Abstract
Aim
Diabetic (type-2) is a metabolic disease characterized by increased blood glucose level from the normal level. In the present study, apigenin (AG) loaded lipid vesicles (bilosomes: BIL) was prepared, optimized and evaluated for the oral therapeutic efficacy.
Experimental
AG-BIL was prepared by a thin-film evaporation method using cholesterol, span 60 and sodium deoxycholate. The prepared formulation was optimized by 3-factor and 3-level Box-Behnken design using particle size, entrapment efficiency and drug release as a response. The selected formulation further evaluated for ex-vivo permeation, in vivo pharmacokinetic and pharmacodynamics study.
Results
The optimized AG bilosomes (AG-BILopt) has shown the vesicle size 183.25 ± 2.43 nm, entrapment efficiency 81.67 ± 4.87%. TEM image showed a spherical shape vesicle with sharp boundaries. The drug release study revealed a significant enhancement in AG release (79.45 ± 4.18%) from AG-BILopt as compared to free AG-dispersion (25.47 ± 3.64%). The permeation and pharmacokinetic studies result revealed 4.49 times higher flux and 4.67 folds higher AUC0-t than free AG-dispersion. The antidiabetic activity results showed significant (P < 0.05) enhancement in therapeutic efficacy than free AG-dispersion. The results also showed marked improvement in biochemical parameters.
Conclusion
Our findings suggested, the prepared apigenin loaded bilosomes was found to be an efficient delivery in the therapeutic efficacy in diabetes.
Keywords: Diabetes, Apigenin, Bilosomes, Optimization, Pharmacokinetic, Anti-diabetic activity
1. Introduction
Diabetes (Type 2) is a disease associated with increased blood glucose level in the body. Due to increased blood glucose level, there are many other symptoms like weight loss, frequent urination, feeling more hunger and altered biochemical parameters (lipid profile, liver and kidney function) found. These symptoms lead to heart attack, kidney failure as well as diabetic retinopathy. Globally up to the year 2019, approximately 463 million people, were associated with type 2 diabetes and may increases up to 700 million by 2045. Its percentage in adult increases mostly in low and middle-income countries (WHO, 2014).
About 40–70% of therapeutic drugs are water-insoluble, having low permeability and absorption to systemic circulation after oral administration. The poor solubility of a drug (lipophilic) directly effects the rate of dissolution as well as absorption (Gupta et al., 2013). Apigenin (AG) is a natural bioactive compound, belongs to flavones category. It is found in various natural sources like vegetables and fruits (Funakoshi-Tago et al., 2011). There are number of pharmacological activities like antidiabetic (Choi, et al., 2014), anticancer (Lu et al., 2020), anti-oxidant (Darabi et al., 2020) and anti-inflammatory activity (Wang et al., 2020) have been reported. It has poor aqueous solubility and belongs to BCS-II class category (Huang et al., 2016). Due to poor aqueous solubility, their bioavailability is limited (~7.1%) and led to poor therapeutic efficacy after single-dose oral administration in the rat (Perez-Moral et al., 2018, Elhennawy and Lin, 2018).
There are several nano-sized lipid vesicles of poorly soluble drugs have been reported to enhance the solubility and bioavailability (Mishra et al., 2016, Paudel et al., 2017, Freag et al., 2018, Pauli et al., 2019, Sze et al., 2019, Rizwanullah et al., 2017). Bilosomes (BIL) is an elastic nano-size vesicle consists of lipid, surfactant and bile salt (Parashar et al., 2019). It is structurally similar to liposome (Yang et al., 2019a, Yang et al., 2019b), and protect the drug molecule from the enzymatic degradation into GIT (Wilkhu et al., 2013). The bile salt present in intestine (GIT) limits the capabilities of the conventional vesicle by causing lysis of vesicle membrane and led to early release of entrapped drug or molecule before reaching to the site of action (Naguib et al., 2020). To overcome the limitations of conventional nano-vesicular drug delivery systems, the application of bilosomes (bile salt stabilized nano-vesicle) as a delivery system has been widely accepted. It is a nanosized vesicle prepared with the addition of bile salt into a lipid bilayer. The bile salt is biologically compatible and having no toxicity (Nuruunabi et al., 2016; Ahmad et al., 2017). It has assimilation enhancing ability and acts as solubilizing and permeation enhancing agent. It may enhance the drug permeability and bioavailability of poorly soluble therapeutics (Deng and Bae, 2020). Bile salts absorbed through apical sodium-dependent bile acid transporter (ASBT) in the GIT, and may also act as an enhancer in the oral delivery (Nurunnabi et al., 2016). The different type of bile salts such as sodium deoxycholate, sodium glycolate and sodium taurocholate were used to formulate BIL. Among them, sodium deoxycholate is most commonly used due to its nontoxic and high permeation enhancing capacity (Niu et al., 2012). There are different AG loaded nano formulations were evaluated for the enhancement of solubility and permeability. The different formulations like nano-crystal (Al-Shaal et al., 2011), phytosomes and nanoemulsion (Telange et al., 2017, Abcha et al., 2010), polymeric micelles (Zhai et al., 2013). But till date, no literature reported for the formulation of AG loaded BIL for the oral delivery.
In present research work, we have prepared and optimized AG loaded nano sized BIL for oral delivery to enhance the therapeutic efficacy. The prepared formulations were optimized using box Behnken design and evaluated for physicochemical parameters. Finally, the selected formulation was further evaluated for ex-vivo permeation, in-vivo anti-diabetic activity, pharmacokinetic and biochemical study to check the potential of developed formulation on murine model.
2. Materials and methods
2.1. Materials
Apigenin was procured from “Beijing Mesochem Technology Co. Pvt. Ltd. (Beijing, China)”. Span 60, diethyl ether, chloroform, HPLC grade ethyl acetate, methanol, acetonitrile and water were procured from Sigma Aldrich (St Louis, MO63103, USA). Formic acid was procured from SD-fine chemical (Mumbai, India). Cholesterol and Sodium deoxycholate were obtained from Thermo Fisher Scientific, India. Dialysis bag (MW-cut off 12000) was purchased from the Sigma Aldrich (St Louis, MO63103, USA).
3. Methods
3.1. Formulation of AG bilosomes
AG loaded bilosomes were prepared by the thin-film hydration method with slight modification (Saifi et al., 2020). The weighed quantity of cholesterol (CHO), surfactant (Span 60), and Apigenin (AG) were taken in a round bottom flask and dissolved in organic solvent (10 ml). The round bottom flask attached to the rotary evaporator (IKA, RV-3 V, Germany) and the organic solvent was evaporated at 55 °C under reduced pressure. After complete removal of the solvent, the flask was kept overnight in desiccator to remove the residual solvent. The dry thin film was hydrated with bile salt (Sodium deoxycholate, SDC) containing distilled water for 45 min. Finally, AG loaded bilosomes (BIL) was collected and stored for further characterization.
4. Formulation optimization
Box-Behnken design (BBD) was used to optimize the prepared AG BIL. This design was used most commonly because it gives lesser number of runs with five centre point (Ameeduzzafar et al., 2014, Khan et al., 2018). The independent variables Cholesterol (A, CHO), surfactant (B, span 60) and bile salt (C, sodium deoxycholate) were used at three level (low, medium, high) as independent variables. There effects were assessed on vesicle size (Y1), entrapment efficiency (Y2, EE) and drug release (Y3) as shown in Table 1. The design showed seventeen experimental runs with five centre point (* same composition) and the detail compositions were expressed in Table 2.
Table 1.
Independent variables and responses used for optimization of bilosomes.
| Factor |
Level |
||
|---|---|---|---|
| Independent variables | Low (−1) | Medium (0) | High (+1) |
| A: Cholesterol (CHO, %) | 10 | 20 | 30 |
| B: Surfactant (Span 60, %) | 50 | 60 | 70 |
| C: Bile Salt (Sodium deoxycholate, SDC) | 10 | 15 | 20 |
| Responses | |||
| Y1: Vesicle size (nm) | Optimum | ||
| Y2: Entrapment efficiency (%) | Maximize | ||
| Y3: Drug release (%) | Maximize | ||
Table 2.
Formulation design and their practical and predicted value of all responses.
| Code |
Process variable |
Responses |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CHO (%) | SP60 (%) | SDC (%) |
Vesicle size (nm) |
Entrapment efficiency (%) |
Drug release (%) |
||||
| Actual value | Predicted value | Actual value | Predicted value | Actual value | Predicted value | ||||
| 1 | 10 | 50 | 15 | 155.98 | 155.87 | 69.54 | 69.56 | 93.08 | 92.97 |
| 2 | 30 | 50 | 15 | 267.32 | 267.05 | 87.74 | 87.78 | 85.20 | 85.14 |
| 3 | 10 | 70 | 15 | 118.93 | 119.19 | 78.63 | 78.59 | 82.39 | 82.44 |
| 4 | 30 | 70 | 15 | 197.34 | 197.45 | 93.04 | 93.02 | 76.34 | 76.44 |
| 5 | 10 | 60 | 10 | 155.32 | 155.24 | 71.62 | 71.69 | 84.98 | 85.04 |
| 6 | 30 | 60 | 10 | 247.15 | 247.23 | 89.31 | 89.36 | 78.09 | 78.11 |
| 7 | 10 | 60 | 20 | 133.45 | 133.37 | 73.75 | 73.7 | 91.32 | 91.30 |
| 8 | 30 | 60 | 20 | 230.76 | 230.83 | 88.74 | 88.67 | 84.45 | 84.38 |
| 9 | 20 | 50 | 10 | 241.65 | 241.84 | 79.13 | 79.04 | 87.23 | 87.27 |
| 10 | 20 | 70 | 10 | 170.66 | 170.47 | 83.74 | 83.71 | 77.23 | 77.11 |
| 11 | 20 | 50 | 20 | 204.28 | 204.47 | 77.21 | 77.24 | 92.87 | 92.99 |
| 12 | 20 | 70 | 20 | 169.76 | 169.57 | 86.75 | 86.84 | 83.97 | 83.92 |
| 13 | 20 | 60 | 15 | 183.25 | 183.67 | 81.67 | 81.67 | 86.98 | 86.46 |
| 14 | 20 | 60 | 15 | 183.65 | 183.67 | 82.04 | 81.67 | 86.76 | 86.46 |
| 15 | 20 | 60 | 15 | 184.89 | 183.67 | 81.12 | 81.67 | 86.02 | 86.46 |
| 16 | 20 | 60 | 15 | 184.02 | 183.67 | 81.56 | 81.67 | 86.12 | 86.46 |
| 17 | 20 | 60 | 15 | 182.54 | 183.67 | 81.98 | 81.67 | 86.45 | 86.46 |
4.1. Characterization
4.1.1. Vesicle characterization
The vesicle size, PDI, and zeta potential of the prepared AG-BIL were determined by size analyzer (Malvern zeta sizer, Malvern., USA). The sample of AG-BIL (0.1 ml) were diluted 100-fold and transferred into cuvette. The sample were scanned at 90 ° scattering angle at room temperature. The morphology of the selected AG-BIL was performed by using high resolution TEM. The sample was stained and evaluated under the electron microscope to evaluate the morphology.
4.1.2. Entrapment efficiency (EE)
The entrapped AG into BIL was estimated by ultracentrifugation method (Ameeduzzafar et al., 2014). The appropriate volume of BIL was taken into centrifugation tube and centrifuged at 18000 rpm for 30 min at 4 °C (Sigma 3-16KL, Osterodeam Harz, Germany). The supernatant was separated and AG concentration was analyzed by using UV–Vis-spectrophotometer (Genesys10S UV–Vis, Thermo Scientific, USA) at 269 nm with appropriate dilution. The study was performed in triplicate and % EE was calculated by below formula: -
4.1.3. Drug release
The release study of AG-BIL formulations were studied by dialysis bag method (Ameeduzzafar et al., 2020). The dialysis bag (mw ~ 12000, St Louis, MO63103, USA) was prepared for the study as per the standard procedure and then soaked with release media (simulated intestinal fluid, SIF). The appropriate volume (~5 mg of AG) was filled into dialysis bag and tied from both side and immersed into released media (900 ml) with tween 80 (0.3% v/v). The release media was rotated at 100 rpm and temperature was maintained at 37 ± 0.5 °C during the study. At predetermine time intervals (30 min, 1, 2, 4, 6, 8, 12 h) a fixed volume (5 ml) sample withdrawn and replaced with fresh release media. In similar experimental condition the release study of AG- dispersion was performed. The collected released sample at each time point was diluted, filtered and drug content was evaluated by UV spectrophotometer at 269 nm. The study was performed in triplicate and the data was fitted into different release kinetic models to assess the release mechanism.
4.1.4. Differential scanning calorimetry
Thermal analysis of AG, cholesterol (CHO), sodium deoxycholate and AG-BILopt were done by using differential scanning calorimetry (DSC, Mettler, Toledo, USA). The samples (4 mg) were placed into aluminium pan and scanned between 50 and 400 °C at heating rate of 10 °C/min under inert nitrogen atmosphere.
4.1.5. Permeation study
The permeation study of AG-BILopt and free AG dispersion was done on rat intestine to check the permeability enhancement. The rat intestine was collected and washed with water to remove the food contents. The test samples AG-BILopt and AG dispersion (~2 mg of AG) were filled into intestinal sac, tightly tied from both end and immersed in Krebs solution (500 ml with tween 80 (0.3% v/v)). The continuous supply of oxygen was done by using aerator and temperature was maintained at 37 ± 0.5 °C throughout the study. At predetermine time interval, 2 ml aliquots were withdrawn and replaced with same volume of fresh Krebs solution. The collected samples were filtered, diluted and drug concentration at each time point was analysed by reported HPLC method (Cai et al., 2006). The permeation flux and apparent permeability was calculated for both the sample to compare the enhancement.
5. Biological study
5.1. Animal handling
The comparative preclinical evaluation of AG-BILopt and free AG dispersion was done on Wistar male albino rat (200–250 gm). The study protocol was approved (04/02/41) by institutional animal ethical committee of Jouf University, Aljouf, Saudi Arabia. The animal was procured from animal house and kept in standard environmental condition (12 h dark/light cycle). The animals were fed with standard high rich fat diet contain normal pellet diet, cholesterol, casein protein, vitamin, coconut oil, sucrose, fructose, sodium chloride and dl- methionine for 15 days. The blood glucose level (BGL) of each animal was measured using glucometer (Accu-Check, Roche, Germany) at zero time and further after treatment with AG-BILopt and free AG dispersion of same dose. The animals were divided into four groups (group 1 considered as normal control (NC); group 2 taken as diabetic control (DC), group 3 and group 4 received Free AG dispersion and AG-BILopt. Each treatment group has six rats as shown in Table 4.
Table 4.
Analysis of variance of response surface quadratic model of each response.
| Model | Source | Vesicle size (nm) | Encapsulation efficiency (%) | Drug release (%) |
|---|---|---|---|---|
| Quadratic | Sum of Squares | 25402.66 | 650.94 | 373.006 |
| df | 9 | 9 | 9 | |
| Mean Square | 2822.518 | 72.32 | 41.44 | |
| F- Value | 5811.195 | 868.50 | 393.03 | |
| P-value, Prob > F | < 0.0001 | < 0.0001 | < 0.0001 | |
| Remark | Suggested, Significant | |||
| Lack of fit | ||||
| Quadratic | Sum of Squares | 0.33 | 0.035425 | 0.068 |
| df | 3 | 3 | 3 | |
| Mean Square | 0.11 | 0.011808 | 0.02 | |
| F- Value | 0.14 | 0.086268 | 0.13 | |
| P-value, Prob > F | 0.92 | 0.96 | 0.93 | |
| Remark | Suggested, not significant | |||
| Residual | ||||
| Quadratic | Sum of Squares | 3.399925 | 0.582945 | 0.73814 |
| df | 7 | 7 | 7 | |
| Mean Square | 0.485704 | 0.083278 | 0.105449 | |
5.2. Induction of diabetes
Streptozotocin (STZ) with high fat diet model was used for induction of hyperglycaemia (Type 2 diabetes) in rats. STZ (0.1 M) was freshly prepared in citrate buffer (pH 4.5) and administered single low dose (35 mg/kg) intraperitoneally (Guo et al., 2018, Ameeduzzafar et al., 2019). The animals were kept for 72 h to stabilized the BGL and after that the fasting BGL was measured. The animal having ≥ 200 mg/dl fasting BGL were marked as hyperglycaemic animals and considered for further experiments.
5.3. Pharmacokinetic study
The pharmacokinetic study of the prepared AG-BILopt and free AG dispersion was performed in Wistar albino rats. The AG-BILopt and free AG-dispersion (~60 mg/kg of AG) was administered into group 3 and group 4 animals (Ding et al., 2014). At predetermine time interval (0, 0.5, 1, 2, 6, 12, and 24 h) animals were anesthetized using diethyl ether inhaler and the blood was collected into EDTA tube. The blood was centrifuged at 5000 rpm for 15 min and plasma was separated. AG was extracted from the plasma by liquid phase extraction technique. Formic acid (2% v/v) was mixed with plasma and vortexed for 10 min. Then ethyl acetate (2 ml) was added, mixed and centrifuged for 15 min (3000 rpm) at 4 °C. The supernatant was collected and dried it under nitrogen evaporator. The sample reconstituted with methanol (500 µl), filtered by membrane filter (0.25 µm) and injected (20 µl) into HPLC system (Auto-sampler) for estimation of AG concentration. The different pharmacokinetic parameters i.e., Cmax, Tmax, T1/2, AUC0-t, AUC0-∞, Kel and AUMC0–24 was determined by using of software (Excel add-on PK solver).
5.4. Evaluation of hyperglycaemic activity
The prepared AG-BILopt and free AG-dispersion formulations were orally administered one time a day in appropriate dose (60 mg/kg) in the treatment animal group 3 and group 4 using oral feeding needle (Ding et al., 2014). At predetermined time interval one drop of blood was taken directly into glucometer strip from rat tail. After some time (5 s), BGL displayed on the screen of glucometer and noted. The reduction (%) BGL was calculated by given formula for both groups
5.5. Biochemical evaluation
The biochemical parameters were evaluated at the end of study. The collected blood sample was kept aside for some time and plasma was separated by centrifuging it at 3000 rpm for 15 min. The blood plasma was stored in refrigerator for the biochemical evaluation. The parametrs like lipid profile, urea, uric acid, serum total protein, creatinine, Serum glutamic–pyruvic transaminase, and serum glutamic oxaloacetic transaminase were evaluated using commercially available standard kits by PAP method.
5.6. Statistical analysis
The study was performed in triplicate and data was represented as mean with standard deviation. The graph pad prism was employed to determine the statistical calculation. The P value < 0.05 considered as statistically significant.
6. Result and discussion
6.1. Optimization
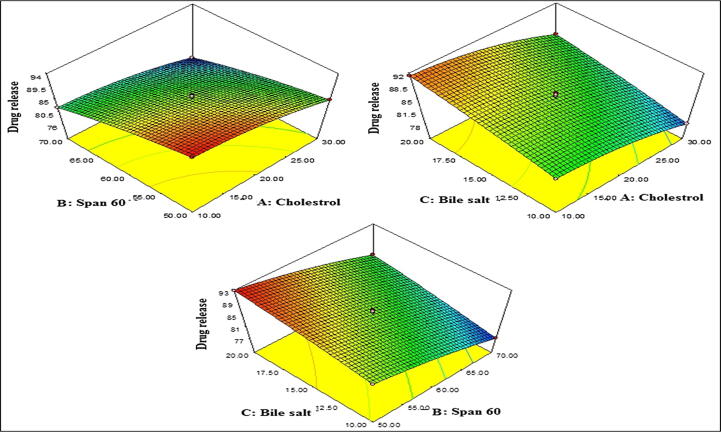
The design showed total seventeen experimental runs from BBD with five centre point (same composition) as represented in Table 2. These experimental results were fitted into the software for optimization. The vesicle size, entrapment efficiency and drug release of AG-BIL formulations were found in the range of 118.93–267.32 nm, 71.62–93.04 %, and 76.34–92.87 %, respectively. The effect of independent variable on dependent variable were assessed graphically i.e., 3D response surface plot (Fig. 1A, Fig. 1B, Fig. 1C, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7). It helps to express the effect of more than one variable over one response at one time. The results of each dependent variable were fitted into various experimental models to evaluate the best fit model. The best model was found to be quadratic model and the maximum regression value (R2) was found than other models. The actual (experimental) and predicted (software) value of each response was found to be very close to each other (Table 3). The effect was also expressed by mathematical polynomial equation and by numerical value of the actual vs predicted value graph (Fig. 2). The analysis of variance for each response was calculated and the value was found to be P < 0.0001 (Table 4).
Fig. 1A.
3D response surface plot showing effect of independent variable over the vesicle size.
Fig. 1B.
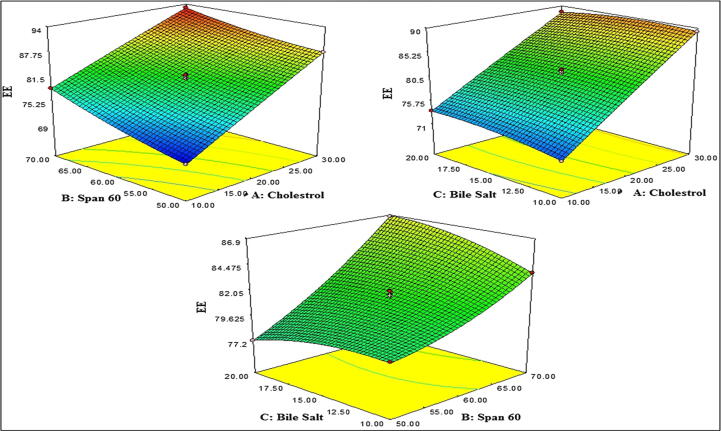
3D response surface plot showing effect of independent variable over the entrapment efficiency.
Fig. 1C.
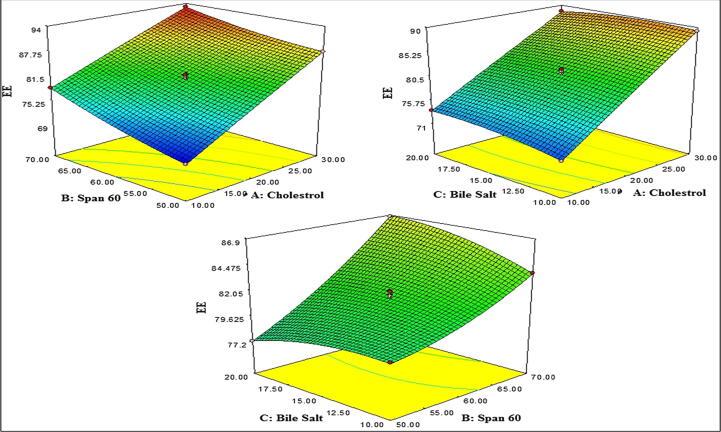
3D response surface plot showing effect of independent variable over the drug release.
Fig. 2.
Actual and predicted value graph of A) Vesicle size, B) Entrapment efficiency, C) Drug release (%).
Fig. 3.
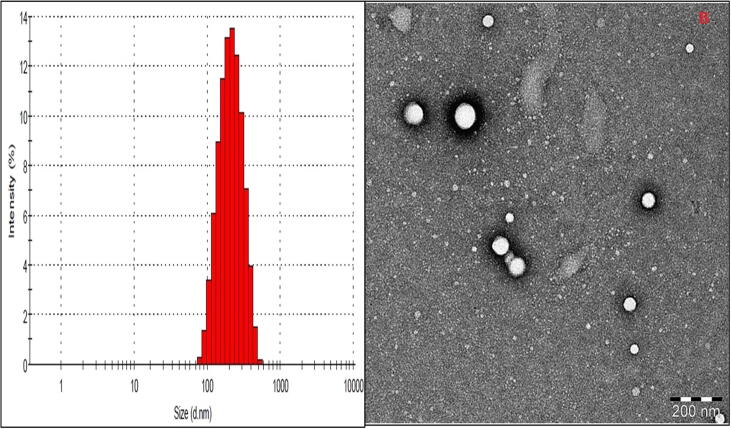
A) vesicle size distribution graph of AG-BIL-Opt, B) TEM image of AG-BIL-Opt.
Fig. 4.
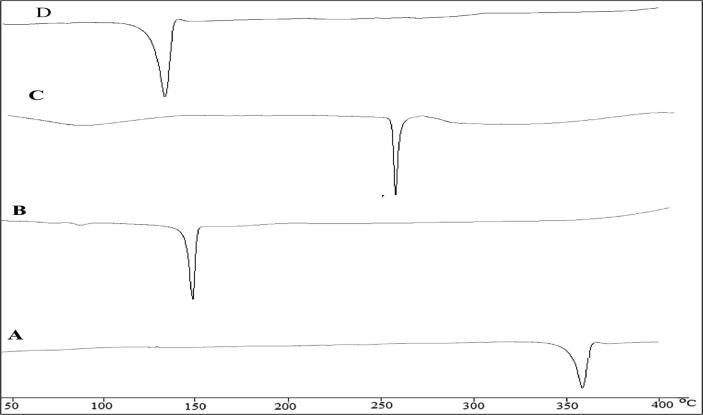
DSC Thermogram of A) Apigenin, B) Cholesterol, C) Sodium deoxycholate, D) optimized bilosome.
Fig. 5.
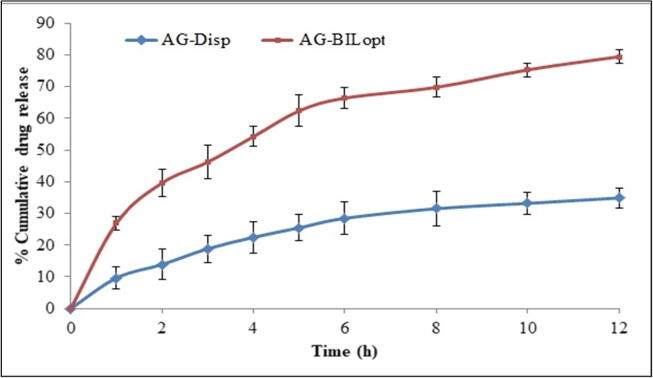
In-vitro drug release study of AG-BILopt and AG-dispersion.
Fig. 6.
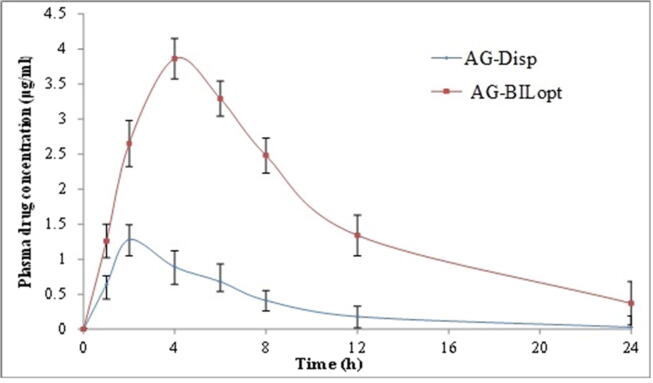
Plasma concentration Vs time graph of oral administration of AG-BILopt and AG-Dispersion in rats.
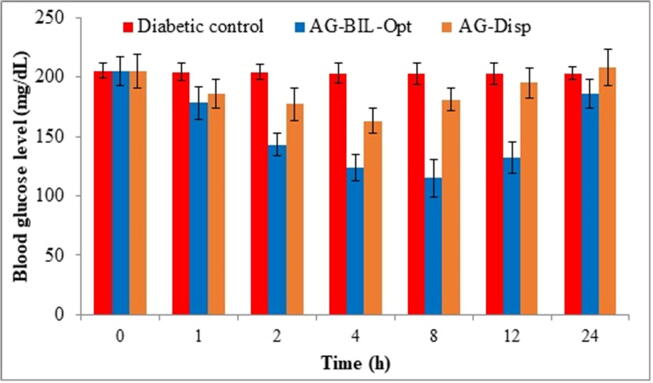
Fig. 7.
Mean fasting blood glucose level at different time point after treatment with AG-BILopt and AG-Dispersion.
Table 3.
Statistical summary of each model for all responses predicted from statistical design software.
|
Vesicle size (Y1) | ||||||
|---|---|---|---|---|---|---|
| Source (Model) | R2 | Adjusted R2 | Predicted R2 | Standard deviation | Coefficient of variance (%) | Remark |
| Linear | 0.9574 | 0.9476 | 0.9161 | 9.12 | --- | --- |
| 2FI | 0.9814 | 0.9703 | 0.9265 | 6.86 | --- | --- |
| Quadratic | 0.9999 | 0.9997 | 0.9996 | 0.69 | 0.37 | Suggested |
| Entrapment efficiency (Y2) | ||||||
| Linear | 0.9755 | 0.9698 | 0.9494 | 1.10 | --- | --- |
| 2FI | 0.9931 | 0.9889 | 0.9680 | 0.67 | --- | --- |
| Quadratic | 0.9991 | 0.9980 | 0.9978 | 0.28 | 0.35 | Suggested |
| Drug release (Y3) | ||||||
| Linear | 0.9613 | 0.9524 | 0.9395 | 1.05 | --- | --- |
| 2FI | 0.9644 | 0.9430 | 0.9019 | 1.15 | --- | --- |
| Quadratic | 0.9980 | 0.9954 | 0.9942 | 0.32 | 0.38 | Suggested |
6.2. Effect of cholesterol, span 60 and sodium deoxycholate on vesicle size
The vesicle size of all the prepared AG-BIL formulations were found in range of 118.93–267.32 nm (Table 2). The polynomial equation and 3D response surface graph (Fig. 1A) showed the effect of independent variables on the size. Cholesterol (CHO) exhibited positive effect on vesicle size. As the CHO concentration increases the vesicle size increases. CHO hindered the compact packing of lipid vesicle and subsequently higher aqueous phases inside the bilosome vesicle and lead to increase in vesicle size (Ameeduzzafar et al., 2020). The surfactant showed the negative effect on vesicle size. The increase in surfactant concentration led to reduction in interfacial tension between CHO and aqueous phase and size decreases. The third variable bile salt also exhibited the negative effect on vesicle size. As the bile salt concentration increases the vesicle size decreases due to reduced surface tension as well as flexibility of BIL. But at high concentration it tends to form aggregates itself (Yang et al., 2019a, Yang et al., 2019b). The computer-generated polynomial equation of quadratic model for vesicle size is given bellow (Eq. (1))
| (1) |
where the A, B, C, AB, AC, BC, A2, B2, and C2 are the significant model term (P < 0.05). The model F value was found to be 5811.20 suggested that model is significant (P < 0.0001). The F-value Lack of fit is 0.15, suggested that not significant expressed that model was well fitted. The predicted R2 of 0.9996 is in reasonable agreement with adjusted R2 of 0.9997 and the adequate precision was found to be > 4 (276.62), represented the model have adequate signal (Table 3). The actual and predicted value was expressed by graphical presentation (Fig. 2A).
6.3. Effect of cholesterol, span 60 and sodium deoxycholate on entrapment efficiency
The entrapment efficiency was found in range of 71.62–93.04 % (Table 2). The effect of independent variables on response was represented by 3D response surface graph (Fig. 1B). From the graph and below polynomial equation, it was observed that CHO (A) showed synergistic effect on entrapment efficiency, i.e., increased CHO concentration lead to increased entrapment efficiency. CHO has the capability to enhance the hydrophobicity, firmness of lipid bilayer membrane. It increases the permeability, stabilized the bilosomes and prevents the leakage of drug from bilosomes (Qumbar et al., 2017, Jain et al., 2014). Moreover, surfactant concentration (B) showed direct effect on entrapment efficiency. The high phase transition temperature of surfactant (span 60, 50 °C) as well as presence of long alkyl chain. The long alkyl chain of span 60 directly influence on entrapment efficiency, increases the solubility and permeability of AG in lipid bilayer thereby increases the entrapment efficiency (Ameeduzzafar et al., 2020). The bile salt (Sodium deoxycholate: SDC) exhibited synergistic effect on entrapment efficiency but less prominent effect than CHO and surfactant. Sodium deoxycholate concentration increases the entrapment efficiency increases because SDC have surface active property and incorporated into bilayer membrane surface, increases the flexibility of lipid membrane and increasing the solubility of drug in the lipid membrane hence increased the entrapment efficiency. The computer generated polynomial equation written below to interpret the results.
| (2) |
In this polynomial equation, the model term A, B, C, AB, AC, BC, B2, and C2 are significant (P < 0.05, i.e., significant effect on the entrapment efficiency) and A2 has shown non-significant model term (P > 0.05). The positive and negative sign of equation indicates the synergistic and antagonistic effect to the tested responses. The model F-value (868.6) implies that model is significant (P < 0.0001) and well fitted. The lack of fit value is very low (F = 0.9) and suggested that lack of fit is non-significant and as well as fitted for quadratic model. The predicted R2 of 0.9996 is in reasonable agreement with the adjusted R2. The adequate precision is 276.619 (<4), and represented the model has adequate signal. The actual and predicted value was expressed by graphical presentation (Fig. 2B).
6.4. Effect of cholesterol, span 60 and sodium deoxycholate on drug release
The drug release of all experimental runs (actual value) is 76.34–92.87% as shown in Table 2. The 3D and contour plot were generated and explained the effect of two factors on one response (Fig. 1C). CHO (A) exhibited negative effect on drug release, as the concentration of CHO (A) increases the drug release gradually decreases. The increases in CHO concentration, the vesicle wall become stiff and impede drug release from the vesicle. The second factor, surfactant (B) has shown the negative effect on drug release. At higher surfactant concentration, AG release from the BIL decreased due to the increased viscosity. The other reason for this type of release behaviour of AG into the dissolution media due to higher transition temperature and low HLB value of surfactant (span 60, B). The third factor bile salt (SDC, C) exhibited positive effect on drug release. The increases in bile salt concentration lead to increased drug release. On increasing the bile salt (SDC) concentration, it incorporated into core of BIL membrane and increases the flexibility. It gives increased release of AG from the formulation but at higher concentration it the drug release decrease.
The software generated polynomial equation of drug release was expressed by Eq. (3)
| (3) |
The polynomial equation showed the A, B, C AB, A2, and B2 are significant (P < 0.05) affected on drug releases and remaining AC, BC, and C2 are insignificantly (P > 0.05) effects on drug release. The positive and negative sign represent the synergistic and antagonistic effect on the drug release. The model F-Value of quadratic model for drug release was found to 393.04 suggested that model is significant (P < 0.05) and well fitted. The lack of fit is non-significant (P > 0.05) and represents ideal for the model. The predicted R2 (0.9943) is in reasonable agreement with adjusted R2 (0.9955). The adequate precision was found to 66.450 (>4) indicates adequate signal. The actual and predicted value difference was expressed by graphical (Fig. 2C).
Finally, the optimized formulation was selected form the point prediction method of the software. The optimized AG bilosomes (AG-BILopt) has shown the vesicle size of 183.25 ± 2.43 nm and encapsulation efficiency of 81.67 ± 3.87% using the composition CHO (15.5%), surfactant (span 60, 70.2%), bile salt (SDC, 12.4%) and used for further study.
6.5. Vesicle characterization
The vesicle size of prepared bilosomes were found in the range of 118.93–267.32 nm (Table 2). The optimized formulation (AG-BILopt) showed vesicle size of 183.25 ± 2.43 nm and graphically represented in Fig. 3A. PDI of AG-BILopt was found to be 0.42 (<0.5), and it indicates homogeneous distribution of bilosomes. The zeta potential value (-31.8 mV) of AG-BILopt indicates higher stability. TEM was used to evaluate the morphology of AG-BILopt and the sample exhibited spherical shape (Fig. 3B).
6.6. Entrapment efficiency (%)
The entrapment efficiency of prepared AG-BILs were determined by centrifugation method and result showed in the range of 71.62–93.04% (Table 2). The optimized formulation AG-BILopt has shown 81.67 ± 3.87% encapsulation efficiency.
6.7. Differential scanning calorimetry
DSC Thermogram of AG, CHO, sodium deoxycholate and AG-BILopt were analysed and depicted in Fig. 4A-D. The thermogram of AG, CHO, and sodium deoxycholate exhibited its characteristic peak at 356 °C, 150 °C and 260 °C, respectively (Fig. 4 A-C). The thermogram of AG-BILopt showed only one characteristic endothermic peak at 145 °C (Fig. 4D), which is closer to the melting point of CHO. There was no any other characteristic peak AG was found in thermogram, indicates that AG was completely encapsulated into lipid bilayer matrix.
6.8. In-vitro release study
The comparative release study of AG from the prepared AG-BILopt and free AG dispersion was expressed graphically in Fig. 5. AG-BILopt showed biphasic release behaviour, an initial burst release (39.58 ± 2.32% in 2 h) and later prolonged release (79.45 ± 3.18%) was found for 12 h. The burst release was found due to the release of adsorb AG from the BIL surface whereas, prolonged release is due to more affinity of AG toward the hydrophobic part of bilosomes (Jain et al., 2014). The free AG dispersion exhibited poor drug release of 25.47 ± 1.64% than AG-BILs formulation. The release data was fitted into different release kinetic model for determination of best fit model on the basis of maximum regression co-efficient value (r2). The release kinetic study showed Korsmeyer-Peppas model as best fit model because it showed highest value (r2 = 0.9538). The n value found to 0.54 (0.45 to 0.85) representing non– Fickian mechanism with dual release i.e., diffusion and swelling release (Wu et al., 2019).
6.9. Ex-vivo permeability study
The ex-vivo permeability of AG-BILopt and free AG dispersion was performed on rat intestine. The permeated amount of AG from AG-BILopt was found to be 83.23 ± 3.42 µg/cm2 which is significantly higher (p < 0.05) than free AG dispersion (18.76 ± 1.54 µg / cm2). The flux value from AG-BILopt was found to be 1.35 µg/cm2/h, and was 4.49 fold higher than free AG dispersion (0.31 µg/cm2/h). The APC of AG-BILopt and free AG dispersion was also calculated and found to be 1.08 × 10-4 cm/min and 2.41 × 10-5 cm/min, respectively. The higher permeation was acheived due to nanosized vesicle, high internalization in lipid matrix and also due to presence of nonionic surfactant. The presence of nonionic surfactant has the permeation enhacing capacity, it opens the tight jouction of intestine due to more hydrostatic pressure. The surfactant also inhibited the Pgp efflux pump and reduced the reticulo-endothelial uptake. It also lessens the formulation efflux and the bilosomes vesicles having the flexible property due to presence of bile salt (Gaba et al., 2015).
7. Biological study
7.1. Pharmacokinetic study
The pharmacokinetic study of AG-BILopt and free AG-dispersion was performed to evaluate bioavailability of AG. AG concentration vs time profile of AG-BILopt and free AG-dispersion was expressed in Fig. 6. AG-BILopt showed significantly higher (3.86 ± 0.26 µg/ml, P < 0.05) Cmax value than AG-dispersion (1.28 ± 0.11 µg/ml). The higher Cmax of AG-BILopt is due to the smaller size, high encapsulation, high solubility in GIT fluid, high permeability as well as avoids the first-pass metabolism. AUC0-24 and AUC0-∞ value of AG-BILopt treated rats showed 39.92 ± 2.65 (µg. h/ml) and 43.01 ± 2.15 (µg. h/ml). These values are found to be significantly (p < 0.05) higher (4.67 and 4.93 fold) than free AG-dispersion (AUC0-248.54 ± 0.98; AUC0-∞ 8.71 ± 1.04). Tmax of AG-BILopt was also found to be high (4 h) as compared to AG-dispersion (2 h). The AUMC0-t of AG-dispersion is 51.06 ± 1.54 µg.h2/ml, whereas AG-BILopt showed 321.01 ± 10.43 µg.h2 /ml. The formulation free AG-dispersion and AG-BILopt depicted AUMC0-∞ value of 56.01 ± 3.48 and 421.19 ± 5.64 µg.h2 /ml, respectively. The high value of AUC and Tmax is due to slow and prolonged release of AG which helps to absorb maximum drug amount. The elimination rate constant (h−1) of AG-BILopt and free AG-dispersion was found to be 0.1195 and 0.173. Half-life (h) was found to be 3.98 ± 0.25 for free AG-dispersion and 5.79 ± 0.23 AG-BILopt. The overall results indicate AG-BILopt enhances the relative bioavailability of ~ 4.67 fold as compare to free AG dispersion. The higher bioavailability was found due to the higher uptake of bilosomes by intestinal M−cell of peyer’s patch and also due to increased solubility in the presence of lipid and surfactant (Elnaggar, 2015).
7.2. Evaluation of hypoglycaemic activity
The antihyperglycaemic effect of AG-BILopt and free AG-dispersion were determined by evaluating average fasting blood glucose level (BGL) as depicted in Fig. 7. The normal and diabetic control group rats showed BGL 102 ± 5.9 mg/dl and 205 ± 7.2 mg/dl, respectively. The data showed a reduction of blood glucose level after 1 hr administration and the reduction was maintained up to 12 h for AG-BILopt and AG-dispersion showed up to 4 h. The highest blood glucose level reduction was found to be 40.49% (122 ± 4.7 mg/dL in 12 h). The AG-BILopt exhibited remarkable (P < 0.001**, P < 0.0001#) effect than free AG-Dispersion and diabetic control. After 12 h, blood glucose level gradually increases and reached up to 156 mg/dl. The free AG-dispersion showed an increase in BGL after 4 h and reached up to 198 ± 6.7 mg/dL in 24 h. The result of the study indicates AG-BILopt treated animals showed a significant reduction of blood glucose level for a longer time due to enhanced solubility.
7.3. Biochemical evaluation
At the end of the study, the various biochemical parameters (TG, TC, HDL-C, uric acid, urea, serum glutamic–pyruvic transaminase (SGPT), and serum glutamic oxaloacetic transaminase (SGOT) were evaluated. The biochemical parameters of normal control, diabetic control, treated AG-dispersion and AG-Bilopt was depicted in Table 5. The blood glucose level significantly changed (P < 0.0001) in STZ-induced DM than normal rats. The alteration in lipid profile causes cardiovascular complaints associated with type-2 DM (Kaur et al., 2013, Shaveta et al., 2020). The streptozotocin also causes liver toxicity due to alteration in serum glutamic–pyruvic transaminase and serum glutamic oxaloacetic. AG-BILopt and AG-dispersion treated group exhibited significant (P < 0.0001) reduction in elevated TC and TG as well as the decreased level of HDL-C as compared to diabetic control rats. The serum creatinine, uric acid, urea, serum glutamic–pyruvic transaminase and serum glutamic oxaloacetic transaminase was also decreased the elevated level (P < 0.0001) by AG-BILopt as compared to diabetic control rats. Also, AG-BILopt significantly decreased the elevated level of total protein in serum as compared to the diabetic control group.
Table 5.
Comparative estimated biochemical parameters result of different treated groups.
| Groups | TG (mg/dl) | TC (mg/dl) | HDL-C (mg/dl) | UA (mg/dl) | UA (mg/dl) | SGPT (U/L) | SGOT (U/L) |
|---|---|---|---|---|---|---|---|
| NC | 47.76 ± 0.87 | 64.62 ± 0.5 | 50.23 ± 0.6 | 35.72 ± 0.6 | 1.28 ± 0.2 | 28.24 ± 1.03 | 72.34 ± 2 0.1 |
| DC | 72.2 ± 1.1 | 103.76 ± 1.8 | 28.52 ± 1.6 | 62.76 ± 2.3 | 2.35 ± 0.4 | 51.25 ± 1.43 | 89.5 ± 2.3 |
| Free-AG-Dispersion | 57.8 ± 1.2 | 82.39 ± 0.7 | 37.59 ± 1.4 | 51.87 ± 1.5 | 1.87 ± 0.5 | 40.38 ± 1.13 | 80.04 ± 2.1 |
| AG-BILopt | 49.2 ± 1.4*** | 66.19 ± 0.9*** | 45.39 ± 1.8*** | 39.65 ± 1.4*** | 1.35 ± 0.4*** | 29.28 ± 1.03*** | 72.34 ± 1.3*** |
***P < 0.0001 as compared to diabetic control group, SGPT: Serum glutamic –pyruvic transaminase, SGOT:- Serum glutamic oxaloacetic transaminase, Uric acid:- UA, HDL-C:- High density lipoprotein- Cholesterol, Triglycerides: TG, Total cholesterol: TC.
8. Conclusion
In the present study, AG- bilosomes was successfully developed with the objective to enhance the oral efficacy for management of type-2 DM. The prepared AG-BILs have shown nanosize range, high entapment efficiency, spherical shape, and higher in-vitro drug release upto 12 h. The thermal analysis study revealed that AG was encapsulated in lipid matrix. The intestinal permeation study revealed that high amount of AG permeated (P < 0.05) than free AG-dispersion. Pharmacokinetic study showed AG-BILopt enhances the systemic bioavailability and residence time than free AG-dispersion. The pharmacodynamic study also revealed a significant (P < 0.05) enhancement in the hypoglyceamic activity and biochemical parameters than free AG. Our findings concluded that oral AG-BILs was found to be better treatment alternative for diabetes with improved therapeutic efficacy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors are very thankful to Jouf University, Saudi Arabia for providing facilites for present research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abcha I., Souilem S., Neves M.A., Wang Z., Nefatti M. Ethyl oleate food-grade O/W emulsions loaded with apigenin: Insights to their formulation characteristics and physico-chemical stability. Food. Res. Int. 2010;116:953–962. doi: 10.1016/j.foodres.2018.09.032. [DOI] [PubMed] [Google Scholar]
- Ahmad J., Singhal M., Amin S., Rizwanullah M., Akhter S. Bile Salt Stabilized Vesicles (Bilosomes): A Novel Nano-Pharmaceutical Design for Oral Delivery of Proteins and Peptides. Curr. Pharm. 2017;23:1575–1588. doi: 10.2174/1381612823666170124111142. [DOI] [PubMed] [Google Scholar]
- Al-Shaal L., Shegokar R., Muller R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011;420(1):133–140. doi: 10.1016/j.ijpharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Ameeduzzafar, Ali, J., Bhatnagar, A., Kumar, N., Ali, A., 2014. Chitosan nanoparticles amplify the ocular hypotensive effect of carteolol in rabbits. Int. J. Biolog. Macromol. 65, 479–491. [DOI] [PubMed]
- Ameeduzzafar, Alruwaili, N.K., Alotaibi, N.H., Imam, S.S., Alhakamy, N.A., et al., 2020. Formulation of chitosan polymeric vesicles of ciprofloxacin for ocular delivery: Box Behnken optimization, in-vitro characterization, HET-CAM irritation, and antimicrobial assessment. AAPS Pharm. Sci. Tech. 21, 5, 167. [DOI] [PubMed]
- Ameeduzzafar, El-Bagory, I., Alruwaili, N.K., Elkomy, M.H., Ahmad, J., et al., 2019. Development of novel dapagliflozin loaded solid self-nanoemulsifying oral delivery system: Physiochemical characterization and in vivo antidiabetic activity. J. Drug. Deliv. Sci. Technol. 54, 101279.
- Cai H., Raynaud D., Steward W.P., Gescher A.J. A simple HPLC method for the determination of apigenin in mouse tissues. Biomed. Chromatogr. 2006;20(10):1038–1042. doi: 10.1002/bmc.634. [DOI] [PubMed] [Google Scholar]
- Darabi P., Khazali H., Natanzi M.M. Therapeutic potentials of the natural plant flavonoid apigenin in polycystic ovary syndrome in rat model: via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecol. Endocrinol. 2020;36:582–587. doi: 10.1080/09513590.2019.1706084. [DOI] [PubMed] [Google Scholar]
- Deng F., Bae Y.H. Bile acid transporter-mediated oral drug delivery. J. Control. Release. 2020;327:100–116. doi: 10.1016/j.jconrel.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Zhang Z., Song J., Cheng X., Jiang J., Jia X. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014;9:2327–2333. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhennawy M.G., Lin H.S. Dose- and time-dependent pharmacokinetics of apigenin trimethyl ether. Eur. J. Pharm. Sci. 2018;118:96–102. doi: 10.1016/j.ejps.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Elnaggar Y.S. Multifaceted applications of bile salts in pharmacy: an emphasis on nanomedicine. Int. J. Nanomed. 2015;10:3955–3971. doi: 10.2147/IJN.S82558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freag, M,S., Saleh, W.M., Abdallah, O.Y., 2018. Self-assembled phospholipid based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int. J. Pharm. 535, 18–26. [DOI] [PubMed]
- Funakoshi-Tago M., Nakamura K., Tago K., Mashino T., Kasahara T. Anti-inflammatory activity of structurally related flavonoids, apigenin, luteolin and fisetin. Int. Immunopharmacol. 2011;11:1150–1159. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Gaba B., Fazil M., Ali A., Baboota S., Sahni J.K., Ali J. Nanostructured lipid (NLCs)carriers as a bioavailability enhancement tool for oral administration. Drug Deliv. 2015;22:691–700. doi: 10.3109/10717544.2014.898110. [DOI] [PubMed] [Google Scholar]
- Guo X.X., Wang Y., Wang K.B.P., Zhou J.F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B. 2018;19(7):559–569. doi: 10.1631/jzus.B1700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Kesarla R., Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013;2013 doi: 10.1155/2013/848043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zu Y., Zhao X., Wu M., Feng Z. Preparation of inclusion complex of apigenin-hydroxypropyl-β-cyclodextrin by using supercritical antisolvent process for dissolution and bioavailability enhancement. Int. J. Pharm. 2016;511(2):921–930. doi: 10.1016/j.ijpharm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Jain S., Harde H., Indulkar A., Agrawal A.K. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine. 2014;10(2):431–440. doi: 10.1016/j.nano.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Kaur R., Afzal M., Kazmi I., Ahamd I., Ahmed Z., Ali B., Ahmad S., Anwar F. Polypharmacy (herbal and synthetic drug combination): a novel approach in the treatment of type-2diabetes and its complications in rats. J. Nat. Med. 2013;67(3):662–671. doi: 10.1007/s11418-012-0720-5. [DOI] [PubMed] [Google Scholar]
- Khan, N., Ameeduzzafar, Khanna, K., Bhatnagar, A., Ahmad, F.J., Ali, A., 2018. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 30, 116, 648–663. [DOI] [PubMed]
- Lu. X., Mohamed, Y.Z., Waleed, Y., Anwar, U., Gehad, R.A., Yingqiu, Z., 2020. The Anticancer Potential of Apigenin Via Immunoregulation. Curr. Pharm. Des. 10.2174/1381612826666200713171137. [DOI] [PubMed]
- Mishra, A., Imam, S.S., Aqil, M., Ahad, A., Sultana, Y., et al., 2016. Carvedilol nano lipid carriers: formulation, characterization and in-vivo evaluation. Drug Deliv. 23(4)1486-94, [DOI] [PubMed]
- Naguib M.J., Kamel A.M., Negmeldin A.T., Elshafeey A.H., Elsayed I. Molecular docking and statistical optimization of taurocholate-stabilized galactose anchored bilosomes for the enhancement of sofosbuvir absorption and hepatic relative targeting efficiency. Drug. Deliv. 2020;27(1):996–1009. doi: 10.1080/10717544.2020.1787557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Lu Y., Hovgaard L., Guan P., Tan Y. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012;2:265–272. doi: 10.1016/j.ejpb.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Nurunnabi M., Khatun Z., Revuri V., Nafiujjaman M., Cha S. Design and strategies for bile acid mediated therapy and imaging. RSC Adv. 2016;6:73986–74002. [Google Scholar]
- Parashar P., Rana P., Dwivedi M., Saraf S.A. Dextrose modified bilosomes for peroral delivery: improved therapeutic potential and stability of silymarin in diethylnitrosamine-induced hepatic carcinoma in rats. J. Liposome. Res. 2019;29(3):251–263. doi: 10.1080/08982104.2018.1551408. [DOI] [PubMed] [Google Scholar]
- Paudel, A., Ameeduzzafar, Ahmad, F.J., Ali, A., 2017. Formulation and optimization of Candesartan Cilexetil Nano Lipid Carrier: in-vitro and in-vivo evaluation. Curr. Drug Deliv. 14, 7, 1005–1015. [DOI] [PubMed]
- Pauli G., Tang W.L., Li S.D. Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics. 2019;11:465. doi: 10.3390/pharmaceutics11090465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moral N., Saha S., Philo M., Hart D.J., Winterbone M.S. Comparative bio-accessibility, bioavailability and bioequivalence of quercetin, apigenin, glucoraphanin and carotenoids from freeze-dried vegetables incorporated into a baked snack versus minimally processed vegetables: Evidence from in vitro models and a human bioavailability study. J. Func. Foods. 2018;48:410–419. doi: 10.1016/j.jff.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qumbar, M., Ameeduzzafar, Imam. S.S., Ali, J., Ahmad, J., Ali, A., 2017. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In-vitro characterization and in-vivo activity. Biomed. Pharmacother. 93, 255–266. [DOI] [PubMed]
- Rizwanullah M., Amin S., Ahmad J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J. Drug Target. 2017;25:58–74. doi: 10.1080/1061186X.2016.1191080. [DOI] [PubMed] [Google Scholar]
- Saifi Z., Rizwanullah M., Mir S.R., Amin S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Tech. 2020;57 [Google Scholar]
- Shaveta, S., Singh, J, Afzal, M., Imam, S.S., Ameeduzzafar et al., 2020. Development of solid lipid nanoparticle as carrier of pioglitazone for amplification of oral efficacy: Formulation design optimization, in-vitro characterization and in-vivo biological evaluation. J. Drug. Deliv. Sci. Technol. 57, 101674.
- Sze L.P., Li H.Y., Lai K.L.A., Chow S.F., Li Q. Oral delivery of paclitaxel by polymeric micelles: A comparison of different block length on uptake, permeability and oral bioavailability. Colloids Surf. B. Biointerfaces. 2019;184 doi: 10.1016/j.colsurfb.2019.110554. [DOI] [PubMed] [Google Scholar]
- Telange D.R., Patil A.T., Pethe A.M., Fegade H., Anand S., Dave V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017;108:36–49. doi: 10.1016/j.ejps.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Wang W., Yue R.F., Jin Z., He L.M., Shen R. Efficiency comparison of apigenin-7-O-glucoside and trolox in antioxidative stress and anti-inflammatory properties. J. Pharm. Pharmacol. 2020 doi: 10.1111/jphp.13347. [DOI] [PubMed] [Google Scholar]
- WHO, Diabetes, [Internet]. Available from: https://www.who.int/news-room/factsheets/detail/diabetes.
- Wilkhu J.S., McNeil S.E., Anderson D.E., Perrie Y. Characterization and optimization of bilosomesfor oral vaccine delivery. J. Drug. Target. 2013;21:291–299. doi: 10.3109/1061186X.2012.747528. [DOI] [PubMed] [Google Scholar]
- Wu I.Y., Bala S., Basnet N.S., Cagno M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019;138 doi: 10.1016/j.ejps.2019.105026. [DOI] [PubMed] [Google Scholar]
- Yang H., Liu Z., Song Y., Hu C. Hyaluronic acid-functionalized bilosomes for targeted delivery of tripterine to inflamed area with enhancive therapy on arthritis. Drug. Deliv. 2019;26:820–830. doi: 10.1080/10717544.2019.1636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Liu Z., Song Y., Hu C. Hyaluronic acid-functionalized bilosomes for targeted delivery of tripterine to inflamed area with enhancive therapy on arthritis. Drug Deliv. 2019;1:820–830. doi: 10.1080/10717544.2019.1636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Gua S., Liu C., Yang C., Doua J., Li L., Zhai G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2013;429:24–30. [Google Scholar]
- Choi J.S., Islam M.N., Ali M.Y., Kim E.J., Kim Y.M., Jung H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer's disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]