Abstract
Background
Therapies for metabolic diseases are numerous, yet improving insulin sensitivity beyond that induced by weight loss remains challenging. Therefore, search continues for novel treatment candidates that can stimulate insulin sensitivity and increase weight loss efficacy in combination with current treatment options. Calcitonin gene-related peptide (CGRP) and amylin belong to the same peptide family and have been explored as treatments for metabolic diseases. However, their full potential remains controversial.
Scope of review
In this article, we introduce this rather complex peptide family and its corresponding receptors. We discuss the physiology of the peptides with a focus on metabolism and insulin sensitivity. We also thoroughly review the pharmacological potential of amylin, calcitonin, CGRP, and peptide derivatives as treatments for metabolic diseases, emphasizing their ability to increase insulin sensitivity based on preclinical and clinical studies.
Major conclusions
Amylin receptor agonists and dual amylin and calcitonin receptor agonists are relevant treatment candidates, especially because they increase insulin sensitivity while also assisting weight loss, and their unique mode of action complements incretin-based therapies. However, CGRP and its derivatives seem to have only modest if any metabolic effects and are no longer of interest as therapies for metabolic diseases.
Keywords: Amylin, CGRP, DACRA, Insulin sensitivity, Diabetes, Obesity, Treatments, Preclinical, Pharmacology, Receptors
Highlights
-
•
Amylin and CGRP are peptide hormones of the calcitonin family that regulate of appetite and energy expenditure.
-
•
Pramlintide, an analogue of amylin, was successfully developed for diabetes; however, its use is limited by low potency.
-
•
CGRP-R agonists have failed to show preclinical efficacy in metabolic conditions and appear to be abandoned.
-
•
DACRAs are Dual Amylin and Calcitonin Receptor Agonists.
-
•
DACRAs have the ability to induce insulin sensitivity and weight loss beyond what is obtained with amylin preclinically.
-
•
DACRAs and other long-acting amylin analogues are complementary to incretin-based therapy.
1. Introduction
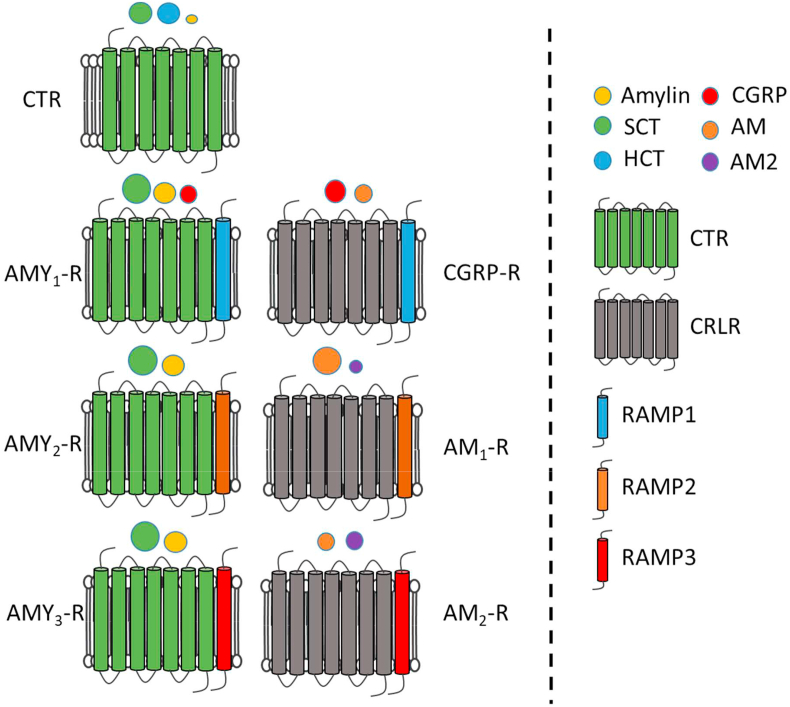
Amylin, calcitonin, and CGRP are members of the calcitonin family of peptides and receptors. This family is unique and has complex pharmacology and diverse physiological roles, so their treatment potential covers a relatively broad range of diseases [1,2]. Although the peptide family is quite well understood, several questions remain unanswered. The family consists of calcitonin, amylin, adrenomedullin, intermedin (adrenomedullin 2), α- and βCGRP, and the calcitonin receptor-stimulating peptides (1–3). The receptors of the family consist of one of the two 7TM GPCR domains, called calcitonin receptors (CTRs) and calcitonin receptor-like receptors (CRLRs); however, only the CTRs are active as stand-alone receptors. The 7TM GPCR domains can interact with one of three different receptor activity-modifying proteins (RAMPs), resulting in a total of 7 different functional receptors as well as the non-functional CRLRs (see Section 2.2) [[1], [2], [3]].
The complexity of the receptor-ligand system in this family has complicated studies of the physiological role of these receptors and ligands, although many reports have shed some light on their physiology and identified several relevant pharmacological targets.
This review provides a thorough description of the metabolic roles and therapeutic applications of amylin and CGRP, underscoring the unique potential of these peptide hormones.
2. The peptide family
2.1. Ligands
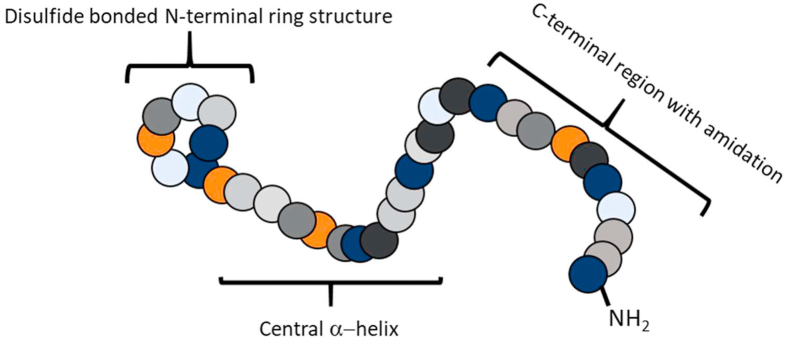
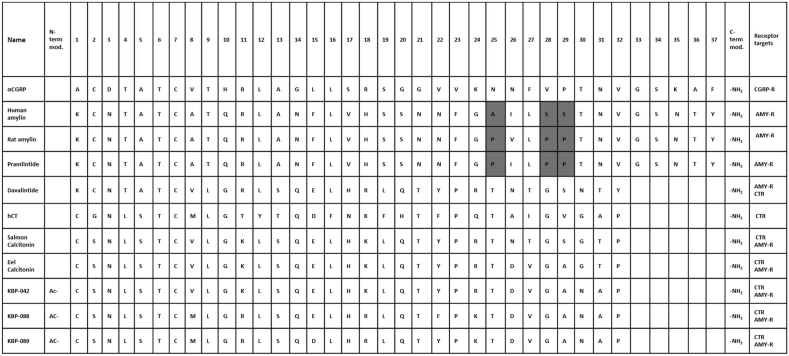
Amylin or islet amyloid polypeptide (IAPP) is a 37-amino acid peptide discovered in 1987 as an amyloid fibril protein of the β cells of the pancreas. Amylin has later shown to be co-secreted with insulin and have anorexigenic effects [4,5]. Amylin is produced from an 89-amino acid precursor that is processed into mature amylin through the removal of N- and C-terminal pro-peptides. Mature amylin is a single peptide chain with an Mw of approximately 4 kDa, an N-terminal disulfide bridge linking cysteines 2 and 7, and C-terminal amidation, which are common features of all members of the family (Figure 1) [2]. Mature human amylin is known to aggregate and fibrillate, an effect associated with the death of pancreatic islets, limiting the pharmacological utility of human amylin [6]. Interestingly, rat amylin does not aggregate due to alterations in key amino acids (positions 25, 28, and 29 substituted to prolines; see Figure 2). Hence, amylin has been extensively studied from a pharmacological perspective that led to the development of pramlintide, a non-fibrillogenic form of human amylin based on rat amylin approved to treat diabetes [6].
Figure 1.
Schematic structure of the peptide ligands of the calcitonin receptor family. Colored circles indicate amino acids, but not a specific type of amino acid.
Figure 2.
Names, sequences of the peptides described in this article, and their target receptors. Gray highlights underscore the amino acid changes in pramlintide inspired by rat amylin.
αCGRP, a 37 amino acid peptide, was discovered in 1982 as an alternatively spliced mRNA product of preprocalcitonin [7]. Whether calcitonin or αCGRP is expressed is tissue-dependent [8]. βCGRP is the product of a second gene revealing no calcitonin-encoding DNA, just a calcitonin pseudogene. It is very similar to αCGRP, as it differs by only three amino acids [7]. Structurally, CGRPs are identical to amylin, with an N-terminal disulfide bridge and C-terminal amidation. CGRPs are expressed throughout the peripheral and central nervous system and are involved in pain transmission [9]. Inhibition of CGRP functionality has been extensively studied, and antagonism of CGRP-CGRP-receptor (CGRP-R) interactions has proven efficacious in migraine [9,10]. Importantly, studies have also indicated a role of CGRPs in the regulation of food intake [11].
Calcitonin is a 32 amino acid peptide that was discovered in 1961. Later it was shown to be secreted by the thyroid gland, and its role in calcium homeostasis was established [[12], [13], [14]]. Calcitonin is generated through the maturation of preprocalcitonin, a 141-amino-acid peptide. Preprocalcitonin is processed into active peptide by removing the signal- and propeptides as well as a C-terminal peptide called katacalcin [15]. Whether katacalcin has a function remains unknown [15]. Mature calcitonin is a single-chain peptide with 32 amino acid residues and a molecular weight of approximately 3.4 kDa, depending on the species. Similar to amylin, it has an N-terminal disulfide bridge connecting the cysteines at positions 1 and 7, an α-helical domain, and natural C-terminal amidation [2]. To date, a host of calcitonins from different species has been identified, although most have not been fully assessed. In addition to human calcitonin, porcine, eel, salmon, and chicken calcitonin have been studied thoroughly due to their potencies, which far exceed those of mammalian calcitonins in human calcitonin. Overall, the calcitonin sequences fall into three categories: 1) human-type, 2) porcine-type, and 3) salmon-type. There are very few differences in terms of potency of these peptides on the target receptor, with the salmon-type exceeding the other groups [2]. Salmon-type calcitonins, salmon and eel calcitonin, are the best studied and were identified in 1967 and 1975, respectively [16,17]. Interestingly, salmon calcitonin and possibly also eel and chicken calcitonin has the same ability as calcitonin and amylin. They are considered dual amylin and calcitonin receptor agonists (DACRAs) and have been studied as amylin receptor agonists in addition to research in which they were used as calcitonin receptor agonists [18,19].
Three additional members of the family are known. Adrenomedullin (AM) is the largest member of the family with 52 amino acids. It was isolated in 1993 from human pheochromocytoma as a cAMP elevating peptide in platelets. The encoding cDNA was elucidated shortly thereafter [[20], [21], [22]]. Intermedin (adrenomedullin 2) was discovered in 2004 and is a 53 amino acid peptide shown to have some overlap in tissue expression with CGRP or AM [[23], [24], [25]]. The final member of the family is the calcitonin receptor-stimulating peptide (CRSP) of which three porcine but no human isoforms have been identified. CRSP, a 38 amino acid peptide, was identified in 2003 and shown to have a sequence similar to CGRPs. However, from a functionality perspective, it appears more related to calcitonin, although only little is known [26]. As the functionalities of these three family members reside outside the metabolic field, they will only be mentioned briefly herein. Adrenomedullin (AM) is a widely expressed peptide hormone that was originally identified as a vasodilator and mediates a series of physiologic effects in the cardiovasculature [22,27]. Intermedin/adrenomedullin 2 is also widely expressed, although it appears to be more mode restricted than AM [28]. Similar to AM, it appears to be functional in the vasculature and heart, where it increases blow flow and contractility, respectively, and the effects are mediated both centrally and peripherally [28].
2.2. Receptors
For a long time after ligand identification, the family's receptors were elusive; however, in 1991, the porcine calcitonin receptor (CTR) was identified [29]. Shortly thereafter, the human homologues of this receptor were cloned [30]. These were shown to be 7-transmembrane (7-TM) G-protein coupled receptors (GPCRs). Studies have demonstrated that two subtypes of human and rat CTR exist, namely CTRa and CTRb, where the CTRa variant has broad tissue expression, while the CTRb variant is more restricted, with expression in the placenta and ovaries, but not in fetal brain and uterus [31]. The calcitonin-receptor like receptor (CRLR) was identified as a 7-TM GPCR; however, it was considered an orphan receptor at its discovery in 1995 [32]. It was not until seminal work by McLatchie et al., in 1998 that the full receptor picture became clear. They described the receptor activity-modifying proteins (RAMPs), of which three were identified. These are critical receptor components for the functionality of this receptor [33]. The amylin receptor was ultimately shown to consist of a CTR and a RAMP [34]. The discovery of the RAMPs and their interactions with the CTRs and CRLRs laid the foundation for the segregation of the receptors into the presently known seven functionally different receptors. The 7 receptors are illustrated in Figure 3 showing the CTR alone or in the presence of one of the three RAMPs, while the CRLR alone is non-functional and therefore not shown. The CRLR becomes functional when combined with one of the RAMPs as illustrated in Figure 3 [3].
Figure 3.
Modified from [2]. Ligands are indicated by spheres with sizes showing their relative potency at each receptor (bigger sphere = higher potency). Abbreviations: AM1-2, adrenomodullin receptor 1–2. AMY1-3, amylin receptor 1–3. AM, adrenomodullin. AM2, adrenomodullin 2 (intermedin). CGRP, calcitonin gene-related peptide. CTR, calcitonin receptor. HCT, human calcitonin. SCT, salmon calcitonin.
2.3. Receptor pharmacology
The receptors for the calcitonin family are class B GPCRs, that is, 7-TM GPCRs. As previously mentioned, the receptors for CGRP, AM, and amylin require interactions between the GPCR and one of the three RAMPs [2]. To form the amylin receptor, one of the three RAMPs interacts with the CTR. For CGRP and AM, the related CRLR interacts with RAMP1 to form the CGRP receptor and RAMP2 or 3 to form the AM1 and AM2 receptors [10,35,36]. Additionally, there are two CTR isoforms a and b that respond with slightly different profiles to stimulation with calcitonin, although overall the functional difference in vivo between these is unclear [10,35,36].
A full review of the downstream signal transduction of these receptors is beyond the scope of this article. However, the classical Gs-induced cascade with cAMP as a critical mediator of receptor activation is common to these receptors and has shed light on the ranking of ligand potencies using engineered reporter cell systems [2]. Recent studies have emerged investigating the potential therapeutic power of biased ligands, that is, ligands that selectively activate parts of the signaling cascades. Andreassen et al. [37] described how optimizing biased ligands or prolonging receptor activation may be critical for evoking better in vivo activities. These studies are early and further research is necessary to determine whether they will result in improved and more selective therapies [[38], [39], [40], [41]]. Together, this warrants the need to understand differences in physiologically and pharmacologically relevant signaling in target tissues and cells.
Careful receptor profiling using radio-ligand binding and cAMP induction in cells engineered to over-express the receptors has helped elucidate the individual peptides' rank order on the receptors (Table 1). Validating these studies in vivo, or even in isolated primary cell systems, in many cases still remains to be published. The most important finding is that each human ligand is relatively selective for its target receptor, although with some overlap between amylin and CGRP, which activate the opposing receptor, but with reduced potency [2]. The amylin receptor subtypes are pharmacologically distinct and AMY1 and AMY3 receptors bind amylin with high affinity, while AMY1 receptors, but not AMY3 receptors bind CGRP with high affinity [2]. A key finding is that salmon calcitonin and other teleost and avian forms of calcitonin such as eel and chicken are not just calcitonin receptor agonists, but also by far the most potent amylin receptor agonists [18] and as such are called dual amylin and calcitonin receptor agonists (DACRAs). Salmon calcitonin has also been shown to bind and activate the receptors for a prolonged period compared to human and rat versions of calcitonin and amylin [37,42]. This prolonged activation is due to a significantly reduced dissociation of the DACRAs from the receptor as well as an ability to maintain the actively signaling receptor following internalization compared to endogenous ligands [37,43].
Table 1.
| Receptor | Calcitonin Receptor | Amylin Receptors | CGRP Receptor |
|---|---|---|---|
| Composition | CTR |
AMY1 -R: CTR + RAMP1 AMY2-R: CTR + RAMP2 AMY3-R: CTR + RAMP3 |
CGRP-R: CRLR + RAMP1 |
| Rank order of potency | SCT > human CT > AMY, CGRP > AM, AM2/IMD |
AMY1 -R: SCT > AMY > CGRP > AM2/IMD > human CT > AM AMY3-R: SCT > AMY > CGRP > AM2/IMD > human CT > AM |
CGRP > AM > AM2/IMD > AMY > SCT |
| Selective agonists | Human CT | AMY | αCGRP |
| Prolonged activation | SCT | SCT/Davalintide |
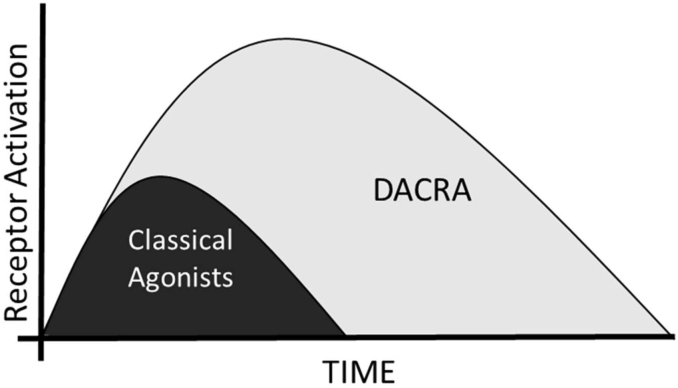
A newer group of synthetically produced DACRAs, also called KBPs, has been developed. These have been studied with respect to both receptor activation profiles and in vivo activities in a series of metabolic models (see Section 4.1). These molecules were developed to identify a peptide with increased potency on both the CTR and AMY-R compared not only to the selective ligands, but also to the natural DACRAs, SCT, and ECT. In in vitro receptor activation tests, they showed 2–4 fold higher potency on both the CTR and AMY3-R, with no activation of the CGRP-R, consistent with originating from SCT [42,44,45]. Second, they were studied with respect to their ability to bind and elicit a prolonged receptor activation, and as described by Gydesen et al. [42], DACRAs activate the receptors for up to 72 h depending on the dose. Importantly, this translates into prolonged effects in vivo and is likely a key part of the explanation of their ability to elicit weight loss and glucose control when dosed as single daily injections in vivo, where selective agonists such as rat amylin require delivery via infusion pumps to be efficacious [46] (see Figure 4). A particular case is seen with davalintide, which functionally is a DACRA, although it activates the CGRP-R at very high concentrations [47,48]. Interestingly, it binds irreversibly to the AMY-R and yet fails to induce weight loss without being delivered via infusion pumps, potentially explaining the lack of improved clinical efficacy compared to pramlintide [48,49]. An important comment to these studies is that the overall ranking of the potencies of these ligands is not based on head-to-head comparisons of all the ligands, but on a series of separate studies comparing a few in one or two selected over-expression systems.
Figure 4.
Schematic illustration of the effect of prolonged receptor activation by DACRAs. Inspired by [43,49].
2.4. The challenges of dissecting expression profiles and thereby the specific effects of individual family members
The complexity of interpreting receptor expression and functionality data is an essential aspect of the receptor family. A series of studies have assessed the expression of the CTR, CRLR, and individual RAMPs to clarify in which tissues the receptors are expressed. As previously mentioned, the CTR receptors are located throughout the body, with a prominent presence in the osteoclasts. They are also in the osteocytes, kidneys, testes, placenta, lungs, and brain [41]. Furthermore, they are expressed in multiple areas of the brain, where the area postrema (AP), nucleus accumbens (NAc), ventral tegmental area (VTA), arcuate nucleus (ARC), ventromedial hypothalamus (VMN), and nucleus of the solitary tract (NTS) as well as others have been identified [41]. Similarly, the CRLRs are expressed at very high levels in lung and adipose tissue, but with expression found in most tissues [52]. Importantly, the expression of CTRs or CRLRs without RAMPs does not indicate which receptor is present; however, assessing RAMP expression at the protein level has proven difficult. Expression profiling has demonstrated RAMP1 mRNA expression of RAMP1 in the lungs, muscle, and brain, with RAMP2 mRNA expression detected in the lungs, heart, skeletal muscle, endothelial tissue, and brain [41]. RAMP3 is primarily found in the brain, although lower levels are observed in the gut, lungs, heart, and kidneys. Detailed studies of the expression have demonstrated the presence of mRNAs for RAMPs in the previously mentioned areas in the brain [41]. However, these expression studies are challenged by the receptor construction, as neither a protein nor an mRNA expression study of the CTR will determine whether it has a RAMP attached. While such studies may indicate the presence of a receptor, they fail to unlock its identity (CTR or AMY1-3-R). Similarly, expression studies of RAMPs do not conclusively tell which receptor is present, as the interactions with the CTRs remain elusive. RAMPs also interact with other GPCRs, further complicating the interpretation of expression studies [3].
The overlap in the coding sequences of the αCGRP and calcitonin genes has limited the utility of knockouts. Furthermore, knocking out the GPCR part (for example, CTR) will inherently remove more than one receptor (the CTRs and AMY1-3-R) unless it is targeted to a cell specifically expressing only one receptor. However, even osteoclasts that are highly responsive to calcitonin and have been studied for decades are still thought to express more than just the CTRs [53]. Knocking out RAMPs is even more complicated as they interact with multiple receptors in numerous tissues, and although not well-described, there are indications that RAMPs compete for binding to the respective GPCR units [3].
While ligands such as amylin and calcitonin are selective for individual receptors, their low potencies and rapid dissociation rates from the individual target receptors compared to the dual agonists such as salmon calcitonin have limited their use in receptor-binding studies [54]. Therefore, salmon calcitonin has been extensively researched due to its high in vivo and in vitro potency; however, many studies were conducted before separate amylin and calcitonin receptors were discovered [33,34]. While antagonists of the receptors exist in the form of N-terminally truncated peptides, these are limited in terms of both selectivity and potency, although AC187 is considered selective for the amylin receptor and has been widely applied in studies of the physiology of amylin [55].
3. Physiological role(s) of amylin, calcitonin, and CGRP
3.1. Amylin
Physiologically, amylin is as a satiation hormone that acts as a meal-ending signal [56]. The concentration of amylin increases 5–6 fold in response to meal size [[57], [58], [59]]. Amylin is rapidly cleared from the circulation via the kidneys [60,61] and has a half-life of approximately 13 min [62]. The pancreas is the primary peripheral tissue secreting amylin although mRNA has been detected in the lungs and gastrointestinal tract as well as the hypothalamus and dorsal root ganglia [[63], [64], [65], [66]]. However, the relevance of amylin derived from non-pancreatic tissues remains unclear.
A few studies have used amylin knockout mice to investigate amylin's physiological role. These studies were consistent with a role of amylin as a regulator of appetite, energy homeostasis, and glucose control, at least partially, through a downregulation of insulin and potentially an increase in insulin sensitivity [67,68]. The satiation properties of amylin have also been investigated using the amylin receptor antagonist AC187 [39]. Both central and intravenous infusion of the antagonist AC187 result in dose-dependent increases in food intake in rats without effects on overall body weight [[69], [70], [71]]. Instead, amylin seems to serve as an adiposity controller given that AC187-infused rats increase body fat relative to lean mass [69]. This was supported by a study in which the amylin receptor core (the CTR) was specifically depleted in the ventromedial hypothalamic nuclei (VMN) [72]. Receptor-depleted rats showed no alterations in food intake, while they gained approximately 30% more weight, predominantly fat mass, consistent with amylin signaling playing a central regulatory role in body weight independent of appetite regulation. Along with higher adiposity, rats infused with AC187 exhibited increased plasma insulin and plasma glucose compared to control rats, data that indicate a physiological role in glucose control and potentially insulin sensitivity [69] and contrast findings in isolated skeletal muscles from rats showing deterioration of glucose control following amylin stimulation [73].
In addition to the previously mentioned insulin secretion, amylin's glucose regulation qualities include a broad range of different downstream effects including reductions in glucagon secretion, gastric emptying, and gastric acid secretion. Endogenous amylin slowed the rate of gastric emptying, delaying the transfer of glucose and other nutrients into the circulation, which helps control blood glucose [74,75].
In addition to reducing the gastric emptying rate, amylin also exerts a direct inhibitory effect on glucagon secretion. When rats were infused with the amylin antagonist AC187, glucagon levels increased compared to vehicle under euglycemic conditions [75]. This was to a large extent supported by the finding that near-physiological (47 pM) but not physiological (13 pM) amylin infusion causes significant reductions in l-arginine-stimulated glucagon increase [76]. The effect seems to be extrinsic to the pancreas, as endogenous amylin does not affect glucagon levels in l-arginine-stimulated perfused rat pancreas or isolated islets [77].
Overall, there is little doubt that most amylin signaling is centrally mediated via the area postrema, which subsequently propagates the signal. Brain nuclei such as the ventral tegmental area and nucleus accumbens are also key players in mediating central amylin signaling. A detailed description of the brain nuclei involved in amylin signaling is outside the scope of this review but was reviewed in detail in [41,78].
3.2. Calcitonin
Calcitonin is secreted by the C cells of the thyroid gland [79]. Attempts at deciphering the physiological function of calcitonin are numerous. However, these have proven complex due to the previously described genetic overlap between calcitonin and αCGRP and the receptor overlap between CTR and AMY-R [2,34].However, the overlap in receptors is the main reason for the inclusion of calcitonin in this review.
Most calcitonin studies focused on bone turnover and calcium homeostasis, where the interaction between calcitonin and the receptor plays a modest role as documented by calcitonin receptor knockout in the osteoclasts, that is, one of the few cells considered to exclusively express CTRs [53,80]. More importantly and relevant for the metabolic focus of this paper, CTR knockout mice have impaired glucose tolerance [81]. However, it remains unclear whether this is due to deficiency in the CTR or AMY-R [34,54,82]. Furthermore, the same group showed that deleting the calcitonin gene led to improved glucose tolerance and protection from diet-induced obesity in male mice, an effect that was less pronounced when only deleting the αCGRP gene exclusively [81]. Moreover, a recent study showed the prevention of obesity and hyperglycemia in aged obese male mice [83] when deleting the calcitonin gene while maintaining the αCGRP gene intact. Thus, data exist to support a role of the calcitonin-CTR interaction in metabolism, although further data are needed. In contrast to these studies, pharmacological administration of the CTR-selective ligand, rat calcitonin, showed an improvement in glucose control in ZDF rats [84]. This improvement further complicates the picture but fits well with the potent glucose regulatory capacities of the DACRA molecules and is in line with CTR knockout mice which indicate a potential role of CTR in glucose homeostasis [81,85,86].
3.3. CGRP
The central nervous system (CNS) has proven critical in regulating energy homeostasis. Studies have demonstrated innervation of the white adipose tissue (WAT) and thereby a cellular source of CGRP release in this highly relevant metabolic tissue. WAT innervation and CGRP release derived from the neurons may play an important role in adipocyte lipid metabolism and thereby whole-body metabolism [87]. CGRP is synthesized by neurons in the central and peripheral nervous system and released by trigeminal and dorsal root ganglia [88,89], although peripheral cells have also been reported to produce CGRP [90]. The main function appears to be vasodilatory effects as a function of different stimuli, including pain, which is well-described [90,91]. However, for a detailed examination of the physiology and pathophysiology of CGRP besides the metabolic functions, we refer to the following excellent reviews [10,90].
While several CGRP knockout mice have been developed for studies of the physiological role, most of these studies focused on vasodilatory effects and some were confounded by simultaneously knocking out the calcitonin gene [90]. Walker et al. demonstrated that αCGRP deficiency was associated with increased β oxidation and energy expenditure and thereby protection against diet-induced obesity, although one model also demonstrated deficient calcitonin [92]. Bartelt et al. and Liu et al. studied specific αCGRP knockout in mice exposed to metabolic disturbance by a high-fat diet [81,93]. In this model, they found a modest weight reduction and small improvement in glucose control and plasma insulin, indicating that αCGRP has a moderately negative effect on these metabolic parameters [81,93]. This was supported by a study showing that antagonism of CGRP signaling improved metabolic parameters and potentially inhibited metabolic decline in aged mice [94]. Hence, it appears that αCGRP is a negative regulator of metabolic health. An interesting observation was that pharmacological dosing with CGRP showed beneficial effects on the metabolism, indicating a mixture of pro- and anti-diabetic/-metabolic effects [90]. However, as pharmacology and physiology in many cases provide different responses, this will be discussed later. Overall, the physiological relevance of CGRP with respect to metabolic functions is somewhat limited.
4. In vivo pharmacology of amylin receptor agonists
4.1. Preclinical
Several agonists with different receptor activation potencies have been used to investigate the pharmacological effects of amylin receptor activation. The most interesting and commonly used will briefly be presented before a description of amylin receptor agonist pharmacology.
4.1.1. Introducing the amylin agonists
In contrast to most species-specific amylins, human amylin has the unfortunate property of forming toxic aggregates in the pancreas [95], which makes the human sequence of amylin unsuited for pharmacological purposes. Therefore, the most frequently used amylin is rat amylin, which does not aggregate due to sequence differences (see Table 1). Pramlintide is a designed amylin analogue in which the non-aggregatory properties of rat amylin are fused with the human amylin sequence for clinical treatment [96]. The amylin analogue, davalintide, was developed for the same purpose, although davalintide also activates the calcitonin receptor and has very low potency on the CGRP-R. However, due to the low potency on the CGRP-R, it is unclear whether this manifests in vivo [47,49]. As previously described, salmon calcitonin is used due to its potent amylin receptor agonism; however, it should be kept in mind that SCT is a potent calcitonin receptor agonist. Peptide derivatives were developed based on the potent nature of teleost-avian calcitonins. As previously mentioned, these were carefully developed to exceed the potency of SCT in terms of both amylin and calcitonin receptor activation in vitro and in vivo. They are called KeyBioscience peptides (KBPs) [18,44] and have demonstrated metabolic effects exceeding those of selective amylin receptor agonists (see Figure 5).
Figure 5.
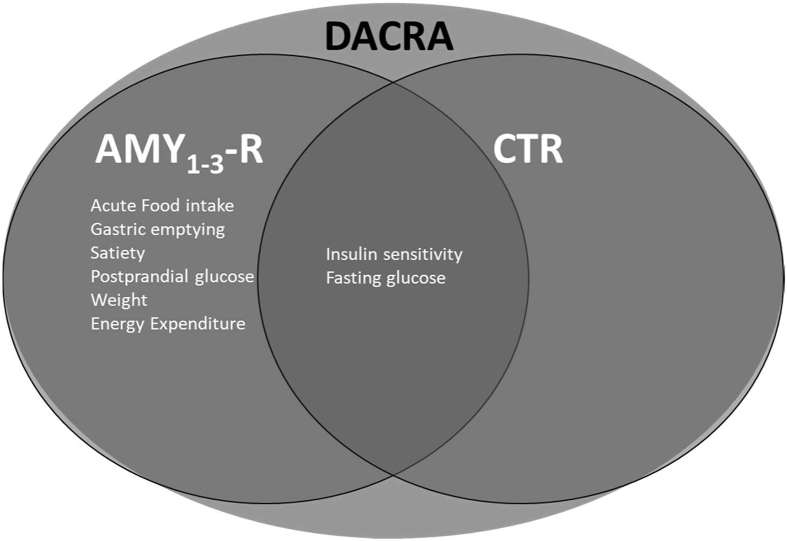
Effects of DACRAs on metabolism and their individual receptors involved in these actions and the overlap in terms of glucose homeostasis, in which CTR has recently been shown to be important. Abbreviations: AMY1-3-R, amylin receptors. CTR, calcitonin receptor. DACRA, dual amylin and calcitonin receptor agonist.
4.1.2. Meal size, taste aversion, nausea, and pica behavior
Pharmacological amylin doses reduce overall food intake in rats by altering meal patterns. The reduction is evident by a reduction in meal size, although meal frequency may also be affected [56,[97], [98], [99], [100]]. Salmon calcitonin dosed centrally also decreases meal size in rats [101] and non-human primates across a dose range while only the highest dose decreased meal frequency [102]. In many cases, amylin is administered by continuous infusion, for example, by subcutaneously implanted osmotic minipumps, ensuring continuous drug availability and compensating for the short half-life. Using this delivery method, amylin reduced food intake for 3–4 days after which food intake returned to control levels [46,103]. When delivering amylin through subcutaneous injections, food intake is also reduced, resulting in weight loss; however, the effects only last 1–2 h [56,[97], [98], [99], [100]]. The mechanism behind reduced food intake does not include conditioned taste aversions [99,[104], [105], [106]] or ingestion of non-nutritive substances such as kaolin, indicating that the molecules, independent of their potency, do not induce toxic effects at pharmacologically relevant doses [47,97,101,107,108].
4.1.3. Food preference
Activation of the amylin receptor leads to a transient reduction in food intake, which normalizes to control levels. The literature supports that the reduction is primarily mediated by a decrease in meal size. In addition, data also point to improved food preference. Rats offered a high-fat diet and chow significantly reduced the percentage of calories obtained from a high-fat diet relative to chow when treated with amylin (300 μg/kg/day) or davalintide (10 μg/kg/day) [47]. The data were corroborated by another amylin study [97] and research using KBPs. In these studies, control rats favored chocolate ingestion, while treated rats switched to regular chow [85]. This improvement in food preference was also observed in long-term studies (5–6 weeks) in both lean and obese rats offered a high-fat diet and chow [108]. Collectively, these studies suggest that amylin agonism leads to an increase in low-energy dense foods at the expense of high-energy dense foods and is not dependent on the lean/obese state.
4.1.4. Weight loss and comparison of agonists
The weight-reducing effect of amylin has received considerable attention in light of the worldwide increase in obesity and concomitant comorbidities. Treating obese rats using infusion pumps with amylin led to a dose-dependent weight loss that reached a plateau at 8–10% of vehicle-corrected weight loss [46,103], although up to 14% has been reported [97]. Dosing with 1000 μg/kg/day amylin failed to produce a greater weight loss [46], supporting that the maximal weight loss was reached with amylin monotherapy at ∼300 μg/kg/day in rats. Dual agonists such as salmon calcitonin, KBPs, or davalintide induce higher weight loss at much lower doses than amylin. Importantly, the increased efficacy of the DACRAs is unrelated to the exposure profile, which is very similar to that of other members of this family of peptides, namely fast elimination (T1/2 < 1 h) [109]. Consistent with their increased in vitro potency and ability to induce prolonged receptor activation, they were superior to known agonists in terms of body weight and glucose improvements when dosed at equimolar concentrations [44,49]. In a study focusing on eliminating differences in potency using the maximum efficacious dose of amylin delivered via continuous infusion pumps, DACRA showed a larger effect on body weight and glycemic control [46,84]. Studies of the dual and/or triple agonists demonstrate an order of potency regarding weight loss in the following order: KBPs > SCT > davalintide > amylin [44,46,49], although it is important to remember that not all of these studies were conducted head to head with all compounds, but in most cases compared only two of the molecules, and future studies comparing all in the same study using the same output are relevant.
4.1.5. Energy expenditure, hyperthermia, and locomotor activity
The weight loss obtained with amylin agonism can be ascribed to the pronounced food suppressive effect observed during the initial phase of the treatment period. However, rats pair-fed to treated rats did not achieve the same weight loss and/or reduction in adipose tissues [103,107,110], and pair-weighed rats [85] must receive significantly less food compared to treated rats to obtain the same weight loss. To understand this, energy expenditure in treated, pair-fed, and vehicle rats was investigated. These studies showed that amylin and salmon calcitonin as a minimum prevented the typical compensatory reduction in energy expenditure induced by food restriction [47,97,103,[111], [112], [113]]. In papers reporting increased energy expenditure [97,111], this may be attributable to a relative increase in lean mass [103]. There was no indication of locomotor activity changes that could theoretically contribute to increased energy expenditure regardless of the type of amylin receptor agonist used [47,97,111]. Whether increased energy expenditure leads to increased body temperature remains unclear, but appears to depend on the administration route [111,112,114].
4.1.6. Glucose control, insulin and glucagon secretion, and gastric emptying
Amylin agonism improves glucose metabolism by affecting different parameters: the gastric emptying rate and insulin and glucagon secretion. Rats dosed continuously with amylin or KBP demonstrated reduced gastric emptying rates during an oral glucose challenge [46]. Similarly, davalintide reduced the gastric emptying rate [48]. The gastric emptying effect of amylin agonism helped control glucose excursions during a glucose challenge and reduced the insulin required for glucose control [107]. Importantly, insulin secretion regulation is independent of the gastric emptying response, as it was also present during an intravenous glucose challenge [85,107].
Another property of amylin agonism is the suppression of glucagon secretion, which is often inappropriately elevated in individuals with insulin resistance [115]. Salmon calcitonin suppressed glucagon secretion during an acute oral glucose tolerance test [116], and davalintide attenuated the rise in glucagon secretion following an l-arginine bolus using a hyperinsulinemic-euglycemic clamp [48].
In Zucker diabetic fatty (ZDF) (fa/fa) rats, amylin and DACRAs showed the ability to reduce fasting blood glucose independent of weight loss [19,46,84]. These data strongly support improvements in insulin action, as was observed in studies of a KBP using the hyperinsulinemic-euglycemic clamp method [107]. Of note, amylin, even at high and continuously delivered doses, was unable to match the KBP in terms of efficacy on glucose regulation, demonstrating that the combination of AMY-R and CTR agonism by DACRAs was essential for the improved blood glucose regulation in rat models [46,84].
Islet histology assessments demonstrated that salmon calcitonin treatment preserved β cells and protected against loss of insulin secretion in the ZDF model [19], although it is unknown whether this was due to prevention of β cell loss through improving glucose homeostasis or a direct protective effect on the β cells.
In summary, these data demonstrate that activation of the amylin receptor increases insulin action by requiring less insulin to control a given glucose challenge; however, the combination of amylin and calcitonin receptor induced effects appears to be responsible for DACRA-induced increases in insulin action and glucose control.
4.1.7. Are the amylin-mediated effects central, peripheral, or both?
It is well known that amylin primarily mediates its effects through central activation of the receptors present in brain regions such as the area postrema, nucleus of the solitary tract, ventral tegmental area, nucleus accumbens, and other regions of the brain [47,98,[117], [118], [119], [120], [121], [122]]. These mechanisms were recently and carefully reviewed by [41,78] and thus will not be further described.
In addition to the centrally mediated effects, several studies shed light on potential peripheral effects of amylin receptor agonism. Interestingly, effects on adipocytes and skeletal muscle cells have been described; however, these were in many cases dependent on the system studied with marked differences between fasted and fed conditions as well as healthy vs obese vs diabetic conditions and as such will not be described in further detail [[123], [124], [125], [126], [127], [128], [129], [130], [131], [132]].
An interesting finding is that most of these studies indicated that amylin agonism induces insulin resistance in rat muscle tissue, a result that clearly contrasts with its anti-diabetic and weight loss effects in long-term preclinical and clinical studies [85,107,[133], [134], [135]]. One interesting aspect is that acute (first dose) treatment with amylin agonists results in an acute rise in blood glucose in humans [[136], [137], [138]] and rats [49,73,107,139], likely by translocating muscle glycogen to the liver using lactate as a substrate [139], a finding that led to an initial idea that both amylin and salmon calcitonin were diabetogenic. However, acute dosing in type 2 diabetic and obese subjects demonstrated the opposite [[136], [137], [138]], and further chronic studies have clearly shown that this is a finding observed only at the first dose and primarily in healthy subjects [85,107,[133], [134], [135]].
4.1.8. Amylin receptor sensitivity
The balance between efficacy and tolerability can be delicate and must be assessed for any given drug. Amylin is generally well-tolerated and food intake is normalized within a few days upon continuous dosing assuming food intake is a direct reflection of tolerability and hence receptor sensitivity [46,103]. Intriguingly, an acute amylin response can still be induced on top of a high dose of chronic amylin treatment, suggesting that the amylin receptor system is not saturated despite normalized food intake [46]. An alternative dosing regimen such as dosing every other day with KBPs was shown to enhance the already known improvement in weight loss. However, it is unclear to what extent this will impact the tolerability [45,85,108]. Nevertheless, these observations indicate that amylin receptor sensitivity is complicated and depends on both the activation and dissociation of the ligands from the receptors, and studies shedding light on this are still warranted.
4.1.9. Combination studies
Leptin is an adipose tissue-derived peptide hormone that regulates appetite and loss of leptin function either through deficiency of the molecule itself or the leptin receptor, leading to obesity and type 2 diabetes [140]. However, obesity causes consistently elevated leptin levels and subsequent loss of leptin sensitivity, and as such leptin therapy alone is limited [141,142]. However, co-administration of amylin or a DACRA acutely potentiates a response to leptin therapy in rodent models [109,143]. In concurrence, amylin- and DACRA-induced weight loss is absent is virtually absent in leptin-deficient models such as the ZDF rats [86,144]. The underlying mechanism behind these effects is not clearly understood, but involves a central relay and IL-6 signaling [145].
Incretin-based therapies are another important combination possibility with amylin. Incretin-based molecules such as liraglutide, dulaglutide, and semaglutide are important therapies for both obesity and type 2 diabetes. Incretins at least partially mediate their effects through regulation of appetite reduction, suppression of gastric emptying, and post-prandial glucose stimulation and as such have attractive characteristics for combination with amylin and/or DACRAs [146,147]. A previous study in non-human primates suggested a synergistic reduction in food intake when combining GLP-1 and amylin agonism [148]. In addition, co-treatment with DACRA, KBP-089, and GLP-1 agonist liraglutide was recently shown to lead to combined effects on weight and glucose homeostasis [45]. Overall, the amylin-mediated mechanism of action is highly interesting as a combination partner, and as is described later, this is already being clinically tested.
4.2. Clinical
The amylin analogue, pramlintide, was approved by the US Food and Drug Administration in 2005 and is currently the only amylin analogue on the market. It is approved for patients with type 1 diabetes in combination with mealtime insulin and has since been approved for patients with type 2 diabetes in combination with insulin, metformin, and/or sulfonylurea. Davalintide was thought to be the next generation of amylin receptor agonists; however, development was halted following a phase 2 study failing to demonstrate effects superior to pramlintide [149]. Salmon calcitonin was approved in 1975 to treat osteoporosis in men and post-menopausal women, but the weight-reducing and glucose-regulatory potential has not been the focus of clinical studies and will not be further addressed in this section. Interestingly, a new long-acting amylin analogue is being studied in clinical trials, and although data have only been shared in the form of press releases and hence have not been under the scrutiny of peer review, they underscore the potential of amylin-receptor agonism at least for weight loss [150].
4.2.1. Weight control
Insulin therapy in patients with type 1 or 2 diabetes is often accompanied by weight gain [151]. Therefore, it is beneficial that pramlintide treatment is associated with a modest placebo-corrected weight loss of up to 3.6 kg in non-insulin-treated obese individuals [133] and insulin-treated type 2 [134] and type I diabetes patients [135]. In addition to the studies of pramlintide as a monotherapy, combination studies with sibutramine, phentermine, or metreleptin have also been published. In these studies of weight loss improvement in obese subjects, it was clearly demonstrated that pramlintide could be combined with either of these drugs/drug candidates and result in increased weight loss [152,153]. Importantly, despite these rather encouraging weight loss data, pramlintide has never been approved as a therapy for obesity. Pramlintide has been shown numerous times to help control post-prandial glucose excursions that are often experienced by individuals with type 1 or type 2 diabetes [154,155]. Three different mechanisms aid in this: modulation of the gastric emptying rate, glucagon secretion, and satiety. Pramlintide reduces gastric emptying, thereby causing a slower transfer of nutrients and glucose into the circulation [156,157]. Interestingly, pramlintide seems to have a “hypoglycemic brake.” In hypoglycemia, the normal pramlintide-induced reduction in gastric emptying is bypassed, thus allowing a more rapid nutrient uptake [158]. Glucagon secretion is often inappropriately elevated in patients with type 1 and type 2 diabetes, which signals the liver to increase glucose production, thereby worsening hyperglycemia [159,160]. In healthy individuals, endogenous insulin is secreted from the pancreas and directly to the hepatic portal vein, which potently suppresses hepatic glucose production. In individuals with impaired or lack of insulin secretion, exogenous insulin does not reach the same high concentration in the portal vein, thus attenuating its suppressive effect. Hence, in terms of glucagon and hepatic glucose production, individuals with diabetes, metaphorically, have one foot on the gas pedal and no brake. Pre-prandial pramlintide partially compensates for this, as it suppresses inappropriate glucagon secretion, thereby avoiding glucagon-induced hyperglycemia [155,161,162]. As has also been shown preclinically, pramlintide therapy in humans promotes long-term satiety [133] and reduces caloric intake in single-injection studies [163].
Daily injections of pramlintide in addition to insulin therapy significantly improve glycemic control in patients with type 1 or 2 diabetes as evident by a reduction in HbA1c by ∼0.5% (placebo corrected) [134,135,164]. In studies designed with flexible insulin therapy, pramlintide causes a relative reduction in daily insulin use, suggesting an overall improvement in insulin action [134,164,165]. Pramlintide is generally well-tolerated and further improves with dose escalation, although transient nausea is observed but lessens over time [133]. The incidence of hypoglycemic events increased during the first weeks of pramlintide therapy [166], particularly in clinical studies in which adjusting the insulin dose was not encouraged [134,135].
4.2.2. Pramlintide and analogues and their future clinical use
The worldwide prevalence of obesity increases the risk of comorbidities such as type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease. Medical treatments preferably targeting multiple diseases are in demand. Pramlintide improves post-prandial glycemic control while causing modest weight loss and possibly improves markers of cardiovascular disease, although the overall risk of cardiovascular incidence is unaffected [167,168]. Cardiovascular disease and non-alcoholic fatty liver disease are both caused primarily by obesity [169,170]. However, pramlintide is not of great use for cost and efficacy reasons, and thus, a search is being conducted for more potent amylin receptor agonists and longer-acting amylin receptor agonists such as DACRAs and different chemically modified versions of pramlintide [49,171]. Interestingly, amylin receptor agonism has presented as complementary to GLP-1 receptor agonism, highlighting this mechanism's potential as an add-on therapy to GLP-1R agonists [18,172,173]. A long-acting amylin agonist has already shown potential as a weight-reducing agent both as monotherapy and combination therapy with a GLP-1 agonist [150]. This discovery may revive the interest in amylin therapy as an approach to treat diabetes, obesity, and associated comorbidities.
4.3. Pharmacology of calcitonin receptor agonists
As mentioned in the section on physiology, there are indications that calcitonin plays a role in metabolism. However, while calcitonin, particularly DACRA salmon calcitonin, has been extensively studied within osteoporosis and osteoarthritis, there are very few studies utilizing a selective calcitonin receptor agonist, that is, human or rat calcitonin, to investigate the effects on metabolic parameters. Salmon calcitonin is widely used as an amylin agonist due to its vastly superior potency on the amylin receptor as previously described [101,102]. However, few studies have considered the possibility that one reason for the potent ability of DACRAs to improve glucose homeostasis beyond amylin-mediated post-prandial glucose regulation could be the activation of the calcitonin receptor. A recent study focused on deconstructing the role of the two target receptors of DACRAs for their ability to regulate blood glucose [84]. In this study, the differences in terms of potency and prolonged receptor activation [37,49] between DACRAs and the natural and receptor-selective ligand rat calcitonin were eliminated by administering rat calcitonin, rat amylin, or the two combined using an infusion pump delivering a daily dose of 300 μg/kg of either molecule alone or in combination and compared to a daily dose of 5 μg/kg of DACRA. Amylin produced the expected anti-diabetic response. Interestingly, rat calcitonin also contributed to glucose-lowering effects, both as a stand-alone therapy and when combined with rat amylin, where the effect matched that of DACRA as schematically illustrated in Figure 6 [84]. These effects were independent of weight loss and specifically observed under diabetic conditions, underscoring the calcitonin receptor's role in insulin action and glucose homeostasis (see Figure 5). They also demonstrated that activation of both CTR and AMY-R can contribute to glucose control [46,85,107]. However, this remains to be studied further as the molecular mechanism and target tissues must be determined. Furthermore, these were rat data with the lack of efficacy on glycemic control by KBP-042 in a phase 2 trial [174], so it remains to be confirmed in humans.
Figure 6.
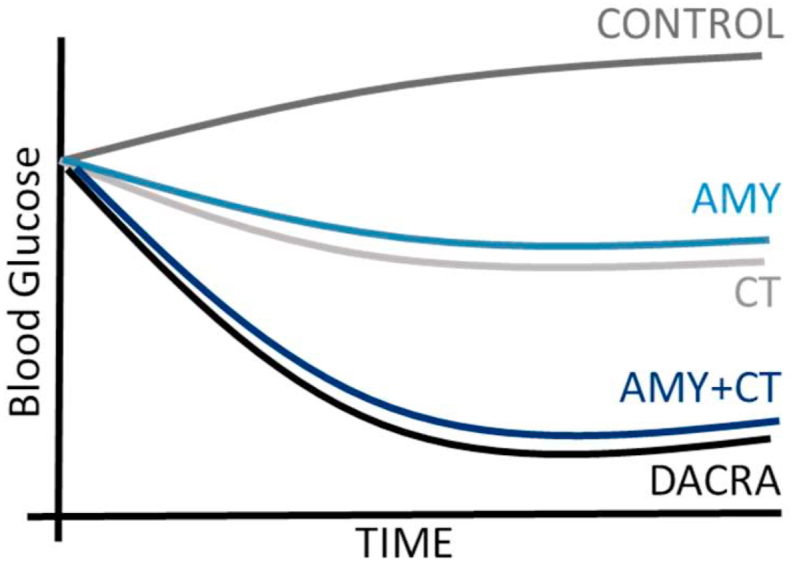
Schematic representation of blood glucose levels in ZDF rats following therapy with either maximally efficacious doses of the selective ligands amylin or calcitonin, the combination of the two selective ligands, or DACRA, all delivered using infusion pumps and thereby eliminating differences in potency, plasma half-life, and prolonged receptor activation. The study showed that calcitonin receptor agonism improved fasting blood glucose and did so in a manner complementary to amylin receptor agonism and that the combination of these two resulted in a DACRA-like response. Inspired by [175].
4.4. Pharmacology of CGRP-R agonists
While genetic data from knockout mice primarily indicate that αCGRP in vivo blunts metabolic responses to a high-fat diet [81,92], pharmacological studies have provided mixed results. Some studies are consistent with the genetic knockout data, while others show improvements in metabolism as a function of treatment [90].
A series of in vivo studies showed that αCGRP administration led to reduced food intake in rodents after peripheral [11] and central administration [176], although it was inferior to amylin in terms of potency [177]. Danaher et al. [178] demonstrated that administering αCGRP induced β oxidation of fatty acids in muscle and as such increased lipid metabolism, all in all indicating the beneficial effects of CGRP administration on the metabolism.
Recent studies investigated the potential mode of action that underlies the beneficial effects of αCGRP administration. Nilsson et al. [179] utilized a long-acting αCGRP and observed improvements in food intake, body weight, and glucose control in preclinical models, effects that may involve the upregulation of GLP-1 secretion. Sanford et al. [180] demonstrated reduced food intake in lean animals using αCGRP and a concomitant reduction in energy expenditure. However, this reduction in energy expenditure was due to the substantial reduction in food intake. They also observed reductions in plasma glucagon, but not insulin, data that are consistent with improvements in metabolism, yet appear highly model dependent [180] and as such require further studies.
Other studies were consistent with the knockout models' data and showed detrimental effects on insulin action in vitro and in vivo [181], thereby illustrating the complicated effects of αCGRP on metabolic parameters. Interestingly, while antagonism of αCGRP is approved as a therapy for migraine and numerous antagonists are still in clinical development [182], there are no consistent reports of either detrimental or beneficial effects on the body weight of these drugs [183]. In terms of agonists, these have not yet reached the level of clinical studies, and hence this is unknown.
The discrepancies between pharmacology and physiology are quite intriguing. One important parameter that may explain some of the discrepancies is the overlapping agonism of αCGRP on CGRP-R and AMY-R [2], rendering it difficult to determine the receptor origin of the downstream effects. For ligands such as the long-acting αCGRP analogue, the potency on the CGRP-receptor is unknown [159], which further complicates the picture. Interestingly, this overlapping agonism seems to be unidirectional, as the ability to induce GLP-1 release, a known CGPR effect, was not observed with potent DACRA or salmon calcitonin, rather the opposite [18].
In summary, while both agonism and antagonism of the CGRP receptor have been studied in terms of metabolic responses, the outcome is not promising for using this mechanism as a potential treatment for metabolic diseases. The beneficial effects observed, if any, were relatively modest, and long-acting αCGRP does not appear to be in clinical development. Combined with the lack of reports on the metabolic benefits of antagonists, it does not appear that CGRP modulation will play a major role in the treatment of metabolic diseases.
5. Conclusion and perspectives
Molecules that can increase insulin sensitivity while reducing weight are a hot commodity as treatments for type 2 diabetes and/or obesity and more so if they are complementary to existing therapies such as incretin-based molecules.
The calcitonin family of peptides is an ancient group of molecules that has been extensively studied in terms of physiology, pathophysiology, and pharmacological applications. This review has focused on the potential of amylin, amylin analogues/derivatives, and CGRP as therapies for metabolic disorders such as obesity and type 2 diabetes. We have emphasized their ability to increase insulin sensitivity and work in combination with existing drugs, particularly incretin-based therapies that demonstrate weight loss and glucose control but would benefit from combination with an insulin-sensitizing entity.
Intriguingly, some peptides demonstrated a disconnect between the physiology and pharmacological effects, as exemplified by αCGRP, wherein knockout mice showed protection against diet-induced obesity but treatment with agonists led to improvements in metabolism [90,179], although not potent enough to warrant clinical testing. However, amylin knockout mice underscored the physiological importance of amylin in appetite regulation [67], consistent with the well-described pharmacology of amylin receptor agonists.
Overall, understanding the effects of this family of peptides is clouded by its complicated nature, with the receptors consisting partially of the same subunits, not just for the GPCR domain, but also for RAMPs. This complexity essentially eliminates the utility of classical expression studies and knockouts of the receptors.
Pharmacologically speaking, CGRP receptor modulators have shown only limited metabolic benefits and do not appear to be useful. However, amylin and amylin analogues are very intriguing and have been extensively studied, resulting in the FDA-approved molecule pramlintide. However, pramlintide is somewhat limited in terms of potency and half-life, and there are various approaches to solving these issues. Although not much has been published, Novo Nordisk shared clinical data on their long-acting analogue, and to date, the weight loss observed with once-weekly dosing is promising [150]. This is particularly true considering that the effects appear to be complimentary with GLP-1R agonism, which brings the induced weight loss to a level not previously observed pharmacologically [18,150,172] and as such presenting amylin agonism as an attractive add-on therapy provided the combination is tolerable.
Another promising approach is DACRAs based on the natural dual amylin and calcitonin receptor agonists salmon calcitonin and eel calcitonin but showing unmatched amylin and calcitonin receptor potencies and an ability to prolong activation of the receptor, reducing the dosing frequency to once daily [44,49,85]. DACRAs also appear to possess a greater potential for glucose control than pure amylin receptor agonism and as such may have broader applications [84]. However, clinical data on the DACRA KBP-042 failed to show an improvement in HbA1c in a three-month trial [174], indicating that more clinical data on DACRAs are needed.
An intriguing aspect of amylin-receptor pharmacology is the potential applications in Alzheimer's disease. Alzheimer's is still poorly understood but preclinical data indicate a benefit of non-fibrillating amylin-receptor agonists [184] not only through the anticipated effects of metabolic improvement [185].
In conclusion, CGRP receptor agonism appears to not provide useful metabolic benefits, with a potential for vasodilation-induced adverse events. This mechanism seems to be improbable for metabolic complications. In contrast, amylin receptor agonism and particularly dual agonism of amylin and calcitonin receptors remain highly interesting, as the mechanism of action involves improved insulin sensitivity. As they perfectly complement other drugs with metabolic actions, they could help treatment-induced weight and glucose control match bariatric surgery-induced effects [186,187].
Acknowledgments
Funding for work on DACRAs was obtained from the Danish Research Foundation (Den Danske Forskningsfond).
Conflict of interest
All authors are employed by Nordic Bioscience and KeyBioscience. MK and KH own stocks in Nordic Bioscience and hold patents on DACRAs.
References
- 1.Hay D.L., Chen S., Lutz T.A., Parkes D.G., Roth J.D. Amylin: pharmacology, physiology, and clinical potential. Pharmacological Reviews. 2015;67:564–600. doi: 10.1124/pr.115.010629. [DOI] [PubMed] [Google Scholar]
- 2.Hay D.L., Garelja M.L., Poyner D.R., Walker C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. British Journal of Pharmacology. 2018;175:3–17. doi: 10.1111/bph.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pioszak A.A., Hay D.L. Elsevier; 2020. RAMPs as allosteric modulators of the calcitonin and calcitonin-like class B G protein-coupled receptors; pp. 115–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westermark P., Andersson A., Westermark G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiological Reviews. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P., Wernstedt C., Wilander E., Hayden D.W., O’Brien T.D., Johnson K.H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proceedings of the National Academy of Sciences. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling W., Huang Y.-M., Qiao Y.-C., Zhang X.-X., Zhao H.-L. Human amylin: from pathology to physiology and pharmacology. Current Protein & Peptide Science. 2019;20:944–957. doi: 10.2174/1389203720666190328111833. [DOI] [PubMed] [Google Scholar]
- 7.Amara S.G., Jonas V., Rosenfeld M.G., Ong E.S., Evans R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 8.Lima W.G., Marques-Oliveira G.H., da Silva T.M., Chaves V.E. Role of calcitonin gene-related peptide in energy metabolism. Endocrine. 2017;58:3–13. doi: 10.1007/s12020-017-1404-4. [DOI] [PubMed] [Google Scholar]
- 9.Gingell J.J., Rees T.A., Hendrikse E.R., Siow A., Rennison D., Scotter J. Distinct patterns of internalization of different calcitonin gene-related peptide receptors. ACS Pharmacology Translational Science. 2020;3:296–304. doi: 10.1021/acsptsci.9b00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gingell J.J., Hendrikse E.R., Hay D.L. New insights into the regulation of CGRP-family receptors. Trends in Pharmacological Sciences. 2019;40:71–83. doi: 10.1016/j.tips.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Lutz T.A., Rossi R., Althaus J., Del Prete E., Scharrer E. Evidence for a physiological role of central calcitonin gene-related peptide (CGRP) receptors in the control of food intake in rats. Neuroscience Letters. 1997;230:159–162. doi: 10.1016/s0304-3940(97)00503-x. [DOI] [PubMed] [Google Scholar]
- 12.Copp D.H., Cameron E.C., Cheney B.A., Davidson A.G.F., Henze K.G. Evidence for calcitonin—a new hormone from the parathyroid that lowers blood calcium. Endocrinology. 1962;70:638–649. doi: 10.1210/endo-70-5-638. [DOI] [PubMed] [Google Scholar]
- 13.Ashwini Kumar M., Foster G.V., Macintyre I. Further evidence for calcitonin a rapid-acting hormone which lowers plasma-calcium. Lancet. 1963;282:480–482. doi: 10.1016/s0140-6736(63)90224-1. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch P.F., Voelkel E.F., Munson P.L. Thyrocalcitonin. Hypocalcemic hypophosphatemic principle of the thyroid gland. Science. 1964;146(80):412–413. doi: 10.1126/science.146.3642.412. [DOI] [PubMed] [Google Scholar]
- 15.Zaidi M., Moonga B.S., Bevis P.J.R., Bascal Z.A., Breimer L.H. The calcitonin gene peptides: biology and clinical relevance. Critical Reviews in Clinical Laboratory Sciences. 1990;28:109–174. doi: 10.3109/10408369009105900. [DOI] [PubMed] [Google Scholar]
- 16.Copp D.H., Cockcroft D.W., Kueh Y. Calcitonin from ultimobranchial glands of Dogfish and chickens. Science. 1967;158(80):924–925. doi: 10.1126/science.158.3803.924. [DOI] [PubMed] [Google Scholar]
- 17.Morikawa T., Munekata E., Sakakibara S., Noda T., Otani M. Synthesis of Eel-Calcitonin and [Asu1,7]-Eel-Calcitonin: contribution of the disulfide bond to the hormonal activity. Experientia. 1976;32:1104–1106. doi: 10.1007/BF01927568. [DOI] [PubMed] [Google Scholar]
- 18.Gydesen S., Andreassen K.V., Hjuler S.T., Hellgren L.I., Karsdal M.A., Henriksen K. Optimization of tolerability and efficacy of the novel dual amylin and calcitonin receptor agonist KBP-089 through dose escalation and combination with a GLP-1 analog. American Journal of Physiology. Endocrinology and Metabolism. 2017;313:E598–E607. doi: 10.1152/ajpendo.00419.2016. [DOI] [PubMed] [Google Scholar]
- 19.Feigh M., Andreassen K.V., Neutzsky-Wulff A.V., Petersen S.T., Hansen C., Bay-Jensen A.C. Oral salmon calcitonin attenuates hyperglycaemia and preserves pancreatic beta-cell area and function in Zucker diabetic fatty rats. British Journal of Pharmacology. 2012;167:151–163. doi: 10.1111/j.1476-5381.2012.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochemical and Biophysical Research Communications. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura K., Sakata J., Kangawa K., Kojima M., Matsuo H., Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochemical and Biophysical Research Communications. 1993;194:720–725. doi: 10.1006/bbrc.1993.1881. [DOI] [PubMed] [Google Scholar]
- 22.Fischer J.-P., Els-Heindl S., Beck-Sickinger A.G. Adrenomedullin – current perspective on a peptide hormone with significant therapeutic potential. Peptides. 2020;131:170347. doi: 10.1016/j.peptides.2020.170347. [DOI] [PubMed] [Google Scholar]
- 23.Roh J., Chang C.L., Bhalla A., Klein C., Hsu S.Y.T. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. Journal of Biological Chemistry. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 24.Takei Y., Inoue K., Ogoshi M., Kawahara T., Bannai H., Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Letters. 2004;556:53–58. doi: 10.1016/s0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- 25.Bell D., McDermott B.J. Intermedin (adrenomedullin-2): a novel counter-regulatory peptide in the cardiovascular and renal systems. British Journal of Pharmacology. 2009;153:S247–S262. doi: 10.1038/sj.bjp.0707494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katafuchi T., Kikumoto K., Hamano K., Kangawa K., Matsuo H., Minamino N. Calcitonin receptor-stimulating peptide, a new member of the calcitonin gene-related peptide family. Journal of Biological Chemistry. 2003;278:12046–12054. doi: 10.1074/jbc.M207970200. [DOI] [PubMed] [Google Scholar]
- 27.Voors A.A., Kremer D., Geven C., Ter Maaten J.M., Struck J., Bergmann A. Adrenomedullin in heart failure: pathophysiology and therapeutic application. European Journal of Heart Failure. 2019;21:163–171. doi: 10.1002/ejhf.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong Y., Hay D.L., Quirion R., Poyner D.R. The pharmacology of adrenomedullin 2/intermedin. British Journal of Pharmacology. 2012;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin H., Harris T., Flannery M., Aruffo A., Kaji E., Gorn A. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991;254(80):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- 30.Gorn A.H., Lin H.Y., Yamin M., Auron P.E., Flannery M.R., Tapp D.R. Cloning, characterization, and expression of a human calcitonin receptor from an ovarian carcinoma cell line. Journal of Clinical Investigation. 1992;90:1726–1735. doi: 10.1172/JCI116046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pondel M. Calcitonin and calcitonin receptors: bone and beyond. International Journal of Experimental Pathology. 2000;81:405–422. doi: 10.1046/j.1365-2613.2000.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fluhmann B., Muff R., Hunziker W., Fischer J.A., Born W. A human orphan calcitonin receptor-like structure. Biochemical and Biophysical Research Communications. 1995;206:341–347. doi: 10.1006/bbrc.1995.1047. [DOI] [PubMed] [Google Scholar]
- 33.McLatchie L.M., Fraser N.J., Main M.J., Wise a., Brown J., Thompson N. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 34.Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Molecular Pharmacology. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- 35.Hendrikse E.R., Bower R.L., Hay D.L., Walker C.S. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia. 2019;39:403–419. doi: 10.1177/0333102418765787. [DOI] [PubMed] [Google Scholar]
- 36.Purdue B.W., Tilakaratne N., Sexton P.M. Molecular pharmacology of the calcitonin receptor. Receptors and Channels. 2002;8:243–255. [PubMed] [Google Scholar]
- 37.Andreassen K.V., Hjuler S.T., Furness S.G., Sexton P.M., Christopoulos A., Nosjean O. Prolonged calcitonin receptor signaling by salmon, but not human calcitonin, reveals ligand bias. PloS One. 2014;9 doi: 10.1371/journal.pone.0092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weston C., Winfield I., Harris M., Hodgson R., Shah A., Dowell S.J. Receptor activity-modifying protein-directed G protein signaling specificity for the calcitonin gene-related peptide family of receptors. Journal of Biological Chemistry. 2016;291:21925–21944. doi: 10.1074/jbc.M116.751362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey R.J., Walker C.S., Ferner A.H., Loomes K.M., Prijic G., Halim A. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. British Journal of Pharmacology. 2012;166:151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker C.S., Conner A.C., Poyner D.R., Hay D.L. Regulation of signal transduction by calcitonin gene-related peptide receptors. Trends in Pharmacological Sciences. 2010;31:476–483. doi: 10.1016/j.tips.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Boccia L., Gamakharia S., Coester B., Whiting L., Lutz T.A., Le Foll C. Amylin brain circuitry. Peptides. 2020;132:170366. doi: 10.1016/j.peptides.2020.170366. [DOI] [PubMed] [Google Scholar]
- 42.Gydesen S., Andreassen K.V., Hjuler S.T., Christensen J.M., Karsdal M.A., Henriksen K. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. American Journal of Physiology. Endocrinology and Metabolism. 2016;310:E821–E827. doi: 10.1152/ajpendo.00514.2015. [DOI] [PubMed] [Google Scholar]
- 43.Furness S.G.B., Liang Y.-L., Nowell C.J., Halls M.L., Wookey P.J., Dal Maso E. Ligand-dependent modulation of G protein conformation alters drug efficacy. Cell. 2016;167:739–749. doi: 10.1016/j.cell.2016.09.021. e11. [DOI] [PubMed] [Google Scholar]
- 44.Andreassen K.V., Feigh M., Hjuler S.T., Gydesen S., Henriksen J.E., Beck-Nielsen H. A novel oral dual amylin and calcitonin receptor agonist (KBP-042) exerts antiobesity and antidiabetic effects in rats. American Journal of Physiology. Endocrinology and Metabolism. 2014;307:E24–E33. doi: 10.1152/ajpendo.00121.2014. [DOI] [PubMed] [Google Scholar]
- 45.Gydesen S., Andreassen K.V., Hjuler S.T., Hellgren L.I., Karsdal M.A., Henriksen K. Optimization of tolerability and efficacy of dual amylin and calcitonin receptor agonist, KBP-089, through dose escalation and combination with a GLP-1 analogue. American Journal of Physiology. Endocrinology and Metabolism. 2017:2016. doi: 10.1152/ajpendo.00419.2016. ajpendo.00419. [DOI] [PubMed] [Google Scholar]
- 46.Larsen A.T., Sonne N., Andreassen K.V., Gehring K., Karsdal M.A., Henriksen K. The dual amylin and calcitonin receptor agonist KBP-088 induces weight loss and improves insulin sensitivity superior to chronic amylin therapy. Journal of Pharmacology and Experimental Therapeutics. 2019;370:35–43. doi: 10.1124/jpet.119.257576. [DOI] [PubMed] [Google Scholar]
- 47.Mack C.M., Soares C.J., Wilson J.K., Athanacio J.R., Turek V.F., Trevaskis J.L. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. International Journal of Obesity. 2010;34:385–395. doi: 10.1038/ijo.2009.238. [DOI] [PubMed] [Google Scholar]
- 48.Mack C.M., Smith P.A., Athanacioa J.R., Xu K., Wilson J.K., Reynolds J.M. Glucoregulatory effects and prolonged duration of action of davalintide: a novel amylinomimetic peptide. Diabetes Obesity Metabolic. 2011;13:1105–1113. doi: 10.1111/j.1463-1326.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- 49.Gydesen S., Andreassen K.V., Hjuler S.T., Christensen J.M., Karsdal M.A., Henriksen K. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. American Journal of Physiology Endocrinology and Metabolism. 2016:2015. doi: 10.1152/ajpendo.00514.2015. ajpendo.00514. [DOI] [PubMed] [Google Scholar]
- 50.Hay D.L. https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=11 No Title.
- 52.Protein atlas. https://www.proteinatlas.org/ENSG00000064989-CALCRL/summary/rna
- 53.Naot D., Musson D.S., Cornish J. The activity of peptides of the calcitonin family in bone. Physiological Reviews. 2019;99:781–805. doi: 10.1152/physrev.00066.2017. [DOI] [PubMed] [Google Scholar]
- 54.Muff R., Bühlmann N., Fischer J.A., Born W. An amylin receptor is revealed following Co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- 55.Hay D.L., Pioszak A.A. Receptor activity-modifying proteins (RAMPs): new insights and roles. Annual Review of Pharmacology and Toxicology. 2016;56:469–487. doi: 10.1146/annurev-pharmtox-010715-103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnelo U., Reidelberger R., Adrian T.E., Larsson J., Permert J. Sufficiency of postprandial plasma levels of islet amyloid polypeptide for suppression of feeding in rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1998;275 doi: 10.1152/ajpregu.1998.275.5.R1537. [DOI] [PubMed] [Google Scholar]
- 57.Lutz T. Pancreatic amylin as a centrally acting satiating hormone. Current Drug Targets. 2005;6:181–189. doi: 10.2174/1389450053174596. [DOI] [PubMed] [Google Scholar]
- 58.Asmar M., Bache M., Knop F.K., Madsbad S., Holst J.J. Do the actions of glucagon-like peptide-1 on gastric emptying, appetite, and food intake involve release of amylin in humans? Journal of Clinical Endocrinology & Metabolism. 2010;95:2367–2375. doi: 10.1210/jc.2009-2133. [DOI] [PubMed] [Google Scholar]
- 59.Lutz T., Pieber T., E S. Proceedings annual meeting of the society for the study of ingestive behavior. 1996. Amylin reduces food intake in rats at doses that cause only slightly supraphysiological plasma amylin levels. [Google Scholar]
- 60.Vine W., Smith P., LaChappell R., Blase E., Lumpkin R., Young A. Nephrectomy decreases amylin and pramlintide clearance in rats. Hormone and Metabolic Research. 1998;30:514–517. doi: 10.1055/s-2007-978923. [DOI] [PubMed] [Google Scholar]
- 61.Watschinger B., Hartter E., Traindl O., Pohanka E., Pidlich J., Kovarik J. Increased levels of plasma amylin in advanced renal failure. Clinical Nephrology. 1992;37:131–134. [PubMed] [Google Scholar]
- 62.Young A.A., Rink T.J., Wang M.-W. Dose response characteristics for the hyperglycemic, hyperlactemic, hypotensive and hypocalcemic actions of amylin and calcitonin gene-related peptide-I (CGRP alpha) in the fasted, anaesthetized rat. Life Sciences. 1993;52:1717–1726. doi: 10.1016/0024-3205(93)90480-q. [DOI] [PubMed] [Google Scholar]
- 63.Li Z., Kelly L., Heiman M., Greengard P., Friedman J.M. Hypothalamic amylin acts in concert with leptin to regulate food intake. Cell Metabolism. 2015;22:1059–1067. doi: 10.1016/j.cmet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Ferrier G.J.M., Pierson A.M., Jones P.M., Bloom S.R., Girgis S.I., Legon S. Expression of the rat amylin (IAPP/DAP) gene. Journal of Molecular Endocrinology. 1989;3 doi: 10.1677/jme.0.003r001. [DOI] [PubMed] [Google Scholar]
- 65.Nicholl C.G., Bhatavdekar J.M., Mak J., Girgis S.I., Legon S. Extra-pancreatic expression of the rat islet amyloid polypeptide (amylin) gene. Journal of Molecular Endocrinology. 1992;9:157–163. doi: 10.1677/jme.0.0090157. [DOI] [PubMed] [Google Scholar]
- 66.Miyazato M., Nakazato M., Shiomi K., Aburaya J., Toshimori H., Kangawa K. Identification and characterization of islet amyloid polypeptide in mammalian gastrointestinal tract. Biochemical and Biophysical Research Communications. 1991;181:293–300. doi: 10.1016/s0006-291x(05)81416-0. [DOI] [PubMed] [Google Scholar]
- 67.Gebre-Medhin S., Mulder H., Pekny M., Westermark G., Törnell J., Westermark P. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin) Biochemical and Biophysical Research Communications. 1998;250:271–277. doi: 10.1006/bbrc.1998.9308. [DOI] [PubMed] [Google Scholar]
- 68.Olsson M., Herrington M.K., Reidelberger R.D., Permert J., Gebre-Medhin S., Arnelo U. Food intake and meal pattern in IAPP knockout mice with and without infusion of exogenous IAPP. Scandinavian Journal of Gastroenterology. 2012;47:191–196. doi: 10.3109/00365521.2011.638392. [DOI] [PubMed] [Google Scholar]
- 69.Rushing P.A., Hagan M.M., Seeley R.J., Lutz T.A., D’Alessio D.A., Air E.L. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142:5035–5038. doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- 70.Mollet A., Gilg S., Riediger T., Lutz T.A. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiology & Behavior. 2004;81:149–155. doi: 10.1016/j.physbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Reidelberger R.D., Haver A.C., Arnelo U., Smith D.D., Schaffert C.S., Permert J. Amylin receptor blockade stimulates food intake in rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2004;287:568–574. doi: 10.1152/ajpregu.00213.2004. [DOI] [PubMed] [Google Scholar]
- 72.Dunn-Meynell A.A., Foll Le C., Johnson M.D., Lutz T.A., Hayes M.R., Levin B.E. Endogenous VMH amylin signaling is required for full leptin signaling and protection from diet-induced obesity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2016;310:R355–R365. doi: 10.1152/ajpregu.00462.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young A.A., Wang M.-W., Gedulin B., Rink T.J., Pittner R., Beaumont K. Diabetogenic effects of salmon calcitonin are attributable to amylin-like activity. Metabolism. 1995;44:1581–1589. doi: 10.1016/0026-0495(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 74.Young A.A., Gedulin B., Vine W., Percy A., Rink T.J. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia. 1995;38:642–648. doi: 10.1007/BF00401833. [DOI] [PubMed] [Google Scholar]
- 75.Gedulin B.R., Jodka C.M., Herrmann K., Young A.A. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regulatory Peptides. 2006;137:121–127. doi: 10.1016/j.regpep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Gedulin Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46:67–70. doi: 10.1016/s0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- 77.Silvestre R.A., Rodri J., Jodka C., Parkes D.G., Pittner R.A., Young A.A. 2001. Selective amylin inhibition of the glucagon response to arginine is extrinsic to the pancreas. [DOI] [PubMed] [Google Scholar]
- 78.Kern K.A., Mietlicki-Baase E.G. Distributed amylin receptor signaling and its influence on motivated behavior. Physiology & Behavior. 2020;222 doi: 10.1016/j.physbeh.2020.112958. [DOI] [PubMed] [Google Scholar]
- 79.Karsdal M.A., Henriksen K., Arnold M., Christiansen C. Calcitonin??? A drug of the past or for the future? BioDrugs. 2008;22:137–144. doi: 10.2165/00063030-200822030-00001. [DOI] [PubMed] [Google Scholar]
- 80.Turner A.G., Tjahyono F., Chiu W.S.M., Skinner J., Sawyer R., Moore A.J. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone. 2011;48:354–361. doi: 10.1016/j.bone.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 81.Bartelt A., Jeschke A., Müller B., Gaziano I., Morales M., Yorgan T. Differential effects of Calca-derived peptides in male mice with diet-induced obesity. PloS One. 2017;12 doi: 10.1371/journal.pone.0180547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armour S.L., Foord S., Kenakin T., Chen W. Pharmacological characterization of receptor-activity-modifying proteins (RAMPs) and the human calcitonin receptor. Journal of Pharmacology and Toxicology. 2000;42:217–224. doi: 10.1016/s1056-8719(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 83.Nakamura M., Nomura S., Yamakawa T., Kono R., Maeno A., Ozaki T. Endogenous calcitonin regulates lipid and glucose metabolism in diet-induced obesity mice. Scientific Reports. 2018;8:1–10. doi: 10.1038/s41598-018-35369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsen A.T., Sonne N., Andreassen K.V., Karsdal M.A., Henriksen K. The calcitonin receptor plays a major role in glucose regulation as a function of dual amylin and calcitonin receptor agonist therapy. Journal of Pharmacology and Experimental Therapeutics. 2020;374:74–83. doi: 10.1124/jpet.119.263392. [DOI] [PubMed] [Google Scholar]
- 85.Gydesen S., Hjuler S.T., Freving Z., Andreassen K.V., Sonne N., Hellgren L.I. A novel dual amylin and calcitonin receptor agonist, KBP-089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. British Journal of Pharmacology. 2017;174:591–602. doi: 10.1111/bph.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hjuler S.T., Gydesen S., Andreassen K.V., Karsdal M.A., Henriksen K. The dual amylin- and calcitonin receptor agonist KBP-042 works as adjunct to metformin on fasting hyperglycaemia and HbA1c in a rat model of type 2 diabetes. Journal of Pharmacology and Experimental Therapeutics. 2017:241281. doi: 10.1124/jpet.117.241281. jpet.117. [DOI] [PubMed] [Google Scholar]
- 87.Nogueiras R., López M., Diéguez C. Regulation of lipid metabolism by energy availability: a role for the central nervous system. Obesity Reviews. 2010;11:185–201. doi: 10.1111/j.1467-789X.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 88.Mason R.T., Peterfreund R.A., Sawchenko P.E., Corrigan A.Z., Rivier J.E., Vale W.W. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature. 1984;308:653–655. doi: 10.1038/308653a0. [DOI] [PubMed] [Google Scholar]
- 89.Gibson S., Polak J., Bloom S., Sabate I., Mulderry P., Ghatei M. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. Journal of Neuroscience. 1984;4:3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russell F.A., King R., Smillie S.-J., Kodji X., Brain S.D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiological Reviews. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poyner D.R. Calcitonin gene-related peptide: multiple actions, multiple receptors. Pharmacology & Therapeutics. 1992;56:23–51. doi: 10.1016/0163-7258(92)90036-y. [DOI] [PubMed] [Google Scholar]
- 92.Walker C.S., Li X., Whiting L., Glyn-Jones S., Zhang S., Hickey A.J. Mice lacking the neuropeptide α-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology. 2010;151:4257–4269. doi: 10.1210/en.2010-0284. [DOI] [PubMed] [Google Scholar]
- 93.Liu T., Kamiyoshi A., Sakurai T., Ichikawa-Shindo Y., Kawate H., Yang L. Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology. 2017;158:1194–1206. doi: 10.1210/en.2016-1510. [DOI] [PubMed] [Google Scholar]
- 94.Riera C.E., Huising M.O., Follett P., Leblanc M., Halloran J., Van Andel R. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 95.Betsholtz C., Svensson V., Rorsman F., Engström U., Westermark G.T., Wilander E. Islet amyloid polypeptide (IAPP): cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Experimental Cell Research. 1989;183:484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- 96.Young A., Vine W., Gedulin B.R., Pittner R., Janes S., Gaeta L.S.L. Preclinical pharmacology of pramlintide in the rat: comparisons with human and rat amylin. Drug Development Research. 1996;37:231–248. [Google Scholar]
- 97.Mack C., Wilson J., Athanacio J., Reynolds J., Laugero K., Guss S. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;293:R1855–R1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- 98.Lutz T., Mollet A., Rushing P., Riediger T., Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postremaanucleus of the solitary tract (APaNTS) lesioned rats. International Journal of Obesity. 2001;25:1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 99.Lutz T.A., Geary N., Szabady M.M., Del Prete E., Scharrer E. Amylin decreases meal size in rats. Physiology & Behavior. 1995;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 100.Arnelo U., Permert J., Adrian T.E., Larsson J., Reidelberger R.D., Larsson J. Chronic infusion of islet amyloid polypeptide causes anorexia in rats. American Journal for Physiology. 1996;271:1654–1659. doi: 10.1152/ajpregu.1996.271.6.R1654. [DOI] [PubMed] [Google Scholar]
- 101.Mietlicki-baase E.G., Rupprecht L.E., Olivos D.R., Zimmer D.J., Alter M.D., Pierce R.C. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology. 2013;38:1685–1697. doi: 10.1038/npp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bello N.T., Kemm M.H., Moran T.H. Salmon calcitonin reduces food intake through changes in meal sizes in male rhesus monkeys. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2008;295 doi: 10.1152/ajpregu.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roth J.D., Hughes H., Kendall E., Baron A.D., Anderson C.M. Antiobesity effects of the β-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147:5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 104.Rushing P.A., Seeley R.J., Air E.L., Lutz T.A., Woods S.C. Acute 3rd-ventricular amylin infusion potently reduces food intake but does not produce aversive consequences. Peptides. 2002;23:985–988. doi: 10.1016/s0196-9781(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 105.Chance W.T., Balasubramaniam A., Chen X., Fischer J.E. Tests of adipsia and conditioned taste aversion following the intrahypothalamic injection of amylin. Peptides. 1992;13:961–964. doi: 10.1016/0196-9781(92)90057-a. [DOI] [PubMed] [Google Scholar]
- 106.Freed W.J., Perlow M.J., Wyatt R.J. Calcitonin: inhibitory effect on eating in rats. Science. 1979;206(80):850–852. doi: 10.1126/science.493987. [DOI] [PubMed] [Google Scholar]
- 107.Hjuler S.T., Gydesen S., Andreassen K.V., Pedersen S.L.K., Hellgren L.I., Karsdal M.A. The dual amylin- and calcitonin-receptor agonist KBP-042 increases insulin sensitivity and induces weight loss in rats with obesity. Obesity (Silver Spring). 2016;24:1712–1722. doi: 10.1002/oby.21563. [DOI] [PubMed] [Google Scholar]
- 108.Larsen A.T., Sonne N., Andreassen K.V., Karsdal M.A., Henriksen K. Dose frequency optimization of the dual amylin and calcitonin receptor agonist KBP-088: long-lasting improvement of food preference and body weight loss. Journal of Pharmacology and Experimental Therapeutics. 2020;373 doi: 10.1124/jpet.119.263400. [DOI] [PubMed] [Google Scholar]
- 109.Hjuler S.T., Andreassen K.V., Gydesen S., Karsdal M.A., Henriksen K. KBP-042 improves bodyweight and glucose homeostasis with indices of increased insulin sensitivity irrespective of route of administration. European Journal of Pharmacology. 2015;762:229–238. doi: 10.1016/j.ejphar.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 110.Issakson B., Wang F., Permert J., Olsson M., Fruin B., Herrington M.K. Chronically administered islet amyloid polypeptide in rats serves as an adiposity inhibitor and regulates energy homeostasis. Pancreatology. 2005;5:29–36. doi: 10.1159/000084488. [DOI] [PubMed] [Google Scholar]
- 111.Wielinga P.Y., Alder B., Lutz T.A. The acute effect of amylin and salmon calcitonin on energy expenditure. Physiology & Behavior. 2007;91:212–217. doi: 10.1016/j.physbeh.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 112.Wielinga P.Y., Löwenstein C., Muff S., Munz M., Woods S.C., Lutz T.A. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiology & Behavior. 2010;101:45–52. doi: 10.1016/j.physbeh.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seth R., Knight W.D., Overton J.M. Combined amylin-leptin treatment lowers blood pressure and adiposity in lean and obese rats. International Journal of Obesity(Lond) 2011;35:1183–1192. doi: 10.1038/ijo.2010.262. [DOI] [PubMed] [Google Scholar]
- 114.Bouali S.M., Wimalawansa S.J., Jolicoeur F.B. In vivo central actions of rat amylin. Regulatory Peptides. 1995;56:167–174. doi: 10.1016/0167-0115(95)00009-z. [DOI] [PubMed] [Google Scholar]
- 115.Færch K., Vistisen D., Pacini G., Torekov S.S., Johansen N.B., Witte D.R. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes. 2016;65:3473–3481. doi: 10.2337/db16-0240. [DOI] [PubMed] [Google Scholar]
- 116.Feigh M. A novel oral form of salmon calcitonin improves homeostasis and reduces body weight in diet-induced obese rats. Diabetes, Obesity and Metabolism. 2011;13:911–920. doi: 10.1111/j.1463-1326.2011.01425.x. [DOI] [PubMed] [Google Scholar]
- 117.Mietlicki-Baase E.G., McGrath L.E., Koch-Laskowski K., Krawczyk J., Reiner D.J., Pham T. Amylin receptor activation in the ventral tegmental area reduces motivated ingestive behavior. Neuropharmacology. 2017;123:67–79. doi: 10.1016/j.neuropharm.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lutz T.A., Coester B., Whiting L., Dunn-Meynell A.A., Boyle C.N., Bouret S.G. Amylin selectively signals onto POMC neurons in the arcuate nucleus of the hypothalamus. Diabetes. 2018;67:805–817. doi: 10.2337/db17-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whiting L., McCutcheonb J.E., Boyle C.N., Roitman M.F., Lutz T.A. The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc) Physiology & Behavior. 2017;176 doi: 10.1016/j.physbeh.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Braegger F.E., Asarian L., Dahl K., Lutz T.A., Boyle C.N. The role of the area postrema in the anorectic effects of amylin and salmon calcitonin: behavioral and neuronal phenotyping. European Journal of Neuroscience. 2014;40:3055–3066. doi: 10.1111/ejn.12672. [DOI] [PubMed] [Google Scholar]
- 121.Wickbom J., Herrington M.K., Permert J., Jansson A., Arnelo U. Gastric emptying in response to IAPP and CCK in rats with subdiaphragmatic afferent vagotomy. Regulatory Peptides. 2008;148:21–25. doi: 10.1016/j.regpep.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 122.Lutz T.A., Del Prete E., Scharrer E. Subdiaphragmatic vagotomy does not influence the anorectic effect of amylin. Peptides. 1995;16:457–462. doi: 10.1016/0196-9781(94)00203-i. [DOI] [PubMed] [Google Scholar]
- 123.Miegueu P., St-Pierre D.H., Munkonda M.N., Lapointe M., Cianflone K. Amylin stimulates fatty acid esterification in 3T3-L1 adipocytes. Molecular and Cellular Endocrinology. 2013;366:99–107. doi: 10.1016/j.mce.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 124.Isaksson B., Wang F., Permert J., Olsson M., Fruin B., Herrington M.K. Chronically administered islet amyloid polypeptide in rats serves as an adiposity inhibitor and regulates energy homeostasis. Pancreatology. 2005;5:29–36. doi: 10.1159/000084488. [DOI] [PubMed] [Google Scholar]
- 125.Moon H.S., Chamberland J.P., Diakopoulos K.N., Fiorenza C.G., Ziemke F., Schneider B. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care. 2011;34:132–138. doi: 10.2337/dc10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cooper G.J.S., Leighton B., Dimitriadis G.D., Parry-Billings M., Kowalchuk J.M., Howland K. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lupien R.J., Young A. No measurable effect of amylin on lipolysis in either white or brown isolated adipocytes from rats. Diabetes, Nutrition Metabolic. 1993;6:13–18. [Google Scholar]
- 128.Pittner R.A., Wolfe-Lopez D., Young A.A., Rink T.J. Amylin and epinephrine have no direct effect on glucose transport in isolated rat soleus muscle. FEBS Letters. 1995;365:98–100. doi: 10.1016/0014-5793(95)00449-j. [DOI] [PubMed] [Google Scholar]
- 129.Young D.A., Deems R.O., Deacon R.W., McIntosh R.H., Foley J.E. Effects of amylin in glucose metabolism and glycogenolysis in vivo and in vitro. American Journal of Physiology. Endocrinology and Metabolism. 1990;259 doi: 10.1152/ajpendo.1990.259.3.E457. [DOI] [PubMed] [Google Scholar]
- 130.Leighton B., Foot E. The effects of amylin on carbohydrate metabolism in skeletal muscle in vitro and in vivo. Biochemical Journal. 1990;269:19–23. doi: 10.1042/bj2690019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kreutter D.K., Orena S.J., Torchia A.J., Contillo L.G., Andrews G.C., Stevenson R.W. Amylin and CGRP induce insulin resistance via a receptor distinct from cAMP-coupled CGRP receptor. American Journal of Physiology. Endocrinology and Metabolism. 1993;264 doi: 10.1152/ajpendo.1993.264.4.E606. [DOI] [PubMed] [Google Scholar]
- 132.Leighton B., Cooper G.J.S. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988;335:632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- 133.Aronne L., Fujioka K., Aroda V., Chen K., Halseth A., Kesty N.C. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. Journal of Clinical Endocrinology & Metabolism. 2007;92:2977–2983. doi: 10.1210/jc.2006-2003. [DOI] [PubMed] [Google Scholar]
- 134.Ratner R.E., Want L.L., Fineman M.S., Velte M.J., Ruggles J.A., Gottlieb A. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technology & Therapeutics. 2002;4:51–61. doi: 10.1089/15209150252924094. [DOI] [PubMed] [Google Scholar]
- 135.Whitehouse F., Kruger D.F., Fineman M., Shen L., Ruggles J.A., Maggs D.G. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724–730. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- 136.Petralito A., Lunetta M., Liuzzo A., Fiore C.E., Heynen G. Effects of salmon calcitonin on blood glucose and insulin levels under basal conditions and after intravenous glucose load. Journal of Endocrinological Investigation. 1979;2:209–211. doi: 10.1007/BF03349315. [DOI] [PubMed] [Google Scholar]
- 137.Passariello N., Giugliano D., Sgambato S., Torella R., D’onofrio F. Calcitonin, A diabetogenic hormone? Journal of Clinical Endocrinology & Metabolism. 1981;53:318–323. doi: 10.1210/jcem-53-2-318. [DOI] [PubMed] [Google Scholar]
- 138.Gattereau A., Bielmann P., Durivage J., Davignon J., Larochelle P. Effect of acute and chronic administration of calcitonin on serum glucose in patients with paget's disease of bone. Journal of Clinical Endocrinology & Metabolism. 1980;51:354–357. doi: 10.1210/jcem-51-2-354. [DOI] [PubMed] [Google Scholar]
- 139.Young A.A., Wang M.W., Cooper G.J.S. Amylin injection causes elevated plasma lactate and glucose in the rat. FEBS Letters. 1991;291:101–104. doi: 10.1016/0014-5793(91)81113-m. [DOI] [PubMed] [Google Scholar]
- 140.Martínez-Sánchez N. There and back again: leptin actions in white adipose tissue. International Journal of Molecular Sciences. 2020;21 doi: 10.3390/ijms21176039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 142.Marazziti D., Rutigliano G., Baroni S., Landi P., Dell'Osso L. Metabolic syndrome and major depression. CNS Spectrums. 2014;19:293–304. doi: 10.1017/S1092852913000667. [DOI] [PubMed] [Google Scholar]
- 143.Kusakabe T., Ebihara K., Sakai T., Miyamoto L., Aotani D., Yamamoto Y. Amylin improves the effect of leptin on insulin sensitivity in leptin-resistant diet-induced obese mice. American Journal of Physiology. Endocrinology and Metabolism. 2012;302 doi: 10.1152/ajpendo.00198.2011. [DOI] [PubMed] [Google Scholar]
- 144.Duffy S., Lutz T.A., Boyle C.N. Rodent models of leptin receptor deficiency are less sensitive to amylin. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2018;315:R856–R865. doi: 10.1152/ajpregu.00179.2018. [DOI] [PubMed] [Google Scholar]
- 145.Levin B.E., Lutz T.A. Amylin and leptin: Co-regulators of energy homeostasis and neuronal development. Trends in Endocrinology and Metabolism. 2017;28:153–164. doi: 10.1016/j.tem.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 146.Roth J.D., Erickson M.R., Chen S., Parkes D.G. GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. British Journal of Pharmacology. 2012;166:121–136. doi: 10.1111/j.1476-5381.2011.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jansson J.O., Palsdottir V. Brain IL-6 - where amylin and GLP-1 antiobesity signaling congregate. Diabetes. 2015;64:1498–1499. doi: 10.2337/db14-1910. [DOI] [PubMed] [Google Scholar]
- 148.Bello N.T., Kemm M.H., Ofeldt E.M., Moran T.H. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. American Journal of Physiology Integrative Comparative Physiology. 2010;299:R945–R952. doi: 10.1152/ajpregu.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.No Title. 2010. http://www.takeda.de/en-us/newsroom/news-releases/
- 150.Nordisk N. 2020. Novo Nordisk successfully completes AM833 phase 2 trial and phase 1 combination trial with AM833 and semaglutide in obesity. [Google Scholar]
- 151.Brown A., Guess N., Dornhorst A., Taheri S., Frost G. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes, Obesity and Metabolism. 2017;19:1655–1668. doi: 10.1111/dom.13009. [DOI] [PubMed] [Google Scholar]
- 152.Aronne L.J., Halseth A.E., Burns C.M., Miller S., Shen L.Z. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity (Silver Spring). 2010;18:1739–1746. doi: 10.1038/oby.2009.478. [DOI] [PubMed] [Google Scholar]
- 153.Ravussin E., Smith S.R., Mitchell J.A., Shringarpure R., Shan K., Maier H. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring). 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maggs D.G., Fineman M., Kornstein J., Burrell T., Schwartz S., Wang Y. Pramlintide reduces postprandial glucose excursions when added to insulin lispro in subjects with type 2 diabetes: a dose-timing study. Diabetes Metabolism Research Review. 2004;20:55–60. doi: 10.1002/dmrr.419. [DOI] [PubMed] [Google Scholar]
- 155.Levetan C., Want L.L., Weyer C., Strobel S.A., Crean J., Wang Y. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8. doi: 10.2337/diacare.26.1.1. [DOI] [PubMed] [Google Scholar]
- 156.Kolterman O.G., Gottlieb A., Moyses C., Colburn W. Reduction of postprandial hyperglycemia in subjects with IDDM by intravenous infusion of AC137, a human amylin analogue. Diabetes Care. 1995;18:1179–1182. doi: 10.2337/diacare.18.8.1179. [DOI] [PubMed] [Google Scholar]
- 157.Kong M.F., Stubbs T.A., King P., Macdonald I.A., Lambourne J.E., Blackshaw P.E. The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia. 1998;41:577–583. doi: 10.1007/s001250050949. [DOI] [PubMed] [Google Scholar]
- 158.Samsom M., Szarka L.A., Camilleri M., Vella A., Zinsmeister A.R., Rizza R.A. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2000;278:G946–G951. doi: 10.1152/ajpgi.2000.278.6.G946. [DOI] [PubMed] [Google Scholar]
- 159.Edelman S.V., Weyer C. Unresolved challenges with insulin therapy in type 1 and type 2 diabetes: potential benefit of replacing amylin, a second b-cell hormone. Diabetes Technology & Therapeutics. 2002;4 doi: 10.1089/15209150260007390. [DOI] [PubMed] [Google Scholar]
- 160.Dinneen S., Alzaid A., Turk D., Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–343. doi: 10.1007/BF00400639. [DOI] [PubMed] [Google Scholar]
- 161.Fineman M., Weyer C., Maggs D.G., Strobel S., Kolterman O.G. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Hormone and Metabolic Research. 2002;34:504–508. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- 162.Nyholm B., Ørskov L., Hove K.Y., Gravholt C.H., Møller N., George K. The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism. 1999;48:935–941. doi: 10.1016/s0026-0495(99)90232-9. [DOI] [PubMed] [Google Scholar]
- 163.Chapman I., Parker B., Doran S., Feinle-Bisset C., Wishart J., Strobel S. Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia. 2005;48:838–848. doi: 10.1007/s00125-005-1732-4. [DOI] [PubMed] [Google Scholar]
- 164.Hollander P.A., Levy P., Fineman M.S., Maggs D.G., Shen L.Z., Strobel S.A. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2003;25:784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- 165.Hollander P., Maggs D.G., Ruggles J.A., Fineman M., Shen L., Kolterman O.G. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obesity Research. 2004;12:661–668. doi: 10.1038/oby.2004.76. [DOI] [PubMed] [Google Scholar]
- 166.Edelman S., Garg S., Frias J., Maggs D., Wang Y., Zhang B. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006;29:2189–2195. doi: 10.2337/dc06-0042. [DOI] [PubMed] [Google Scholar]
- 167.Wysham C., Lush C., Zhang B., Maier H., Wilhelm K. 2007. Effect of pramlintide as an adjunct to basal insulin on markers of cardiovascular risk in patients with type 2 diabetes. [DOI] [PubMed] [Google Scholar]
- 168.Herrmann K., Zhou M., Wang A., De Bruin T.W.A. 2016. Cardiovascular safety assessment of pramlintide in type 2 diabetes: results from a pooled analysis of five clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.MacMahon S., Baigent C., Duffy S., Rodgers A., Tominaga S., Chambless L. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kalafateli A.L., Vestlund J., Raun K., Egecioglu E., Jerlhag E. Effects of a selective long-acting amylin receptor agonist on alcohol consumption, food intake and body weight in male and female rats. Addiction Biology. 2020 doi: 10.1111/adb.12910. [DOI] [PubMed] [Google Scholar]
- 172.Liberini C.G., Koch-Laskowski K., Shaulson E., McGrath L.E., Lipsky R.K., Lhamo R. Combined Amylin/GLP-1 pharmacotherapy to promote and sustain long-lasting weight loss. Scientific Reports. 2019;9:8447. doi: 10.1038/s41598-019-44591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nascimento C.V.M.F., Sinezia C., Sisnande T., Lima L.M.T.R., Lacativa P.G.S. BZ043, a novel long-acting amylin analog, reduces gastric emptying, food intake, glycemia and insulin requirement in streptozotocin-induced diabetic rats. Peptides. 2019;114:44–49. doi: 10.1016/j.peptides.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 174.https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-001061-24/results No Title.
- 175.Larsen Anna Thorsø, Sonne Nina, Andreassen Kim Vietz, Karsdal, Asser Morten, Henriksen K. Deconstruction of the role of the amylin and calcitonin receptors in the regulation of body weight and glucose tolerance. American Diabetes Association. 2019:1993. doi: 10.2337/db19-1993-P. [DOI] [Google Scholar]
- 176.Krahn D.D., Gosnell B.A., Levine A.S., Morley J.E. Effects of calcitonin gene-related peptide on food intake. Peptides. 1984;5:861–864. doi: 10.1016/0196-9781(84)90107-4. [DOI] [PubMed] [Google Scholar]
- 177.Lutz T., Rossi R., Althaus J., Del Prete E., Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides. 1998;19:1533–1540. doi: 10.1016/s0196-9781(98)00114-4. [DOI] [PubMed] [Google Scholar]
- 178.Danaher R.N., Loomes K.M., Leonard B.L., Whiting L., Hay D.L., Xu L.Y. Evidence that α-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology. 2008;149:154–160. doi: 10.1210/en.2007-0583. [DOI] [PubMed] [Google Scholar]
- 179.Nilsson C., Hansen T.K., Rosenquist C., Hartmann B., Kodra J.T., Lau J.F. Long acting analogue of the calcitonin gene-related peptide induces positive metabolic effects and secretion of the glucagon-like peptide-1. European Journal of Pharmacology. 2016;773:24–31. doi: 10.1016/j.ejphar.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 180.Sanford D., Luong L., Gabalski A., Oh S., Vu J.P., Pisegna J.R. An intraperitoneal treatment with calcitonin gene-related peptide (CGRP) regulates appetite, energy intake/expenditure, and metabolism. Journal of Molecular Neuroscience. 2019;67:28–37. doi: 10.1007/s12031-018-1202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rossetti L., Farrace S., Choi S.B., Giaccari A., Sloan L., Frontoni S. Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. American Journal Physiology Metablism. 1993;264:E1–E10. doi: 10.1152/ajpendo.1993.264.1.E1. [DOI] [PubMed] [Google Scholar]
- 182.Tepper S.J. History and review of anti-calcitonin gene-related peptide (CGRP) therapies: from translational research to treatment. Headache: The Journal of Head and Face Pain. 2018;58:238–275. doi: 10.1111/head.13379. [DOI] [PubMed] [Google Scholar]
- 183.Deen M., Correnti E., Kamm K., Kelderman T., Papetti L., Rubio-Beltrán E. Blocking CGRP in migraine patients – a review of pros and cons. The Journal of Headache and Pain. 2017;18:96. doi: 10.1186/s10194-017-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Servizi S., Corrigan R.R., Casadesus G. The importance of understanding amylin signaling mechanisms for therapeutic development in the treatment of Alzheimer's disease. Current Pharmaceutical Design. 2020;26:1345–1355. doi: 10.2174/1381612826666200318151146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Neergaard J.S., Dragsbæk K., Christiansen C., Nielsen H.B., Brix S., Karsdal M.A. Metabolic syndrome, insulin resistance, and cognitive Dysfunction: does your metabolic profile Affect your brain? Diabetes. 2017;66:1957–1963. doi: 10.2337/db16-1444. [DOI] [PubMed] [Google Scholar]
- 186.Bettini S., Belligoli A., Fabris R., Busetto L. Diet approach before and after bariatric surgery. Reviews in Endocrine & Metabolic Disorders. 2020 doi: 10.1007/s11154-020-09571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Buchwald H., Avidor Y., Braunwald E., Jensen M.D., Pories W., Fahrbach K. Bariatric surgery. Journal of the American Medical Association. 2004;292:1724. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]