This cross-sectional study evaluates the independent associations of chronological age and frailty (physiological age) with outcomes following vestibular schwannoma resection.
Key Points
Question
What are the independent prognostic associations of chronological age and frailty (physiological age) with outcomes following vestibular schwannoma (VS) resection?
Findings
In this population-based, cross-sectional analysis of outcomes following VS resection, an assessment of 27 313 patients using the National Inpatient Sample demonstrated that mortality and extended hospital lengths of stay were independently associated with increasing frailty and not with increasing age.
Meaning
Although these findings warrant prospective validation, frailty may be more accurate for predicting surgical outcomes and guiding treatment decisions than advanced patient age alone following VS resection.
Abstract
Importance
Although numerous studies have evaluated the influence of advanced age on surgical outcomes following vestibular schwannoma (VS) resection, few if any large-scale investigations have assessed the comparative prognostic effects of age and frailty. As the population continues to age, it is imperative to further evaluate treatment and management strategies for older patients.
Objective
To conduct a population-based evaluation of the independent associations of chronological age and frailty (physiological age) with outcomes following VS resection.
Design, Setting, and Participants
In this large-scale, multicenter, cross-sectional analysis, weighted discharge data from the National Inpatient Sample were searched to identify adult patients (≥18 years old) who underwent VS resection from 2002 through 2017 using International Classification of Diseases, Ninth Revision, Clinical Modification and Tenth Revision, Clinical Modification codes. Data collection and analysis took place September to December 2020.
Main Outcomes and Measures
Complex samples regression models and receiver operating characteristic curve analysis were used to evaluate the independent associations of frailty and age (along with demographic confounders) with complications and discharge disposition. Frailty was evaluated using the previously validated 11-point modified frailty index (mFI).
Results
Among the 27 313 patients identified for VS resection, the mean (SEM) age was 50.4 (0.2) years, 15 031 (55.0%) were women, and 4720 (21.0%) were of non-White race/ethnicity, as determined by the National Inpatient Sample data source. Of the included patients, 15 090 (55.2%) were considered robust (mFI score = 0), 8204 (30.0%) were prefrail (mFI score = 1), 3022 (11.1%) were frail (mFI score = 2), and 996 (3.6%) were severely frail (mFI score ≥3). On univariable analysis, increasing frailty was associated with development of postoperative hemorrhagic or ischemic stroke (odds ratio [OR], 2.44 [95% CI, 2.07-2.87]; area under the curve, 0.73), while increasing age was not. Following multivariable analysis, increasing frailty and non-White race/ethnicity were independently associated with both mortality (adjusted OR [aOR], 2.32 [95% CI, 1.70-3.17], and aOR, 3.05 [95% CI, 1.02-9.12], respectively) and extended hospital stays (aOR, 1.54 [95% CI, 1.41-1.67], and aOR, 1.71 [95% CI, 1.42-2.05], respectively), while increasing age was not. Increasing frailty (aOR, 0.61 [95% CI, 0.56-0.67]), age (aOR, 0.98 [95% CI, 0.97-0.99]), and non-White race/ethnicity (aOR, 0.62 [95% CI 0.51-0.75]) were all independently associated with routine discharge.
Conclusions and Relevance
In this cross-sectional study, findings suggest that frailty may be more accurate for predicting outcomes and guiding treatment decisions than advanced patient age alone following VS resection.
Introduction
Frailty, broadly defined as decreased physiological reserve due to the cumulative effects of comorbid conditions, has been validated as a robust predictor of postoperative morbidity and mortality across various surgical disciplines.1,2,3,4 Although advanced age has been shown to predispose patients to operative complications and worse outcomes following certain surgical procedures, a growing body of evidence demonstrates that older patients in many cases represent good candidates for surgery and share similar rates of favorable outcomes compared with younger individuals.5,6,7
Resection of vestibular schwannoma (VS), a benign, slow-growing neoplasm of the eighth cranial nerve, represents one such surgical intervention in which patients of advanced age perform well. A previous study8 evaluating the surgical treatment of VS found no difference in the development of postoperative complications, facial nerve disorders, or hearing loss between older and younger patients, which suggests that advanced age alone should not be an absolute contraindication for surgery. Given the increased aging of the population,9 it is imperative to explore the dichotomy between physiological and chronological age to better inform management strategies practiced in older patients. We hypothesized that frailty would serve as a better prognostic tool than age alone for patients following VS resection. To address this, we used the National Inpatient Sample (NIS) to conduct a large-scale retrospective inquiry into outcomes following VS resection.
Methods
Data Source
The NIS, which was developed and is maintained by the Healthcare Cost and Utilization Project (HCUP), is among the largest publicly accessible inpatient care databases in the United States. Yearly unweighted data approximate 7 000 000 patients, reflecting a 20% stratified sample of all HCUP-participating community hospitals nationally. The large sample size afforded by the NIS allows for substantive inquiry into health care utilization, access, charges, quality, and outcomes, as well as reliable reproduction of national estimates annually. Data elements include demographic characteristics, hospital and regional information, diagnoses, procedures, and discharge disposition for all documented patients. More information regarding the NIS and data access can be found at http://www.hcup-us.ahrq.gov. Owing to the use of publicly accessible and deidentified data in this analysis, the institutional review board of the University of New Mexico deemed the study exempt from approval. For the same reasons, patient consent was neither sought nor necessary.
Patient Selection
All NIS discharge data from 2002 through 2017 were searched using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes for vestibular schwannoma (ICD-9-CM 225.1 and ICD-10-CM D33.3) and corresponding procedure codes for resection (ICD-9-CM 04.01 and ICD-10-CM 00BN0ZZ). Patients lacking age, those younger than 18 years, and those missing diagnosis or procedure data were excluded from the analysis. Patient demographic characteristics included age, sex, and race/ethnicity. The NIS/HCUP makes 2 comments concerning data collection for race/ethnicity: (1) the database retains information on the race of the patient as provided by the data source, and (2) ethnicity takes precedence over race in setting the HCUP value for race. Given the prevalence of White patients in this analysis (82.4% of the cohort), race/ethnicity was dichotomized into White and non-White categories.
Modified Frailty Index
The modified frailty index10 (mFI) is composed of 11 baseline factors present on admission: history of diabetes, chronic obstructive pulmonary disease or recent pneumonia, congestive heart failure, myocardial infarction, percutaneous coronary stent/intervention or angina, hypertension, impaired sensorium, cerebrovascular accident without neurological deficit, cerebrovascular accident with neurological deficit, peripheral vascular disease or rest pain, and dependent functional status. Determination of functional status was made using billing codes encompassing impairments to activities of daily living, fall risk assessment, bed-ridden status, use of cane or wheelchair, and chronic mobility impairments. Comorbid conditions, each assigned a value of 1, were summed for each patient to create a composite score. The cohort was subsequently stratified into 4 subcohorts based on increasing frailty: robust (mFI score = 0), prefrail (mFI score = 1), frail (mFI score = 2), and severely frail (mFI score ≥3).
Clinical End Points
The primary clinical end points of this investigation were those related to outcome, including in-hospital mortality, routine discharge (home or to self-care), and extended hospital length of stay (defined as >6 days, the value corresponding to the 75th percentile of length of stay in the cohort). Postoperative complications were evaluated as a secondary end point. Neurological complications included postoperative cerebrovascular infarction or hemorrhage, abducens nerve paralysis, trochlear nerve paralysis, facial nerve disorder, vocal cord paralysis, dysphagia, tinnitus, hearing loss, acute hydrocephalus, cerebral edema, meningitis, placement of external ventricular drain or ventriculoperitoneal shunt, and cerebrospinal fluid leak, including rhinorrhea or otorrhea. Medical complications evaluated included pneumonia, urinary tract infection, deep venous thrombosis or pulmonary embolism, acute myocardial infarction, sepsis, acute kidney injury, and mechanical ventilation.
Statistical Analysis
All analyses were performed within a complex samples function with appropriate stratum and cluster variables and discharge weights per HCUP guidelines to account for NIS sampling design and to ultimately yield accurate national estimates.11 Descriptive statistics were performed to measure variation in baseline demographic characteristics as well as in clinical complications and outcomes among the frailty subcohorts. Dichotomous variables were assessed using Pearson χ2 test, while estimated marginal means for continuous baseline parameters were computed by t test within a complex samples general linear model and reported with SEM.
Simple univariable regression models were used to evaluate unadjusted associations of age and frailty, respectively, with development of postoperative cerebrovascular infarction or hemorrhage. Complex samples multivariable logistic regression models were constructed to evaluate the adjusted, independent associations of age, frailty (by mFI score), non-White race/ethnicity, and female sex with in-hospital mortality, routine discharge, and extended hospital stays (corresponding odds ratios [ORs] reported with 95% CIs). A Bonferroni correction was applied to all 3 models to account for multiple comparisons.
A bias assessment was undertaken to evaluate differences in frailty and age distribution between patients excluded from the analysis owing to missing documentation of race/ethnicity and those who were included (compared using χ2 test; eTable 1 in the Supplement). Sensitivity analysis was conducted in which frailty was evaluated as a binary (robust [mFI score = 0] in comparison to frail [mFI score ≥1]) and ordinal (robust [mFI score = 0], prefrail [mFI score = 1], frail [mFI score = 2], and severely frail [mFI score ≥3]) parameter for the 3 clinical end points analyzed in the primary multivariable analytical models (eTable 2 in the Supplement). Similarly, the 3 models were also evaluated with race/ethnicity as a categorical variable comprising all racial/ethnic groups (White, Black, Hispanic, Asian or Pacific Islander, Native American, and other). Further sensitivity testing was performed with propensity-score matching using a nearest-neighbor approach without replacement and a match sensitivity of 0.01. In observational studies, propensity-score matching is used to mimic the effect of randomization while minimizing confounding by indication. Matching parameters included age, sex, and race/ethnicity for frailty as a binary variable as defined previously (eTable 2 in the Supplement).
Receiver operating characteristic (ROC) curve analysis was undertaken to evaluate the comparative discrimination of age and frailty (by mFI score) for postoperative cerebrovascular infarction or hemorrhage and in-hospital mortality. Areas under the curve (AUC) were reported with 95% CIs. A combined frailty–race/ethnicity model for prediction of in-hospital mortality was constructed based on predicted probabilities of a multivariable logistic regression model subsequently evaluated using ROC curve analysis. The AUCs were documented for this model along with sensitivity and specificity based on the Youden index, the point on the ROC curve corresponding to the greatest combination of these 2 parameters. All statistical analyses were performed in SPPS, version 26 (IBM), and statistical significance was evaluated at 2-sided P < .05.
Results
Frailty Distribution and Baseline Demographic Characteristics
Among the 27 313 patients identified for VS resection, 15 090 (55.2%) were robust (mFI score = 0), 8204 (30.0%) were prefrail (mFI score = 1), 3022 (11.1%) were frail (mFI score = 2), and 996 (3.6%) were severely frail (mFI score ≥3). The complete cohort (mean [SEM] age, 50.4 [0.2] years) was 55.0% (n = 15 031) female, and 21.0% (n = 4720) were of non-White race/ethnicity (race/ethnicity was documented for 82.4% of the cohort [n = 22 494]) (Table 1). Among those of non-White race/ethnicity, 1016 (21.5%) were African American, 1745 (37.0%) were Hispanic, 893 (18.9%) were Asian or Pacific Islander, 79 (1.7%) were Native American, and 986 (20.9%) identified as a race other than those listed.
Table 1. Stratified Comparisons of Demographic Characteristics, Complications, and Outcomes Among Subcohorts of Patients Undergoing Vestibular Schwannoma Resection.
| Characteristic | No. (%)a,b | P value | ||||
|---|---|---|---|---|---|---|
| Total cohortc | Robust | Prefrail | Frail | Severely frail | ||
| Total reported | 27 313 (100) | 15 090 (55.2) | 8204 (30.0) | 3022 (11.1) | 996 (3.6) | NA |
| Age, mean (SEM), y | 50.4 (0.2) | 46.5 (0.3) | 53.3 (0.3) | 58.0 (0.4) | 61.2 (0.8) | <.001d |
| 18-29 | 2175 (8.0) | 1659 (11.0) | 443 (5.4) | 59 (1.9) | 14 (1.5) | |
| 30-39 | 3582 (13.1) | 2726 (18.1) | 737 (9.0) | 99 (3.3) | 20 (2.0) | |
| 40-49 | 6428 (23.5) | 4205 (27.9) | 1646 (20.1) | 469 (15.5) | 108 (10.9) | |
| 50-59 | 8259 (30.2) | 4279 (28.4) | 2681 (32.7) | 1039 (34.4) | 259 (26.0) | |
| 60-69 | 4972 (18.2) | 1770 (11.7) | 1958 (23.9) | 905 (29.9) | 339 (34.1) | |
| 70-79 | 1650 (6.0) | 410 (2.7) | 656 (8.0) | 373 (12.4) | 211 (21.1) | |
| ≥80 | 246 (0.9) | 40 (0.3) | 83 (1.0) | 79 (2.6) | 45 (4.5) | |
| Female sex | 15 031 (55.0) | 8625 (57.2) | 4378 (53.4) | 1545 (51.1) | 483 (48.5) | .002d |
| Non-White race/ethnicitye | 4720 (21.0) | 2364 (19.2) | 1499 (22.0) | 645 (25.3) | 212 (25.8) | .003d |
| Neurological complications | ||||||
| Postoperative cerebrovascular infarction or hemorrhage | 368 (1.3) | 46 (0.3) | 144 (1.8) | 88 (2.9) | 90 (9.0) | <.001d |
| Nerve paralysis | ||||||
| Abducens | 341 (1.2) | 193 (1.3) | 94 (1.1) | 37 (1.3) | 15 (1.5) | .96 |
| Trochlear | 30 (0.1) | 16 (0.1) | 0 | NR | NR | .08 |
| Facial nerve disorder | 3725 (13.6) | 2023 (13.4) | 1174 (14.3) | 391 (12.9) | 138 (13.8) | .81 |
| Vocal cord paralysis | 334 (1.2) | 164 (1.1) | 120 (1.5) | 50 (1.7) | 0 | .23 |
| Dysphagia | 1150 (4.2) | 390 (2.6) | 433 (5.3) | 203 (6.7) | 124 (12.4) | <.001d |
| Tinnitus | 1974 (7.2) | 1129 (7.5) | 609 (7.4) | 191 (6.3) | 44 (4.4) | .31 |
| Hearing loss | 10 189 (37.3) | 5265 (34.9) | 3256 (39.7) | 1302 (43.1) | 366 (36.7) | <.001d |
| Acute hydrocephalus | 1484 (5.4) | 545 (3.6) | 519 (6.3) | 266 (8.8) | 153 (15.4) | <.001d |
| Cerebral edema | 1555 (5.7) | 562 (3.7) | 579 (7.1) | 211 (7.0) | 203 (20.4) | <.001d |
| Meningitis | 167 (0.6) | 45 (0.3) | 89 (1.1) | NR | NR | .01d |
| External ventricular drain or ventriculoperitoneal shunt | 505 (1.8) | 170 (1.1) | 180 (2.2) | 95 (3.1) | 60 (6.0) | <.001d |
| Cerebrospinal fluid leak (rhinorrhea or otorrhea) | 580 (2.1) | 349 (2.3) | 167 (2.0) | NR | NR | .35 |
| Medical complications | ||||||
| Pneumonia | 285 (1.0) | 0 | 118 (1.4) | 92 (3.0) | 75 (7.6) | <.001d |
| Urinary tract infection | 558 (2.0) | 181 (1.2) | 235 (2.9) | 79 (2.6) | 64 (6.4) | <.001d |
| Deep venous thrombosis/pulmonary embolism | 236 (0.9) | 83 (0.6) | 84 (1.0) | 45 (1.5) | 24 (2.5) | .01d |
| Acute myocardial infarction | 56 (0.2) | 25 (0.2) | 26 (0.3) | NR | NR | .61 |
| Sepsis | 49 (0.2) | 0 | 34 (0.4) | NR | NR | <.001d |
| Acute kidney injury | 99 (0.4) | NR | NR | 39 (1.3) | 15 (1.5) | <.001d |
| Mechanical ventilation | 668 (2.4) | 164 (1.1) | 225 (2.7) | 159 (5.3) | 119 (12.0) | <.001d |
| Discharge disposition | ||||||
| Routine discharge | 22 108 (80.9) | 13 208 (87.5) | 6355 (77.5) | 2017 (66.7) | 529 (53.1) | <.001d |
| In-hospital mortality | 75 (0.3) | NR | NR | 24 (0.8) | 15 (1.6) | <.001d |
| Extended hospital stay (>6 d, 75th percentile) | 4861 (17.8) | 2035 (13.5) | 1689 (20.6) | 777 (25.7) | 360 (36.1) | <.001d |
Abbreviations: mFI, modified frailty index; NA, not applicable; NR, not reported owing to small sample size.
Values presented as No. (%) for categorical data (compared by χ2 test) and mean (SEM) for continuous data (compared by t test).
Subcohorts are defined by score on the 11-point mFI as robust (mFI score = 0), prefrail (mFI score = 1), frail (mFI score = 2), and severely frail (mFI score ≥3).
Data from 2002 through 2017 comprise the study cohort, which is inclusive of limited data from the study years 2007 through 2010.
Statistical significance was evaluated at P < .05 for univariable comparison.
Discrepancies in percentages reflect missing data for race.
The robust, prefrail, frail, and severely frail subcohorts differed significantly on the basis of age (46.5 vs 53.3 vs 58.0 vs 61.2 mean years, respectively; P < .001), sex (57.2% vs 53.4% vs 51.1% vs 48.5% female, respectively; P = .002), and race/ethnicity (19.2% vs 22.0% vs 25.3% vs 25.8% non-White, respectively; P = .003) (Table 1). A bias assessment demonstrated that patients excluded from the analysis owing to lack of documentation of race/ethnicity did not differ on the basis of frailty or age distribution (eTable 1 in the Supplement).
Among comorbidities composing the mFI, 8.3% (n = 2274) of the cohort presented with diabetes, 9.9% (n = 2699) with chronic obstructive pulmonary disease or recent pneumonia, 0.7% (n = 196) with congestive heart failure, 2.0% (n = 560) with a previous percutaneous coronary intervention/stent or angina, 1.3% (n = 342) with a previous myocardial infarction, 34.5% (n = 9433) with hypertension, 0.3% (n = 74) with impaired sensorium, 1.1% (n = 300) with a previous cerebrovascular accident without a neurological deficit, 2.3% (n = 619) with a previous cerebrovascular accident with a neurological deficit, 0.7% (n = 188) with peripheral vascular disease or rest pain, and 2.9% (n = 780) with a dependent functional status (Table 2).
Table 2. Distribution of Characteristics of the Modified Frailty Index Among the Study Cohort (N = 27 313).
| Characteristic | No. (%) |
|---|---|
| Diabetes | 2274 (8.3) |
| Chronic obstructive pulmonary disease or recent pneumonia | 2699 (9.9) |
| Congestive heart failure | 196 (0.7) |
| Myocardial infarction | 342 (1.3) |
| Percutaneous coronary intervention/stent or angina | 560 (2.0) |
| Hypertension | 9433 (34.5) |
| Impaired sensorium | 74 (0.3) |
| Cerebrovascular accident without neurological deficit | 300 (1.1) |
| Cerebrovascular accident with neurological deficit | 619 (2.3) |
| Peripheral vascular disease or rest pain | 188 (0.7) |
| Dependent functional status | 780 (2.9) |
Frailty and Complications
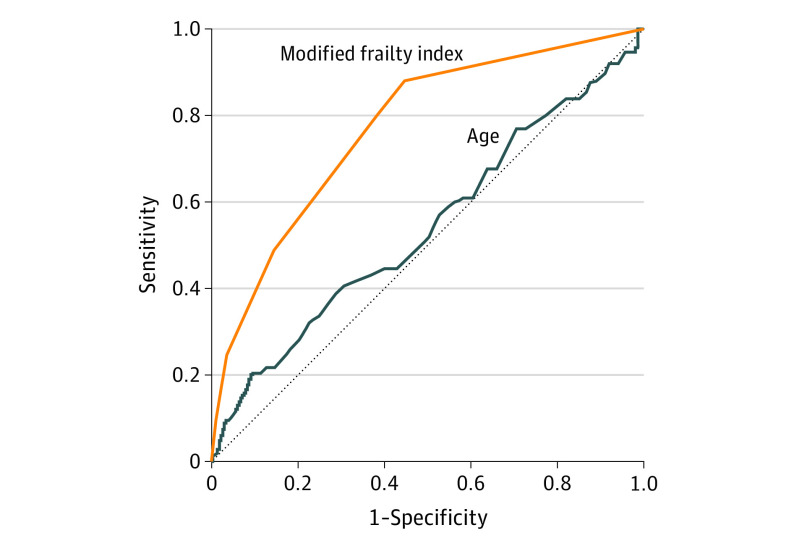
Increasing frailty was associated with numerous neurological and medical complications during hospitalization (Table 1). On univariable analysis, increasing frailty was associated with development of postoperative cerebrovascular infarction or hemorrhage (OR, 2.44 [95% CI, 2.07-2.87]; AUC, 0.73 [95% CI, 0.72-0.83]), while increasing age was not (OR, 1.01 [95% CI, 0.97-1.01]; AUC, 0.54 [95% CI, 0.47-0.61]) (Figure 1).
Figure 1. Receiver Operating Characteristic Curve Analysis.
Comparison of areas under the curve of modified frailty index (orange) and age (blue) for postoperative cerebrovascular infarction or hemorrhage.
Frailty and Outcome
Following VS resection, 80.9% (n = 22 108) of the cohort was routinely discharged, while 0.3% (n = 75) of patients died postoperatively (Table 1). A hospital length of stay of 6 days corresponded with the 75th percentile for patients in the cohort. Following multivariable logistic regression analysis, increasing frailty (adjusted OR [aOR], 2.32 [95% CI, 1.70-3.17]) and non-White race/ethnicity (aOR, 3.05 [95% CI, 1.02-9.12]) were independently associated with in-hospital mortality, while age was not. Increasing frailty and non-White race/ethnicity were similarly the only 2 parameters independently associated with extended hospital stays (aOR, 1.54 [95% CI, 1.41-1.67], and aOR, 1.71 [95% CI 1.42-2.05], respectively). Age (aOR, 0.98 [95% CI, 0.97-0.99]), non-White race/ethnicity (aOR, 0.62 [95% CI, 0.51-0.75]), and increasing frailty (aOR, 0.61 [95% CI, 0.56-0.67]) were all independently associated with routine discharge (Table 3). Sensitivity analysis evaluating frailty as a binary and ordinal parameter demonstrated similar associations with respect to clinical outcome (eTable 2 in the Supplement). Following propensity matching to account for residual confounding by age, frailty remained independently associated with clinical outcomes (eTable 2 in the Supplement). Multivariable models for clinical outcomes evaluating race/ethnicity as a categorical parameter are presented in eTable 3 in the Supplement.
Table 3. Comparative Evaluation of Frailty and Age on Clinical Outcomea.
| Clinical Outcome | Odds ratio (95% CI) | P value |
|---|---|---|
| In-hospital mortality | ||
| Age | 0.99 (0.93-1.05) | .65 |
| Female sex | 1.78 (0.56-5.61) | .33 |
| Non-White race/ethnicity | 3.05 (1.02-9.12) | .05b |
| Modified frailty index score | 2.32 (1.70-3.17) | <.001b |
| Routine discharge | ||
| Age | 0.98 (0.97-0.99) | <.001b |
| Female sex | 0.74 (0.63-0.87) | <.001b |
| Non-White race/ethnicity | 0.62 (0.51-0.75) | <.001b |
| Modified frailty index score | 0.61 (0.56-0.67) | <.001b |
| Hospital length of stay (>6 d, 75th percentile) | ||
| Age | 1.01 (0.99-1.01) | .10 |
| Female sex | 1.04 (0.89-1.21) | .62 |
| Non-White race/ethnicity | 1.71 (1.42-2.05) | <.001b |
| Modified frailty index score | 1.54 (1.41-1.67) | <.001b |
Significant associations of age (continuous parameter), female sex, non-White race/ethnicity, and modified frailty index score (continuous parameter) with clinical outcome were evaluated using complex samples multivariable logistic regression models.
Statistical significance following Bonferroni correction for multiple comparisons.
ROC Curve Analysis and Combined Frailty–Race/Ethnicity Model for In-Hospital Mortality
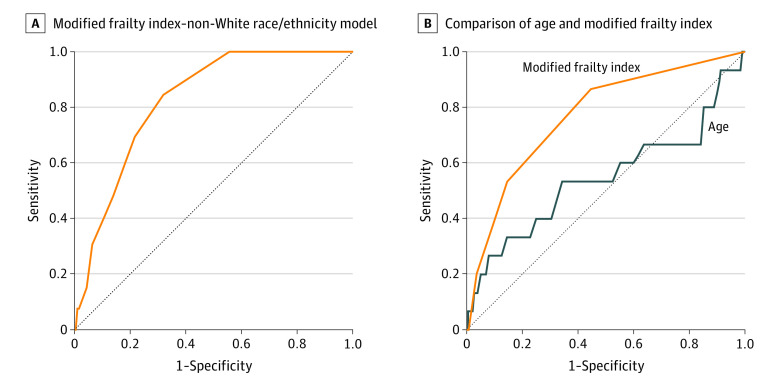
Age (as a continuous parameter) achieved an AUC of 0.55 (95% CI, 0.37-0.73) (sensitivity, 0.53; specificity, 0.66) for in-hospital mortality, while frailty (by mFI score) achieved an AUC of 0.77 (95% CI, 0.65-0.89) (sensitivity, 0.87; specificity, 0.55). Optimal discrimination of frailty for in-hospital mortality occurred at a threshold of an mFI score of 1 based on the Youden index. A combined frailty–non-White race/ethnicity model achieved an AUC of 0.83 (95% CI, 0.75-0.90) (sensitivity, 0.85; specificity, 0.68) for in-hospital mortality (Figure 2).
Figure 2. Receiver Operating Characteristic Curve Analysis for In-Hospital Mortality.
Discussion
This investigation represents 1 of few population-based evaluations of outcomes following VS resection in the literature and, to our knowledge, is the only large-scale, multicenter study to evaluate the comparative efficacy of frailty and age as prognostic indicators of morbidity and mortality. The primary findings demonstrate independent associations of increasing frailty, quantified using the mFI, and not increasing age with in-hospital mortality, extended lengths of stay, and development of postoperative stroke.
Although previous studies by McCutcheon et al12 and McClelland et al13 demonstrated increasing mortality rates in older patients and an association between increasing age and mortality following VS resection, respectively, neither considered comorbidities or other metrics of illness severity as potential confounders in multivariable analyses. Although concomitant consideration of comorbidities and age in assessing VS resection outcomes were undertaken by 2 previous studies14,15 using the NIS, the present analysis is, to our knowledge, the first to use a systematically validated frailty index on a large scale. Also to our knowledge, the influence of frailty on pertinent outcomes using the NIS for patients undergoing VS resection has not been evaluated previously. Sonig et al14 manually extracted 28 comorbid conditions that they deemed important for the analysis (not based on a validated index of any kind). Importantly, these likely encompassed events that occurred during the hospitalization, such as fluid or electrolyte disturbances or infectious complications. Sylvester et al15 evaluated individual comorbidities as confounders in a multivariable analysis. Neither of these analyses appropriately evaluate frailty, which is the cumulative physiological effect of comorbid baseline conditions present on admission. This distinction is crucial because it not only highlights the novelty of the present study, but also establishes the importance of the premise for the comparison of chronological and physiological age, which neither of the aforementioned studies assesses. Additionally, to the best of our knowledge, we present the largest and most up-to-date evaluation of short-term outcomes and clinical complications following VS resection in the literature.
Only 2 previous studies have used an mFI to evaluate admission predictors of VS outcomes. A single-center retrospective study conducted by Casazza et al16 found increasing frailty (by mFI score), and not age, independently associated with increasing hospital length of stay—a finding further bolstered by the present analysis. A recent study17 using the American College of Surgeons National Surgical Quality Improvement Program database arrived at the same finding using a mFI with 5 baseline factors but did not use multivariable analysis to evaluate the comparative influences of age and frailty on complications or clinical outcome. Taken collectively, the body of literature examining outcomes following VS resection demonstrates a significant prognostic value of frailty relative to increasing age alone with respect to clinical outcome.
Consideration of the cumulative effects of comorbid conditions provides added insight into the nuances of patient sociocultural factors and their influence on medical care. Remarkably, the present analysis found that non-White patients were 3 times more likely to experience in-hospital mortality and less likely (aOR, approximately 0.6) to be routinely discharged in comparison with White individuals following VS resection. This finding merits considerable attention given the hypothesis that racial or ethnic disparities in outcome following treatment may be explained by the variance in the distribution of comorbidities among these groups and that controlling for this would presumably remove the downstream variance observed in outcomes.18 Yet, in the present analysis, the association between non-White race/ethnicity and in-hospital mortality (as well as nonroutine discharge disposition) persists even after controlling for both frailty and age.
Unfortunately, this finding is not unprecedented: a previous study13 using NIS data to evaluate postoperative outcomes following VS resection demonstrated that African American patients were 9 times more likely to die following surgery compared with White patients over a decade-long interval. Moreover, an investigation by Muhlestein et al19 found that minority (non-White) race/ethnicity independently increased the risk of extended length of stay and nonroutine discharge following craniotomy for intracranial neoplasm resection, which suggests that this phenomenon is observed more broadly in the field of neurosurgery. The present demonstration of a similar pattern of racial disparities in post-VS resection outcomes over a period of nearly 2 decades ought to serve as motivation to examine why this may be the case and to initiate focused efforts in improving outcomes for these patients. A current national discussion regarding implicit bias, which suggests that some health care professionals may display biases toward minority patients with the potential to influence diagnosis and treatment decisions,20 may be an appropriate starting point.
Limitations
The primary limitations of this study are owed to its retrospective design and to other common limitations associated with use of administrative databases, such as errors in the post hoc assignment of diagnostic and procedural codes following discharge. Because frailty parameters were determined using these billing codes, it is ultimately not possible to ascertain the reliability of all potential comorbid conditions recorded for a given patient and consequent influence on frailty index calculation. Added inconsistency may be precipitated by working across the ICD-9-CM to ICD-10-CM coding transition, which occurred in 2015. Moreover, the NIS lacks the granularity to evaluate important prognostic factors associated with VS outcomes, including tumor size, location, and surgical approach, which could have been evaluated as potential confounders to the association between frailty and outcome. Finally, although propensity-score matching was used in sensitivity analyses, unmeasured confounding by indication and residual confounding cannot be ruled out for potential influence on the derived estimates of this analysis.
Conclusions
Results of this cross-sectional study demonstrate an independent association of frailty, and not increasing age, with mortality and extended hospital lengths of stay following VS resection. Although these retrospective findings warrant prospective validation, they provide preliminary evidence to suggest that frailty may be more accurate for predicting VS resection outcomes than advanced patient age alone. This distinction is crucial for the evaluation of decisions regarding treatment and for prognostication of outcome.
eTable 1. Bias Assessment – Comparison of Frailty Stratification between Included and Excluded Patients for Multivariable Analysis
eTable 2. Sensitivity Analysis – Significant Associations of Frailty with Clinical Outcome
eTable 3. Significant Associations of Frailty with Clinical Outcome – Additional Assessment of Racial Categories
References
- 1.Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139(8):783-789. doi: 10.1001/jamaoto.2013.3969 [DOI] [PubMed] [Google Scholar]
- 2.Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27(7):904-908. doi: 10.1016/j.avsg.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 3.Runner RP, Bellamy JL, Vu CCL, Erens GA, Schenker ML, Guild GN III. Modified frailty index is an effective risk assessment tool in primary total knee arthroplasty. J Arthroplasty. 2017;32(9S):S177-S182. doi: 10.1016/j.arth.2017.03.046 [DOI] [PubMed] [Google Scholar]
- 4.Mogal H, Vermilion SA, Dodson R, et al. Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2017;24(6):1714-1721. doi: 10.1245/s10434-016-5715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobran M, Marini A, Nasi D, et al. Clinical outcome of patients over 90 years of age treated for chronic subdural hematoma. J Korean Neurosurg Soc. 2019. doi: 10.3340/jkns.2018.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassman SD, Carreon LY, Dimar JR, Campbell MJ, Puno RM, Johnson JR. Clinical outcomes in older patients after posterolateral lumbar fusion. Spine J. 2007;7(5):547-551. doi: 10.1016/j.spinee.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 7.Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2006;88(12):2714-2720. doi: 10.2106/00004623-200612000-00019 [DOI] [PubMed] [Google Scholar]
- 8.Bowers CA, Gurgel RK, Brimley C, et al. Surgical treatment of vestibular schwannoma: does age matter? World Neurosurg. 2016;96:58-65. doi: 10.1016/j.wneu.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 9.Wroe PC, Finkelstein JA, Ray GT, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis. 2012;205(10):1589-1592. doi: 10.1093/infdis/jis240 [DOI] [PubMed] [Google Scholar]
- 10.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104-110. doi: 10.1016/j.jss.2013.01.021 [DOI] [PubMed] [Google Scholar]
- 11.Khera R, Angraal S, Couch T, et al. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318(20):2011-2018. doi: 10.1001/jama.2017.17653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCutcheon BA, Grauberger J, Murphy M, et al. ; Mayo Clinic Neuro-Informatics Laboratory . Is patient age associated with perioperative outcomes after surgical resection of benign cranial nerve neoplasms? World Neurosurg. 2016;89:101-107. doi: 10.1016/j.wneu.2016.01.089 [DOI] [PubMed] [Google Scholar]
- 13.McClelland S III, Guo H, Okuyemi KS. Morbidity and mortality following acoustic neuroma excision in the United States: analysis of racial disparities during a decade in the radiosurgery era. Neuro Oncol. 2011;13(11):1252-1259. doi: 10.1093/neuonc/nor118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonig A, Khan IS, Wadhwa R, Thakur JD, Nanda A. The impact of comorbidities, regional trends, and hospital factors on discharge dispositions and hospital costs after acoustic neuroma microsurgery: a United States nationwide inpatient data sample study (2005-2009). Neurosurg Focus. 2012;33(3):E3. doi: 10.3171/2012.7.FOCUS12193 [DOI] [PubMed] [Google Scholar]
- 15.Sylvester MJ, Shastri DN, Patel VM, et al. Outcomes of vestibular schwannoma surgery among the elderly. Otolaryngol Head Neck Surg. 2017;156(1):166-172. doi: 10.1177/0194599816677522 [DOI] [PubMed] [Google Scholar]
- 16.Casazza GC, McIntyre MK, Gurgel RK, et al. Increasing frailty, not increasing age, results in increased length of stay following vestibular schwannoma surgery. Otol Neurotol. 2020;41(10):e1243-e1249. doi: 10.1097/MAO.0000000000002831 [DOI] [PubMed] [Google Scholar]
- 17.Goshtasbi K, Abouzari M, Soltanzadeh-Zarandi S, et al. The association of age, body mass index, and frailty with vestibular schwannoma surgical morbidity. Clin Neurol Neurosurg. 2020;197:106192. doi: 10.1016/j.clineuro.2020.106192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opara F, Hawkins K, Sundaram A, Merchant M, Rasmussen S, Holmes L. Impact of comorbidities on racial/ethnic disparities in hypertension in the United States. ISRN Public Health. 2013;2013:967518. doi: 10.1155/2013/967518 [DOI] [Google Scholar]
- 19.Muhlestein WE, Akagi DS, Chotai S, Chambless LB. The impact of race on discharge disposition and length of hospitalization after craniotomy for brain tumor. World Neurosurg. 2017;104:24-38. doi: 10.1016/j.wneu.2017.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi: 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Bias Assessment – Comparison of Frailty Stratification between Included and Excluded Patients for Multivariable Analysis
eTable 2. Sensitivity Analysis – Significant Associations of Frailty with Clinical Outcome
eTable 3. Significant Associations of Frailty with Clinical Outcome – Additional Assessment of Racial Categories