Abstract
Aims:
Friedreich Ataxia (FRDA) is the most commonly inherited ataxia; nearly 60% of deaths are cardiac in nature, with 1 in 8 deaths due to arrhythmia. Additional or irregular heartbeats, measured as ectopy, can be quantified using portable heart rhythm monitoring. We sought to describe the ectopic burden in FRDA.
Methods:
Using a natural history study of FRDA patients at a single center, we analyzed portable heart rhythm monitors (Holters). Ectopic burden was defined as the proportion of atrial or ventricular ectopic beats over total beats.
Results
Of 456 patients, 131 had Holters. Sixty-eight (52.0%) were male, median age of symptom onset was 8.0 years (5.0–13.0, n=111), median age at time of Holter was 17.3 years (IQR 12.9–22.8, n=129), and median duration of illness was 8.7 years (IQR 5.3–11.6, n=110). Median GAA length on the shorter FXN allele was 706.0 (IQR 550.0–840.0, n=112). Eight (7.8%, n=103) had diminished cardiac function and 74 (74.0%, n=100) had ventricular hypertrophy. Ninety patients (83.0%) had atrial ectopy (SVE): 85 (78.0%) with rare SVE (>0–5%) and 5 (5.0%) with frequent SVE (>10%). Twenty-five (19.0%) had supraventricular runs and 1 (0.8%) had atrial fibrillation/flutter. Forty-five (41.0%) had ventricular ectopy (VE): 43 (39.0%) with rare VE (05%) and 2 (2.0%) with moderate VE (5–10%). Compared to patients with none and rare SVE, patients with frequent SVE had longer disease duration (18.3 vs 4.6 vs 9.0 years, p=0.0005).
Conclusion:
Patients with longer disease duration had higher rates of SVE. Heart rhythm monitoring may be considered for risk stratification, however longitudinal analysis is needed.
Introduction
Friedreich Ataxia (FRDA) is an autosomal recessive neurodegenerative disorder that typically presents in childhood. It is the most common form of hereditary ataxia and affects roughly 1 in 50,000 people [1]. Patients are affected by progressive gait disturbance, loss of proprioception, scoliosis, diabetes and cardiomyopathy. Nearly 96% of cases are due to a homozygous GAA triplet repeat expansion leading to a frataxin protein deficiency [1,2]. In 4% of cases, there is a point mutation or deletion in one allele and a triplet repeat expansion in the second allele.
Frataxin is expressed in a variety of tissues and within the cell is localized to the mitochondria. It is involved in the assembly of enzymes containing iron-sulfur clusters and has been implicated in a variety of important cellular functions, including adenosine triphosphate (ATP) synthesis and oxidative phosphorylation [3–5]. Frataxin deficiency also leads to a maldistribution of iron [6,7]. While the exact pathology of how frataxin deficiency leads to FRDA has not been clearly defined, it is hypothesized that a deficiency in frataxin leads to dysfunction of iron-sulfur containing enzymes, deficient ATP production and possibly increased levels of reactive oxygen species.
The clinical hallmarks of the disease are predominantly neurologic, but cardiac dysfunction accounts for up to 60% of deaths [8]. Cardiac hypertrophy represents the most common early phenotype, with development of ventricular dilation and fibrosis in later disease stages [9–11]. It is estimated that cardiac arrhythmias account for up to 16% of deaths [8]. While nonspecific electrocardiogram (ECG) abnormalities such as T wave inversion have been described in a significant number of patients, there has been limited assessment of the irregular heart beat, or ectopy, burden in the FRDA population using portable rhythm monitoring [10–13]. Several have suggested that supraventricular ectopy (SVE), which arises from the atria, is relatively common [10] and more frequent than ventricular ectopy (VE) [11], but none have explored whether SVE burden is associated other features of this rare disease.
Thus, in the present study, we evaluated portable rhythm monitors with the goal of describing the ectopic burden in patients with FRDA. For this study ectopic burden was defined as a proportion (number of atrial or ventricular ectopic beats over total number of beats).We then compared differences in characteristics between patients with different ectopic burden and sought to describe patient and disease characteristics amongst patients with frequent ectopy. We hypothesized that markers of increased disease severity, including shorter GAA repeat length and diminished cardiac function, would be associated with a higher SVE burden.
Methods
Cohort
Using a natural history study of consecutive patients seen at a single center for FRDA between October 1998 and March 2018, we performed an analysis of portable arrhythmia monitoring as assessed by 24–48 Holter monitors. To be included in the study, patients needed to have a diagnosis of Friedreich Ataxia. Holter studies were performed either by the Children’s Hospital of Philadelphia or by the patient’s primary cardiologist. If performed by the patient’s cardiologist, the Holter report was forwarded to our institution. For patients with more than one Holter, the most recent Holter monitor was used. Echocardiographic data provided by the patient’s cardiologist was also collected if the study was performed within 6 months prior to or 1 month after the Holter.
All protocols were approved by the Institutional Review Board at the Children’s Hospital of Philadelphia. In this manuscript, the term “portable” is used as the majority of this population is not ambulatory in the conventional use of the word.
Variables
Baseline demographics included sex and age at time of Holter. Disease-specific characteristics were also recorded and included age of onset of symptoms, length of the GAA repeat on the shorter frataxin (FXN) allele, and the Friedreich Ataxia Rating Scale (FARS) score, which is used to measure disease progress with a range of 0 to 159 (higher scores represent greater disability). Duration of illness was defined as time from age of onset of symptoms to age at time of Holter. Cardiac medications (diuretics, beta blockers, angiotensin converting enzyme inhibitors, or angiotensin II receptor blockers) in use at the time of the Holter monitor were captured.
Echocardiographic data were also collected. For patients ≤18 years of age, the reported interventricular septal thickness at end-diastole (IVSd) and left ventricular posterior wall thickness was expressed as a Z score relative to the distribution of measurements vs. body surface area [14]. Hypertrophy was defined as a Z score >2. For patients ≥18 years, hypertrophy for males was defined as >1 cm and for females >0.9 cm [15]. The biplane ejection fraction (EF), and if not reported, a 4 chamber ejection fraction or the shortening fraction, was used to determine cardiac function. Diminished function was defined as an EF <50%. If an EF was not reported, then diminished function was determined as a shortening fraction (SF) <25%.
Outcome
Ectopic burden was defined as a proportion, calculated as the number of (supraventricular or ventricular) ectopic beats over total number of beats. Patients were then grouped into categories based on the burden of ectopy as follows: none (0%), rare (>0–5%), moderate (>5%10%), and frequent (>10%) [16–20]. A run is defined as three or more consecutive ectopic beats categorized as tachycardia. A supraventricular run is ectopy originating from the atria and a ventricular run is ectopy originating from the ventricles.
Statistical Analysis
Continuous data are reported as median (interquartile range) and categorical data as counts (percentages). Chi-square and Wilcoxon signed-rank tests were used to assess differences between patients with and without Holter monitors. Fisher’s and Kruskal-Wallis tests were used to test for differences in patient characteristics across SVE groups, followed by pairwise comparisons between the individual groups. To adjust for multiple comparisons, a Bonferroni correction was made by comparing P-values to the α level (0.05) divided by 3. Correlation between supraventricular runs and duration of illness was evaluated with Pearson’s correlation analysis. All statistical analyses was performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Of 456 patients enrolled in the FRDA cardiac study, 131 (28.7%) had a Holter available for review (Table 1). Compared to patients who did not have a Holter available to review, patients with a Holter had an earlier onset of symptoms (median 7.0 years [IQR 5.0–13.0] vs 11.0 years [IQR 7.0–15.0], p <0.001). There was no difference with respect to shorter GAA repeat length.
Table 1.
Patient Characteristics
| Variable | Holter Group (N = 131) | Non-Holter Group (N = 325) | P-value |
|---|---|---|---|
| Sex, N (%) (Holter=130, No Holter=308) | 0.6600 | ||
| Female | 62 (47.7) | 154 (50.0) | |
| Male | 68 (52.3) | 154 (50.0) | |
| Age of Onset, Median (IQR) (Holter=111, No Holter=307) | 7.0 (5.0–13.0) | 11.0 (7.0–15.0) | <.0001 |
| Age at Time of Holter (years), (N = 130), Median (IQR) | 17.3 (12.9–22.8) | NA | |
| Duration of Illness (years), Median (IQR) (N = 110) | 8.7 (5.3–11.6) | NA | |
| Short GAA Repeat Length, Median (IQR) (Holter=114, No Holter=302) | 706.0 (558.0–833.0) | 690.0 (500.0–800.0) | 0.1400 |
| FARS Score, Median (IQR) (N = 58) | 59.9 (46.7–74.0) | NA |
Patient and disease characteristics among those who had Holter data available and those who did not. Abbreviation: FARS, Friedreich Ataxia Rating Scale; IQR, interquartile range
Among patients with a Holter, 68 (52.0%) were male, median duration of illness was 8.7 years (IQR 5.3–11.6), and the median shorter GAA repeat length was 706.0 [IQR 558.0–833.0]. Median age at time of most recent Holter analysis was 17.3 years [IQR 12.9–22.8 years] and median FARS score was 59.9 [IQR 46.7–74.0]. Of those patients who had echo imaging available to review, 74 (74.0%, n=100) had evidence of hypertrophy and 8 (7.8%, n=103) had diminished function. 33 (33.3%, n=99) were taking cardiac medications at the time of the Holter.
Characteristics of Holter Monitors
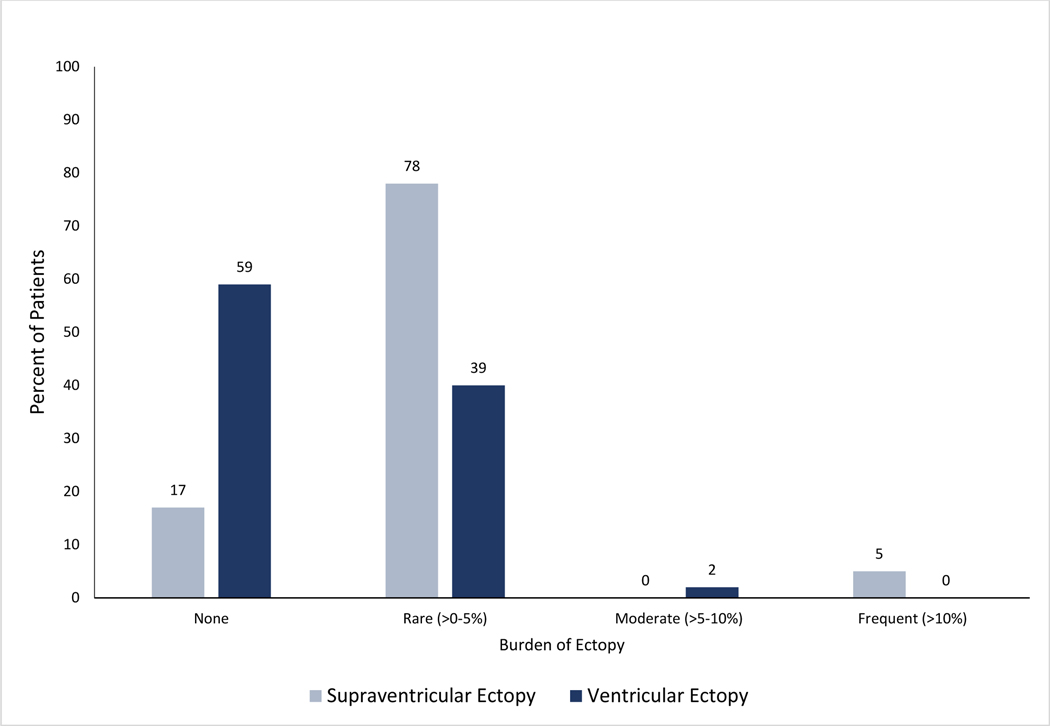
The majority of patients had 24-hour Holter monitors (128, 98.0%); the remainder had 48-hour Holters. With respect to ectopic burden, 90 (83.0%, n=109) had SVE, including 85 (78.0 %) with rare (>0–5%) SVE and 5 (5.0%) with frequent (>10%) SVE (Figure 1). 25 (19.1%) had supraventricular runs and 1 (0.8%) patient had atrial fibrillation/flutter. 45 (41.0%, n=109) patients had VE including 43 (39.0%) with rare (>0–5%) VE and 2 (2.0%) with moderate (510%) VE. There was 1 (0.8%) patient with an episode of ventricular tachycardia. 7 (7.0%, n=100) patients had a pause of 2.0 seconds or longer (Table 2).
Figure 1.
Burden of Supraventricular and Ventricular Ectopy by Group
Table 2.
Holter Characteristics
| Characteristics | N (%) |
|---|---|
| Presence of Atrial Couplets/Pairs, (N = 131) | 36 (27.5) |
| Presence of Supraventricular Runs, (N = 131) | 25 (19.1) |
| Presence of Atrial Fibrillation/Atrial Flutter, (N = 130) | 1 (0.8) |
| Presence of VE Couplets, (N = 130) | 8 (6.2) |
| Presence of VE Triplets, (N = 129) | 1 (0.8) |
| Presence of Ventricular Runs, (N = 129) | 1 (0.8) |
| Longest R To R Interval ≥ 2.0 Seconds, (N = 100) | 7 (7.0) |
Holter Characteristics and frequency of observations among patients. Abbreviation: VE, ventricular ectopy
Comparison of patient characteristics by SVE Group
Study findings comparing patient characteristics overall by SVE group (none, rare, frequent) are shown in Table 3. There was a significant difference in distribution of sex (p=0.0041) and disease duration (p=0.0005) amongst the three SVE groups. However, upon correction for multiple pairwise comparisons, this difference in SVE between males and females was not significantly different between frequent SVE vs none or frequent vs rare SVE groups. With respect to duration of illness, those with frequent ectopy had a longer duration of illness (18.3 years [IQR 14.8–18.4]) compared to those with no ectopy (4.6 years [IQR 2.8–8.7], p= 0.014) or rare ectopy (9.0 years [IQR 5.8–12.5], p=0.018). There was no statistically significant difference in shorter GAA repeat length, FARS score, ventricular function or hypertrophy amongst the 3 groups. For the 110 patients with non-missing data, there was a low positive correlation (r=0.31, p<0.001) between supraventricular runs and duration of illness.
Table 3.
Descriptive analysis of patient characteristics among Supraventricular Ectopy groups
| Characteristics | None (N = 19) | Rare (N = 85) | Frequent (N = 5) | P-value |
|---|---|---|---|---|
| Sex, N (%) | 0.0041 | |||
| Female | 15 (79.0) | 36 (42.3) | 1 (20.0) | |
| Male | 4 (21.0) | 49 (57.7) | 4 (80.0) | |
| Age of Onset, Median (IQR) | 7.0 (4.0–10.5) | 7.5 (5.0–13.5) | 7.0 (5.0–9.0) | 0.4334 |
| Duration of Illness (years), Median (IQR) | 4.6 (2.8–8.7) | 9.0 (5.8–12.5) | 18.3 (14.8–18.4) | 0.0005 |
| Short GAA Repeat Length, Median (IQR) | 721.0 (550.0–900.0) | 700.0 (546.0–833.0) | 840.0 (771.0–850.0) | 0.3152 |
| FARS Score, Median (IQR) | 52.5 (44.5–58.0) | 67.0 (46.7–78.0) | 100.0 (100.0–100.0) | 0.0656 |
| Diminished Function, N (%) | 2 (14.3) | 4 (5.9) | 1 (33.3) | 0.0857 |
| Hypertrophy, N (%) | 11 (84.6) | 50 (71.4) | 2 (66.7) | 0.5855 |
Patient and Disease characteristics by SVE Group. Abbreviation: FARS, Friedreich Ataxia Rating Scale; IQR, interquartile range
Discussion
This is the largest observational study to date of portable cardiac rhythm monitoring in FRDA. We found a remarkably high burden of SVE in this cohort (83%), including 5% with frequent SVE and 19% with supraventricular runs. Although sustained arrhythmia was rare, nearly one fifth of patients had non-sustained supraventricular runs, and 5% had frequent SVE. Additionally, we found that SVE burden was associated with longer disease duration. VE was observed less frequently in our series. Due to this low prevalence, we did not assess for patient or disease characteristics associated with VE.
It does not appear that we can attribute the degree of ectopic burden in this study to an older or sicker cohort than typical FRDA patients. The median age of neurologic symptom onset in this cohort was 7 years (IQR 5–13 years), which is similar to some previously described studies, but slightly younger than others where the average age of onset was early to mid-adolescence [9,21–23]. The majority of patients had normal cardiac function and evidence of hypertrophy by echocardiography, which aligns with previous descriptions regarding the burden of cardiac disease in FRDA [9,22].
There have been few description of portable monitoring in FRDA. In a prospective study of patients with FRDA receiving a co-enzyme Q10 analog (Idebenone), Ribai et al found that 13 of 62 (21%) patients with Holter monitors had SVE [10]. Similar to the present study, the majority of those patients had normal cardiac function and 70% had cardiac hypertrophy. Follow-up Holter analysis was not described. Weidemann et al. performed an analysis of 32 patients, and similar to the present study, observed SVE occurring more frequently than VE [11]. In both of the aforementioned studies, the average age of the cohort was older than the present study (mean 19.3 years +/− 10.4 years vs Ribai 32 +/− 11 years vs Wiedemann 33 years +/− 13 years). Disease duration was also longer (Ribai mean 16 +/− 8 years vs mean 11 years +/− 7.5). Thus, our observations suggest that even younger patients with shorter disease duration than previously described may present with SVE.
When compared to studies in the normal population, our observation suggests that the SVE burden in FRDA may be higher. In an observational study of 50 male medical students, Brodsky et al found that 56% had SVE, which is slightly less than in our study [24]. However, Folarin et al. reviewed the Holter monitors of 303 asymptomatic male aviators without structural heart disease [20]. In that study, 78.2% demonstrated SVE; however only 5.2% had >0.1% ectopy and 0.3% had very frequent ectopy (>10%), which is a notably smaller proportion than the 5.4% in our study with >10% ectopy. With respect to VE, Folarin et al. observed a VE burden of >0.1% in 52% of participants, with no participants with >10% VE.
Prior studies have demonstrated an association between GAA repeat length, disease severity, and cardiac manifestations. Amongst a group of 67 FRDA patients, Filla et al observed that a longer GAA repeat length on the shorter allele was associated with a younger age of onset and the presence of cardiomyopathy [25]. Durr et al found a similar association between GAA length and age of onset, loss of ambulation, and cardiomyopathy [1].
While we had initially hypothesized that shorter GAA repeat length and diminished cardiac function would be associated with SVE, we did not find this to be the case. Likewise, we did not find any significant relationship between SVE burden and the presence or absence of cardiac hypertrophy. However, we did find an association between duration of illness and SVE, which has not been described previously [10,11,8]. This suggests that SVE burden increases with prolonged duration of illness, although not necessarily in lock step with the trajectory of hypertrophy (which can be seen at various stages of disease) or decline in systolic function (which typically does not occur until late in disease). Other etiologies for ectopy should also be considered such as atrial myocardial fibrosis and scarring, which could predispose patients to supraventricular ectopy [26]. In this study we also identified a positive correlation between the presence of SVE runs and duration of illness. Although there was a low correlation, this finding suggests that duration of illness is not only associated with overall ectopic burden, but may also be associated with SVE runs and the small effect size may be due to other unmeasured modifiers. Thus, if supraventricular ectopic burden is influenced by the same disease process but precedes cardiac dysfunction, overt arrhythmias, and heart failure symptoms, then an improvement in SVE burden could potentially serve as an outcome measure for clinical trials in early stage disease.
Limitations of this study include the retrospective and cross-sectional nature of the data collection. The relatively young age of this cohort may have also prevented the ability to capture frank arrhythmias. The reason for obtaining portable rhythm monitoring was also not always provided, therefore there may be selection bias in those patients that did undergo testing. Longitudinal rhythm monitoring with serial Holters and use of cardiac medications as anti-arrhythmics were not captured. Interestingly, while patients in this study who had Holters were younger than those who did not, their GAA repeat length was not different between the groups.
In conclusion, the data in this study suggests that the majority of patients with FRDA in this study have SVE on portable heart rhythm monitoring. Timely and serial Holter monitoring in FRDA patients with longer disease duration may aid in assessing progression of ectopy burden. Supraventricular ectopic burden, in conjunction with additional testing such as cardiac magnetic resonance imaging, may prove to be a useful marker for assessing disease severity. Further work involving longitudinal analysis is needed to understand the development and progression of arrhythmias in FRDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, Koenig M (1996) Clinical and genetic abnormalities in patients with Friedreich’s ataxia. The New England journal of medicine 335 (16):1169–1175. doi: 10.1056/NEJM199610173351601 [DOI] [PubMed] [Google Scholar]
- 2.Schols L, Amoiridis G, Przuntek H, Frank G, Epplen JT, Epplen C (1997) Friedreich’s ataxia. Revision of the phenotype according to molecular genetics. Brain 120 ( Pt 12):2131–2140. doi: 10.1093/brain/120.12.2131 [DOI] [PubMed] [Google Scholar]
- 3.Pandolfo M, Pastore A (2009) The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol 256 Suppl 1:9–17. doi: 10.1007/s00415-009-1003-2 [DOI] [PubMed] [Google Scholar]
- 4.Babcock M, de Silva D, Oaks R, Davis-Kaplan S, Jiralerspong S, Montermini L, Pandolfo M, Kaplan J (1997) Regulation of mitochondrial iron accumulation by Yfh1p, a putative homolog of frataxin. Science 276 (5319):1709–1712. doi: 10.1126/science.276.5319.1709 [DOI] [PubMed] [Google Scholar]
- 5.Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P (1997) Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17 (2):215–217. doi: 10.1038/ng1097-215 [DOI] [PubMed] [Google Scholar]
- 6.Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH (2000) Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum Mol Genet 9 (2):275–282. doi: 10.1093/hmg/9.2.275 [DOI] [PubMed] [Google Scholar]
- 7.Ramirez RL, Qian J, Santambrogio P, Levi S, Koeppen AH (2012) Relation of cytosolic iron excess to cardiomyopathy of Friedreich’s ataxia. The American journal of cardiology 110 (12):1820–1827. doi: 10.1016/j.amjcard.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsou AY, Paulsen EK, Lagedrost SJ, Perlman SL, Mathews KD, Wilmot GR, Ravina B, Koeppen AH, Lynch DR (2011) Mortality in Friedreich ataxia. J Neurol Sci 307 (1–2):46–49. doi: 10.1016/j.jns.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 9.Kipps A, Alexander M, Colan SD, Gauvreau K, Smoot L, Crawford L, Darras BT, Blume ED (2009) The longitudinal course of cardiomyopathy in Friedreich’s ataxia during childhood. Pediatric cardiology 30 (3):306–310. doi: 10.1007/s00246-008-9305-1 [DOI] [PubMed] [Google Scholar]
- 10.Ribai P, Pousset F, Tanguy ML, Rivaud-Pechoux S, Le Ber I, Gasparini F, Charles P, Beraud AS, Schmitt M, Koenig M, Mallet A, Brice A, Durr A (2007) Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch Neurol 64 (4):558–564. doi: 10.1001/archneur.64.4.558 [DOI] [PubMed] [Google Scholar]
- 11.Weidemann F, Liu D, Hu K, Florescu C, Niemann M, Herrmann S, Kramer B, Klebe S, Doppler K, Uceyler N, Ritter CO, Ertl G, Stork S (2015) The cardiomyopathy in Friedreich’s ataxia - New biomarker for staging cardiac involvement. International journal of cardiology 194:50–57. doi: 10.1016/j.ijcard.2015.05.074 [DOI] [PubMed] [Google Scholar]
- 12.Harding AE (1981) Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104 (3):589–620 [DOI] [PubMed] [Google Scholar]
- 13.Schadt KA, Friedman LS, Regner SR, Mark GE, Lynch DR, Lin KY (2012) Cross-sectional analysis of electrocardiograms in a large heterogeneous cohort of Friedreich ataxia subjects. J Child Neurol 27 (9):1187–1192. doi: 10.1177/0883073812448461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez L, Colan S, Stylianou M, Granger S, Trachtenberg F, Frommelt P, Pearson G, Camarda J, Cnota J, Cohen M, Dragulescu A, Frommelt M, Garuba O, Johnson T, Lai W, Mahgerefteh J, Pignatelli R, Prakash A, Sachdeva R, Soriano B, Soslow J, Spurney C, Srivastava S, Taylor C, Thankavel P, van der Velde M, Minich L, Pediatric Heart Network I (2017) Relationship of Echocardiographic Z Scores Adjusted for Body Surface Area to Age, Sex, Race, and Ethnicity: The Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging 10 (11). doi: 10.1161/CIRCIMAGING.117.006979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16 (3):233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 16.Lee A, Walters TE, Gerstenfeld EP, Haqqani HM (2019) Frequent Ventricular Ectopy: Implications and Outcomes. Heart Lung Circ 28 (1):178–190. doi: 10.1016/j.hlc.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 17.Bertels RA, Harteveld LM, Filippini LH, Clur SA, Blom NA (2017) Left ventricular dysfunction is associated with frequent premature ventricular complexes and asymptomatic ventricular tachycardia in children. Europace 19 (4):617–621. doi: 10.1093/europace/euw075 [DOI] [PubMed] [Google Scholar]
- 18.Gunda S, Akyeampong D, Gomez-Arroyo J, Jovin DG, Kowlgi NG, Kaszala K, Tan AY, Koneru JN, Kron J, Ellenbogen KA, Huizar JF (2019) Consequences of chronic frequent premature atrial contractions: Association with cardiac arrhythmias and cardiac structural changes. J Cardiovasc Electrophysiol 30 (10):1952–1959. doi: 10.1111/jce.14067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F Jr., Crawford T, Ebinger M, Oral H, Morady F, Bogun F (2010) Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 7 (7):865–869. doi: 10.1016/j.hrthm.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 20.Folarin VA, Fitzsimmons PJ, Kruyer WB (2001) Holter monitor findings in asymptomatic male military aviators without structural heart disease. Aviat Space Environ Med 72 (9):836–838 [PubMed] [Google Scholar]
- 21.Harding AE, Hewer RL (1983) The heart disease of Friedreich’s ataxia: a clinical and electrocardiographic study of 115 patients, with an analysis of serial electrocardiographic changes in 30 cases. Q J Med 52 (208):489–502 [PubMed] [Google Scholar]
- 22.Meyer C, Schmid G, Gorlitz S, Ernst M, Wilkens C, Wilhelms I, Kraus PH, Bauer P, Tomiuk J, Przuntek H, Mugge A, Schols L (2007) Cardiomyopathy in Friedreich’s ataxia-assessment by cardiac MRI. Mov Disord 22 (11):1615–1622. doi: 10.1002/mds.21590 [DOI] [PubMed] [Google Scholar]
- 23.Cook A, Giunti P (2017) Friedreich’s ataxia: clinical features, pathogenesis and management. Br Med Bull 124 (1):19–30. doi: 10.1093/bmb/ldx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM (1977) Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. The American journal of cardiology 39 (3):390–395. doi: 10.1016/s00029149(77)80094-5 [DOI] [PubMed] [Google Scholar]
- 25.Filla A, De Michele G, Cavalcanti F, Pianese L, Monticelli A, Campanella G, Cocozza S (1996) The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am J Hum Genet 59 (3):554–560 [PMC free article] [PubMed] [Google Scholar]
- 26.Hanson E, Sheldon M, Pacheco B, Alkubeysi M, Raizada V (2019) Heart disease in Friedreich’s ataxia. World J Cardiol 11 (1):1–12. doi: 10.4330/wjc.v11.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]