: 10.7573/dic.212537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisone D, Friedlaender A, Malapelle U, Banna G, Addeo A. A BRAF new world. Crit Rev Oncol Hematol. 2020;152:103008. doi: 10.1016/j.critrevonc.2020.103008. [DOI] [PubMed] [Google Scholar]
- 11.Friedlaender A, Drilon A, Weiss GJ, Banna GL, Addeo A. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer Treat Rev. 2020;85:101978. doi: 10.1016/j.ctrv.2020.101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlaender A, Banna GL, Buffoni L, Addeo A. Poor-Performance Status Assessment of Patients with Non-small Cell Lung Cancer Remains Vague and Blurred in the Immunotherapy Era. Curr Oncol Rep. 2019;21:107. doi: 10.1007/s11912-019-0852-9. [DOI] [PubMed] [Google Scholar]
- 13.Friedlaender A, Banna G, Malapelle U, Pisapia P, Addeo A. Next Generation Sequencing and Genetic Alterations in Squamous Cell Lung Carcinoma: Where Are We Today? Front Oncol. 2019;9:166. doi: 10.3389/fonc.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, Lew M, Pantelas J, Ramalingam SS, Reck M, Saqi A, Simoff M, Singh N, Sundaram B. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36:911–919. doi: 10.1200/JCO.2017.76.7293. [DOI] [PubMed] [Google Scholar]
- 15.Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIH Study to Compare AMG 510 "Proposed INN Sotorasib" With Docetaxel in Non Small Cell Lung Cancer (NSCLC) (CodeBreak 200). 2021. [cited 10 January 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04303780 .
- 18.Jnne PA, Rybkin II, Spira AI, Riely GJ, Ou S. KRYSTAL-1: activity and safety of adagrasib (mrtx849) in advanced/metastatic non–small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Cancer . 2020;138:S1–S2. [Google Scholar]
- 19.NIH First-in-Human Study of JNJ-74699157 in Participants With Tumors Harboring the KRAS G12C Mutation. [cited 8 January 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04006301?cond=KRAS+G12C&draw=1&rank=1 .
- 20.NIH A Study to Evaluate the Safety, Pharmacokinetics, and Activity of GDC-6036 Alone or in Combination in Participants With Advanced or Metastatic Solid Tumors With a KRAS G12C Mutation. [cited 10 January 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04449874?cond=KRAS+G12C&draw=1&rank=3 .
- 21.Jiao D, Yang S. Overcoming Resistance to Drugs Targeting KRASG12C Mutation. Innovation (N Y) 2020;1 doi: 10.1016/j.xinn.2020.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindoli A, Rigobello MP, Scutari G, Gabbiani C, Casini A, Messori L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord Chem Reviews. 2009;253:1692–1707. [Google Scholar]
- 23.Misale S, Fatherree JP, Cortez E, Li C, Bilton S, Timonina D, Myers DT, Lee D, Gomez-Caraballo M, Greenberg M, Nangia V, Greninger P, Egan RK, McClanaghan J, Stein GT, Murchie E, Zarrinkar PP, Janes MR, Li LS, Liu Y, Hata AN, Benes CH. KRAS G12C NSCLC Models Are Sensitive to Direct Targeting of KRAS in Combination with PI3K Inhibition. Clin Cancer Res. 2019;25:796–807. doi: 10.1158/1078-0432.CCR-18-0368. [DOI] [PubMed] [Google Scholar]
- 24.Kordiak J, Szemraj J, Grabska-Kobylecka I, Bialasiewicz P, Braun M, Kordek R, Nowak D. Intratumor heterogeneity and tissue distribution of KRAS mutation in non-small cell lung cancer: implications for detection of mutated KRAS oncogene in exhaled breath condensate. J Cancer Res Clin Oncol. 2019;145:241–251. doi: 10.1007/s00432-018-2779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue JY, Zhao Y, Aronowitz J, Mai TT, Vides A, Qeriqi B, Kim D, Li C, de Stanchina E, Mazutis L, Risso D, Lito P. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577:421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty A. KRASG12C inhibitor: combing for combination. Biochem Soc Trans. 2020;48:2691–2701. doi: 10.1042/BST20200473. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Lu H, Wang H, Loo A, Zhang X, Yang G, Kowal C, Delach S, Wang Y, Goldoni S, Hastings WD, Wong K, Gao H, Meyer MJ, Moody SE, LaMarche MJ, Engelman JA, Williams JA, Hammerman PS, Abrams TJ, Mohseni M, Caponigro G, Hao HX. Combinations with Allosteric SHP2 Inhibitor TNO155 to Block Receptor Tyrosine Kinase Signaling. Clin Cancer Res. 2021;27:342–354. doi: 10.1158/1078-0432.CCR-20-2718. [DOI] [PubMed] [Google Scholar]
- 28.Cullis J, Das S, Bar-Sagi D. Kras and Tumor Immunity: Friend or Foe? Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a031849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, Lanman BA, Werner J, Rapaport AS, San Miguel T, Ortiz R, Osgood T, Sun JR, Zhu X, McCarter JD, Volak LP, Houk BE, Fakih MG, O'Neil BH, Price TJ, Falchook GS, Desai J, Kuo J, Govindan R, Hong DS, Ouyang W, Henary H, Arvedson T, Cee VJ, Lipford JR. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 30.MRRATI Therapeutics-Reports-Investigational-Adagrasib-MRTX849-Preliminary-Data-Demonstrating-Tolerability-and-Durable-Anti-Tumor-Activity-as-well-as-Initial-MRTX1133-Preclinical-Data/default.aspx. [cited 8 January 2021]. Available from: https://ir.mirati.com/news-releases/news-details/2020/Mirati .
- 31.NIH A Study to Test Different Doses of BI 1701963 Alone and Combined With Trametinib in Patients With Different Types of Advanced Cancer (Solid Tumours With KRAS Mutation). 2021. [cited 10 December 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04111458 .
- 32.NIH A Study of mRNA-5671/V941 as Monotherapy and in Combination With Pembrolizumab (V941-001). [cited 10 December 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT03948763 .
- 33.KRAS vaccine (mRNA-5671) Moderna’s mRNA vaccines elicit Tcells required for curative cancer therapy. [cited 6 January 2021]. Available from: https://investors.modernatx.com/static-files/12f15d58-0a61-4ca4-a3e1-61d06ab28e0c .
- 34.Mass RD. The HER receptor family: a rich target for therapeutic development. Int J Radiat Oncol Biol Phys. 2004;58:932–940. doi: 10.1016/j.ijrobp.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 35.Cooper WA, Lam DC, O'Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis. 2013;5 Suppl 5:S479–S490. doi: 10.3978/j.issn.2072-1439.2013.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 37.Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, Park K, Soo RA, Chang JWC, Alip A, Peters S, Douillard JY. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 39.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, Wei Y, Chou CK, Wang SC, Yan M, Tu CY, Hsia TC, Chiang SF, Chao KSC, Wistuba II, Hsu JL, Hortobagyi GN, Hung MC. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019; 36: 168-178. :e4. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R, Zhou C, Nakagawa K, Thongprasert S, Fukuoka M, Lord S, Marschner I, Tu YK, Gralla RJ, Gebski V, Mok T, Yang JC. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw279. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Cao R, Zhang X, Huang L, Sun L, Zhao J, Ma J, Han C. Recent Progress in Rare Oncogenic Drivers and Targeted Therapy For Non-Small Cell Lung Cancer. Onco Targets Ther. 2019;12:10343–10360. doi: 10.2147/OTT.S230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. 2018;15:694–708. doi: 10.1038/s41571-018-0081-4. [DOI] [PubMed] [Google Scholar]
- 43.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 44.Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, Crino L, Satouchi M, Chu Q, Hida T, Han JY, Juan O, Dunphy F, Nishio M, Kang JH, Majem M, Mann H, Cantarini M, Ghiorghiu S, Mitsudomi T. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 45.Skrzypski M, Szymanowska-Narloch A, Dziadziuszko R. Osimertinib - effective treatment of NSCLC with activating EGFR mutations after progression on EGFR tyrosine kinase inhibitors. Contemp Oncol (Pozn) 2017;21:254–258. doi: 10.5114/wo.2017.70116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, Shah R, Cobo M, Lee KH, Cheema P, Tiseo M, John T, Lin MC, Imamura F, Kurata T, Todd A, Hodge R, Saggese M, Rukazenkov Y, Soria JC FLAURA Investigators. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Yang Z, Wang Z. Are VEGFR-TKIs effective or safe for patients with advanced non-small cell lung cancer? Oncotarget. 2015;6:18206–18223. doi: 10.18632/oncotarget.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drilon A, Cappuzzo F, Ou SI, Camidge DR. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J Thorac Oncol. 2017;12:15–26. doi: 10.1016/j.jtho.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, Van den Heuvel M, Neal J, Peled N, Früh M, Ng TL, Gounant V, Popat S, Diebold J, Sabari J, Zhu VW, Rothschild SI, Bironzo P, Martinez-Marti A, Curioni-Fontecedro A, Rosell R, Lattuca-Truc M, Wiesweg M, Besse B, Solomon B, Barlesi F, Schouten RD, Wakelee H, Camidge DR, Zalcman G, Novello S, Ou SI, Milia J, Gautschi O. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camidge DR, Ou S-HI, Shapiro G. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC) ASCO . 2014 [Google Scholar]
- 51.Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Van Meerbeeck J, Raskin J, Reinmuth N, Conte P, Kowalski D, Cho BC, Patel JD, Horn L, Griesinger F, Han JY, Kim YC, Chang GC, Tsai CL, Yang JC, Chen YM, Smit EF, van der Wekken AJ, Kato T, Juraeva D, Stroh C, Bruns R, Straub J, Johne A, Scheele J, Heymach JV, Le X. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan J, Umapathy G, Yamazaki Y, Wolfstetter G, Mendoza P, Pfeifer K, Mohammed A, Hugosson F, Zhang H, Hsu AW, Halenbeck R, Hallberg B, Palmer RH. FAM150A and FAM150B are activating ligands for anaplastic lymphoma kinase. Elife. 2015;4:e09811. doi: 10.7554/eLife.09811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Tang Y, Wilner KD, Blackhall F, Mok TS. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:2251–2258. doi: 10.1200/JCO.2017.77.4794. [DOI] [PubMed] [Google Scholar]
- 54.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, Thurm H, Calella AM, Peltz G, Solomon BJ CROWN Trial Investigators. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 55.Lin JJ, Schoenfeld AJ, Zhu VW, Yeap BY, Chin E, Rooney M, Plodkowski AJ, Digumarthy SR, Dagogo-Jack I, Gainor JF, Ou SI, Riely GJ, Shaw AT. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J Thorac Oncol. 2020;15:258–265. doi: 10.1016/j.jtho.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 57.Drilon A, Jenkins C, Iyer S, Schoenfeld A, Keddy C, Davare MA. ROS1-dependent cancers - biology, diagnostics and therapeutics. Nat Rev Clin Oncol. 2021;18:35–55. doi: 10.1038/s41571-020-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ou SH, Zhu VW. Catalog of 5′ fusion partners in RET+ NSCLC circa 2020. JTO Clin Res Rep . 2020;1:100037. doi: 10.1016/j.jtocrr.2020.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsao MS, Hirsch FR, Yatabe Y. IASLC atlas of ALK and ROS1 testing in lung cancer. International Association for the Study of Lung Cancer; 2016. [cited 10 January 2021]. Available from: https://www.iaslc.org/meetings-webinars/iaslc-atlas-alk-and-ros1-testing-lung-cancer .
- 60.Lin JJ, Ritterhouse LL, Ali SM, Bailey M, Schrock AB, Gainor JF, Ferris LA, Mino-Kenudson M, Miller VA, Iafrate AJ, Lennerz JK, Shaw AT. ROS1 Fusions Rarely Overlap with Other Oncogenic Drivers in Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:872–877. doi: 10.1016/j.jtho.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Q, Ouyang D, Anwar M, Xie N, Wang S, Fan P, Qian L, Chen G, Zhou E, Guo L, Gu X, Ding B, Yang X, Liu L, Deng C, Xiao Z, Li J, Wang Y, Zeng S, Hu J, Zhou W, Qiu B, Wang Z, Weng J, Liu M, Li Y, Tang T, Wang J, Zhang H, Dai B, Tang W, Wu T, Xiao M, Li X, Liu H, Li L, Yi W, Ouyang Q. Effectiveness and Safety of Pyrotinib, and Association of Biomarker With Progression-Free Survival in Patients With HER2-Positive Metastatic Breast Cancer: A Real-World, Multicentre Analysis. Front Oncol. 2020;10:811. doi: 10.3389/fonc.2020.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang D, Huemer F, Rinnerthaler G, Horner A, Wass R, Brehm E, Akbari K, Granitz M, Hutarew G, Kaiser B, Greil R, Lamprecht B. Therapy Line and Associated Predictors of Response to PD-1/PD-L1-Inhibitor Monotherapy in Advanced Non-small-Cell Lung Cancer: A Retrospective Bi-centric Cohort Study. Target Oncol. 2019;14:707–717. doi: 10.1007/s11523-019-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, Zheng Z, Tan W, Stephenson P, Shreeve SM, Tye LM, Christensen JG, Wilner KD, Clark JW, Iafrate AJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, Varella-Garcia M, Iafrate AJ, Shapiro GI, Usari T, Wang SC, Wilner KD, Clark JW, Ou SI. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30:1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moro-Sibilot D, Faivre L, Zalcman G, Pérol M, Barlesi F, Otto J, Monnet I, Cortot AB, Wislez M, Lena H: Crizotinib in patients with advanced ROS1-rearranged non-small cell lung cancer (NSCLC) Preliminary results of the ACSé phase II trial. J Clin Oncol . 2015;33:8065. [Google Scholar]
- 66.Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, Yamanaka T, Kemner A, Roychowdhury D, Paolini J, Usari T, Wilner KD, Goto K. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:1405–1411. doi: 10.1200/JCO.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 67.Patil T, Smith DE, Bunn PA, Aisner DL, Le AT, Hancock M, Purcell WT, Bowles DW, Camidge DR, Doebele RC. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J Thorac Oncol. 2018;13:1717–1726. doi: 10.1016/j.jtho.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaw AT, Solomon BJ, Chiari R, Riely GJ, Besse B, Soo RA, Kao S, Lin CC, Bauer TM, Clancy JS, Thurm H, Martini JF, Peltz G, Abbattista A, Li S, Ou SI. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 69.Lim SM, Kim HR, Lee JS, Lee KH, Lee YG, Min YJ, Cho EK, Lee SS, Kim BS, Choi MY, Shim HS, Chung JH, La Choi Y, Lee MJ, Kim M, Kim JH, Ali SM, Ahn MJ, Cho BC. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol. 2017;35:2613–2618. doi: 10.1200/JCO.2016.71.3701. [DOI] [PubMed] [Google Scholar]
- 70.Drilon A, Siena S, Dziadziuszko R, Barlesi F, Krebs MG, Shaw AT, de Braud F, Rolfo C, Ahn MJ, Wolf J, Seto T, Cho BC, Patel MR, Chiu CH, John T, Goto K, Karapetis CS, Arkenau HT, Kim SW, Ohe Y, Li YC, Chae YK, Chung CH, Otterson GA, Murakami H, Lin CC, Tan DSW, Prenen H, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Doebele RC trial investigators. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yun MR, Kim DH, Kim SY, Joo HS, Lee YW, Choi HM, Park CW, Heo SG, Kang HN, Lee SS, Schoenfeld AJ, Drilon A, Kang SG, Shim HS, Hong MH, Cui JJ, Kim HR, Cho BC. Repotrectinib Exhibits Potent Antitumor Activity in Treatment-Naïve and Solvent-Front-Mutant ROS1-Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res. 2020;26:3287–3295. doi: 10.1158/1078-0432.CCR-19-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho BC, Drilon AE, Doebele RC, Kim D-W, Lin JJ, Lee J, Ahn M-J, Zhu VW, Ejadi S, Camidge DR. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study) J Clin Oncol. 2019;37:9011. [Google Scholar]
- 73.Watanabe J, Furuya N, Fujiwara Y. Appearance of a BRAF Mutation Conferring Resistance to Crizotinib in Non-Small Cell Lung Cancer Harboring Oncogenic ROS1 Fusion. J Thorac Oncol. 2018;13:e66–e69. doi: 10.1016/j.jtho.2017.11.125. [DOI] [PubMed] [Google Scholar]
- 74.Lin JJ, Johnson T, Lennerz JK, Lee C, Hubbeling HG, Yeap BY, Dagogo-Jack I, Gainor JF, Shaw AT. Resistance to lorlatinib in ROS1 fusion-positive non-small cell lung cancer. ASCO. 2020 [Google Scholar]
- 75.Leonetti A, Facchinetti F, Rossi G, Minari R, Conti A, Friboulet L, Tiseo M, Planchard D. BRAF in non-small cell lung cancer (NSCLC): Pickaxing another brick in the wall. Cancer Treat Rev. 2018;66:82–94. doi: 10.1016/j.ctrv.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Palanisamy N, Ateeq B, Kalyana-Sundaram S, Pflueger D, Ramnarayanan K, Shankar S, Han B, Cao Q, Cao X, Suleman K, Kumar-Sinha C, Dhanasekaran SM, Chen YB, Esgueva R, Banerjee S, LaFargue CJ, Siddiqui J, Demichelis F, Moeller P, Bismar TA, Kuefer R, Fullen DR, Johnson TM, Greenson JK, Giordano TJ, Tan P, Tomlins SA, Varambally S, Rubin MA, Maher CA, Chinnaiyan AM. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, Giannone V, D'Amelio AM Jr, Zhang P, Mookerjee B, Johnson BE. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 78.Casadei Gardini A, Chiadini E, Faloppi L, Marisi G, Delmonte A, Scartozzi M, Loretelli C, Lucchesi A, Oboldi D, Dubini A, Frassineti GL, Ulivi P. Efficacy of sorafenib in BRAF-mutated non-small-cell lung cancer (NSCLC) and no response in synchronous BRAF wild type-hepatocellular carcinoma: a case report. BMC Cancer. 2016;16:429. doi: 10.1186/s12885-016-2463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sereno M, Moreno V, Moreno Rubio J, Gómez-Raposo C, García Sánchez S, Hernández Jusdado R, Falagan S, Zambrana Tébar F, Casado Sáenz E. A significant response to sorafenib in a woman with advanced lung adenocarcinoma and a BRAF non-V600 mutation. Anticancer Drugs. 2015;26:1004–1007. doi: 10.1097/CAD.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 80.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 81.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchiò C, Scaltriti M, Ladanyi M, Iafrate AJ, Bibeau F, Dietel M, Hechtman JF, Troiani T, López-Rios F, Douillard JY, Andrè F, Reis-Filho JS. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol. 2019;30:1417–1427. doi: 10.1093/annonc/mdz204. [DOI] [PubMed] [Google Scholar]
- 84.Tognon C, Garnett M, Kenward E, Kay R, Morrison K, Sorensen PH. The chimeric protein tyrosine kinase ETV6-NTRK3 requires both Ras-Erk1/2 and PI3-kinase-Akt signaling for fibroblast transformation. Cancer Res. 2001;61:8909–8916. [PubMed] [Google Scholar]
- 85.Haratake N, Seto T. NTRK Fusion-positive Non-small-cell Lung Cancer: The Diagnosis and Targeted Therapy. Clin Lung Cancer. 2021;22:1–5. doi: 10.1016/j.cllc.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32:147–153. doi: 10.1038/s41379-018-0118-3. [DOI] [PubMed] [Google Scholar]
- 87.Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siena S, Doebele RC, Shaw AT, Karapetis CS, Tan DS-W, Cho BC, Kim D-W, Ahn M-J, Krebs M, Goto K. Efficacy of entrectinib in patients (pts) with solid tumors and central nervous system (CNS) metastases: Integrated analysis from three clinical trials. ASCO . 2019 [Google Scholar]
- 89.FDA FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. [cited 26 December 2020]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc .
- 90.FDA FDA approves larotrectinib for solid tumors with NTRK gene fusions. [cited 26 December 2020]. Available from: https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions .
- 91.Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, Smith S, Lauriault V, Kolakowski GR, Brandhuber BJ, Larsen PD, Bouhana KS, Winski SL, Hamor R, Wu WI, Parker A, Morales TH, Sullivan FX, DeWolf WE, Wollenberg LA, Gordon PR, Douglas-Lindsay DN, Scaltriti M, Benayed R, Raj S, Hanusch B, Schram AM, Jonsson P, Berger MF, Hechtman JF, Taylor BS, Andrews S, Rothenberg SM, Hyman DM. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017;7:963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hyman D, Kummar S, Farago A, Geoerger B, Mau-Sorensen M, Taylor M, Garralda E, Nagasubramanian R, Natheson M, Song L. Abstract CT127, Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) AACR. 2019 [Google Scholar]
- 93.Drilon A, Zhai D, Deng W, Zhang X, Lee D, Rogers E, Whitten J, Huang Z, Graber A, Liu J. Repotrectinib, a next generation TRK inhibitor, overcomes TRK resistance mutations including solvent front, gatekeeper and compound mutations. In AACR Annual Meeting. 2019. [cited 26 December 2020]. Available from: https://www.researchgate.net/publication/335047459_Abstract_442_Repotrectinib_a_next_generation_TRK_inhibitor_overcomes_TRK_resistance_mutations_including_solvent_front_gatekeeper_and_compound_mutations .
- 94.Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30:viii23–viii30. doi: 10.1093/annonc/mdz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.NIH A Study of Repotrectinib (TPX-0005) in Patients With Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1). [cited 26 December 2020]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03093116?cond=repotrectinib&draw=2&rank=2 .
- 96.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bazley L, Gullick WJ. The epidermal growth factor receptor family. Endocr Relat Cancer . 2005;12:S17–S27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- 99.Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban T, Moro-Sibilot D, Dansin E, Chouaid C, Wislez M, Diebold J, Felip E, Rouquette I, Milia JD, Gautschi O. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 100.Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, Barton C, Ghahramani P, Hirsh V. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15:19–27. doi: 10.1093/annonc/mdh031. [DOI] [PubMed] [Google Scholar]
- 101.Mazières J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, Peters S, Dansin E, Früh M, Pless M, Rosell R, Wislez M, Fournel P, Westeel V, Cappuzzo F, Cortot A, Moro-Sibilot D, Milia J, Gautschi O. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27:281–286. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 102.Drope J, Liber AC, Cahn Z, Stoklosa M, Kennedy R, Douglas CE, Henson R, Drope J. Who's still smoking? CA Cancer J Clin. 2018;68:106–115. doi: 10.3322/caac.21444. [DOI] [PubMed] [Google Scholar]
- 103.Smit EF, Nakagawa K, Nagasaka M, Felip E, Goto Y, Li BT, Pacheco JM, Murakami H, Barlesi F, Saltos AN. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. ASCO. 2020 [Google Scholar]
- 104.NIH DS-8201a in Human Epidermal Growth Factor Receptor 2 (HER2)-Expressing or -Mutated Non-Small Cell Lung Cancer (DESTINY-Lung01). [cited 10 January 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03505710 .
- 105.NIH Trastuzumab Deruxtecan in Participants With HER2-mutated Metastatic Non-small Cell Lung Cancer (NSCLC) (DESTINY-LUNG02). [cited 10 January 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04644237 .
- 106.Muscarella LA, Rossi A. NRG1: a cinderella fusion in lung cancer? Lung Cancer Manag. 2017;6:121–123. doi: 10.2217/lmt-2017-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin DH, Jo JY, Han JY. Dual Targeting of ERBB2/ERBB3 for the Treatment of SLC3A2-NRG1-Mediated Lung Cancer. Mol Cancer Ther. 2018;17:2024–2033. doi: 10.1158/1535-7163.MCT-17-1178. [DOI] [PubMed] [Google Scholar]
- 108.Jonna S, Feldman RA, Swensen J, Gatalica Z, Korn WM, Borghaei H, Ma PC, Nieva JJ, Spira AI, Vanderwalde AM, Wozniak AJ, Kim ES, Liu SV. Detection of NRG1 Gene Fusions in Solid Tumors. Clin Cancer Res. 2019;25:4966–4972. doi: 10.1158/1078-0432.CCR-19-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fernandez-Cuesta L, Plenker D, Osada H, Sun R, Menon R, Leenders F, Ortiz-Cuaran S, Peifer M, Bos M, Daßler J, Malchers F, Schöttle J, Vogel W, Dahmen I, Koker M, Ullrich RT, Wright GM, Russell PA, Wainer Z, Solomon B, Brambilla E, Nagy-Mignotte H, Moro-Sibilot D, Brambilla CG, Lantuejoul S, Altmüller J, Becker C, Nürnberg P, Heuckmann JM, Stoelben E, Petersen I, Clement JH, Sänger J, Muscarella LA, la Torre A, Fazio VM, Lahortiga I, Perera T, Ogata S, Parade M, Brehmer D, Vingron M, Heukamp LC, Buettner R, Zander T, Wolf J, Perner S, Ansén S, Haas SA, Yatabe Y, Thomas RK. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014;4:415–422. doi: 10.1158/2159-8290.CD-13-0633. [DOI] [PubMed] [Google Scholar]
- 110.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 111.Shin DH, Lee D, Hong DW, Hong SH, Hwang JA, Lee BI, You HJ, Lee GK, Kim IH, Lee YS, Han JY. Oncogenic function and clinical implications of SLC3A2-NRG1 fusion in invasive mucinous adenocarcinoma of the lung. Oncotarget. 2016;7:69450–69465. doi: 10.18632/oncotarget.11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cheema PK, Doherty M, Tsao MS. A Case of Invasive Mucinous Pulmonary Adenocarcinoma with a CD74-NRG1 Fusion Protein Targeted with Afatinib. J Thorac Oncol. 2017;12:e200–e202. doi: 10.1016/j.jtho.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 113.Ferrara R, Auger N, Auclin E, Besse B. Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer. J Thorac Oncol. 2018;13:27–45. doi: 10.1016/j.jtho.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 114.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 115.Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kääriäinen H. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 116.Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 117.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, Lim Choi Y, Satoh Y, Okumura S, Nakagawa K, Mano H, Ishikawa Y. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 118.Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, Peled N, Weiss J, Kim YJ, Ohe Y, Nishio M, Park K, Patel J, Seto T, Sakamoto T, Rosen E, Shah MH, Barlesi F, Cassier PA, Bazhenova L, De Braud F, Garralda E, Velcheti V, Satouchi M, Ohashi K, Pennell NA, Reckamp KL, Dy GK, Wolf J, Solomon B, Falchook G, Ebata K, Nguyen M, Nair B, Zhu EY, Yang L, Huang X, Olek E, Rothenberg SM, Goto K, Subbiah V. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.FDA FDA approves selpercatinib for lung and thyroid cancers with RET gene mutations or fusions. [cited 10 January 2021]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-selpercatinib-lung-and-thyroid-cancers-ret-gene-mutations-or-fusions . [DOI] [PubMed]
- 120.Solomon BJ, Zhou CC, Drilon A, Park K, Wolf J, Elamin Y, Davis HM, Soldatenkova V, Sashegyi A, Lin AB, Lin BK, F Loong HH, Novello S, Arriola E, Pérol M, Goto K, Santini FC. Phase III study of selpercatinib versus chemotherapy ± pembrolizumab in untreated RET positive non-small-cell lung cancer. Future Oncol. 2021;17:763–773. doi: 10.2217/fon-2020-0935. [DOI] [PubMed] [Google Scholar]
- 121.Gainor JF, Curigliano G, Kim D-W, Lee DH, Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DS-W. Registrational dataset from the phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET fusion+ non-small cell lung cancer (NSCLC) ASCO. 2020 [Google Scholar]
- 122.NIH AcceleRET Lung Study of Pralsetinib for 1L RET Fusion-positive, Metastatic NSCLC. [cited 10 January 2021]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04222972?cond=RET+fusion+NSCLC&draw=2&rank=4 .
- 123.Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, Van Voorthuysen M, Somwar R, Smith RS, Montecalvo J, Plodkowski A, Ginsberg MS, Riely GJ, Rudin CM, Ladanyi M, Kris MG. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17:1653–1660. doi: 10.1016/S1470-2045(16)30562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, Camidge R, Narayanan V, Doebele RC, Besse B, Remon-Masip J, Janne PA, Awad MM, Peled N, Byoung CC, Karp DD, Van Den Heuvel M, Wakelee HA, Neal JW, Mok TSK, Yang JCH, Ou SI, Pall G, Froesch P, Zalcman G, Gandara DR, Riess JW, Velcheti V, Zeidler K, Diebold J, Früh M, Michels S, Monnet I, Popat S, Rosell R, Karachaliou N, Rothschild SI, Shih JY, Warth A, Muley T, Cabillic F, Mazières J, Drilon A. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, Tuch BB, Yoda S, Gainor JF, Sequist LV, Oxnard GR, Gautschi O, Drilon A, Subbiah V, Khoo C, Zhu EY, Nguyen M, Henry D, Condroski KR, Kolakowski GR, Gomez E, Ballard J, Metcalf AT, Blake JF, Dawson SJ, Blosser W, Stancato LF, Brandhuber BJ, Andrews S, Robinson BG, Rothenberg SM. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J Thorac Oncol. 2020;15:541–549. doi: 10.1016/j.jtho.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin JJ, Liu SV, McCoach CE, Zhu VW, Tan AC, Yoda S, Peterson J, Do A, Prutisto-Chang K, Dagogo-Jack I, Sequist LV, Wirth LJ, Lennerz JK, Hata AN, Mino-Kenudson M, Nardi V, Ou SI, Tan DS, Gainor JF. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Ann Oncol. 2020;31:1725–1733. doi: 10.1016/j.annonc.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 128.Ramalingam SS, Gray JE, Ohe Y, Cho B, Planchard D. Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis. Ann Oncol. 2019;30:v914–v915. [Google Scholar]
- 129.Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, Geater SL, Orlov S, Cortinovis D, Yu CJ, Hochmair M, Cortot AB, Tsai CM, Moro-Sibilot D, Campelo RG, McCulloch T, Sen P, Dugan M, Pantano S, Branle F, Massacesi C, de Castro G Jr. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 130.Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee KH, Gridelli C, Delmonte A, Garcia Campelo R, Kim DW, Bearz A, Griesinger F, Morabito A, Felip E, Califano R, Ghosh S, Spira A, Gettinger SN, Tiseo M, Gupta N, Haney J, Kerstein D, Popat S. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 131.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 132.Moro-Sibilot D, Cozic N, Pérol M, Mazières J, Otto J, Souquet PJ, Bahleda R, Wislez M, Zalcman G, Guibert SD, Barlési F, Mennecier B, Monnet I, Sabatier R, Bota S, Dubos C, Verriele V, Haddad V, Ferretti G, Cortot A, De Fraipont F, Jimenez M, Hoog-Labouret N, Vassal G. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol. 2019;30:1985–1991. doi: 10.1093/annonc/mdz407. [DOI] [PubMed] [Google Scholar]
- 133.Duruisseaux M. Lorlatinib: a new treatment option for ROS1-positive lung cancer. Lancet Oncol. 2019;20:1622–1623. doi: 10.1016/S1470-2045(19)30716-8. [DOI] [PubMed] [Google Scholar]
- 134.Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, Chiari R, Bearz A, Lin CC, Gadgeel SM, Riely GJ, Tan EH, Seto T, James LP, Clancy JS, Abbattista A, Martini JF, Chen J, Peltz G, Thurm H, Ou SI, Shaw AT. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 135.Planchard D, Besse B, Groen HJ, Souquet P-J, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol . 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.D'Arcangelo M, Tassinari D, De Marinis F, Delmonte A, Cappuzzo F. P2.01-15 Phase II Single Arm Study of CABozantinib in Non-Small Cell Lung Cancer Patients with MET Deregulation (CABinMET) J Thorac Oncol. 2019;14:S644. [Google Scholar]
- 137.Wolf J, Overbeck TR, Han JY, Hochmair M, Heist RS. Capmatinib in patients with high-level MET-amplified advanced non–small cell lung cancer (NSCLC): results from the phase 2 GEOMETRY mono-1 study. J Clin Oncol. 2020;38 (15_suppl):9509–9509. [Google Scholar]
- 138.Shitara K, Yamazaki K, Tsushima T, Naito T, Matsubara N, Watanabe M, Sarholz B, Johne A, Doi T. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol. 2020;50:859–866. doi: 10.1093/jjco/hyaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, Nagasubramanian R, Davis JL, Rudzinski E, Feraco AM, Tuch BB, Ebata KT, Reynolds M, Smith S, Cruickshank S, Cox MC, Pappo AS, Hawkins DS. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19:705–714. doi: 10.1016/S1470-2045(18)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drilon A, Oxnard G, Wirth L, Besse B, Gautschi O, Tan S, Loong H, Bauer T, Kim Y, Horiike A. PL02. 08 registrational results of LIBRETTO-001: a phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol . 2019;14:S6–S7. [Google Scholar]
- 142.Kim DW, Lee DH, Han JY, Lee J, Cho BC, Kang JH, Lee KH, Cho EK, Kim JS, Min YJ, Cho JY, An HJ, Kim HG, Kim BS, Jang IJ, Yoon S, Han O, Noh YS, Hong KY, Park K. Safety, tolerability, and anti-tumor activity of olmutinib in non-small cell lung cancer with T790M mutation: A single arm, open label, phase 1/2 trial. Lung Cancer. 2019;135:66–72. doi: 10.1016/j.lungcan.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 143.Johnson ML, Ou S-HI, Barve M, Rybkin II, Papadopoulos KP, Leal TA, Velastegui K, Christensen JG, Kheoh T, Chao RC. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Eur J Cancer . 2020 [Google Scholar]
- 144.Smit EF, Nakagawa K, Nagasaka M, Felip E, Janne PA. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J Clin Oncol. 2020;38:9504–9504. [Google Scholar]