Abstract
Background
Multiple studies over the past 4 decades have shown the significant benefit of breast cancer screening (BCS) in reducing mortality rates from breast cancer (BC). However, significant debate exists about the role of BCS in this regard, with some studies also showing no benefit in terms of mortality along with issues such as overdiagnosis, health care utilisation costs, psychological distress or overtreatment. To date, no BCS study has focused on disability. Hence the aim of this study is to evaluate the relative contribution of BCS approaches to age-standardized mortality and disability-adjusted life years (DALYs) rates along with other related risk factors, from a country-level perspective.
Patients and methods
This study created a country-dataset by merging information from the Global Burden of Disease study regarding female age-standardized BC mortality, DALYs rates and other risk factors with the BCS programme availability at the national or regional level (versus no or only pilot such programme), BCS type (mammography, digital screening, breast self-examination and clinical breast examination) and other BCS-related information among 130 countries. Mixed-effect multilevel regression models were run to examine the associations of interest.
Results
The most important factor predictive of lower mortality was the more advanced type of BCS programme availability [mammography: −4.16, 95% CI −6.76 to −1.55; digital mammography/ultrasound: −3.64, 95% CI −6.59 to −0.70] when compared with self- or clinical breast examinations. High levels of low-density lipoprotein cholesterol (LDL-c) and smoking were also related to higher mortality and DALYs from BC. In terms of BC DALYs, BCS had a 21.9 to 22.3-fold increase in the magnitude of effect compared with that in terms of mortality. Data on mortality and DALYs in relation to BCS programmes were also calculated for high-, middle- and low-income countries.
Conclusions
These data further support the positive effects of BCS in relation to age-standardized BC mortality rates, and for the first time show the impact of BCS on DALYs too. Additional factors, such as diabetes, high levels of LDL-c or smoking seemed to be related to BC mortality and disability, and could be considered as additional components of possible interventions to be used alongside BCS to optimize the BCS benefit on patients.
Key words: breast cancer, screening, mortality, disability, risk factors, global
Highlights
-
•
A key factor predictive of lower age-standardized BC mortality was breast cancer screening (BCS).
-
•
This was the case with national-level BCS programmes as well as the availability of different types of BCS.
-
•
LDL-c and smoking among others, were related to mortality and disability.
-
•
BCS with mammography or digital screening was related to less age-standardized BC disability.
-
•
These data support, for the first time, the relation of BCS on disability.
Introduction
Breast cancer (BC) carries a significant disease burden. In 2017, the incident rate of BC was reported to be 1.9 million. In the same period, BC was also the leading cause of cancer deaths and disability-adjusted life years (DALYs) in females with 601 000 deaths and 17.4 million life years, respectively.1 Positive evidence on the effectiveness of breast cancer screening (BCS) has been reported for nearly 4 decades. A 2020 systematic review of 60 cohort and case–control studies from Europe reaffirmed that BC mortality is reduced in all regions where formal screening programmes exist, and reductions between attenders and nonattenders of BCS programmes varied from 12% to 58% in different regions.2 A meta-analysis of 27 cohort studies also concluded that BCS programme invitations were linked to a 22% reduction in BC mortality.3 In a UK study, nearly 1 million women aged 49-64 were followed up for 15 years; invitation to the national screening programme was associated with a 21% reduction in BC mortality after adjustment for age, socioeconomic status and lead time.4 In the UK Age randomized trial, which involves screening women from the age of 40 or 41, yearly mammography before the age of 50 was associated with a 25% reduction in mortality at the 10-year follow-up.5 BCS can have an impact on other clinical outcomes as well. One study showed that mammographic screening was associated with higher chances of receiving less invasive surgery and shorter hospital stay.6 Another review of 17 trend studies in Europe also showed a 25%-31% reduction in BC mortality in those invited to screening, with a higher value (38%-48%) in those that were actually screened.7 Cost-effectiveness of BCS programmes has also been demonstrated.8,9 It is estimated that if all countries in Europe achieve full screening coverage, an additional 12 434 BC deaths could be prevented, translating to an additional 23% benefit.10
However, there remains significant and ongoing controversy surrounding the role of BCS in reducing BC mortality. A Cochrane systematic review of trials that included some 600 000 women showed that BCS was not effective in reducing all cancer mortality after 10 years and all-cause mortality after 13 years, although there was more breast surgery carried out and more use of radiotherapy in those screened.11 Overdiagnosis, anxiety, financial hardship, health care costs and a small risk of morbidity and mortality in those overdiagnosed, methodological shortcomings and limitations are observed in the early randomized trials and in case–control or cohort population studies; overtreatment, psychological distress from false-positive findings, problems with the criteria decided for evaluating screening effectiveness or unverified statistical assumptions in modelling work have been discussed elsewhere.11, 12, 13
Most data on BCS effectiveness are derived from the assessment of national screening programmes conducted in individual countries. Data from a global perspective would allow for a stronger argument beyond country differences. Finally, BCS effectiveness data have traditionally focused on mortality as an outcome. So far, there are no studies linking BCS to any impact on disability, which is also an important outcome to consider. Hence, this paper aims to evaluate the relative contribution of BCS approaches to age-standardized mortality and DALY rates alongside other related risk factors, from a country-level perspective. To contextualize the effect of risk factors, morbidity and BCS programme-related information with BC mortality and DALYs, we performed mixed-effect multilevel regression analyses of female age-standardized BC mortality and DALYs, while we also explored country-level income differences (high, middle and low income).
Methods
Breast cancer mortality and Global Burden of Disease measures
We used the results of Global Burden of Disease (GBD) 2017 to evaluate temporal and regional trends in age-standardized female BC mortality and DALY rates, to assess temporal patterns of risk factors in a country's income on a global and regional scale and also their relation to risk factors and comorbidities.14 Additional details on methods used to estimate mortality rates, years of life lost (YLLs), years lived with disability (YLDs) and DALYs including all other analytical approaches for assessment of relative morbidity and mortality from individual diseases and injuries are available in the GBD 2017 publications.14,15
Specifically, in the GBD, death due to BC is defined based on the International Classification of Disease (ICD) coding system (ICD-9 and ICD-10 codes)16 and BC-specific mortality is estimated using standardized modelling processes.16 DALYs are a summary measure of health that GBD study has calculated for each age–sex–year–state–cause strata by summing up the fatal (YLL) and nonfatal (YLD) components.15
The GBD study uses epidemiological data from systematic literature reviews, health surveys, and various other sources to generate cause- and each sequelae prevalence and incidence estimates. The study generated these estimates using a variety of modelling approaches, among which the Bayesian meta-regression compartmental modelling in DisMod-MR 2.1 was the most common.17 In addition, the GBD study derived and applied disability weights for each unique health state, as reported in previous publications.18,19 The study used a microsimulation framework for adjustment of comorbidities and calculated YLDs for each cause by multiplying prevalence and corresponding disability weights for each sequela of each cause.15 For the current study, the aforementioned methodology was used for the extraction of YLDs and DALYs causes, such as, cardiovascular diseases, diabetes and kidney diseases, and neoplasms.
Socioeconomic indicators, risk factors estimation and uncertainty levels
Each country's income level (classified as high, middle and low) was based on the World Bank's classification in 2018-19.20
The GBD 2017 comparative risk assessment classified all the risk factors and their respective risk factor clusters into one of three categories: behavioural, environmental/occupational or metabolic. Risk factor exposure levels data were assessed, and modelled using approaches similar to nonfatal models, with added emphasis on accurately fitting distributions of exposure among continuous and polytomous risk factors. Quantitative relative risk was estimated for each risk–outcome pair, and population-attributable fraction statistics calculated using standard GBD comparative risk assessment methods.21 Risk factors were expressed as summary exposure values (SEVs), which reflect the measure of a population's exposure to a risk factor taking into account the extent of exposure by risk level as well as the severity of that risk's contribution to disease burden. The SEV score varies from 0 to 1. A SEV score of ‘0’ indicates that no excess risk for a population exists, whereas a score of ‘1’ reflects the highest risk level. SEV is expressed on a scale from 0% to 100% to reflect the risk-weighted prevalence. We focused our risk factor analysis (see analysis below) on specific BC risk factors,22, 23, 24, 25, 26 including high low-density lipoprotein-cholesterol (LDL-c), smoking habits and high body mass index (BMI), as defined by the latest GBD 2017 methodology. Regarding uncertainty levels, the GBD study reports 95% uncertainty intervals that are derived from 1000 draws from the posterior distribution of each step in the estimation process.
Breast cancer screening information
BCS information, including among others the type of screening method applied [only breast self-examination (BSE) and/or clinical breast examination (CBE) versus BSE/CBE with mammographic screening availability versus BSE/CBE/mammographic with digital mammographic and/or ultrasound (US) breast screening availability] and whether there is no or only pilot or opportunistic BCS programme, or regional and national programme, was collected and cross-checked from a number of sources including the Global Health Observatory of the World Health Organization (WHO),27 the WHO cancer country profiles (https://www.who.int/cancer/country-profiles/en/), OECD Health Statistics 2020 data on BCS (http://www.oecd.org/health/health-data.htm), International Agency for Research on Cancer/WHO IARC Handbooks of Cancer Prevention,28 literature29, 30, 31 and internet searches on BCS for each country. Where information was incomplete or not available, WHO collaborating centres in a country were approached to clarify the data. Of the 194 countries that information was collected, 130 had complete data. The complete list is available in Supplementary File S1, available at https://doi.org/10.1016/j.esmoop.2021.100111.
Primary analysis
Descriptive statistics (boxplots) were used to show the trends of age-standardized BC mortality rates across countries, by country income level and by BCS type of screening, between 1990 and 2017.
Secondary analysis and trends assessment
Linear regression mixed-model analysis
Mixed-effect multilevel regression models based on the literature32 were carried out to assess whether female age-adjusted BC mortality and DALYs rates (as outcomes) were associated with the presence of BCS screening programmes at regional and national levels and the type of BCS tests applied in each country (independent variables), after adjustment for various confounders (i.e., smoking habits and others). The analysis was also repeated by country-income level. Maximum likelihood estimation was used in the multilevel analysis, where country-year data were considered the first level of analysis, and the repeated measures of countries as aggregated data were considered as the second level. Linear mixed-model analyses were performed with the R package lme4.
Ethical approval and consent to participate were not required as this is an analysis of a secondary publicly available data.
Results
Mortality from breast cancer
Among all variables assessed as potentially predictive of mortality, by far the most important factor was breast screening examination, where the availability of mammography in addition to SBE/CBE (as opposed to only SBE or CBE examination) was linked to a lower mortality rate [−4.16, 95% confidence interval (CI) −6.76 to −1.55]. Active smoking was also linked to BC mortality as well as high LDL-c, diabetes and cardiovascular diseases. Interestingly, BMI was inversely related to age-standardized BC mortality. Details on the predictor variables in relation to age-standardized BC mortality are presented in Table 1.
Table 1.
Age-standardized female breast cancer mortality in relation to bioclinical factors and breast cancer screening programmes
| Coefficient | 95% CI | |
|---|---|---|
| Cardiovascular diseases | 0.025 | 0.02 to 0.03 |
| Diabetes and kidney diseases | 0.013 | 0.01 to 0.02 |
| Neoplasms | 0.04 | 0.02 to 0.05 |
| High LDL-c | 0.63 | 0.48 to 0.78 |
| Smoking | 1.55 | 1.34 to 1.75 |
| Secondary smoke | −0.02 | −0.05 to 0.01 |
| High BMI | −0.13 | −0.21 to −0.06 |
| Only SBE/CBE tests | Reference | |
| MM and/or SBE/CBE tests | −4.16 | −6.76 to −1.55 |
| DMM/US and/or the previous tests | −3.64 | −6.59 to −0.70 |
| No country/pilot screening programmea | Reference | |
| Regional-wise screening programme | −1.36 | −4.89 to 2.18 |
| National-wise screening programme | −4.41 | −8.14 to −0.68 |
Cardiovascular diseases, diabetes and kidney disease, and neoplasms are expressed as age-standardized years lived with disability. Neoplasms estimates exclude breast cancer. High LDL-c, smoking, secondary smoke and high BMI are expressed as age-standardized summary exposure values (SEVs; range 0-100).
95 CIs, 95% confidence intervals; BMI, body mass index; CBE, clinical breast examination; DMM, digital mammography; DMM/US, digital mammography and/or ultrasound; LDL-c, low-density lipoprotein-cholesterol; MM, mammography; SBE, self-breast examination; SBE/CBE tests, self-breast and/or clinical breast examination; SEV, summary exposure value; US, ultrasound.
No country programme or existence of an opportunistic or pilot screening programme.
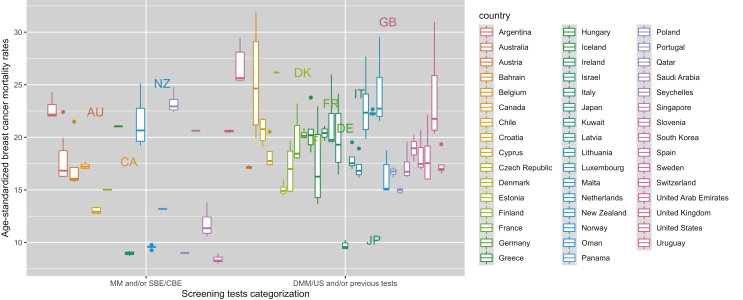
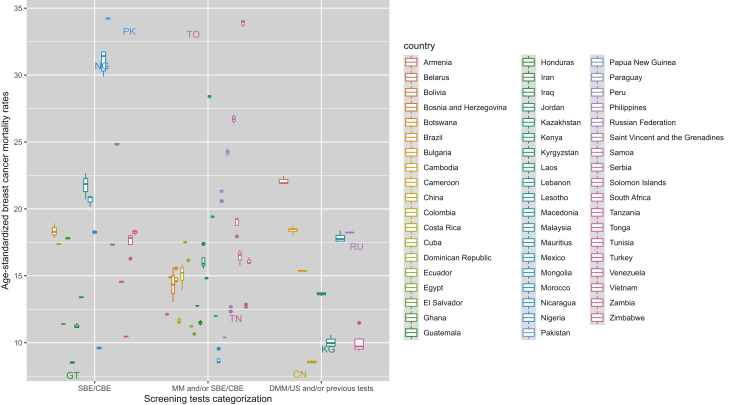
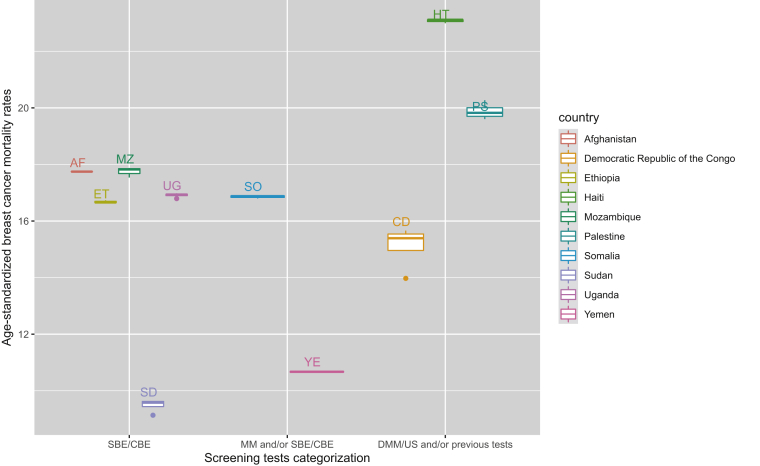
The addition of digital mammography and/or the use of US on top of the rest of screening tests in comparison with only SBE and/or CBE examinations was related to a similar reduction in age-standardized mortality (−3.64, 95% CI −6.59 to −0.70). Furthermore, only the presence of a national screening programme in comparison with no or pilot/opportunistic programme was related to less BC mortality rates (−4.41, 95% CI −8.14 to −0.68; Table 1). This BCS impact on mortality (BSE/CBE versus mammography and/or BSE/CBE versus digital screening tests and/or the previous tests) is further illustrated by country, among high-income countries, middle-income countries and low-income countries, showing also some variability in the mortality outcomes from one country to another (Figure 1, Figure 2, Figure 3).
Figure 1.
Age-standardized female breast cancer mortality by type of screening test among high-income countries/regions, 1990-2017.
CBE, clinical breast examination; DMM, digital mammography; DMM/US, digital mammography and/or ultrasound; MM, mammography; SBE, self-breast examination; SBE/CBE tests, self-breast and/or clinical breast examination; US, ultrasound.
Figure 2.
Age-standardized breast female cancer mortality by type of screening test among middle-income countries/regions, 1990-2017.
CBE, clinical breast examination; DMM, digital mammography; DMM/US, digital mammography and/or ultrasound; MM, mammography; SBE, self-breast examination; SBE/CBE tests, self-breast and/or clinical breast examination; US, ultrasound.
Figure 3.
Age-standardized breast female cancer mortality by type of screening test and among low-income countries/regions, 1990-2017.
CBE, clinical breast examination; DMM, digital mammography; DMM/US, digital mammography and/or ultrasound; MM, mammography; SBE, self-breast examination; SBE/CBE tests, self-breast and/or clinical breast examination; US, ultrasound.
Breast cancer disability adjusted life years
When the analysis was conducted on the same variables in relation to age-standardized BC disability, the findings, although overall similar with the mortality ones, changed in magnitude. The availability of BCS examinations with mammography as well as with digital mammography and US (when compared with only SBE/CBE tests) decreased DALYs by 21.9- to 22.3-fold, compared with the mortality data. However, availability of national or regional BCS programmes was not linked to age-standardized BC disability. In addition, other factors positively predicting age-standardized BC DALYs were actual smoking habits, high LDL-c, high BMI and diabetes, whereas secondary smoking appeared to be inversely related with DALYs. Details on the predictor variables in relation to BC DALYs are presented in Table 2.
Table 2.
Age-standardized female breast cancer disability (DALYs) in relation to bioclinical factors and breast cancer screening programmes
| Coefficient | 95% CI | |
|---|---|---|
| Cardiovascular diseases | 0.004 | −0.00 to 0.01 |
| Diabetes and kidney diseases | 0.07 | 0.06 to 0.08 |
| Neoplasms | 0.08 | 0.06 to 0.10 |
| High LDL-c | 23.72 | 19.58 to 27.87 |
| Smoking | 42.94 | 37.22 to 48.67 |
| Secondary smoke | −0.89 | −1.71 to −0.07 |
| High BMI | 2.81 | 0.62 to 5.00 |
| Only SBE/CBE tests | Reference | |
| MM and/or SBE/CBE tests | −91.27 | −158.17 to −24.37 |
| DMM/US and/or the previous tests | −81.31 | −156.06 to −6.56 |
| No country/pilot screening programmea | Reference | |
| Regional-wise screening programme | −51.37 | −141.65 to 38.92 |
| National-wise screening programme | −91.15 | −186.32 to 4.02 |
Cardiovascular diseases, diabetes and kidney disease, and neoplasms are expressed as age-standardized disability adjusted years of life. Neoplasms estimates exclude breast cancer. High LDL-c, smoking, secondary smoke and high BMI are expressed as age-standardized summary exposure values (SEVs; range 0-100).
95 CIs, 95% confidence intervals; BMI, body mass index; CBE, clinical breast examination; DMM, digital mammography; DMM/US, digital mammography and/or ultrasound; LDL-c, low-density lipoprotein-cholesterol; MM, mammography; SBE, self-breast examination; SBE/CBE tests, self-breast and/or clinical breast examination; SEV, summary exposure value; US, ultrasound.
No country programme or existence of an opportunistic or pilot screening programme.
The aforementioned analysis was further stratified by country income level to explore different patterns among the BCS cancer programmes. A significant effect between the implementation of national programmes in comparison with no or only pilot/opportunistic programmes was noted only among high-income regions in terms of age-standardized BC mortality and DALYs rates. The relationship between age-standardized female breast cancer mortality and disability and breast cancer screening programmes by country-income levels is shown in Table 3.
Table 3.
Mixed-effect multilevel regression to assess the relationship between age-standardized female breast cancer mortality and disability and breast cancer screening (BCS) programmes, by country-income levels (high-, middle-, low-)
| Coefficient |
95% CI |
Coefficient |
95% CI |
Coefficient |
95% CI |
|
|---|---|---|---|---|---|---|
| HICs | MICs | LICs | ||||
| Mortality | ||||||
| No country/pilot screening programmea | Reference | |||||
| Regional-wise screening programme | −7.81 | −13.39 to −2.23 | −1.49 | −5.86 to 2.88 | 3.85 | −2.48 to 10.17 |
| National-wise screening programme | −9.71 | −14.70 to −4.72 | −2.90 | −7.77 to 1.97 | −0.66 | −7.52 to 6.20 |
| Disability (DALYs) | ||||||
| No country/pilot screening programmea | Reference | |||||
| Regional-wise screening programme | −184.50 | −315.47 to −53.54 | −55.72 | −184.06 to 72.62 | 78.07 | −55.04 to 211.17 |
| National-wise screening programme | −245.12 | −362.28 to −127.96 | −53.76 | −195.55 to 88.03 | 49.58 | −94.56 to 193.73 |
Models are equally adjusted as previous tables.
95 CIs, 95% confidence intervals; DALYs, disability-adjusted life years; HICs, high-income countries; LICs, low-income countries; MICs, middle-income countries.
No country programme or existence of an opportunistic or pilot screening programme.
Discussion
This study reaffirms the role of availability of mammographic (digital or conventional) and US BCS tests in relation to mortality from BC, also highlighting the effect of risk factors and morbidities such as smoking, diabetes and high LDL-c in shaping mortality and disability in BC. Areas with established national BCS programmes reported lower age-standardized BC mortality. Furthermore, this study shows for the first time the immense impact of types of BCS tests in relation to age-standardized disability.
This study examined BC mortality in relation to BCS programs availability providing stronger evidence of its effect from an international perspective compared with single-country and specific health care system-based data as per most of the related studies published previously. Despite the acknowledged shortcomings of various BCS studies in the past,11,13 our data further support the notion that BCS (either through mammography or digital mammography and US when added to self- and clinical examinations) does have a strong contribution to decreases in BC mortality. Digital mammography and/or US examinations when available with other BCS examinations showed also reductions in BC mortality. It was also shown that the implementation of national-only BCS examination programmes was related to reductions in BC mortality when compared with no or pilot BCS programmes. The current country-based data showed also the association of other risk factors and comorbidities with BC mortality, such as of diabetes (possibly through the pathway of fasting plasma glucose), cardiovascular diseases and high LDL-c, in line with previous studies.33, 34, 35 As expected, active smoking was related to increased BC mortality. Interestingly, high BMI levels were inversely related to BC mortality, a result that is supported by a recent study on the role of the adipose tissue in premenopausal BC,36 along other studies showing no significance of high BMI in BC.37 Considering the role of lifestyle factors in BC mortality, it may be an opportune moment during BCS to focus also on interventions to decrease smoking as well as to control diabetes and cholesterol levels. With significant improvements in treatments for BC, it is further argued that a combination of BCS and more effective treatments is the ‘winning weapon’ in decreasing BC mortality.38
The data we present on disability are new. Some of the factors that are linked to mortality and disease burden in previous studies, such as active smoking or high LDL-c,34,39 were also associated with significant impact on age-adjusted DALYs in this study. Although not expected, secondary smoking was inversely related to BC disability. However, mixed results have been reported in the literature regarding secondary smoking and BC, indicating the necessity for further research on this topic.40 Besides these factors, the more advanced BCS tests available in a country, the more the benefit related to age-standardized BC disability. The impact of BCS availability on disability is ~22 times higher than in relation to mortality rates. This signifies another dimension that has not been usually focused on in BSC outcomes, acknowledging that mortality and disability are not easily comparable outcomes. Unfortunately there is no similar previous research to compare our results with but data obtained are striking. Traditionally the effectiveness of screening programmes is based on mortality statistics, but as people live longer with improved treatments after the diagnosis of cancer, DALYs should be considered as a significant outcome in such evaluations. Furthermore, the identified factors that impact on disability and morbidity need to be incorporated in preventative efforts, and again the BCS programme may be an opportune moment to start introducing such interventions.
It is also interesting to observe that when we added data on availability of digital screening and US tests to BCS programmes, the results for both mortality and disability were also favourable. This result indicates the beneficial effect of advanced BCS examinations as adds-on to mammography and other BCS examinations (SBE and/or CBE). However, until today mixed evidence has been reported about screening examinations that practice shift from film-screen mammography to digital mammography and their relations to intervals and detection of cancer rates as well as their impact on health benefits in women who were screened.41
Low-income countries and lower-middle-income countries often lack the infrastructure for providing population-based mammography. Our stratified analysis by country income level did not show significant results among low- to middle-income countries but only in high-income countries. Despite our findings, BSE and CBE should be offered in such countries with weak health care systems, as on some occasions the results may be comparable with that of mammography, low and middle income countries are presented in Figures 2 and 3. In such a case, breast self-examination and CBE should be the more cost-effective option to screen for BC, although issues around low health literacy, raising awareness through health education and minimizing stigma need to be considered.42,43 However, we have no concrete data on the effectiveness of such screening approaches, and these are urgently needed to maximize the impact of BCS practices where it is needed the most.
This analysis raises several new questions. What is the added value of using digital screening methods and film-screen mammography compared with only self- and CBE? Can a risk-based breast screening44,45 (with lifestyle and health data as shown in this analysis) lead to improved BC-related mortality and disability outcomes, particularly in countries where a population-based screening programme cannot be offered? Better head-to-head data on the effectiveness of different breast screening approaches are further needed. Furthermore, BCS programmes can be used as an opportunity to provide health and lifestyle improvement messages and interventions, alongside social media-based and mobile health (mHealth)/electronic health (eHealth) interventions.
Limitations of this study are a lack of comprehensive information on availability of BCS programmes in some countries, which we tried to resolve by asking personal contacts in these countries. However, some of these data may be inaccurate, as we do not know whether an available BCS programme on paper is actually implemented on the ground and what is the uptake of BCS in each country/region. Taking into account the nature of the data, causality cannot be established. There may also be other risk factors related to mortality and disability; we have included a reasonable number of them in the earlier analysis (such as plasma glucose and others); however, as their contribution was close to ‘0’ and/or due to model collinearity, we have excluded them from our final analysis, to create a stronger outcome model. There were also significantly improved changes in treatments for BC over the past 2 decades that have not been captured in the current analysis and will contribute to the mortality rates shown. Another limitation is that the data analysis could not adjust for other factors39 influencing BC population mortality and disability (due to the GBD study's population-based nature), such as variance in genetic factors, adherence to specific treatments and invasive surgeries, that could also affect the presented estimations. Any limitations in previous publications of the GBD are also applicable to this study,16 mostly the challenges in capturing sources of uncertainty,39 lags in BC and other related data availability, variation in coding practices and other biases, and limitations of existing analytical tools, which may not fully capture temporal trends in BC mortality and disability.
Conclusions
The data from the current cross-country analysis add to the argument of the positive effects of BCS in the age-standardized female mortality and DALYs rates in BC. The proposition from this discussion is to not only have organized mammographic BCS programmes, implemented at the national level, but also to introduce the BCS interventions to address effectively risk factors and comorbidities related to BC mortality and disability. Besides, BCS needs to optimize benefits, reduce morbidity and balance adequately false-positive and false-negative rates, as highlighted by the European guidelines for BCS and diagnosis.46 Finally, data are presented in simple terms to allow policy makers to set up their own priorities to improve BC mortality and disability within the central focus of organized BCS programmes.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
GBD data are available on the Global Health Data Exchange GBD 2020 website (http://ghdx.healthdata.org/). The data used on the BCS per country can be found in Supplementary File S1, available at https://doi.org/10.1016/j.esmoop.2021.100111 or can be obtained through the corresponding author.
Supplementary data
References
- 1.Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zielonke N., Gini A., Jansen E.E.L., EU-TOPIA consortium Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: a systematic review. Eur J Cancer. 2020;127:191–206. doi: 10.1016/j.ejca.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Dibden A., Offman J., Duffy S.W., Gabe R. Worldwide review and meta-analysis of cohort studies measuring the effect of mammography screening programmes on incidence-based breast cancer mortality. Cancers (Basel) 2020;12(4):976. doi: 10.3390/cancers12040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns L.E., Coleman D.A., Swerdlow A.J., Moss S.M. Effect of population breast screening on breast cancer mortality up to 2005 in England and Wales: an individual-level cohort study. Br J Cancer. 2017;116(2):246–252. doi: 10.1038/bjc.2016.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy S.W., Vulkan D., Cuckle H. Effect of mammographic screening from age 40 years on breast cancer mortality (UK age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21(9):1165–1172. doi: 10.1016/S1470-2045(20)30398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fancellu A., Sanna V., Sedda M.L. Benefits of organized mammographic screening programs in women aged 50 to 69 years: a surgical perspective. Clin Breast Cancer. 2019;19(5):e637–e642. doi: 10.1016/j.clbc.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Broeders M., Moss S., Nyström L., EUROSCREEN Working Group The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14–25. doi: 10.1258/jms.2012.012078. [DOI] [PubMed] [Google Scholar]
- 8.Rim S.H., Allaire B.T., Ekwueme D.U. Cost-effectiveness of breast cancer screening in the National Breast and Cervical Cancer Early Detection Program. Cancer Causes Control. 2019;30(8):819–826. doi: 10.1007/s10552-019-01178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuniar P., Robinson S., Moorin R., Norman R. Economic evaluation of breast cancer early detection strategies in Asia: a systematic review. Value Health Reg Issues. 2020;21:252–263. doi: 10.1016/j.vhri.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Zielonke N., Kregting L.M., Heijnsdijk E.A.M., EU-TOPIA collaborators The potential of breast cancer screening in Europe. Int J Cancer. 2021;148:406–418. doi: 10.1002/ijc.33204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gøtzsche P.C., Jørgensen K.J. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;2013(6):CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jatoi I., Pinsky P.F. Breast cancer screening trials: endpoints and over-diagnosis. J Natl Cancer Inst. 2020:djaa140. doi: 10.1093/jnci/djaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Autier P., Boniol M. Mammography screening: a major issue in medicine. Eur J Cancer. 2018;90:34–62. doi: 10.1016/j.ejca.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Roth G.A., Johnson C., Abajobir A. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaxman A.D., Vos T., Murray C.J.L. University of Washington; Seattle, WA: 2015. An Integrative Metaregression Framework for Descriptive Epidemiology. [Google Scholar]
- 18.Salomon J.A., Vos T., Hogan D.R. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomon J.A., Haagsma J.A., Davis A. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous. New country classifications by income level: 2018-2019. World Bank Blogs. https://blogs.worldbank.org/opendata/new-country-classifications-income-level-2018-2019 Available at:
- 21.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youn H.J., Han W. A review of the epidemiology of breast cancer in Asia: focus on risk factors. Asian Pac J Cancer Prev. 2020;21(4):867–880. doi: 10.31557/APJCP.2020.21.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellingjord-Dale M., Vos L., Vik Hjerkind K. Number of risky lifestyle behaviors and breast cancer risk. JNCI Cancer Spectr. 2018;2(3):pky030. doi: 10.1093/jncics/pky030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellingjord-Dale M., Vos L., Hjerkind K.V. Alcohol, physical activity, smoking, and breast cancer subtypes in a large, nested case-control study from the Norwegian Breast Cancer Screening Program. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1736–1744. doi: 10.1158/1055-9965.EPI-17-0611. [DOI] [PubMed] [Google Scholar]
- 25.Neuhouser M.L., Aragaki A.K., Prentice R.L. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol. 2015;1(5):611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lofterød T., Frydenberg H., Flote V. Exploring the effects of lifestyle on breast cancer risk, age at diagnosis, and survival: the EBBA-Life study. Breast Cancer Res Treat. 2020;182(1):215–227. doi: 10.1007/s10549-020-05679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Global Health Observatory Existence of national screening program for breast cancer. Geneva, Switzerland: World Health Organization. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4689 Available at:
- 28.International Agency for Research on Cancer/WHO . Breast Cancer Screening Volume 15. IARC; Lyon France: 2016. Chapter 3, Screening Programmes; pp. 165–236. [Google Scholar]
- 29.Altobelli E., Lattanzi A. Breast cancer in European Union: an update of screening programmes as of March 2014. Int J Oncol. 2014;45(5):1785–1792. doi: 10.3892/ijo.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peintinger F. National breast screening programs across Europe. Breast Care (Basel) 2019;14(6):354–358. doi: 10.1159/000503715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altobelli E., Rapacchietta L., Angeletti P.M., Barbante L., Profeta F.V., Fagnano R. Breast cancer screening programmes across the WHO European region: differences among countries based on national income level. Int J Environ Res Public Health. 2017;14(4):452. doi: 10.3390/ijerph14040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caballero F.F., Soulis G., Engchuan W. Advanced analytical methodologies for measuring healthy ageing and its determinants, using factor analysis and machine learning techniques: the ATHLOS project. Sci Rep. 2017;7:43955. doi: 10.1038/srep43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradshaw P.T., Stevens J., Khankari N., Teitelbaum S.L., Neugut A.I., Gammon M.D. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cedó L., Reddy S.T., Mato E., Blanco-Vaca F., Escolà-Gil J.C. HDL and LDL: potential new players in breast cancer development. J Clin Med. 2019;8(6):853. doi: 10.3390/jcm8060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michels K.B., Solomon C.G., Hu F.B. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26(6):1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 36.Premenopausal Breast Cancer Collaborative Group. Schoemaker M.J., Nichols H.B., Wright L.B. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018;4(11):e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mubarik S., Wang F., Malik S.S., Shi F., Wang Y., Nawsherwan Yu C. A hierarchical age-period-cohort analysis of breast cancer mortality and disability adjusted life years (1990-2015) attributable to modified risk factors among Chinese women. Int J Environ Res Public Health. 2020;17(4):1367. doi: 10.3390/ijerph17041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trimboli R.M., Giorgi Rossi P., Battisti N.M.L. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging. 2020;11(1):105. doi: 10.1186/s13244-020-00905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji P., Gong Y., Jin M.L., Hu X., Di G.H., Shao Z.M. The burden and trends of breast cancer from 1990 to 2017 at the global, regional, and national levels: results from the Global Burden of Disease Study 2017. Front Oncol. 2020;10:650. doi: 10.3389/fonc.2020.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y., Zhang F., Skrip L., Wang Y., Liu S. Lack of an association between passive smoking and incidence of female breast cancer in non-smokers: evidence from 10 prospective cohort studies. PLoS One. 2013;8(10):e77029. doi: 10.1371/journal.pone.0077029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farber R., Houssami N., Wortley S. Impact of full-field digital mammography versus film-screen mammography in population screening: a meta-analysis. J Natl Cancer Inst. 2020;113:16–26. doi: 10.1093/jnci/djaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutnik L.A., Matanje-Mwagomba B., Msosa V. Breast cancer screening in low- and middle-income countries: a perspective from Malawi. J Glob Oncol. 2015;2(1):4–8. doi: 10.1200/JGO.2015.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey S. Preventing breast cancer in LMICs via screening and/or early detection: the real and the surreal. World J Clin Oncol. 2014;5(3):509–519. doi: 10.5306/wjco.v5.i3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimitrova N., Parkinson Z.S., Bramesfeld A. JRC Technical Reports (EUR 28360 EN) 2016. European Guidelines for Breast Cancer Screening and Diagnosis—the European Breast Guidelines.https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines 2016. Available at: [Google Scholar]
- 45.Van Veen E., Brentnall A.R., Byers H. Improving classical breast cancer risk prediction with single nucleotide polymorphisms and mammographic density. JAMA Oncol. 2018;4:476–482. doi: 10.1001/jamaoncol.2017.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rainey L., van der Waal D., Broeders M.J.M. Dutch women's intended participation in a risk-based breast cancer screening and prevention programme: a survey study identifying preferences, facilitators and barriers. BMC Cancer. 2020;20:965. doi: 10.1186/s12885-020-07464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.