Abstract
Nodular fasciitis is a benign tumor of soft tissues originating from the proliferation of fibroblasts and myofibroblasts, generally developing between the subcutaneous tissue and the underlying muscular layer. Nodular fasciitis predominantly localizes in the upper extremities, trunk, head and neck. Biomolecular and immunohistochemical analyses result essential to demonstrate the benign origin of the process, also confirmed by very low recurrence rate after complete excision, which represents the gold standard for treatment. We report the case of a 36 years-old man who developed a nodular protuberance clinically evident in the upper-left side of the thorax. We further, highlight the main characteristics of this rare neoplasm trough a thorough review of the literature.
Keywords: Nodular fasciitis, Ultrasound Magnetic resonance imaging, Soft tissue mass, Mesenchymal tissue
Introduction
Nodular fasciitis (NF) represents a benign tumor of soft tissues typical of young-middle age adults (third-fifth decade), substantially equally distributed through both sexes [1]. In a recent retrospective study, Lu et al. [2] evaluated 272 patients (160 males and 112 females) affected by this condition, with a range of age from newborn up to 77 years (mean and median ages: 36 years; peak incidence: fourth decade) and a minimal male predominance (male-to-female ratio: 1.4:1).
It originates from the proliferation of fibroblasts and myofibroblasts [3] and generally develops between the subcutaneous tissue and the underlying muscular layer, although other localizations - intramuscular, intradermal and periosteal - have been described [2].
The most frequent clinical presentation of nodular fasciitis consists in a solid, painless, well-defined solitary nodule [4], usually hard at palpation [5].
With the rare exception of giant nodular fasciitis [6,7], dimensions are usually below 3-4 centimeters [7]; history of previous trauma can be referred in a low percentage of cases (10%) [8].
Histological features are strictly related to the inherent phlogistic reaction: immature fibroblast-like spindle cells are mixed with chronic inflammatory cells, capillary proliferation, myxoid matrix and extravasated erythrocytes [9].
Biomolecular analysis must be assessed to demonstrate the typical nuclear chromatin pattern indicating the benign origin of nodular fasciitis [10].
Microscopic features present a widely heterogeneous scenario : cellular-stroma ratio can be very different between cases, ranging from hypercellular lesions to ones with prevalent hyalinized stromal matrix [2].
Immunohistochemical assessment reveals a statistically significant expression of smooth muscle actin, calponin, muscle specific actin and CD10; moreover, positivity for CD68 marker was observed for intralesional histiocytes and small multinucleated giant cells [2].
Because of its high cellularity and mitotic activity - as expression of rapid growth – nodular fasciitis is often erroneously placed in the spectrum of muscular malignant neoplasms (sarcoma or metastasis) [4]. However, the aforementioned features are not specific and differential diagnosis includes also a multitude of benign lesions, i.e. eosinophilic granuloma, aneurysmal bone cyst, hamartoma, osteoma, osteochondroma or chondroma [11], [12], [13].
Nodular fasciitis may occur in every part of the body, presenting elective localization in the upper extremities and trunk [2,7,14], as well as head and neck [15,16]- the latter, recognizes a particular involvement of infants and children [1].
Other rarer locations regard salivary glands [17,18], mesentery [19], vulva [20] and breast [8,21,22]. Localizations and frequency with percentage for each site are summarized in Table 1.
Table 1.
Most common localizations of nodular fasciitis , modified from Lu et al. (2).
| Localization | Prevalence |
|---|---|
| Upper extremities | 34% |
| Head and neck | 24% |
| Trunk | 21% |
| Lower extremities | 14% |
| Breast | 3.3% |
| Groin | 2.9% |
| Vulva | 0.8% |
Nodular fasciitis has a low recurrence rate after complete excision, which represents the gold standard for diagnosis and treatment [13].
We report the case of a 36- years old man who developed a nodular protuberance clinically evident in the upper-left side of thorax. We further, highlight the main characteristics of this rare neoplasm trough a thorough review of the literature.
Case report
A 36-years old man presented with a solid, asymptomatic nodule localized in the left parasternal region of the thorax, at the level of the second sternocostal joint, progressively increased in volume in the last four months.
At the clinical examination the mass appeared firm, with dimensions of 3 × 2.5 cm, without macroscopic signs of inflammation.
Anamnesis was negative for trauma or cancer; standard blood tests didn't reveal significant alterations of basic parameters.
A targeted ultrasound (US) was performed using a high-frequency linear array transducer (11 MHz) (Esaote MyLab E, Milan, Italy). US showed, in correspondence with the clinically palpable tumefaction, within sub-fascial layer of left pectoralis major muscle, a hyperechoic nodular mass of 32 × 18 × 29 mm, with some internal septa and an incomplete external capsule (Figs. 1A andB). Neither signs of invasions of surrounding structures (Fig. 1C), nor abnormal lymph nodes were found in the explored region (Fig. 1D).
Fig. 1.
Ultrasound assessment shows an iso-hyperechoic nodular mass of 32 × 18 × 29 mm, partially encapsulated and provided with a median septum (A), with no signs of invasions of surrounding structures (B); itdevelops superficially within sub-fascial layer of left major pectoralis muscle (C), in correspondence with second sternochondral joint. No adenopathies were found in the explored region (D).
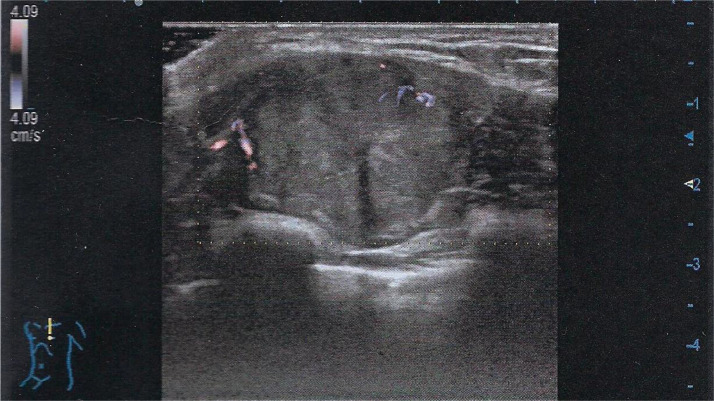
The color Doppler examination revealed weak signals in the peripheral area of the nodule (Fig. 2).
Fig. 2.
Color Doppler examination reveals weak peripheral signals.
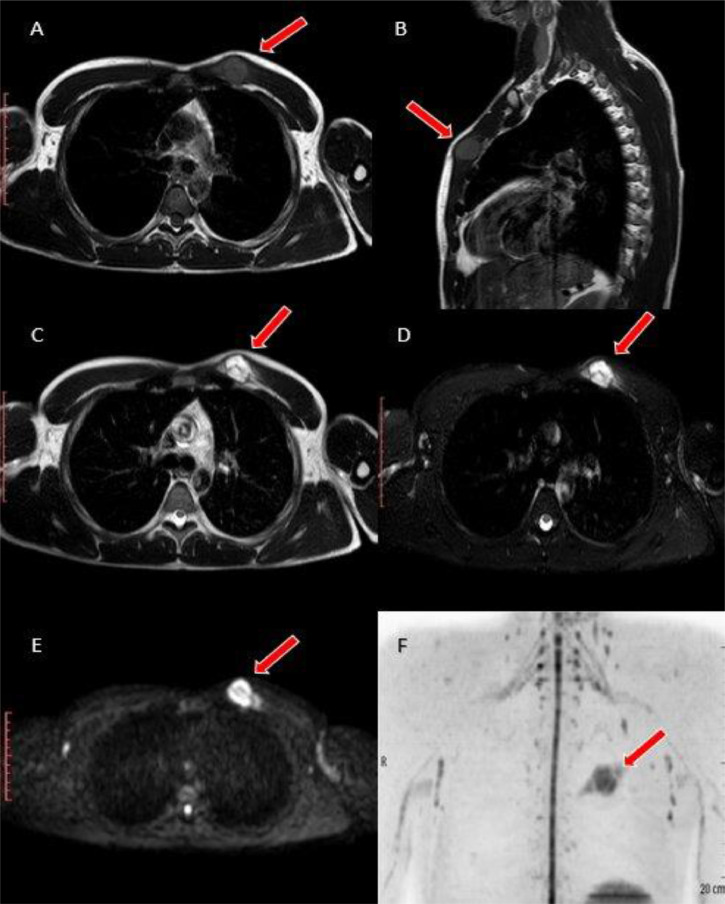
The patient was therefore scheduled for a MRI scan (Philips 1.5 T Achieva Dstream, Netherlands), that confirmed the presence of a circumscribed round mass, slightly hyperintense in T1-W sequences, with inhomogeneous hyperintensity in T2-W ones and high restricted diffusion in DWI, with development within fatty tissue underlying the muscolo-tendinous junctions of pectoralis major muscle. No evidence of macroscopic invasion of adjacent musculoskeletal compartments was identified (Fig. 3).
Fig. 3.
MRI scan confirms the presence of a circumscribed nodular lesion, tenuously hyperintense compared to adjacent muscular structures in T1-W sequences (A: axial, B: sagittal), inhomogeneously hyperintense in T2-W ones (C) and SPAIR (D), that moreover shows elevated restriction of water diffusion in DWI (E: axial, F: coronal MIP with background signal suppression). It's located within fatty tissue between minor and major pectoralis muscles: no macroscopic local invasion of the adjacent compartments was observed.
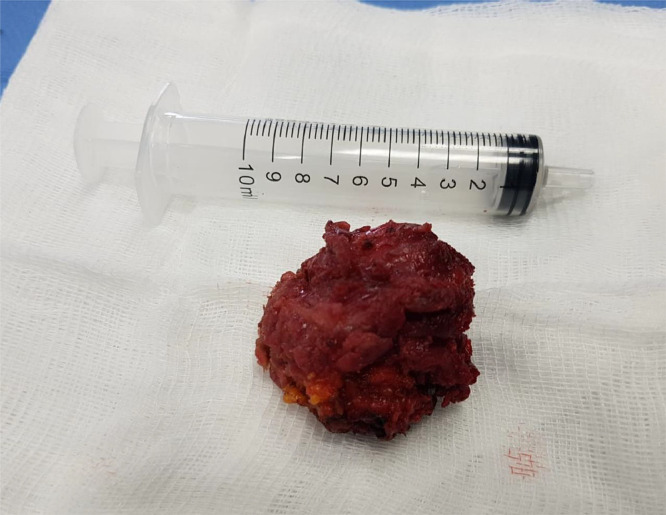
Finally, the patient underwent surgical en-bloc excision of the nodule for a microscopic evaluation (Fig. 4).
Fig. 4.
Gross Pathology of nodular fasciitis demonstrates a relatively well-circumscribed lesion, without a clear capsule.
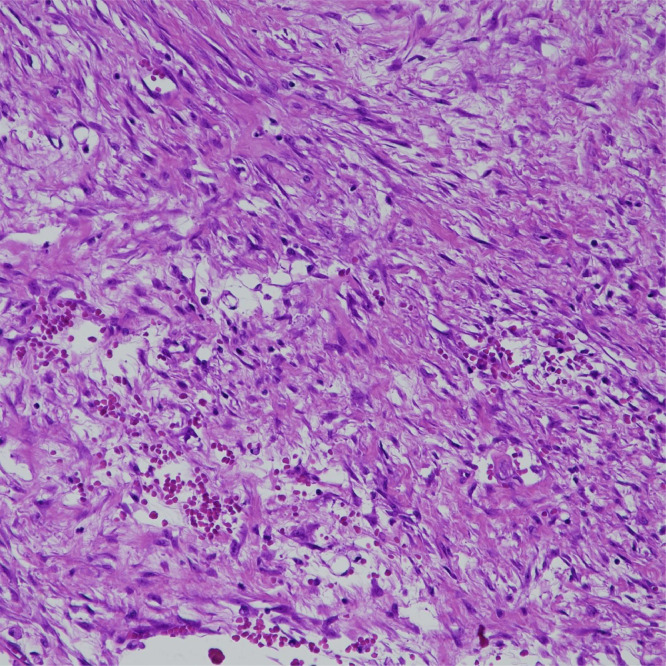
Histopathological examination showed expression of Vimentin and AML and negativity for S100, CD34 and Desmin, with MIB1 less than 5%. The first report leaned to a myxofibrosarcoma; a second view demonstrated instead the nature of nodular fasciitis (Fig. 5).
Fig. 5.
Histopathological examination (Hematoxylin-Eosin stain, 20X magnification): microscopically striated muscle appeared infiltrated by well-circumscribed nodular proliferation along the fascia. Marginal areas presented more fibrous stroma and the proliferating cells closely remembered immature fibroblasts and myofibroblasts of granulation tissue. There were scattered microhemorrhages between spindle cells and some lymphocytes and sidero-phages. The diagnosis of nodular fasciitis, intramuscular type, was made.
Therefore, the diagnosis of nodular fasciitis was made. The patient underwent a clinical follow-up and no appreciable signs suggestive for local recurrences have been identified after nearly 2 years.
Discussion
Nodular fasciitis is a benign tumor originating from mesenchymal tissue.
This condition has been described for the first time in 1955 by Konwaler et al. [23], who initially referred to as “Pseudosarcomatous fibromatosis”.
Price et al. [15] firstly used the term “nodular fasciitis” because of the origin of tumor mainly from superficial and deep fascial layers.
Bochaton-Piallat et al. [24] highlighted the role of myofibroblasts in fibrosis and main molecular patterns of basic inflammatory process; indeed, nodular fasciitis shares lots of intrinsic characteristics with sarcoma, and this aspect leads often to misdiagnosis. Namely, nodular fasciitis is characterized by the presence of a rapid-growing solid nodule with scarce and unspecific symptoms.
Local excision guarantees complete resolution, with a minimal recurrence rate after surgical treatment. Moreover, it can sporadically show a spontaneous slow regression [16]. Metastatic spread has never been demonstrated.
Diagnostic gold standard is represented by hematoxylin-eosin evaluation: in our case, which shows a morphologic overlap to low-grade myxofibrosarcoma, the rarity of incidence of this last one provides an essential issue orienting towards a correct diagnosis.
Due to the concomitant expression by myofibroblasts of the same markers both in nodular fasciitis and in myxofibrosarcoma, immunohistochemical analysis is not diriment: however, it results essential to evaluate the proliferation and to rule out other similar mimicking conditions.
Nodular fasciitis has been pathologically classified into 3 subtypes - according to predominant histological features [5]: myxoid (type 1), cellular (type 2), and fibrous (type 3). Type 1 lesions are composed of spindle, plump or stellate fibroblast-like cells embedded in myxomatous stroma rich in hyaluronidase-digestible acid mucopolysaccharide. This type is contradistinguished by abundance of immature capillaries running in a parallel direction, frequent red cells extravasation and considerable inflammatory changes. Type 2 lesions show a higher cellularity and less plentiful ground substances. The fibroblast-like spindle cells have been revealed to be large and plump with vesicular nuclei. Type 3 lesions demonstrate an increased collagen production, and fibroblast-like cells are slenderer and spindle-shaped [5].
The histological appearance of nodular fasciitis may mutate from active myxoid to cellular, before changing lastly to the mature fibrous type. Myxoid type of nodular fasciitis should be differentiated from the myxoid variant of malignant fibrous histiocytoma, which usually occurs in older patients.
By the way, these classifications should not be conceived as each other independent, but rather continuous: indeed, it's not rare to observe the coexistence of different types in the same lesion, sometimes representing one the evolution of the other [5].
Imaging remarks are not specific for nodular fasciitis. US findings consist in some typical patterns that well correlate to clinical and pathological evidences, with predominant subdivision into solid and cystic lesions.
CT evaluation shows iso-hypodensity compared with adjacent muscles, with peripheral rim-like enhancement in some contrast-enhanced scans [2].
On MRI, this condition usually demonstrates homogenous hypo-isointensity of signal on T1-W scans and heterogeneous hyperintensity on T2-W, with variable grade of contrast enhancement appreciable on the border [2]. However, the lesion can sometimes appear hyperintense even in T1-W sequences [25].
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) reveals frequently a significantly increased uptake of 18F-FDG in the areas characterized by rich cellularity, as expression of high mitotic activity [26].
Difficulties in the correct characterization and treatment of nodular fasciitis has been reported throughout the literature. Focusing on thoracic localization, this condition may show clinical features and imaging findings similar to those ones of chest wall sarcoma. Indeed, nodular fasciitis presents often a rapid growth and can be easily mistaken for a malignancy also because of its microscopic characteristics, concerning rich cellularity and high mitotic activity, thus making histopathologic assessment very challenging.
Abnormal elevated proliferative activity can be explained even by molecular rearrangement, as demonstrated by Erickson-Johnson et al. [27], who focused on the role of MYH9-USP6 hybrid gene fusion, expressed in 92% of nodular fasciitis' cases, thus suggesting the intrinsic neoplastic nature of this nosological entity.
A study of Son et al. [28] stated the role of some specific molecules in pathological evaluation. HuR and COX-2 expression results useful in differentiating nodular fasciitis from low-grade sarcoma and may be used to determine the unknown biologic behavior of proliferative spindle cell lesions, particularly in those of a borderline nature. In low-grade sarcoma, there are moderate or strong COX-2 immunoreactivity correlated with nuclear or cytoplasmic HuR expression; these aspects are not identified in nodular fasciitis.
Conclusion
Our case shows a typical clinical and diagnostic picture of a nodular fasciitis. We point out the particular localization of this case to include this entity in the possible differential diagnoses of thoracic lesions - as chest is rarely involved, and the upper limb shows the highest incidence.
In light of its unspecific characteristics, a correct diagnosis can be reached only evaluating all the related features: in particular, a detailed clinico-pathological correlation carried out through an accurate collection of anamnestic data, associated to the criterion of consensual temporal stability, result pivotal to accurately discern the process.
For these reasons, further work is needed to discover other specific immunohistochemical markers, in order to develop codified protocols in anatomopathological assessment with the goal of accurate diagnosis and proper intervention, thus avoiding overdiagnoses of malignant.
The patient gave consent for images or other clinical information relating to his case to be referred in a medical publication. All provided data were reported anonymously.
Patient consent
The patient gave consent for images or other clinical information relating to his case to be referred in a medical publication. All provided data were reported anonymously.
Footnotes
Competing interests: The Authors declare no conflict of interest and/or commercial involvement in this manuscript.
References
- 1.Stout AP. Pseudosarcomatous fascitis in children. Cancer. 1961;14:1216–1222. doi: 10.1002/1097-0142(196111/12)14:6<1216::aid-cncr2820140611>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Lu L, Lao IW, Liu X, Yu L, Wang J. Nodular fasciitis: a retrospective study of 272 cases from China with clinicopathologic and radiologic correlation. Ann Diagn Pathol. 2015;19(3):180–185. doi: 10.1016/j.anndiagpath.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Wagner LM, Gelfand MJ, Laor T, Ryckman FC, Al-Ghawi H, Bove KE. A welcome surprise: nodular fasciitis presenting as soft tissue sarcoma. J Pediatr Hematol Oncol. 2011;33(4):316–319. doi: 10.1097/MPH.0b013e3181e88649. [DOI] [PubMed] [Google Scholar]
- 4.Plaza JA, Mayerson J, Wakely PE. Nodular fasciitis of the hand: a potential diagnostic pitfall in fine-needle aspiration cytopathology. Am J Clin Pathol. 2005;123(3):388–393. doi: 10.1309/PWD0-HB51-1L3V-R56W. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology (Phila) 1984;16(2):161–166. doi: 10.3109/00313028409059097. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49(8):1668–1678. doi: 10.1002/1097-0142(19820415)49:8<1668::aid-cncr2820490823>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita H, Yonemoto T, Kamoda H. Giant protruding nodular fasciitis of the anterior chest wall clinically mimicking a soft tissue sarcoma. Case Rep Orthop. 2019 doi: 10.1155/2019/4174985. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6636556/ Availbale at: Accessed March 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paliogiannis P, Cossu A, Palmieri G. Breast nodular fasciitis: A comprehensive review. Breast Care. 2016;11(4):270–274. doi: 10.1159/000448185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown V, Carty NJ. A case of nodular fascitis of the breast and review of the literature. The Breast. 2005;14(5):384–387. doi: 10.1016/j.breast.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Dahl I, Akerman M. Nodular fasciitis a correlative cytologic and histologic study of 13 cases. Acta Cytol. 1981;25(3):215–223. [PubMed] [Google Scholar]
- 11.Nagano H, Kiyosawa T, Aoki S, Azuma R. A case of nodular fasciitis that was difficult to distinguish from sarcoma. Int J Surg Case Rep. 2019;65:27–31. doi: 10.1016/j.ijscr.2019.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee SJ, Ryu JK, Kim JH, Lim SJ. Nodular fasciitis of the breast: two cases with a review of imaging findings. Clin Imaging. 2014;38(5):730–733. doi: 10.1016/j.clinimag.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Kessels LW, Simsek S, Van Hattum AH, Stam F, Comans EFI. Nodular fasciitis: an unexpected finding on computed tomography and positron emission tomography. Eur J Intern Med. 2004;15(3):183–185. doi: 10.1016/j.ejim.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Suh JH, Yoon JS, Park CB. Nodular fasciitis on chest wall in a teenager: a case report and review of the literature. J Thorac Dis. 2014;6(6) doi: 10.3978/j.issn.2072-1439.2014.05.18. E108–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price EB., Silliphant WM, Shuman R. Nodular fasciitis: a clinicopathologic analysis of 65 cases. Am J Clin Pathol. 1961;35:122–136. doi: 10.1093/ajcp/35.2.122. [DOI] [PubMed] [Google Scholar]
- 16.Allen PW. Nodular fasciitis. Pathology (Phila) 1972;4(1):9–26. doi: 10.3109/00313027209068920. [DOI] [PubMed] [Google Scholar]
- 17.Gibson TC, Bishop JA, Thompson LDR. Parotid gland nodular fasciitis: A clinicopathologic series of 12 Cases with a review of 18 cases from the literature. Head Neck Pathol. 2015;9(3):334–344. doi: 10.1007/s12105-014-0594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen KTK, Bauer V. Nodular fasciitis presenting as parotid tumor. Am J Otolaryngol. 1987;8(3):179–181. doi: 10.1016/s0196-0709(87)80043-1. [DOI] [PubMed] [Google Scholar]
- 19.Shiga M, Okamoto K, Matsumoto M. Nodular fasciitis in the mesentery, a differential diagnosis of peritoneal carcinomatosis. World J Gastroenterol. 2014;20(5):1361–1364. doi: 10.3748/wjg.v20.i5.1361. Baishideng Publishing Group Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connell JX, Young RH, Nielsen GP, Rosenberg AE, Bainbridge TC, Clement PB. Nodular fasciitis of the vulva: a study of six cases and literature review. Int J Gynecol Pathol. 1997;16(2):117–123. doi: 10.1097/00004347-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Ozben V, Aydogan F, Karaca FC, Ilvan S, Uras C. Nodular fasciitis of the breast previously misdiagnosed as breast carcinoma. Breast Care. 2009;4(6):401–402. doi: 10.1159/000261502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Chishima T, Adachi S. Nodular fasciitis of the breast mimicking breast cancer. Case Rep Surg. 2014 doi: 10.1155/2014/747951. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4055069/ Available at: at: Accessed March 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konwaler BE, Keasbey L, Kaplan L. Subcutaneous Pseudosarcomatous fibromatosis (fasciitis) Am J Clin Pathol. 1955;25(3):241–252. doi: 10.1093/ajcp/25.3.241. [DOI] [PubMed] [Google Scholar]
- 24.Bochaton-Piallat M L, Gabbiani G, Hinz B. The myofibroblast in wound healing and fibrosis: answered and unanswered questions. F1000Research. 2016;5 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4847562/. Accessed March 27, 2020. [DOI] [PMC free article] [PubMed]
- 25.Khanna V, Rajan M, Reddy T, Alexander N, Surendran P. Nodular fasciitis mimicking a soft tissue sarcoma - a case report. Int J Surg Case Rep. 2018;44:29–32. doi: 10.1016/j.ijscr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JY, Park J, Choi YY, Lee S, Paik SS. Nodular fasciitis mimicking soft tissue metastasis on 18F-FDG PET/CT during surveillance. Clin Nucl Med. 2015;40(2):172–174. doi: 10.1097/RLU.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 27.Erickson-Johnson MR, Chou MM, Evers BR. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91(10):1427–1433. doi: 10.1038/labinvest.2011.118. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 28.Son HJ, Baek TH, Lee SY. Expression of HuR and Cyclooxygenase-2 in nodular fasciitis and low-grade sarcoma: an immunohistochemical study. Korean J Pathol. 2014;48(4):270–275. doi: 10.4132/KoreanJPathol.2014.48.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]