Abstract
This case series investigates the frequency and type of SARS-CoV-2 nasopharyngeal test complications in Helsinki, Finland.
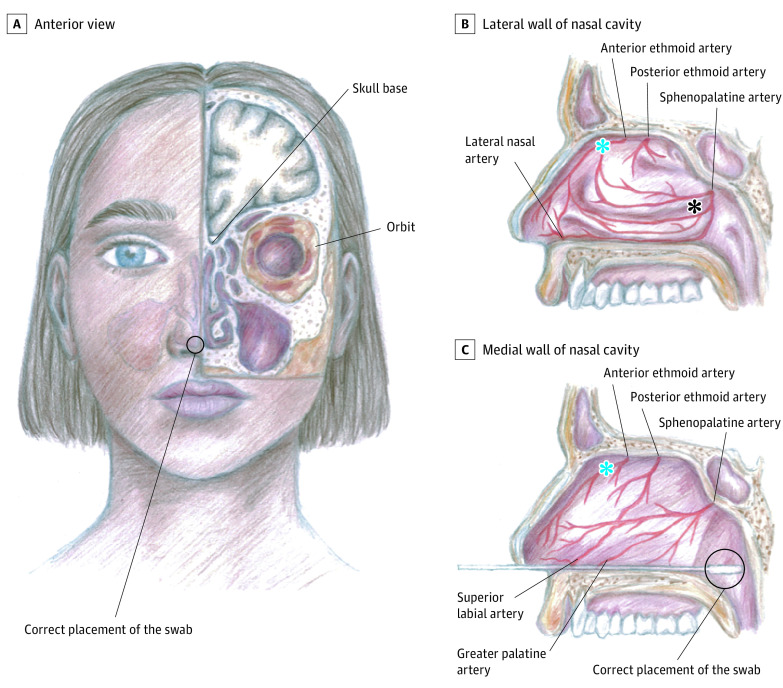
During the COVID-19 pandemic, numerous swab samples have been taken for SARS-CoV-2 reverse transcriptase–polymerase chain reaction (RT-PCR) testing. Nasopharyngeal sampling is considered safe, despite adjacent vital structures (eg, orbit, skull base, rich vasculature; Figure). However, single case reports1,2,3,4 and clinical observations indicate the possibility of severe complications. This case series investigated the frequency and type of SARS-CoV-2 nasopharyngeal test complications.
Figure. Anatomical Structures Related to Nasopharyngeal Sampling.
Correct placement of the swab and origins of the nasal bleeds in this study. The life-threatening bleeds resulted from the anterior ethmoid artery, 1 from the lateral and 1 from the medial nasal wall (blue asterisks). One bleed resulted from the sphenopalatine artery (black asterisk), and in 1 case, the bleeding site could not be identified. A, Anterior view; B, Lateral wall of nasal cavity; C, Medial wall of nasal cavity.
Methods
All patients presenting to the dedicated otorhinolaryngology emergency department (ED) of Helsinki University Hospital Department of Otorhinolaryngology–Head and Neck Surgery between March 1 and September 30, 2020, were retrospectively screened for complications after SARS-CoV-2 nasopharyngeal swab sampling. Those experiencing sampling complications underwent medical record review.
The number of SARS-CoV-2 tests performed in the catchment population (1.6 million people) of the Helsinki University Hospital during the same time period was obtained from the Finnish Institute for Health and Welfare. This study was approved by the Research Administration of Helsinki University Hospital (HUS/58/2020). As this was a retrospective registry study with no patient intervention, ethics committee approval and informed consent were not required by Finnish national legislation in accordance with the Medical Research Act of Finland 488/1999.
Results
During the 7-month study period, 643 284 SARS-CoV-2 RT-PCR tests were performed. Eight complication-related visits (7 females, 1 male; age range, 14.0-78.6 years; mean [SD] age, 39.5 [20.9] years) were identified in 2899 otorhinolaryngology ED patients—4 nasal bleeds and 4 broken swabs, all occurring immediately after sampling (Table). None of these 8 patients tested positive for COVID-19.
Table. Treatment and Sequelae of 8 Patients Treated for Complications After SARS-CoV-2 Nasopharyngeal Swab Test.
| Clinical event | Specific occurrence | Measure of occurrence | |||
|---|---|---|---|---|---|
| Predisposing condition | Broken swab (n = 4) | Epistaxis (n = 4) | |||
| Previous rhinosurgery | Septoplasty | 1 | 1 | ||
| Rhinologic disorder | Nasal congestion | 1 | 1 | ||
| Septal deviation | 1 | 1 | |||
| Hematologic disorder | Idiopathic thrombocytopenic purpura | 0 | 1 | ||
| Cardiovascular disease | Coronary heart disease | 1 | 1 | ||
| Medication | Anticoagulant | 1 | 1 | ||
| Other | Pregnancy | 0 | 1 | ||
| Treatment and sequelae in patients with broken swabs | Patient | ||||
| 1 | 2 | 3 | 4 | ||
| Procedure (local anesthesia) | Removal of the broken swab | 1 | 1 | 0a | 1 |
| Complications | NA | No | No | No | No |
| Diagnostic, treatment, and sequelae in patients with epistaxis | Patient | ||||
| 5 | 6 | 7 | 8 | ||
| Blood loss | Hemoglobin level, g/dL | 6.4 | 6.4 | 9.6 | 10.2 |
| Procedure (local anesthesia) | Anterior nasal packing | 3 | 3 | 7 | 0 |
| Posterior nasal packing | 2 | 0 | 0 | 0 | |
| Bipolar coagulation | 1 | 0 | 3 | 0 | |
| Surgical (general anesthesia) | Anterior ethmoidal artery ligation | 1 | 0 | 0 | 0 |
| Posterior nasal packing | 0 | 1 | 0 | 0 | |
| Bipolar coagulation | 0 | 1 | 0 | 0 | |
| Endovascular procedures | Sphenopalatine artery embolization | 0 | 0 | 1 | 0 |
| Medication | Local hemostatic | 0 | 3 | 1 | 1 |
| Systemic antibiotics | Yes | Yes | Yes | 0 | |
| Local antibiotics | 0 | Yes | 0 | 0 | |
| Iron supplements (oral or intravenous) | Yes | Yes | Yes | No | |
| Blood transfusion | Red blood cells, 49 g Hb/unit | 6 | 2 | 1 | 0 |
| Complication | Local infection | Yes | Yes | Yes | No |
| Systemic infection | No | No | Yesb | No | |
| Septum perforation, scarring | 0 | 1 | 0 | 0 | |
Abbreviations: Hb, hemoglobin; NA, not applicable.
Patient swallowed the broken tip of the swab during the procedure.
Staphylococcus aureus sepsis.
The frequency of complications requiring treatment in the ED was 1.24 per 100 000 performed SARS-CoV-2 tests. The broken swabs were removed via nasal endoscopy under local anesthesia, whereas the nasal bleeds required medication, numerous nasal packings, and surgical and endovascular procedures and led to fetal risk, sepsis, and blood transfusions (Table). Half of the bleeds were potentially life threatening (hemoglobin level fell below 6.5 g/dL [to convert to g/L, multiply by 10.0]). Massive bleeding complicated localization of the bleeds (shown in Figure). Infections, as well as intranasal adhesions and septal perforations, likely resulted from the repetitive nasal packings.
Discussion
Timely and reliable testing is important in controlling the COVID-19 pandemic. Nasopharyngeal swab RT-PCR testing is often used as the main diagnostic test method because it yields early results with moderate sensitivity and excellent specificity.5
The frequency of complications was extremely low in this study. All complications seemed to involve an incorrect sampling technique: excess use of force or an overly cranial direction of the swab. While the patients who experienced broken swabs fared well, the patients with epistaxis had rockier recuperations. The complications also exposed personnel to the risk of an aerosol-generating procedure.
Literature regarding SARS-CoV-2 sampling complications is scarce. Breaking of the swab tip has resulted in a foreign body in the nasal cavity,1 the esophagus2 and, after sampling through tracheostomy, the bronchus.3 A case of test-related cerebrospinal fluid leak, probably owing to preexisting encephalocele, has been reported.4
Sampling should always be performed bearing in mind the anatomical structures of the nasal cavity and its surroundings to ensure safe sampling and correct results.5,6 Force should never be used, especially in patients with known prior operations of the nose or skull base. The sampling swab should be directed along the nasal floor, not too laterally nor too cranially, until resistance is encountered (Figure).6
The retrospective setting is a limitation of this study. It should be noted that Finland has a national public health service. Of the Helsinki University Hospital’s catchment population (1.6 million), all severe acute otorhinolaryngology problems are treated solely in our 1 ED. Patients presenting with minor complications may have been treated at other facilities, but we did not have access to this information. Furthermore, no private otorhinolaryngologist offices have been open for patients with suspected COVID-19. Nevertheless, this study is an apt representation of patients with SARS-CoV-2 nasopharyngeal swab test complications in a large tertiary care referral center.
Based on the results, the risk for a severe complication requiring specialist-level care after SARS-CoV-2 nasopharyngeal swab testing is extremely low. Nonetheless, complications involve anatomically challenging locations and may be life threatening. To avoid complications, correct sampling techniques are crucial.
References
- 1.Mughal Z, Luff E, Okonkwo O, Hall CEJ. Test, test, test—a complication of testing for coronavirus disease 2019 with nasal swabs. J Laryngol Otol. 2020;134(7):646-649. doi: 10.1017/S0022215120001425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Luca L, Maltoni S. Is naso-pharyngeal swab always safe for SARS-CoV-2 testing? an unusual, accidental foreign body swallowing. Clin J Gastroenterol. 2021;14(1):44-47. doi: 10.1007/s12328-020-01236-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain MH, Siddiqui S, Mahmood S, Valsamakis T. Tracheal swab from front of neck airway for SARS-CoV-2; a bronchial foreign body. BMJ Case Rep. 2020;13(8):e237787. doi: 10.1136/bcr-2020-237787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan CB, Schwalje AT, Jensen M, et al. Cerebrospinal fluid leak after nasal swab testing for coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(12):1179-1181. doi: 10.1001/jamaoto.2020.3579 [DOI] [PubMed] [Google Scholar]
- 5.Green DA, Zucker J, Westblade LF, et al. Clinical performance of SARS-CoV-2 molecular tests. J Clin Microbiol. 2020;58(8):e00995-20. doi: 10.1128/JCM.00995-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins TS, Wu AW, Ting JY. SARS-CoV-2 nasopharyngeal swab testing—false-negative results from a pervasive anatomical misconception. JAMA Otolaryngol Head Neck Surg. 2020;146(11):993-994. doi: 10.1001/jamaoto.2020.2946 [DOI] [PubMed] [Google Scholar]