Graphical abstract
Keywords: Bioassay, Fume, Galvanic, Metals, Microalgae, Particulate matter
Highlights
-
•
The toxicity of galvanic exhausts mostly depends on particle solubility rather than their number.
-
•
The emissions of the aluminum cleaning process were the most toxic for microalgae.
-
•
Electroplating exhaust samples caused microalgae membrane hyperpolarization.
-
•
Low concentrations of electroplating exhaust particles can have chronic toxicity.
-
•
Low concentrations can stimulate algal blooms in the environment.
Abstract
Electroplating is a widely used group of industrial processes that make a metal coating on a solid substrate. Our previous research studied the concentrations, characteristics, and chemical composition of nano- and microparticles emitted during different electroplating processes. The objective of this study was to evaluate the environmental toxicity of particulate matter obtained from five different electrochemical processes. We collected airborne particle samples formed during aluminum cleaning, aluminum etching, chemical degreasing, nonferrous metals etching, and nickel plating. The toxicity of the particles was evaluated by the standard microalgae growth rate inhibition test. Additionally, we evaluated membrane potential and cell size changes in the microalgae H. akashiwo and P. purpureum exposed to the obtained suspensions of electroplating particles. The findings of this research demonstrate that the aquatic toxicity of electroplating emissions significantly varies between different industrial processes and mostly depends on particle chemical composition and solubility rather than the number of insoluble particles. The sample from an aluminum cleaning workshop was significantly more toxic for both microalgae species compared to the other samples and demonstrated dose and time-dependent toxicity. The samples obtained during chemical degreasing and nonferrous metals etching processes induced depolarization of microalgal cell membranes, demonstrated the potential of chronic toxicity, and stimulated the growth rate of microalgae after 72 h of exposure. Moreover, the sample from a nonferrous metals etching workshop revealed hormetic dose-response toxicity in H. akashiwo, which can lead to harmful algal blooms in the environment.
1. Introduction
Electrochemical processes are widely used in making coatings for industry, electronics, decoration, etc. [[1], [2], [3]]. Recent studies have shown that particulate matter (PM) containing metal components even at low concentrations may cause adverse health effects [4,5]. Continuous occupational exposure to harmful substances used in electroplating production, such as nickel, hexavalent chromium, and other toxic metals, can cause serious health effects, including cancer, asthma, and other diseases of respiratory, cardiovascular, and musculoskeletal system [[6], [7], [8]]. In the environment, industrial PM accumulated in soil and water bodies can be a significant source of metallic contamination [9], which will lead to the degradation of ecosystems and the potential implementation of toxic components in trophic chains [10]. A known fact that low concentrations of PM, metals, metalloids, and ions can induce positive effects on algae and higher plants stimulating their growth [11], which can upset the balance of the environment. It is therefore important to evaluate the impact of emissions from the electroplating industry on the environment and human health.
The aerodynamic size is one of the most important parameters in determining the atmospheric lifetime of particles and their deposition onto soil or water [12,13]. PM has been classified by particle size as PM10 (particles ≤10 μm in diameter), also called coarse particles, PM2.5 (particles ≤2.5 μm in diameter), also called fine particles, and PM0.1 (particles ≤0.1 μm in diameter), also called ultrafine particles [13]. Our previous work has shown that the number of PM of median aerodynamic diameter less than 0.3 μm (PM0.3), also called quasi-ultrafine particles, is over 10,000 times larger than the number of PM10 particles in the air of industrial electroplating workshop [14]. This fraction of atmospheric particles has recently attracted scientists’ attention due to their abundance in the air of urban areas [15] and potential toxicity to human health [16,17]. The comprehensive study of Sicard et al. [18] demonstrated that despite the existing air quality standards and limitations, PM levels in urban areas of the EU widely exceeding the WHO limit values for the protection of human health.
Longer residence in the atmosphere allows fine, quasi-ultrafine, and ultrafine PM a large geographic distribution [19]. These particles can travel far beyond the working area of an electroplating production line, workshop, or protection zone of an enterprise. The smallest industrial particles have been found at considerable distances from manufacturing buildings [20]. The precipitation of industrial PM through rain, production of industrial wastewater, or electroplating sludge disposal can introduce toxic electroplating products into soil and water bodies [[21], [22], [23]]. Intensified human activities in coastal regions [24,25] lead to progressive degradation of coastal and marine ecosystems [26] and can facilitate the transfer of toxic metals and persistent organic pollutants through food chains to seafood, fish, and eventually to humans [27,28]. The regulation of the negative impact of pollutant emissions on the aquatic environment requires the consideration of standards for different types of pollutants.
The current US Environmental Protection Agency (EPA) regulation called National Emission Standards for Hazardous Air Pollutants (NESHAP) includes a category of Plating and Polishing Operations, which requires minimizing, control, and capture of exhaust emissions. The EU Industrial Emissions Directive (2010/75/EU, IED) requires an application and regular update of the best available techniques in its Surface Treatment of Metals and Plastics by Electrolytic and Chemical Processes. The existing standards and regulations are generally based on mass concentrations of PM, and do not take into account particle characteristics, composition, the toxicity of different components, or their combination [12] which may be crucial for electroplating emissions.
Several studies have shown that the smaller the size, the greater the solubility of PM is because of the increased surface-to-volume ratio [29,30]. Other studies have shown that most toxic metals accumulate in fractions of PM2.5 or less [[31], [32], [33]], and the solubility of trace metals from PM mostly depend on chemical composition rather than particle size [34]. In practice, the population and the environment are never exposed to one single chemical but are subject to constant multi-chemical exposure from many different sources, usually below regulatory limits, and may exert synergistic, combined, or competitive action [[35], [36], [37]]. Therefore, even small emissions of the electroplating industry present a potential risk to the environment and human health, because of the combined toxic action of multi-component pollution by PM and metals with different characteristics and compositions [[38], [39], [40]].
The wastes generated in different electroplating procedures vary in characteristics and possible environmental impact [41]. Electroplating includes different steps, such as degreasing, rinsing, etching, etc. [42]. Despite the existing body of research on galvanic wastewater treatment [[43], [44], [45]], there is little published data on the toxic properties of different electroplating aerosols [46,47]. Considering the possible release of industrial exhaust gases and the need for regulation development, the properties of galvanic aerosols required investigation in terms of environmental toxicity.
This study aims to assess the aquatic toxicity of airborne particles emitted by five common electrochemical processes, namely aluminum cleaning (AC), aluminum etching (AE), chemical degreasing (CDG), nonferrous metals etching (NME), and nickel plating (NP). PM toxicity was evaluated by the standard microalgae growth-rate inhibition test using flow cytometry. Moreover, we evaluated membrane potential and cell size changes in the marine microalgae Heterosigma akashiwo and Porphyridium purpureum exposed to PM emitted by five electroplating processes. This study examined the general differences in acute toxic effects of different electroplating emissions in microalgae cells. The establishing of chronic exposure was beyond the scope of this study. The findings of this work provide a basic level for further environmental risk assessment of electroplating emissions in real-life conditions, and they should make an important contribution to the field of the environmental regulation of the electroplating industry.
2. Materials and methods
2.1. Collection of electrochemical exhaust particles
To study the toxicity of airborne particles formed during different electrochemical processes, we collected samples in real workshop conditions. The chemical composition and quantitative distribution of aerosol particles having an average diameter between 0.3 and 10 μm in the air of an electroplating workshop are described in our previous work [14]. The sampling procedure was performed according to the previously reported method by [48,49]: 2.7-liter sterile plastic containers with distilled water were placed on the floor of the workshop during its operation. Before the experiment, the containers were thoroughly washed, namely one time with running water and two times with distilled water. After that, the containers were filled with 800 mL of distilled water obtained with the DE-4−02-EMO water distiller (Electromedoborudovanie, Russia).
The duration of sample collection was eight hours in line with the operating shift of the workshop. The containers were placed near the still baths and opened at the beginning of the work shift. At the end of the work shift, the containers were tightly closed, marked, and transported to the laboratory for further analyses, and were stored at room temperature for 24 h. The samples obtained during the operation of five electrochemical processes were allocated for the toxicity bioassay (Table 1).
Table 1.
Sampling information and particle characteristics.
| Coded name | Sampling point (electrochemical process) | Electrolyte composition | Chemical composition of the particlesa | Morphology of the particlesa |
|---|---|---|---|---|
| AC | Aluminum cleaning | HNO3 | high content of potassium compounds: Al, Ca, Na, Mg, Fe, Zn (chemical salts); Zn particles. | spheroidal and lamellar structure |
| AE | Aluminum etching | NaOH | high content of magnesium and potassium compounds: Al, Ca, Na, Cl, Fe with inclusions of Br and S. High content of Ba particles. | acicular |
| CDG | Chemical degreasing | Detergent Labomid 203 (Chempack, Russia) | compounds of Na, S, and K; Zn, Cu and Cr, oxides of Fe and Al, aluminosilicates, and NaCl. | agglomerates and drop-shaped clusters |
| NME | Nonferrous metals etching | HNO3; H2SO4; HCl | inclusions of Cu, Zn, Pb, Ni, Fe, Al, SnO, and PbS. | dendritic aggregates, spherical particles of tin (8–24 %) |
| NP | Nickel plating | NiSO4; MgSO4; Na2SO4; NaCl; H3BO3 | the bulk was represented by fakes of Na, S, Al, Cu, Na, and Cl; inclusions of Ni, Co, Cu, Zn, Ag, Fe, Cr, W, S. | polygons with relatively smooth surface |
Results of electron microscopy and energy dispersive X-ray analysis were reported in previous work [14].
The negative impact of aerosol particles on organisms and human health is often associated with particle size [50,51]. In this study, we only used quasi-ultrafine particles. To obtain the required size fractions, the samples were filtered through 0.45 μm Whatman PVDF syringe filters (Sigma-Aldrich, USA).
Additionally, we carried out particle size analysis of the filtered samples by a CytoFLEX flow cytometer (Beckman Coulter, USA) using the data of forward scattering of a violet laser (405 nm). The cytometer was calibrated with a mixture of fluorescent particles Megamix-Plus SSC and Megamix-Plus FSC (BioCytex, France). Each sample was measured in triplicate at a flow rate of 25 μL/min for 60 s. The registration settings, including acquisition mode, threshold, and cleaning between the samples, were performed according to [52], and flow rate was adjusted empirically to achieve stable acquisition.
2.2. Microalgae cultures
We assessed the toxicity and morphological and biochemical changes in microalgae cells with the microalgae growth-rate inhibition test. Two species of marine microalgae, namely a golden-brown algae Heterosigma akashiwo (Y.Hada) Y.Hada ex Y.Hara & M.Chihara, 1987. (Ochrophyta) and a red alga Porphyridium purpureum (Bory de Saint-Vincent) Drew et Ross, 1965 (Rhodophyta) originally isolated from the Peter the Great Bay (Sea of Japan, Far Eastern Russia) were provided by the Resource Collection Marine Biobank of the National Scientific Center of Marine Biology, Far Eastern Branch of the Russian Academy of Sciences (NSCMB FEB RAS). The microalgae model was chosen as a sensitive bioindicator [53], and as a crucial element of all aquatic trophic chains which is the main producer of organic matter in the aquatic environment [54]. The microalgae species were selected based on their abundance among microalgae in the Sea of Japan [55] and their suitability as test-organisms in ecotoxicology [56,57]. Their relevance as test-organisms in toxicity bioassay of welding fume particles has been previously confirmed [48].
Microalgae culturing and bioassay conditions were according to OECD No. 201 guidance [58], with minor modifications as previously described [59]. Microalgae cultures were cultivated in f/2 medium [60] under a temperature of 20 ± 2 °C, with 12:12 h light:dark irradiation cycle. For bioassays, microalgae cultures were used in the exponential growth phase.
2.3. Bioassays
Toxicity bioassays were performed in 24-well plates. Each well was filled with 1 mL of microalgal culture and 1 mL of sample suspension diluted by distilled water to obtain the final concentrations of 10, 25, and 50 % of the tested sample in microalgae culturing media. The same volume of distilled water was added to the control groups. All the experiments were performed in quadruplicate.
A CytoFLEX flow cytometer with CytExpert v.2.4 software package (Beckman Coulter, USA) was used to determine the number of microalgae cells and their morphological and biochemical changes. During the growth-rate inhibition measurement, dead cells were excluded from the count by propidium iodide (PI) staining, according to the standard procedure [61]. The membrane potential of microalgal cells was assessed by a lipophilic, positively charged fluorescent dye 3,3′-dihexyloxacarbocyanine iodide (DiOC6) [62]. The emission filters were selected according to the characteristics of the maximum emission of dyes provided by the manufacturer (Molecular Probes, USA). A blue laser (488 nm) of the CytoFLEX flow cytometer was chosen as the source of excitation light. To determine the size of microalgal cells, a size calibration kit (batch F13838, Molecular probes, USA) with a certified size distribution of 1, 2, 4, 6, 10, 15 μm was used for the FSC (forward scattering of a blue laser) channel. The toxicity bioassay endpoints used in this work and conditions for their registration are shown in Table 2. Each sample was measured at a flow rate of 100 μL/min for 30 s.
Table 2.
Toxicity bioassay endpoints and their registration conditions.
| Endpoint | Registration time, h | Biomarker | CytoFLEX emission filter, nm |
|---|---|---|---|
| Growth-rate inhibition | 24, 72 | PI | ECD, 610 |
| Membrane potential | 24, 72 | DIOC6 | FITC, 525 |
| Cell size | 72 | Forward scattering intensity | FSC |
To determine the optimal concentration of fluorescent dyes and the optimal duration of staining, a series of preliminary measurements were made for each microalgae species before the assessment of growth-inhibition and membrane potential of cells [59]. The exposure periods of 24 and 72 h were selected according to the standard methods commonly used to assess the toxicity of test substances and materials in aqueous systems with microalgae [63,64].
2.4. Statistical analysis
Statistical analyses were performed using the software package GraphPad Prism 8.0.2 (GraphPad Software, USA). Normality was checked using the Shapiro-Wilk test. The one-way ANOVA test was used for analysis. A value of р ≤ 0.05 was considered statistically significant.
3. Results
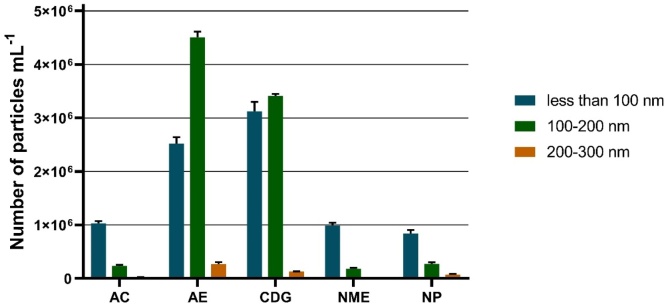
The results of particle size analysis performed by flow cytometer CytoFLEX are presented in Fig.1 as a number of particles per one mL in three different size ranges.
Fig. 1.
Particle size distribution in galvanic particle suspensions. AC, Aluminum cleaning; AE, Aluminum etching; CDG, Chemical degreasing; NME, Nonferrous metals etching; NP, Nickel plating.
Among the tested samples, a similarity in particle size distribution can be observed for AC, NME, and NP samples. The particles obtained from these galvanic processes had about one million particles of an average diameter less than 100 nm per one mL. Sample AE had 2.5 and 4.5 million particles per mL in the size ranges less than 100 nm and 100–200 nm, respectively. Sample CDG had 3.2 and 3.4 million particles per mL in the size ranges less than 100 nm and 100–200 nm, respectively. Moreover, samples AE and CDG had 2.8 and 1.3 thousand particles per mL in the size range of 200–300 nm, respectively.
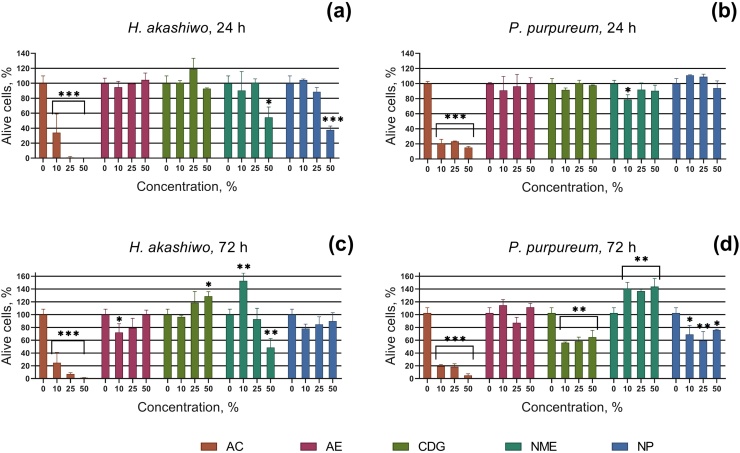
The changes in the growth rate of microalgae H. akashiwo and P. purpureum exposed to particle suspensions representing five electroplating processes are presented in Fig. 2. Statistical analysis demonstrated that all datasets have passed the Shapiro-Wilk normality test. The statistical significance of each treatment was computed by One-way ANOVA with multiple comparisons of the mean data of each treatment to the mean data of a control group.
Fig. 2.
Growth inhibition of microalgae H. akashiwo and P. purpureum exposed to galvanic particle suspensions. (a) H. akashiwo after 24 h of exposure, (b) P. purpureum after 24 h of exposure, (c) H. akashiwo after 72 h of exposure, (d) P. purpureum after 72 h of exposure. AC, Aluminum cleaning; AE, Aluminum etching; CDG, Chemical degreasing; NME, Nonferrous metals etching; NP, Nickel plating. * p < 0.05, ** p < 0.001, *** p < 0.0001.
The most significant growth rate inhibition and death of both microalgae species was caused by sample AC, obtained at the workshop of an aluminum cleaning process. At a concentration of 50 %, this sample caused the death of all H. akashiwo cells and about 80 % of P. purpureum cells after 24 h of exposure (Fig. 2a, b). The negative effect slightly increased over time between 24 and 72 h of exposure (Fig. 2c, d). For the red alga P. purpureum, only the AC sample caused a growth rate inhibition of more than 20 % after 24 h of exposure (Fig. 2b).
Samples AE and CDG had no significant effect on the growth rate of both microalgae species after 24 h of exposure (Fig. 2a, b). However, after 72 h of exposure, sample CDG stimulated the growth rate of H. akashiwo (Fig. 1c) and caused about 40 % growth rate inhibition in P. purpureum. Sample AE demonstrated did not show a dose-dependent growth rate inhibition for either microalgae species after 72 h of exposure (Fig. 2c, d).
Sample NME caused significant growth rate inhibition and death of H. akashiwo cells after 72 h of exposure at the highest used concentration. Interestingly, after 72 h of exposure to the low concentration (10 %) of this sample (Fig. 2c), the growth rate of H. akashiwo was stimulated up to 60 %, which indicates biphasic dose-response relationship. All concentrations of the NME sample stimulated the growth rate of red microalgae P. purpureum after 72 h of exposure (Fig. 2d).
Sample NP inhibited the growth rate of H. akashiwo at the highest concentration (about 60 %) after 24 h of exposure (Fig. 2a), and after 72 h the negative effect was not observed (Fig. 1c). On the contrary, this sample inhibited the growth rate of P. purpureum (about 40 %) only after 72 h of exposure (Fig. 2d).
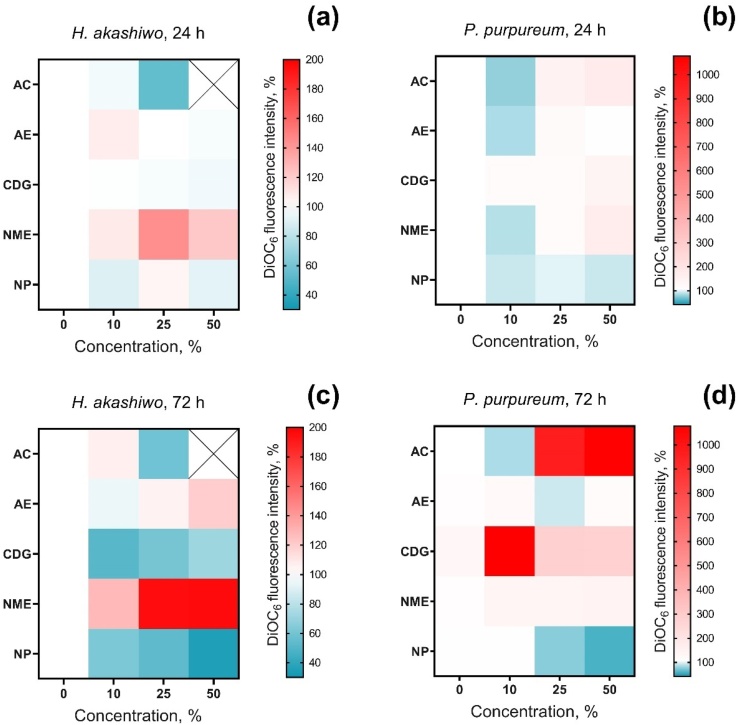
The membrane polarization changes in the cells of H. akashiwo and P. purpureum exposed to particle suspensions obtained from five electroplating workshops are presented in Fig. 3.
Fig. 3.
Changes of microalgae membrane potential after 24 h and 72 h of exposure to galvanic particle suspensions (a) H. akashiwo after 24 h of exposure, (b) P. purpureum after 24 h of exposure, (c) H. akashiwo after 72 h of exposure, (d) P. purpureum after 72 h of exposure. AC, Aluminum cleaning; AE, Aluminum etching; CDG, Chemical degreasing; NME, Nonferrous metals etching; NP, Nickel plating.
After 24 h of exposure, the CDG sample had almost no effect on membrane polarization of both microalgae species (Fig. 3a, b). NP demonstrated membrane depolarization at concentrations 10 and 50 % in both microalgae species after 24 h (Fig. 3a, b). AC, AE, and NME caused depolarization of membranes of the red microalgae P. purpureum only at the 10 % concentrations (Fig. 3b), but for H. akashiwo, AC caused the highest membrane depolarization at a concentration of 25 %, and NME caused membrane hyperpolarization after 24 h of the exposure (Fig. 3a). The influence of AC at the concentration of 50 % on membrane polarization of H. akashiwo (Fig. 3a) is not shown because almost 100 % of the cells were dead, according to the results of growth rate inhibition (Fig. 1a).
After 72 h of the exposure, almost all samples caused depolarization of H. akashiwo cell membranes (Fig. 3c). However, the membrane hyperpolarization of H. akashiwo under the influence of NME increased after 72 h compared to 24 h of exposure. Membrane hyperpolarization was also observed in H. akashiwo cells after 72 h of exposure to AE at the highest concentration. At the same time, the red microalgae P. purpureum responded with membrane hyperpolarization after 72 h of exposure to AC and CDG, and with dose-dependent depolarization after 72 h of exposure to NP (Fig. 3d). NME had no effect on membrane polarization of P. purpureum after 72 h of exposure.
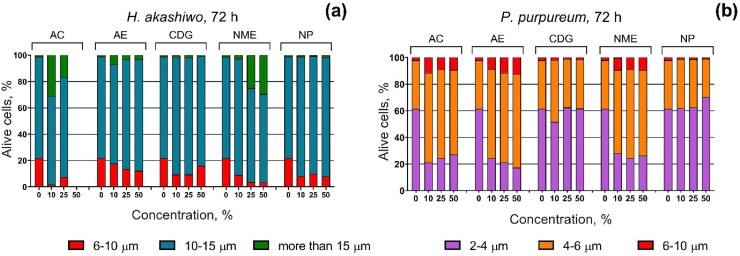
The changes in the size of H. akashiwo and P. purpureum cells after 72 h of exposure to galvanic particle suspensions are presented in Fig. 4.
Fig. 4.
Changes in microalgae cell size 72 h of exposure to galvanic particle suspensions (a) H. akashiwo after 72 h of exposure, (b) P. purpureum after 72 h of exposure. AC, Aluminum cleaning; AE, Aluminum etching; CDG, Chemical degreasing; NME, Nonferrous metals etching; NP, Nickel plating.
In the control group, H. akashiwo had 20 % of 6−10 μm cells and 80 % of 10−15 μm cells. All the tested samples caused enlargement of H. akashiwo cells after 72 h of exposure (Fig. 4a). The most pronounced effect was observed for the cells of H. akashiwo that were exposed to the AC and NME samples, where 17 % and 29 % of the cells, respectively, increased by more than 15 μm at the highest used concentration (the data of H. akashiwo exposed to AC at the concentration of 50 % are not shown, because almost all the cells were dead).
In the control group, the red alga P. purpureum had 62 % of 2−4 μm cells, 36 % of 4−6 μm cells, and 2% of 6−10 μm cells. The enlargement of the algal cells was evident after 72 h of exposure to the AC, AE, and NME samples (Fig. 4b). In the case of P. purpureum, the NP sample caused cell size mitigation at the highest used concentration, where 71 % of the cells were in the size range 2−4 μm (Fig. 4b).
4. Discussion
Aquatic toxicity studies are an important part of environmental risk assessment [59,65]. However, the results of galvanic particle size and quantity distribution in prepared suspensions (Fig. 1) are not correlated with the number of particles in the air near the same electrochemical processes compared to our previous study [14]. In that study, the quantity of PM0.3 particles measured by the AeroTrak Handheld Particle Counter 9306 (TSI Incorporated, USA) in the air near the five workshops studied in current work was in the following (descending) order: NP (8.4∙108) > AC (5.1∙108) > AE (5.0∙108) > CDG (4.5∙108) > NME (3.0∙108). However, the results from this study (Fig.1) showed that the highest number of particles of this size range were in the AE and CDG sample suspensions. This observation leads us to assume that ultrafine particles emitted from NP and AC processes tend to dissociate in water more than the particles from the other processes under study. Therefore, the higher amount of toxic and bioavailable metal ions from dissolved particles may be the reason behind the significantly higher toxicity of sample AC compared to the other samples (Fig. 2). Several studies [[66], [67], [68]] have demonstrated that apart from the chemical composition of particulate matter, the type of interaction between metals and organic compounds plays a significant role in their toxicity, which is synergistic at the lower concentrations of metals.
The influence of industrial particulate matter on membrane polarization and the size of microalgae cells is also often associated with the toxicity of metal-containing particles and released metal ions [69,70]. The increase in cell size of both microalgae species after 72 h of the exposure to samples AC, AE, and NME (Fig. 4) was most probably caused by increased intracellular and extracellular ROS production and further metabolic disorders in microalgal cells affected by toxic metals and metal ions. Thus, an increase in the number of large cells of both microalgae species indicates either the adaptation of microalgae to toxic influence or the disruption of metabolic processes associated with carbon sequestration and cell division [3].
Growth inhibition and change in cell size of microalgae should be assessed as an endpoint of direct cytotoxic action. At the same time, changes of membrane potential can indicate either the initial stage of toxic action or activation of the adaptive capacity of organisms [71,72]. Damage, deformation, and violation of the cell wall integrity are very dangerous for cell life. In animal cells, the intact cell membrane is a vital factor because the membranes accomplish the barrier, mechanical, and matrix properties of organisms [73,74]. Decreased membrane potential (depolarization) may be accompanied by changes in membrane elasticity, loss of lipid microdomains, and changes in ion permeability [75]. Increased polarization (hyperpolarization) of microalgal membranes can lead to membrane dysfunction, disruption of metabolic processes in cells, and subsequent cell death [73].
It should be noted that the H. akashiwo and P. purpureum cells responded differently to the galvanic suspensions with regards to the membrane polarization alterations (Fig. 3). Microalgal cell-wall is composed of lipids, polysaccharides, and glycoproteins, and can form different chemical bonds with metal-containing particulate matter depend on the nature of the metal ion and donor atoms in biomolecules [76]. Moreover, P. purpureum has a higher adherence to more hydrophobic components due to a mucous covering of the cells [77].
A stronger cell hyperpolarization of red microalgae P. purpureum (Fig. 3d) was caused by the most toxic sample AC, according to the growth rate inhibition data (Fig. 2). This indicates a significant time and dose-dependent toxicity of this sample, and the possibility of chronic toxicity, which may represent a risk for aquatic organisms even at relatively low concentrations after long-term exposure. The high membrane hyperpolarization of P. purpureum after 72 h of exposure to the low concentration of CDG sample (Fig. 3d) correlates with the growth rate inhibition data (Fig. 2d). Therefore, decreased toxicity and decreased cell hyperpolarization with increased concentration of sample CDG may indicate the activation of algal cell adaptation mechanisms. A similar effect was observed earlier for P. purpureum when exposed to metal-based nanoparticles [59]. For H. akashiwo, cell membrane hyperpolarization and, therefore, potential chronic toxicity can be highlighted for sample NME (Fig. 3c). Moreover, pronounced biphasic dose-response toxicity of this sample with a low dose stimulation and a high dose inhibition (Fig. 2c) indicates hormesis commonly observed in algae and higher plants [11,78]. The hormetic effect can lead to algal bloom and to further death of living organisms by the hypoxic aquatic condition and cyanotoxin poisoning [79,80]. This observation directly applicable to H. akashiwo which can form harmful blooms [81].
The observed toxic effects of electroplating emissions on microalgae confirm their serious threat to aquatic organisms. Long term low dose exposure of multi-component pollutants such as galvanic wastes combined with existing pollution sources can be dramatic for coastal and marine ecosystems. The fact that the effects of toxic stimuli combinations are not taken into account in setting regulatory exposure limits warrants a more detailed approach for further toxicity studies [82]. The application of a real-life risk simulation framework [[83], [84], [85]] is a good opportunity for further environmental toxicity assessment of electroplating exhausts with long-term low dose experiments.
5. Conclusions
In general, the toxicity of PM suspensions obtained in galvanic workshop significantly varies between different electroplating processes. The most hazardous effect and direct cytotoxicity in both microalgae species were caused by the sample collected during the aluminum cleaning process. The toxic effects of galvanic suspensions were expressed in a considerable growth rate inhibition and death of microalgae cells, hyperpolarization of cell membranes, and increase of microalgae cell size. Sample CDG collected during the chemical degreasing process demonstrated chronic toxicity potential at lower concentrations on the red algae P. purpureum and stimulated growth-rate of bloom-forming golden-brown algae H. akashiwo. Sample NME collected during nonferrous metals etching stimulated the growth-rate of H. akashiwo at low concentration and inhibited at high concentration, revealing the hormetic concentration-response relationship. Based on the results of the current study we can conclude that the toxicity of tested suspensions does not correlate with the number of insoluble PM and is associated with the influence of metal ions released in water, which can be a reason for high toxicity to aquatic organisms. These findings highlight the need for more stringent regulation of electroplating exhausts not only in terms of human health but also in the context of environmental safety. Therefore, further research with different test-organisms, a broader set of toxicity biomarkers, and the application of a real-life risk simulation approach should be performed to address the problem of galvanic exhaust environmental regulation. Further studies in this area will facilitate the regulation of emissions produced by the electroplating industry and prevent the negative impact on human health and the environment.
CRediT authorship contribution statement
Konstantin Pikula: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Writing - review & editing. Konstantin Kirichenko: Resources, Writing - original draft. Igor Vakhniuk: Resources, Writing - original draft. Olga-Ioanna Kalantzi: Writing - review & editing. Aleksei Kholodov: Resources, Writing - original draft. Tatiana Orlova: Resources. Zhanna Markina: Resources. Aristidis Tsatsakis: Supervision, Writing - review & editing. Kirill Golokhvast: Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The work was supported by the Russian Foundation for Basic Research (RFBR), project number 20-53-56041. The authors are grateful to the FEFU Collective Use Center for providing scientific equipment.
Edited by Dr. A.M Tsatsaka
References
- 1.Deepak J., Raja V.B., Kaliaraj G.S. Mechanical and corrosion behavior of Cu, Cr, Ni and Zn electroplating on corten A588 steel for scope for betterment in ambient construction applications. Results Phys. 2019;14 [Google Scholar]
- 2.Giurlani W., Zangari G., Gambinossi F., Passaponti M., Salvietti E., Di Benedetto F. Electroplating for decorative applications: recent trends in research and development. Coatings. 2018;8:260. [Google Scholar]
- 3.Launay H., Receveur-Bréchot V., Carrière F., Gontero B. Orchestration of algal metabolism by protein disorder. Arch. Biochem. Biophys. 2019;672 doi: 10.1016/j.abb.2019.108070. [DOI] [PubMed] [Google Scholar]
- 4.Li H., Wu H., Qg Wang, Yang M., Li F., Sun Y. Chemical partitioning of fine particle-bound metals on haze–fog and non-haze–fog days in Nanjing, China and its contribution to human health risks. Atmos. Res. 2017;183:142–150. [Google Scholar]
- 5.Onishi K., Otani S., Yoshida A., Mu H., Kurozawa Y. Adverse health effects of Asian dust particles and heavy metals in Japan. Asia Pacific J. Public Health. 2015;27:NP1719–NP1726. doi: 10.1177/1010539511428667. [DOI] [PubMed] [Google Scholar]
- 6.Deng Q., Deng L., Miao Y., Guo X., Li Y. Particle deposition in the human lung: health implications of particulate matter from different sources. Environ. Res. 2019;169:237–245. doi: 10.1016/j.envres.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Li H., Cai D., Li P., Jin J., Jiang X. Chronic oral exposure to cadmium causes liver inflammation by NLRP3 inflammasome activation in pubertal mice. Food Chem. Toxicol. 2021;148 doi: 10.1016/j.fct.2020.111944. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H., Reynolds M. Cadmium exposure in living organisms: a short review. Sci. Total Environ. 2019;678:761–767. doi: 10.1016/j.scitotenv.2019.04.395. [DOI] [PubMed] [Google Scholar]
- 9.Souza I.D.C., Morozesk M., Mansano A.S., Mendes V.A., Azevedo V.C., Matsumoto S.T. Atmospheric particulate matter from an industrial area as a source of metal nanoparticle contamination in aquatic ecosystems. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141976. [DOI] [PubMed] [Google Scholar]
- 10.Karimi M., Sadeghi R., Kokini J. Human exposure to nanoparticles through trophic transfer and the biosafety concerns that nanoparticle-contaminated foods pose to consumers. Trends Food Sci. Technol. 2018;75:129–145. [Google Scholar]
- 11.Agathokleous E., Feng Z., Iavicoli I., Calabrese E.J. The two faces of nanomaterials: a quantification of hormesis in algae and plants. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.105044. [DOI] [PubMed] [Google Scholar]
- 12.Geiger A., Cooper J. Environmental Protection Agency, Air Quality; Washington, DC, USA: 2010. Overview of Airborne Metals Regulations, Exposure Limits, Health Effects, and Contemporary Research. [Google Scholar]
- 13.Schraufnagel D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020;52:311–317. doi: 10.1038/s12276-020-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirichenko K.Y., Vakhniuk I., Ivanov V., Tarasenko I., Kosyanov D.Y., Medvedev S. Complex study of air pollution in electroplating workshop. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-67771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argyropoulos G., Besis A., Voutsa D., Samara C., Sowlat M.H., Hasheminassab S. Source apportionment of the redox activity of urban quasi-ultrafine particles (PM0. 49) in Thessaloniki following the increased biomass burning due to the economic crisis in Greece. Sci. Total Environ. 2016;568:124–136. doi: 10.1016/j.scitotenv.2016.05.217. [DOI] [PubMed] [Google Scholar]
- 16.Gao R., Sang N. Quasi-ultrafine particles promote cell metastasis via HMGB1-mediated cancer cell adhesion. Environ. Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113390. [DOI] [PubMed] [Google Scholar]
- 17.Ohlwein S., Kappeler R., Joss M.K., Künzli N., Hoffmann B. Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int. J. Public Health. 2019;64:547–559. doi: 10.1007/s00038-019-01202-7. [DOI] [PubMed] [Google Scholar]
- 18.Sicard P., Agathokleous E., De Marco A., Paoletti E., Calatayud V. Urban population exposure to air pollution in Europe over the last decades. Environ. Sci. Eur. 2021;33(1):1–12. doi: 10.1186/s12302-020-00450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon H.-S., Ryu M.H., Carlsten C. Ultrafine particles: unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020;52:318–328. doi: 10.1038/s12276-020-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golokhvast K.S., Shvedova A.A. Galvanic manufacturing in the cities of Russia: potential source of ambient nanoparticles. PLoS One. 2014:9. doi: 10.1371/journal.pone.0110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramteke P.W., Sagar A., Singh M. Assessment of wastewater toxicity by Vibrio fischeri bioassay. Int. J. Ecol. Environ. Sci. 2019;45:15–17. [Google Scholar]
- 22.Tseng C.-H., Lee I.-H., Chen Y.-C. Evaluation of hexavalent chromium concentration in water and its health risk with a system dynamics model. Sci. Total Environ. 2019;669:103–111. doi: 10.1016/j.scitotenv.2019.03.103. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Wu B., Gan Y., Chen Z., Zhang S. Sludge reduction and cost saving in removal of Cu (II)-EDTA from electroplating wastewater by introducing a low dose of acetylacetone into the Fe (III)/UV/NaOH process. J. Hazard. Mater. 2020;382 doi: 10.1016/j.jhazmat.2019.121107. [DOI] [PubMed] [Google Scholar]
- 24.Chen X., Qian W. Effect of marine environmental regulation on the industrial structure adjustment of manufacturing industry: an empirical analysis of China’s eleven coastal provinces. Mar. Policy. 2020;113 [Google Scholar]
- 25.Hu B., Zhao R., Chen S., Zhou Y., Jin B., Li Y. Heavy metal pollution delineation based on uncertainty in a coastal industrial city in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health. 2018;15:710. doi: 10.3390/ijerph15040710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y., Yuan J., Lu X., Su C., Zhang Y., Wang C. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environ. Pollut. 2018;239:670–680. doi: 10.1016/j.envpol.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Sun T., Wu H., Wang X., Ji C., Shan X., Li F. Evaluation on the biomagnification or biodilution of trace metals in global marine food webs by meta-analysis. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2019.113856. [DOI] [PubMed] [Google Scholar]
- 28.Tonhá M.S., Garnier J., Araújo D.F., Cunha B.C., Machado W., Dantas E. Behavior of metallurgical zinc contamination in coastal environments: a survey of Zn from electroplating wastes and partitioning in sediments. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140610. [DOI] [PubMed] [Google Scholar]
- 29.Jiang S.Y., Kaul D.S., Yang F., Sun L., Ning Z. Source apportionment and water solubility of metals in size segregated particles in urban environments. Sci. Total Environ. 2015;533:347–355. doi: 10.1016/j.scitotenv.2015.06.146. [DOI] [PubMed] [Google Scholar]
- 30.Jiang S.Y., Yang F., Chan K.L., Ning Z. Water solubility of metals in coarse PM and PM2. 5 in typical urban environment in Hong Kong. Atmos. Pollut. Res. 2014;5:236–244. [Google Scholar]
- 31.Sarti E., Pasti L., Rossi M., Ascanelli M., Pagnoni A., Trombini M. The composition of PM1 and PM2. 5 samples, metals and their water soluble fractions in the Bologna area (Italy) Atmos. Pollut. Res. 2015;6:708–718. [Google Scholar]
- 32.Wiseman C.L., Zereini F. Characterizing metal (loid) solubility in airborne PM10, PM2. 5 and PM1 in Frankfurt, Germany using simulated lung fluids. Atmos. Environ. 2014;89:282–289. [Google Scholar]
- 33.Frydas I.S., Kermenidou M., Tsave O., Salifoglou A., Sarigiannis D.A. Unraveling the blood transcriptome after real-life exposure of Wistar-rats to PM2.5, PM1 and water-soluble metals in the ambient air. Toxicol. Rep. 2020;7:1469–1479. doi: 10.1016/j.toxrep.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birmili W., Allen A.G., Bary F., Harrison R.M. Trace metal concentrations and water solubility in size-fractionated atmospheric particles and influence of road traffic. Environ. Sci. Technol. 2006;40:1144–1153. doi: 10.1021/es0486925. [DOI] [PubMed] [Google Scholar]
- 35.Tsatsakis A.M., Docea A.O., Tsitsimpikou C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36(6):554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 37.Sergievich A.A., Khoroshikh P.P., Artemenko A.F., Zakharenko A.M., Chaika V.V., Kodintsev V.V. Behavioral impacts of a mixture of six pesticides on rats. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138491. [DOI] [PubMed] [Google Scholar]
- 38.Fakhri Y., Bjørklund G., Bandpei A.M., Chirumbolo S., Keramati H., Pouya R.H. Concentrations of arsenic and lead in rice (Oryza sativa L.) in Iran: a systematic review and carcinogenic risk assessment. Food Chem. Toxicol. 2018;113:267–277. doi: 10.1016/j.fct.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Niu Y., Jiang X., Wang K., Xia J., Jiao W., Niu Y. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci. Total Environ. 2020;700 doi: 10.1016/j.scitotenv.2019.134509. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J., Du N., Li D., Qin J., Li H., Chen G. Combined effects of perchlorate and hexavalent chromium on the survival, growth and reproduction of Daphnia carinata. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2020.144676. [DOI] [PubMed] [Google Scholar]
- 41.Reis M.T.A., Ismael M.R.C. Electroplating wastes. Phys. Sci. Rev. 2018:3. [Google Scholar]
- 42.Burgess J. Electroplating onto aluminium and its alloys. Trans. IMF. 2019;97:285–288. [Google Scholar]
- 43.Sofińska-Chmiel W., Kołodyńska D. Application of ion exchangers for the purification of galvanic wastewater from heavy metals. Sep. Sci. Technol. 2018;53:1097–1106. [Google Scholar]
- 44.Xu H., Liu Y., Liang H., Gao C., Qin J., You L. Adsorption of Cr (VI) from aqueous solutions using novel activated carbon spheres derived from glucose and sodium dodecylbenzene sulfonate. Sci. Total Environ. 2021;759 doi: 10.1016/j.scitotenv.2020.143457. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J., Boada R., Cibin G., Palet C. Enhancement of selective adsorption of Cr species via modification of pine biomass. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143816. [DOI] [PubMed] [Google Scholar]
- 46.Malaeva E. Environmental problem in organization of works related to electroplating of parts. J. Econ. Soc. Sci. 2020 [Google Scholar]
- 47.Setyaningsih Y., Husodo A.H., Astuti I. Work environment factors and their influence on urinary chromium levels in informal electroplating workers. E3S Web of Conferences. 31. EDP Sciences. 2018:06007. [Google Scholar]
- 48.Kirichenko K., Zakharenko A., Pikula K., Chaika V., Markina Z., Orlova T. Dependence of welding fume particle toxicity on electrode type and current intensity assessed by microalgae growth inhibition test. Environ. Res. 2019;179 doi: 10.1016/j.envres.2019.108818. [DOI] [PubMed] [Google Scholar]
- 49.Kirichenko K.Y., Drozd V.A., Gridasov A.V., Kobylyakov S.P., Kholodov A., Chaika V.V. 3D-modeling of the distribution of welding aerosol nano-and microparticles in the working area. Nano Hybrid. Comp. 2017;13:232–238. Trans Tech Publ. [Google Scholar]
- 50.Haghani A., Johnson R., Safi N., Zhang H., Thorwald M., Mousavi A. Toxicity of urban air pollution particulate matter in developing and adult mouse brain: comparison of total and filter-eluted nanoparticles. Environ. Int. 2020;136 doi: 10.1016/j.envint.2020.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raftis J.B., Miller M.R. Nanoparticle translocation and multi-organ toxicity: a particularly small problem. Nano Today. 2019;26:8–12. doi: 10.1016/j.nantod.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brittain G.C., Chen Y.Q., Martinez E., Tang V.A., Renner T.M., Langlois M.-A. A novel semiconductor-based flow cytometer with enhanced light-scatter sensitivity for the analysis of biological nanoparticles. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-52366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng G., Lu L., Yang Y., Wei J., Han B., Zhang Q. Development of microfluidic dilution network-based system for lab-on-a-chip microalgal bioassays. Anal. Chem. 2018;90:13280–13289. doi: 10.1021/acs.analchem.8b02597. [DOI] [PubMed] [Google Scholar]
- 54.Falkowski P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosyn. Res. 1994;39:235–258. doi: 10.1007/BF00014586. [DOI] [PubMed] [Google Scholar]
- 55.Orlova T.Y., Stonik I., Shevchenko O. Flora of planktonic microalgae of Amursky Bay, Sea of Japan. Russ. J. Mar. Biol. 2009;35:60–78. [Google Scholar]
- 56.Pikula K.S., Chernyshev V.V., Zakharenko A.M., Chaika V.V., Waissi G., Hai L.H. Toxicity assessment of particulate matter emitted from different types of vehicles on marine microalgae. Environ. Res. 2019;179 doi: 10.1016/j.envres.2019.108785. [DOI] [PubMed] [Google Scholar]
- 57.Özhan K., Bargu S. Responses of sympatric Karenia brevis, Prorocentrum minimum, and Heterosigma akashiwo to the exposure of crude oil. Ecotoxicology. 2014;23:1387–1398. doi: 10.1007/s10646-014-1281-z. [DOI] [PubMed] [Google Scholar]
- 58.OECD . 2011. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. [Google Scholar]
- 59.Pikula K., Mintcheva N., Kulinich S.A., Zakharenko A., Markina Z., Chaika V. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species. Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109513. [DOI] [PubMed] [Google Scholar]
- 60.Guillard R.R., Ryther J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Q., Chen A.-N., Hu S.-X., Liu Q., Chen A.-N., Liu L. Microalgal microscale model for microalgal growth inhibition evaluation of marine natural products. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-28980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabnis R.W., Deligeorgiev T.G., Jachak M.N., Dalvi T.S. DiOC(6)(3): a useful dye for staining the endoplasmic reticulum. Biotech. Histochem. 1997;72:253–258. doi: 10.3109/10520299709082249. [DOI] [PubMed] [Google Scholar]
- 63.Grégori G., Denis M., Lefèvre D., Beker B. Advanced Flow Cytometry: Applications in Biological Research. Springer Netherlands; Dordrecht: 2003. A flow cytometric approach to assess phytoplankton respiration; pp. 99–106. [Google Scholar]
- 64.Prado R., Rioboo C., Herrero C., Cid Á. Screening acute cytotoxicity biomarkers using a microalga as test organism. Ecotoxicol. Environ. Saf. 2012;86:219–226. doi: 10.1016/j.ecoenv.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Hjorth R., Coutris C., Nguyen N.H., Sevcu A., Gallego-Urrea J.A., Baun A. Ecotoxicity testing and environmental risk assessment of iron nanomaterials for sub-surface remediation–recommendations from the FP7 project NanoRem. Chemosphere. 2017;182:525–531. doi: 10.1016/j.chemosphere.2017.05.060. [DOI] [PubMed] [Google Scholar]
- 66.Badran G., Ledoux F., Verdin A., Abbas I., Roumie M., Genevray P. Toxicity of fine and quasi-ultrafine particles: focus on the effects of organic extractable and non-extractable matter fractions. Chemosphere. 2020;243 doi: 10.1016/j.chemosphere.2019.125440. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Puthussery J.V., Yu H., Verma V. Synergistic and antagonistic interactions among organic and metallic components of the ambient particulate matter (PM) for the cytotoxicity measured by Chinese hamster ovary cells. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139511. [DOI] [PubMed] [Google Scholar]
- 68.Dai Y.L., Jiang Y.F., Lu Y.A., Yu J.B., Kang M.C., Jeon Y.J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021;8:349–358. doi: 10.1016/j.toxrep.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franklin N.M., Rogers N.J., Apte S.C., Batley G.E., Gadd G.E., Casey P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ. Sci. Technol. 2007;41:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 70.Hurtado-Gallego J., Pulido-Reyes G., González-Pleiter M., Salas G., Leganés F., Rosal R. Toxicity of superparamagnetic iron oxide nanoparticles to the microalga Chlamydomonas reinhardtii. Chemosphere. 2020;238 doi: 10.1016/j.chemosphere.2019.124562. [DOI] [PubMed] [Google Scholar]
- 71.Li J., Ou D.Y., Zheng L.L., Gan N.Q., Song L.R. Applicability of the fluorescein diacetate assay for metabolic activity measurement of Microcystis aeruginosa (Chroococcales, Cyanobacteria) Phycological Res. 2011;59:200–207. [Google Scholar]
- 72.Perry S.W., Norman J.P., Barbieri J., Brown E.B., Gelbard H.A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galotti A., Jiménez‐Gómez F., Parra G. Flow cytometry assessment of microalgae physiological alterations under CO2 injection. Cytom. Part A. 2020 doi: 10.1002/cyto.a.24028. [DOI] [PubMed] [Google Scholar]
- 74.Prado R., García R., Rioboo C., Herrero C., Cid Á. Suitability of cytotoxicity endpoints and test microalgal species to disclose the toxic effect of common aquatic pollutants. Ecotoxicol. Environ. Saf. 2015;114:117–125. doi: 10.1016/j.ecoenv.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 75.Melegari S.P., Perreault F., Costa R.H.R., Popovic R., Matias W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013;142:431–440. doi: 10.1016/j.aquatox.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 76.Kumar D., Pandey L.K., Gaur J. Metal sorption by algal biomass: from batch to continuous system. Algal Res. 2016;18:95–109. [Google Scholar]
- 77.Oakley B.R., Dodge J.D. The ultrastructure and cytochemistry of microbodies in Porphyridium. Protoplasma. 1974;80:233–244. doi: 10.1007/BF01666362. [DOI] [PubMed] [Google Scholar]
- 78.Calabrese E.J. Hormesis: a fundamental concept in biology. Microb. Cell. 2014;1(5):145. doi: 10.15698/mic2014.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen F., Xiao Z., Yue L., Wang J., Feng Y., Zhu X. Algae response to engineered nanoparticles: current understanding, mechanisms and implications. Environ. Sci. Nano. 2019;6(4):1026–1042. [Google Scholar]
- 80.Zhang Y., Calabrese E.J., Zhang J., Gao D., Qin M., Lin Z. A trigger mechanism of herbicides to phytoplankton blooms: from the standpoint of hormesis involving cytochrome b559, reactive oxygen species and nitric oxide. Water Res. 2020;173 doi: 10.1016/j.watres.2020.115584. [DOI] [PubMed] [Google Scholar]
- 81.Kempton J., Keppler C.J., Lewitus A., Shuler A., Wilde S. A novel Heterosigma akashiwo (Raphidophyceae) bloom extending from a South Carolina bay to offshore waters. Harmful Algae. 2008;7(2):235–240. [Google Scholar]
- 82.Kostoff R.N., Aschner M., Goumenou M., Tsatsakis A. Setting safer exposure limits for toxic substance combinations. Food Chem. Toxicol. 2020;140 doi: 10.1016/j.fct.2020.111346. [DOI] [PubMed] [Google Scholar]
- 83.Margina D., Nițulescu G.M., Ungurianu A., Mesnage R., Goumenou M., Sarigiannis D.A. Overview of the effects of chemical mixtures with endocrine disrupting activity in the context of real‑life risk simulation (RLRS): an integrative approach. World Acad. Sci. J. 2019;1:157–164. doi: 10.3892/wasj.2019.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsatsakis A., Tyshko N.V., Docea A.O., Shestakova S.I., Sidorova Y.S., Petrov N.A. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes–a real-life risk simulation approach. Toxicol. Lett. 2019;315:96–106. doi: 10.1016/j.toxlet.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 85.Hernández A.F., Docea A.O., Goumenou M., Sarigiannis D., Aschner M., Tsatsakis A. Application of novel technologies and mechanistic data for risk assessment under the real-life risk simulation (RLRS) approach. Food Chem. Toxicol. 2020;137 doi: 10.1016/j.fct.2020.111123. 111123-111123. [DOI] [PubMed] [Google Scholar]