Abstract
The treatment of large-area bone defects still faces many difficulties and challenges. Here, we developed a blood clot delivery platform loaded with BMP-2 protein (BMP-2@BC) for enhanced bone regeneration. Blood clot gel platform as natural biomaterials can be engineered from autologous blood. Once implanted into the large bone defect site, it can be used for BMP-2 local delivery, as well as modulating osteoimmunology by recruiting a great number of macrophages and regulating their polarization at different stages. Moreover, due to the deep-red color of blood clot gel, mild localized hyperthermia under laser irradiation further accelerated bone repair and regeneration. We find that the immune niche within the bone defect microenvironment can be modulated in a controllable manner by the blood clots implantation and laser treatment. We further demonstrate that the newly formed bone covered almost 95% of the skull defect area by our strategy in both mice and rat disease models. Due to the great biocompatibility, photothermal potential, and osteoimmunomodulation capacity, such technology shows great promise to be used in further clinical translation.
Keywords: Blood clot, BMP-2, Osteoimmunology, Photothermal therapy, Bone repair
Graphical abstract
Highlights
-
•
BMP-2@BC-based osteoimplant is developed for photothermal cranial defect therapy.
-
•
Blood clot not only serves as a drug delivery carrier but also works as an effective thermogenic biomaterial.
-
•
BMP-2@BC-based hyperthermia therapy effectively regulates the osteoimmunology associated with the healing of bone defects.
1. Introduction
Bone defect is an orthopedic condition that refers to the destruction of bone caused by injury or disease, resulting in local dysfunction, delayed healing, and bone nonunion [[1], [2], [3]]. Bone grafting is the gold standard for clinical large bone defects treatment including autologous bone graft implants (from the patient) [4] and allogeneic bone graft implants (from the donor) [5]. However, autologous bone transplantation can cause unnecessary pain and trauma to the patient, and it is easy to cause complications such as injury, pain, and infection at the donor site [6]. Allogeneic bone graft has potential immune rejection and the risk of disease transmission [7]. The limitation of treatment for bone defects results in patients living with a reduced quality of life [8].
Osteoimmunology is an evolving interdisciplinary research field that is focused on the interaction between skeletal and immune systems [9,10]. The process of bone healing involves three successive but interdependent stages: inflammation, repair and remodeling [11]. Bone healing is initiated by the inflammatory response after injury, which also influences the subsequent bone repair and remodeling phases [12]. Bone healing as a regenerative process involves the participation of a variety of active molecules and cells, such as immune cells, precursor cells and stem cells [13]. Many immune cells, such as neutrophils, monocytes/macrophages and T cells, infiltrate to the local injury site to modulate bone homeostasis, accompany by the production of multiple cytokines [14]. Given the critical roles of the immune cells in bone repair, manipulation of osteoimmunomodulation provides a promising way in bone regeneration.
The recent development of advanced biomaterials including inorganic materials [[15], [16], [17]], organic polymers [[18], [19], [20], [21], [22], [23], [24]], and their composites [25,26] has shown great promise for accelerating bone reconstruction [27,28], providing alternatives bone remodeling methods [[29], [30], [31], [32]]. Despite the outcomes of biomaterials for bone repair, many issues still hinder the clinical transformation from several aspects. For example, the clinical indications are limited, the majority of biomaterials are used as bone void fillers for implant fixation and only a few of them can be used in non-union fractures [33]. In addition, biocompatibility is another concern. Although the early design of the materials is biologically inert, they are still prone to induce foreign body reactions (FBR), including the formation of granulation tissue components and the fiber encapsulation of implants [34]. Besides, most of the bone implants lack immunoregulation capacities within the defect site to promote bone healing. With the in-depth understanding of the interaction between the immune system and biomaterials, there is cognition that immune response is an important part of the biomaterials-mediated tissue reconstruction, which can be regulated by implanted biomaterials. The next generation for bone repair focuses on applying immunomodulatory biomaterials with osteoconductive, osteoinductive and osteoimmunomodulation ability [35,36].
Blood clot gel is a novel kind of biomaterials that can be generated from autologous blood to the superior gel-like state ex vivo with excellent biocompatibility. There are several unique and unprecedented properties of blood clots for bone defect regeneration. Firstly, the innate immune response can be effectively initiated and regulated by the blood clot locally, resulting in the recruitment of many various immune cells as we previously reported [37]; Secondary, the composition of platelets and plasma in the blood clot containing various growth factors makes blood clot benefit for the bone reparation; At last, due to the deep-red color, blood clots could be easily heated by near-infrared light (NIR) irradiation [38] to induce mild hyperthermia in the site of defect, which promotes the bone generation as well [38,39].
Accordingly, here we developed an implantable bone morphogenetic protein-2 (BMP-2)-loaded blood clot platform for bone repair (Fig. 1). BMP-2 as a conventional therapeutic agent in clinical practice [[40], [41], [42], [43]], is generally administered by oral or systemic injection, which is likely to cause systemic exposure and low concentration in local lesions. In this study, ex vivo blood was mixed with BMP-2 in the customized model, followed by the coagulation cascade process that triggers the formation of BMP-2 contained blood clot ex vivo. Then blood clot gels were implanted into the large bone defect site for BMP-2 local delivery, as well as modulating osteoimmunology by recruiting macrophages and regulating their polarization. We demonstrated that the immune niche within the bone defect microenvironment can be modified by the blood clot implantation in a controllable manner. Moreover, under laser irradiation, mild localized hyperthermia further accelerates bone repair and regeneration. Such technology shows great promise to be used in further clinical translation. The blood clot platform can be made by mixing the autologous blood from the patient and BMP-2 ex vivo in a model according to the shape of bone defect, followed by implanted into the same patient for the personalized bone generation therapy.
Fig. 1.
The scheme of an implantable blood clot to generate mild localized heat and regulate osteoimmunology for bone repair. a. Computer tomography technology is used to determine the size and shape of bone defect, and custom models were prepared according to the detected damage morphology. b. BMP-2@BC can be customized into different shapes and sizes according to the local defect, which is effective for bone regeneration. It can produce a mild thermal effect under the NIR irradiation. After treatment, the recruited macrophages play the role of pro-inflammatory in the early stage and anti-inflammatory in the later stage during the whole process of bone repair and produce high-efficiency immunoregulatory effects in the damaged area to promote the progress of bone damage.
2. Results
2.1. Preparation and characterization of BMP-2@BC hydrogel
Implantable BMP-2@BC hydrogel was prepared by dispersing BMP-2 into fresh blood in a designed model, followed by gentle drying to avoid the seepage of serum and agents (Fig. 1). The size and shape of the blood clot could be tailored according to the specific conditions of the fracture injury site individually (Fig. 2a). The prepared BMP-2@BC hydrogel was composed of fiber-encased red blood cells as shown in scanning electron microscope (SEM) imaging (Fig. 2b). The loading efficiency of BMP-2 was more than 90% determined by enzyme-linked immunoabsorbent assay (ELISA) (Fig. 2c). Loaded drug can be evenly distributed in the blood clot according to the results of the confocal image (Fig. 2d). To investigate the in vitro degradability, hydrogel was incubated with PBS at 37 °C and observed for gradual degradation over time (Fig. 2e). The release of BMP-2 shows an initial burst release in the first two days, and followed by a slower, and sustained release in the next couple of days (Fig. 2f). The mechanical properties of blood clots and BMP-2@BC further indicated their hydrogel properties (Fig. 2g-i). Likewise, the blood clot-based hydrogel significantly prolonged the retention of loaded DiD in vivo at least for two weeks compared to the free DiD (Fig. 2j and k), suggesting a long-term sustained drug release behavior in vivo. Meanwhile, the degradation of blood clot gel in vivo was similar to the results we previously reported [37]. First, the degradation of blood clot was relatively slow in the first three days, and the subsequent degradation speed increased, and it was completely metabolized and cleared up in about 15 days (Appendix A, Appendix A). Besides, the blood clots we prepared also detected a variety of growth factors similar to those in the serum, including transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF) and and platelet-derived growth factor-AB (PDGF-AB) (Appendix A, Appendix A), indicating its potential ability to promote bone repair. Compared with clinical used platelet-rich plasma, the blood clots describe here are also rich in platelet-derived growth factors such as VEGF and PDGF. In addition, the formed blood clot had good morphology and mechanical strength, which can be used as the implant to the affected bone site [44].
Fig. 2.
Formation and characterization of BMP-2@BC gel. a. The morphology of BMP-2@BC tailored according to different models. b. SEM image of BMP-2@BC. Arrows pointing to fiber. Scale bar, 2 μm. c. The loading efficiency of BMP-2 into the BC gel. d. Confocal image of drug distribution in blood clot. Scale bar, 10 μm. e. The degradation of BMP-2@BC in vitro. f. The release curve of BMP-2 from BC gel. g. Amplitude test of fixed frequency. h. Frequency test of fixed strain (stress). i. Compressing strength and modulus. j. Fluorescence image of the retention and degradation of BC in the mice skull defect sites and k. corresponding fluorescence quantitative data. Data are presented in means ± SEM. (n = 3–5).
2.2. The ability of BMP-2@BC to promote bone formation
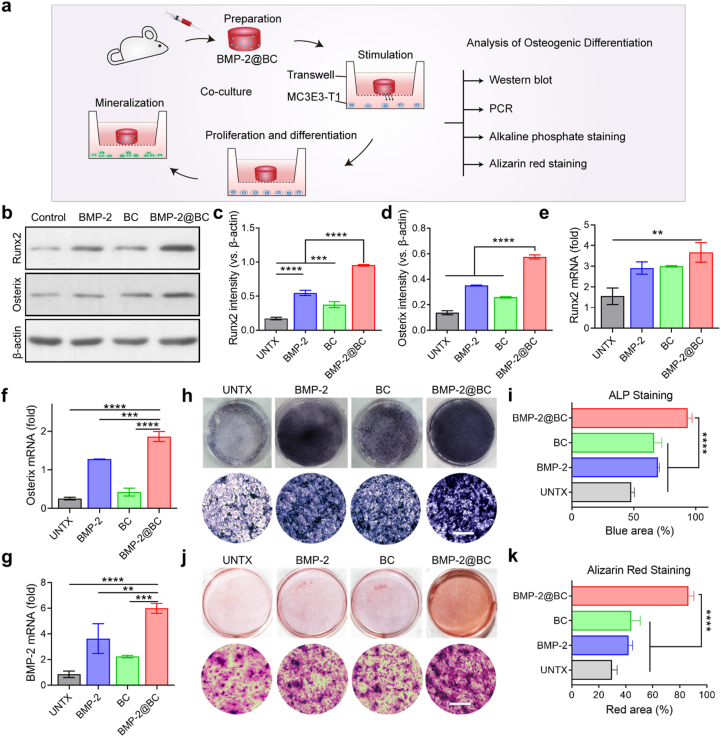
The osteogenic potential of BMP-2@BC was evaluated in vitro (Fig. 3a). MC3T3-E1 cells were cultured in the lower compartment and BMP-2@BC were added to the upper compartment in a transwell system. We first evaluated its ability to induce osteogenesis from protein levels. After stimulating with BMP-2@BC for 24 h, cells at the bottom were collected for western blot analysis (WB). As the western blot results showed, BMP-2@BC upregulated the expression of Runx2 and Osterix (Fig. 3b), which are two key transcription factors for bone formation [45]. Runx2, known as a core-binding factor, is necessary for bone marrow mesenchymal stem cell differentiation and bone development [46]. Osterix is a newly discovered transcription factor containing zinc-finger structure, which is located downstream of Runx2 in the differentiation pathway of osteoblasts and regulates the production of osteoblasts [47]. Quantitative analysis of Runx2 and Osterix showed that the BMP-2@BC group was about five times and four times higher than the PBS group, respectively (Fig. 3c and d), indicating the BMP-2@BC could significantly induce osteogenic differentiation. Besides, BMP-2-loaded blood clots tended to stimulate more osteogenic protein expression than free BMP-2. The reason for this enhancement may be the endogenous factors of the blood clot that itself can promote osteoblasts to express Runx2 and Ostetrix, probably due to the growth factors released by the activation of platelets during the clotting process, such as PDGF, VEGF, TGF-β and so on (Appendix A, Appendix A) [48].
Fig. 3.
The effects of BMP-2@BC intervention on the osteogenic differentiation of MC3E3-T1 cells. a. The diagram of the culture system by using transwell. b. Western blot analysis of Runx2, Osterix expressed in MC3E3-T1 cells, β-Actin was used as a reference protein and corresponding quantification of the c. Runx2 level and d. Ostetrix level. e-g. PCR analysis of representative osteogenesis gene including e. Runx2, f. Osterix, and g. BMP-2. h. The alkaline phosphate staining of osteoblasts and i. the corresponding quantitative data of generated blue nodules. j. The alizarin red staining of osteoblasts and k. the corresponding quantitative data of generated red nodules. Scale bar, 200 μm. Data are presented in means ± SD. Statistical significance was analyzed by one-way ANOVA using the Tukey post-test. P-value: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (n = 3–5). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Then, real-time quantitative PCR was used to analyze the mRNA expression levels of osteogenesis-related genes (Runx2, Osterix, and BMP-2) in MC3T3-E1 [49]. The results showed BMP-2@BC treatment could remarkably upregulate the osteogenic mRNA expression during the treatment, while free BMP-2 had relatively limited effects on stimulating bone regeneration (Fig. 3e–g). Compared with the PBS group, the gene expression of Runx2 was increased about three times (Fig. 3e), and the gene expression of Osterix and BMP-2 was up to seven times (Fig. 3f and g). In addition, blood clots significantly induced the expression of Runx2, and the mRNA expression of Osterix and BMP-2 also showed an upward trend.
Alkaline phosphatase (ALP) is an early osteogenic marker of cell maturation and calcification. In consistence with previous data, the ALP activity of the cells treated with BMP-2@BC was significantly enhanced compared to all other controls, which was an important indicator of BMP-2@BC promoting the osteogenic differentiation of MC3T3-E1 (Fig. 3h and i). Alizarin red staining is another way to determine mineralized nodules in osteoblasts. As shown in Fig. 3j and k, the number of calcium nodules and calcium salt deposition in the BMP-2@BC group detected was more than those in the control groups. Notably, the blank blood clot also tended to stimulate differentiation in both protein and mRNA levels, as well as ALP staining and alizarin red staining results. These results suggest that the BMP-2@BC could induce differentiation of MC3T3-E1 cells in vitro from pre-osteoblasts to osteoblasts.
2.3. Photothermal treatment accelerated bone growth
Mild hyperthermia (40–43 °C) has been proven to promote bone repair [38]. Interestingly, blood clot gel with the reddish-brown color can be used as a novel photothermal agent to generate mild hyperthermia under NIR laser exposure. The photothermal potential of blood clot gel was tested using an 808 nm laser with different power intensity and monitored with an infrared camera (Fig. 4a). As shown in Fig. 4a and b, the temperature of blood clot increased as the laser intensity increased from 0 W to 0.4 W. When implanted in vivo, the blood clot gel could be heating up to about 41–43 °C at the local implantation site (Fig. 4c and d), indicating the blood clot could serve as a photothermal agent for potential combined photothermal therapy to accelerate bone healing. Besides, we also investigated the release of BMP-2 from BC gel under near-infrared irradiation in vitro. The mild heat generated by NIR irradiation promoted the release of the BMP-2 from gel, however, there was non-significant difference compared to unirradiated group.
Fig. 4.
The mild photothermal effect of BMP-2@BC further stimulated bone formation. a. IR thermal images of BMP-2@BC in vitro. b. Photothermal heating curves of BMP-2@BC under different power of 808 nm laser irradiation. c. IR thermal images of BMP-2@BC in local skull defect site, and d. corresponding heating curves of BMP-2@BC in vivo. e. Western blot analysis of Hsp47, Runx2, and Osterix expressed in MC3E3-T1 cells after irradiation, β-Actin was used as a reference protein and the corresponding quantification of the f. Hsp47, g. Runx2, and h. Ostetrix. i-l. mRNA expression including i. Collagen I, j. Runx2, k. Osterix, and l. BMP-2. m. The alkaline phosphate staining of osteoblasts and n. the corresponding quantitative data of generated blue nodules. o. The alizarin red staining of osteoblasts under mild heat stimulation and p. the corresponding quantitative data of generated red nodules. Scale bar, 200 μm. Data are presented in means ± SD. Statistical significance was analyzed by one-way ANOVA using the Tukey post-test. P-value: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (n = 3–5). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To validate the performance of BMP-2@BC on osteoblasts when combined with laser-induced hyperthermia treatment, transwell system also used in this part of the cell experiment. MC3T3-E1 cells were incubated in 6-well plates and cocultured with PBS and BMP-2@BC. For irradiation, BMP-2@BC in the upper compartment was irradiated with 808 nm laser (0.4 W/cm2) for about 10 min. The laser irradiation group alone was used as a control. After 24 h, treated cells collected from experimental groups including PBS, Irradiation, BMP-2@BC, and BMP-2@BC with NIR irradiation were evaluated for the expressions of Hsp47, Collagen I, Runx2 and Osterix by WB (Fig. 4e). Heat shock protein 47 (Hsp47) as a kind of Hsps in mammals could regulate the cross-linking of collagen to produces a unique ECM, which is essential for the biosynthesis and molecular maturation of type I collagen related to osteogenesis [50]. Irradiation alone had no stimulation effects on Hsp47 expression of MC3T3-E1. Interestingly, when exposed to the NIR laser, the mild heat generated by BMP-2@BC could effectively activate the expression of Hsp47 (Fig. 4f) compared to the control groups. The Runx2 expression was three to four times than that of laser alone and the BMP-2@BC (Fig. 4g), respectively. Similarly, combination treatment promoted the expression of Ostetrix as well (Fig. 4h).
Then, we carried out a qPCR assay to test the photothermal effects at the mRNA level. Similar to WB results, NIR irradiation alone cannot induce the expression of osteogenesis-related genes in MC3T3-E1 cells. However, when treated with BMP-2@BC plus NIR laser exposure, there was remarkably elevations of the mRNA in Collagen I, Runx2, Osterix and BMP-2 than BMP-2@BC alone and other controls (Fig. 4i–l). Similarly, the depositions of mineralized sites were increased as characterized by ALP staining (Fig. 4m and n) and Alizarin red staining (Fig. 4o and p). These findings indicated that BMP-2@BC with irradiation further promotes osteoblast proliferation and differentiation.
2.4. BMP-2@BC with laser stimulation for the treatment of bone defects in mice
Encouraged by in vitro results, we further studied the therapeutic effects of BMP-2@BC-based photothermal therapy on promoting bone healing in mice models (Fig. 5a). The bone defects (4 mm in diameter) were established on the cranial bone [51]. After the large-area skull defect model was built, the local defect sites were treated in the following way: (1) 100 μL Sterile PBS as the control group, (2) 100 μL Sterile PBS + Laser irradiation, (3) 100 μL Free BMP-2 (500 ng), (4) 100 μL Blood clot, (5) 100 μL BMP-2@BC or (6) 100 μL BMP-2@BC + Laser irradiation. At 1 h postsurgery, mice in need of laser treatment were anesthetized with isoflurane, the entire surgical site was irradiated with an 808 nm laser, and the local temperature was monitored with an infrared imaging camera. During the experiment period, mice were irradiated once every three days, a total of 3 times (Fig. 5a).
Fig. 5.
BMP-2@BC-based photothermal stimulation of bone defects after local implantation in mice model. a. Schematic illustration of the BMP-2@BC-based PTT to accelerate bone healing. b. Micro-CT 3D reconstruction of cranial defect sites. c. Hematoxylin and eosin staining and d. Masson staining for the evaluation of bone regeneration. Scale bar: 1 mm. e-f. Quantitative analysis of bone healing effect after different treatments, including e. new bone volume (BV) and f. bone tissue volume/total tissue volume (BV/TV). Data are means ± SD. Statistical significance was calculated by one-way ANOVA with Tukey's post hoc test. P-value: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (n = 4).
After 6 weeks of treatment, the local bone defect site was harvested for micro-CT imaging and 3D construction [52]. As shown in Fig. 5b, Free BMP-2 or blood clot treatment had a moderate promotion of bone healing. Mice receiving BMP-2@BC showed more pronounce healing effects. Notably, when the mice implanted with BMP-2@BC were irradiated with a laser to generate mild hyperthermia, the best therapeutic efficacy to accelerate osteogenesis was achieved in vivo. In particular, the fusion degree of the skull defect was the highest, the defects treated with BMP-2@BC with laser were closed by >95%, which was significantly higher than the defects treated with the other treatments (Fig. 5b).
To further investigate the influence of BMP-2@BC with laser on bone formation, we performed H&E and Masson's staining to analyze harvested skull samples (Fig. 5c and d). The BMP-2@BC with irradiation group showed intramembranous bone formation. Two ends of the cranial fracture had been bridged by newly formed bone tissue, and the defect area had almost been reshaped to its original shape. Quantitative analysis of the microstructure of bone formation at the defect site further supported the results of tissue regeneration with different treatments [53]. Irradiation alone couldn't increase new bone volumes (BV) and bone tissue volume per total tissue volume (BV/TV) BMP-2 and/or BC moderately increased BV and BV/TV. In contrast, BMP-2@BC with laser irradiation could maximize the value of BV and BV/TV (Fig. 5e and f). The above results demonstrated that BMP-2@BC with laser irradiation could effectively promote bone formation in mice bone defect model.
2.5. BMP-2@BC with irradiation regulated osteoimmunology for bone repair
The initial inflammatory phase of bone defect is closely related to bone union, which is affected by the systemic and local inflammatory situations. This inflammatory response triggers the recruitment, proliferation, and activation of multiple immune cells and hematopoietic cells. Accumulating evidence suggests that macrophages are highly active throughout the bone repair period owing to their diversity and plasticity [54]. The obvious phenotype and functional changes of resident and recruited macrophages are in response to the cytokines and growth factors released in the local microenvironment [55]. During the inflammation phase, classically M1 macrophages migrate and engulf debris, releasing high levels of pro-inflammatory cytokines [56]. Local progenitor cells and stem cells can be activated to involve in the bone repair process [57]. When the inflammation subsides, macrophages increase their population through migration and activate M1 and M2 phenotypes under cytokine stimuli. In this process, the two phenotypes can also transfer between each other [58,59]. During the repair phase, macrophages with anti-inflammatory phenotypes become the ascendant population, which modulate the inflammation and promote tissue repair [60]. Blood clot is well known to recruit many various immune cells including macrophages to initiate innate immune response at the first stage. Macrophages help clean and engulf the blood clot. After that, macrophages also send out growth factors that help repair the area at the later stage [57,61]. To study the local osteoimmunological situation of BMP-2@BC after irradiation, we compared the changes in macrophage number and phenotype in local bone defects on days 3, 7, 14 of treatment. From Fig. 6a, the implanted BMP-2@BC in the treated group can fit well to the defect site. The healing of skull defects in the treatment group was visually obviously higher than that of the control group. Next, we removed the blood clot and the tissue adjacent to the defect area, then digested it into the single-cell suspension. The local macrophage composition before and after treatment was analyzed by flow cytometry. The results showed local trauma caused a certain degree of inflammation with the accumulation of macrophages. At the three tested points, the treated group tended to recruit more macrophages than the control group (Fig. 6b). Besides, the local macrophage number has a dynamic process from increasing to decreasing with the development of bone defect healing. Consistent with the flow cytometry results, immunofluorescence staining of blood clot indicated that macrophages in the blood clot gel also increased in the early stage and decreased in the later stage (Fig. 6c).
Fig. 6.
Local osteoimmunological changes of BMP-2@BC after irradiation. a. Optical image of BMP-2@BC in the skull defect sites at days 3, 7 and 14. b. Flow cytometric analysis of the proportion of macrophages at the injury site. c. The immunofluorescence images of F4/80 in cryosection of BMP-2@BC after treatment. Scale bars, 50 μm. d. The percentage of CD80 in infiltrated macrophages at days 3, 7 and 14 and e. the representative flow cytometry diagram. f. The proportion of CD206 in infiltrated macrophages at day 3, 7 and 14 respectively and g. the representative flow cytometry diagram. h-k. The concentration of pro-inflammatory and anti-inflammatory related cytokines, h. TNF-α, i. IL-6, j. IL-10, and k. IL-4. Data are means ± SD. l. Schematic diagram of the macrophage changes in the immune microenvironment of the bone defect after BMP-2@BC-based hyperthermia therapy. Statistical significance was calculated by the Two-tailed Student's t-test. P-value: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, not significant. (n = 3–5).
Macrophages equip with the ability to change the polarized phenotype flexibly, accompanied by changes in the local microenvironment. We further analyzed the phenotype of macrophages infiltrated at different time points. Compared with the untreated group, the infiltrating macrophages in the BMP-2@BC with laser group showed increased expression of CD80 (Fig. 6d and Appendix A, Appendix A), CD86 and MHC II (Appendix A, Appendix A) on the 3rd and 7th day, indicating more M1 polarization of infiltrated macrophage at the early stages of bone healing. Interestingly, the frequency of macrophages was reversed on the 14th day (Fig. 6e). More M2 anti-inflammatory macrophages were induced at the repair sites (Fig. 6f, g and Appendix A, Appendix A). By analyzing the local cytokines, we found that the local pro-inflammatory factors also showed a trend of first increasing stage and then decreasing stage. Inflammatory cytokines TNF-a and IL-6 increased significantly in the first seven days, which was about twice and four times that of the control group (Fig. 6h and i). On the 14th day, the concentration of inflammatory factors showed a downward trend. In addition, the concentration of anti-inflammatory cytokine IL-10 gradually increased (Fig. 6j), and the concentration of IL-4 was maintained at a relatively high level (Fig. 6k). This observation confirmed our supposition that blood clot could attract many more macrophages and boost the inflammatory and anti-inflammatory response in the bone defect at two stages, respectively. Besides, we also evaluated the safety of this treatment by performing routine blood analysis on the 14th day after the treatment and pathological section analysis of the main organs. The mental and physical conditions of the mice did not change significantly compared with the control group (Appendix A, Appendix A). Collectively, in the initial osteoimmune environment, early inflammation acted as an essential part in promoting bone repair and remodeling, accelerating the aggregation of macrophages and pro-inflammatory polarization in the early stage of treatment (Fig. 6l). In the later stage, primary macrophages and some M1 type macrophages were transformed into M2 anti-inflammatory macrophages which play a role in bone repair to promote the healing of bone tissue around the osteoimplant. Our strategy showed effective regulation of the bone osteoimmunological microenvironment by promoting the early-stage pro-inflammatory and potentiating late-stage anti-inflammatory response in a controllable manner, significantly accelerating the healing of the bone defect site without any toxicity.
2.6. BMP-2@BC with laser stimulation for the treatment of bone defects in rat
To further evaluate the potency of the BMP-2@BC on bone regeneration in other models, the critical-sized skull defects were developed in rats and then treated in the same way as the mice bone defect model (Fig. 7a and b). After 12 weeks of treatment, micro-CT imaging and 3D reconstruction were used to evaluate bone regeneration (Fig. 7c). Similar results were obtained in the rat model, BMP-2@BC with NIR irradiation could effectively promote the healing of bone defects without obvious toxicity (Fig. 7c). HE and Masson staining as well as several indexes also indicated the improved regeneration efficiency of our strategy compared to the control treatments (Fig. 7e–g). Collectively, these results suggest that blood clot-based scaffolds serve as a therapeutic depot to enhance repair and regeneration after bone defects.
Fig. 7.
In vivo bone regeneration of BMP-2@BC with local irradiation in the rat model. a. Schematic diagram of the BMP-2@BC-based PTT to promote cranial defect regeneration. b. Optical image before and after blood clot implantation. The minimum unit of the ruler is 1 mm. c. Micro-CT 3D reconstruction of treated sites. d. Hematoxylin and eosin staining and e. Masson staining for the evaluation of bone regeneration. Scale bar: 1 mm. f-g. Quantitative analysis of bone healing effect in rat model after different treatments, including f. bone volume (BV) and g. bone tissue volume/total tissue volume (BV/TV). Data are means ± SD. Statistical significance was calculated by one-way ANOVA with Tukey's post hoc test. P-value: *, P < 0.05; ***, P < 0.001; ****, P < 0.0001. (n = 4).
3. Conclusion
In summary, we developed a BMP-2@BC-based osteoimplant for photothermal cranial defect therapy. This multifunctional erythrocyte gel platform has a high degree of plasticity and can be tailored to the shape of the damaged area. In addition, the red blood cells used to construct BMP-2@BC are derived from the patient's own body or from a donor of the same blood type, which exhibits higher biosafety than other synthetic biomaterials. BMP-2@BC can effectively promote osteogenic differentiation and regeneration both in vitro and in vivo. Importantly, in addition to being a carrier, the blood clot is also an effective thermogenic photosensitizer under the 808 nm laser irradiation. The mild localized hyperthermia generated by BMP-2@BC significantly accelerates bone growth and healing. We demonstrated that the immune niche within the bone defect microenvironment can be modified by the BMP-2@BC with laser treatment. BMP-2@BC-based hyperthermia therapy effectively regulates the osteoimmunology associated with the healing of bone defects. After treatment, the proportion of macrophages dynamic changed with two-stage during the whole process of bone repair. Overall, considering the great biocompatibility, photothermal potential, and osteoimmunomodulation capacity, our BMP-2@BC implant showed great prospects for clinical transformation in bone regeneration. In the future, 3D printing technology can be used to adjust our delivery platform for individual clinical patients' bone defect therapy, envision high plasticity of our technology for clinical use.
4. Materials and method
4.1. Materials
The blood was taken from the venous plexus at the fundus of the mouse. Bone morphogenetic protein-2 (PeproTech, catalog no.120-02-50) was obtained from Dakewe Biotech Co. Ltd. Fluorescent dye DiD (Beyotime, catalog no. C1038) and BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, catalog no. C3206) were purchased from Shanghai Beyotime Biotechnology Co., Ltd. Ultrapure RNA Kit (CWBIO, catalog no. CW0581S) and HiFiScript gDNA Removal cDNA Synthesis Kit (CWBIO, catalog no. CW2582 M) were purchased from Suzhou Mingde Biological Technology Co., Ltd. Calcium salt stain kit (Solarbio, catalog no. G3280) was purchased from Beijing Solarbio Science & Technology Co., Ltd.
4.2. Cell lines and mice
Mouse embryonic osteoblast precursor MC3T3-E1 were purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. MC3T3-E1 cells were cultured in a 5% CO2, 37 °C incubator, and the cell culture medium was modified α-MEM containing 10% fetal bovine serum. The medium was changed every 2–3 days according to the cell growth and culture conditions, and the cells were passaged when the cell density reached 70–80%. Balb/c mice about 6–8 weeks old were purchased from the laboratory animal center of Soochow University. Animal experiments have been approved by the Ethics Committee of Soochow University. All experimental operations and animal welfare complied with ethical principles and comply with relevant national regulations.
4.3. Preparation
Blood clot carrier was prepared by cascade coagulation behavior and mild drying. In detail, 150 μL blood was taken out from mice and placed in a designed mold. After preliminarily agglomerating at room temperature for about 10 min, sample was transferred to the drying cabinet containing phosphorus pentoxide at 37 °C and dried for about 20 min. For the preparation of BMP-2-loaded blood clot, BMP-2 (500 ng or 1 μg) was mixed with blood evenly and then placing it in a sterile mold. The subsequent processing steps were the same as the preparation of blank blood clots. The demonstrated models in this manuscript were purchased from supplier.
4.4. Characterization
The external structure and internal microscopic appearance of the drug-loaded blood clot were characterized by a camera and a SEM (ZEISS G500), respectively. To test the loading efficiency of BC, the concentration of untrapped BMP-2 in the supernatant was measured by the microplate reader (Bio-Tek, Synergy H1) after determining the volume of the extra precipitated liquid. The loading efficiency of BMP-2 in blood clot was calculated according to the following equation: Loading efficiency of BMP-2 (%) = (1 – the weight of unloaded BMP-2/weight of added BMP-2) × 100%. FITC-BSA was used to simulate the distribution of BMP-2 in a blood clot. FITC-BSA was loaded into a blood clot gel according to the above preparation method, wrapped with OCT-gel and frozen overnight at −80 °C, and then stained after frozen sectioning. For analysis, rhodamine was used to mark cell membranes in blood clots (dilution: 1:100). In order to characterize the in vitro degradation of BMP-2@BC, we placed 150 μL of the sample in 2 mL of PBS at 37 °C, and took pictures on the day 0, 1, 3, and 7. To study the drug release behavior, BMP-2-loaded blood clots were incubated with 1 mL sterilized neutral PBS at 37 °C. 100 μL liquids were collected at different time points for quantitative analysis, then added with the equal volume of PBS. Collected samples were centrifuged at high speed under 4 °C and then tested by ELISA. The rheological properties of the BMP-2@BC were tested by Thermo HAAKE MARS 60 rotational rheometer and mechanical testing machine (CMT6103) according to the standard protocols. To characterize the degradation of the blood clot in the skull defect area, the blood clot loaded with fluorescent dye DiD was implanted and small animal imaging was used to observe the degradation of the blood clot every three days. The same amount of free DiD was set as control. In addition, blood clot gels were implanted under the skin. We measured the volume of blood clots with digital caliper, calculated following the formula: short diameter2 × long diameter × 0.5. and took photos to visually characterize the degradation.
4.5. Western blot assay
Protein expression was analyzed by western blot according to the standard instructions [30]. MC3T3-E1 cells were treated with PBS, 25 ng BMP-2, 100 μL BC, 100 μL BMP-2@BC containing the same amount of BMP-2 for 24 h. In detail, MC3T3-E1 cells were cultured in the lower compartment and BMP-2@BC were added to the upper compartment in a transwell system. For laser irradiation stimulation, 808 nm laser (0.4 W/cm2) was used to irradiate cells or BMP-2@BC for about 10 min. The total protein amount of samples was determined by the BCA protein kit. 20 μg proteins were used for each well. Then, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore, MA). After incubation with the primary antibody Runx2 (CST, Cat# 12556S), Osterix (Abcam, Cat# ab209484), Hsp47 (Abcam, Cat# ab109117), and β-actin (ABclonal, Cat# AC026), followed by incubating with secondary antibody (BOSTER, Cat# BA1054). Chemiluminescence reagents (Proteintech, Cat# PK10001) were added to the membrane. The targeted protein bands were visualized on X-ray film. The total gray of the interested band was quantified by the Image J software.
4.6. qPCR assay
MC3T3-E1 cells were cultured according to the above-mentioned culture method. 2 × 105/per well cells were incubated in 6-well plates. After the cells adhered to the wall, the culture medium was changed and added with a concentration of 10 mmol/L sodium β-glycerophosphate and 50 μg/mL ascorbic acid. Different factors were used to stimulate the cells for about 24 h. Total RNA was extracted from MC3T3-E1 cells by Trizol (Total RNA Extraction Reagent) according to the manufacturer's manual. The purity and concentration of total RNA were measured by DS-11 Series Spectrophotometer/Fluorometer (Denovix). The first strand of cDNA was synthesized by reverse transcription according to the instructions of the RevertAid First Strand cDNA Synthesis Kit and amplified by fluorescence quantitative PCR. The related thermocycler parameters were set as follows: 95 °C for 10min, 95 °C for 10s, 55 °C for 1min, 45 cycles, 95 °C for 5s. The primer sequences used were as follows: Mouse β-actin primers (internal control): forward primer 5′-GTGACGTTGACATCCGTAAAGA-3′ and reverse primer 5′-GCCGGACTCATCGTACTCC-3′; Mouse Cola 1 primers: forward primer 5′-GCTCCTCTTAGGGGCCACT-3′ and reverse primer 5′-ATTGGGGACCCTTAGGCCAT-3′; Mouse Runx2 primers: forward primer 5′-GACTGTGGTTACCGTCATGGC-3′ and reverse primer 5′-ACTTGGTTTTTCATAACAGCGGA-3′; Mouse BMP-2 primers: forward primer 5′-ACACAGCTGGTCACAGATAAG-3′ and reverse primer 5′-CTTCCGCTGTTTGTGTTTGG-3′. The relative expression levels of the above genes were calculated according to the Ratio = 2−ΔΔCT method. Mouse β-actin was used as the internal control.
4.7. Alizarin red staining
MC3T3-E1 cells were cultured on 12-well plates with osteogenic differentiation medium (low glucose-DMEM, 10% FBS, 100 U/mL penicillin-streptomycin, 10 nM dexamethasone, 10 mM sodium-β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate), and treated with PBS, 25 ng BMP-2, 100 μL BC, 100 μL BMP-2@BC containing the same amount of BMP-2 for 14 days. For laser irradiation stimulation, 808 nm laser (0.4 W/cm2 was used to irradiate cells or BMP-2@BC for about 10 min. Samples were fixed in 4% PFA for 15–30 min and washed twice with PBS (calcium and magnesium-free). Calcium deposits in osteoblasts were detected by the Alizarin Red Staining Kit (Solarbio, Cat# G3280) according to the manufacturer's instructions. (Alizarin red staining solution was dripped onto the samples for 5 min. After washed slightly with PBS, samples were rinsed with McGee-Russell differentiation solution for a few seconds. Mayer's hematoxylin staining solution lightly stained the nucleus for 1–2 min, then wash three times with PBS. The prepared samples were photographed under the LED light and observed by microscopy (Nikon ECLIPSE Ts2R/Ts2R-FL). The areas of alizarin red staining were then quantified using ImageJ software and determined as staining area/total area × 100%.
4.8. Alkaline phosphate staining
For alkaline phosphate staining assay, cells were stimulated under the same condition of alizarin red staining. Samples were fixed in 4% PFA for 15–30 min and washed twice with PBS (calcium and magnesium-free). Staining working solution (Beyotim, Cat# C3206) was prepared by mixing 3 mL alkaline phosphatase coloring buffer, 10 μL BCIP solution (300X), 20 μL NBT solution (150X), and 3.03 mL of BCIP/NBT staining working solution. Samples were incubated in the dark at room temperature for 12 h, then removed the staining working solution and washed 1–2 times with deionized water to stop the color reaction. The prepared samples were photographed under the LED light and observed by microscopy (Nikon ECLIPSE Ts2R/Ts2R-FL). The areas of alkaline phosphatase-positive nodules were then quantified using ImageJ software and determined as staining area/total area × 100%.
4.9. Photothermal performance of blood clot
To investigate the photothermal performance of blood clots, PBS and 200 μL blood clots were illuminated for 10 min by a continuous 808 nm laser (0.4 W/cm2). To investigate the effect of laser intensity on blood clot heat conversion, blood clots were irradiated by 808 lasers at different power (0 W/cm2, 0.2 W/cm2, 0.3 W/cm2, and 0.4 W/cm2) for about 10 min. IR thermal camera (Fortric 225) was used to monitor the temperature of blood clot and recorded. To study the experiment of the release of BMP-2 from BC gel under near-infrared irradiation. BMP-2-loaded blood clots were incubated with 1 mL sterilized neutral PBS at 37 °C. 100 μL liquids were collected at different time points for quantitative analysis, then added with the equal volume of PBS. BMP-2@BC gel were irradiated under 808 nm laser (0.4 W/cm2) at 0.3 h and 24 h. Samples should be collected before and after irradiation.
4.10. In vivo treatment experiments
For the establishment of skull defect model in mice, the severe circular defect was created in the skull of experimental Balb/c mice with the drill, without piercing the brain dura. Then the skull defect sites were injected with 100 μL free BMP-2 (500 ng/per mice) or implanted 100 μL blood clots/BMP-2-loaded blood clots. The surgical wound was carefully closed to avoid loss. 24 experimental mice were randomly divided into 6 groups as Group 1: Control, Group 2: Free BMP-2, Group 3: Irradiation (0.7 W/cm2, 10 min), Group 4: Blood clot, Group 5: Blood clot@BMP-2, and Group 6: Blood clot@BMP-2 with irradiation (0.7 W/cm2, 10 min) post-surgery. The frequency of irradiation was once every three days, a total of three times during the experiment period. All experiments are performed in a sterile environment, and the experimental protocols comply with animal ethics. The mice were anesthetized with 2.5% isoflurane inhalation, and the physiological conditions of the mice were monitored throughout the experiment. 6 weeks after surgery. The skulls of model mice were harvested and fixed in neutral formalin for subsequent micro-computed tomography (micro-CT) to evaluate regenerated new bone, and histological analysis was performed after decalcification.
For the preparation of the rat skull model, SD rats were fixed, anesthetized with isoflurane inhalation, and disinfected the surgery site with iodophor. An electric drill was used to create a circular full-thickness defect with 6–8 mm diameter in the cranial bone. The grouping and treatment of animals are consistent with the experiments of mice: Group 1: Control, Group 2: Free BMP-2 (1 μg/per rat), Group 3: Irradiation (0.7 W/cm2, 10 min), Group 4: Blood clot, Group 5: Blood clot@BMP-2, and Group 6: Blood clot@BMP-2 with irradiation (0.7 W/cm2, 10 min) post-surgery. An infrared thermal imaging camera was used to monitor the dynamic temperature of the defect sites under irradiation. The frequency of irradiation was once every three days, a total of three times during the experiment period. At 12 weeks after treatment, the cranial bones were collected and analyzed by micro-CT to evaluate the regeneration of newly-formed bone in the defect area after animal euthanasia. The quality of the new-formed bone was evaluated after establishing the 3D images of the critical calvarial defect. After decalcifying with 10% ethylenediaminetetraacetic acid (EDTA), the skull samples were dehydrated with graded ethanol solutions. Prepared samples were cut into 4 μm sections and stained with hematoxylin-eosin staining and Masson's trichrome staining kit according to the manufacture's protocol.
4.11. Immune evaluation
To evaluate the local osteoimmunology after treatment, mice with cranial defection were treated with BMP-2@BC + NIR irradiation or PBS. The heads were photographed on the third day, seventh day and the fourteenth day after treatment. The blood clots and adjacent local tissues in the defect of the mouse skull were removed for flow cytometric analysis. Flow cytometry antibodies included: FITC anti-mouse F4/80 Antibody (BioLegend, Cat# 123108), PE anti-mouse CD80 Antibody (BioLegend, Cat# 104708), APC anti-mouse CD206 Antibody (BioLegend, Cat# 141708), PE anti-mouse CD86 Antibody (BioLegend, Cat# 105008) and PE anti-mouse I-A/I-E (BioLegend, Cat# 107608). Local cytokines were measured through enzyme-linked immunosorbent kits (ELISA) including Mouse IL-6 ELISA MAX™ Deluxe (BioLegend, Cat# 431304), Mouse TNF-α ELISA MAX™ Deluxe (BioLegend, Cat# 430904), Mouse IL-10 ELISA MAX™ Deluxe (BioLegend, Cat# 431414), and Mouse IL-4 ELISA MAX™ Deluxe (BioLegend, Cat# 431104).
Micro-CT
The collected samples were taken out and fixed in neutralized formalin. SkyScan 1176 Micro-computed tomography (Aartselaar, Belgium) was used to measure the new bone formation. The scanning parameters were as follows: voltage 65 kV, current 385 μA and resolution 18 μm. The 3D models of the local site were reconstructed through Skyscan software. Regions of interest were outlined in new bone formation. The following parameters were tested: bone volume (BV; mm3) and bone volume/total volume (BV/TV; %)
4.12. Histology examination
To investigate the safety of the therapeutic method, the major organ of mice (heart, liver, lung, spleen, and kidney) were harvested after treatment about six weeks and fixed in 4% paraformaldehyde. Samples embedded routinely into paraffin were cut into 4 μm slices, stained with hematoxylin and eosin (H&E), and observed by Leica microscope (DM4000). At 6 weeks and 12 weeks, the specimens were collected and treated for histological analysis. The specimens were decalcified with 10% EDTA at room temperature according to previous procedures. Then, the decalcified specimens were dehydrated with gradient alcohol solutions (75%, 85%, 90%, 95%, and 100%) and embedded in paraffin. After being sectioned, the specimen sections were stained with H&E and Masson's trichrome to reveal the tissue ingrowth and new bone formation at defect sites.
4.13. Small animal imaging
After the mice were gas anesthetized with isoflurane, they were imaged with a small animal in vivo fluorescence imaging system (Lumina III) and observed every three days.
4.14. Statistical analysis
All analysis results in this work were presented as mean ± SD or mean ± SEM. Two-tailed Student's t-test was used to calculate the statistical differences between the two groups. One-way ANOVA was performed to compare the difference between more than two groups.
Author contributions
C.W., X. Z. and Q. F. designed the project. Q. F. and J. B. performed the experiments and collected the data. Q. F. and J. B. analyzed and interpreted the data. All authors contributed to the writing of the manuscript, discussed the results and implications, and edited the manuscript at all stages.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgment
This work was supported by the Program for Jiangsu Specially-Appointed Professors to C. W. This work was also supported by National Natural Science Foundation of China (No. 32022043, 81873995),the Preponderant Discipline Supporting Program of Discipline Construction Supporting Project of the Second Affiliated Hospital of Soochow University (XKTJ-XK202003), Suzhou Special Foundation of Clinical Key Diseases Diagnosis and Therapy (LCZX201904, LCZX201708), the Social Development Program for Clinical Advanced Technology in Jiangsu Province (BE2019662, BE2018656) and the Key Laboratory for Peripheral Nerve Injury Repair Research of Suzhou (SZS201720). The Advanced Ph.D. research project of the Second Affiliated Hospital of Soochow University (SDFEYBS2011) and The Open Project of Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices (KJS1905). This work is partly supported by Collaborative Innovation Center of Suzhou Nano Science and Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.04.008.
Contributor Information
Qin Fan, Email: qfan@stu.suda.edu.cn.
Jinyu Bai, Email: baijy@suda.edu.cn, baijinyu770@foxmail.com.
Huajian Shan, Email: 20184033005@stu.suda.edu.cn.
Ziying Fei, Email: zyfei@stu.suda.edu.cn.
Hao Chen, Email: 20194133090@stu.suda.edu.cn.
Jialu Xu, Email: jlxu7@stu.suda.edu.cn.
Qingle Ma, Email: qlma@stu.suda.edu.cn.
Xiaozhong Zhou, Email: zhouxz@suda.edu.cn.
Chao Wang, Email: cwang@suda.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Loi F., Cordova L.A., Pajarinen J., Lin T.H., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang D., Tare R.S., Yang L.Y., Williams D.F., Ou K.L., Oreffo R.O. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–382. doi: 10.1016/j.biomaterials.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R., Garcia A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan C.M., Shi Y.Y., Aalami O.O., Chou Y.F., Mari C., Thomas R., Quarto N., Contag C.H., Wu B., Longaker M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004;22(5):560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 5.Kloss F.R., Offermanns V., Kloss-Brandstatter A. Comparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects-A 12-month retrospective radiographic evaluation. Clin. Oral Implants Res. 2018;29(11):1163–1175. doi: 10.1111/clr.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhumiratana S., Bernhard J.C., Alfi D.M., Yeager K., Eton R.E., Bova J., Shah F., Gimble J.M., Lopez M.J., Eisig S.B., Vunjak-Novakovic G. Tissue-engineered autologous grafts for facial bone reconstruction. Sci. Transl. Med. 2016;8(343) doi: 10.1126/scitranslmed.aad5904. 343ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shegarfi H., Reikeras O. Review article: bone transplantation and immune response. J. Orthop. Surg. 2009;17(2):206–211. doi: 10.1177/230949900901700218. [DOI] [PubMed] [Google Scholar]
- 8.Mills L.A., Aitken S.A., Simpson A. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017;88(4):434–439. doi: 10.1080/17453674.2017.1321351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunetti G., Mori G., D'Amelio P., Faccio R. The crosstalk between the bone and the immune system: osteoimmunology. Clin. Dev. Immunol. 2013;2013:1–2. doi: 10.1155/2013/617319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arron J.R., Choi Y. Bone versus immune system. Nature. 2000;408(6812):535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 11.Takayanagi H. New developments in osteoimmunology. Nat. Rev. Rheumatol. 2012;8(11):684–689. doi: 10.1038/nrrheum.2012.167. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama M., Rhee C., Utsunomiya T., Zhang N., Ueno M., Yao Z., Goodman S.B. Modulation of the inflammatory response and bone healing. Front. Endocrinol. 2020;11:386. doi: 10.3389/fendo.2020.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono T., Okamoto K., Nakashima T., Nitta T., Hori S., Iwakura Y., Takayanagi H. IL-17-producing gammadelta T cells enhance bone regeneration. Nat. Commun. 2016;7:10928. doi: 10.1038/ncomms10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenblatt M.B., Shim J.H. Osteoimmunology: a brief introduction. Immune Netw. 2013;13(4):111–115. doi: 10.4110/in.2013.13.4.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parent M., Baradari H., Champion E., Damia C., Viana-Trecant M. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: a review of the parameters affecting the loading and release of the therapeutic substance. J. Contr. Release. 2017;252:1–17. doi: 10.1016/j.jconrel.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Deng C., Li J., Yao Q., Chang J., Wang L., Wu C. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials. 2019;196:138–150. doi: 10.1016/j.biomaterials.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Wei J., Jia J., Wu F., Wei S., Zhou H., Zhang H., Shin J.-W., Liu C. Hierarchically microporous/macroporous scaffold of magnesium–calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31(6):1260–1269. doi: 10.1016/j.biomaterials.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Clark A.Y., Martin K.E., Garcia J.R., Johnson C.T., Theriault H.S., Han W.M., Zhou D.W., Botchwey E.A., Garcia A.J. Integrin-specific hydrogels modulate transplanted human bone marrow-derived mesenchymal stem cell survival, engraftment, and reparative activities. Nat. Commun. 2020;11(1):114. doi: 10.1038/s41467-019-14000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann P., Willuweit A., Neffe A.T., Geisler S., Gebauer T.P., Beer S., Coenen H.H., Fischer H., Hermanns-Sachweh B., Lendlein A., Shah N.J., Kiessling F., Langen K.J. Bone regeneration induced by a 3D architectured hydrogel in a rat critical-size calvarial defect. Biomaterials. 2017;113:158–169. doi: 10.1016/j.biomaterials.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Li L., Li J., Guo J., Zhang H., Zhang X., Yin C., Wang L., Zhu Y., Yao Q. 3D molecularly functionalized cell‐free biomimetic scaffolds for osteochondral regeneration. Adv. Funct. Mater. 2018;29(6):1807356. doi: 10.1002/adfm.201807356. [DOI] [Google Scholar]
- 21.Deng C., Lin R., Zhang M., Qin C., Yao Q., Wang L., Chang J., Wu C. Micro/nanometer-structured scaffolds for regeneration of both cartilage and subchondral bone. Adv. Funct. Mater. 2019;29(4):1806068. doi: 10.1002/adfm.201806068. [DOI] [Google Scholar]
- 22.Bao M., Lou X., Zhou Q., Dong W., Yuan H., Zhang Y. Electrospun biomimetic fibrous scaffold from shape memory polymer of PDLLA-co-TMC for bone tissue engineering. ACS Appl. Mater. Interfaces. 2014;6(4):2611–2621. doi: 10.1021/am405101k. [DOI] [PubMed] [Google Scholar]
- 23.Shi W., Sun M., Hu X., Ren B., Cheng J., Li C., Duan X., Fu X., Zhang J., Chen H., Ao Y. Structurally and functionally optimized silk-fibroin–gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater. 2017;29(29):1701089. doi: 10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., Ma B., Liu F., Duan J., Wang S., Qiu J., Li D., Sang Y., Liu C., Liu D., Liu H. Polylactic acid nanopillar array-driven osteogenic differentiation of human adipose-derived stem cells determined by pillar diameter. Nano Lett. 2018;18(4):2243–2253. doi: 10.1021/acs.nanolett.7b04747. [DOI] [PubMed] [Google Scholar]
- 25.Okuchi Y., Reeves J., Ng S.S., Doro D.H., Junyent S., Liu K.J., El Haj A.J., Habib S.J. Wnt-modified materials mediate asymmetric stem cell division to direct human osteogenic tissue formation for bone repair. Nat. Mater. 2021;20(1):108–118. doi: 10.1038/s41563-020-0786-5. [DOI] [PubMed] [Google Scholar]
- 26.Bai S., Zhang X., Lv X., Zhang M., Huang X., Shi Y., Lu C., Song J., Yang H. Bioinspired mineral–organic bone adhesives for stable fracture fixation and accelerated bone regeneration. Adv. Funct. Mater. 2020;30(5):1908381. doi: 10.1002/adfm.201908381. [DOI] [Google Scholar]
- 27.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5(8):584–603. doi: 10.1038/s41578-020-0204-2. [DOI] [Google Scholar]
- 28.Wei W., Ma Y., Yao X., Zhou W., Wang X., Li C., Lin J., He Q., Leptihn S., Ouyang H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021;6(4):998–1011. doi: 10.1016/j.bioactmat.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekaran A., Garcia A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. 2011;96(1):261–272. doi: 10.1002/jbm.a.32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai J., Zhang Y., Fan Q., Xu J., Shan H., Gao X., Ma Q., Sheng L., Zheng X., Cheng W., Li D., Zhang M., Hao Y., Feng L., Chen Q., Zhou X., Wang C. Reactive oxygen species-scavenging scaffold with rapamycin for treatment of intervertebral disk degeneration. Adv. Healthc. Mater. 2020;9(3) doi: 10.1002/adhm.201901186. e1901186. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Huang H., Hao G., Zhang Y., Ding H., Fan Z., Sun L. 3D printing hydrogel scaffolds with nanohydroxyapatite gradient to effectively repair osteochondral defects in rats. Adv. Funct. Mater. 2020:2006697. doi: 10.1002/adfm.202006697. [DOI] [Google Scholar]
- 32.Xu C., Su P., Chen X., Meng Y., Yu W., Xiang A.P., Wang Y. Biocompatibility and osteogenesis of biomimetic Bioglass-Collagen-Phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials. 2011;32(4):1051–1058. doi: 10.1016/j.biomaterials.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 33.Simpson C.R., Kelly H.M., Murphy C.M. Synergistic use of biomaterials and licensed therapeutics to manipulate bone remodelling and promote non-union fracture repair. Adv. Drug Deliv. Rev. 2020;160:212–233. doi: 10.1016/j.addr.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z., Wu C., Xiao Y. Convergence of osteoimmunology and immunomodulation for the development and assessment of bone biomaterials. In: Corradetti B., editor. The Immune Response to Implanted Materials and Devices. Springer International Publishing; Cham: 2017. pp. 107–124. [Google Scholar]
- 35.Franz S., Rammelt S., Scharnweber D., Simon J.C. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32(28):6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 36.Gaharwar A.K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020;5(9):686–705. doi: 10.1038/s41578-020-0209-x. [DOI] [Google Scholar]
- 37.Fan Q., Ma Q., Bai J., Xu J., Fei Z., Dong Z., Maruyama A., Leong K.W., Liu Z., Wang C. An implantable blood clot-based immune niche for enhanced cancer vaccination. Sci. Adv. 2020;6(39) doi: 10.1126/sciadv.abb4639. eabb4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Cheng G., Xing X., Liu J., Cheng Y., Ye T., Wang Q., Xiao X., Li Z., Deng H. Near-infrared light-triggered porous AuPd alloy nanoparticles to produce mild localized heat to accelerate bone regeneration. J. Phys. Chem. Lett. 2019;10(15):4185–4191. doi: 10.1021/acs.jpclett.9b01735. [DOI] [PubMed] [Google Scholar]
- 39.Tong L., Liao Q., Zhao Y., Huang H., Gao A., Zhang W., Gao X., Wei W., Guan M., Chu P.K., Wang H. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11. doi: 10.1016/j.biomaterials.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Bouyer M., Guillot R., Lavaud J., Plettinx C., Olivier C., Curry V., Boutonnat J., Coll J.L., Peyrin F., Josserand V., Bettega G., Picart C. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials. 2016;104:168–181. doi: 10.1016/j.biomaterials.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herberg S., McDermott A.M., Dang P.N., Alt D.S., Tang R., Dawahare J.H., Varghai D., Shin J.Y., McMillan A., Dikina A.D., He F., Lee Y.B., Cheng Y., Umemori K., Wong P.C., Park H., Boerckel J.D., Alsberg E. Combinatorial morphogenetic and mechanical cues to mimic bone development for defect repair. Sci. Adv. 2019;5(8) doi: 10.1126/sciadv.aax2476. eaax2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mi M., Jin H., Wang B., Yukata K., Sheu T.-j., Ke Q.H., Tong P., Im H.-J., Xiao G., Chen D. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene. 2013;512(2):211–218. doi: 10.1016/j.gene.2012.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei F., Zhou Y., Wang J., Liu C., Xiao Y. The immunomodulatory role of BMP-2 on macrophages to accelerate osteogenesis. Tissue Eng. 2018;24(7–8):584–594. doi: 10.1089/ten.TEA.2017.0232. [DOI] [PubMed] [Google Scholar]
- 44.Cavallo C., Roffi A., Grigolo B., Mariani E., Pratelli L., Merli G., Kon E., Marcacci M., Filardo G. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. BioMed Res. Int. 2016:6591717. doi: 10.1155/2016/6591717. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu X., Li Y., Huang T., Yu Z., Ma K., Yang M., Liu Q., Pan H., Wang H., Wang J., Guan M. Runx2/Osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Adv. Sci. 2018;5(4):1700755. doi: 10.1002/advs.201700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida C.A., Furuichi T., Fujita T., Fukuyama R., Kanatani N., Kobayashi S., Satake M., Takada K., Komori T. Core-binding factor β interacts with Runx2 and is required for skeletal development. Nat. Genet. 2002;32(4):633–638. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- 47.Sinha K.M., Zhou X. Genetic and molecular control of osterix in skeletal formation. J. Cell. Biochem. 2013;114(5):975–984. doi: 10.1002/jcb.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anitua E., Andia I., Ardanza B., Nurden P., Nurden A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemostasis. 2004;91(1):4–15. doi: 10.1160/Th03-07-0440. [DOI] [PubMed] [Google Scholar]
- 49.Jang W.G., Kim E.J., Kim D.K., Ryoo H.M., Lee K.B., Kim S.H., Choi H.S., Koh J.T. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 2012;287(2):905–915. doi: 10.1074/jbc.M111.253187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masago Y., Hosoya A., Kawasaki K., Kawano S., Nasu A., Toguchida J., Fujita K., Nakamura H., Kondoh G., Nagata K. The molecular chaperone Hsp47 is essential for cartilage and endochondral bone formation. J. Cell Sci. 2012;125(Pt 5):1118–1128. doi: 10.1242/jcs.089748. [DOI] [PubMed] [Google Scholar]
- 51.Li Y., Chen S.K., Li L., Qin L., Wang X.L., Lai Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 2015;3(3):95–104. doi: 10.1016/j.jot.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 53.Klintstrom E., Smedby O., Klintstrom B., Brismar T.B., Moreno R. Trabecular bone histomorphometric measurements and contrast-to-noise ratio in CBCT. Dentomaxillofacial Radiol. 2014;43(8):20140196. doi: 10.1259/dmfr.20140196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sima C., Glogauer M. Macrophage subsets and osteoimmunology: tuning of the immunological recognition and effector systems that maintain alveolar bone. Periodontol. 2000;63(1):80–101. doi: 10.1111/prd.12032. 2013. [DOI] [PubMed] [Google Scholar]
- 55.Jenkins S.J., Ruckerl D., Cook P.C., Jones L.H., Finkelman F.D., van Rooijen N., MacDonald A.S., Allen J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Sci. 2011;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlundt C., Schell H., Goodman S.B., Vunjak-Novakovic G., Duda G.N., Schmidt-Bleek K. Immune modulation as a therapeutic strategy in bone regeneration. J. Exp. Orthop. 2015;2(1):1. doi: 10.1186/s40634-014-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front. Immunol. 2014;5(514):514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 60.Maruyama M., Rhee C., Utsunomiya T., Zhang N., Ueno M., Yao Z., Goodman S.B. Modulation of the inflammatory response and bone healing. Front. Endocrinol. 2020;11:386. doi: 10.3389/fendo.2020.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trejo I., Kojouharov H., Chen-Charpentier B. Modeling the macrophage-mediated inflammation involved in the bone fracture healing process. Math. Comput. Appl. 2019;24(1):12. doi: 10.3390/mca24010012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.