Abstract
Background
GLP-1 receptor agonists (GLP-1 RAs) with exenatide b.i.d. first approved to treat type 2 diabetes in 2005 have been further developed to yield effective compounds/preparations that have overcome the original problem of rapid elimination (short half-life), initially necessitating short intervals between injections (twice daily for exenatide b.i.d.).
Scope of review
To summarize current knowledge about GLP-1 receptor agonist.
Major conclusions
At present, GLP-1 RAs are injected twice daily (exenatide b.i.d.), once daily (lixisenatide and liraglutide), or once weekly (exenatide once weekly, dulaglutide, albiglutide, and semaglutide). A daily oral preparation of semaglutide, which has demonstrated clinical effectiveness close to the once-weekly subcutaneous preparation, was recently approved. All GLP-1 RAs share common mechanisms of action: augmentation of hyperglycemia-induced insulin secretion, suppression of glucagon secretion at hyper- or euglycemia, deceleration of gastric emptying preventing large post-meal glycemic increments, and a reduction in calorie intake and body weight. Short-acting agents (exenatide b.i.d., lixisenatide) have reduced effectiveness on overnight and fasting plasma glucose, but maintain their effect on gastric emptying during long-term treatment. Long-acting GLP-1 RAs (liraglutide, once-weekly exenatide, dulaglutide, albiglutide, and semaglutide) have more profound effects on overnight and fasting plasma glucose and HbA1c, both on a background of oral glucose-lowering agents and in combination with basal insulin. Effects on gastric emptying decrease over time (tachyphylaxis). Given a similar, if not superior, effectiveness for HbA1c reduction with additional weight reduction and no intrinsic risk of hypoglycemic episodes, GLP-1RAs are recommended as the preferred first injectable glucose-lowering therapy for type 2 diabetes, even before insulin treatment. However, GLP-1 RAs can be combined with (basal) insulin in either free- or fixed-dose preparations. More recently developed agents, in particular semaglutide, are characterized by greater efficacy with respect to lowering plasma glucose as well as body weight. Since 2016, several cardiovascular (CV) outcome studies have shown that GLP-1 RAs can effectively prevent CV events such as acute myocardial infarction or stroke and associated mortality. Therefore, guidelines particularly recommend treatment with GLP-1 RAs in patients with pre-existing atherosclerotic vascular disease (for example, previous CV events). The evidence of similar effects in lower-risk subjects is not quite as strong. Since sodium/glucose cotransporter-2 (SGLT-2) inhibitor treatment reduces CV events as well (with the effect mainly driven by a reduction in heart failure complications), the individual risk of ischemic or heart failure complications should guide the choice of treatment. GLP-1 RAs may also help prevent renal complications of type 2 diabetes. Other active research areas in the field of GLP-1 RAs are the definition of subgroups within the type 2 diabetes population who particularly benefit from treatment with GLP-1 RAs. These include pharmacogenomic approaches and the characterization of non-responders. Novel indications for GLP-1 RAs outside type 2 diabetes, such as type 1 diabetes, neurodegenerative diseases, and psoriasis, are being explored. Thus, within 15 years of their initial introduction, GLP-1 RAs have become a well-established class of glucose-lowering agents that has the potential for further development and growing impact for treating type 2 diabetes and potentially other diseases.
Keywords: Glucagon-like peptide-1 receptor agonists, Exenatide, Lixisenatide, Liraglutide, Dulaglutide, Albiglutide, Semaglutide, Type 2 diabetes, Cardiovascular disease, Body weight
Highlights
-
•
The GLP-1 receptor agonists class comprises seven compounds/preparations with a similar mode of action.
-
•
GLP-1 receptor agonists differ with respect to pharmacokinetic properties, duration of action, and clinical effectiveness.
-
•
Plasma glucose is lowered by effects on insulin and glucagon secretion, and by decelerating gastric emptying.
-
•
GLP-1 receptor agonists lower body weight by their influence on the central nervous system.
-
•
GLP-1 R reduce cardiovascular events (myocardial infarction, stroke, and associated mortality).
1. Development of GLP-1 RAs
The identification of gut-derived glucagon-like peptide-1 (GLP-1), putatively belonging to the family of incretin hormones (i.e. gastrointestinal hormones released after nutrient intake with the ability to glucose-dependently augment insulin secretory responses during periods characterized by hyperglycemia) triggered the development of GLP-1 receptor agonists (GLP-1 RAs). The groups around Jens Holst (Copenhagen, Denmark) [1] and Joel Habener (Boston, MA, USA) [2] were the first to correctly identify “truncated” GLP-1 (GLP-1 [7–36 amide], the amidated form [1], or GLP-1 [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]], the glycine-extended form [2]), as the product(s) of proglucagon translational processing in mammalian gut mucosa (L cells) as published in 1987. Based on the proglucagon nucleotide sequence, prior assumptions regarding processing enzymes led to an erroneous GLP-1 sequence longer by 6 N-terminal amino acid residues [3]. However, “truncated” GLP-1 was clearly insulinotropic at much lower (picomolar) concentrations compared to the extended GLP-1 sequence [1,2]. Initial studies with rodent models indicated that GLP-1 is highly effective as an insulinotropic agent in non-diabetic, metabolically healthy animals, but shared substantially reduced biological activity in diabetic animals with the previously identified incretin glucose-dependent insulinotropic polypeptide (GIP) [4]. Nevertheless, studies in human subjects with type 2 diabetes surprisingly showed well-preserved insulinotropic activity of both GLP-1 [7–36 amide] [5] and GLP-1 [7–36] that was accompanied by a short-term reduction in plasma glucose in the normal fasting range in patients previously characterized by persistent hyperglycemia [6,7]. However, GLP-1 was found to be proteolytically degraded and inactivated by the ubiquitous protease dipeptidyl peptidase-4 (DPP-4) [8] and both the intact GLP-1 molecule and DPP-4-generated metabolites (GLP-1 [9–36 amide] or [9–36]) were subject to rapid elimination from the circulation, with an elimination half-life of approximately 2 min [9]. Therefore, GLP-1 allowed the “proof-of-principle” that GLP-1 receptor stimulation is a suitable method of reducing plasma glucose in subjects with type 2 diabetes. It also helped clarify the three main mechanisms leading to reductions in plasma glucose concentrations: (a) glucose-dependent insulinotropic actions [5], (b) suppression of glucagon hypersecretion [7] except during episodes characterized by hypoglycemia [10], and (c) a deceleration of gastric emptying, which was found to be associated with marked effects on post-meal glycemic excursions [11]. Relatively early acute changes in appetite, satiety, and prospective food consumption by pharmacological doses of GLP-1 were described, resulting in a corresponding reduction in caloric intake [12], thus increasing the motivation to develop compounds mimicking the physiology of GLP-1 resistant to the proteolytic inactivation by DPP-4 and with slower elimination kinetics to allow for reasonable administration frequencies. As a product of serendipity, the peptide exendin-4 from the saliva of a venomous lizard (Heloderma suspectum, the Gila monster) was found to be homologous to mammalian GLP-1 and able to bind and activate GLP-1 receptors [13,14]. Synthetic exendin-4 was named exenatide and, without further modification, was the first GLP-1 receptor agonist approved to treat type 2 diabetes. The detailed background of the (patho)physiology of the incretin system and the history of the development of incretin-based glucose-lowering medications have recently been reviewed [15,16].
2. GLP-1 RAs available in 2020 and their pharmacokinetic properties (Table 1)
Table 1.
Characteristics of GLP-1 RAs that have been approved to treat type 2 diabetes as of 2020.
| GLP-1 RA | First approved (date) | Molecular weight (Da)c | Reference amino acid sequence | Other important components | Elimination half-life | Administration schedule | Pharmaceutical company | Reference |
|---|---|---|---|---|---|---|---|---|
| For subcutaneous injection | ||||||||
| Short-acting compounds | ||||||||
| Exenatide b.i.d. | 2005 (USA); 2006 (Europe); Byetta |
4186.6 | Exendin-4 | None | 3.3–4.0 h | Twice daily | AstraZenecai | [21] |
| Lixisenatide | 2013 (Europe); Lyxumia; 2016 (USA); Adlyxin |
4858.5 | Exendin-4 | Poly-lysine tail | 2.6 h | Once daily | Sanofi | [22] |
| Long-acting compounds/preparations | ||||||||
| Liraglutide | 2009 (Europe); 2010 (USA); Victoza |
3751.2 | Mammalian GLP-1 | Free fatty acide | 12.6–14.3 h | Once daily | Novo Nordisk | [23] |
| Once-weekly exenatide | 2012; BYDUREONa | 4186.6 | Exendin-4 | Active ingredient encapsulated in microspheres of poly-(d,l-lactide-co-glycolide) | 3.3–4.0 hf | Once weekly | AstraZenecai | [21] |
| Dulaglutide | 2014; Trulicity | 59670.6 | Mammalian GLP-1 | Immunoglobulin Fc fragment | 4.7–5.5 d | Once weekly | Eli Lilly and Company | [24] |
| Albiglutide | 2014 (Europe); Eperzan Tanzeum (USA)b | 72971.3 | Mammalian GLP-1 | Albumin | 5.7–6.8 d | Once weekly | GlaxoSmithKline | [25] |
| Semaglutide | 2017 (USA); 2019 (Europe); Ozempic |
4113.6 | Mammalian GLP-1 | Free fatty acide | 5.7–6.7 d | Once weekly | Novo Nordisk | [26] |
| For oral administration | ||||||||
| Semaglutide (long-acting) | 2020; Rybelsus | 4113.6 | Mammalian GLP-1 | Free fatty acide | 5.7–6.7 d | Once daily | Novo Nordisk | [27] |
| Fixed-dose combinations | ||||||||
| With basal insulin (for subcutaneous injection) | ||||||||
| Liraglutide/insulin degludec (iDegLira) | 2014 (Europe); 2016 (USA); Xultophy |
3751.2d | Mammalian GLP-1 | Basal insulin | 12.6–14.3 h | Once daily (anytimeg) | Novo Nordisk | [28] |
| Lixisenatide/insulin glargine (iGlarLixi) | 2016 (USA); Soliqua 100/33; 2017 (Europe); Suliqua |
4858.5d | Exendin-4 | Basal Insulin | 2.6 h | Once dailyh | Sanofi | [29] |
Improved once-weekly auto-injector BYDUREON BCise was approved in 2018.
Marketing was discontinued in 2018.
Mammalian GLP-1: 3297.7.
For the GLP-1 RA component only.
Promoting binding to albumin.
Identical to the short-acting preparation.
Approximately the same time every day.
Before meals with the highest expected glycemic excursion.
Previously Amylin Pharmaceuticals, Eli Lilly and Company, and Bristol Myers Squibb.
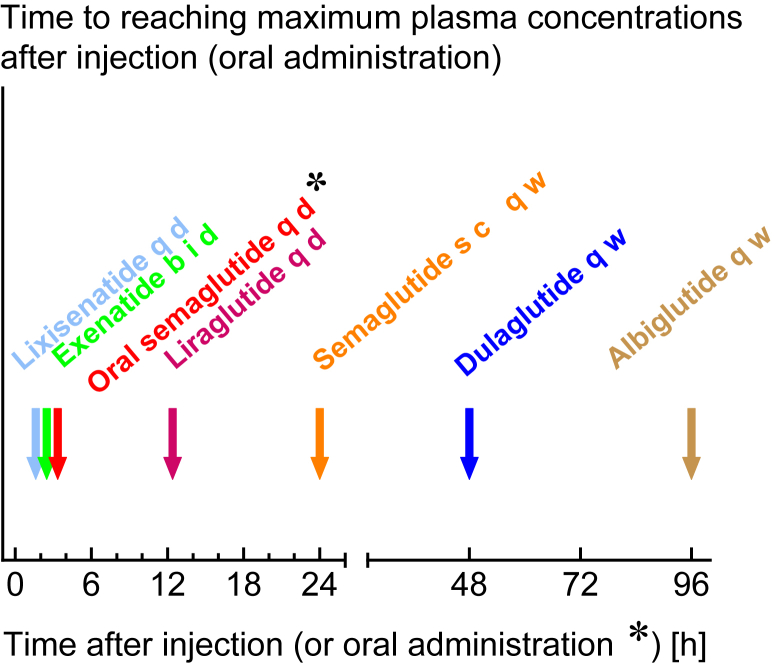
Following the approval of exenatide to treat type 2 diabetes (USA: 2005; Europe: 2006), several pharmaceutical companies started diverse developments aiming at GLP-1 receptor stimulation with greater effectiveness and longer duration of action. Exenatide needs to be injected at least twice daily, which mainly provides active circulating concentrations covering two major meals every day, with low levels between the two injections. Liraglutide, approved in 2009, was designed to provide a nearly unchanged amino acid sequence compared to mammalian GLP-1. A free fatty acid side chain was coupled to the peptide, which promotes binding to albumin in plasma and interstitial fluid. Only a minor proportion (estimated 1–2%) of liraglutide circulates in a free (non-albumin-bound) form, ready to diffuse into tissues and bind receptors. The albumin-bound bulk forms a reservoir promoting prolonged action. Overall, the elimination half-life is approximately 13 h, making it a suitable preparation for once-daily injection. The next step was aiming at once-weekly injections of GLP-1 RAs. Exenatide was developed as a novel preparation with the active ingredient slowly released after subcutaneous injection from a matrix dissolving over time. Thus, the onset of action was very much delayed, and a steady state was not reached until 8–10 weeks of treatment [17,18]. Other approaches followed the strategy to couple (modified) GLP-1 to large proteins such as an immunoglobulin Fc fragment (dulaglutide or efpeglenatide) or albumin (albiglutide). These compounds appear to slowly degrade, with half-lives of approximately one week. After subcutaneous injection, they reach effective circulating concentrations relatively early, thus beginning to lower plasma glucose soon after initiating such treatment. Semaglutide is another compound with a structure generally similar to liraglutide (GLP-1 with a free fatty acid side chain) but with a much longer half-life, apparently mediated by even tighter coupling to albumin. Semaglutide is presently available for once-weekly subcutaneous injection. More recently, semaglutide was co-formulated with sodium N-(8-(2-hydroxybenzoyl) amino) caprylate (SNAC) for oral treatment. To account for the relatively low bioavailability of semaglutide when absorbed through the gastrointestinal tract, oral semaglutide needs to be administered daily. This is the first GLP-1 RA approved for oral administration. At equivalent doses, subcutaneous and oral semaglutide seem to have similar effects on HbA1c, body weight, and adverse events [19]. Details regarding the molecular structures of various GLP-1 RAs and additional pharmacokinetic information were summarized by Nauck and Meier in 2019 [20].The time between subcutaneous (or oral) administration and the occurrence of peak concentrations is displayed in Figure 1.
Figure 1.
Arrows indicate the time from injection (or oral administration in the case of oral semaglutide) to peak plasma concentrations (Cmax) for GLP-1 RAs (Tmax). For references, please see [20]. Peak plasma concentrations may determine the time when nausea and vomiting are observed with GLP-1 RA treatment. The extremely slow absorption of once-weekly exenatide does not allow identification of a peak.
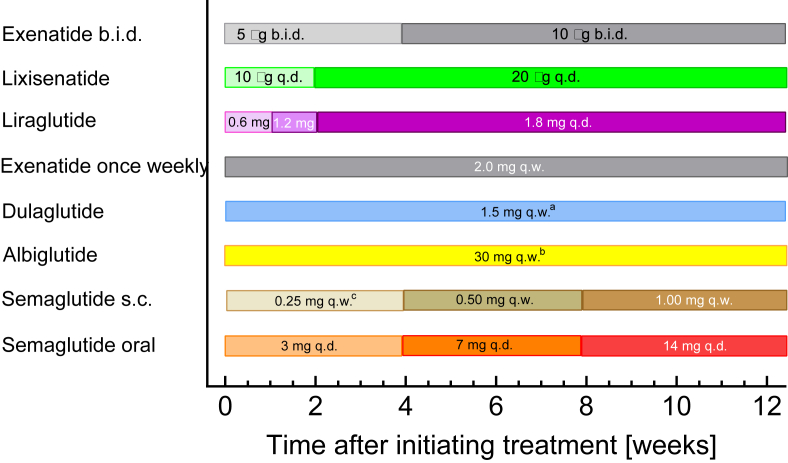
2.1. Recommendations for initial up-titration (Figure 2)
Figure 2.
Recommendations issued in official package inserts regarding the necessity for slow up-titration of approved GLP-1 receptor agonists.
All GLP-1 RAs developed to date have been designed for standardized dosage recommendations applicable to most if not all patients. Nausea and vomiting were noticed as common side effects, mainly occurring after the initiation of injection treatment or after increasing the dose. Peak plasma concentrations may determine the time when these symptoms most likely occur. In the early stages, a strategy of starting exenatide with a lower than maintenance dose, slowly increasing to the desired steady state, was found to reduce problems with gastrointestinal adverse events. Since then, recommendations have been developed for such an up-titration (dose escalation) approach to induce tolerance before patients are exposed to higher doses of GLP-1 RAs (Figure 2). Whether or not initial up-titration has to be recommended for a given compound/preparation depends on these agents’ pharmacokinetic properties. This is not necessary for preparations such as once-weekly exenatide because the protracted action is the result of slow absorption, while the elimination of circulating exenatide follows the same kinetics as known for un-retarded (b.i.d.) exenatide (Table 1). Among those agents that have a long duration of action mainly through their slow elimination (long elimination half-life, see Table 1), those with a relatively rapid time to peak concentration (Tmax < 24 h; applies to short-acting GLP-1 RAs, liraglutide, and semaglutide [20]) are those with recommended dose escalation schedules, while those with slower absorption (dulaglutide and albiglutide; Tmax ≥ 48 h) (Figure 1) can be initiated at their final dose. This could be explained by the fact that the GLP-1 RAs characterized by a free fatty acid side chain are injected as “free” (non-albumin-bound) compounds and that it takes some time to reach a steady–state equilibrium for binding to albumin. Only after reaching this equilibrium, most of the compound is bound to albumin, and, as such, is unable to diffuse into tissues and elicit effects (including adverse events).
Choosing the appropriate initial dose escalation schedule can have consequences for dose selection in phase 2 of clinical development programs, since doses carried on into phase 3 and suggested for approval have to be effective as well as tolerable and safe. Less than optimal up-titration regimens may lead to (avoidable) side effects and will most likely limit the upper dose range that is considered to have a beneficial efficacy-side effect relationship.
Another question related to initial up-titration is whether it is needed when switching from one agent to another (e.g., for increasing efficacy or avoiding side effects). This is an issue that is not normally clarified by dedicated clinical trials. Therefore, recommendations mainly based on pharmacokinetic modeling are available [30].
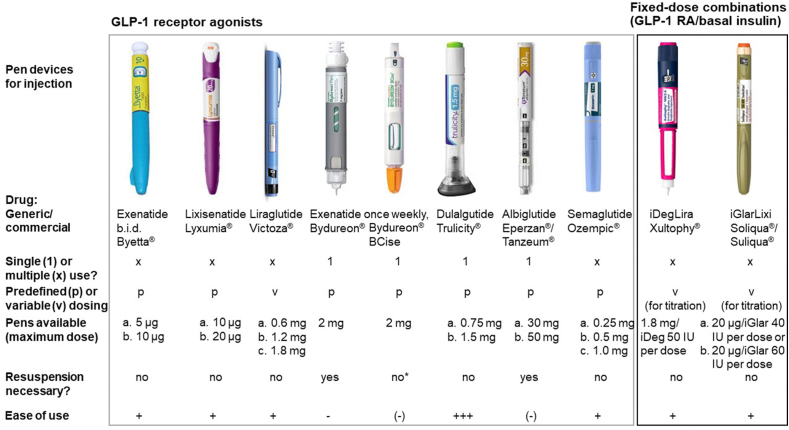
2.2. Injection devices (Figure 3)
Figure 3.
Optical appearance and properties of pen injection devices for approved GLP-1 receptor agonists (as mono substances or fixed-dose combinations with basal insulin). Modified from Nauck and Meier 2019 [20]. ∗Thorough shaking was necessary to evenly resuspend the active ingredient. The ease of use was estimated semi-quantitatively based on informal feedback from patients using these pen injection devices.
All GLP-1 RAs are delivered from pre-filled, dedicated pen injection devices developed for each particular product. However, details are considerably different for various products. They vary with respect to one time (mainly once-weekly GLP-1 RAs) vs multiple usage and in their ability to deliver one predetermined dose or whether it can be used to choose between several dose settings. For once-weekly exenatide, the microspheres containing the active drug need to be resuspended in buffer. Originally, this meant reconstitution of the active ingredient in vehicle solutions, which are stored in different vessels. An improved dual-chamber device has simplified this procedure. The dulaglutide pen injection device has received attention because of its single-use design and the needle, which is never visible throughout the injection procedure. Figure 3 depicts the visual appearance and some essential properties as well as the authors’ evaluation of their ease of use of all available pen injection devices for GLP-1 RAs (free and fixed-dose combinations).
2.3. Classification as short- and long-acting GLP-1 RAs
Since the parent compound of GLP-1 RAs, GLP-1, has a very short elimination half-life that precluded its clinical use outside settings characterized by continuous administration, compounds/preparations with longer intervals between injections have been developed over time (Table 1). While this at first was thought to be mainly relevant with respect to the injection frequency, thus representing a convenience issue, essential pharmacological differences were later identified that suggested that both short- and long-acting GLP-1 RAs may have specific advantages and indications [31]. By definition, short-acting GLP-1 RAs (exenatide b.i.d. and lixisenatide) are characterized by short-lived peaks in plasma drug concentrations following each injection, with intermittent periods of near-zero concentrations. Thus, the time–action profile changes between periods (lasting a few hours) during which patients are exposed to effective circulating drug concentrations, and “resting” periods, during which GLP-1 receptors are not activated. In contrast, long-acting GLP-1 RAs, once at a steady state, are characterized by constantly elevated drug concentrations in a range leading to substantial GLP-1 receptor stimulation and only minor fluctuations between injections (e.g., a 24-h period for liraglutide and a week-long period for semaglutide). Of note, this definition does not rest on the injection frequency alone but on the pharmacological kinetics. Consequently, once-daily lixisenatide is a short-acting compound, whereas once-daily liraglutide is a long-acting GLP-1 RA (Table 1).
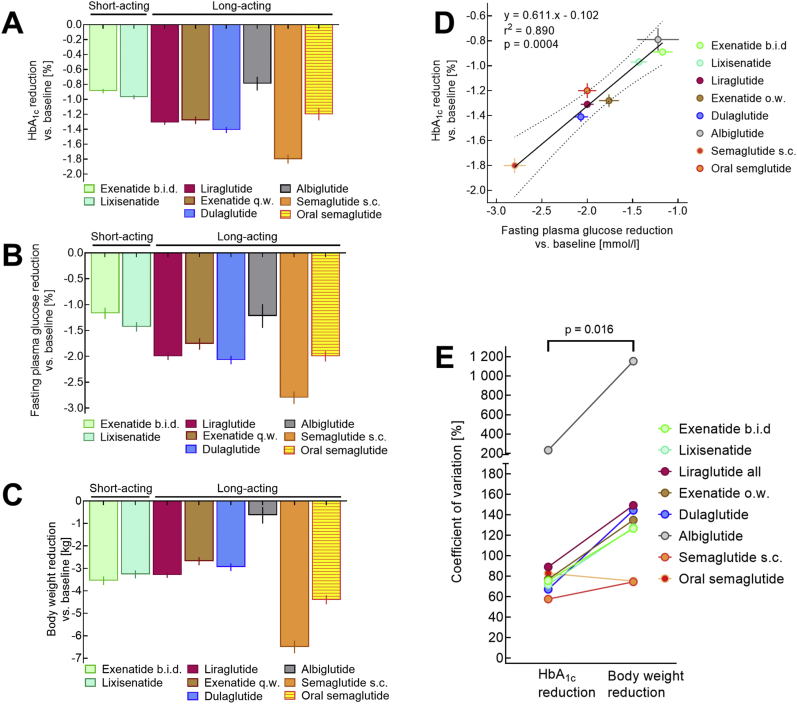
One obvious consequence of the different temporal patterns of short- and long-acting GLP-1 RAs with reduced exposure during the night in short-acting compounds is the ability of long-acting GLP-1 RAs to more profoundly lower fasting plasma glucose than short-acting GLP-1 RAs. This was best exemplified by a study comparing un-retarded (b.i.d.) and long-acting release (once-weekly) exenatide [18], although the differences were valid for the comparison of any short- and long-acting GLP-1 RA (Figure 4).
Figure 4.
Comparison of approved GLP-1 RAs with respect to their effectiveness in reducing HbA1C (A), fasting plasma glucose (B), and body weight (C). A linear regression analysis relating reductions in fasting plasma glucose to reductions in HbA1c is shown in panel D. A comparison of the reported coefficients of variation for reducing HbA1c and body weight is displayed in panel E. All data are from clinical trials reporting head-to-head comparisons between various GLP-1 RAs (exenatide b.i.d. vs lixisenatide [36], exenatide b.i.d. vs liraglutide [37], lixisenatide vs liraglutide [38], exenatide once-weekly vs liraglutide [39], albiglutide vs liraglutide [40], dulaglutide vs liraglutide [41], subcutaneous semaglutide vs dulaglutide [42], and oral semaglutide vs liraglutide [43]) on a background of oral glucose-lowering agents. Data concerning the same GLP-1 RA were pooled using conventional equations to calculate common means and their standard deviations.
Another peculiarity relates to the effectiveness of GLP-1 RAs to slow gastric emptying in light of tachyphylaxis: while intermittent stimulation of GLP-1 receptors (short-acting GLP-1 RAs) is associated with preserved effects on gastric motility, even long-term continuous stimulation leads to desensitization, which probably begins early (within 4–24 h) and reaches its full expression after several weeks or months [32]. Since the velocity of gastric emptying is tightly coupled to the absorption of nutrient carbohydrates, slowed gastric emptying means reduced and/or delayed glycemic increases after meals. For short-acting GLP-1 RAs, delayed gastric emptying is the main mechanism for post-meal reductions in plasma glucose rises [33]. It has been claimed that short-acting GLP-1 RAs act preferentially on post-meal glycemic rises through their effect on gastric emptying, which are preserved over time [18,33,34], while there is substantial tachyphylaxis for long-acting compounds [18,34]. First, long-acting GLP-1 RAs reduce post-prandial glucose as well, mainly through increasing insulin and suppressing glucagon [31]. The effect on gastric emptying relates only to meals, before which the short-acting GLP-1 RA has been administered (once daily with lixisenatide and twice daily with exenatide b.i.d.), with minor effects at most for other meals [33]. Whether this translates into a net advantage is far from clear. In a recent meta-analysis comparing short- and long-acting GLP-1 RAs on a basal insulin background, post-prandial glucose increases were not significantly different [35]. Conditions under which a reduction in post-meal glycemic excursions through a lasting deceleration of gastric emptying cause an obvious advantage of short-over long-acting GLP-1 RAs still need to be defined.
The effectiveness of short- and long-acting GLP-1 RAs for controlling fasting plasma glucose and HbA1c in patients with type 2 diabetes otherwise treated with oral glucose-lowering agents was compared in relatively large head-to-head comparison trials conducted in patients receiving oral glucose-lowering medications as background therapy. Figure 5 shows representative data from these clinical trials. The reduction in fasting plasma glucose was systematically more pronounced with long-acting compounds. Consequently, HbA1c values were reduced significantly more by long-acting GLP-1 RAs (since the overnight period represented one-third of the 24 h period). Efficacy regarding reductions in fasting plasma glucose and HbA1c were highly correlated (Figure 4D), underscoring the importance of controlling fasting plasma glucose to achieve acceptable overall glycemic control based on commonly recommended target ranges. Similar conclusions were derived from specifically assessing 4 head-to-head clinical trials comparing short- and long-acting GLP-1 RAs (depicted in Nauck and Meier 2019 [20]).
Figure 5.
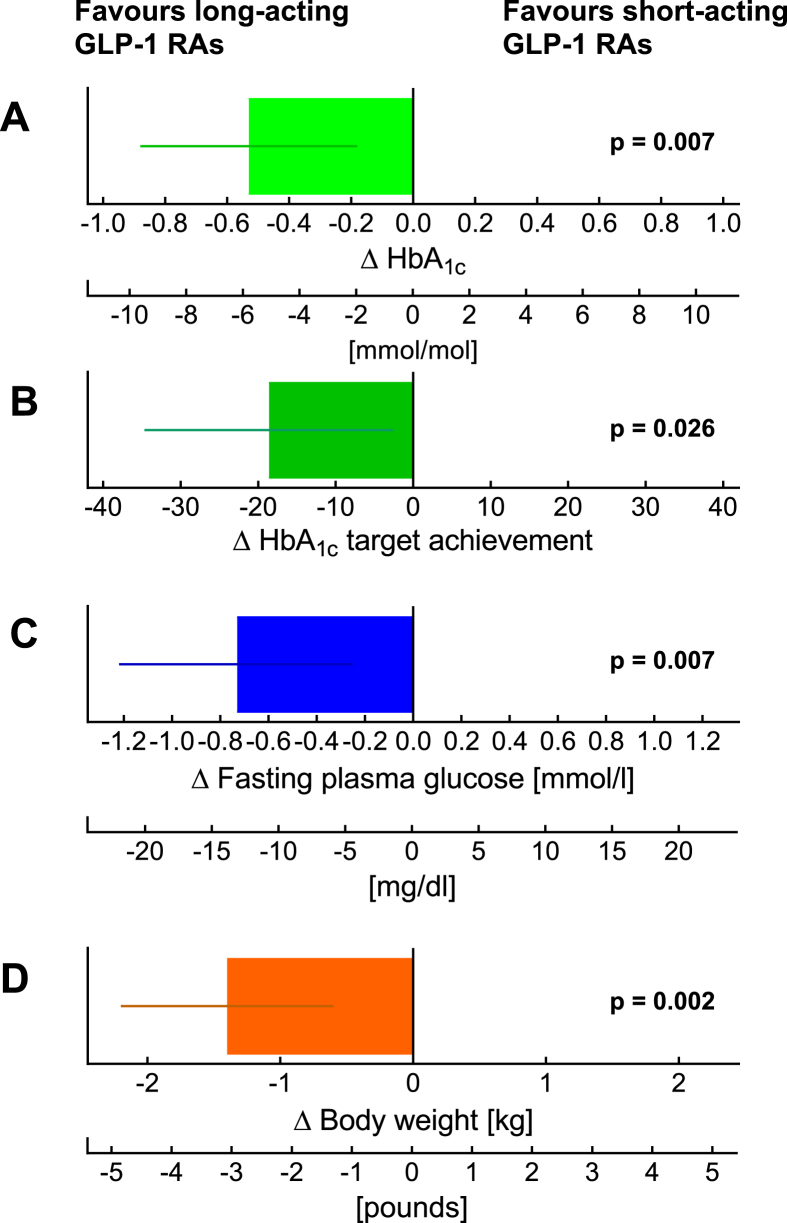
Meta-analysis comparing effects of short- and long-acting GLP-1 receptor agonists added to basal insulin in HbA1c (A), HbA1c target (≤7.0%) achievement (B), fasting plasma glucose (C), and body weight (D). For each variable, the results were significantly better for long-acting compounds (liraglutide, once-weekly exenatide, dulaglutide, and semaglutide based on 6 studies) compared to short-acting compounds (exenatide b.i.d. and lixisenatide based on 8 studies). Both studies with free and fixed-dose combinations were analyzed. Modified from [50].
2.4. Comparison between GLP-1 RA and insulin therapy
According to current recommendations, recently diagnosed type 2 diabetes should be treated with patient education instructing in favor of a healthy lifestyle including nutrition avoiding excess calories and rapidly absorbed carbohydrates and physical exercise. At this early stage or later, single (mostly metformin) or combination therapy with oral glucose-lowering agents is recommended until injectable therapy with more effective drugs (insulin or GLP-1 receptor agonists) becomes necessary. It was surprising that when meta-analyzing studies directly comparing insulin treatment (mainly basal insulin combined with oral agents) with any of the GLP-1 receptor agonists, there was, at most, a minor difference in glycemic effectiveness [44,45]. If anything, GLP-1 receptor agonist had a slightly better effect on reducing HbA1c. In addition, they uniformly led to some weight loss, and were only associated with hypoglycemic episodes when combined with sulfonylureas or insulin. As a factor contributing to more convenience, GLP-1 RAs can be employed using more or less standardized dosing instructions (including initial up-titration), while insulin needs to be individually titrated, with effective doses spread across a wide range. Some features of (basal) insulin and GLP-1 RA therapy in combination with oral glucose-lowering agents are summarized in Table 2. Of note, basal insulin and GLP-1 RAs are similarly effective in patients starting at very high baseline HbA1c values (although patients selected by this criterion often fail to reach conventional target ranges for HbA1c) [46]. Overall, these reasons form the basis of the ADA/EASD recommendation to preferentially use GLP-1 RAs in type 2 diabetes patients failing on oral agents alone [47]. Exceptions are circumstances suggesting type 1 diabetes or latent autoimmune diabetes in adults (LADA) with severe insulin deficiency.
Table 2.
Comparison of injectable treatments for type 2 diabetes with basal insulin or GLP-1 receptor agonists (based on meta-analyses of head-to-head comparisons [44,45]).
| Criterion | Treatment with |
Commentary | |
|---|---|---|---|
| Basal insulin | GLP-1 receptor agonists | ||
| Glycemic control | |||
| Fasting plasma glucose (FPG) | After meticulous titration, FPG concentrations in the target range (for example, 80–110 mg/dl) can often be reached | Substantial reduction can be achieved. Overall, slightly less effective than insulin | An exception is semaglutide for once-weekly injection, which lowered FPG more than insulin glargine [48] |
| Prandial glycemic excursions | Can be reduced with appropriately dosed basal insulin | Reduced through deceleration of gastric emptying (short-acting GLP-1 RAs) and the influence on insulin and/or glucagon secretion [31] | Short-acting GLP-1 RAs maintain their effect on gastric emptying with continued administration, while there is tachyphylaxis over days/weeks with long-acting GLP-1 RAs [18] |
| HbA1c | Substantial reduction, often into the target range | Substantial reduction, often into the target range | A slightly better reduction was shown with GLP-1 RAs, which might have been caused by insufficient titration of basal insulin; long-acting GLP-1 RAs achieve lower HbA1c concentrations [44] |
| Dosing | By titration, often starting with approximately 10 IU/d. Effective doses are somewhere between 15 and 200 IU/d and cannot be precisely predicted based on clinical characteristics (for example, BMI)a | Standard dosage recommendations are available for individual GLP-1 RAs (often including some slow up-titration during the initial period) | Hypoglycemia may be dose-limiting for insulin, while nausea and vomiting may suggest using lower doses than generally recommended for GLP-1 RAs |
| Frequency | Usually once daily (“bedtime” insulin) | Between twice daily (exenatide b.i.d.) and once weekly | Variable for GLP-1 RAs because of their differing elimination kinetics |
| Changes in body weight | Increases by 1–2.5 kg on average | Decreases by 2–6 kg on average | Within the range typical for each GLP-1 RA, individual weight loss is highly variable |
| Risk of hypoglycemic episodes | Hypoglycemic episodes are reported in approximately 43% of patients, in part depending on the proportion receiving sulfonylurea treatment [44] | Hypoglycemic episodes are reported in approximately 23% of patients, very much depending on the proportion receiving sulfonylurea treatment [44] | Clinically meaningful hypoglycemia with GLP-1 RAs heavily depends on a co-medication with sulfonylureas [44] |
| Nausea and vomiting as adverse events | Rare | Nausea (up to 20%) and vomiting (up to 10%) mainly occur after initiating treatment or associated with increases in dosage | Gastrointestinal side effects lead to medication withdrawal in approximately 5–10% [49] |
Algorithms are available that aid the titration process.
2.5. Combination with (basal) insulin therapy
Therapy with basal insulin may fail because it may be successful in controlling fasting plasma glucose but does not sufficiently limit post-prandial glycemic excursions. Treatment intensification can mean adding one to three prandial insulin injections per day or adding a GLP-1 RA to ongoing insulin treatment. Nevertheless, GLP-1 RA therapy with a background of oral glucose-lowering medications may fail to achieve glycemic targets as well. In this case, combining it with insulin (mainly basal insulin) is a well-documented method of improving fasting, post-prandial, and overall (HbA1c) glycemic control [[51], [52], [53], [54], [55], [56], [57]]. The combination of (basal) insulin with a GLP-1 RA is a highly effective treatment even for advanced stages of type 2 diabetes. It should only be used in patients needing a combination of two injectable treatments, especially considering the costs of such a combination.
When a GLP-1 RA is added to (basal) insulin, the combination is as effective as an intensified (basal bolus) insulin regime in terms of HbA1c control, but with a much lower risk of hypoglycemia and weight gain [58].
When insulin is added to a GLP-1 RA, it helps control fasting plasma glucose. In combination with post-prandial effects of GLP-1 RAs (through decelerating gastric emptying, stimulating insulin, or suppressing glucagon secretion [31]), this provides excellent chances to achieve the target ranges for fasting, post-prandial, and overall (HbA1c) glycemic control. In studies comparing basal insulin and GLP-1 RAs alone and in combination with each other, the combination achieved the lowest HbA1c or highest HbA1c reduction and a body weight transformation in between GLP-1 RA alone (lowest) and insulin alone (highest) [59]. There is a risk of hypoglycemic episodes with this combination, which is higher than treating with GLP-1 RAs alone, but lower compared to insulin treatment alone [59].
The fact that a combination of a GLP-1 RA with basal insulin is a highly efficacious glucose-lowering treatment regime for advanced stages of type 2 diabetes has led to the development of fixed-dose combinations. GLP-1 RAs that are usually injected once daily (liraglutide or lixisenatide) were combined with basal insulin designed for once-daily injection (insulin degludec or insulin glargine), resulting in the fixed-dose combinations iDegLira [28,59] and iGlarLixi [60,61]. Since insulin must be titrated slowly as part of the dose-finding process, the GLP-1 RA component of these fixed-dose combinations is titrated slowly as well. This approach for introducing GLP-1 RA therapy has resulted in fewer problems with nausea, vomiting, or diarrhea. Apparently smaller steps of increasing GLP-1 RA exposure better support an adaptation process increasing patients’ tolerance to such adverse reactions.
It has been postulated that short-acting GLP-1 RAs are particularly suited for combination with basal insulin because the strength of long-acting compounds, a greater effect on fasting plasma glucose, is not needed in this combination since the role of basal insulin would be to control fasting plasma glucose. However, the effect of slowing gastric emptying leading to slower absorption of nutrients (which is preserved over time with short-acting GLP-1 RAs) is a mechanism limiting post-meal glycemic excursion [31]. Notably, with short-acting GLP-1 RAs, this effect only applies to the meal before which the agent has been injected. A recent meta-analysis described the advantages of combining long-acting GLP-1 RAs (compared to short-acting GLP-1 RAs) with basal insulin [50]. This applied to free combinations (dosage determined separately for the GLP-1 RAs and basal insulin) as well as fixed-dose combinations [50]. As depicted in Figure 5, not only HbA1c was lowered significantly more and HbA1c targets were achieved in a higher proportion of patients, but also fasting plasma glucose concentrations and body weight were controlled better with long-acting GLP-1 RAs [50]. In addition, the risk of hypoglycemic episodes and gastrointestinal side effects was slightly, but significantly lower with long-acting GLP-1 RAs [50].
2.6. Weight loss induced by GLP-1 RAs
Intracerebroventricular [62] and peripheral administration of GLP-1 [12] and GLP-1 RAs [20,63] reduces appetite and prospective food consumption, increases satiety and a feeling of abdominal fullness, and limits caloric intake under conditions of ad libitum feeding. All GLP-1 RAs after longer-term treatment lead to weight loss but in varying degrees (Figure 4). Thus, GLP-1 RAs are unique in promoting weight loss while reducing the glycemia level, which in turn limits glucosuria (energy lost through urinary glucose excretion) and therefore should be associated with weight gain. Most other glucose-lowering agents, except for sodium/glucose cotransporter-2 (SGLT-2) inhibitors, usually lead to some weight gain (sulfonylureas, insulin, or thiazolidinediones) or are weight neutral (metformin, DPP-4 inhibitors, or α-glucosidase inhibitors) [47]. Liraglutide (at doses somewhat higher than used to treat diabetes mellitus) is also approved for pharmacological obesity therapy [64,65]. Semaglutide, the GLP-1 RA with the highest efficacy regarding weight loss in clinical trials of type 2 diabetes patients (Figure 4), is also undergoing evaluation as a weight-loss agent in obese subjects without diabetes mellitus [[66], [67], [68]].
The quantitative differences in body weight reduction typically achieved with different GLP-1 RAs critically depend on the respective doses selected in phase 2 studies. Since the primary indication for using GLP-1 RAs is type 2 diabetes, dose selection has mainly addressed glycemic control (HbA1c reduction). Some data suggest that while HbA1c reduction plateaus at relatively lower doses, higher doses may still be more effective for weight loss [69,70]. This is one important reason for testing higher doses, for example, dulaglutide [71], to seek approval for more effective, higher doses of GLP-1 RAs for those who tolerate them.
The fact that some GLP-1 RAs have particularly weak effects with respect to body weight (e.g., albiglutide), whereas other compounds seem to have more pronounced effects (e.g., semaglutide) even if their glucose-lowering effects are similar, has sparked interest in characterizing the mechanism of action. It is obvious that appetite-and weight-reducing effects involve uptake into specific brain regions and interaction with CNS neural circuits involved in the homeostatic or hedonic [77] regulation of energy household and food intake. Table 3 summarizes recent insights gained from comprehensive studies characterizing semaglutide's (and liraglutide) effects on diet-induced obesity in rodents [72,73]. These findings point to a role of the arcuate nucleus within the hypothalamus, area postrema (AP), and nucleus tractus solitarii (NTS) for the influence of systemically administered GLP-1 RAs on appetite, satiety, calorie intake, and body weight as schematically summarized in Figure 6. In this model, GLP-1 RAs seem to be effective at preventing meal initiation by suppressing the activity of NPY/agouti-related peptide (AgRP) producing neurons in the arcuate nucleus and inducing meal termination in the lateral parabrachial nucleus (PB). Signals reaching the PB originate from the arcuate nucleus of the hypothalamus and brain stem (AP and NTS). POMC/CART neurons expressing GLP-1 receptors activate PB neurons and directly or indirectly suppress NPY/AgRP neurons [72,73], leading to disinhibition of suppressive signals to the PB (Figure 6). Recent data indicated subtle differences in how the brain interacts with liraglutide and semaglutide [73], which may help explain why these two GLP-1 RAs differ in their efficacy to reduce body weight (Figure 4). This information may guide the design of GLP-1 RAs or related pharmacological agents with even more pronounced weight loss efficacy. It still remains unclear why albiglutide has a weaker weight-lowering efficacy than other GLP-1 RAs (Figure 4).
Table 3.
Mechanisms involved in GLP-1 RA-associated appetite and weight reduction as reported in a recently published comprehensive study focusing on the effects of semaglutide (compared to liraglutide) on diet-induced obesity in mice (based on Secher et al., 2014 and [72] and Gabery et al., 2020 [73]).
| Aspects of the mechanism of GLP-1 RA-induced weight loss | Findings | Explanation/commentary |
|---|---|---|
| Access of peripherally circulating GLP-1 RAs into the central nervous system |
|
|
| Access of GLP-1 RAs to GLP-1 receptors in the brain |
|
|
| Direct effects of GLP-1 RAs on the hypothalamus (nucleus arcuatus) |
|
|
| Neuronal activation in brain areas accessible for GLP-1 RAs |
|
|
| Neuronal activation in brain areas not directly accessible for GLP-1 RAs (“secondary activation”) |
|
|
| Food intake and body weight |
|
|
| Food preference |
|
|
| Energy expenditure |
|
|
BBB: blood–brain barrier, GLP-1 R: glucagon-like peptide-1 receptor, GLP-1 RA: GLP-1 receptor agonist, POMC/CART: proopiomelanocortin/cocaine- and amphetamine-regulated transcript, NPY/AgRP: neuropeptide Y/agouti-related peptide.
Figure 6.
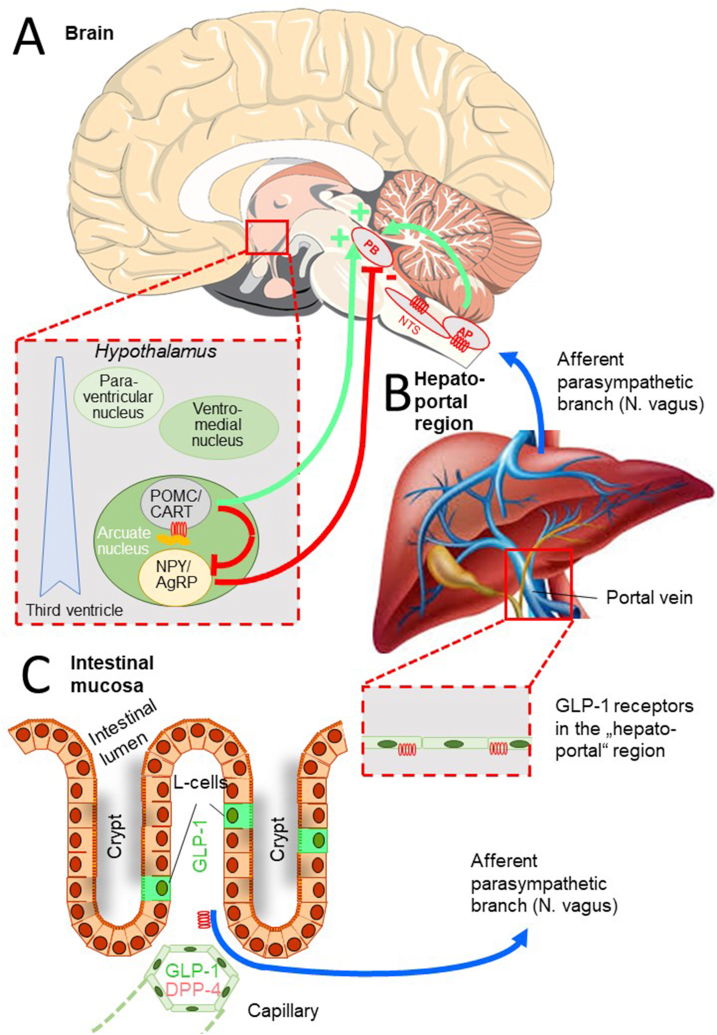
Schematic diagram demonstrating how various methods of GLP-1 or GLP-1 RA administration into the general circulation can reach and influence brain areas involved in the regulation of energy intake and expenditure [72,73]. (A) Evidence also suggests that GLP-1 receptors in the hepatoportal region [75] (B) and on afferent parasympathetic nerve endings in the intestinal mucosa (C) [76] may generate central nervous system signals influencing insulin secretion and metabolism. Stimulatory signals (+) are shown in green, inhibitory (−) signals are depicted in red, and afferent parasympathetic (vagal) signals are denoted in blue. See the text for a more detailed explanation of the mechanisms.
Human studies have confirmed the ability of GLP-1 RAs to influence food choices (toward a selection of less energy-dense healthier foods) [66,78]. However, in contrast to some recent findings in rodents [73], studies in human subjects did not observe any interference of semaglutide treatment with a reduction in energy expenditure that usually accompanies weight loss (as one important mechanism for maintaining body weight close to a pre-determined “set point”) [66,79]. Twelve weeks may not be sufficient to reach a steady state of weight reduction and possibly compensatory mechanisms. Of interest are the results of questionnaires indicating that obese subjects had fewer food cravings and could better resist food cravings while treated with semaglutide [66]. The answers point to the fact that eating was considered less pleasurable during treatment with semaglutide [66]. This could be in line with functional magnetic resonance imaging showing that GLP-1 R activation decreases anticipatory food reward (the anticipated pleasure of eating certain meals) and increases consummatory food reward (the pleasure offered by eating a meal) [80]. The regulation of energy intake thus is not only subject to homeostatic regulation (nervous system circuits attempting to maintain unchanged body weight), but also interacts with the brain reward system [[80], [81], [82], [83], [84]].
The robust effects of GLP-1 RAs to reduce body weight, usually by 2–7 kg (or % of initial body weight) on average in type 2 diabetes, have led to the exploration of GLP-1 RAs as a novel pharmacological treatment in obese but non-diabetic subjects often with impaired fasting glucose or glucose tolerance (“prediabetes”). Based on the observation that dose–response relationships have shown a plateau for glycemic control at lower doses than for body weight reduction, higher doses have been employed to treat obese subjects. Daily doses up to 3.0 mg are approved for liraglutide (1.8 mg is the maximum dose for treating type 2 diabetes) [65], and clinical trials have tested semaglutide at up to 0.4 mg per day (that is, corresponding to 2.8 vs 1.0 mg per week for type 2 diabetes) [68]. Doses of up to 4.5 mg per week (vs a maximum of 1.5 mg for type 2 diabetes) are being explored for dulaglutide [85]. In subjects tolerating these higher doses of GLP-1 RAs, substantially greater reductions in body weight were observed than with “conventional” doses typically employed to treat type 2 diabetes. Impaired glucose tolerance often improves while subjects receive this type of treatment, most likely explained by the glucose-lowering properties of GLP-1 RAs [65,86]. Whether this means a true interference with or a delay in the progression to diabetes needs to be studied in trials assessing the long-term consequences of withdrawing GLP-1 RA treatment. GLP-1 RAs need to be continuously administered after induction of weight loss. After discontinuation of this pharmacological treatment, body weight will revert to baseline values or at least close to baseline values within a few months [87].
It has often been overlooked that the individual weight reduction response of patients with type 2 diabetes treated with GLP-1 RAs is more variable than the reduction in HbA1c. This is obvious when treatment-related weight and HbA1c changes are plotted individually [18,88]. It can also be concluded from a higher coefficient of variation (the standard deviation divided by the mean value expressed as a percentage) depicted in Figure 4E. Why some patients do not reduce their body weight at all when treated with GLP-1 RAs while others respond with weight loss very much exceeding the mean values reported in clinical trials (for example, Figure 4) can only partially be answered with current knowledge. Schlogel et al. [89] examined responders and non-responders (with respect to exenatide's effect on energy intake) and found hypothalamic effects only in responders. This hints at a biological reason most likely related to the mechanisms summarized in Table 3 and could be the result of genetic polymorphisms, for example, regarding GLP-1 receptors or other components of the signal transduction pathway.
However, the weight-lowering effects of GLP-1 RAs can probably be modulated by lifestyle measures aiming at reduced calorie intake [90], although a systematic examination of the combined efficacy of initiating treatment with GLP-1 RAs and patient education aiming at optimizing the weight-reducing effects of GLP-1 RAs is still lacking (or has failed to provide convincing benefits [91]). Obese patients with type 2 diabetes often tried various dietary approaches to lose weight and failed. One possible explanation for the wide spectrum of weight loss observed with initiating treatment with GLP-1 RAs could be that some patients feel motivated for further attempts to improve their eating behavior and lifestyle because of a realistic chance of success. Other patients may instead believe that the GLP-1 RA will ameliorate their obesity problem without them contributing by willingly restricting caloric intake and engaging in physical activity. This is a hypothesis worth sparking clinical studies, as would be developing a dedicated patient education program aiming at optimizing weight reduction with GLP-1 RAs in type 2 diabetes and in particular when using them in obese, prediabetic subjects to prevent progression to type 2 diabetes [68,86,92].
2.7. Gastrointestinal and other adverse events
Side effects most reported with GLP-1 RAs are nausea, vomiting, and diarrhea, often summarized as gastrointestinal adverse events. They are typically most prominent when initiating treatment with (any) GLP-1 RA or after increasing the dose (e.g., during recommended up-titration regimens). Since these symptoms can occur in fasting subjects, they are probably not related to the effects of GLP-1 RA treatment on gastrointestinal functions (e.g., deceleration of gastric emptying) but instead are caused by direct interactions with CNS GLP-1 receptors (Figure 6) most likely located in the brain stem (area postrema). Nausea is typically reported in up to 25% and vomiting or diarrhea in up to 10% of subjects treated with GLP-1 RAs [20,49]. For most patients, these are short, self-limited episodes that cease spontaneously, even with continued treatment. The time point of occurrence is probably related to the time characterized by maximum drug concentrations typically occurring at Tmax following several hours to days after each injection (Figure 1). The probability of these side effects varies with sudden incremental exposure to GLP-1 RAs. An often-used recommendation to avoid these adverse events is a standardized, slowly increased exposure through up-titration regimens (Figure 2), which have been shown to mitigate gastrointestinal side effects. Experience with fixed-dose combinations with basal insulin (which must be titrated much more slowly) underscore the effectiveness of this approach.
Summarizing adverse event reporting from clinical trials examining GLP-1 RAs discloses subtle differences in the risk of these side effects depending on the short- (worse) vs long-acting nature (better) background medication (worse in combination with metformin or insulin) that are also related to the individual compound/preparation [49].
In part related to adverse events, patients randomized to GLP-1 RA treatment often discontinue this medication. Table 4 shows reported figures from cardiovascular (CV) outcome trials with GLP-1 RAs, the largest trials available reporting the longest durations of exposure to GLP-1 RAs. The proportions of patients reporting adverse events were not generally different from shorter clinical trials [49]. This indicates that while the frequency and severity of side effects can be successfully modulated through optimized up-titration regimens, a certain percentage of patients does not tolerate this treatment with the current regimens of initiating GLP-1 RA. Interestingly, in a recent study allowing individual titration of oral semaglutide, most patients discontinuing this treatment did so after exposure to the lowest (initial) dose of 3 mg per day [93]. This may indicate that the sensitivity of patients toward developing gastrointestinal adverse events is considerably heterogeneous, such that some patients fail to tolerate low doses, while for others, higher doses than currently used may offer better effectivity without increasing side effects. Along these lines, higher doses of some GLP-1 RAs are being explored, especially to further reduce body weight [65,67,68,71,86]. The reported nausea, vomiting, and diarrhea rates are generally lower in Japanese than Caucasian populations, suggesting that the cultural background and eating behaviors may also have an impact on the induction of nausea with GLP-1 RAs [94].
Table 4.
Proportions of patients randomized to GLP-1 RA treatment in CV outcome trials discontinuing study drug treatment, proportion of the follow-up period during which patients were exposed to the study drug, and proportions discontinuing due to adverse events.
| GLP-1 RA | Proportion of patients permanently discontinuing the study drug [%]a | Proportion of follow-up period during which the study drug was taken [%] | Proportion of patients discontinuing the study drug because of adverse events [%] | Trial/reference |
|---|---|---|---|---|
| Lixisenatide | 27.5 | 90.5 | 11.4 | ELIXA [95] |
| Liraglutide | n.p. | 84 | 9.5 | LEADER [96] |
| Once-weekly exenatide | 43.0 | 76 | 4.5c | EXSCEL [97] |
| Dulaglutide | 26.8 | 82.2 | 9.1 | REWIND [98] |
| Albiglutide | 24.5 | 87 | 8.6 | HARMONY Outcomes [99] |
| Semaglutide s.c. | 21.3 | 86.5 | 13.2 | SUSTAIN-6 [100] |
| Oral semaglutide | 15.3 | n.p.b | 11.6 | PIONEER-6 [101] |
n.p.: Not presented.
Not counting transient “drug holidays.”
75% received the study medication for more than 1 year (total follow-up of 15.3 months).
Counting only gastrointestinal adverse events. No CV outcome trial has been reported for exenatide b.i.d. (approved before these studies became mandatory).
When GLP-1 RAs were introduced as novel agents to treat type 2 diabetes, there was uncertainty about several potential adverse effects such as acute pancreatitis, pancreatic cancer, and thyroid cancer [102,103]. The availability of large databases from randomized CV outcome studies that defined pancreatitis, pancreatic cancer, and thyroid cancer as “adverse events of special interest” with protocols carefully adjudicating suspected cases has reduced these concerns since they uniformly reported hazard ratios of these adverse events not significantly different from 1.0 [104]. In retrospect, an elevation in amylase and/or lipase activity commonly observed with GLP-1 RAs [105,106] together with abdominal symptoms typically triggered by GLP-1 RAs may have led to the suspicion of pancreatitis. Since 2 diagnostic criteria are sufficient for this diagnosis, pancreatitis may have been diagnosed even in the absence of imaging results supporting this diagnosis [105]. Nevertheless, thyroid C cells express GLP-1 receptors [107], and subjects at risk of (rare) medullary thyroid cancer (e.g., based on personal or family history or genetic testing) should not be treated with GLP-1 RAs. These subjects were consequently excluded from clinical trials with GLP-1 RAs.
3. Cardiovascular outcome studies
All GLP-1 RAs were approved for treating type 2 diabetes patients after 2008 (except for exenatide b.i.d., which was approved in 2005). Therefore, all of the compounds/preparations had to provide results of dedicated cardiovascular outcome studies supporting at least the cardiovascular safety of these medications in the target population and compared to placebo both on a background of standard of care (allowing any additional glucose-lowering medication necessary to meet targets recommended by current guidelines). The typical primary endpoint was major adverse cardiovascular events (MACE: time to first event of either CV death or non-fatal myocardial infarction or stroke). According to guidelines by the US Food and Drug Administration, definite proof of CV safety would be a hazard ratio for MACE near or below 1.0 with a confidence interval not exceeding 1.3 (equivalent to a 30% elevation in risk). If a study provides preliminary proof of safety (upper limit of the confidence interval below 1.8), another CV outcome study aiming at definite proof is required. Depending on the ambitions, studies with different patient numbers and durations are needed. This explains the heterogeneity in study designs, sample sizes, and follow-up periods between the trials summarized in Figure 7 [20,108]. Another differentiator is the proportion of patients with pre-existing cardiovascular damage, albeit defined by previous events or supported by functional testing and/or imaging, which ranged from 31% (REWIND [98]) to 100% (ELIXA [95] and HARMONY Outcomes [99]) and obviously had an important impact on the CV event rate observed during the trials.
Figure 7.
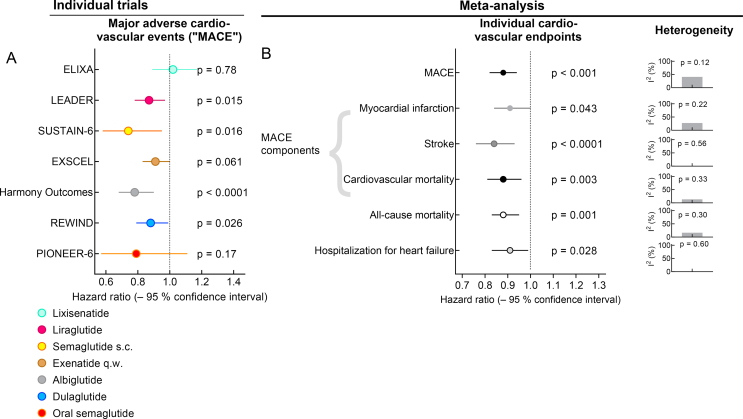
Results of cardiovascular outcome studies comparing GLP-1 RAs with placebo on a background of standard of care. (A) Reduction in major adverse cardiovascular events (MACE: time to first event) in published individual clinical trials. (B) Results of a published meta-analysis [108] analyzing various cardiovascular endpoints across all of the clinical trials shown in panel A. MACE (a combination of either cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) was the primary endpoint in all studies. Meta-analysis results are supplemented with I2 and related p values indicating the heterogeneity of the analysis of individual endpoints (column of panels to the far right) as reported in [108].
3.1. Heterogeneity regarding principal results from CV outcome trials comparing GLP-1 RAs with placebo
Figure 7A displays hazard ratios (active treatment vs placebo) for MACE and their 95% confidence intervals for all published CV outcome trials with GLP-1 RAs. With the exception of lixisenatide, all other GLP-1 RAs at least show a trend of a reduced incidence of MACE events, which was significant in four studies and not significant in 2 additional studies. Hence, the results are, from a clinical perspective, quite heterogeneous and suggest that some GLP-1 RAs are more suitable to prevent CV events than others. Assessing heterogeneity mathematically as part of the meta-analysis, however, resulted in I2 values suggesting at most moderate heterogeneity. Our interpretation is that comparing the various trials indicates a common mechanism of action, but important differences related to pharmacokinetic properties (one injection per day of lixisenatide does not fully cover a 24 h period), optimized dosages as a result of phase 2 dose-finding studies (probably applies to 2 mg per week of once-weekly exenatide), and drug discontinuation rates impact the degree of CV benefit that can be achieved with individual compounds/preparations as suggested by Caruso et al. [109]. Remarkably, the reduction in MACE events with albiglutide is very much comparable if not more pronounced than with other effective GLP-1 RAs (Figure 7A) despite its reduced ability to lower HbA1c, fasting plasma glucose, and body weight in clinical trials (Figure 4) [40]. When choosing a GLP-1 RA to prevent CV events, one of the compounds significantly reducing MACE should be selected. Liraglutide (LEADER trial) was unique in not only significantly reducing MACE events, but also CV and all-cause mortality [96]. Semaglutide (subcutaneous, SUSTAIN-6 [100], and oral, PIONEER-6 [101]) trials showed impressive results, especially considering their small sample sizes and short durations. This was due to their primary ambition to demonstrate safety, the minimum requirement for approval, which requires smaller patient numbers, a shorter trial duration, and fewer events. This preliminary nature makes additional larger trials necessary to fully characterize the potential to prevent CV complications of type 2 diabetes (oral semaglutide: SOUL, ClinicalTrials.gov NCT 03914326) or obesity (once-weekly semaglutide s.c.: SELECT ClinicalTrials.gov NCT03574597).
Individual CV outcome trials were not powered to assess single CV endpoints, but a composite endpoint such as MACE. However, a meta-analysis pooling results from all individual trials provided some insight that CV events can generally be prevented by GLP-1 RA treatment [108]. As shown in Figure 7B, across all of the trials, significant reductions by 9–16% in the incidence of acute myocardial infarction, stroke, cardiovascular, and even all-cause death could be achieved for the GLP-1 RA class as a whole, while in the individual trials, these effects on individual cardiovascular outcomes were only occasionally significant. However, the number of such events (myocardial infarction, stroke, CV death, etc.) in individual trials was too low to provide the power to detect significant differences. This also applied to a reduction in the hospitalization for heart failure, which was not significant in any of the individual trials, but in the meta-analysis (hazard ratio, 0.91; 95% confidence interval, 0.83–0.99). This figure contrasts with the consistent ≈35% risk reduction for hospitalization for heart failure in all studies employing SGLT-2 inhibitors [110,111]. Of note, patients with NYHA IV heart failure were excluded from the CVOTs with GLP-1 RAs, such that no firm conclusions could be drawn regarding these patients. In light of the dedicated studies of liraglutide in patients with advanced heart failure, which not only failed to prove benefits, but suggested some potential for harm caused by GLP-1 RAs [112,113], GLP-1 RAs are usually not recommended as first choice if the objective is to prevent heart failure complications. Indeed, the small increase in heart rate observed with GLP-1 RA treatment may represent an unfavorable mechanism in patients with advanced (NYHA III/IV) heart failure [34]. Instead, the pattern of effects observed in CV outcome trials suggests a primary mode of action preventing complications of atherosclerosis such as ischemic events (myocardial infarction and stroke) and associated mortality (vide infra).
Most CV outcome trials with GLP-1 RAs recruited patients with type 2 diabetes characterized by established CV disease (e.g., previous CV events) or indicators of a high risk of CV events. These studies were originally primarily designed as safety trials, and accruing a large number of CV events in high-risk patients was one strategy to limit the sample size and duration of these trials. Therefore, the results of these trials cannot be extrapolated to the general population of type 2 diabetes patients including those with short disease duration and lack of CV comorbidities. The REWIND study (employing dulaglutide as the GLP-1 RA) was exceptional in having recruited a mixed population with 31.5% with and 68.5% without pre-existing atherosclerotic vascular damage [98]. Subgroup analyses of the REWIND trial (dulaglutide vs placebo, both on a background of standard of care) highlighted that dulaglutide was able to induce a significant MACE reduction in the overall study population and quantitatively similar regardless of the patients' history of CV events (p for interaction was 0.97). Those with or without CV co-morbidities at baseline had identical risk reductions (that for both subgroups just missed statistical significance) [98]. These data suggest a potential to prevent CV complications even in lower-risk type 2 diabetes patients, yet fall short of definite proof.
Along the same lines, a subgroup analysis within the meta-analysis by Kristensen et al. [108] identified no statistically significant heterogeneity for the effect of GLP-1 RAs on MACE between primary vs secondary prevention (p = 0.24) The more recent meta-analysis by Marsico et al. [114] strengthened this conclusion. However, since the absolute risk reduction was smaller in the primary prevention population, it remains to be ascertained whether this intervention would be cost-effective in lower-risk patients.
3.2. Mediation analyses aiming to define the mechanism(s) leading to beneficial cardiovascular effects of GLP-1 RAs
As previously demonstrated in detail [115], GLP-1 RAs modify a number of risk factors for cardiovascular complications, including body weight reduction, lower systolic blood pressure, reduced plasma LDL cholesterol and triglyceride concentrations, and improved glycemic control (reductions in fasting and post-meal plasma glucose resulting in lower HbA1c; see Figure 4). Thus, a reduction in the incidence of ischemic events could be the consequence of a more beneficial risk profile under treatment with GLP-1 RAs. Mediation analysis is an approach to identify potential mediators that might explain the findings observed in terms of endpoints. While several mathematical approaches have been developed, their common aim is to show that taking into account the changes in a potential mediator reduces the effect size with respect to the endpoint of interest. Potential mediators are variables measured in the trial that are differentially affected by active drugs and placebo. For example, GLP-1 RAs reduce systolic blood pressure by 2–4 mmHg compared to placebo treatment [115]. Considering this reduction in systolic blood pressure, if the difference in MACE outcomes is reduced, it can be concluded that a reduction in systolic blood pressure mediates the prevention of MACE. If the effect is nullified, this mechanism is responsible for 100% of the effect, but partial mediation is also possible.
Using slightly different approaches, mediation analyses have been published on the effects of liraglutide in the LEADER trial [116] and the effects of dulaglutide in the REWIND study [117]. Interestingly, both analyses concluded that HbA1c reduction was a potential mediator, responsible for up to 82% of the total effect. A reduction in urinary albumin excretion was found to be another potential mediator in the LEADER trial (responsible for up to 33% of the total effect). Of note, any potential mechanism that does not leave a measurable trace or has not been assessed in a given trial will never be identified as a potential mediator using this approach. This applies to intravascular changes associated with the progression of atherosclerosis unless they are accompanied by, for example, inflammatory responses, which can be identified by measuring C-reactive protein or inflammatory cytokines (which was not done in any of the CV outcome trials of GLP-1 RAs). Hence, identifying HbA1c reduction as a potential mediator of CV benefits induced by GLP-1 RAs leaves a number of open questions, especially since it has been difficult to establish a relationship of HbA1c reduction with cardiovascular benefits in other glucose-lowering medications [118].
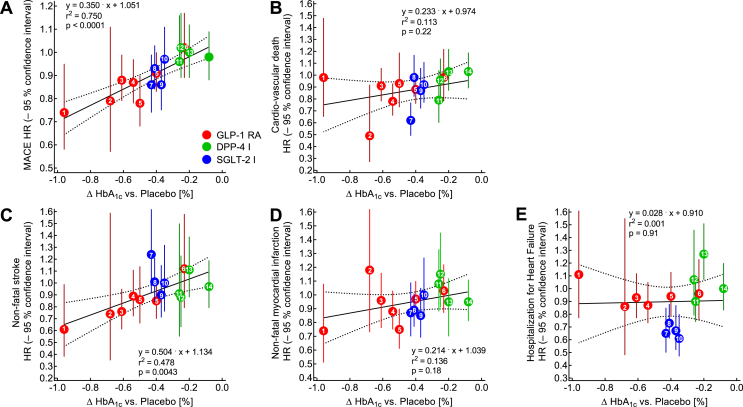
For CV outcome studies of GLP-1 RAs, a relationship that links the magnitude of the HbA1c reduction achieved (versus placebo) to the hazard ratio for major adverse CV events was suggested by Caruso et al. [119]. In particular, they identified a relationship between the mean reduction in HbA1c in individual trials and the corresponding hazard ratio for stroke [119]. Figure 8 presents a similar analysis, however, including CV outcome studies with DPP-4 and SGLT-2 inhibitors. Remarkably, these additional data points were positioned along the same regression lines, and the relationship between the reduction in HbA1c and MACE (Figure 8A) or stroke (Figure 8C) remained significant. Similar trends of non-fatal acute myocardial infarction and CV death were non-significant. A significant reduction in hospitalizations for heart failure was restricted to SGLT-2 inhibitors and independent from reductions in HbA1c (Figure 8F). Such an analysis may not only confirm the relationship initially observed by Caruso et al. [119], but may also explain why DPP-4 inhibitors and SGLT-2 inhibitors did not consistently reduce MACE [110,111,115]. Given that all of the CV outcome trials aimed at glycemic equipoise (similar if not identical glycemic control for active drug and placebo treatment), only trials with potent glucose-lowering medications such as GLP-1 RAs, which failed to achieve glycemic equipoise, underscored the potential for a CV benefit.
Figure 8.
Regression analysis of differences achieved in HbA1c concentrations between patients treated with placebo and active drug vs hazard ratios for major adverse cardiovascular outcomes (MACE; A), cardiovascular death (B), non-fatal stroke (C), non-fatal myocardial infarction (D), and hospitalization for heart failure (E) reported from cardiovascular outcome studies with GLP-1 receptor agonists (red), SGLT-2 inhibitors (blue), and DPP-4 inhibitors (green). Significant associations are shown for MACE (A) and non-fatal stroke (C) with similar slopes of the regression lines, while for cardiovascular death (B) and non-fatal myocardial infarction (D), a less prominent, non-significant correlation resulted from the analysis. Regarding hospitalization for heart failure (E), hazard ratios did not vary with HbA1c reduction. Analyzing GLP-1 receptor agonists only resulted in significant correlations for MACE and stroke as well as previously reported by Caruso et al. [119] but not for the other endpoints. Numbers in symbols identify the clinical trials: 1: SUSTAIN-6 (subcutaneous semaglutide) [100], 2: PIONEER-6 (oral semaglutide) [101], 3: REWIND (dulaglutide) [98], 4: LEADER (liraglutide) [96], 5: EXCSEL (once-weekly exenatide) [97], 6: ELIXA (lixisenatide) [95], 7: EMPA-REG Outcomes (empagliflozin) [120], 8: DECLARE-TIMI-58 (dapagliflozin) [121], 9: CANVAS program (canagliflozin) [122]; 10: VERTIS-CV (ertugliflozin, presented at the 80th scientific session of the American Diabetes Association); 11: EXAMINE (alogliptin) [123], 12 CARMELINA (linagliptin) [124], 13: SAVOR-TIMI-53 (saxagliptin) [125], and 14: TECOS (sitagliptin) [126].
3.3. Mechanisms explaining cardiovascular benefits
Understanding the robust interference of GLP-1 RAs with the progression and complications of atherosclerosis requires detailed knowledge of the pathomechanisms involved and consequences of GLP-1 receptor stimulation. Various steps and mechanisms involved in atherogenesis [127] are displayed in Figure 9A, while the effects of GLP-1 receptor stimulation in arterial vessel walls are shown in complementary Figure 9B.
Figure 9.
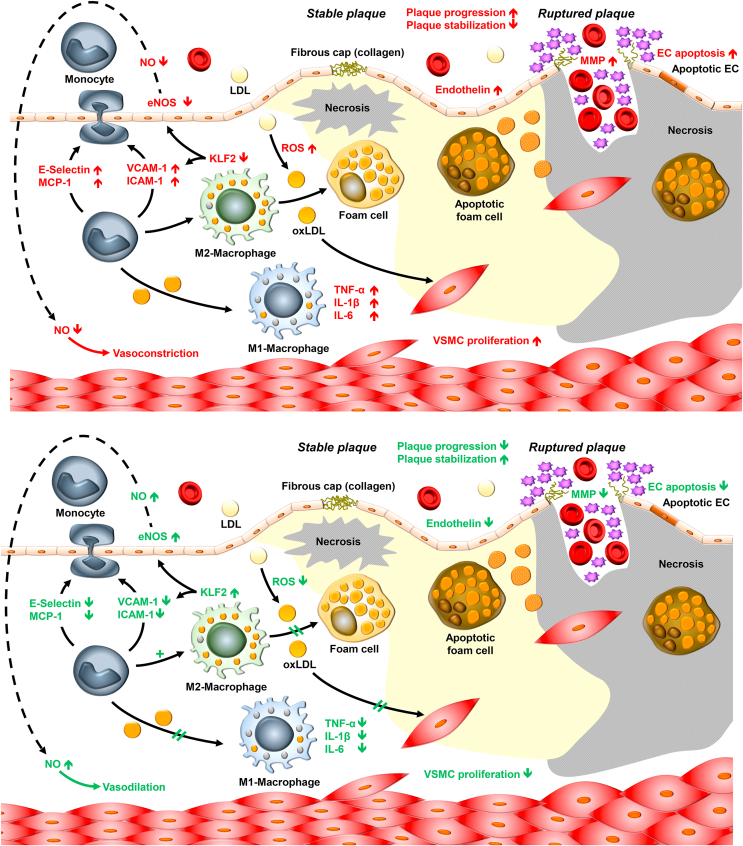
Mechanisms driving the development of atherosclerotic lesions in patients with type 2 diabetes (A) and effects of GLP-1 RAs on the progression of atherogenesis and the development of its complications (B). See the text for further details on the mechanisms involved and references to the supporting literature. EC: endothelial cell, eNOS: endothelial nitrous oxide synthase, ICAM-1: intercellular adhesion molecule-1, IL: interleukin, KLF-2: Krüppel-like factor-2, LDL: low-density lipoprotein, MCP-1: monocyte chemoattractant protein-1, NO: nitrous oxide, oxLDL: oxidized low-density lipoprotein, ROS: reactive oxygen species, TNF-α: tumor necrosis factor, VCAM-1: vascular cell adhesion protein 1, VSMC: vascular smooth muscle cell.
3.4. Atherogenesis in patients with type 2 diabetes (Figure 9A)
LDL cholesterol is transported across the intima layer of arterial blood vessels and in part oxidized to oxidized LDL particles (oxLDL) through reactive oxygen species (ROS). Contact of monocytes and macrophages with oxLDL and ROS promotes further infiltration of monocytes by secreting adhesion molecules such as vascular cell adhesion protein 1 (VCAM-1), monocyte chemoattractant protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin. Stimulated by oxLDL, monocytes transform into macrophages. M1 macrophages produce pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin (IL)-6, and IL-1β. M2 macrophages take up lipid particles through phagocytosis and suppress the formation of Krüppel-like factor 2 (KLF-2), which in turn suppresses endothelial NO synthase (eNOS), leading to lower NO production and preventing vasodilation through NO-mediated vascular smooth muscle relaxation. In an environment dominated by ROS and oxLDL, M2 macrophages transform into foam cells that can undergo apoptosis and release their lipid content into the lipid core of nascent atherosclerotic plaques. Stable plaques are characterized by a dense fibrous cap mainly composed of collagen that helps prevent rupture. However, as atherogenesis progresses, larger necrotic areas form, endothelial cells (EC) undergo apoptosis, and matrix metalloproteinases (MMP) proteolytically destroy the fibrous cap. This results in plaque rupture, thrombus formation, and bleeding into necrotic plaque areas.
3.5. Interference of GLP-1 RAs with the atherogenesis process (Figure 9B)
As demonstrated in animal studies and experiments using human cells, GLP-1 receptors expressed in endothelial cells, monocytes, macrophages, and vascular smooth muscle cells produce numerous effects potentially interfering with the process of atherosclerotic plaque formation or rupture. First, ROS production is reduced by GLP-1 [[128], [129], [130]], exenatide [131], liraglutide [130,[132], [133], [134], [135], [136]], and semaglutide [137]. The oxLDL-mediated activation of monocytes and macrophages and the consecutive activation of adhesion molecules such as VCAM-1, MCP-1, E-selectin, and ICAM-1 is successfully reduced by GLP-1 receptor stimulation (e.g., GLP-1 [138], exenatide [[138], [139], [140]], dulaglutide [141], and liraglutide [142]). This results in a reduction of monocyte accumulation in the vascular wall, as shown for example with exenatide [143]. Endothelial cells express more eNOS, produce more NO, and suppress endothelin formation that overall lead to vascular smooth muscle relaxation and endothelium-derived vasodilation (e.g., GLP-1 [130,144], exenatide [144], and liraglutide [130,133,145]). M2 macrophages instead of M1 macrophages preferentially form from monocytes (e.g., lixisenatide [146] and liraglutide [147]) and the otherwise suppressed KLF-2 formation instead increases (e.g., by lixisenatide [146], liraglutide [147], and dulaglutide [141]). The reduced exposure to ROS after GLP-1 receptor stimulation slows the process of foam cell formation (e.g., GLP-1 [148,149] and liraglutide [150]) and reduces caspase-mediated apoptosis of foam cells (e.g., GLP-1 [151] and semaglutide [152]) and the formation of necrosis in the core of atherosclerotic plaques (e.g., GLP-1 [153] and lixisenatide [154]). Furthermore, GLP-1 receptor stimulation reduces vascular smooth muscle proliferation (e.g., exenatide [155] and liraglutide [156]) and possible migration into plaques (liraglutide [157]). The integrity of endothelial cells was shown to be stabilized by exenatide [158,159]. Plaque hemorrhage was reduced by semaglutide [160]. The reduced expression of MMP preserves intact fibrous caps and prevents plaque rupture (e.g., GLP-1 [153], exenatide [161] and semaglutide) [160]. The overall result is a slowing of plaque progression and plaque stabilization. The formation, extent, and vulnerability of atherosclerotic lesions in animal models characterized by rapidly progressive atherosclerosis was substantially reduced by GLP-1 RA [160]. Studies in humans have partially confirmed anti-inflammatory [162] and anti-atherosclerotic actions of GLP-1 RAs [163].
4. Renal effects of GLP-1 RAs
The discovery of beneficial renal effects using GLP-1 RAs is a recent achievement mainly based on the observations that GLP-1 RAs prevented new-onset macroalbuminuria [99,164,165], reduced urinary albumin excretion [164,165], or slowed the decline in the estimated glomerular filtration rate (eGFR) over time [[164], [165], [166]]. The mechanisms leading to these renal benefits are largely unknown. While significant reductions in achieving renal composite outcomes were reported [99,164,165], they heavily relied on dominating effects preventing new-onset persistent macro-albuminuria. Clinical events indicating progression to end-stage renal disease (doubling in serum creatinine, major reduction by 30–50% in eGFR, achieving eGFR below 15 ml/min per 1.73 m2, necessary to initiate renal replacement therapy or perform renal transplantation, or death due to renal causes) have rarely been reported in numbers allowing a meaningful analysis. This is due in part to the fact that the populations studied had fairly good renal function at baseline. Studies of selected patients with prominent or advanced renal disease are lacking. A dedicated trial studying the effects of semaglutide on renal outcomes in type 2 diabetic patients with chronic kidney disease is underway to clarify these issues: FLOW (ClinicalTrials.gov NCT03819153). Since most GLP-1 RAs can be used in chronic kidney disease, while SGLT-2 inhibitors lose some of their glucose-lowering efficiency with reduced glomerular filtration rates, further studies appear to be needed, especially since patients with reduced eGFR at baseline seem to benefit most in terms of preventing rapid declines in eGFR [164]. For the time being, more robust effects have been reported for SGLT-2 inhibitors, which are preferred glucose-lowering medications interfering with the progression of diabetic renal disease even in patients with moderately reduced eGFR [110,111,167].
5. Adherence and persistence (observational studies)
While initiating GLP-1 RA treatment in clinical practice is already discrepant from current guidelines, suboptimal treatment persistence and adherence are additional important issues [168]. A recent study suggested that HbA1c reductions with GLP-1 RAs observed in real-world studies were ∼0.5% below those observed in controlled clinical trials [169]. The authors attributed approximately three-fourths of this gap to poor medication adherence in clinical practice. In a retrospective analysis comparing different GLP-1 RAs, once-weekly injectable dulaglutide demonstrated greater adherence rates than once-weekly exenatide or once-daily injectable liraglutide [170]. Of note, over a six-month treatment period, 26.2% of dulaglutide and 48.4% of once-weekly exenatide patients discontinued treatment, and in a direct comparison of dulaglutide and liraglutide, the respective discontinuation rates were 28.0% and 35.6% [170]. When the proportion of days covered (PDC) was compared between once-weekly exenatide and liraglutide, the proportions of patients with good adherence (PDC > 0.80) after 6 months were 53.4% and 48.1%, respectively [171]. Likewise, an analysis of Medicare recipients in the US reported a PDC >80% of 43.2% in patients receiving exenatide QW, 39.0% in patients receiving exenatide b.i.d., and 35% in patients receiving liraglutide [172]. Hence, no consistent data on differences in adherence between short- and long-acting GLP-1 RAs could be found. A recent real-world retrospective observational study showed that dulaglutide users were less likely to interrupt treatment than semaglutide and exenatide BCise users [173]. In a pairwise meta-analysis comparing treatment adherence and persistence between GLP-1 RAs and long-acting insulin analogues, the odds ratio for non-adherence was 1.95, suggesting better adherence with the insulin analogs [174]. As a general trend from these comparisons, adherence to GLP-1 RAs seems to be better with lower injection frequencies.
However, these studies must be interpreted with caution because of the retrospective study designs and partially incomplete data assessment. Furthermore, observation periods of 6–12 months are still too short to judge the long-term adherence to GLP-1 RAs.
6. Discontinuation rates in randomized CV outcome trials
As presented in Table 4, some patients randomized to GLP-1 RA treatment discontinued the assigned medication. The proportion withdrawing from GLP-1 RA treatment in CV outcome trials ranged from 15% (oral semaglutide) to approximately 25%; an exceptionally high withdrawal rate was observed with once-weekly exenatide (43%), possibly related to the less comfortable pen injection device requiring resuspension of the active ingredient in buffer (Figure 3) or the occurrence of subcutaneous nodules at injection sites [175]. Approximately one-half of the discontinuations were reported to be associated with adverse events (Table 4). In the trials reporting discontinuation because of any adverse events and those specifically due to gastrointestinal side effects, the latter were responsible for approximately one-half of the withdrawals. Another potential reason contributing to withdrawals was a perception of ineffective glycemic and body weight control achieved (including a suspicion to have been randomized to placebo), perhaps as a consequence of the progression of the type 2 diabetes mellitus disease process [176]. Whether or not GLP-1 RA treatment counters this progression (e.g., through β cell-preserving effects [177]) remains an open question. In rodents, these effects are restricted to earlier periods in life [178] when β cells have a propensity to proliferate, which they lose in adult animals [179]. Overall, randomized controlled clinical trials showed that high persistence regarding GLP-1 RA treatment could be maintained for periods up to 5 years, which contrasts with data from observational studies (as previously described). Efforts to encourage persistent use of GLP-1 RA, as successful in clinical trials, may be necessary to achieve better persistence in clinical practice as well.
7. Guideline recommendations and clinical reality
The current ADA/EASD consensus algorithm suggests that GLP-1 RAs should be preferentially used after metformin failure in (a) patients with established atherosclerotic cardiovascular disease and (b) patients without established cardiovascular disease with high-risk indicators, such as age ≥ 55 years, carotid, lower extremity or coronary artery stenosis >50%, left ventricular hypertrophy, eGFR < 60 ml/min, or albuminuria [180]. GLP-1 RAs may also be used to prevent hypoglycemia or weight gain. The ESC guidelines have gone even further in recommending GLP-1 RAs (or SGLT-2 inhibitors) as first-line therapy in patients with established atherosclerotic cardiovascular disease or in those at high or very high risk (that is, three or more major risk factors or diabetes duration ≥ 10 years without target organ damage, plus any other additional risk factors) [181]. According to these international recommendations, ∼30–60% of patients with type 2 diabetes would qualify for a GLP-1 RA. However, in clinical reality, the percentage of patients receiving GLP-1 RA treatment remains low, ranging between 1% and 10% in different countries [182].
The reasons for this apparent gap between guideline recommendations and clinical reality are heterogeneous: first, the cost of treatment with GLP-1 RAs is considerably higher than most oral glucose-lowering drugs, but instead comparable to the cost of an intensified insulin treatment regimen (including glucose-monitoring costs). Although various cost-effectiveness analyses suggested that the overall benefits associated with GLP-1 RA treatment outweigh the direct treatment costs [183], the price of the currently available GLP-1 RAs remains a major barrier in most countries. Second, the need for daily or weekly injections discourages some patients from initiating GLP-1 RAs [184]. Third, contraindications (i.e., history of pancreatitis, diabetic retinopathy, or medullary thyroid cancer) may prevent the use of GLP-1 RAs in affected patients [185]. Finally, gastrointestinal adverse events remain an important limitation of GLP-1 RA treatment [49].
8. Opportunities for future development of GLP-1 RAs
Since 2005, when exenatide was first approved, rapid development began that has yielded progress with respect to GLP-1 RAs pharmacokinetics, with the obvious consequence that instead of 2 (or more) injections per day, now once-weekly injections are available. While advances making GLP-1 RA treatment more comfortable are welcome, it should not be overlooked that the effectiveness of GLP-1 RAs has increased in large steps (e.g., going from exenatide to liraglutide, the first long-acting GLP-1 RA, but also advancing to semaglutide, which clearly has superior efficacy than other GLP-1 RAs, especially with respect to body weight reduction as depicted in Figure 4). These significant advances, occurring in substantial leaps, suggest that this development has not yet come to an end.
8.1. Oral administration of GLP-1 RAs
One development worth noting is that, despite the peptide nature of all of the GLP-1 RAs, semaglutide is now available for oral administration. An absorption enhancer molecule (SNAC; see Section 2) must be part of the oral preparation to promote absorption through the gastric mucosa. Low bioavailability after oral administration makes daily administration of a semaglutide tablet necessary to avoid wide fluctuations in drug exposure. It must be taken on an empty stomach, and for 30 min after taking oral semaglutide, no other food, drink, or medication should be administered to allow undisturbed absorption. With these precautions, in principle, quantitatively similar effects can be achieved with respect to glycemic control and lowering body weight [19]. The phase 3 PIONEER program, however, was conducted with somewhat lower doses (maximum, 14 mg/d) than would be necessary to match the effectiveness of subcutaneous semaglutide at 0.5 or 1.0 mg/week [43].
In addition to developing peptide-based GLP-1 RAs for oral administration, some reports described small molecules with GLP-1 receptor agonist properties that should be suitable for oral administration without additives and/or sensitive procedures. To date, the binding affinities of these compounds has been too low to support further development as clinically effective drugs [[186], [187], [188], [189]].
8.2. Use in patients with type 1 diabetes
Effects of GLP-1 or GLP-1 RAs on residual insulin secretion [190], glucagon suppression [190,191], gastric emptying delay [190], and plasma glucose [192,193] in type 1 diabetic subjects were described starting in the early stages of GLP-1 discovery. Clinical trials employing liraglutide or exenatide (un-retarded preparation usually administered b.i.d.) in addition to intensified insulin regimens, however, did not demonstrate convincing benefits (e.g., with respect to optimized glycemic control or the frequency of hypoglycemic episodes) or described potential adverse outcomes such as a higher risk of ketoacidosis. Only body weight and insulin doses were consistently reduced [[194], [195], [196], [197]]. However, these results do not rule out benefits for specific subgroups (e.g., obese patients with type 1 diabetes or subjects at high risk of cardiovascular complications) or with dosage recommendations that may differ from those used to treat type 2 diabetes.
8.3. Individualized use in well-defined type 2 diabetes subtypes
Cluster analysis was applied to define subgroups within the type 2 diabetes population that differ with respect to insulin secretory capacity, insulin sensitivity, age at diagnosis, and the presence of autoimmune markers [[198], [199], [200]]. These subgroups display significant differences in the development of CV and renal complications [[198], [199], [200]]. Thus, given the beneficial actions of GLP-1 RAs on preventing CV events (and on the progression of nephropathy), they may turn out to be particularly effective in those presenting a high a priori risk of these complications. Identifying a central pathophysiological defect (e.g., reduced insulin secretory capacity) may also help select a specific therapy addressing this point (e.g., GLP-1 RAs augmenting insulin secretion triggered by hyperglycemia). While prospective studies comparing various therapies in type 2 diabetes patients belonging to different subgroups are lacking, this sub-classification promises to be a helpful tool assisting in a more individualized approach toward selecting glucose-lowering medications for a given patient.
8.4. Combination treatment with GLP-1 RAs plus SGLT-2 inhibitors
In addition to GLP-1 RAs, SGLT-2 inhibitors are another class of glucose-lowering medications that have proven beneficial CV effects, especially regarding the prevention of heart failure complications (Figure 6 [110,111]). This raises the question of differential indications [201]. Based on the pattern of effects on various CV endpoints, GLP-1 RAs seem to better prevent ischemic events potentially resulting from anti-atherogenic effects (as previously described). The mechanisms of action of SGLT-2 inhibitors differ and aim to prevent heart failure complications (using hospitalization as an indicator) and the progression toward end-stage renal failure [110,111]. Therefore, if a patient seems to be at risk of ischemic events (e.g., because of previous events), GLP-1 RAs appear to be the better option. However, if the risk of congestive heart failure complications is considered the primary problem, SGLT-2 inhibitors are the better choice.
Since the severalfold elevated risk of CV events that type 2 diabetes demonstrates is only partially reduced by both GLP-1 RAs and SGLT-2 inhibitors, it may be necessary to combine medications from both classes to further improve their effectiveness. Combining dapagliflozin with exenatide once weekly lowers plasma glucose and body weight more than any of the single agents alone [202], even for prolonged periods of time [203]. Similar results were observed after adding empagliflozin to liraglutide in Japanese patients [204] and when adding canagliflozin to liraglutide treatment [205]. The weight loss induced by the combination compared to the single agents appeared to be additive, but HbA1c reduction was less than additive. When adding dulaglutide to pre-existing treatment with SGLT-2 inhibitors, HbA1c decreased substantially, while body weight declined by only 1 kg (at a higher dose of 1.5 mg) [206]. Systolic blood pressure was also lowered substantially by this combination [[204], [205], [206], [207], [208]]. The effects of combining GLP-1 RAs with SGLT-2 inhibitors were corroborated in a meta-analysis by Castellana et al. [209], confirming this combination's potential.
This leads to the essential question, will combining GLP-1 RAs and SGLT-2 inhibitors result in even better CV outcomes? No data are available to estimate the effects on CV outcomes using this combination. It is uncertain whether a large enough clinical trial addressing this question will ever be conducted. Real-world studies analyzing existing databases documenting medication use and clinical outcomes may help in this respect, but no such analysis seems to be currently available.
8.5. Unimolecular oligo-hormonal agonists address more than just GLP-1 receptors
One avenue of further increasing the potency of GLP-1 RAs is developing molecules that address not only GLP-1 receptors, but a second (co-agonist) or even a third (tri-agonist) receptor (choosing from glucagon, glucose-dependent insulinotropic polypeptide [GIP], or peptide YY [PYY] receptors). Preliminary findings suggest that highly effective compounds (e.g., tirzepatide [210]) can be developed this way, in particular providing weight loss far exceeding that reported with pure GLP-1 RAs. These developments will be the focus of another manuscript on the present supplement volume (Baggio et al. [211]).
8.6. Pharmacogenomics
Since GLP-1 RAs exert their biological effects by interacting with the GLP-1 receptor, interindividual differences in the expression of these receptors or polymorphisms at the GLP-1 receptor gene may modify biological responses [[212], [213], [214], [215]]. Along these lines, certain polymorphisms regarding the TCF7L2 gene (probably involved in determining β cell mass and the expression of GLP-1 receptors) impair insulin responses to exogenous GLP-1 [216]. One study described a modification of the in vitro effects of GLP-1 RAs for the GLP-1 receptor variant T149M (methionine instead of threonine in position 149) on β cells [217]. However, a preliminary clinical study did not describe differences in pharmacological effects in response to short-term treatment with exenatide [218]. Given the potential of selecting patients with a predicted greater clinical effectiveness [219], the issue of pharmacogenomics regarding GLP-1 RA still appears to be an under-studied research area. Furthermore, patients not responding to GLP-1 RAs as expected, either when initially exposed to GLP-1 RAs (primary non-responders) or after a satisfactory response period (secondary non-responders), have often been observed in clinical practice. Systematic studies elucidating the mechanisms of a potential non-response to GLP-1 RA treatment (such as genetics or lifestyle issues) remain lacking.
8.7. Potential novel indications: neurodegenerative diseases and psoriasis
Interest in using GLP-1 RAs to treat neurodegenerative diseases emerged from preclinical studies showing that GLP-1 receptor signaling is involved in cognitive functions [220] and GLP-1 RAs can induce neuronal growth and synaptic plasticity and reduce apoptosis and oxidative stress [221].
In Alzheimer's disease, the most prevalent form of dementia, animal studies have shown the positive effects of GLP-1 RAs on cognitive impairment [222,223]. In a clinical trial of patients with prediabetes and type 2 diabetes, memory function improved after 4 months of liraglutide administration [224]. However, there was no placebo control. In another trial with non-diabetes subjects at increased risk of Alzheimer's disease, administration of liraglutide for 3 months improved brain region connectivity (assessed by functional MRI), but cognitive functions did not improve [225]. Another trial in non-diabetes patients with Alzheimer's disease found that 6 months of liraglutide treatment prevented a further decline in brain glucose uptake (assessed by positron emission tomography), but did not change cognitive function tests [226]. A larger clinical trial is currently ongoing that is investigating the effects of liraglutide on mild Alzheimer's disease using comprehensive neurological and cognitive assessment [227].
Parkinson's disease is another neurodegenerative disease for which GLP-1 RAs are being explored as treatment options [221,228]. In a mouse model of Parkinson's disease, a novel GLP-1 RA protected dopaminergic neurons and ameliorated behavioral deficits, most likely by blocking the formation of a neurotoxic astrocyte variant [229]. In clinical trials, exenatide improved the MDS-UPDRS score (a standardized assessment scale for patients with Parkinson's disease) [230,231]. Nevertheless, a recent systematic Cochrane Database review declared the evidence of improved motor impairment in GLP-1 RA-treated patients with Parkinson's disease as “low certainty” [232].
In an animal model of Huntington's disease, mice treated with exendin-4 for 9 weeks presented with reduced huntingtin protein aggregates in the cortex compared to placebo and had longer life spans [233]. We are not aware of any clinical trials in human patients with Huntington's disease.
Psoriasis is associated with type 2 diabetes [234]. Two case reports generated interest in using GLP-1 RAs as a potentially novel treatment option for psoriasis [235,236]. Two subsequent prospective studies found positive effects on psoriasis severity scores in type 2 diabetes patients treated with GLP-1 RAs [237,238], a finding that could not be confirmed in non-diabetes subjects [239]. This possibly suggests a clinical effectiveness that may differ in diabetes and non-diabetes patients.
9. Conclusions
Clinical research conducted over the past 30 years has established GLP-1 RAs as a widely recommended class of glucose-lowering agents. The best representatives of this class are capable of lowering plasma glucose comparable to insulin regimens, but with a lower risk of hypoglycemia and the added benefit of weight loss. The ability to prevent CV events in high-risk patients has re-emphasized the particular benefits that GLP-1 RAs may generate in type 2 diabetes therapy. Despite these past achievements, there is a potential for further increasing effectivity, optimizing molecules and dosing regimens, and exploring specific patient groups that will particularly benefit from GLP-1 RAs.
Acknowledgments
We thank Laura Kupfer and Birgit Baller for assisting with retrieving the literature and Nina Schenker, Dr. Mirna Abd El Aziz, and Dr. Oscar Cahyadi for helpful discussions.
Conflict of interest
M.A.N. has been a member of advisory boards or consulted with AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Menarini/Berlin-Chemie, Merck, Sharp & Dohme, and Novo Nordisk. He has received grant support from AstraZeneca, Eli Lilly and Company, Menarini/Berlin-Chemie, Merck, Sharp & Dohme, and Novo Nordisk. He has also served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Menarini/Berlin-Chemie, Merck, Sharp & Dohme, and Novo Nordisk. J.J.M. has received consulting and speaker honoraria from AstraZeneca, Eli Lilly and Company, Merck, Sharp & Dohme, Novo Nordisk, Sanofi, and Sevier. He has received research support from Boehringer Ingelheim, Eli Lilly and Company, Merck, Sharp & Dohme, Novo Nordisk, and Sanofi. J.W. and D.Q. have nothing to declare.
References
- 1.Holst J.J., Ørskov C., Vagn-Nielsen O., Schwartz T.W. Truncated glucagon-like peptide 1, an insulin-releasing hormone from the distal gut. FEBS Letters. 1987;211:169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 2.Mojsov S., Weir G.C., Habener J.F. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. Journal of Clinical Investigation. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell G.I., Sanchez-Pescador R., Laybourn P.J., Najarian R.C. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S., Kawai K., Ohashi S., Mukai H., Murayama Y., Yamashita K. Reduced insulinotropic effects of glucagonlike peptide 1-(7-36)-amide and gastric inhibitory polypeptide in isolated perfused diabetic rat pancreas. Diabetes. 1990;39:1320–1325. doi: 10.2337/diab.39.11.1320. [DOI] [PubMed] [Google Scholar]
- 5.Nauck M.A., Heimesaat M.M., Ørskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. Journal of Clinical Investigation. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauck M.A., Weber I., Bach I., Richter S., Ørskov C., J.J. H. Normalization of fasting glycaemia by intravenous GLP-1 ([7-36 amide] or [7-37]) in Type 2-diabetic patients. Diabetic Medicine. 1998;15:937–945. doi: 10.1002/(SICI)1096-9136(1998110)15:11<937::AID-DIA701>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Nauck M.A., Kleine N., Ørskov C., Holst J.J., Willms B., Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 8.Deacon C.F., Nauck M.A., Toft-Nielsen M., Pridal L., Willms B., Host J.J. Both subcutaneously and intravenously administered glucagon-like peptide 1 are rapidly degraded from the NH2-terminus in type 2-diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 9.Deacon C.F., Pridal L., Klarskov L., Olesen M., Holst J.J. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. American Journal of Physiology. 1996;271:E 458–E 464. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- 10.Nauck M.A., Heimesaat M.M., Behle K., Holst J.J., Nauck M.S., Ritzel R. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. Journal of Clinical Endocrinology & Metabolism. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 11.Wettergren A., Schjoldager B., Mortensen P.E., Myhre J., Christiansen J., Holst J.J. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Digestive Diseases and Sciences. 1993;38:665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- 12.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide-1 promotes satiety and suppresses energy intake in humans. Journal of Clinical Investigation. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng J., Kleinman W.A., Singh L., Singh G., Raufman J.P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from Guinea pig pancreas. Journal of Biological Chemistry. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 14.Göke R., Fehmann H.C., Linn T., Schmidt H., Krause M., Eng J. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. Journal of Biological Chemistry. 1993;268:19650–19655. [PubMed] [Google Scholar]
- 15.Nauck M.A., Meier J.J. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinology. 2016;4:525–536. doi: 10.1016/S2213-8587(15)00482-9. [DOI] [PubMed] [Google Scholar]
- 16.Nauck M.A. The rollercoaster history of using physiological and pharmacological properties of incretin hormones to develop diabetes medications with a convincing benefit-risk relationship. Metabolism. 2020;103:154031. doi: 10.1016/j.metabol.2019.154031. [DOI] [PubMed] [Google Scholar]
- 17.Kim D., MacConell L., Zhuang D., Kothare P.A., Trautmann M., Fineman M. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 18.Drucker D.J., Buse J.B., Taylor K., Kendall D.M., Trautmann M., Zhuang D. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 19.Davies M., Pieber T.R., Hartoft-Nielsen M.L., Hansen O.K.H., Jabbour S., Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. Journal of the American Medical Association. 2017;318:1460–1470. doi: 10.1001/jama.2017.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nauck M.A., Meier J.J. Management of endocrine disease: are all GLP-1 agonists equal in the treatment of type 2 diabetes? European Journal of Endocrinology. 2019;181:R 211–R 234. doi: 10.1530/EJE-19-0566. [DOI] [PubMed] [Google Scholar]
- 21.Kolterman O.G., Kim D.D., Shen L., Ruggles J.A., Nielsen L.L., Fineman M.S. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. American Journal of Health-System Pharmacy. 2005;62:173–181. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 22.Becker R.H., Stechl J., Steinstraesser A., Golor G., Pellissier F. Lixisenatide reduces postprandial hyperglycaemia via gastrostatic and insulinotropic effects. Diabetes Metabolism Research Reviews. 2015;31:610–618. doi: 10.1002/dmrr.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damholt B., Golor G., Wierich W., Pedersen P., Ekblom M., Zdravkovic M. An open-label, parallel group study investigating the effects of age and gender on the pharmacokinetics of the once-daily glucagon-like peptide-1 analogue liraglutide. The Journal of Clinical Pharmacology. 2006;46:635–641. doi: 10.1177/0091270006288215. [DOI] [PubMed] [Google Scholar]
- 24.Geiser J.S., Heathman M.A., Cui X., Martin J., Loghin C., Chien J.Y. Clinical pharmacokinetics of dulaglutide in patients with type 2 diabetes: analyses of data from clinical trials. Clinical Pharmacokinetics. 2016;55:625–634. doi: 10.1007/s40262-015-0338-3. [DOI] [PubMed] [Google Scholar]
- 25.Matthews J.E., Stewart M.W., De Boever E.H., Dobbins R.L., Hodge R.J., Walker S.E. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2008;93:4810–4817. doi: 10.1210/jc.2008-1518. [DOI] [PubMed] [Google Scholar]
- 26.Marbury T.C., Flint A., Jacobsen J.B., Derving Karsbol J., Lasseter K. Pharmacokinetics and tolerability of a single dose of semaglutide, a human glucagon-like peptide-1 analog, in subjects with and without renal impairment. Clinical Pharmacokinetics. 2017;56:1381–1390. doi: 10.1007/s40262-017-0528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granhall C., Donsmark M., Blicher T.M., Golor G., Sondergaard F.L., Thomsen M. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clinical Pharmacokinetics. 2019;58:781–791. doi: 10.1007/s40262-018-0728-4. [DOI] [PubMed] [Google Scholar]
- 28.Gough S.C., Jain R., Woo V.C. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Review of Endocrinology and Metabolism. 2016;11:7–19. doi: 10.1586/17446651.2016.1113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies M.J., Leiter L.A., Guerci B., Grunberger G., Ampudia-Blasco F.J., Yu C. Impact of baseline glycated haemoglobin, diabetes duration and body mass index on clinical outcomes in the LixiLan-O trial testing a titratable fixed-ratio combination of insulin glargine/lixisenatide (iGlarLixi) vs insulin glargine and lixisenatide monocomponents. Diabetes, Obesity and Metabolism. 2017;19:1798–1804. doi: 10.1111/dom.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overgaard R.V., Lindberg S.O., Thielke D. Impact on HbA1c and body weight of switching from other GLP-1 receptor agonists to semaglutide: a model-based approach. Diabetes, Obesity and Metabolism. 2019;21:43–51. doi: 10.1111/dom.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nature Reviews Endocrinology. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 32.Umapathysivam M.M., Lee M.Y., Jones K.L., Annink C.E., Cousins C.E., Trahair L.G. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes. 2014;63:785–790. doi: 10.2337/db13-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier J.J., Menge B.A., Schenker N., Erdmann S., Kahle-Stephan M., Schliess F. Effects of sequential treatment with lixisenatide, insulin glargine, or their combination on meal-related glycemic excursions, insulin and glucagon secretion, and gastric emptying in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2020;22:599–611. doi: 10.1111/dom.13935. [DOI] [PubMed] [Google Scholar]
- 34.Meier J.J., Rosenstock J., Hincelin-Mery A., Roy-Duval C., Delfolie A., Coester H.V. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care. 2015;38:1263–1273. doi: 10.2337/dc14-1984. [DOI] [PubMed] [Google Scholar]
- 35.Huthmacher J.A., Meier J.J., Nauck M.A. Efficacy and safety of short- and long-acting glucagon-like peptide 1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care. 2020;43:2303–2312. doi: 10.2337/dc20-0498. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstock J., Raccah D., Koranyi L., Maffei L., Boka G., Miossec P. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36:2945–2951. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buse J.B., Rosenstock J., Sesti G., Schmidt W.E., Montanya E., Brett J.H. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 38.Nauck M., Rizzo M., Johnson A., Bosch-Traberg H., Madsen J., Cariou B. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: a 26-week randomized controlled clinical trial. Diabetes Care. 2016;39:1501–1509. doi: 10.2337/dc15-2479. [DOI] [PubMed] [Google Scholar]
- 39.Buse J.B., Nauck M., Forst T., Sheu W.H., Shenouda S.K., Heilmann C.R. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 40.Pratley R.E., Nauck M.A., Barnett A.H., Feinglos M.N., Ovalle F., Harman-Boehm I. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinology. 2014;2:289–297. doi: 10.1016/S2213-8587(13)70214-6. [DOI] [PubMed] [Google Scholar]
- 41.Dungan K.M., Povedano S.T., Forst T., Gonzalez J.G., Atisso C., Sealls W. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–1357. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 42.Pratley R.E., Aroda V.R., Lingvay I., Lüdemann J., Andreassen C., Navarria A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinology. 2018;6:275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 43.Pratley R., Amod A., Hoff S.T., Kadowaki T., Lingvay I., Nauck M. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 44.Abd El Aziz M.S., Kahle M., Meier J.J., Nauck M.A. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes, Obesity and Metabolism. 2017;19:216–227. doi: 10.1111/dom.12804. [DOI] [PubMed] [Google Scholar]
- 45.Singh S., Wright E.E., Jr., Kwan A.Y., Thompson J.C., Syed I.A., Korol E.E. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes, Obesity and Metabolism. 2017;19:228–238. doi: 10.1111/dom.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buse J.B., Peters A., Russell-Jones D., Furber S., Donsmark M., Han J. Is insulin the most effective injectable antihyperglycemic therapy? Diabetes, Obesity and Metabolism. 2015;17:145–151. doi: 10.1111/dom.12402. [DOI] [PubMed] [Google Scholar]
- 47.Davies M.J., D'Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 48.Aroda V.R., Bain S.C., Cariou B., Piletic M., Rose L., Axelsen M. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinology. 2017;5:355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 49.Bettge K., Kahle M., Abd El Aziz M.S., Meier J.J., Nauck M.A. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes, Obesity and Metabolism. 2017;19:336–347. doi: 10.1111/dom.12824. [DOI] [PubMed] [Google Scholar]
- 50.Huthmacher J.A., Meier J.J., M.A. N. Efficacy and safety of short- and long-acting GLP-1 receptor agonists on a background of basal insulin in type 2 diabetes: a meta-analysis. Diabetes Care. 2020;43:2303–2312. doi: 10.2337/dc20-0498. [DOI] [PubMed] [Google Scholar]
- 51.Nauck M.A., Meier J.J. Pharmacotherapy: GLP-1 analogues and insulin: sound the wedding bells? Nature Reviews Endocrinology. 2011;7:193–195. doi: 10.1038/nrendo.2011.30. [DOI] [PubMed] [Google Scholar]
- 52.Buse J.B., Bergenstal R.M., Glass L.C., Heilmann C.R., Lewis M.S., Kwan A.Y. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Annals of Internal Medicine. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 53.Ahmann A., Rodbard H.W., Rosenstock J., Lahtela J.T., de Loredo L., Tornoe K. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2015;17:1056–1064. doi: 10.1111/dom.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pozzilli P., Norwood P., Jodar E., Davies M.J., Ivanyi T., Jiang H. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9) Diabetes, Obesity and Metabolism. 2017;19:1024–1031. doi: 10.1111/dom.12937. [DOI] [PubMed] [Google Scholar]
- 55.Leiter L.A., Gross J.L., Chow F., Miller D., Johnson S., Ahren B. Once weekly glucagon-like peptide-1 receptor agonist albiglutide vs. prandial insulin added to basal insulin in patients with type 2 diabetes mellitus: results over 52 weeks. Journal of Diabetic Complications. 2017;31:1283–1285. doi: 10.1016/j.jdiacomp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Rodbard H.W., Lingvay I., Reed J., de la Rosa R., Rose L., Sugimoto D. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. Journal of Clinical Endocrinology & Metabolism. 2018;103:2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinman B., Aroda V.R., Buse J.B., Cariou B., Harris S.B., Hoff S.T. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42:2262–2271. doi: 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamant M., Nauck M.A., Shaginian R., Malone J.K., Cleall S., Reaney M. Glucagon-like Peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37:2763–2773. doi: 10.2337/dc14-0876. [DOI] [PubMed] [Google Scholar]
- 59.Gough S.C., Bode B., Woo V., Rodbard H.W., Linjawi S., Poulsen P. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinology. 2014;2:885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 60.Frias J., Puig Domingo M., Meneghini L., Napoli R., Liu M., Soltes Rak E. More patients reach glycemic control with a fixed-ratio combination of insulin glargine and lixisenatide (iGlarLixi) than with basal insulin at 12 weeks of treatment: a post hoc time-to-control analysis of LixiLan-O and LixiLan-L. Diabetes, Obesity and Metabolism. 2018;20:2314–2318. doi: 10.1111/dom.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabak A.G., Anderson J., Aschner P., Liu M., Saremi A., Stella P. Efficacy and safety of iGlarLixi, fixed-ratio combination of insulin glargine and lixisenatide, compared with basal-bolus regimen in patients with type 2 diabetes: propensity score matched analysis. Diabetes Therapeutics. 2020;11:305–318. doi: 10.1007/s13300-019-00735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turton M.D., D O.S., Gunn I., Beak S.A., Edwards C.M., Meeran K. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 63.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 64.Astrup A., Rossner S., Van Gaal L., Rissanen A., Niskanen L., Al Hakim M. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 65.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 66.Blundell J., Finlayson G., Axelsen M., Flint A., Gibbons C., Kvist T. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obesity and Metabolism. 2017;19:1242–1251. doi: 10.1111/dom.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kushner R.F., Calanna S., Davies M., Dicker D., Garvey W.T., Goldman B. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP Trials 1 to 5. Obesity (Silver Spring) 2020;28:1050–1061. doi: 10.1002/oby.22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Neil P.M., Birkenfeld A.L., McGowan B., Mosenzon O., Pedersen S.D., Wharton S. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 69.Nauck M., Marre M. Adding liraglutide to oral antidiabetic drug monotherapy: efficacy and weight benefits. Postgraduate Medical Journal. 2009;121:5–15. doi: 10.3810/pgm.2009.05.1997. [DOI] [PubMed] [Google Scholar]
- 70.Davies M.J., Bergenstal R., Bode B., Kushner R.F., Lewin A., Skjoth T.V. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Journal of the American Medical Association. 2015;314:687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 71.Frias J.P., Bonora E., Nevarez Ruiz L.A., Li G., You Z., Milicevic Z. Efficacy and safety of dulaglutide 3 mg and 4.5 mg vs. dulaglutide 1.5 mg: 52-week results from AWARD-11 (abstract 357-OR) Diabetes. 2020;69(Suppl. 1) [Google Scholar]
- 72.Secher A., Jelsing J., Baquero A.F., Hecksher-Sorensen J., Cowley M.A., Dalboge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Ronne J., Alanentalo T., Baquero A.F. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5 doi: 10.1172/jci.insight.133429. epub Mar 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campos C.A., Bowen A.J., Schwartz M.W., Palmiter R.D. Parabrachial CGRP neurons control meal termination. Cell Metabolism. 2016;23:811–820. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishizawa M., Nakabayashi H., Uehara K., Nakagawa A., Uchida K., Koya D. Intraportal GLP-1 stimulates insulin secretion predominantly through the hepatoportal-pancreatic vagal reflex pathways. American Journal of Physiology. 2013;305:E 376–E 387. doi: 10.1152/ajpendo.00565.2012. [DOI] [PubMed] [Google Scholar]
- 76.Hansen L., Deacon C.F., Ørskov C., Holst J.J. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 77.Di Marzo V., Ligresti A., Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. International Journal of Obesity (Lond) 2009;33(Suppl 2):S18–S24. doi: 10.1038/ijo.2009.67. [DOI] [PubMed] [Google Scholar]
- 78.Kadouh H., Chedid V., Halawi H., Burton D.D., Clark M.M., Khemani D. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. Journal of Clinical Endocrinology & Metabolism. 2020;105 doi: 10.1210/clinem/dgz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 80.van Bloemendaal L., Veltman D.J., Ten Kulve J.S., Groot P.F., Ruhe H.G., Barkhof F. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes, Obesity and Metabolism. 2015;17:878–886. doi: 10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- 81.Daniele G., Iozzo P., Molina-Carrion M., Lancaster J., Ciociaro D., Cersosimo E. Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes. 2015;64:3406–3412. doi: 10.2337/db14-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dickson S.L., Shirazi R.H., Hansson C., Bergquist F., Nissbrandt H., Skibicka K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. Journal of Neuroscience. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richard J.E., Anderberg R.H., Goteson A., Gribble F.M., Reimann F., Skibicka K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PloS One. 2015;10 doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Bloemendaal L., Rg I.J., Ten Kulve J.S., Barkhof F., Konrad R.J., Drent M.L. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 85.Frias J.P., Wynne A.G., Matyjaszek-Matuszek B., Bartaskova D., Cox D.A., Woodward B. Efficacy and safety of an expanded dulaglutide dose range: a phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes, Obesity and Metabolism. 2019;21:2048–2057. doi: 10.1111/dom.13764. [DOI] [PubMed] [Google Scholar]
- 86.le Roux C.W., Astrup A., Fujioka K., Greenway F., Lau D.C.W., Van Gaal L. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 87.Kelly A.S., Auerbach P., Barrientos-Perez M., Gies I., Hale P.M., Marcus C. A randomized, controlled trial of liraglutide for adolescents with obesity. New England Journal of Medicine. 2020;382:2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 88.Diamant M., Van Gaal L., Stranks S., Northrup J., Cao D., Taylor K. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–2243. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 89.Schlogl H., Kabisch S., Horstmann A., Lohmann G., Muller K., Lepsien J. Exenatide-induced reduction in energy intake is associated with increase in hypothalamic connectivity. Diabetes Care. 2013;36:1933–1940. doi: 10.2337/dc12-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Boer S.A., Lefrandt J.D., Petersen J.F., Boersma H.H., Mulder D.J., Hoogenberg K. The effects of GLP-1 analogues in obese, insulin-using type 2 diabetes in relation to eating behaviour. International Journal of Clinical Pharmacy. 2016;38:144–151. doi: 10.1007/s11096-015-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jorgensen P.G., Jensen M.T., Mensberg P., Storgaard H., Nyby S., Jensen J.S. Effect of exercise combined with glucagon-like peptide-1 receptor agonist treatment on cardiac function: a randomized double-blind placebo-controlled clinical trial. Diabetes, Obesity and Metabolism. 2017;19:1040–1044. doi: 10.1111/dom.12900. [DOI] [PubMed] [Google Scholar]
- 92.Wadden T.A., Tronieri J.S., Sugimoto D., Lund M.T., Auerbach P., Jensen C. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring) 2020;28:529–536. doi: 10.1002/oby.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pieber T.R., Bode B., Mertens A., Cho Y.M., Christiansen E., Hertz C.L. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinology. 2019;7:528–539. doi: 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed] [Google Scholar]
- 94.Miyagawa J., Odawara M., Takamura T., Iwamoto N., Takita Y., Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes, Obesity and Metabolism. 2015;17:974–983. doi: 10.1111/dom.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pfeffer M.A., Claggett B., Diaz R., Dickstein K., Gerstein H.C., Kober L.V. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. New England Journal of Medicine. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 96.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holman R.R., Bethel M.A., Mentz R.J., Thompson V.P., Lokhnygina Y., Buse J.B. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gerstein H.C., Colhoun H.M., Dagenais G.R., Diaz R., Lakshmanan M., Pais P. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 99.Hernandez A.F., Green J.B., Janmohamed S., D'Agostino R.B., Sr., Granger C.B., Jones N.P. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 100.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 101.Husain M., Birkenfeld A.L., Donsmark M., Dungan K., Eliaschewitz F.G., Franco D.R. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 102.Butler P.C., Elashoff M., Elashoff R., Gale E.A. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care. 2013;36:2118–2125. doi: 10.2337/dc12-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nauck M.A., Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36(Suppl. 2):S 245–S 252. doi: 10.2337/dcS13-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abd El Aziz M., Cahyadi O., Meier J.J., Schmidt W.E., Nauck M.A. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: a meta-analysis based on cardiovascular outcomes trials. Diabetes, Obesity and Metabolism. 2019;22:699–704. doi: 10.1111/dom.13924. [DOI] [PubMed] [Google Scholar]
- 105.Steinberg W.M., Buse J.B., Ghorbani M.L.M., Orsted D.D., Nauck M.A., Committee L.S. Amylase, lipase, and acute pancreatitis in people with type 2 diabetes treated with liraglutide: results from the LEADER randomized trial. Diabetes Care. 2017;40:966–972. doi: 10.2337/dc16-2747. [DOI] [PubMed] [Google Scholar]
- 106.Nauck M.A., Frossard J.L., Barkin J.S., Anglin G., Hensley I.E., Harper K.D. Assessment of pancreas safety in the development program of once-weekly GLP-1 receptor agonist dulaglutide. Diabetes Care. 2017;40:647–654. doi: 10.2337/dc16-0984. [DOI] [PubMed] [Google Scholar]
- 107.Bjerre Knudsen L., Madsen L.W., Andersen S., Almholt K., de Boer A.S., Drucker D.J. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 108.Kristensen S.L., Rorth R., Jhund P.S., Docherty K.F., Sattar N., Preiss D. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinology. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 109.Caruso I., Cignarelli A., Giorgino F. Heterogeneity and similarities in GLP-1 receptor agonist cardiovascular outcomes trials. Trends in Endocrinology and Metabolism. 2019;30:578–589. doi: 10.1016/j.tem.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 110.Zelniker T.A., Wiviott S.D., Raz I., Im K., Goodrich E.L., Bonaca M.P. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 111.Zelniker T.A., Wiviott S.D., Raz I., Im K., Goodrich E.L., Furtado R.H.M. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 112.Jorsal A., Kistorp C., Holmager P., Tougaard R.S., Nielsen R., Hanselmann A. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. European Journal of Heart Failure. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 113.Margulies K.B., Hernandez A.F., Redfield M.M., Givertz M.M., Oliveira G.H., Cole R. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. Journal of the American Medical Association. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marsico F., Paolillo S., Gargiulo P., Bruzzese D., Dell'Aversana S., Esposito I. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. European Heart Journal. 2020 doi: 10.1093/eurheartj/ehaa082. [DOI] [PubMed] [Google Scholar]
- 115.Nauck M.A., Meier J.J., Cavender M.A., Abd El Aziz M., Drucker D.J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 116.Buse J.B., Bain S.C., Mann J.F.E., Nauck M.A., Nissen S.E., Pocock S. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43:1546–1552. doi: 10.2337/dc19-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Colhoun H.M., Hasnour C., Riddle M.C., Brancj K., M K., Atisso C. Exploring potential mediators of the cardiovascular benefit of dulaglutide in REWIND (abstract 924-P) Diabetes. 2020;69(Suppl. 1) doi: 10.1186/s12933-021-01386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ray K.K., Seshasai S.R., Wijesuriya S., Sivakumaran R., Nethercott S., Preiss D. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 119.Caruso I., Cignarelli A., Natalicchio A., Perrini S., Laviola L., Giorgino F. Commentary: glucose control: not just a bystander in GLP-1RA-mediated cardiovascular protection. Metabolism. 2020;109:154272. doi: 10.1016/j.metabol.2020.154272. [DOI] [PubMed] [Google Scholar]
- 120.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 121.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 122.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 123.White W.B., Cannon C.P., Heller S.R., Nissen S.E., Bergenstal R.M., Bakris G.L. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. New England Journal of Medicine. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 124.Rosenstock J., Perkovic V., Johansen O.E., Cooper M.E., Kahn S.E., Marx N. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. Journal of the American Medical Association. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scirica B.M., Bhatt D.L., Braunwald E., Steg P.G., Davidson J., Hirshberg B. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. New England Journal of Medicine. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 126.Green J.B., Bethel M.A., Armstrong P.W., Buse J.B., Engel S.S., Garg J. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 127.Spartalis M., Spartalis E., Athanasiou A., Paschou S.A., Kontogiannis C., Georgiopoulos G. The role of the endothelium in premature atherosclerosis: molecular mechanisms. Current Medicinal Chemistry. 2020;27:1041–1051. doi: 10.2174/0929867326666190911141951. [DOI] [PubMed] [Google Scholar]
- 128.Ku H.C., Chen W.P., Su M.J. DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes. PloS One. 2013;8 doi: 10.1371/journal.pone.0054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alharby H., Abdelati T., Rizk M., Youssef E., Gaber N., Moghazy K. Association of fasting glucagon-like peptide-1 with oxidative stress and subclinical atherosclerosis in type 2 diabetes. Diabetes, Metabolic Syndrome. 2019;13:1077–1080. doi: 10.1016/j.dsx.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 130.Barale C., Buracco S., Cavalot F., Frascaroli C., Guerrasio A., Russo I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation. Thrombosis & Haemostasis. 2017;117:1115–1128. doi: 10.1160/TH16-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang S.T., Zhang Q., Tang H.Q., Wang C.J., Su H., Zhou Q. Effects of glucagon-like peptide-1 on advanced glycation endproduct-induced aortic endothelial dysfunction in streptozotocin-induced diabetic rats: possible roles of Rho kinase- and AMP kinase-mediated nuclear factor kappaB signaling pathways. Endocrine. 2016;53:107–116. doi: 10.1007/s12020-015-0852-y. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y.C., Wang W.T., Lee S.S., Kuo Y.R., Wang Y.C., Yen S.J. Glucagon-like peptide-1 receptor agonist attenuates autophagy to ameliorate pulmonary arterial hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3beta pathways. International Journal of Molecular Sciences. 2019;20 doi: 10.3390/ijms20143435. epub 25 July 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Helmstädter J., Frenis K., Filippou K., Grill A., Dib M., Kalinovic S. Endothelial GLP-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020;40:145–158. doi: 10.1161/atv.0000615456.97862.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dai Y., Mercanti F., Dai D., Wang X., Ding Z., Pothineni N.V. LOX-1, a bridge between GLP-1R and mitochondrial ROS generation in human vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2013;437:62–66. doi: 10.1016/j.bbrc.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 135.Shiraki A., Oyama J., Komoda H., Asaka M., Komatsu A., Sakuma M. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 136.Cai X., She M., Xu M., Chen H., Li J., Chen X. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. International Journal of Biological Sciences. 2018;14:1696–1708. doi: 10.7150/ijbs.27774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Q., Tuo X., Li B., Deng Z., Qiu Y., Xie H. Semaglutide attenuates excessive exercise-induced myocardial injury through inhibiting oxidative stress and inflammation in rats. Life Sciences. 2020;250:117531. doi: 10.1016/j.lfs.2020.117531. [DOI] [PubMed] [Google Scholar]
- 138.Dorecka M., Siemianowicz K., Francuz T., Garczorz W., Chyra A., Klych A. Exendin-4 and GLP-1 decreases induced expression of ICAM-1, VCAM-1 and RAGE in human retinal pigment epithelial cells. Pharmacological Reports. 2013;65:884–890. doi: 10.1016/s1734-1140(13)71069-7. [DOI] [PubMed] [Google Scholar]
- 139.Erdogdu Ö., Nathanson D., Sjöholm Å., Nyström T., Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Molecular and Cellular Endocrinology. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 140.Wei R., Ma S., Wang C., Ke K., Yang J., Li W. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. American Journal of Physiology. 2016;310:E 947–E 957. doi: 10.1152/ajpendo.00400.2015. [DOI] [PubMed] [Google Scholar]
- 141.Chang W., Zhu F., Zheng H., Zhou Z., Miao P., Zhao L. Glucagon-like peptide-1 receptor agonist dulaglutide prevents ox-LDL-induced adhesion of monocytes to human endothelial cells: an implication in the treatment of atherosclerosis. Molecular Immunology. 2019;116:73–79. doi: 10.1016/j.molimm.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 142.Dai Y., Mehta J.L., Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear Factor-kappa B activation. Cardiovascular Drugs and Therapy. 2013;27:371–380. doi: 10.1007/s10557-013-6463-z. [DOI] [PubMed] [Google Scholar]
- 143.Arakawa M., Mita T., Azuma K., Ebato C., Goto H., Nomiyama T. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding L., Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacologica Sinica. 2012;33:75–81. doi: 10.1038/aps.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dai Y., Mehta J.L., Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovascular Drugs and Therapy. 2013;27(5):371–380. doi: 10.1007/s10557-013-6463-z. [DOI] [PubMed] [Google Scholar]
- 146.Vinué Á., Navarro J., Herrero-Cervera A., García-Cubas M., Andrés-Blasco I., Martínez-Hervás S. The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia. 2017;60:1801–1812. doi: 10.1007/s00125-017-4330-3. [DOI] [PubMed] [Google Scholar]
- 147.Bruen R., Curley S., Kajani S., Lynch G., O'Reilly M.E., Dillon E.T. Liraglutide attenuates preestablished atherosclerosis in apolipoprotein E-deficient mice via regulation of immune cell phenotypes and proinflammatory mediators. Journal of Pharmacology and Experimental Therapeutics. 2019;370:447–458. doi: 10.1124/jpet.119.258343. [DOI] [PubMed] [Google Scholar]
- 148.Hirano T., Mori Y. Anti-atherogenic and anti-inflammatory properties of glucagon-like peptide-1, glucose-dependent insulinotropic polypepide, and dipeptidyl peptidase-4 inhibitors in experimental animals. Journal of Diabetes Investigations. 2016;7(Suppl. 1):80–86. doi: 10.1111/jdi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nagashima M., Watanabe T., Terasaki M., Tomoyasu M., Nohtomi K., Kim-Kaneyama J. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tashiro Y., Sato K., Watanabe T., Nohtomi K., Terasaki M., Nagashima M. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides. 2014;54:19–26. doi: 10.1016/j.peptides.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 151.Zhan Y., Sun H.L., Chen H., Zhang H., Sun J., Zhang Z. Glucagon-like peptide-1 (GLP-1) protects vascular endothelial cells against advanced glycation end products (AGEs)-induced apoptosis. Medical Science Monitor. 2012;18:BR 286–BR 291. doi: 10.12659/MSM.883207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang X., Feng P., Zhang X., Li D., Wang R., Ji C. The diabetes drug semaglutide reduces infarct size, inflammation, and apoptosis, and normalizes neurogenesis in a rat model of stroke. Neuropharmacology. 2019;158:107748. doi: 10.1016/j.neuropharm.2019.107748. [DOI] [PubMed] [Google Scholar]
- 153.Burgmaier M., Liberman A., Möllmann J., Kahles F., Reith S., Lebherz C. Glucagon-like peptide-1 (GLP-1) and its split products GLP-1(9-37) and GLP-1(28-37) stabilize atherosclerotic lesions in apoe⁻/⁻ mice. Atherosclerosis. 2013;231:427–435. doi: 10.1016/j.atherosclerosis.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 154.Sudo M., Li Y., Hiro T., Takayama T., Mitsumata M., Shiomi M. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 2017;265:283–291. doi: 10.1016/j.atherosclerosis.2017.06.920. [DOI] [PubMed] [Google Scholar]
- 155.Hirata Y., Kurobe H., Nishio C., Tanaka K., Fukuda D., Uematsu E. Exendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. European Journal of Pharmacology. 2013;699:106–111. doi: 10.1016/j.ejphar.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 156.Jojima T., Uchida K., Akimoto K., Tomotsune T., Yanagi K., Iijima T. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis. 2017;261:44–51. doi: 10.1016/j.atherosclerosis.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 157.Rizzo M., Rizvi A.A., Patti A.M., Nikolic D., Giglio R.V., Castellino G. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovascular Diabetology. 2016;15:162. doi: 10.1186/s12933-016-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang S.T., Tang H.Q., Su H., Wang Y., Zhou Q., Zhang Q. Glucagon-like peptide-1 attenuates endothelial barrier injury in diabetes via cAMP/PKA mediated down-regulation of MLC phosphorylation. Biomedicine & Pharmacotherapy. 2019;113:108667. doi: 10.1016/j.biopha.2019.108667. [DOI] [PubMed] [Google Scholar]
- 159.Krasner N.M., Ido Y., Ruderman N.B., Cacicedo J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PloS One. 2014;9 doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rakipovski G., Rolin B., Nohr J., Klewe I., Frederiksen K.S., Augustin R. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(-/-) and LDLr(-/-) mice by a mechanism that includes inflammatory pathways. JACC Basic Translational Science. 2018;3:844–857. doi: 10.1016/j.jacbts.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Garczorz W., Gallego-Colon E., Kosowska A., Kłych-Ratuszny A., Woźniak M., Marcol W. Exenatide exhibits anti-inflammatory properties and modulates endothelial response to tumor necrosis factor α-mediated activation. Cardiovascular Therapeutics. 2018;36(2) doi: 10.1111/1755-5922.12317. [DOI] [PubMed] [Google Scholar]
- 162.Anholm C., Kumarathurai P., Pedersen L.R., Samkani A., Walzem R.L., Nielsen O.W. Liraglutide in combination with metformin may improve the atherogenic lipid profile and decrease C-reactive protein level in statin treated obese patients with coronary artery disease and newly diagnosed type 2 diabetes: a randomized trial. Atherosclerosis. 2019;288:60–66. doi: 10.1016/j.atherosclerosis.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 163.Balestrieri M.L., Rizzo M.R., Barbieri M., Paolisso P., D'Onofrio N., Giovane A. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes. 2015;64:1395–1406. doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 164.Mann J.F.E., Orsted D.D., Brown-Frandsen K., Marso S.P., Poulter N.R., Rasmussen S. Liraglutide and renal outcomes in type 2 diabetes. New England Journal of Medicine. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 165.Gerstein H.C., Colhoun H.M., Dagenais G.R., Diaz R., Lakshmanan M., Pais P. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 166.Tuttle K.R., Lakshmanan M.C., Rayner B., Busch R.S., Zimmermann A.G., Woodward D.B. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinology. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 167.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 168.Giorgino F., Penfornis A., Pechtner V., Gentilella R., Corcos A. Adherence to antihyperglycemic medications and glucagon-like peptide 1-receptor agonists in type 2 diabetes: clinical consequences and strategies for improvement. Patient Preference and Adherence. 2018;12:707–719. doi: 10.2147/PPA.S151736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carls G.S., Tuttle E., Tan R.D., Huynh J., Yee J., Edelman S.V. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469–1478. doi: 10.2337/dc16-2725. [DOI] [PubMed] [Google Scholar]
- 170.Alatorre C., Fernandez Lando L., Yu M., Brown K., Montejano L., Juneau P. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes, Obesity and Metabolism. 2017;19:953–961. doi: 10.1111/dom.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qiao Q., Ouwens M.J., Grandy S., Johnsson K., Kostev K. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metabolism Syndrome Obesity. 2016;9:201–205. doi: 10.2147/DMSO.S99732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen H., Dufour R., Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Advances in Therapy. 2017;34:658–673. doi: 10.1007/s12325-016-0470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mody R., Yu M., Nepal B., Konig M., Grabner M. Adherence and persistence among patients with type 2 diabetes initiating dulaglutide compared with semaglutide and exenatide BCise: 6-month follow-up from US real-world data. Diabetes, Obesity and Metabolism. 2020 doi: 10.1111/dom.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McGovern A., Tippu Z., Hinton W., Munro N., Whyte M., de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes, Obesity and Metabolism. 2018;20:1040–1043. doi: 10.1111/dom.13160. [DOI] [PubMed] [Google Scholar]
- 175.Jones S.C., Ryan D.L., Pratt V.S., Niak A., Brinker A.D. Injection-site nodules associated with the use of exenatide extended-release reported to the U.S. Food and drug administration adverse event reporting system. Diabetes Spectrum. 2015;28:283–288. doi: 10.2337/diaspect.28.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Turner R.C., Cull C.A., Frighi V., Holman R.R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) group. Journal of the American Medical Association. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 177.Brubaker P.L., Drucker D.J. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 178.Tschen S.I., Dhawan S., Gurlo T., Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58:1312–1320. doi: 10.2337/db08-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Meier J.J., Butler A.E., Saisho Y., Monchamp T., Galasso R., Bhushan A. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European Heart Journal. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 182.Laux G., Berger S., Szecsenyi J., Kaufmann-Kolle P., Leutgeb R. Prescribing differences in family practice for diabetic patients in Germany according to statutory or private health insurance: the case of DPP-4-inhibitors and GLP-1-agonists. BMC Family Practice. 2016;17:146. doi: 10.1186/s12875-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hong D., Si L., Jiang M., Shao H., Ming W.K., Zhao Y. Cost effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors: a systematic review. PharmacoEconomics. 2019;37:777–818. doi: 10.1007/s40273-019-00774-9. [DOI] [PubMed] [Google Scholar]
- 184.Kruger D.F., LaRue S., Estepa P. Recognition of and steps to mitigate anxiety and fear of pain in injectable diabetes treatment. Diabetes Metabolism Syndrome Obesity. 2015;8:49–56. doi: 10.2147/DMSO.S71923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aroda V.R., Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metabolism Research Reviews. 2011;27:528–542. doi: 10.1002/dmrr.1202. [DOI] [PubMed] [Google Scholar]
- 186.Tibaduiza E.C., Chen C., Beinborn M. A small molecule ligand of the glucagon-like peptide 1 receptor targets its amino-terminal hormone binding domain. Journal of Biological Chemistry. 2001;276:37787–37793. doi: 10.1074/jbc.M106692200. [DOI] [PubMed] [Google Scholar]
- 187.Teng M., Johnson M.D., Thomas C., Kiel D., Lakis J.N., Kercher T. Small molecule ago-allosteric modulators of the human glucagon-like peptide-1 (hGLP-1) receptor. Bioorganic & Medicinal Chemistry Letters. 2007;17:5472–5478. doi: 10.1016/j.bmcl.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 188.Sloop K.W., Willard F.S., Brenner M.B., Ficorilli J., Valasek K., Showalter A.D. Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes. 2010;59:3099–3107. doi: 10.2337/db10-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fan H., Gong N., Li T.F., Ma A.N., Wu X.Y., Wang M.W. The non-peptide GLP-1 receptor agonist WB4-24 blocks inflammatory nociception by stimulating beta-endorphin release from spinal microglia. British Journal of Pharmacology. 2015;172:64–79. doi: 10.1111/bph.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kielgast U., Holst J.J., Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual beta-cell function. Diabetes. 2011;60:1599–1607. doi: 10.2337/db10-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Creutzfeldt W.O., Kleine N., Willms B., Ørskov C., Holst J.J., Nauck M.A. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–586. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- 192.Dupré J., Behme M.T., Hramiak M., McFarlane P., Williamson M.P., Zabel P. Glucagon-like peptide 1 reduces postprandial glycemic excursions in IDDM. Diabetes. 1995;44:626–630. doi: 10.2337/diab.44.6.626. [DOI] [PubMed] [Google Scholar]
- 193.Dupré J., Behme M.T., McDonald T.J. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. Journal of Clinical Endocrinology & Metabolism. 2004;89:3469–3473. doi: 10.1210/jc.2003-032001. [DOI] [PubMed] [Google Scholar]
- 194.Dejgaard T.F., Knop F.K., Tarnow L., Frandsen C.S., Hansen T.S., Almdal T. Efficacy and safety of the glucagon-like peptide-1 receptor agonist liraglutide added to insulin therapy in poorly regulated patients with type 1 diabetes-a protocol for a randomised, double-blind, placebo-controlled study: the Lira-1 study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dejgaard T.F., Frandsen C.S., Hansen T.S., Almdal T., Urhammer S., Pedersen-Bjergaard U. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinology. 2016;4:221–232. doi: 10.1016/S2213-8587(15)00436-2. [DOI] [PubMed] [Google Scholar]
- 196.Dejgaard T.F., Schmidt S., Frandsen C.S., Vistisen D., Madsbad S., Andersen H.U. Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: the Lira Pump trial-a randomized, double-blinded, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2020;22:492–500. doi: 10.1111/dom.13911. [DOI] [PubMed] [Google Scholar]
- 197.Johansen N.J., Dejgaard T.F., Lund A., Schluntz C., Frandsen C.S., Forman J.L. Efficacy and safety of meal-time administration of short-acting exenatide for glycemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinology. 2020;8:313–324. doi: 10.1016/S2213-8587(20)30030-9. [DOI] [PubMed] [Google Scholar]
- 198.Ahlqvist E., Storm P., Karajamaki A., Martinell M., Dorkhan M., Carlsson A. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinology. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 199.Dennis J.M., Shields B.M., Henley W.E., Jones A.G., Hattersley A.T. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinology. 2019;7:442–451. doi: 10.1016/S2213-8587(19)30087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zaharia O.P., Strassburger K., Strom A., Bonhof G.J., Karusheva Y., Antoniou S. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinology. 2019;7:684–694. doi: 10.1016/S2213-8587(19)30187-1. [DOI] [PubMed] [Google Scholar]
- 201.Giorgino F., Caruso I., Moellmann J., Lehrke M. Differential indication for SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with established atherosclerotic heart disease or at risk for congestive heart failure. Metabolism. 2020;104:154045. doi: 10.1016/j.metabol.2019.154045. [DOI] [PubMed] [Google Scholar]
- 202.Frias J., Guja C., hardy E., Ahmed A., Dong F., Öhmann P. Combination of exenatide once weekly and dapagliflozin once daily versus exenatide and dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a phase 3, 28-week, double-blind, randomised controlled study. Lancet Diabetes Endocrinology. 2016 doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 203.Jabbour S.A., Frias J.P., Hardy E., Ahmed A., Wang H., Ohman P. Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care. 2018;41:2136–2146. doi: 10.2337/dc18-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Terauchi Y., Utsunomiya K., Yasui A., Seki T., Cheng G., Shiki K. Safety and efficacy of empagliflozin as add-on therapy to GLP-1 receptor agonist (liraglutide) in Japanese patients with type 2 diabetes mellitus: a randomised, double-blind, parallel-group phase 4 study. Diabetes Therapeutics. 2019;10:951–963. doi: 10.1007/s13300-019-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ali A.M., Martinez R., Al-Jobori H., Adams J., Triplitt C., DeFronzo R. Combination therapy with canagliflozin plus liraglutide exerts additive effect on weight loss, but not on HbA1c, in patients with type 2 diabetes. Diabetes Care. 2020;43:1234–1241. doi: 10.2337/dc18-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ludvik B., Frias J.P., Tinahones F.J., Wainstein J., Jiang H., Robertson K.E. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinology. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 207.Frias J.P., Guja C., Hardy E., Ahmed A., Dong F., Ohman P. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinology. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 208.Jabbour S.A., Frias J.P., Guja C., Hardy E., Ahmed A., Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes, Obesity and Metabolism. 2018;20(6):1515–1519. doi: 10.1111/dom.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Castellana M., Cignarelli A., Brescia F., Perrini S., Natalicchio A., Laviola L. Efficacy and safety of GLP-1 receptor agonists as add-on to SGLT2 inhibitors in type 2 diabetes mellitus: a meta-analysis. Scientific Reports. 2019;9:19351. doi: 10.1038/s41598-019-55524-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 211.Baggio L.L., Drucker D.J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Molecular Metabolism. 2020 doi: 10.1016/j.molmet.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sathananthan A., Man C.D., Micheletto F., Zinsmeister A.R., Camilleri M., Giesler P.D. Common genetic variation in GLP1R and insulin secretion in response to exogenous GLP-1 in nondiabetic subjects: a pilot study. Diabetes Care. 2010;33:2074–2076. doi: 10.2337/dc10-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li W., Li P., Li R., Yu Z., Sun X., Ji G. GLP1R single-nucleotide polymorphisms rs3765467 and rs10305492 affect beta cell insulin secretory capacity and apoptosis through GLP-1. DNA and Cell Biology. 2020 doi: 10.1089/dna.2020.5424. epub 27 Jul 2020. [DOI] [PubMed] [Google Scholar]
- 214.Yau A.M.W., McLaughlin J., Maughan R.J., Gilmore W., Ashworth J.J., Evans G.H. A pilot study investigating the influence of glucagon-like peptide-1 receptor single nucleotide polymorphisms on gastric emptying rate in caucasian men. Frontiers in Physiology. 2018;9:1331. doi: 10.3389/fphys.2018.01331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chedid V., Vijayvargiya P., Carlson P., Van Malderen K., Acosta A., Zinsmeister A. Allelic variant in the glucagon-like peptide 1 receptor gene associated with greater effect of liraglutide and exenatide on gastric emptying: a pilot pharmacogenetics study. Neuro-Gastroenterology and Motility. 2018;30 doi: 10.1111/nmo.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schäfer S.A., Tschritter O., Machicao F., Thamer C., Stefan N., Gallwitz B. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–2450. doi: 10.1007/s00125-007-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Beinborn M., Worrall C.I., McBride E.W., Kopin A.S. A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regulatory Peptides. 2005;130:1–6. doi: 10.1016/j.regpep.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 218.Lin C.H., Lee Y.S., Huang Y.Y., Hsieh S.H., Chen Z.S., Tsai C.N. Polymorphisms of GLP-1 receptor gene and response to GLP-1 analogue in patients with poorly controlled type 2 diabetes. Journal of Diabetes Research. 2015;2015:176949. doi: 10.1155/2015/176949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Karras S.N., Rapti E., Koufakis T., Kyriazou A., Goulis D.G., Kotsa K. Pharmacogenetics of glucagon-like peptide-1 agonists for the treatment of type 2 diabetes mellitus. Current Clinical Pharmacology. 2017;12:202–209. doi: 10.2174/1574884713666180221121512. [DOI] [PubMed] [Google Scholar]
- 220.During M.J., Cao L., Zuzga D.S., Francis J.S., Fitzsimons H.L., Jiao X. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nature Medicine. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 221.Holst J.J., Burcelin R., Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Current Medical Research and Opinion. 2011;27:547–558. doi: 10.1185/03007995.2010.549466. [DOI] [PubMed] [Google Scholar]
- 222.Batista A.F., Forny-Germano L., Clarke J.R., Lyra E.S.N.M., Brito-Moreira J., Boehnke S.E. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer's disease. The Journal of Pathology. 2018;245:85–100. doi: 10.1002/path.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Grieco M., Giorgi A., Gentile M.C., d'Erme M., Morano S., Maras B. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Frontiers in Neuroscience. 2019;13:1112. doi: 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vadini F., Simeone P.G., Boccatonda A., Guagnano M.T., Liani R., Tripaldi R. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. International Journal of Obesity (Lond) 2020;44:1254–1263. doi: 10.1038/s41366-020-0535-5. [DOI] [PubMed] [Google Scholar]
- 225.Watson K.T., Wroolie T.E., Tong G., Foland-Ross L.C., Frangou S., Singh M. Neural correlates of liraglutide effects in persons at risk for Alzheimer's disease. Behavioural Brain Research. 2019;356:271–278. doi: 10.1016/j.bbr.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 226.Nordberg A., Rinne J.O., Kadir A., Langstrom B. The use of PET in Alzheimer disease. Nature Reviews Neurology. 2010;6(2):78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- 227.Femminella G.D., Frangou E., Love S.B., Busza G., Holmes C., Ritchie C. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer's disease: study protocol for a randomised controlled trial (ELAD study) Trials. 2019;20:191. doi: 10.1186/s13063-019-3259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Foltynie T., Athauda D. Repurposing anti-diabetic drugs for the treatment of Parkinson's disease: rationale and clinical experience. Progress in Brain Research. 2020;252:493–523. doi: 10.1016/bs.pbr.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 229.Yun S.P., Kam T.I., Panicker N., Kim S., Oh Y., Park J.S. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nature Medicine. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Aviles-Olmos I., Dickson J., Kefalopoulou Z., Djamshidian A., Ell P., Soderlund T. Exenatide and the treatment of patients with Parkinson's disease. Journal of Clinical Investigation. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Athauda D., Maclagan K., Skene S.S., Bajwa-Joseph M., Letchford D., Chowdhury K. Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mulvaney C.A., Duarte G.S., Handley J., Evans D.J., Menon S., Wyse R. GLP-1 receptor agonists for Parkinson's disease. Cochrane Database of Systematic Reviews. 2020;7:Cd 012990. doi: 10.1002/14651858.CD012990.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Martin B., Golden E., Carlson O.D., Pistell P., Zhou J., Kim W. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yeung H., Takeshita J., Mehta N.N., Kimmel S.E., Ogdie A., Margolis D.J. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatology. 2013;149:1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hogan A.E., Tobin A.M., Ahern T., Corrigan M.A., Gaoatswe G., Jackson R. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54:2745–2754. doi: 10.1007/s00125-011-2232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Buysschaert M., Tennstedt D., Preumont V. Improvement of psoriasis during exenatide treatment in a patient with diabetes. Diabetes & Metabolism. 2012;38:86–88. doi: 10.1016/j.diabet.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 237.Ahern T., Tobin A.M., Corrigan M., Hogan A., Sweeney C., Kirby B. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. Journal of the European Academy of Dermatology and Venereology. 2013;27:1440–1443. doi: 10.1111/j.1468-3083.2012.04609.x. [DOI] [PubMed] [Google Scholar]
- 238.Buysschaert M., Baeck M., Preumont V., Marot L., Hendrickx E., Van Belle A. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal γδ T-cell number: a prospective case-series study. British Journal of Dermatology. 2014;171:155–161. doi: 10.1111/bjd.12886. [DOI] [PubMed] [Google Scholar]
- 239.Faurschou A., Gyldenløve M., Rohde U., Thyssen J.P., Zachariae C., Skov L. Lack of effect of the glucagon-like peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients-a randomized placebo-controlled trial. Journal of the European Academy of Dermatology and Venereology. 2015;29:555–559. doi: 10.1111/jdv.12629. [DOI] [PubMed] [Google Scholar]