Abstract
Background
Mitochondrial uncouplers shuttle protons across the inner mitochondrial membrane via a pathway that is independent of adenosine triphosphate (ATP) synthase, thereby uncoupling nutrient oxidation from ATP production and dissipating the proton gradient as heat. While initial toxicity concerns hindered their therapeutic development in the early 1930s, there has been increased interest in exploring the therapeutic potential of mitochondrial uncouplers for the treatment of metabolic diseases.
Scope of review
In this review, we cover recent advances in the mechanisms by which mitochondrial uncouplers regulate biological processes and disease, with a particular focus on metabolic associated fatty liver disease (MAFLD), nonalcoholic hepatosteatosis (NASH), insulin resistance, and type 2 diabetes (T2D). We also discuss the challenges that remain to be addressed before synthetic and natural mitochondrial uncouplers can successfully enter the clinic.
Major conclusions
Rodent and non-human primate studies suggest that a myriad of small molecule mitochondrial uncouplers can safely reverse MAFLD/NASH with a wide therapeutic index. Despite this, further characterization of the tissue- and cell-specific effects of mitochondrial uncouplers is needed. We propose targeting the dosing of mitochondrial uncouplers to specific tissues such as the liver and/or developing molecules with self-limiting properties to induce a subtle and sustained increase in mitochondrial inefficiency, thereby avoiding systemic toxicity concerns.
Keywords: Mitochondrial uncouplers, Metabolic syndrome, Diabetes, NAFLD/NASH, MAFLD, Liver fibrosis, Insulin resistance
Abbreviations
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- AMPK
AMP-activated protein kinase
- BAM15
2-fluorophenyl)6-[(2-fluorophenyl)amino](1,2,5-oxadiazolo [3,4-3]pyrazine 5-yl)amine
- BAT
brown adipose tissue
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- CCl4
carbon tetrachloride
- CDAA/c
choline deficient L-amino acid diet supplemented with 1% cholesterol
- CRMP
controlled release mitochondrial protonophore
- CVD
cardiovascular disease
- DAG
diacylglycerol
- DKD
diabetic kidney disease
- DNP
2,4-dinitrophenol
- DNP-LC
DNP-liquid crystal gel
- DNPME
DNP-methyl ether
- ETC
electron transport chain
- FCCP
Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- HbA1c
hemoglobin A1c
- HCC
hepatocellular carcinoma
- IMM
inner mitochondrial membrane
- IP
intraperitoneal
- LD50
50% lethal dose
- LDL
low density lipoprotein
- MAFLD
metabolic associated fatty liver disease
- MTTP
microsomal triglyceride transfer protein
- NAFLD
Non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- NEN
niclosamide ethanolamine
- NPP
niclosamide peprazine
- NTZ
nitazoxanide
- nPKC
novel protein kinase C
- OXPHOS
oxidative phosphorylation
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- ROS
reactive oxygen species
- SGLT2
sodium glucose transport protein 2
- SR4
1, 3-bis(dichlorophenyl)urea
- STZ
streptozotocin
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- UCP
uncoupling protein
- VLDL
very low density lipoprotein
- ZDF
Zucker diabetic fatty
1. Introduction
Over the last two decades, the obesity pandemic has triggered a rise in the prevalence of non-alcoholic fatty liver disease (NAFLD), the most common chronic liver disease worldwide [[1], [2]]. NAFLD is characterized by lipid deposition in hepatocytes in the absence of excessive alcohol consumption and encompasses a continuum of pathologies, ranging from simple steatosis, non-alcoholic steatohepatitis (NASH), fibrosis, and cirrhosis [3]. NAFLD also increases the risk of developing hepatocellular carcinoma (HCC), cardiovascular disease (CVD), and type 2 diabetes (T2D) [[4], [5], [6]], which was recently renamed metabolic associated fatty liver disease (MAFLD) to reflect the hepatic manifestation of this systemic metabolic disorder [[7], [8], [9]]. In particular, the new definition establishes MAFLD as a disorder with evidence of hepatic steatosis accompanied by obesity, T2D, and/or metabolic dysregulation [[7], [8], [9]].
Although not completely understood, a mechanistic picture of disease progression is emerging. It is generally thought that over-accumulation of liver fat in MAFLD patients leads to the production of cytotoxic lipid oxidation side products and the establishment of the chronic necro-inflammatory state that defines NASH [10]. Importantly, MAFLD patients do not always transition to NASH based on steatosis alone; numerous factors, including mitochondrial dysfunction, as characterized by impaired oxidative phosphorylation (OXPHOS) and reactive oxygen species (ROS) production, contribute to disease progression [11]. The necro-inflammatory environment of NASH ultimately triggers the activation of hepatic stellate cells and the development of fibrosis, which if severe enough can lead to scarring, cirrhosis, and eventually liver failure [10, 12].
Reduced mitochondrial function and lipid catabolism are associated with increases in ectopic fat content and insulin resistance often seen in patients with MAFLD [4,[7], [8], [9],13]. While several lipid metabolites have been implicated [14], a substantial body of work supports the role of diacylglycerols (DAGs) and ceramides in promoting hepatic insulin resistance (for a review, see [15]). In one model, sn-1-2-diacylglycerol (DAG) accumulation in the plasma membrane activates novel protein kinase C epsilon (PKCε), which in turn impairs insulin receptor tyrosine kinase activity and all downstream arms of hepatic insulin signaling, including stimulation of glycogen synthesis and transcriptional downregulation of gluconeogenic genes [16,17]. Explanations of ceramide-associated hepatic insulin resistance are less clear, but include alterations in insulin signaling (through AKT inactivation), mitochondrial function, and inflammatory pathways [18]. Based on this mechanistic view, numerous therapies are currently being developed for the treatment of MAFLD/NASH and T2D, with compounds focused on reducing hepatic fat accumulation, lowering oxidative stress, altering the intestinal microbiome, and reducing hepatic fibrosis. Despite this, there are no FDA-approved medications for MAFLD/NASH. Indeed, lifestyle management remains the cornerstone treatment, with moderate weight reduction being highly effective at decreasing hepatic fat content and improving hepatic insulin sensitivity in MAFLD patients [[19], [20], [21], [22], [23], [24], [25], [26]]. However, few patients adhere to the life-long diet and exercise programs required to reverse MAFLD/NASH and T2D, with ∼70–95% of those who lose significant weight subsequently regaining it [[27], [28], [29], [30]]. Safe and effective pharmacological interventions are therefore needed.
Liver-targeted mitochondrial uncoupling agents represent a unique therapeutic strategy to increase hepatic lipid utilization and reduce hepatic steatosis and its ensuing metabolic complications. Small molecule mitochondrial uncouplers such as the classical mitochondrial protonophore 2,4 dinitrophenol (DNP) increase mitochondrial inefficiency, resulting in enhanced nutrient oxidation to produce a given amount of adenosine triphosphate (ATP) [31]. While DNP was shown to have anti-obesity effects in the early 1930s [32], its toxicity concerns precluded its clinical use and most likely contributed to the lack of interest in further exploring its metabolic actions [33]. Recently, however, there has been increased interest in treating MAFLD/NASH by increasing mitochondrial energy expenditure via chemical and natural mitochondrial uncouplers [34]. Herein, we review the most promising mitochondrial uncouplers for the treatment of MAFLD/NASH and T2D and discuss the challenges that remain to be addressed for their successful clinical implementation.
2. Oxidative metabolism and mitochondrial uncoupling
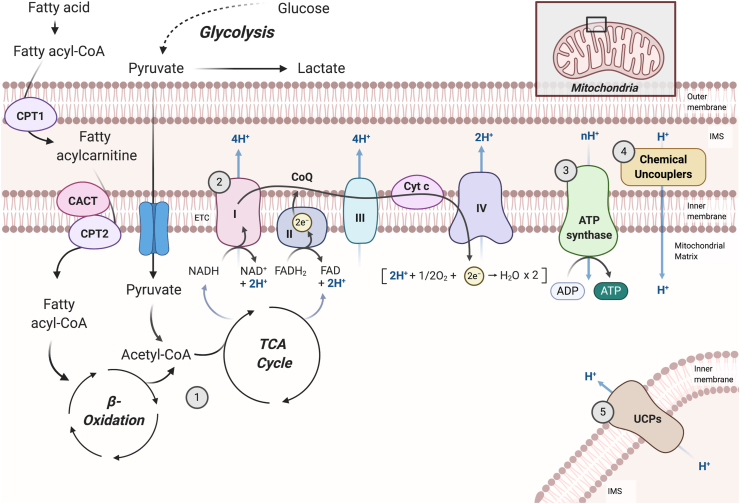
Mitochondria are essential organelles responsible for cellular respiration, the process through which cells convert nutrients into the common bioenergy currency ATP [35]. OXPHOS is the final stage of cellular respiration in aerobic organisms and is driven by the energy released during stepwise oxidation-reduction chain reactions in electron transporting systems [36,37] (Figure 1). Specifically, substrate oxidation yields electrons in the form of reduced hydrogen carriers (NADH and FADH2) that are donated to a series of enzyme complexes embedded in the inner mitochondrial membrane (IMM), ultimately reducing oxygen to water [36,37]. This electron flow leads to the pumping of protons (H+) across the IMM, thereby generating a membrane potential and proton motive force that are subsequently used to drive the synthesis of ATP from adenosine diphosphate (ADP) and inorganic phosphate [38]. Not all available energy in the electrochemical gradient is coupled to ATP production, however, as protons pumped out of the matrix are able to return to the mitochondrial matrix independently of ATP synthase [39,40]. This energy-dissipating cycle, termed “mitochondrial uncoupling,” occurs in all eukaryotic cells and accounts for 20–30% of the basal metabolic rate depending on the tissue type [41].
Figure 1.
Mitochondrial respiration and uncoupling. During cellular respiration, the oxidation of substrates such as glucose and fatty acids [1] yields electrons (e−) in the form of reduced hydrogen carriers, NADH and FADH2, which are donated to a series of enzyme complexes embedded in the inner mitochondrial membrane (IMM), ultimately reducing oxygen to water [2]. As electrons are transferred along the electron transport chain (ETC), a fixed number of protons (H+) are pumped across the IMM, generating a membrane potential (Δψm) and proton motive force that is subsequently used to drive the synthesis of ATP from ADP and inorganic phosphate [3]. Proton leak induced by chemical uncouplers [4] or uncoupling proteins (UCPs) [5] competes for the same proton gradient, resulting in lower Δψm and diminished production of ATP, accelerating mitochondrial respiration to maintain energy homeostasis. This figure was adapted from “Electron Transport Chain” by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates.
In mammals, physiological uncoupling is typically unregulated (basal proton leak via diffusion) or mediated by a dedicated set of proteins such as adenine nucleotide translocases and uncoupling proteins [42]. A wide variety of natural and synthetic compounds have also been reported to be uncouplers of OXPHOS in mitochondria, including fatty acids, capsaicins, thyroid hormone (T3), carbonyl cyanide 4-(trifluoro-methoxy)phenylhydrazone (FCCP), carbonyl cyanide-3-chlorophenylhydrazone (CCCP), and DNP [43]. While most of these molecules are hydrophobic weak acids with protonophoric activities (hydrophobic enough to enable passage through biological membranes and acidic enough to enable partial and reversible pH-dependent ionization [[44], [45], [46]]), some compounds indirectly cause uncoupling by regulating the activity of uncoupling proteins and/or altering mitochondrial function.
By allowing protons to leak across the IMM and circumvent ATP synthase, mitochondrial uncouplers decrease the coupling efficiency of cellular respiration to ATP synthesis, releasing the potential energy generated from substrate oxidation in the form of heat (reviewed in [47]). This creates a thermogenic futile cycle whereby the electron transport chain (ETC) and TCA cycle work faster to pump protons out of the mitochondria, which requires more ATP hydrolysis and associated thermogenesis [48]. Heat can be released from collisional interactions as protons move out of the mitochondria or from the amount of energy required to maintain the appropriate concentration of enzymes to drive futile cycling [48]. This is perhaps best exemplified by the mitochondrial uncoupling protein in brown adipocytes (UCP1 and its role in thermogenesis (reviewed in [49]) and the classical protonophore, DNP, which can directly stimulate cellular respiration and a consequent rise in body temperature in rodents [50] and humans [51]. Despite this, not all synthetic uncouplers increase body temperature, suggesting that uncoupling-mediated increases in thermogenesis may be compound, dose, and/or tissue specific [52]. Alternatively, DNP-mediated increases in body temperature may be related to its ability to uncouple non-mitochondrial membranes [[53], [54], [55], [56]].
3. Therapeutic relevance of mitochondrial uncouplers
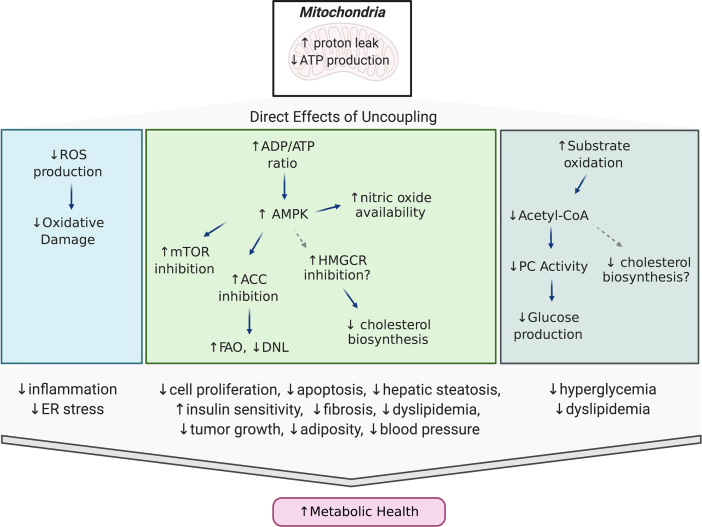
Mild uncoupling of OXPHOS provides beneficial effects that permit adaptations to the increased hepatic fatty acid supply and ensuing lipotoxicity that is characteristic of MAFLD/NASH patients. Specifically, its therapeutic potential is often linked to 1) increased β-oxidation of fatty acids to compensate for inefficient ATP production, 2) a decrease in ROS production from the electron transport chain, and/or 3) activation of AMP protein kinase (AMPK) expression, thereby steering metabolic pathways from energy accumulation to energy expenditure and fuel preference from glucose to fatty acids [57] (Figure 2). Herein, we highlight how these beneficial effects can be leveraged to treat metabolic syndrome, with a particular focus on compounds that have proven therapeutic efficacy for managing MAFLD/NASH and T2D.
Figure 2.
Metabolic effects of mitochondrial uncouplers. Diagram highlighting the beneficial metabolic effects of mild mitochondrial uncoupling and how uncouplers can be leveraged to treat obesity and associated comorbidities.
3.1. 2,4-Dinitrophenol and its modified forms
Since the beginning of the twentieth century, several attempts have been made to develop pharmacological agents that increase mitochondrial uncoupling. The most notable, DNP, was initially used to synthesize explosives prior to World War I and later considered a promising anti-obesity drug after Maurice Tainter at Stanford University demonstrated the efficacy of this approach in obese humans [31,32,58]. By the early 1930s, DNP was widely taken as an over-the-counter medication for rapid weight loss in the US; however, reports of toxic side effects, including hyperthermia, cataract formation, and death, led to its withdraw from the market by the US Food and Drug Administration in 1938 [51,59,60]. Nonetheless, Russian soldiers continued to take DNP to stay warm on the Eastern Front during the frigid winters of World War II, and in recent years, there has been a resurgence in the illicit use of DNP by bodybuilders and athletes trying to shed fat and sculpt muscle [61].
Despite DNP's turbulent history, the research community has continued to explore its therapeutic potential in model organisms under well-controlled conditions. For example, Samuel et al. showed that three days of low-dose oral DNP administration (16 mg/kg/day) effectively reversed diet-induced obesity and hepatic insulin resistance in high-fat-fed male Sprague–Dawley rats without toxic side effects [62]. Longer low-dose DNP administration has also proven to be safe and effective at improving metabolic parameters. When administered in drinking water (1 mg/L DNP; ∼100 μg/kg/day), five months of DNP intake decreased oxidative stress, improved metabolic syndrome, and extended the lifespan of chow-fed female Swiss Webster outbred mice [63]. Higher doses of DNP (800 mg/L in drinking water; ∼15–54 mg/kg/day) have also been reported to safely reduce high-fat diet-induced weight gain, hepatic steatosis, and insulin resistance in female C57BL/6 mice housed at thermoneutrality (30 °C). Interestingly, housing mice below thermoneutrality (22 °C) abrogated DNP's effects on body weight, adiposity, and glucose tolerance due to reductions in compensatory brown adipose tissue (BAT) thermogenesis and possibly increased food intake [64]. Given the fundamental differences between thermal biology in mice and humans [65], these studies warrant further investigation into the effect of ambient temperature on DNP's anti-obesity effects.
3.1.1. Liver-targeted and sustained-release DNP formulations
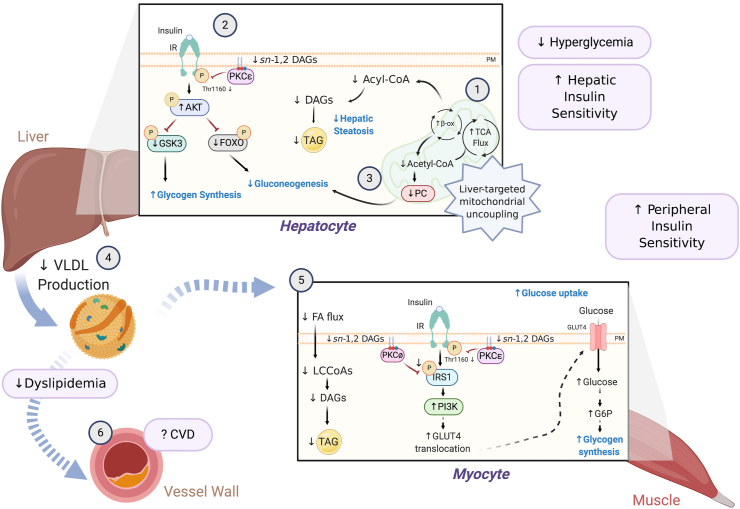
Hyperthermia, due to systemic mitochondrial uncoupling, is a major contributing factor to the low therapeutic index of DNP and other systemic mitochondrial uncouplers [59]. To increase the therapeutic window of DNP, Perry et al. developed prodrugs that target DNP to the liver [50] and a functionally liver-targeted, slow-release DNP formulation, thereby avoiding peak (Cmax) plasma concentrations, promoting selective mitochondria uncoupling in the liver, and minimizing systemic exposure and hyperthermia [66]. These agents have been shown to increase the therapeutic window over DNP by 50–200 fold and have been effectively used to treat metabolic disorders including MAFLD/NASH, insulin resistance, hyperlipidemia, T2D, and CVD in rodents and non-human primate models of metabolic syndrome. In this section, we discuss the therapeutic potential of these modified DNP formulations and highlight the mechanism(s) by which these formulations exert their therapeutic benefits (Figure 3).
Figure 3.
Mechanism(s) by which liver-targeted mitochondrial uncouplers prevent metabolic syndrome. Liver-targeted mitochondrial uncoupling agents hold therapeutic promise for the treatment of MAFLD, NASH, and T2D by promoting increased hepatic cellular energy expenditure [1]. Uncoupling-mediated increases in hepatic fat oxidation lower hepatic triglycerides (TAGs), plasma membrane (PM) sn-1,2-DAG content, and PKCε translocation, which increase hepatic insulin sensitivity [2]. Subtle sustained increases in hepatic mitochondrial inefficiency also reduce hepatic acetyl-CoA content, pyruvate carboxylase (PC) activity, and gluconeogenesis [3]. Collectively, this leads to reduced fasting and postprandial hyperglycemia. Liver-targeted uncoupling also leads to reduced hepatic VLDL production [4], reducing intramyocellular plasma membrane sn-1,2-DAG content and PKCθ/PKCε activity and reversing muscle insulin resistance [5]. Overall, these improvements in dyslipidemia and whole-body insulin sensitivity in rodent and non-human primate models of MAFLD/NASH and T2D reduce the risk of developing atherosclerotic CVD [6] and suggest that liver-targeted mitochondrial uncoupling agents may be a viable therapy for treating cardiometabolic disease in humans.
3.1.1.1. MAFLD/NASH, insulin resistance, and T2D
To increase the therapeutic window of DNP, Perry et al. developed a liver-targeted methyl-ether derivative (DNPME) that is preferentially metabolized by the hepatic cytochrome P450 system to the active protonophore, DNP [50]. Importantly, by utilizing a prodrug, they were able to reduce the 50% lethal dose (LD50) by almost 10-fold, while still achieving in vivo target engagement. Remarkably, intraperitoneal delivery of DNPME (5 mg/kg) was able to prevent and reverse hepatic steatosis, hyperglycemia, and insulin resistance in diet-induced rat models of MAFLD and T2D independently of changes in body weight or systemic toxicities [50]. Improvements in hyperglycemia and whole-body insulin sensitivity were attributed to subtle sustained increases in hepatic mitochondrial fat oxidation, resulting in lower hepatic triglyceride/DAG content and reduced very low density lipoprotein (VLDL) production, which in turn decreased plasma triglycerides and lowered skeletal muscle ectopic lipid content [50]. Interestingly, while low levels of DNP (<3 μM) accumulated in extrahepatic tissues (skeletal muscle, heart, and brain) following DNPME administration, these levels were not sufficient to increase mitochondrial respiration in vitro [50], suggesting that the beneficial effects of DNPME were mostly due to uncoupling in the liver.
To further improve the safety profile of DNP, Perry et al. next developed an extended-release formulation (controlled-release mitochondrial protonophore, CRMP), which increased the toxic to effective dose of DNP by more than 200-fold [66]. Similar to DNPME, CRMP (by virtue of its first pass uptake by the liver following oral ingestion) was able to reverse whole-body insulin resistance, hepatic inflammation, and hepatic fibrosis in rodent models of insulin resistance, T2D, and NASH due to subtle sustained increases in hepatic mitochondrial inefficiency and reductions in ectopic lipid content [66]. Importantly, these findings were also shown to be effective in two different non-human primate models of metabolic syndrome. Specifically, Goedeke et al. recently demonstrated that oral administration of CRMP (0.8 mg/kg BID x 6 weeks) significantly reduced hepatic steatosis in high-fat high-fructose fed cynomolgus monkeys independently of changes in hepatic/renal toxicity [67]. CRMP was also shown to be efficacious in a larger cohort of spontaneously obese dysmetabolic non-human primates. Six weeks of oral CRMP treatment (5 mg/kg/day) increased rates of hepatic mitochondrial fat oxidation by 40% and reduced plasma triglycerides, plasma low density lipoprotein (LDL) cholesterol, and hepatic triglyceride content in dysmetabolic rhesus monkeys without changes in body weight, food intake, hyperthermia, or oxidative stress [67]. Daily oral administration of CRMP also significantly lowered endogenous glucose production, which could be attributed to a 20% reduction in hepatic acetyl-CoA content and pyruvate carboxylase flux [67]. While longer-term safety data are needed before CRMP enters the clinic, collectively, these studies provide important proof-of-concept data to support the development of controlled-release formulations of DNP to treat MAFLD/NASH and metabolic syndrome in humans. Indeed, Wei et al. recently developed a liquid crystal (LC) gel formulation (DNP-LC-gel) with extended-release properties and showed that it was able to maintain DNP plasma concentrations at an effective and non-toxic level to reduce dyslipidemia and hepatic steatosis in a rat model of MAFLD [68]. Importantly, local administration of DNP-LC-gel (up to 50 mg/kg) was not associated with hyperthermia or skin irritation [68], further supporting controlled-release formulations for clinical use.
3.1.1.2. Cardiovascular disease
CVD due to atherosclerosis represents the leading cause of death worldwide [69]. Progress in preventing CVD has been stalled by the growing epidemic of obesity, insulin resistance, and T2D [70], which increases the relative risk of developing atherosclerotic vascular disease and its complications four-fold compared to non-diabetic individuals [[71], [72], [73], [74]]. Given CRMP's ability to safely and effectively reduce systemic risk factors associated with developing CVD (dyslipidemia and insulin resistance), Goedeke et al. assessed the therapeutic potential of liver-targeted mitochondrial uncouplers to treat CVD in a murine Ldlr−/− model of atherosclerosis and metabolic syndrome [75]. Consistent with previous reports, oral administration of CRMP (30 mg/kg/day x 12 weeks) to high-fat cholesterol diet-fed mice significantly reduced plasma triglycerides and improved hepatic and peripheral insulin sensitivity due to reductions in hepatic and muscle ectopic lipid content [75]. Intriguingly, CRMP-treated mice also displayed a 30% reduction in aortic root plaque area, neutral lipid accumulation, and necrotic core areas [75]. While the mechanism by which CRMP attenuates plaque progression remains to be determined, collectively, these results support an anti-atherogenic role for CRMP, at least in part through the systemic modulation of circulating lipid levels and improvements in whole-body insulin sensitivity, and suggest the therapeutic potential of liver-targeted mitochondrial uncouplers for treating cardiometabolic disorders.
3.1.1.3. Orphan disease indications
CRMP has recently been shown to be efficacious at treating several orphan disease indications, including lipodystrophy, a rare genetic disorder characterized by the complete or partial loss of adipose tissue [76]. Patients with lipodystrophy exhibit hypertriglyceridemia, severe insulin resistance, T2D, and NASH, and although leptin replacement therapy has been effective at treating some of these conditions, it is not effective in patients with partial lipodystrophy [77]. As such, new therapeutics to treat lipodystrophy-associated MAFLD/NASH are needed. In line with this, Abulizi et al. demonstrated that oral administration of CRMP (2 mg/kg/day) safely and effectively reversed hypertriglyceridemia, hepatic steatosis, and diabetes in a mouse model of severe lipodystrophy (fatless AZIP/F-1) through subtle sustained increases in hepatic mitochondrial oxidation and reductions in ectopic lipid content [78]. Intriguingly, 4 weeks of oral CRMP treatment was also sufficient to reduce hepatic inflammation and apoptosis through reductions in the IRE-1α branch of the unfolded protein response [78], suggesting that liver-directed mitochondrial uncoupling may be a useful therapeutic strategy for lipodystrophy-associated NASH.
In addition to lipodystrophy, Abulizi et al. also demonstrated that liver-targeted mitochondrial uncoupling by CRMP may be a novel therapeutic treatment for abetalipoproteinemia, a rare autosomal recessive disorder resulting from loss-of-function mutations in microsomal triglyceride transfer protein (MTTP) and characterized by hypocholesterolemia and increased incidence of hepatic steatosis [79]. Intriguingly, treatment of liver-specific Mttp−/- mice with CRMP (2 mg/kg/day) protected them from developing hepatic steatosis and hepatic insulin resistance [79]. Similar to CRMP's mechanism of action in other metabolic diseases, these improvements were attributed to increases in hepatic fat oxidation and reductions in hepatic triglycerides, plasma membrane sn-1,2-DAG content, and PKCε membrane to cytosol translocation [79]. As pharmacological inhibitors of MTTP are currently being developed to lower LDL cholesterol levels in patients with homozygous familial hypercholesterolemia [80], CRMP (and other liver-targeted mitochondrial uncouplers) may represent a novel co-therapeutic to offset hepatic toxicities (hepatic steatosis) associated with MTTP inhibition. More recently, MP201, a DNP prodrug developed by Mitochon Pharmaceuticals, Inc., is currently in clinical development for treating optic neuritis and other neurological disorders [81].
3.2. FDA-approved drugs
Notwithstanding the sphere of mitochondrial uncouplers, an effective strategy for drug development is to repurpose FDA-approved compounds for new indications. For example, several anesthetics, including bupivacaine, are known to influence OXPHOS and have been validated as mitochondrial protonophores in vitro and in vivo [82,83]. More recently, niclosamide, nitazoxanide, sorafenib, and salsalate have been validated as mitochondrial uncouplers and repurposed for treating MAFLD/NASH, insulin resistance, diabetes, and liver fibrosis.
3.2.1. Niclosamide ethanolamine (NEN)
Niclosamide, an FDA-approved oral anthelmintic drug, is used to treat parasitic infections in millions of people worldwide [84]. Among the underlying pathways associated with niclosamide's mechanism of action is inhibition of parasitic OXPHOS and ATP production [84,85]. Recently, water-soluble salt forms of niclosamide (niclosamide ethanolamine [NEN] and niclosamide piperazine [NPP]) have been developed and found to uncouple mitochondrial respiration in mammalian cells at high nanomolar concentrations [86,87]. When administered orally to mice, NEN and NPP (120–150 mg/kg/day) prevented and reversed diet-induced hyperglycemia, hyperinsulinemia, and hepatic steatosis [86,87]. Similar results were also observed in db/db diabetic mice, in which diabetes develops due to a mutation in the leptin receptor gene [87]. Mechanistically, these beneficial effects were attributed to reductions in hepatocellular ATP concentrations, subsequent increases in the ADP/ATP ratio, and enhanced AMPK activation, resulting in increased lipid oxidation and reductions in hepatic fat content. The effect of NEN on hyperglycemia and whole-body glucose metabolism was later attributed to its attenuation of glucagon signaling and hepatic glucose production [88].
Interestingly, unlike other orally available liver-targeted mitochondrial uncouplers, NEN-mediated improvements in insulin sensitivity were associated with increases in whole-body energy expenditure and mild reductions in body weight [87], suggesting that NEN's beneficial effects may also be due to enhanced systemic mitochondrial uncoupling. Indeed, a more recent study examining the effects of NEN in streptozotocin (STZ)-induced type 1 diabetic (T1D) mice and db/db mice found that oral administration of NEN protected against hyperglycemia and delayed the progression of diabetic kidney disease (DKD) due to suppression of renal cortical activation of mTOR/4E-BP1 signaling [89]. Whether these effects are due to NEN's uncoupling activity in the kidney or its role as a direct signaling molecule remain to be determined. Nonetheless, these results are consistent with other reports demonstrating that niclosamide impedes renal fibrosis in murine models of adriamycin nephropathy [90,91], consistent with niclosamide having a direct renal effect. Intriguingly, NEN treatment (120–150 mg/kg/day) in 6- to 24-week-old BKS-db/db mice had little effect on the development of T2D and diabetic complications [92], suggesting that NEN's beneficial effects may be dose-, strain- and site-specific. Further pharmacokinetic studies investigating the various formulations of niclosamide are therefore needed before this uncoupler is translated to humans.
3.2.2. Nitazoxanide (NTZ)
In addition to niclosamide, other anthelmintic drugs, including nitazoxanide (NTZ), have recently been found to have mitochondrial uncoupling activity [93]. Intriguingly, NTZ is not known to have anti-obesity effects; instead, it was discovered to have anti-fibrotic activity in human primary hepatic stellate cells. Moreover, oral NTZ administration (10–100 mg/kg/day) significantly reduced hepatic collagen content in carbon tetrachloride (CCl4) and choline-deficient l-amino acid diet supplemented with 1% cholesterol (CDAA/c) murine models of fibrosis [94]. While NTZ's anti-fibrotic mechanisms of action remain to be determined, it is currently in phase 2 clinical trials for NASH-associated fibrosis (ClinicalTrials.gov identifier NCT03656068).
3.2.3. Sorafenib
The ERK-kinase inhibitor, sorafenib, is currently the only first-line therapy approved for advanced HCC [95]. Sorafenib's potent anti-tumor effects have previously been attributed to its kinase inhibitor properties [96], and while effective at decreasing tumor cell proliferation, inducing apoptosis, and suppressing angiogenesis, are associated with adverse side effects, compromising its beneficial effects [97]. Low-dose sorafenib (10–30 mg/kg every other day) was sufficient to reduce tumorigenesis and pathological features of NASH, including hepatic steatosis, inflammation, and fibrosis, in two independent mouse models of NASH and HCC, without significant side effects [98]. Intriguingly, the mechanisms underlying the anti-NASH effects of sorafenib at these low doses (one-tenth of the clinical dose used in HCC patients) were independent of its effect on receptor tyrosine kinases and instead could be attributed to its hepatic activation of AMPK and subsequent suppression of mTOR. Moreover, sorafenib-mediated increases in AMPK activation were attributed to a reduction in hepatocellular ATP due to a significant increase in state IV respiration [98]. In line with its mitochondrial uncoupling properties, high-fat high-cholesterol-fed sorafenib-treated mice displayed increased whole-body energy expenditure compared to controls [98].
To further validate the clinical significance of low-dose sorafenib treatment for NASH/HCC, the authors next treated dysmetabolic cynomolgus monkeys with NASH intraperitoneally with 1 mg/kg sorafenib every 3 days for 24 weeks [98]. Consistent with previous murine studies, sorafenib-treated monkeys displayed significant reductions in hepatic steatosis, inflammation, and fibrosis independently of changes in body weight or systemic toxicities [98]. Importantly, these beneficial effects were associated with activation of hepatic AMPK and phosphorylation of ACC, suggesting that sorafenib's beneficial effects were due to hepatic mitochondrial uncoupling. Although the tissue-specific and direct mitochondrial effects of low-dose sorafenib remain to be determined, these studies warrant further investigation into its uncoupling properties to demonstrate the efficacy of subtle sustained increases in mitochondrial inefficiency for treating NASH and HCC.
3.2.4. Salsalate
Salsalate, the prodrug of salicylate, is an oral non-steroidal anti-inflammatory that has previously been utilized to treat hyperglycemia in patients with T2D [99,100]. Moreover, recent studies have highlighted its therapeutic potential for treating MAFLD and T2D in mice, but the mechanisms by which salsalate exerts these anti-diabetic affects remain unclear [101]. Although supra-therapeutic doses of salicylate (200 mg/kg, ip) directly activate AMPK via the β1 subunit, Gregory Steinberg's group showed that daily oral administration of salsalate at clinically relevant doses was able to improve whole-body glucose homeostasis, hepatic steatosis, and adipose tissue inflammation independently of AMPKβ1 [102]. In an attempt to identify the mechanism by which low-dose salsalate achieves its beneficial effects, his group found that salicylate was able to increase mitochondrial respiration and reduce membrane potential in isolated primary hepatocytes from wild-type and Ampkβ1 knockout hepatocytes, skeletal muscle fibers, and adipose tissue [102]. Moreover, acute administration of subclinical doses of salsalate inhibited de novo lipogenesis and increased whole-body energy expenditure, indicative of a mitochondrial uncoupling-driven mechanism for salsalate-mediated improvements in MAFLD and T2D. As salsalate is also shown to stimulate BAT mitochondrial activity independently of UCP1 [102], salsalate's uncoupling effects are likely pleiotropic and further work is thus required to dissect the tissue- and dose-specific effects of this anti-inflammatory drug.
3.3. Synthetic mitochondrial uncouplers
Given the toxicity concerns associated with the chemical uncoupler DNP, researchers have long been interested in identifying novel uncouplers, either by repurposing existing chemicals or discovering novel compounds through high-throughput chemical screens. In this section, we highlight several recently identified compounds, focusing on those chemical uncouplers that we believe have therapeutic promise for treating obesity and associated metabolic disorders.
3.3.1. SR4
The compound 1, 3-bis(dichlorophenyl)urea (SR4) was initially discovered in a chemical screen for anti-cancer agents and later shown to be an activator of AMPK in vitro and in vivo [103,104]. Given AMPK's prominent role in metabolism, Figarola et al. investigated its therapeutic potential for metabolic syndrome. Oral administration of S4R (5 mg/kg/day x 6 weeks) to high-fat diet-fed mice prevented hyperlipidemia, reduced hepatic lipid content, and improved glucose metabolism and insulin sensitivity compared to vehicle-treated controls due to increases in hepatic mitochondrial uncoupling and subsequent activation of the AMPK pathway [105]. Despite this, S4R's selectivity for mitochondria has not been established, and mice treated with higher doses of SR4 exhibited almost a 50% reduction in body weight, raising concerns about possible systemic toxicities associated with this chemical uncoupler.
3.3.2. CZ5
When screening a collection of mitochondrial membrane potential depolarizing compounds in isolated skeletal muscle mitochondria, researchers identified the chemical uncoupler, CZ5, and showed that it increased mitochondrial respiration in L6 myotubes and 3T3-L1 adipocytes at concentrations as low as 3 μM without cytotoxicity [106]. After confirming favorable bioavailability following oral dosing, Fu et al. further showed that oral dosing of CZ5 (30 mg/kg/day) reduced food intake, body weight, and total fat mass compared to controls. CZ5 also significantly increased whole-body energy expenditure and reduced hypertriglyceridemia, intramyocellular lipids, and fasting blood glucose and insulin concentrations [106], suggesting that CZ5 may be a promising target for treating metabolic syndrome. Interestingly, these changes were independent of CZ5-mediated increases in hepatic uncoupling and reductions in hepatic triglyceride content, supporting the muscle- and fat-specific uncoupling properties of CZ5. While this makes CZ5 an attractive tissue-specific mitochondrial uncoupling agent, CZ5-mediated reductions in food intake raise toxicity concerns.
3.3.3. OPC-163493
Discovered by Otsuka Pharmaceuticals, the protonophore OPC-163493 was recently shown to have strong anti-diabetic effects in multiple rodent models [107]. Similar to other mitochondrial protonophores, OPC-163493 augments oxygen consumption rates in cultured hepatocytes and isolated liver homogenates. Interestingly, OPC-163493 had no ex vivo uncoupling effect on skeletal muscle or brain homogenates; combined with its liver-enriched tissue distribution, this suggests that OPC-163493's main target organ is the liver. Impressively, oral delivery of OPC-163493 (2–10 mg/kg/day) was able to lower fasting plasma glucose and hemoglobin A1c (HbA1c), reduce hepatic steatosis, and prevent oxidative stress in numerous rodent models of T2D and T1D including Zucker diabetic fatty (ZDF) rats, Akita mice, Otsuka Long-Evans Tokushima fatty rats, ob/ob mice, and HFD-fed mice [107]. Consistent with its liver-targeted role, these beneficial effects were independent of any changes in food intake, whole-body energy expenditure, or body weight and postulated to be related to OPC-163493's ability to enhance AMPK activity, increase hepatic fat oxidation, and improve hepatic insulin sensitivity.
Intriguingly, OPC-163493 was also able to lower blood pressure, delay stroke onset, and ameliorate albuminuria in salt-loaded hypertensive rats [107] presumably due to its ability to indirectly activate AMPK and/or lower ROS production. Alternatively, OPC-163493 may affect blood pressure by activating AMPK and regulating endothelial function as the vessel wall would constantly be exposed to OPC-163493. Indeed, pretreatment of aortas with OPC-163493 was able to enhance the sensitivity of aortas against nitroprusside-mediated relaxation [107], suggesting that OPC-163493 may also enhance nitric oxide availability. Taken together, these studies suggest that OPC-163493 has cardiovascular benefits in addition to its anti-diabetic effects, and given its wide safety range in 4- and 13-week preclinical toxicology studies, is a promising novel mitochondrial uncoupler for treating metabolic disorders.
3.3.4. Compound 6j
Lactic acidosis can occur with toxic levels of any mitochondrial uncoupler when the degree of mitochondrial uncoupling exceeds the ability of the mitochondria to maintain ATP concentrations and the cell switches to mostly anaerobic glycolysis for ATP production [51]. To relieve these adverse effects, Jiang et al. sought to synthesize a novel compound (6j) with both mitochondrial uncoupling activity and pyruvate dehydrogenase (PDH)-activation effects [108]. Activation of PDH would theoretically increase pyruvate oxidation and suppress lactate synthesis, while still maintaining 6j′s uncoupling-mediated reductions in hyperglycemia. Remarkably, chronic oral 6j administration (40 mg/kg) improved insulin sensitivity, glucose tolerance, and liver steatosis in mice with diet-induced obesity without inducing lactic acidosis or other systemic toxicities such as hyperthermia [108]. Interestingly, lactic acidosis has not been reported with oral administration of other liver-directed mitochondrial protonophores [50,66,67,87], suggesting that mitochondrial uncouplers with dual PDH-activation effects may not be necessary to avoid long-term toxicity concerns. Nevertheless, given the promising anti-diabetic action of compound 6j, future studies are warranted to understand its mechanism(s) of action/target tissues.
3.3.5. BAM15 and derivatives
Clinical limitations of classical mitochondrial uncouplers such as DNP have been partially attributed to their ability to induce plasma membrane depolarization, resulting in off-target effects. As such, Kenwood et al. sought to identify novel chemical uncouplers that increased mitochondrial respiration over a broad effective range (0.1–50 μM) independently of changes in plasma membrane electrophysiology [109]. In their study, 2-fluorophenyl)6-[(2-fluorophenyl)amino](1,2,5-oxadiazolo [3,4-3]pyrazine 5-yl) amine, more commonly referred to as BAM15, emerged as a top hit from this screen and was shown to have in vivo bioactivity, as it dose-dependently protected mice against kidney ischemic-reperfusion injury. Since then, structure–activity relationship (SAR) studies have established that furazan, pyrazine, and aniline rings are crucial for BAM15's uncoupling activity [110] and more recently, BAM15 has been leveraged for its anti-obesity and insulin-sensitizing effects. In particular, Alexopoulos et al. demonstrated that BAM15 is orally bioavailable and reduces body weight due to increases in whole-body energy expenditure and reductions in fat mass. BAM15 administration to Western diet-fed mice led to reductions in hypertriglyceridemia, hepatic steatosis, and hepatic inflammation as well as improvements in whole-body insulin sensitivity without altering body temperature or renal/hepatic toxicity [111]. While the exact mechanisms of BAM15's anti-obesity effects remain to be determined, the authors attributed these beneficial effects to BAM15-mediated uncoupling in hepatocytes and increases in hepatic substrate utilization. Given BAM15's ability to increase mitochondrial respiration across multiple cell types, it is plausible that its uncoupling action in extrahepatic tissues may contribute to its anti-obesity effects. Indeed, hyperinsulinemic-euglycemic clamp studies demonstrated that BAM15 enhanced insulin sensitivity in skeletal muscle and white adipose tissue [111]. In support of this, oral dosing of BAM15 (∼85 mg/kg/day) was independently shown to prevent diet-induced obesity and improve glycemic control through alterations in whole-body energy expenditure, reduced adiposity, and AMPK-mediated suppression of WAT lipogenesis [112], supporting a crucial role of adipose tissue in mediating BAM15's beneficial effects. Interestingly, previous studies did not demonstrate an adipose-targeted depot for BAM15-mediated uncoupling [111]; however, differences in dose routes and pharmacokinetic study designs could explain these discrepancies.
Moving forward, the tissue distribution and uncoupling effects of BAM15 need to be carefully assessed to understand the cellular and molecular mechanism(s) by which BAM15 prevents diet-induced obesity. Nevertheless, its pKa (7.5) and mitochondria selectively make it an ideal uncoupler [57] and several groups are exploring improved BAM15 derivatives for treating MAFLD/NASH. In particular, work from the Santos and Hoehn groups has recently demonstrated that two BAM15 derivatives, 10b and 12i (SHS4121705), are efficacious in a STAM murine model of NASH [53,113]. Specifically, oral administration of compound 10b and SHS4121705 (25 mg/kg/day x 21 days) decreased hepatic triglyceride content and improved markers of liver toxicity including plasma ALT, hepatic inflammation, fibrosis, and NAFLD activity score (NAS) independently of changes in food intake, body weight, or body temperature [53,113]. Interestingly, these compounds did not substantially alter hepatic AMPK activity or the expression of genes involved in fibrosis or hepatic stellate cell transitions [53,113]. Nonetheless, as 10b and SHS4121705 were both shown to improve NAS without evidence of toxicity, future studies are warranted to assess the therapeutic potential of BAM15 derivatives to reduce fibrosis.
3.3.6. HU6 and MB-X01Y03
Several additional mitochondrial uncouplers are currently being developed for NASH, including HU6 (Sanyal Biotechnology, LLC/Gencia) and MB-X01Y03 (Protheragen). While the chemical properties of the Gencia mitochondrial uncoupler HU6 remain to be disclosed, promising preclinical data suggest that it would be efficacious in treating NASH. In a diet-induced mouse model of metabolic syndrome (DIAMOND mice), 8 weeks of oral HU6 treatment (1–5 mg/kg) remarkably prevented the progression from hepatic steatosis to NASH in all but one mouse, significantly lowering liver transaminases, oxidative stress, inflammation, and steatosis with no toxicities [114]. Interestingly, mice treated with 5 mg/kg of HU6 weighed significantly less than control-treated groups [114], suggesting that high doses of HU6 may lead to systemic mitochondrial uncoupling and increases in whole-body energy expenditure.
Unlike HU6, the Protheragen uncoupler, MB-X01Y03, is a lipophilic weak acid that exhibits enhanced liver distribution. By causing subtle increases in hepatic mitochondrial inefficiency, MB-X01Y03 was able to reduce fasting plasma glucose, triglycerides, and non-HDL cholesterol as well as improve hepatic steatosis and insulin sensitivity in a HFD-fed mouse model of obesity [115]. Furthermore, similar to CRMP [66], MB-X01Y03 also reduced hepatic inflammation and fibrosis in a mouse model of CCl4-induced liver fibrosis [115], suggesting its therapeutic potential for MAFLD/NASH and metabolic syndrome. While further characterization of MB-X01Y03 is needed, preclinical toxicology reports have been completed and, pending IND approval [115], MB-X01Y03 is slated for phase I clinical trials in obese patients with MAFLD/NASH.
3.4. Natural products
Notwithstanding the major endogenous mitochondrial uncouplers (for example, fatty acids and thyroid hormone T3), natural product isolates have resulted in the discovery of numerous compounds with uncoupling properties including (+)-usnic acid and turmeric. In this section, we discuss how the uncoupling properties of these two natural products have been utilized to treat obesity. Readers are referred to [43] for a more detailed summary of the uncoupling properties associated with fatty acids and thyroid hormone T3.
3.4.1. (+)-usnic acid
Isolated from lichen acid Usnea articulata, (+)-usnic acid is a natural compound found in crude medicines, including LipoKinetix, a dietary supplement marketed as a common weight-loss agent [116]. Intriguingly, (+)-usnic acid was shown to inhibit OXPHOS in isolated murine liver mitochondria, dose-dependently increasing oligomycin-inhibited oxygen consumption and reducing ATP production [116]. Unfortunately, treatment of rats with high doses of (+)-usnic acid (200 mg/kg x 5 days) caused hepatic toxicity by triggering oxidative stress [117,118]. Coupled with the increased incidence of liver injury with LipoKinetix use, (+)-usnic acid [119] is not viable as an anti-obesity drug in its current form. Future studies are warranted to examine alternative formulations and dosing paradigms to determine whether its therapeutic window can be improved.
3.4.2. Theracurmin (curcumin)
Curcumin, a natural phytochemical isolated from the rhizome of turmeric, has been shown to possess anti-oxidant, anti-inflammatory, and anti-tumor properties through altering the AMPK, mTOR, and STAT-3 signaling pathways [120,121]. Proof of its uncoupling properties came from later studies in isolated rat liver mitochondria, wherein curcumin was shown to increase mitochondrial respiration, thereby lowering cellular ATP and activating AMPK [120]. Unfortunately, while curcumin has previously been shown to protect against dyslipidemia and hepatic steatosis, its clinical use is limited by low oral bioavailability. As such, many researchers have developed alternative delivery mechanisms to enhance curcumin's intestinal absorption. Specifically, Sasaki et al. developed Theracurmin, which exhibited 30-fold higher absorption than commercially available curcumin [122]. Moreover, oral administration (150–600 mg/kg active dose) was recently shown to prevent hypertriglyceridemia and hepatic steatosis in high-fat diet-fed mice by reducing hepatic triglyceride content and inhibiting diet-induced lipid peroxidation [123]. Whether these beneficial effects were due to curcumin-mediated hepatic uncoupling remain to be determined; nonetheless, Theracurmin is currently in clinical trials (UMIN000007361) for the treatment of T2D. While initial reports show minimal effects on plasma glucose, six months of Theracurmin treatment was shown to reduce plasma-oxidized LDL in patients with impaired glucose tolerance and T2D [124], supporting additional studies assessing the cardiometabolic benefits of curcumin.
4. Challenges and future considerations
As summarized in this review, rodent and non-human primate studies suggest that a myriad of mitochondrial uncouplers can safely reverse MAFLD, NASH, liver fibrosis, diabetes, and CVD with a wide therapeutic index (Supplementary Table 1). While DNP was banned for human use almost a century ago [51], studies such as these are beginning to overturn the misconception that all mitochondrial uncouplers are toxic. Indeed, mitochondrial agents are now being developed by several pharmaceutical companies, with an open IND granted to Mitochon Pharmaceuticals for pharmacokinetic and safety studies with MP101 and MP202 (DNP prodrugs) in humans [125] and Genfit for NTZ phase 2 clinical trials in NASH patients with fibrosis (ClinicalTrials.gov identifier NCT03656068). Despite this, several challenges remain and warrant further discussion.
4.1. Safety considerations
One of the main barriers for the clinical use of mitochondrial uncouplers is safety, and in the era of high-throughput chemical screens and a plethora of formulation options, the question remains: which uncoupler has the best toxic-to-effective dose and can we learn anything from its mechanism of action to further improve the safety profiles of existing protonophores? In short, only time will tell; however, improving mitochondrial selectivity to avoid off-target actions in non-mitochondrial organelles and developing molecules with self-limiting properties such that they only partially depolarize mitochondrial membranes is of interest [57]. Some of these issues have already begun to be worked out, with groups developing cell- and organelle-targeting scaffolds. Furthermore, as chemical uncouplers continue to be characterized, several favorable properties have emerged. Interestingly, some of the first mitochondrial uncouplers were more acidic, with pKa values below 6.8 (for example, DNP). Recently reported mitochondrial uncouplers, however, are weakly acidic (pKa 6.8–8.1), suggesting that off-target effects may be mitigated in non-mitochondrial organelles with lower pH values [57].
4.2. Mitochondrial dysfunction and MAFLD/NASH
Further characterization of tissue-specific mitochondrial function during various pathological conditions will be crucial, as the effect of uncoupling in different tissue types and its interaction with cell signaling pathways is predicted to be altered during disease progression. For example, while multiple studies suggested that mitochondrial function is impaired in mouse and human models of MAFLD/NASH and T2D [[126], [127], [128]], recent reports demonstrated that mitochondrial activity is enhanced in obese insulin-resistant mice and humans with and without MAFLD [[129], [130], [131]], calling into question whether liver-targeted mitochondrial uncoupling would be beneficial in these settings. In particular, Sunny et al. using [13C3]propionate as a metabolic tracer found that rates of hepatic mitochondrial oxidation and hepatic pyruvate cycling were two-to three-fold greater in subjects with MAFLD compared to healthy control subjects [131]. In contrast, work from our group has shown that rates of hepatic mitochondrial oxidation are similar between NAFLD and control subjects using both in vivo 13C magnetic resonance spectroscopy and PINTA methodologies [[132], [133], [134]], suggesting that targeting uncoupling agents to the liver would be beneficial in the setting of MAFLD/NASH. Indeed, the numerous mitochondrial uncouplers mentioned in this review support the notion of increasing hepatic fat oxidation to treat MAFLD/NASH and T2D in rodent and non-human models of metabolic syndrome.
4.3. Mitochondrial uncoupling and weight loss
Prodrug and controlled-release formulations of DNP have proven safe and effective at reversing metabolic syndrome in rodent and non-human primate models of obesity without inducing hyperthermia [50,[66], [67], [68],78]. Intriguingly, this was all in the absence of weight loss and raises an important point concerning the anti-obesity actions of chemical uncouplers. Short-term caloric restriction, with minimal changes in body weight, has long been known to lower liver triglycerides and effectively reverse MAFLD, hepatic insulin resistance, and T2D in human [26,135] and rodent models of obesity [136], suggesting that uncoupling-mediated weight loss may not be necessary to improve many of the detrimental effects associated with metabolic syndrome. Instead we propose to target dosing of mitochondrial uncouplers with known systemic toxicities to the liver to induce a weight-neutral, subtle, sustained increase in hepatic mitochondrial inefficiency, thereby avoiding systemic toxicity concerns. Alternatively, novel uncouplers specifically targeted to mitochondrial membranes with self-limiting properties may be useful as anti-obesity drugs. Indeed, BAM15 and its derivatives have recently been shown to decrease body fat mass and reverse diet-induced obesity and insulin resistance in mice without altering food intake, lean body mass, body temperature, or biochemical and hematological markers of toxicity [111].
5. Conclusions
To date, our understanding of the pathogenesis of MAFLD/NASH and its downstream consequences has improved and new pharmacological targets have emerged. Unfortunately, developing new treatments has proved to be challenging due to the complexity of insulin resistance and the presence of multiple feedback loops that make it difficult to manipulate genetic pathways [137,138]. Moving forward, small-molecule mitochondrial uncouplers represent an alternative strategy to non-genomically increase cellular energy expenditure and improve metabolic syndrome. While the further characterization of mitochondrial uncouplers and their cell- and tissue-type mechanisms of action remains to be fully discovered, the future is promising with new mitochondria-specific uncoupling agents continuously being developed and mitochondrial research accelerating.
Acknowledgments
This study was funded by grants from the National Institutes of Health: K99 HL150234 (LG), R01 DK113984 (GIS), R01 DK114793 (GIS), R01 DK116774 (GIS), R01 DK119968 (GIS), RC2 DK120534 (GIS), and P30 DK045735 (GIS). The figures were created using BioRender.com. The authors apologize to those colleagues whose work could not be cited due to space limitations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101178.
Contributor Information
Leigh Goedeke, Email: leigh.goedeke@yale.edu.
Gerald I. Shulman, Email: gerald.shulman@yale.edu.
Conflict of interest
LG declares no competing interests. GIS serves on the scientific advisory boards of Merck, Novo Nordisk, AstraZeneca, Gilead Sciences, Esperion, 89bio, and Janssen Research and Development. GIS receives investigator-initiated support from AstraZeneca, Gilead Sciences, and Merck. GIS is an inventor on Yale patents for liver-targeted mitochondrial uncoupling agents and controlled-release mitochondrial uncoupling agents for the treatment of MAFLD, NASH, T2D and related metabolic disorders and is a scientific cofounder of TLC Inc.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Anstee Q.M., Reeves H.L., Kotsiliti E., Govaere O., Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nature Reviews Gastroenterology & Hepatology. 2019;16(7):411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 4.Ballestri S., Zona S., Targher G., Romagnoli D., Baldelli E., Nascimbeni F. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. Journal of Gastroenterology and Hepatology. 2016;31(5):936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 5.Mittal S., El-Serag H.B., Sada Y.H., Kanwal F., Duan Z., Temple S. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2016;14(1):124–131. doi: 10.1016/j.cgh.2015.07.019. e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. New England Journal of Medicine. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 7.Zheng K.I., Fan J.G., Shi J.P., Wong V.W., Eslam M., George J. From NAFLD to MAFLD: a "redefining" moment for fatty liver disease. Chinese Medical Journal. 2020;133(19):2271–2273. doi: 10.1097/CM9.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eslam M., Sanyal A.J., George J., International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1991. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M., George J. Reply to: correspondence regarding "A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement": bringing evidence to the NAFLD-MAFLD debate. Journal of Hepatology. 2020 doi: 10.1016/j.jhep.2020.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Romero F.A., Jones C.T., Xu Y., Fenaux M., Halcomb R.L. The race to bash NASH: emerging targets and drug development in a complex liver disease. Journal of Medicinal Chemistry. 2020;63(10):5031–5073. doi: 10.1021/acs.jmedchem.9b01701. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs M., Schnabl B. Editors' introduction to the NAFLD and NASH special issue. Digestive Diseases and Sciences. 2016;61(5):1211–1213. doi: 10.1007/s10620-016-4152-z. [DOI] [PubMed] [Google Scholar]
- 12.Ekstedt M., Hagstrom H., Nasr P., Fredrikson M., Stal P., Kechagias S. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 13.Sangwung P., Petersen K.F., Shulman G.I., Knowles J.W. Mitochondrial dysfunction, insulin resistance, and potential genetic implications. Endocrinology. 2020;161(4) doi: 10.1210/endocr/bqaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel V.T., Shulman G.I. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen M.C., Shulman G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends in Pharmacological Sciences. 2017;38(7):649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyu K., Zhang Y., Zhang D., Kahn M., Ter Horst K.W., Rodrigues M.R.S. A membrane-bound diacylglycerol species induces PKC-mediated hepatic insulin resistance. Cell Metabolism. 2020;32(4):654–664. doi: 10.1016/j.cmet.2020.08.001. e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. Journal of Clinical Investigation. 2016;126(11):4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagadala M., Kasumov T., McCullough A.J., Zein N.N., Kirwan J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends in Endocrinology and Metabolism. 2012;23(8):365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isbell J.M., Tamboli R.A., Hansen E.N., Saliba J., Dunn J.P., Phillips S.E. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campos G.M., Rabl C., Peeva S., Ciovica R., Rao M., Schwarz J.M. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. Journal of Gastrointestinal Surgery. 2010;14(1):15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laferrere B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plum L., Ahmed L., Febres G., Bessler M., Inabnet W., Kunreuther E. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity. 2011;19(11):2149–2157. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry R.R., Scheaffer L., Olefsky J.M. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 1985;61(5):917–925. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 24.Lim E.L., Hollingsworth K.G., Aribisala B.S., Chen M.J., Mathers J.C., Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackness C., Karmally W., Febres G., Conwell I.M., Ahmed L., Bessler M. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stunkard A., Mc L.-H.M. The results of treatment for obesity: a review of the literature and report of a series. AMA Archives of Internal Medicine. 1959;103(1):79–85. doi: 10.1001/archinte.1959.00270010085011. [DOI] [PubMed] [Google Scholar]
- 28.Wing R.R., Phelan S. Long-term weight loss maintenance. American Journal of Clinical Nutrition. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 29.McGuire M.T., Wing R.R., Hill J.O. The prevalence of weight loss maintenance among American adults. International Journal of Obesity and Related Metabolic Disorders. 1999;23(12):1314–1319. doi: 10.1038/sj.ijo.0801075. [DOI] [PubMed] [Google Scholar]
- 30.Kraschnewski J.L., Boan J., Esposito J., Sherwood N.E., Lehman E.B., Kephart D.K. Long-term weight loss maintenance in the United States. International Journal of Obesity. 2010;34(11):1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tainter M.L., Cutting W.C., Hines E. Effects of moderate doses of dinitrophenol on the energy exchange and nitrogen metabolism of patients under conditions of restricted dietary. Journal of Pharmacology and Experimental Therapeutics. 1935;55:326–353. [Google Scholar]
- 32.Tainter M.L., Stockton A.B., Cutting W.C. Use of dinitrophenol in obesity and related conditions. Journal of the American Medical Association. 1933;101:1472–1475. [Google Scholar]
- 33.Parascandola J. Dinitrophenol and bioenergetics: an historical perspective. Molecular and Cellular Biochemistry. 1974;5(1–2):69–77. doi: 10.1007/BF01874175. [DOI] [PubMed] [Google Scholar]
- 34.Goedeke L., Perry R.J., Shulman G.I. Emerging pharmacological targets for the treatment of nonalcoholic fatty liver disease, insulin resistance, and type 2 diabetes. Annual Review of Pharmacology and Toxicology. 2019;59:65–87. doi: 10.1146/annurev-pharmtox-010716-104727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terada H. Uncouplers of oxidative phosphorylation. Environmental Health Perspectives. 1990;87:213–218. doi: 10.1289/ehp.9087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell P., Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature. 1967;213(5072):137–139. doi: 10.1038/213137a0. [DOI] [PubMed] [Google Scholar]
- 37.Papa S., Martino P.L., Capitanio G., Gaballo A., De Rasmo D., Signorile A. The oxidative phosphorylation system in mammalian mitochondria. Advances in Experimental Medicine & Biology. 2012;942:3–37. doi: 10.1007/978-94-007-2869-1_1. [DOI] [PubMed] [Google Scholar]
- 38.Schultz B.E., Chan S.I. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annual Review of Biophysics and Biomolecular Structure. 2001;30:23–65. doi: 10.1146/annurev.biophys.30.1.23. [DOI] [PubMed] [Google Scholar]
- 39.Loomis W.F., Lipmann F. Reversible inhibition of the coupling between phosphorylation and oxidation. Journal of Biological Chemistry. 1948;173(2):807. [PubMed] [Google Scholar]
- 40.Weinbach E.C., Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221(5185):1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- 41.Rolfe D.F., Newman J.M., Buckingham J.A., Clark M.G., Brand M.D. Contribution of mitochondrial proton leak to respiration rate in working skeletal muscle and liver and to SMR. American Journal of Physiology. 1999;276(3):C692–C699. doi: 10.1152/ajpcell.1999.276.3.C692. [DOI] [PubMed] [Google Scholar]
- 42.Divakaruni A.S., Brand M.D. The regulation and physiology of mitochondrial proton leak. Physiology. 2011;26(3):192–205. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 43.Demine S., Renard P., Arnould T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 2019;8(8) doi: 10.3390/cells8080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuno-Yagi A., Hatefi Y. Uncoupling of oxidative phosphorylation: different effects of lipophilic weak acids and electrogenic ionophores on the kinetics of ATP synthesis. Biochemistry. 1989;28(10):4367–4374. doi: 10.1021/bi00436a037. [DOI] [PubMed] [Google Scholar]
- 45.Hemker H.C. Lipid solubility as a factor influencing the activity of uncoupling phenols. Biochimica et Biophysica Acta. 1962;63:46–54. doi: 10.1016/0006-3002(62)90337-2. [DOI] [PubMed] [Google Scholar]
- 46.Casey J.R., Grinstein S., Orlowski J. Sensors and regulators of intracellular pH. Nature Reviews Molecular Cell Biology. 2010;11(1):50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 47.Dulloo A.G., Samec S. Uncoupling proteins: their roles in adaptive thermogenesis and substrate metabolism reconsidered. British Journal of Nutrition. 2001;86(2):123–139. doi: 10.1079/bjn2001412. [DOI] [PubMed] [Google Scholar]
- 48.Chen K.Y., Brychta R.J., Abdul Sater Z., Cassimatis T.M., Cero C., Fletcher L.A. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. Journal of Biological Chemistry. 2020;295(7):1926–1942. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousset S., Alves-Guerra M.C., Mozo J., Miroux B., Cassard-Doulcier A.M., Bouillaud F. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130–S135. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 50.Perry R.J., Kim T., Zhang X.M., Lee H.Y., Pesta D., Popov V.B. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metabolism. 2013;18(5):740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grundlingh J., Dargan P.I., El-Zanfaly M., Wood D.M. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. Journal of Medical Toxicology. 2011;7(3):205–212. doi: 10.1007/s13181-011-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuart J.A., Cadenas S., Jekabsons M.B., Roussel D., Brand M.D. Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochimica et Biophysica Acta. 2001;1504(1):144–158. doi: 10.1016/s0005-2728(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 53.Childress E.S., Salamoun J.M., Hargett S.R., Alexopoulos S.J., Chen S.Y., Shah D.P. [1,2,5]Oxadiazolo[3,4-b]pyrazine-5,6-diamine derivatives as mitochondrial uncouplers for the potential treatment of nonalcoholic steatohepatitis. Journal of Medicinal Chemistry. 2020;63(5):2511–2526. doi: 10.1021/acs.jmedchem.9b01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y., Ivica N.A., Dong T., Papageorgiou D.P., He Y., Brown D.R. MFSD7C switches mitochondrial ATP synthesis to thermogenesis in response to heme. Nature Communications. 2020;11(1):4837. doi: 10.1038/s41467-020-18607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buckler K.J., Vaughan-Jones R.D. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. Journal of Physiology. 1998;513(Pt 3):819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brismar T., Collins V.P. Effect of external cation concentration and metabolic inhibitors on membrane potential of human glial cells. Journal of Physiology. 1993;460:365–383. doi: 10.1113/jphysiol.1993.sp019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Childress E.S., Alexopoulos S.J., Hoehn K.L., Santos W.L. Small molecule mitochondrial uncouplers and their therapeutic potential. Journal of Medicinal Chemistry. 2018;61(11):4641–4655. doi: 10.1021/acs.jmedchem.7b01182. [DOI] [PubMed] [Google Scholar]
- 58.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regulatory Toxicology and Pharmacology. 2007;48(2):115–117. doi: 10.1016/j.yrtph.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Bianchetti A., Pugliatti C., Jori A. On the hyperthermia induced by 2,4-dinitrophenol. Pharmacology. 2004;17:401–408. doi: 10.1159/000137108. [DOI] [PubMed] [Google Scholar]
- 60.Poole F.E., Haining R.B. Sudden death from dinitrophenol poisoning. Journal of the American Medical Association. 1934;46:251–254. [Google Scholar]
- 61.Petroczi A., Ocampo J.A., Shah I., Jenkinson C., New R., James R.A. Russian roulette with unlicensed fat-burner drug 2,4-dinitrophenol (DNP): evidence from a multidisciplinary study of the internet, bodybuilding supplements and DNP users. Substance Abuse Treatment, Prevention, and Policy. 2015;10:39. doi: 10.1186/s13011-015-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuel V.T., Liu Z.X., Qu X., Elder B.D., Bilz S., Befroy D. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. Journal of Biological Chemistry. 2004;279(31):32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 63.Caldeira da Silva C.C., Cerqueira F.M., Barbosa L.F., Medeiros M.H., Kowaltowski A.J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7(4):552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 64.Goldgof M., Xiao C., Chanturiya T., Jou W., Gavrilova O., Reitman M.L. The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Journal of Biological Chemistry. 2014;289(28):19341–19350. doi: 10.1074/jbc.M114.568204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Overton J.M. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. International Journal of Obesity. 2010;34(Suppl 2):S53–S58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- 66.Perry R.J., Zhang D., Zhang X.M., Boyer J.L., Shulman G.I. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science. 2015;347(6227):1253–1256. doi: 10.1126/science.aaa0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goedeke L., Peng L., Montalvo-Romeral V., Butrico G.M., Dufour S., Zhang X.M. Controlled-release mitochondrial protonophore (CRMP) reverses dyslipidemia and hepatic steatosis in dysmetabolic nonhuman primates. Science Translational Medicine. 2019;11(512) doi: 10.1126/scitranslmed.aay0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei G., Song X., Fu Y., Gong T., Zhang Q. Sustained-release mitochondrial protonophore reverses nonalcoholic fatty liver disease in rats. International Journal of Pharmacy. 2017;530(1–2):230–238. doi: 10.1016/j.ijpharm.2017.07.072. [DOI] [PubMed] [Google Scholar]
- 69.Callow A.D. Cardiovascular disease 2005--the global picture. Vascular Pharmacology. 2006;45(5):302–307. doi: 10.1016/j.vph.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Behn A., Ur E. The obesity epidemic and its cardiovascular consequences. Current Opinion in Cardiology. 2006;21(4):353–360. doi: 10.1097/01.hco.0000231406.84554.96. [DOI] [PubMed] [Google Scholar]
- 71.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Journal of the American Medical Association. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 72.Gore M.O., McGuire D.K., Lingvay I., Rosenstock J. Predicting cardiovascular risk in type 2 diabetes: the heterogeneity challenges. Current Cardiology Reports. 2015;17(7):607. doi: 10.1007/s11886-015-0607-7. [DOI] [PubMed] [Google Scholar]
- 73.Haffner S.M., Lehto S., Ronnemaa T., Pyorala K., Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New England Journal of Medicine. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 74.Hayward R.A., Reaven P.D., Emanuele N.V., Investigators V. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2015;373(10):978. doi: 10.1056/NEJMc1508386. [DOI] [PubMed] [Google Scholar]
- 75.Goedeke L., Rotllan N., Toussaint K., Nasiri A.R., Zhang X., Lee J. ADA 80th scientific sessions. Diabetes, virtual meeting. 2020. Liver-Targeted mitochondrial uncoupling by CRMP improves whole-body insulin sensitivity and attenuates athersclerosis in a LDLR-/- mouse model of metabolic syndrome. [Google Scholar]
- 76.Garg A. Lipodystrophies. Americas Journal of Medicine. 2000;108(2):143–152. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 77.Safar Zadeh E., Lungu A.O., Cochran E.K., Brown R.J., Ghany M.G., Heller T. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. Journal of Hepatology. 2013;59(1):131–137. doi: 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abulizi A., Perry R.J., Camporez J.P.G., Jurczak M.J., Petersen K.F., Aspichueta P. A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice. The FASEB Journal. 2017;31(7):2916–2924. doi: 10.1096/fj.201700001R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abulizi A., Vatner D.F., Ye Z., Wang Y., Camporez J.P., Zhang D. Membrane bound diacylglycerols explain the dissociation of hepatic insulin resistance from steatosis in MTTP(-/-) mice. The Journal of Lipid Research. 2020 doi: 10.1194/jlr.RA119000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bell D.A., Hooper A.J., Watts G.F., Burnett J.R. Mipomersen and other therapies for the treatment of severe familial hypercholesterolemia. Vascular Health and Risk Management. 2012;8:651–659. doi: 10.2147/VHRM.S28581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan R.S., Dine K., Geisler J.G., Shindler K.S. Mitochondrial uncoupler prodrug of 2,4-dinitrophenol, MP201, prevents neuronal damage and preserves vision in experimental optic neuritis. Oxidative medicine and cellular longevity. 2017;2017:7180632. doi: 10.1155/2017/7180632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rottenberg H. Uncoupling of oxidative phosphorylation in rat liver mitochondria by general anesthetics. Proceedings of the National Academy of Sciences of the U S A. 1983;80(11):3313–3317. doi: 10.1073/pnas.80.11.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dabadie P., Bendriss P., Erny P., Mazat J.P. Uncoupling effects of local anesthetics on rat liver mitochondria. FEBS Letters. 1987;226(1):77–82. doi: 10.1016/0014-5793(87)80554-9. [DOI] [PubMed] [Google Scholar]
- 84.Sheth U.K. Mechanisms of anthelmintic action. Progress In Drug Research. 1975;19:147–157. doi: 10.1007/978-3-0348-7090-0_19. [DOI] [PubMed] [Google Scholar]
- 85.Hecht G., Gloxhuber C. Studies on the tolerance of 5,2'-dichloro-4'-nitrosalicylanilide ethanolamine salt. Zeitschrift fuer Tropenmedizin und Parasitologie. 1962;13:1–8. [PubMed] [Google Scholar]
- 86.Guo J., Tao H., Alasadi A., Huang Q., Jin S. Niclosamide piperazine prevents high-fat diet-induced obesity and diabetic symptoms in mice. Eating and Weight Disorders. 2019;24(1):91–96. doi: 10.1007/s40519-017-0424-7. [DOI] [PubMed] [Google Scholar]
- 87.Tao H., Zhang Y., Zeng X., Shulman G.I., Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nature Medicine. 2014;20(11):1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chowdhury M.K., Turner N., Bentley N.L., Das A., Wu L.E., Richani D. Niclosamide reduces glucagon sensitivity via hepatic PKA inhibition in obese mice: implications for glucose metabolism improvements in type 2 diabetes. Scientific Reports. 2017;7:40159. doi: 10.1038/srep40159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han P., Zhan H., Shao M., Wang W., Song G., Yu X. Niclosamide ethanolamine improves kidney injury in db/db mice. Diabetes Research and Clinical Practice. 2018;144:25–33. doi: 10.1016/j.diabres.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Chang X., Zhen X., Liu J., Ren X., Hu Z., Zhou Z. The antihelmenthic phosphate niclosamide impedes renal fibrosis by inhibiting homeodomain-interacting protein kinase 2 expression. Kidney International. 2017;92(3):612–624. doi: 10.1016/j.kint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 91.Han P., Yuan C., Wang Y., Wang M., Weng W., Zhan H. Niclosamide ethanolamine protects kidney in adriamycin nephropathy by regulating mitochondrial redox balance. American Journal of Translational Research. 2019;11(2):855–864. [PMC free article] [PubMed] [Google Scholar]
- 92.Hinder L.M., Sas K.M., O'Brien P.D., Backus C., Kayampilly P., Hayes J.M. Mitochondrial uncoupling has no effect on microvascular complications in type 2 diabetes. Scientific Reports. 2019;9(1):881. doi: 10.1038/s41598-018-37376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amireddy N., Puttapaka S.N., Vinnakota R.L., Ravuri H.G., Thonda S., Kalivendi S.V. The unintended mitochondrial uncoupling effects of the FDA-approved anti-helminth drug nitazoxanide mitigates experimental parkinsonism in mice. Journal of Biological Chemistry. 2017;292(38):15731–15743. doi: 10.1074/jbc.M117.791863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belanger C., Foucart C., Negro E., Degallaix N., Delataille P., Dubernet M. EASL ILC; Amsterdam, The Netherlands: 2017. Drug repurposing screen identifies novel small molecule compounds with potent antifibrotic properties. [Google Scholar]
- 95.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 96.Liu L., Cao Y., Chen C., Zhang X., McNabola A., Wilkie D. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Research. 2006;66(24):11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 97.Strumberg D., Clark J.W., Awada A., Moore M.J., Richly H., Hendlisz A. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. The Oncologist. 2007;12(4):426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 98.Jian C., Fu J., Cheng X., Shen L.J., Ji Y.X., Wang X. Low-dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis. Cell Metabolism. 2020;31(5):892–908. doi: 10.1016/j.cmet.2020.04.011. e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dromgoole S.H., Cassell S., Furst D.E., Paulus H.E. Availability of salicylate from salsalate and aspirin. Clinical Pharmacology & Therapeutics. 1983;34(4):539–545. doi: 10.1038/clpt.1983.211. [DOI] [PubMed] [Google Scholar]
- 100.Goldfine A.B., Silver R., Aldhahi W., Cai D., Tatro E., Lee J. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1(1):36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang W., Verschuren L., Mulder P., van der Hoorn J.W., Verheij J., van Dam A.D. Salsalate attenuates diet induced non-alcoholic steatohepatitis in mice by decreasing lipogenic and inflammatory processes. British Journal of Pharmacology. 2015;172(22):5293–5305. doi: 10.1111/bph.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith B.K., Ford R.J., Desjardins E.M., Green A.E., Hughes M.C., Houde V.P. Salsalate (salicylate) uncouples mitochondria, improves glucose homeostasis, and reduces liver lipids independent of AMPK-beta1. Diabetes. 2016;65(11):3352–3361. doi: 10.2337/db16-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Figarola J.L., Weng Y., Lincoln C., Horne D., Rahbar S. Novel dichlorophenyl urea compounds inhibit proliferation of human leukemia HL-60 cells by inducing cell cycle arrest, differentiation and apoptosis. Investigational New Drugs. 2012;30(4):1413–1425. doi: 10.1007/s10637-011-9711-8. [DOI] [PubMed] [Google Scholar]
- 104.Figarola J.L., Rahbar S. Smallmolecule COH-SR4 inhibits adipocyte differentiation via AMPK activation. International Journal of Molecular Medicine. 2013;31(5):1166–1176. doi: 10.3892/ijmm.2013.1313. [DOI] [PubMed] [Google Scholar]
- 105.Figarola J.L., Singhal P., Rahbar S., Gugiu B.G., Awasthi S., Singhal S.S. COH-SR4 reduces body weight, improves glycemic control and prevents hepatic steatosis in high fat diet-induced obese mice. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0083801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu Y.Y., Zhang M., Turner N., Zhang L.N., Dong T.C., Gu M. A novel chemical uncoupler ameliorates obesity and related phenotypes in mice with diet-induced obesity by modulating energy expenditure and food intake. Diabetologia. 2013;56(10):2297–2307. doi: 10.1007/s00125-013-2987-9. [DOI] [PubMed] [Google Scholar]
- 107.Kanemoto N., Okamoto T., Tanabe K., Shimada T., Minoshima H., Hidoh Y. Antidiabetic and cardiovascular beneficial effects of a liver-localized mitochondrial uncoupler. Nature Communications. 2019;10(1):2172. doi: 10.1038/s41467-019-09911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang H., Jin J., Duan Y., Xie Z., Li Y., Gao A. Mitochondrial uncoupling coordinated with PDH activation safely ameliorates hyperglycemia via promoting glucose oxidation. Diabetes. 2019;68(12):2197–2209. doi: 10.2337/db19-0589. [DOI] [PubMed] [Google Scholar]
- 109.Kenwood B.M., Weaver J.L., Bajwa A., Poon I.K., Byrne F.L., Murrow B.A. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Molecular Metabolism. 2014;3(2):114–123. doi: 10.1016/j.molmet.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kenwood B.M., Calderone J.A., Taddeo E.P., Hoehn K.L., Santos W.L. Structure-activity relationships of furazano[3,4-b]pyrazines as mitochondrial uncouplers. Bioorganic & Medicinal Chemistry Letters. 2015;25(21):4858–4861. doi: 10.1016/j.bmcl.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexopoulos S.J., Chen S.Y., Brandon A.E., Salamoun J.M., Byrne F.L., Garcia C.J. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nature Communications. 2020;11(1):2397. doi: 10.1038/s41467-020-16298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Axelrod C.L., King W.T., Davuluri G., Noland R.C., Hall J., Hull M. BAM15-mediated mitochondrial uncoupling protects against obesity and improves glycemic control. EMBO Molecular Medicine. 2020;12(7) doi: 10.15252/emmm.202012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salamoun J.M., Garcia C.J., Hargett S.R., Murray J.H., Chen S.Y., Beretta M. 6-Amino[1,2,5]oxadiazolo[3,4-b]pyrazin-5-ol derivatives as efficacious mitochondrial uncouplers in STAM mouse model of nonalcoholic steatohepatitis. Journal of Medicinal Chemistry. 2020;63(11):6203–6224. doi: 10.1021/acs.jmedchem.0c00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Portell F., Eaton S., Dennis J., Caffrey R., Marioneaux J., Onyango I. EASL: European Association for the Study of the Liver; Paris, France: 2018. Mitochondrial uncoupler HU6 reduces hepatic oxidative stress in the DIAMOND mouse model thus Abrogating NASH Development. [Google Scholar]
- 115.Protheragen. A mitochondrial uncoupler for NASH treatment, secondary A mitochondrial uncoupler for NASH treatment 2005-2020, https://www.protheragen.com/available-projects/a-mitochondrial-uncoupler-for-nash-treatment/
- 116.Abo-Khatwa A.N., al-Robai A.A., al-Jawhari D.A. Lichen acids as uncouplers of oxidative phosphorylation of mouse-liver mitochondria. Natural Toxins. 1996;4(2):96–102. doi: 10.1002/19960402nt7. [DOI] [PubMed] [Google Scholar]
- 117.Pramyothin P., Janthasoot W., Pongnimitprasert N., Phrukudom S., Ruangrungsi N. Hepatotoxic effect of (+)usnic acid from Usnea siamensis Wainio in rats, isolated rat hepatocytes and isolated rat liver mitochondria. Journal of Ethnopharmacology. 2004;90(2–3):381–387. doi: 10.1016/j.jep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 118.Joseph A., Lee T., Moland C.L., Branham W.S., Fuscoe J.C., Leakey J.E. Effect of (+)-usnic acid on mitochondrial functions as measured by mitochondria-specific oligonucleotide microarray in liver of B6C3F1 mice. Mitochondrion. 2009;9(2):149–158. doi: 10.1016/j.mito.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Sanchez W., Maple J.T., Burgart L.J., Kamath P.S. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clinic Proceedings. 2006;81(4):541–544. doi: 10.4065/81.4.541. [DOI] [PubMed] [Google Scholar]
- 120.Lim H.W., Lim H.Y., Wong K.P. Uncoupling of oxidative phosphorylation by curcumin: implication of its cellular mechanism of action. Biochemical and Biophysical Research Communications. 2009;389(1):187–192. doi: 10.1016/j.bbrc.2009.08.121. [DOI] [PubMed] [Google Scholar]
- 121.Farzaei M.H., Zobeiri M., Parvizi F., El-Senduny F.F., Marmouzi I., Coy-Barrera E. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients. 2018;10(7) doi: 10.3390/nu10070855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sasaki H., Sunagawa Y., Takahashi K., Imaizumi A., Fukuda H., Hashimoto T. Innovative preparation of curcumin for improved oral bioavailability. Biological & Pharmaceutical Bulletin. 2011;34(5):660–665. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- 123.Yang J.W., Yeo H.K., Yun J.H., Lee J.U. Theracurmin (highly bioavailable curcumin) prevents high fat diet-induced hepatic steatosis development in mice. Toxicology Research. 2019;35(4):403–410. doi: 10.5487/TR.2019.35.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Funamoto M., Shimizu K., Sunagawa Y., Katanasaka Y., Miyazaki Y., Kakeya H. Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. J Diabetes Res. 2019;2019:8208237. doi: 10.1155/2019/8208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Geisler J.G. 2,4 dinitrophenol as medicine. Cells. 2019;8(3) doi: 10.3390/cells8030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanyal A.J., Campbell-Sargent C., Mirshahi F., Rizzo W.B., Contos M.J., Sterling R.K. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 127.Schmid A.I., Szendroedi J., Chmelik M., Krssak M., Moser E., Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34(2):448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Szendroedi J., Chmelik M., Schmid A.I., Nowotny P., Brehm A., Krssak M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009;50(4):1079–1086. doi: 10.1002/hep.23093. [DOI] [PubMed] [Google Scholar]
- 129.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metabolism. 2015;21(5):739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Buchner D.A., Yazbek S.N., Solinas P., Burrage L.C., Morgan M.G., Hoppel C.L. Increased mitochondrial oxidative phosphorylation in the liver is associated with obesity and insulin resistance. Obesity. 2011;19(5):917–924. doi: 10.1038/oby.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sunny N.E., Parks E.J., Browning J.D., Burgess S.C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metabolism. 2011;14(6):804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Befroy D.E., Perry R.J., Jain N., Dufour S., Cline G.W., Trimmer J.K. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nature Medicine. 2014;20(1):98–102. doi: 10.1038/nm.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perry R.J., Peng L., Cline G.W., Butrico G.M., Wang Y., Zhang X.M. Non-invasive assessment of hepatic mitochondrial metabolism by positional isotopomer NMR tracer analysis (PINTA) Nature Communications. 2017;8(1):798. doi: 10.1038/s41467-017-01143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Petersen K.F., Befroy D.E., Dufour S., Rothman D.L., Shulman G.I. Assessment of hepatic mitochondrial oxidation and pyruvate cycling in NAFLD by (13)C magnetic resonance spectroscopy. Cell Metabolism. 2016;24(1):167–171. doi: 10.1016/j.cmet.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taylor R., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L. Clinical and metabolic features of the randomised controlled diabetes remission clinical trial (DiRECT) cohort. Diabetologia. 2018;61(3):589–598. doi: 10.1007/s00125-017-4503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perry R.J., Peng L., Cline G.W., Wang Y., Rabin-Court A., Song J.D. Mechanisms by which a very-low-calorie diet reverses hyperglycemia in a rat model of type 2 diabetes. Cell Metabolism. 2018;27(1):210–217. doi: 10.1016/j.cmet.2017.10.004. e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Altaf Q.A., Barnett A.H., Tahrani A.A. Novel therapeutics for type 2 diabetes: insulin resistance. Diabetes, Obesity and Metabolism. 2015;17(4):319–334. doi: 10.1111/dom.12400. [DOI] [PubMed] [Google Scholar]
- 138.Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology. 2018;68(6):2197–2211. doi: 10.1002/hep.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.