Abstract
Site directed drug delivery with high efficacy is the biggest challenge in the area of current pharmaceuticals. Biodegradable polymer-based controlled release nanoparticle platforms could be beneficial for targeted delivery of therapeutics and contrast agents for a myriad of important human diseases. Biodegradable nanoparticles, which can be engineered to load multiple drugs with varied physicochemical properties, contrast agents, and cellular or intracellular component targeting moieties, have emerged as potential alternatives for tracking and treating human diseases. In this review, we will highlight the current advances in the design and execution of such platforms for their potential application in the diagnosis and treatment of variety of diseases ranging from cancer to Alzheimer’s and we will provide a critical analysis of the associated challenges for their possible clinical translation.
Keywords: Active targeting, biomaterials, blood-brain barrier, cancer, cardiovascular diseases, liposome, neurodegenerative diseases, nanomedicine, nanotechnology, polymer, passive targeting
INTRODUCTION
Several diseases, including but not limited to cancers, cardiovascular and neurodegenerative diseases, and viral infections, are the most common causes of death worldwide. The search for the efficacious therapeutics for these diseases continues. It is now well accepted that early diagnosis, accurate visualization of administered therapeutics, and their effective delivery to the target organ/tissue play an essential role to improve therapeutic modalities.
Nanotechnology and Nanomedicine:
“Nanomedicine” is an umbrella term, which is defined as nanotechnology-based delivery vehicles that can be used in tracking and treating diseases at the molecular level [1]. Use of nanotechnology-based drug delivery [2-3] allows one to monitor the drug distribution, release, response, and efficacy of an on-demand controlled drug release that can in turn help overcome both over and under dosing; a common flaw associated with conventional free drug treatments. In a broader sense, nanomedicine is the application of nanotechnologies to the clinical practice for the diagnosis, prevention, and treatment of various diseases. According to the National Nanotechnology Initiative (NNI), nanotechnology is defined as the research and development carried out at the atomic, to macromolecular levels in the length scale of 1- 100 nm range. Although the US Food and Drug Administration (FDA) and the US Patent and Trademark Office follow this size restriction definition imposed by the NNI, the size limitation is not critical to a pharmaceutical company from an efficacy perspective. Instead the most important properties for transition of nanotechnology to nanomedicine is driven by the critical properties of improved solubility, physiological availability and reduction in higher doses and toxicity of drug, which may be achieved in a broader size range greater than 100 nm. There are several nanomaterials that are widespread and broadly marketed; some of these materials have yet to find their way into clinical practices. Metal based nanoparticles (NPs) drug loaded micelles and liposomal nano composites, dendrimers, lipid hybrid and polymeric NPs are considered as the basic nanoscale size materials (Fig. 1) [4]. NPs consist of polymers [5] or lipids [6], has colloidal particle nature and can be administrated intravenously. Since the first FDA approval of nanomedicine, a liposomal formulation of amphotericin B [7], early forecasts for commercialization of nanotechnology-driven medicine are encouraging (Fig. 2) [8].
Fig. (1).
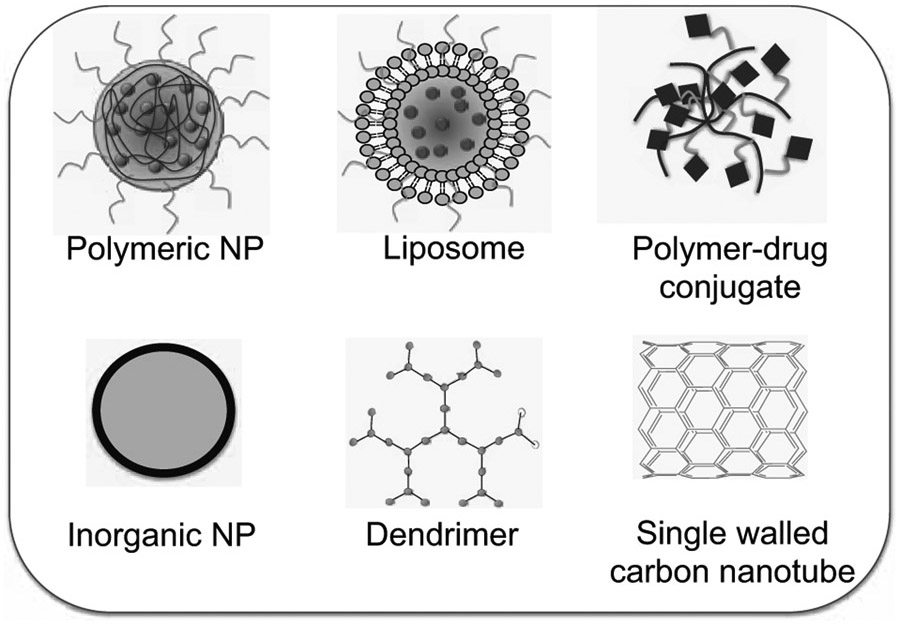
Selective nanomaterials that play important roles in the nanomedicine field.
Fig. (2).
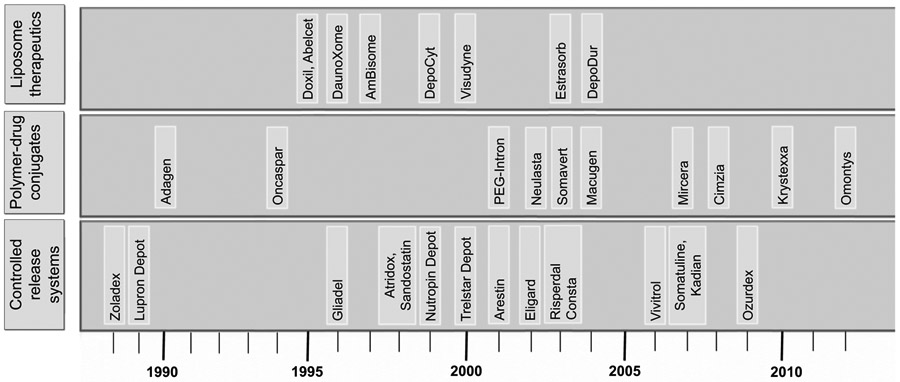
Timeline of FDA approved nanomedicine in the market.
Among the various nanotechnology-based delivery vehicles (Fig. 1), liposomes, polymer-drug conjugates, and polymeric NPs are the most accepted platforms for diagnosis and therapy of different diseases [4]. Extensive research exploited these platforms based on their abilities to improve drug efficacy over free payloads through ameliorated drug encapsulation, increased circulation half-life, sustained and activated payload release. Enhanced permeability and retention (EPR) effect [9-10] is a passive targeting approach used by most of the liposomal and polymeric NPs to accumulate preferentially at the active site of the targeted disease, whereas active targeting can be achieved through surface modification of the NPs with specific targeting ligands [11]. A variety of therapeutics based on liposomes, polymer-drug conjugates, and polymeric NPs which are either clinically approved or have entered clinical trials are shown in Tables 1 and 2.
Table 1.
NP-Based Therapeutics which Follow Passive Targeting
| Trade name | NP Type | Company | Disease | Payload | Current Status / Clinical Trial No. |
|---|---|---|---|---|---|
| CRLX101 | Cyclodextrin-based polymer | Cerulean Pharma Inc. | Solid tumor of various types including pancreatic cancer, ovarian cancer, and non small cell lung cancer | Camptothecin | Phase II/NCT01380769 |
| Doxil® | PEGylated liposome | Janssen Pharmaceuticals | AIDS-related Kaposi’s sarcoma, metastatic ovarian cancer, and multiple myeloma | Doxorubicin | Approved |
| Genexol-PM | Polymer | Samyang Corporation | Breast cancer | Paclitaxel | Phase III/NCT00876486 |
| CPX-1 | Liposome | Celator Pharmaceuticals | Advanced solid tumors in colorectal cancer | Irinotecan- Floxuridine | Phase II/NCT00361842 |
| DaunoXome | Liposome | Galen | AIDS-related Kaposi’s sarcoma | Daunorubicin | Approved |
| Myocet | Non-PEGylated liposome | Enzon Pharmaceuticals | Advanced metastatic breast cancer, ovarian cancer | Doxorubicin | Approved in Europe and Canada |
| LE-SN38 | Liposome | NeoPharm | Metastatic colorectal cancer | SN-38 | Phase III in USA/NCT00294996 |
| Onco TCS | Liposome | INEX and ENZON | Non-Hodgkin’s lymphoma | Vincristine | Approved |
| SPI-77 | Liposome | SEQUUS Pharmaceuticals | Ovarian cancer | Cisplatin | Phase II/NCT00004083 |
| Abraxane | Albumin | Celgene | Metastatic breast cancer | Paclitaxel | Approved |
| NC6004 | Micelle | NanoCarrier | Pancreatic cancer | Cisplatin | Phase I/II /NCT00910741 |
| NK105 | Micelle | Nippon Kayaku Co, Ltd. | Breast cancer | Paclitaxel | Phase III/NCT01644890 |
| NK012 | Polymer Micelle | Nippon Kayaku Co, Ltd. | Solid tumors, Breast cancer | SN-38 | Phase II/NCT00951054 |
Table 2.
Targeted-NP-Based Therapeutics which Follow Active Targeting
| Trade name | NP Type | Company | Targeting Ligand | Disease | Payload | Current Status |
Clinical Trial No. |
|---|---|---|---|---|---|---|---|
| SGT-53 | Liposome | SynerGene Therapeutics, Inc. | Transferrin single-chain antibody fragment | Advanced solid tumors | Gene 53 | Phase Ib | NCT00470613 |
| MBP-426 | Liposome | Mebiopharm | Transferrin | Solid tumors and esophagus disorders | Oxaliplatin | Phase II | NCT00355888, NCT00964080 |
| SEL-068 | Polymeric | Selecta Biosciences | Small molecule | Smoking cessation vaccine | Nicotine antigen T-helper cell peptide, TLR agonist | Phase I | NCT01478893 |
| CALAA-01 | Polymeric | Calando | Human Transferrin | Solid tumors | siRNA | Phase Ib | NCT00689065 |
| BIND-014 | Polymeric | Bind Biosciences | PSMA peptide | Metastatic cancer | Docetaxel | Phase I | NCT01300533 |
| SGT-94 | Liposome | SynerGene Therapeutics, Inc | Anti-transferrin receptor single chain antibody fragment | Solid tumors | RB94 gene | Phase I | NCT01517464 |
Liposomes:
Liposomes are biodegradable and essentially non-toxic drug delivery vehicles [12]. Liposomes are biocompatible and biodegradable nanomaterials, which consist of an aqueous core entrapped by one or more lipid bilayers. Liposomes have the ability to encapsulate both hydrophilic and hydrophobic payloads by utilizing a combination of the hydrated compartment inside the liposome and the lipid core into which especially hydrophobic molecules can be incorporated. The outer lipid leaflet of a liposome can be used to attach functionalities such as targeting ligands to achieve active targeting for liposome uptake. Liposomes are constituted of natural and/or synthetic lipids or other bilayer constituents, such as cholesterol and hydrophilic polymer conjugated lipids. Based on the size and lamellarity, liposomes are classified into multilamellar vesicles, large unilamellar vesicles, and small unilamellar vesicles. Liposomes can be constructed with varied sizes ranging from ~25 nm to ~2.5 μm. Liposome size plays a critical role in determining its blood circulation time. Both size and the number of lipid bilayers significantly alter drug encapsulation efficacy of liposomes. Most conventional liposomes are trapped by the reticuloendothelial system (RES) [13], and the delivery of liposomes to target organs other than RES requires surface modification to result in long-circulating properties.
Biodegradable Polymeric NPs:
In 1964, Folkman and Long invented the concept of therapeutic loading into an implantable controlled release polymeric vehicle [14]. The remarkable work by Langer and Folkman in 1976 led to launch of the field of “controlled release drug delivery” [15]. Since then, a number of polymeric materials and devices for therapeutic delivery enriched this area of research [16-17]. Huge developments in recent years regarding controlled drug delivery systems included polymeric NPs based on biodegradable and biocompatible polymers. These polymers degrade spontaneously in a predetermined and controlled manner into bio-friendly end products that can be eliminated from the body [2-4, 18-35]. Examples of biocompatible and biodegradable polymers include natural or synthetic polymers (Table 3). The most commonly used controlled release polymers include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), their copolymer poly(D,L-lactide-co-glycolide) (PLGA), poly(caprolactone) (PCL), N-(2-hydroxypropyl)-methacrylate copolymers, and poly (amino acids). Biodegradable and biocompatible polymers PLA, PGA, and their co-polymer PLGA are used in FDA approved medical devices for applications such as controlled release of therapeutics and contrast agents, tissue engineering, etc. Polymeric nanocarriers can be constructed by conjugating payloads to soluble macromolecules and polymeric NPs can be synthesized by co-polymer self-assembly. In a self-assembled material, the polymeric NP matrix can be loaded with a variety of payloads [36]. Degradation of these types of NPs follows a drop in polymer molecular weight leading to the erosion of the biomaterial as a result of chemical and/or enzymatic cleavage of the polymeric backbone, which depends on catalysts in the physiological environment or catalysts embedded within the polymer itself, or hydrolytic degradation. The release of payloads from the NP matrix is regulated through the diffusing ability of the drug as well as the eroding nature of the matrix. Erosion can be of homogenous bulk disintegration or heterogeneous surface erosion. Biodegradable polymers abundantly use both type of degradation process. Bulk erosion results from degradation takes place in the entire polymeric matrix caused by the faster water penetration than whole polymer degradation. However, the surface erosion is caused by only degrading the surface of the polymeric matrix, which occurs when degradation rate is faster than water penetration. Release of drugs from bulk degrading polymers is primarily dependent on drug diffusion occurring prior to or concurrent with polymer degradation. Surface-degrading polymers rely on the relative contributions between drug diffusion and degradation of the matrix. On the contrary, conjugation of payload to the polymer backbone allows for predetermined drug loading with control over drug release profiles, and opportunities to load multiple therapeutics with spatio temporal release profiles for combination therapy [30]. Polymeric NPs can be further coated with a hydrophilic polymer for inhibition of opsonization and to enhance water solubility. A well accepted practice to control the stability and immunogenicity of polymeric NPs includes incorporation of polyethylene glycol (PEG), which is a biocompatible non-ionic hydrophilic polymer with stealth behavior [37]. NP formulations with increased stability are usually obtained by incorporation of PEG, which provides suitable steric stabilization and thereby reduces the tendency to aggregate. The unique properties of PEG can influence the pharmacokinetic (PK) profiles of therapeutics as well as the polymeric NP carrier. The presence of PEG in polymeric NPs results in increased blood circulation times [20]. Most of the polymer-based delivery systems that are in the market contain PEG functionalized products (PEGylated). The NP surface can be used to further introduce targeting moieties.
Table 3.
Polymers for Biodegradable Polymeric NPs.
| Natural Polymers | Synthetic Polymers |
|---|---|
| Polysaccharides: Hyaluronate, Dextran, Chitosan, Alginate, Agarose etc. Protein-based polymers: Fibrin, Collagen, Ferritin |
Poly (glycolic acid) (PGA), Poly (lactic acid) (PLA), and copolymer poly(lactide-co-glycolides) (PLGA), polycaprolactone (PCL), Poly (ethylene glycol) (PEG) and its derivatives and copolymers, Polyamides, Polyanhydrides, Poly (vinyl alcohol) PVA, Polypeptides |
In this review, we discuss “nanomedicine” focused on the recent developments in the field of polymeric NP carriers. We also review advances in the use of polymeric NP-based imaging and therapeutics to track and treat cancers, cardiovascular diseases, and neurodegenerative diseases.
POLYMERIC NPs IN TRACKING DISEASES
Polymeric NPs provide opportunities to incorporate contrast agents, which are commonly used in the clinic that include magnetic resonance imaging (MRI), radionuclide imaging, fluorescence imaging, X-ray computed tomography (CT), positron emission tomography (PET), and single photon emission computed tomography (SPECT) [39-40]. Super paramagnetic iron oxide (SPIO) NPs are often utilized for diagnostics with MRI [41-42]. Gold NPs are most often employed for CT [43-44]. Optical probes such as quantum dots and fluorophore-tagged or -encapsulated NPs are used in fluorescence imaging. PET and SPECT use radiolabeled NPs. In every diagnostic case, it is necessary that the imaging agent reach the area of interest in the body at sufficient concentration and time scale. Polymeric NPs are extensively used as vehicles for imaging agents [45]. Coating inorganic-based NPs, such as gold NPs, quantum dots, SPIOs with biocompatible polymers increases stability and blood half-life, while minimizing and/or delaying RES uptake [21, 46]. This kind of modification also increases contrast agent loading and targeting properties.
Polymeric NPs can be particularly useful for delivering imaging probes because of their unique physicochemical properties, apparent lack of toxicity, surface area to volume ratio, and the scope for incorporation of surface functionality for targeting [47]. The size and surface properties of polymeric NPs can be altered, which can play important roles for their biodistribution (bioD) in the circulation [35]. Suitable alterations in these properties can lead the NPs to traverse even the blood-brain barrier (BBB), indicating that the polymeric NPs can be useful for targeting and diagnosing a specific organ of interest. If more than one imaging technique is compatible in resolution or sensitivity, multimodal NPs can take advantage of this increased diagnostic potential by incorporating two signaling modalities [48-49]. Combining imaging techniques that have high sensitivity with ones that have high-resolution can help overcome their individual limitations [50-51].
Cancer Diagnosis:
As the instances of cancer across the world continue to increase, it is becoming important to be able to detect it at an early stage. An early diagnosis makes it possible to treat the diseases allowing for a better chance of survival. The use of polymeric NPs in cancer imaging gained significant interest [47]. The leaky vasculature formed by a solid tumor during angiogenesis provides a unique opportunity for circulating NPs in the bloodstream to get trapped in the blood vessels and eventually leak into the surrounding tissue. This passive accumulation of NPs in the tumor due to the EPR effect can be observed in most cancers [52-53]. However, one can take this passive accumulation technique a step further by introducing targeting ligands that can target the NPs to the diseased cells specifically [54-55].
Polymeric NPs can be used effectively to carry MRI contrast imaging agents such as SPIOs and gadolinium (Gd)-based molecules. There are several different types of polymeric NP platforms that can be utilized for the imaging of cancer cells. PCL-b-poly(methacrylic acid) (PCL-b-PMAA) is an amphiphilic block co-polymer that can be used to generate water soluble nanocarriers for certain payloads, such as fluorophores and magnetic NPs. A recent work involves creation of a multimodal imaging platform by coupling PCL-b-PMAA to pyrene and encapsulating magnetic MnFe2O4 NPs via emulsification [56]. This allows for both fluorescence and MR imaging of cancer cells. This system offers further improvement by utilizing the multiple carboxyl groups on the NP surface for the attachment of targeting moieties. The main drawback of this system is that the fluorophore pyrene has an emission of ~470 nm. At this wavelength, one can expect to have extensive background noise due to auto-fluorescence from the tissues. Contrast agents that fluoresce in the near infrared region (NIR) have attracted much attention due to the fact that NIR fluorescence technology allows elimination of background noise caused by biological tissue autofluorescence and the scattered light from the excitation source is greatly reduced since the scattering intensity is proportional to the inverse fourth power of the wavelength. Wagh et al. used PLGA-b-PEG-NPs to encapsulate a NIR fluorescence resonance energy transfer (FRET) pair that has an emission wavelength of 795 nm [57]. These NPs assembled from PLGA-b-PEG and maleimide-PEG contain encapsulated donor (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocya-nine) and acceptor (1,1′-dioctadecyl-3,3,3′,3′-tetramethyli-ndotricarbocyanine) fluorophores. The optimized formulation showed a higher brightness than quantum dots, a promising stability profile in biological media, and demonstrated favorable bioD properties.
Ye et al. modified the side chains of poly(L-glutamic acid) of different molecular weights with gadolinium-(1,4,7,10-tetraazacyclododecane-1,4,7-trisacetic acid) (Gd-DO3A) to obtain three polymer-Gd-DO3A conjugates of molecular weights of 87, 50 and 28 kDa [58]. These conjugates were studied in mice bearing MDA-MB-231 human breast cancer xenografts. Real-time MRI resulted in a three-dimensional visualization of blood circulation, PK, bioD, and tumor accumulation of the conjugates. The low molecular weight conjugate was rapidly cleared from the circulation and showed a relatively lower tumor accumulation. The higher molecular weight conjugates demonstrated a more prolonged blood circulation and higher tumor accumulation. This study shows the effect of molecular weights of polymers, which can play significant roles in altering PK, and bioD properties of contrast agents in vivo.
Multiple contrast agents can also be encapsulated in a single polymeric NP platform. A recent work shows the use of PLGA polymer to co-encapsulate a positive MR contrast agent, gadolinium diethylentriaminepentaacetic acid (Gd-DTPA) and a fluorescent rhodamine dye using a double emulsification technique (Fig. 3) [59]. These particles were further coated with silver using a photoreduction method allowing for photoacoustic ultrasound (PAUS) imaging [59]. The MRI contrast agent Gd-DTPA and the exogenous dye rhodamine were shown to enhance MR and fluorescence contrast, respectively, and the silver nanocage exhibited strong NIR light absorption.
Fig. (3).
Multimodal imaging based on PLGA-based NPs. Redrawn based on reference 59.
Tracking Cardiovascular Diseases:
Cardiovascular disease (CVD), which includes but is not limited to coronary and periphery artery disease, high blood pressure, stroke, and congestive heart failure, is the number one killer in the industrialized world [60]. Even with the high prevalence of CVD, current techniques for the early detection and treatments are limited. For instance, the most recent technology to significantly help patients suffering from CVD is the coronary stent which was approved by FDA in 1994 [61]. Nanomedicine offers methods to control the high death rates resulting from CVD. Currently, there are only three FDA approved NP formulations, which are used for imaging CVD: AMI-121 (Ferumoxsil), OMP50, and AMI-25 (Feridex) for imaging gastrointestinal tract, and the liver and spleen [35, 62]. The problem with these systems, however, is that they are IO based formulations that typically do not offer optimal circulation half-life on their own. As previously mentioned, incorporation of contrast agents within polymeric NPs improves the PK and bioD properties of contrast agents [21].
An excellent improvement on the detection of CVD using nanomedicine is the detection of atherosclerotic plaques and neointima in the vessels. Atherosclerosis is a chronic condition where plaques composed of cholesterol, fat, calcium, and elements of blood build up inside the arteries limiting the oxygen-enriched blood flow to the organs. Neointima is the formation of a thick layer of arterial innermost layers either in prosthesis or in atherosclerosis through the proliferation of cells. In the early stages of atherosclerosis, integrins are produced as a widespread response to cholesterol feeding. One of these integrins, ανβ3, has shown to be an excellent target for the early detection of atherosclerosis. Winter and coworkers designed an early atherosclerosis targeted NP by coupling a ανβ3 targeting peptide to PEG which was then used to incorporate Gd MRI contrast agents into a NP formulation [62]. This system was able to successfully image ανβ3 buildups on the aortic walls of cholesterol-fed rabbits.
Peters et al. used a similar liposomal approach for targeting atherosclerosis [63]. However, they used a clot-binding peptide, cysteine-arginine-glutamic acid-lysine-alanine (CREKA), in order to successfully target the abundance of clotting that occurs on the atherosclerotic plaques. By modifying one end of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG (DSPE-PEG) with a carboxyfluoroscein dye, they were able to achieve targeted liposomes which can be tracked by fluorescence microscopy [63]. Pan and coworkers designed a system based on a soft colloidal, radio-opaque, and metal-encapsulated polymeric (cROMP) NP [64]. They synthesized an amphiphilic polystyrene-b-polyacrylic acid (PS-b-PAA) di-block copolymer that was used to encapsulate iodine or bismuth for CT imaging. This work demonstrated the feasibility of using polymeric NP system for both in vitro and in vivo CT imaging. cROMP NPs showed several-fold CT signal enhancement in suspension. The PAA backbone allowed for free carboxyl moieties for attachment of targeting ligands [64]. One of the main problems with this system, however, is the cytotoxicity of the polystyrene moiety. One possible way to combat this is to utilize biopolymers.
Nahrendorf et al. developed a dextran based NP system for imaging macrophages in inflammatory atherosclerosis (Fig. 4) [65]. Dextran is a natural, biocompatible polysaccharide composed of many complex branched sugar molecules, which is biocompatible. In this system, dextran was used to create a trimodal NP reporter by encapsulating IONP and a fluorescent reporter (fluorochrome). The design also allowed for incorporation of the nuclear tracer 64Cu moiety on the surface [65]. This multifunctional dextranated NP of size ~20 nm allowed for PET, MRI, and optical imaging. Intravenous administration of these NPs and analysis 24 h post injection into mice deficient in apolipoprotein E was able to identify areas of high plaque load by CT and the results were correlated with MR and optical imaging. This multifunctional NP was shown to be predominantly in macrophages by fluorescence microscopy and flow cytometry of cells from aortas.
Fig. (4).

Schematic view of a macrophage-targeted long-circulating, dextran-coated NP-based trimodality reporter. Redrawn based on reference 65.
Diagnosis of Neurodegenerative Disorders:
Neurodegenerative diseases, such as Alzheimer’s diseases (AD), Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis (ALS), and prion diseases are characterized by the folding and aggregation of various proteins [66]. Neurodegenerative diseases are also associated with their trademark levels of cognitive impairment. As the diseases progress, their level of impairment transverse a no impairment, to mild, to moderate, to severe, and ultimately death because of the inability to move crucial muscles and the loss of vital functions. Disease detection at an early stage would allow better prognostication and appropriate therapy. The biggest challenge posed by neuroimaging is the BBB or the division of the tissues of the brain from the blood [67-68]. The BBB is the homeostatic defense mechanism of the brain against pathogens and toxins; the BBB screens the physico and/or biochemical and structural properties of solutes at its periphery and thus provides barrier selectivity in the passage of molecules into the brain. Polymeric NPs are not suitable to cross the BBB in order to reach the brain parenchyma due to their size ranging from 10-1000 nm. However, polymeric NPs can be tuned to exhibit similarity in their topological features with that of natural carriers such as viruses and serum lipoproteins, and therefore offer specific characteristics useful for theranostic applications in neurodegenerative diseases. Polymeric NPs thus represent a potential means to transport payloads across the BBB [69-71]. The ideal properties required for contrast agent delivery to the brain include: (i) NP size < 100 nm, (ii) biocompatible and biodegradable, (iii) stability in biological milieu, (iv) ability to avoid the mononuclear phagocytic system with prolonged blood circulation time, and (v) receptor-mediated transcytosis across brain capillary endothelial cells.
Polybutylcyanoacrylate (PBCA)-based NPs coated with the nonionic surfactant polysorbate 80 are extensively investigated to deliver payloads to the brain [72]. Koffie et al. constructed a biodegradable NP system using PBCA-dextran polymers coated with polysorbate 80 (PBCA NPs) in order to deliver the BBB-impermeable molecular imaging probes into the brain for targeted molecular neuroimaging [73]. PBCA NPs were successfully utilized in targeted delivery of BBB impermeable contrast agents and staining reagents for electron microscopy, multiphoton optical imaging, and whole brain MRI. PBCA NPs were shown to have the ability to deliver a number of BBB-impermeable fluorophores of different sizes and properties ranging from 500-Da polar molecules to 150,000-Da immunoglobulins in vivo. PBCA NPs are believed to interact with plasma apolipoprotein E for BBB crossing. In addition to the toxicity issue and detailed mechanism of action, PBCA NPs should be evaluated in terms of benefit/risk ratio and duration of the pharmacological effect after administration for in vivo imaging of chronic brain diseases.
A number of limitations with the PBCA NPs preclude, or at least limit their potential applications in the clinic. Biodegradable PLA or PLGA-based NPs have good central nervous system (CNS) biocompatibility [74-75]. The majority of the literature reported in the field of brain targeting with polymeric NPs relies on the receptor-mediated transcytosis to access the brain from blood to deliver the payload into the brain parenchyma without disrupting the BBB [76-77]. This approach demands presence of receptor-specific targeting ligands on the NP surface. In a recent example, a PLGA-based NP was engineered with a known brain targeting simil-opioid glycopeptide (g7) [78]. For in vivo imaging, these NPs with a NIR probe DY-675 and g7 targeting moiety were administrated in mice. The optical imaging confirmed brain localization of these biodegradable polymeric NPs. This study was further extended to investigate the ability of the NP system in promoting the mouse BBB crossing via optical imaging.
A very interesting recent work investigated the movements of NPs varying in diameter and surface “properties” within mouse brain in vivo and fresh human and rat brain tissue ex vivo [79]. An interesting observation from this work was NPs as large as 114 nm and also densely coated with PEG only diffused within the human and rat brain. This work showed the ability of larger NPs to achieve brain penetration with the potential to allow more long-lasting, and effective delivery of payloads within the brain.
Potential Obstacles for using NPs in Imaging:
Many NPs, which are used to date, face limitations including sensitivity and resolution that one needs to consider when designing potential probes for in vivo imaging. NPs can potentially interact with plasma proteins that alter their surface properties, or they can be recognized by phagocytic cells and consequently accumulate in macrophages or the RES of the liver, spleen, or lymph nodes [35]. These parameters should be considered when incorporating sophisticated yet simple surface modifications that would satisfy the requirements of the regulatory authorities for diagnosis of human diseases.
POLYMERIC NPs IN THERAPY
The therapeutic effect of a medication largely depends on how it is formulated and administered. Most therapeutic agents are poorly soluble because of the way they are designed to achieve maximum specificity and efficacy. Improving the performance of a medication depends on four main factors: solubility, sufficient lipophilicity, absorption, and controlled release. Solubility controls the method by which it can be administered to the systemic circulation. Lipophilicity allows the drug to partition from the aqueous intestinal contents for solubilization in the intestinal milieu. Finally, the slow and controlled release properties allow for prolonged effect and reduced dose frequency. It is extremely difficult to incorporate these features in a chemical structure, thus use of delivery vehicles plays important roles in achieving such properties without compromising the drug activity. Advancements in drug delivery vehicles have the potential to revolutionize the efficacy and safety of the old FDA approved drugs. Targeted drug delivery, a concept first used by Paul Ehrlich by introducing the term “magic bullet”, is based on directing therapeutics coupled to a homing device that could result in their selective accumulation at the diseases site, enhancing efficacy while reducing toxicity [80]. An ideal drug delivery vehicle is required to deliver the appropriate quantity of a therapeutic at the right time and for the requisite period directly to the target site of interest. Controlled release biodegradable polymeric NPs are especially promising candidates as drug delivery vehicles [5, 16, 26-27]. In particular, the use of biopolymers PLA, PGA, PLGA, and PEG makes these biomaterials ideal for the development of new therapeutics. In general, controlled-release polymeric NPs increase the efficacy of therapeutics and maximize patient compliance by providing an optimum range of drug levels over a longer period of time than other drug delivery methods. While these advantages can be significant, the potential toxicity or non-biocompatibility in some cases limited their translation into clinical practice [81]. Despite decades of research, only in recent years the development of biocompatible and biodegradable controlled release drug delivery vehicles become practical. A few examples of controlled release systems in clinical use today include Atridox, Lupron Depot, Gliadel, Sandostatin LAR, Risperdal CONSTA, and Vivitrol [82].
Therapeutics for Cancer:
Polymeric NP-based delivery of age-old cancer therapeutics is focused on “passive” and “active” targeting strategies. “Passive” targeting utilizes the unique properties of the tumor microenvironment, such as (i) leaky tumor neovasculature, which is highly permeable to macromolecules relative to the normal tissue, and (ii) a dysfunctional lymphatic drainage system, which results in enhanced fluid retention in the tumor interstitial space [10, 83]. The leaky neovascular and the lack of intact lymphatic system together result in the EPR effect and passive cancer targeting through the accumulation of NPs in the tumor at a higher concentration [84]. The ability of NPs to extravasate the neovasculature depends on the size of open inter-endothelial gap junctions and trans-endothelial channels. The pores in these transport pathways have cutoff sizes reported between 400-600 nm, suggesting a cutoff size of approximately 400 nm [85]. Generally, NPs with diameters less than 200 nm should be the most effective for extravasating the tumor microvasculature [85-87]. Currently approved nanotechnology therapeutic products for cancer therapy operate through the EPR effect [10]. While these passively targeted drug delivery systems are clinically efficacious, there is contradicting data for the added benefit of active targeting [28, 88-91]. Accumulation of NPs in tumor interstitial space requires a long-circulating half-life to facilitate time-dependent extravasations of NPs [92]. This process is largely mediated by the bio-physicochemical properties of the NPs and not by active targeting [20], signifying that drug delivery systems can be engineered to a specific target tissue even in the absences of targeting ligands [93-94]. However, once particles extravasate out of the vasculature into the tumor tissue, active targeting facilitates their retention and specific uptake by cancer cells and receptor-mediated endocytosis [90]. There are several examples of targeted polymeric NPs for cancer targeting in vitro and in vivo [19, 22-23, 25-28, 30, 33-34].
Among the clinically accepted drug delivery systems of old drugs, liposomal formulation of doxorubicin was the first nanotherapeutic to get FDA approval for the treatment of AIDS associated with Kaposi’s sarcoma [95]. Numerous developments since then have resulted in several other clinically approved liposomal formulations and many are in the various stages of clinical trials (Tables 1 and 2).
In the taxane family, paclitaxel and docetaxel are two FDA approved chemotherapeutics with known clinical benefits in the adjuvant and metastatic setting [96]. This huge clinical success, however, is associated with significant side effects and primary as well as secondary resistance. The poor solubility of this family of compounds plays additional difficulty in their administration. Thus these compounds necessitate the inclusion of delivery vehicles in their commercial formulations. There are few liposome-based formulations of paclitaxel [97]. In 2005, FDA approved an albumin stabilized-NP formulation of paclitaxel, Abraxane that is safe and effective in the treatment of metastatic breast cancer [98]. Surprisingly, the only new formulation for docetaxel is BIND-014, which is the first targeted polymeric NP to enter human clinical trial [29]. BIND-014 is a docetaxel encapsulated targeted PLGA-b-PEG polymer-based NP that is targeted to prostate-specific membrane antigen (PSMA), a well-validated antigen expressed on prostate cancer cells and on the neovasculature of most non-prostate solid tumors (Fig. 5). PK studies indicated prolonged circulation and controlled drug release with plasma concentrations remaining up to at least 100-fold higher than conventional docetaxel for over 24 h. These NPs also exhibited significantly enhanced tumor uptake and prolonged tumor growth suppression in multiple tumor models compared with conventional docetaxel. Preliminary clinical data with 17 patients who had advanced or metastatic solid tumor indicated that BIND-014 has pharmacological characteristics consistent with preclinical findings.
Fig. (5).

Schematic illustration of BIND-014 depicting the payload and the targeting moiety in the PLGA-b-PEG-NP.
Cis-diamminedichloroplatinum(II) or cisplatin [99-102], is a first line chemotherapeutic agent which is used to treat 50% of all cancers [103]. Of the numerous derivatives of the cisplatin chemotherapeutic family, only carboplatin and oxaliplatin are in clinical use today, other compounds such as nedaplatin, lobaplatin, and heptaplatin are only approved in Japan, China, and South Korea, respectively. Several attempts to circumvent the side effects and tumor resistance of cisplatin resulted new platinum-based complexes. However, none of these complexes have superior efficacy, reduced toxicity, and less cross-resistance compared to cisplatin. The conventional formulation of cisplatin administered as intravenous infusion shows low bioavailability to the target tissue. Thus, a clinical formulation of cisplatin that can increase and retain the drug concentration in the plasma within the therapeutic window for prolonged duration would be beneficial to reduce the side effects and prevent frequent administration of doses. Controlled release polymeric NPs have the potential to address the above-mentioned factors. However, incorporation of cisplatin into traditional PLGA-based polymeric NPs is a challenge because of its physico-chemical properties [25-26, 104-105]. Dhar et al. devised a unique strategy to deliver cisplatin by constructing Pt(IV)-prodrug encapsulated PLGA-b-PEG-based NPs which were actively targeted to prostate cancer cells by using PSMA targeting aptamer (Fig. 6). In vitro studies indicated that this system is selective in targeting PSMA-overexpressing prostate cancer cells and the NP formulation of cisplatin prodrug has enhanced cytotoxicity in cell culture compared to conventional cisplatin [25]. A follow up publication showed that targeted delivery of the prodrug formulation of cisplatin using a polymeric NP delivery system could markedly improve its tolerability and efficacy in vivo [26].
Fig. (6).

Schematic representation of aptamer-functionalized Pt(IV) encapsulated PLGA-b-PEG-based biodegradable NPs and the chemistry of the intracellular release of cisplatin active drug. Aptamers are nucleic acid-based ligands with the ability to bind to various molecular targets.
Karve et al. sketched out a method to use biodegradable lipid-polymer NP platform to deliver a clinically abandoned phosphatidylinositol 3′ kinase-related inhibitor, wortmannin [106]. It was speculated that the possible reason of wortmannin clinical failure is due to drug delivery challenges. However, higher solubility and lower toxicity was shown by the engineered NP formulation of wortmannin compared to the conventional free form. The therapeutic potential of this new formulation was evaluated in vivo in two murine xenograft models of cancer and the results demonstrated that polymer-based delivery system has the potential to overcome the limitations shown by the abandoned free drug.
Several other pathways are involved in the progression of cancer and targeting only one pathway often results in an insufficient approach to provide an effective anticancer regimen. Combination drug therapy is an attractive strategy and is commonly used to treat diseases like malaria [107], HIV/AIDS [31, 108], diabetes [109], and cancer [30, 110]. Unlike single-agent chemotherapy, combination therapy can attack several different pathways in cancer cells, allowing for the optimization of therapeutic efficacy against individual targets and overcoming resistance. Combination therapy can potentially improve the prognosis for a positive outcome, such as the diminution of side effects [110]. However, administration of drug combinations present therapeutic challenges, including the choice of dosages to reduce side effects, the accurate delivery of the correct drug ratio, and exposure to the targets of interest which is problematic when individually administering drugs. Polymeric NPs can be promising as non-targeted or targeted drug delivery vehicles for combination therapy of cancer. In a recent work, Kolishetti et al. engineered a PSMA-targeted PLGA-b-PEG-NP system for co-delivery of cisplatin and docetaxel, two chemotherapeutics with very different physicochemical properties, to prostate cancer cells. In this work, a functionalized PLA decorated with a Pt(IV)-prodrug was constructed and the resultant polymer was blended with PLGA-b-PEG, and docetaxel was incorporated in the NP matrix during the synthesis of the NPs (Fig. 7) [30]. Based on in vitro studies, the targeted dual-drug encapsulated-NPs were shown to be more effective than the single drug encapsulating NPs. Aryal et al. followed a very similar method and developed a drug-polymer conjugate with two different chemotherapeutics, doxorubicin and camptothecin [111]. The combination formulation developed showed superior in vitro efficacy. These recent works on combination therapy using polymeric NPs share a common feature, functionalization of the backbone of the common biodegradable polymers, PLA, PGA, and their copolymer PLGA. The rationale behind this is lack of functional groups on the aliphatic backbones, which limits the number of sites for conjugation and incorporation of multiple drugs. NPs comprised of functionalized biodegradable polymers are attractive as combination therapy delivery vehicles; however, it has to be emphasized that considerable challenges exist on a case-by-case basis when developing this type of NP therapeutics to address their safety issues.
Fig. (7).

A Pt(IV)-functionalized PLA was blended with PLGA-b-PEG-COOH in presence of docetaxel to form biodegradable polymeric NPs with the ability to deliver a combination of cisplatin and docetaxel in a spatio temporal manner to prostate cancer cells by using surface functionalization with PSMA aptamer.
A very recent example showed the use of controlled release polymer-based NP drug delivery approaches to combine two different therapeutic modalities. In an effort to combine photodynamic therapy (PDT) with immune system, Marrache et al. engineered a photosensitizer zinc phthalocyanine (ZnPc) and a single-stranded DNA-based immunostimulant CpG-ODN loaded PLGA-b-PEG-based NP system (Fig. 8) [112]. In vitro studies indicated that this combination of PDT with a synergistic immunostimulant in a single NP system results in significant immune response and enhanced efficacy compared to the free ZnPc and its NP formulation, or a combination of ZnPc and the CpG-ODN in their free form.
Fig. (8).

A PLGA-b-PEG-based hybrid NP platform that combines phototherapeutic effect of a ZnPc-based photosensitizer with immunostimulatory effect of a DNA-based immunoadjuvant.
Therapeutics For Cardiovascular Diseases
As mentioned earlier, CVD represents a global medical and economic problem. According to the World Health Organization (WHO), CVD is the number one killer worldwide [113]. Depending on the affected organs, CVD is categorized into coronary artery disease, cerebrovascular disease, peripheral arterial disease, and aortic atherosclerosis. In general, CVD is characterized by narrowing of the blood supply and is most commonly caused by atherosclerosis [114]. Management of CVD mainly focuses on lifestyle tuning in the early stage and medications and surgical procedures in the advanced stage. Novel innovations and rapid expansions of nanotherapeutics opened several possible medical alternatives to the treatment of CVDs. Therapeutic uses of NPs in the cardiovascular medicinal field include nanocarriers for delivery of drugs, bioactive molecules, and other technologies for cholesterol reduction [115] and clot dissolution [116-117].
In the current scenario, the primary intervention for coronary diseases is expandable intra-arterial stent, a small mesh tube, replacement; however, this procedure, occasionally leads to in-stent restenosis [118-120]. Clinical trials with systemic administration of anti-proliferative or antithrombotic agents were unsuccessful in the prevention of restenosis [121-124]. Effective prevention of restenosis requires high therapeutic concentrations of drug for a prolonged period of time. Thus, controlled release drug delivery vehicles based on biodegradable polymers, which can provide local drug release at a sustained rate, can be extremely beneficial. PLGA-coated stents have the potential to effectively deliver therapeutics/genes to vessel walls in a controlled manner. Klugherz et al. demonstrated the possibility of such a technology by delivering DNA in a controlled release fashion from a PLGA-coated expandable stent in coronary arteries [125]. In a follow-up work, this was extended to utilize denatured collagen in the PLGA composite vascular stent coating formulation to enhance gene transfer due to adhesion molecule interactions and actin-related mechanisms [126]. The presence of denatured collagen in the vascular stent coating showed improved DNA controlled release and enhanced plasmid DNA transfection through mechanisms involving β3-integrin receptor interactions and associated changes in actin dynamics. Another interesting approach explored the possibility of incorporation of an anti-angiogenic agent, such as angio-statin, in a PLGA-PEG-coated stent [119]. Controlled release of intravascular angiostatin from a stent restricts plaque progression and in-stent restinosis. Using a rabbit model, it was demonstrated that at 7 days, neovascularization was significantly decreased in the angio-statin groups versus the control group. Another possibility includes the localized intravascular infusion of anti-proliferative-loaded NPs to prevent neointimal formation [127]. Mei et al. incorporated paclitaxel in PLGA-based NPs and the surface was coated with a cationic surfactant didodecyldimethylammonium bromide. Treatment of balloon injured rabbit carotid arteries with single infusion of paclitaxel-loaded NPs demonstrated complete inhibition of intimal proliferation [127]. Margolis et al. presented the first human safety trial of a systemic paclitaxel-albumin-based NP, nab-paclitaxel, for in stent restenosis [128]. However, till now, there is no update for the clinical utility of nab-paclitaxel or any other nano formulations for the suppression of coronary in stent restenosis [129]. The field of biodegradable polymer-based materials in stents opened up several promising areas for future refinement of bioactive stent designs and clinical strategies.
Polymeric NP-based therapeutics for neurodegenerative disease
Neurodegenerative diseases of CNS are complicated due to the fact that the BBB is a significant impediment for a variety of molecules to enter into the CNS tissue. The therapeutic potential of polymeric NPs for neurodegenerative diseases includes both neuroprotective and neuroregenerative approaches. Polymer-based nanomedicines that can overcome CNS related barriers when administered systemically would be a tremendous advancement in diagnosis and treatment of neurodegenerative disorders, brain cancer, and trauma [130].
At present, one of the most commonly used polymers for construction of NPs for CNS drug delivery, polyalkylcyanoacrylate (PACA) [131], is not a FDA approved polymer [132-133]. Polymeric NPs constructed from PLA or PLGA undergo sudden removal when injected into the peripheral circulation due to RES activity [133-134]. However, tuning NP surface characteristics, geometry, and size can control the activation of this defensive system. NPs with a hydrophobic surface and negative charges activate the complement system and promote protein adsorption [135]. In general, NPs < 10 nm are rapidly removed after an extensive extravasation and renal clearance, NPs < 100 nm have a lower possibility of being uptaken by macrophages, NPs > 200 nm are rapidly filtered by the spleen and removed by the RES system, and NPs > 5 μm induce capillary blockades. However, polymeric NPs from biodegradable polymers can be engineered to increase their potential crossing of the BBB by specific mechanisms, such as absorptive-mediated transcytosis or receptor-mediated endocytosis. A few specific receptors identified on the brain capillaries for transferring, insulin (INS), and INS-like growth factors can be used to avail BBB targeting.
In an effort to deliver oligonucleotides (ODN) to the CNS for the development of therapeutic modalities for the treatment of neurodegenerative disorders, Vinogradov et al. developed “nanogels” based on a nanoscale network of cross-linked PEG and polyethylenimine [135]. These nanogels were shown to bind and encapsulate spontaneously negatively charged ODN, allowing for the formation of a stable aqueous dispersion of polyelectrolyte complex with particle sizes < 100nm. Using an in vitro model, this study demonstrated that ODN incorporated into nanogel can be effectively transported across the BBB and transport efficacy can be further enhanced by nanogel surface modification with transferrin or INS. Mechanistic investigations showed that the ODN is transported across the brain microvessel cells through the trans cellular pathway, the ODN remains in the nanogel, and displays little degradation compared to the free ODN. In vivo bioD studies in mouse model following ODN distribution 1 h post intravenous administration, demonstrated that the use of nanogel as a delivery vehicle increased ODN accumulation by over 15-fold in the brain but this accumulation in the liver and spleen decreased by 2-fold compared to the free ODN. This study implied that nanogel could be a beneficial system for delivery of therapeutics to the brain.
Aktas et al. utilized targeted –chitosan-based-NPs in the delivery of a peptide-based inhibitor of caspase-3 enzyme to the brain using a mouse monoclonal antibody, OX26, against a rat transferrin receptor as a targeting ligand [136]. The inhibition of the caspase-3 enzyme can increase neuronal cell survival after cerebral ischemia and a peptide Z-DEVD-FMK is a specific caspase inhibitor, which significantly reduced vulnerability to the neuronal cell death. However, the inability of this peptide to cross the BBB and to diffuse into the brain tissue requires an effective delivery vehicle for its successful use. Using the avidin-biotin technology, Aktas et al. developed chitosan-PEG-OX26 nanospheres, the affinity of OX26 for the transferrin receptor helped trigger receptor-mediated transport across the BBB. In vivo distribution of fluorescently labeled targeted-chitosan NPs showed that a significant amount of the NPs were located outside of the intravascular compartment and in the brain. Electron microscopic examination of the brain tissue indicated that these targeted-NPs were able to translocate into the brain tissue after intravenous administration.
CURRENT CHALLENGES FOR CLINICAL TRANSLATION OF NPs
Cancer medicine remains the biggest share of the nanomedicine research. Increasing acceptance of nanotechnologies in the clinic along with the aggressiveness of cancer, are the two most important factors for the success of nanomedicine. Doxil and Abraxane are prime examples of nanomedicine success. Numerous clinical trials, proof-of-concept studies in cell cultures, in small-animal models are under way with several nanomedicine platforms. In the drug delivery segment, nanomedicine dominates with 76% of the publications and 59% of the patents. Despite the leaps and bounds made in the development of polymer-based nanocarriers, there are still several barriers that limit these carriers in clinical trials. BIND-014 is the first targeted polymeric NP to enter human clinical trial. The current challenges for most nanotherapeutics for transition from the bench to the bedside are numerous. Polymeric NPs face additional challenges related to polymer toxicity, premature drug release, poor drug and/or contrast agent loading, and scale-up challenges.
The EPR effect contributes to the success of polymeric NPs to target solid-tumor. Only NPs within a restricted size range can diffuse through the endothelium of tumor and take advantage of the EPR effect. The specific size of tumor vasculature defects depends on the cancer type, tumor site, and stage. NPs below 10 nm undergo first-pass elimination in the kidney and larger than 150-200 nm are cleared by the liver and spleen. A size range of 20-100 nm is essential for NPs to take the advantage of the EPR effect. Therefore, for polymeric NPs to be successful in the clinic, an extensive tuning to obtain suitable size properties, functionalization is a prerequisite.
A few technical challenges of clinical translation include the following: the ability to exhaustively analyze each batch of NP to ensure that it is chemically correct, the control of the release kinetics of the payload, the exact positioning of the payload within the polymeric NP, and the inability to introduce sufficient targeting ligands and payloads without compromising the size.
FDA regulations and long approval procedures are different for polymeric NP-based therapeutics compared to those of other industries. Although polymeric NPs from biodegradable polymers are not inevitably toxic, many of their unique properties, which are not well studied, raise several questions regarding their safety. FDA faces exceptional challenges in efficiently regulating polymeric-NP based therapeutics due to lack of strong scientific knowledge and better understanding of the potential risk factors in the body. Validation of numerous nanotherapeutic platforms for safety and efficacy presents an enormous challenge for FDA.
There are several reasons which include low expected returns, the exaggerated optimism in undertaking an investment on breakthrough projects, and the uncertainty and risk associated with these projects. These reasons make attracting investments from pharmaceutical and biotechnology companies for polymeric NP-based products and in general for “nanomedicine research” particularly challenging. The expenditure to translate a nanotherapeutic product to the clinical market is so huge that companies prefer to focus only on the blockbusters platforms that can please the stockholders.
FUTURE OUTLOOK
The field of nanomedicine has many unique properties that can potentially provide novel solutions to improve health care. This statement is supported by the fact that a number of therapeutics, medical devices, imaging agents, and diagnostic devices, which contain nano-components approved by the FDA, are improving the treatment options of many diseases. Despite several challenges that exist, nanomedicine is finishing the race at a rapid pace to attain a place as an integral part of mainstream future medicine. In his prescient talk “There’s Plenty of Room at the Bottom,” for the application of nanotechnology in medicine and the importance of being small to treat diseases, Feynman prophetically concluded that this is “a development which I think cannot be avoided,” which is indeed very true for the field of nanomedicine and future health care will experience many advancements from this rich field.
ACKNOWLEDGEMENTS
A start-up fund from the National Institutes of Health (P30GM092378) and by the Office of the Vice President for Research, University of Georgia to S.D; financial support from the Ralph E. Powe Junior faculty award (S.D.) and Department of Defense Prostate Cancer Idea award (W81XWH-12-1-0406) (S.D.) are greatly appreciated.
ABBREVIATIONS
- FDA
Food and Drug Administration
- NNI
National Nanotechnology Initiative
- NP
Nanoparticle
- EPR
Enhanced permeability and retention
- RES
Reticuloendothelial system
- PGA
Poly(glycolic acid)
- PLA
Poly(lactic acid)
- PLGA
Poly(lactic-co-glycolic acid)
- PCL
Polycaprolactone
- PEG
Poly(ethylene glycol)
- PVA
Poly vinyl alcohol
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- PET
Positron emission tomography
- SPECT
Single photon emission computed tomography
- SPIO
Super paramagnetic iron oxide
- bioD
Biodistribution
- BBB
Blood-brain barrier
- Gd
Gadolinium
- PCL-b-PMAA
Polycaprolactone -b-poly methacrylic acid
- NIR
Near infrared region
- FRET
Fluorescence resonance energy transfer
- Gd-DO3A
Gadolinium-(1,4,7,10-tetraazacyclododecane-1,4,7-trisacetic acid)
- Gd-DTPA
Gadolinium dietheylentriaminepentaacetic acid
- PAUS
Photoacoustic ultrasound
- CVD
Cardiovascular disease
- CREKA
Cysteine-arginine-glutamic acid-lysine-alanine
- DSPE
1,2-distearoyl-sn-glycero-3-phosphoethanolamine
- cROMP
Colloidal, radio-opaque, and metal-encapsulated polymeric
- PS-b-PAA
Polystyrene-b-polyacrylic acid
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- PBCA
Polybutylcyanoacrylate
- CNS
Central nervous system
- PSMA
Prostate specific membrane antigen
- PDT
Photodynamic therapy
- ZnPc
Zinc phthalocyanine
- WHO
World Health Organization
- PACA
Polyalkylcyanoacrylate
- INS
Insulin
- ODN
Oligonucleotides
Footnotes
CONFLICT OF INTEREST
The authors have no financial conflict with the subject discussed in this review. No writing assistance was used in the production of this manuscript.
REFERENCES
- [1].Freitas RA Jr. What is nanomedicine? Nanomed. Nanotechnol, 2005, 7(1), 2–9. [Google Scholar]
- [2].Farokhzad OC; Langer R Impact of nanotechnology on drug delivery. ACS Nano, 2009, 3(1), 16–20. [DOI] [PubMed] [Google Scholar]
- [3].Shi J; Votruba AR; Farokhzad OC; Langer R Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett., 2010, 70(9), 3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kamaly N; Xiao Z; Valencia PM; Radovic-Moreno AF; Farokhzad OC Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev, 2012, 41(7), 2971–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soppimath KS; Aminabhavi TM; Kulkarni AR; Rudzinski WE Biodegradable polymeric nanoparticles as drug delivery devices. J. Control Release, 2001, 70(1-2), 1–20. [DOI] [PubMed] [Google Scholar]
- [6].Wissing SA; Kayser O; Muller RH Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev, 2004, 56(9), 1257–1272. [DOI] [PubMed] [Google Scholar]
- [7].Marks S Liposome battle for antifungal market shifts into high gear. J. Int. Assoc. Physicians AIDS Care, 1996, 2(4), 46–47. [PubMed] [Google Scholar]
- [8].Ventola CL The nanomedicine revolution: part 2: current and future clinical applications. P T., 2012, 37(10), 582–591. [PMC free article] [PubMed] [Google Scholar]
- [9].Maeda H Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate Chem., 2010, 21(5), 797–802. [DOI] [PubMed] [Google Scholar]
- [10].Matsumura Y; Maeda H A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res., 1986, 46(12), 6387–6392. [PubMed] [Google Scholar]
- [11].Leserman LD; Barbet J; Kourilsky F; Weinstein JN Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature, 1980, 288(5791), 602–604. [DOI] [PubMed] [Google Scholar]
- [12].Torchilin VP Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov, 2005, 4(2), 145–160. [DOI] [PubMed] [Google Scholar]
- [13].Oku N; Namba Y Long-circulating liposomes. Crit. Rev. Ther. Drug, 1994, 11(4), 231–270. [PubMed] [Google Scholar]
- [14].Folkman J; Long DM The use of silicone rubber as a carrier for prolonged drug therapy. J. Surg. Res, 1964, 4, 139–142. [DOI] [PubMed] [Google Scholar]
- [15].Langer R; Folkman J Polymers for the sustained release of proteins and other macromolecules. Nature, 1976, 263(5580), 797–800. [DOI] [PubMed] [Google Scholar]
- [16].Langer R Drug delivery. Drugs on target. Science, 2001, 293(5527), 58–59. [DOI] [PubMed] [Google Scholar]
- [17].Richards Grayson AC; Choi IS; Tyler BM; Wang PP; Brem H; Cima MJ; Langer R Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat. Mater, 2003, 2(11), 767–772. [DOI] [PubMed] [Google Scholar]
- [18].Marrache S; Dhar S Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. USA, 2012, 109(40), 16288–16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Alexis F; Basto P; Levy-Nissenbaum E; Radovic-Moreno AF; Zhang L; Pridgen E; Wang AZ; Marein SL; Westerhof K; Molnar LK; Farokhzad OC HER-2-targeted nanoparticle-affibody bioconjugates for cancer therapy. Chem. Med. Chem, 2008, 3, 1839–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alexis F; Pridgen E; Molnar LK; Farokhzad OC Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmaceut, 2008, 5(4), 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marrache S; Dhar S Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc. Natl. Acad. Sci. USA, 2013, 110(23), 9445–9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chan JM; Zhang L; Yuet KP; Liao G; Rhee JW; Langer R; Farokhzad OC PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials, 2009, 30(8), 1627–1634. [DOI] [PubMed] [Google Scholar]
- [23].Cheng J; Teply BA; Sherifi I; Sung J; Luther G; Gu FX; Levy-Nissenbaum E; Radovic-Moreno AF; Langer R; Farokhzad OC Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials, 2007, 28(5), 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng J; Teply BA; Sherifi I; Sung J; Luther G; Gu FX; Levy-Nissenbaum E; Radovic-Moreno AF; Langer R; Farokhzad OC Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials, 2008, 28, 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dhar S; Gu FX; Langer R; Farokhzad OC; Lippard SJ Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA, 2008, 105(45), 17356–17361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dhar S; Kolishetti N; Lippard SJ; Farokhzad OC Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA, 2011, 108(5), 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farokhzad OC; Jon SY; Khadelmhosseini A; Tran TNT; LaVan DA; Langer R Nanopartide-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res., 2004, 64(21), 7668–7672. [DOI] [PubMed] [Google Scholar]
- [28].Gu F; Zhang L; Teply BA; Mann N; Wang A; Radovic-Moreno AF; Langer R; Farokhzad OC Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA, 2008, 105(7), 2586–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hrkach J; Von Hoff D; Mukkaram Ali M; Andrianova E; Auer J; Campbell T; De Witt D; Figa M; Figueiredo M; Horhota A; Low S; McDonnell K; Peeke E; Retnarajan B; Sabnis A; Schnipper E; Song JJ; Song YH; Summa J; Tompsett D; Troiano G; Van Geen Hoven T; Wright J; LoRusso P; Kantoff PW; Bander NH; Sweeney C; Farokhzad OC; Langer R; Zale S Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med, 2012, 4(128), 128ra139. [DOI] [PubMed] [Google Scholar]
- [30].Kolishetti N; Dhar S; Valencia PM; Lin LQ; Karnik R; Lippard SJ; Langer R; Farokhzad OC Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. USA, 2010, 107(42), 17939–17944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mamo T; Moseman EA; Kolishetti N; Salvador-Morales C; Shi J; Kuritzkes DR; Langer R; von Andrian U; Farokhzad OC Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine-UK, 2010, 5(2), 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang AZ; Gu F; Zhang L; Chan JM; Radovic-Moreno A; Shaikh MR; Farokhzad OC Biofunctionalized targeted Nanoparticles for therapeurtic applications. Expert Opin. Biol. Th, 2008, 8(8), 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang L; Chan JM; Gu FX; Rhee JW; Wang AZ; Radovic-Moreno AF; Alexis F; Langer R; Farokhzad OC Self-assembled lipid--polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano, 2008, 2(8), 1696–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang L; Radovic-Moreno AF; Alexis F; Gu FX; Basto PA; Bagalkot V; Jon S; Langer RS; Farokhzad OC Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle-aptamer bioconjugates. ChemMedChem, 2007, 2(9), 1268–1271. [DOI] [PubMed] [Google Scholar]
- [35].Kolishetti N; Alexis F; Pridgen EM; Farokhzad OC, In: Nanoplatform-Based Molecular Imaging, John Wiley & Sons, Inc.; 2011; pp. 75–104. [Google Scholar]
- [36].Acharya S; Sahoo SK PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev, 2011, 63(3), 170–183. [DOI] [PubMed] [Google Scholar]
- [37].Knop K; Hoogenboom R; Fischer D; Schubert US Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed, 2010, 49(36), 6288–6308. [DOI] [PubMed] [Google Scholar]
- [38].Jokerst JV; Lobovkina T; Zare RN; Gambhir SS Nanoparticle PEGylation for imaging and therapy. Nanomedicine-UK, 2011, 6(4), 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Davis ME; Chen ZG; Shin DM Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov, 2008, 7(9), 771–782. [DOI] [PubMed] [Google Scholar]
- [40].Lammers T; Kiessling F; Hennink WE; Storm G Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol. Pharmaceut, 2010, 7(6), 1899–1912. [DOI] [PubMed] [Google Scholar]
- [41].Jain TK; Morales MA; Sahoo SK; Leslie-Pelecky DL; Labhasetwar V Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharmaceut, 2005, 2(3), 194–205. [DOI] [PubMed] [Google Scholar]
- [42].Wassel RA; Grady B; Kopke RD; Dormer KJ Dispersion of super paramagnetic iron oxide nanoparticles in poly(d,l-lactide-co-glycolide) microparticle. Colloids Surface A, 2007, 292, 125–130. [Google Scholar]
- [43].Rosi NL; Mirkin CA Nanostructures in biodiagnostics. Chem. Rev, 2005, 105, 1547–1562. [DOI] [PubMed] [Google Scholar]
- [44].Katz E; Willner I Integrated nanoparticle–biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed, 2004, 43(45), 6042–6108. [DOI] [PubMed] [Google Scholar]
- [45].Puri A; Blumenthal R Polymeric lipid assemblies as novel theranostic tools. Acc. Chem. Res, 2011, 44(10), 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Smith AM; Dave S; Nie S; True L; Gao X Multicolor quantum dots for molecular diagnostics of cancer. Expert Rev. Mol. Diagn, 2006, 6(2), 231–244. [DOI] [PubMed] [Google Scholar]
- [47].Morgan B Opportunities and pitfalls of cancer imaging in clinical trials. Nat. Rev. Clin. Oncol, 2011, 8(9), 517–527. [DOI] [PubMed] [Google Scholar]
- [48].Kircher MF; Mahmood U; King RS; Weissleder R; Josephson L A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res., 2003, 63(23), 8122–8125. [PubMed] [Google Scholar]
- [49].Jokerst JV; Gambhir SS Molecular imaging with theranostic nanoparticles. Acc. Chem. Res, 2011, 44(10), 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mulder WJ; Griffioen AW; Strijkers GJ; Cormode DP; Nicolay K; Fayad ZA Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomedicine-UK, 2007, 2(3), 307–324. [DOI] [PubMed] [Google Scholar]
- [51].Mulder WJ; Strijkers GJ; van Tilborg GA; Cormode DP; Fayad ZA; Nicolay K Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc. Chem. Res, 2009, 42(7), 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brannon-Peppas L; Blanchette JO Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev, 2004, 56(11), 1649–1659. [DOI] [PubMed] [Google Scholar]
- [53].Jain RK Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng, 1999, 1, 241–263. [DOI] [PubMed] [Google Scholar]
- [54].Sokolov K; Follen M; Aaron J; Pavlova I; Malpica A; Lotan R; Richards-Kortum R Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res., 2003, 63(9), 1999–2004. [PubMed] [Google Scholar]
- [55].Byrne JD; Betancourt T; Brannon-Peppas L Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev, 2008, 60(15), 1615–1626. [DOI] [PubMed] [Google Scholar]
- [56].Yanga J; Lima E-K; Leeb HJ; Parka J; Leeb SC; Leec K; Yoond H-G; Suhe J-S; Huhe Y-M; Haama S Fluorescent magnetic nanohybrids as multimodal imaging agents for human epithelial cancer detection. Biomaterials, 2008, 29(16), 2548–2555. [DOI] [PubMed] [Google Scholar]
- [57].Wagh A; Qian SY; Law B Development of biocompatible polymeric nanoparticles for in vivo NIR and FRET imaging. Bioconjugate Chem., 2012, 23, 981–992. [DOI] [PubMed] [Google Scholar]
- [58].Ye FR; Ke TY; Jeong EK; Wang XL; Sung YG; Johnson M; Lu ZR Noninvasive visualization of in vivo drug delivery of poly(L-glutamic acid) using contrast-enhanced MRI. Mol. Pharmaceut, 2006, 3(5), 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Doiron AL; Homan KA; Emelianov S; Brannon L Poly(lactic-co-glycolic) acid as a carrier for imaging contrast agents. Pharma. Res, 2009, 26(3), 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lloyd-Jones D; Adams RJ; Brown TM; Carnethon M; Dai S; Simone GD; Ferguson TB; Ford E; Furie K; Gillespie C; Go A; Greenlund K; Haase N; Hailpern S; Ho PM; Howard V; Kissela B; Kittner S; Lackland D; Lisabeth L; Marelli A; McDermott MM; Meigs J; Mozaffarian D; Mussolino M; Nichol G; Roger VL; Rosamond W; Sacco R; Sorlie P; Stafford R; Thom T; Wasserthiel-Smoller S; Wong ND; Wylie-Rosett J Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation, 2010, 121, 46–215. [DOI] [PubMed] [Google Scholar]
- [61].Godin B; Sakamoto JH; Serda RE; Grattoni A; Bouamrani A; Ferrari M Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Cell, 2010, 31(5), 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Winter PM; Morawski AM; Caruthers SD; Fuhrhop RW; Zhang H; Williams TA; Allen JS; Lacy EK; Robertson JD; Lanza GM; Wickline SA Molecular imaging of angiogenesis in early-stage atherosclerosis with ανβ3-intergrin-targeted nanoparticles. Circulation, 2003, 108, 2270–2274. [DOI] [PubMed] [Google Scholar]
- [63].Peters D; Kastantin M; Kotamraju VR; Karmali PP; Gujraty K; Tirrell M; Ruoslahti E Targeting atherosclerosis by using modular, multifunctional micelles. Proc. Natl. Acad. Sci. USA, 2009, 106(24), 9815–9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pan D; Williams TA; Senpan A; Allen JS; Scott MJ; Gaffney PJ; Wickline SA; Lanza GM Detecting vascular biosignatures with a colloidal, radio-opaque polymeric nanoparticle. J. Am. Chem. Soc, 2009, 131(42), 15522–15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nahrendorf M; Zhang H; Hembrador S; Panizzi P; Sosnovik DE; Aikawa E; Libby P; Swirski FK; Weissleder R Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation, 2007, 117, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ross CA; Poirier MA Protein aggregation and neurodegenerative disease. Nat. Med, 2004, 10 Suppl, S10–17. [DOI] [PubMed] [Google Scholar]
- [67].Patel MM; Goyal BR; Bhadada SV; Bhatt JS; Amin AF Getting into the brain: Approaches to enhance brain drug delivery. CNS Drugs, 2009, 23(1), 35–58. [DOI] [PubMed] [Google Scholar]
- [68].Re F; Moresco R; Masserini M Nanoparticles for neuroimaging. J. Phys. D Appl. Phys, 2012, 45(7), 073001/073001–073001/073012. [Google Scholar]
- [69].Kreuter J Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev, 2001, 47(1), 65–81. [DOI] [PubMed] [Google Scholar]
- [70].Kreuter J; Shamenkov D; Petrov V; Ramge P; Cychutek K; Koch-Brandt C; Alyautdin R Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target, 2002, 10(4), 317–325. [DOI] [PubMed] [Google Scholar]
- [71].Popovic N; Brundin P Therapeutic potential of controlled drug delivery systems in neurodegenerative diseases. Int. J. Pharm, 2006, 314(2), 120–126. [DOI] [PubMed] [Google Scholar]
- [72].Kreuter J; Alyautdin RN; Kharkevich DA; Ivanov AA Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res., 1995, 674(1), 171–174. [DOI] [PubMed] [Google Scholar]
- [73].Koffie RM; Farrar CT; Saidi L-J; William CM; Hyman BT; Spires-Jones TL Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA, 2011, 108(46), 18837–18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Menei P; Daniel V; Montero-Menei C; Brouillard M; Pouplard-Barthelaix A; Benoit JP Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials, 1993, 14(6), 470–478. [DOI] [PubMed] [Google Scholar]
- [75].Emerich DF; Tracy MA; Ward KL; Figueiredo M; Qian R; Henschel C; Bartus RT Biocompatibility of poly (DL-lactide-co-glycolide) microspheres implanted into the brain. Cell Transplant., 1999, 8(1), 47–58. [DOI] [PubMed] [Google Scholar]
- [76].Shi N; Pardridge WM Noninvasive gene targeting to the brain. Proc. Natl. Acad. Sci. USA, 2000, 97(13), 7567–7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Shi N; Zhang Y; Zhu C; Boado RJ; Pardridge WM Brain-specific expression of an exogenous gene after i.v. administration. Proc. Natl. Acad. Sci. USA, 2001, 98(22), 12754–12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tosi G; Bondioli L; Ruozi B; Badiali L; Severini GM; Biffi S; De Vita A; Bortot B; Dolcetta D; Forni F; Vandelli MA NIR-labeled nanoparticles engineered for brain targeting: in vivo optical imaging application and fluorescent microscopy evidences. J. Neural Transm, 2011, 118(1), 145–153. [DOI] [PubMed] [Google Scholar]
- [79].Nance EA; Woodworth GF; Sailor KA; Shih TY; Xu Q; Swaminathan G; Xiang D; Eberhart C; Hanes J A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med, 2012, 4(149), 149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Strebhardt K; Ullrich A Paul Ehrlich's magic bullet concept: 100 years of progress. Nat. Rev. Cancer, 2008, 8(6), 473–480. [DOI] [PubMed] [Google Scholar]
- [81].Brigger I; Morizet J; Laudani L; Aubert G; Appel M; Velasco V; Terrier-Lacombe MJ; Desmaele D; d'Angelo J; Couvreur P; Vassal G Negative preclinical results with stealth nanospheres-encapsulated Doxorubicin in an orthotopic murine brain tumor model. J. Control. Release, 2004, 100(1), 29–40. [DOI] [PubMed] [Google Scholar]
- [82].Jain JP; Yenet Ayen W; Domb AJ; Kumar N, In: Biodegradable Polymers in Clinical Use and Clinical Development, John Wiley & Sons, Inc.; Hoboken, NJ, USA, 2011; pp. 1–58. [Google Scholar]
- [83].Maeda H; Matsumura Y Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drug, 1989, 6(3), 193–210. [PubMed] [Google Scholar]
- [84].Maeda H; Sawa T; Konno T Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release, 2001, 74(1-3), 47–61. [DOI] [PubMed] [Google Scholar]
- [85].Yuan F; Dellian M; Fukumura D; Leunig M; Berk DA; Torchilin VP; Jain RK Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res., 1995, 55(17), 3752–3756. [PubMed] [Google Scholar]
- [86].Yuan F; Leunig M; Huang SK; Berk DA; Papahadjopoulos D; Jain RK Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res., 1994, 54(13), 3352–3356. [PubMed] [Google Scholar]
- [87].Kong G; Braun RD; Dewhirst MW Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res., 2000, 60(16), 4440–4445. [PubMed] [Google Scholar]
- [88].Pun SH; Tack F; Bellocq NC; Cheng J; Grubbs BH; Jensen GS; Davis ME; Brewster M; Janicot M; Janssens B; Floren W; Bakker A Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther, 2004, 3(7), 641–650. [DOI] [PubMed] [Google Scholar]
- [89].Pirollo KF; Chang EH Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol., 2008, 26(10), 552–558. [DOI] [PubMed] [Google Scholar]
- [90].Kirpotin DB; Drummond DC; Shao Y; Shalaby MR; Hong K; Nielsen UB; Marks JD; Benz CC; Park JW Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res., 2006, 66(13), 6732–6740. [DOI] [PubMed] [Google Scholar]
- [91].Hussain S; Pluckthun A; Allen TM; Zangemeister-Wittke U Antitumor activity of an epithelial cell adhesion molecule targeted nanovesicular drug delivery system. Mol. Cancer Ther, 2007, 6(11), 3019–3027. [DOI] [PubMed] [Google Scholar]
- [92].Jain RK Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev, 2001, 46(1-3), 149–168. [DOI] [PubMed] [Google Scholar]
- [93].Verma A; Uzun O; Hu Y; Hu Y; Han HS; Watson N; Chen S; Irvine DJ; Stellacci F Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater, 2008, 7(7), 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gratton SE; Ropp PA; Pohlhaus PD; Luft JC; Madden VJ; Napier ME; DeSimone JM The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA, 2008, 105(33), 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Barenholz Y Doxil(R)--the first FDA-approved nano-drug: lessons learned. J. Control. Release, 2012, 160(2), 117–134. [DOI] [PubMed] [Google Scholar]
- [96].Esteva FJ; Valero V; Pusztai L; Boehnke-Michaud L; Buzdar AU; Hortobagyi GN Chemotherapy of metastatic breast cancer: what to expect in 2001 and beyond. Oncologist, 2001, 6(2), 133–146. [DOI] [PubMed] [Google Scholar]
- [97].Koudelka S; Turanek J Liposomal paclitaxel formulations. J. Control. Release, 2012, 163(3), 322–334. [DOI] [PubMed] [Google Scholar]
- [98].Gradishar WJ Albumin-bound paclitaxel: a next-generation taxane. Expert Opin. Pharmaco, 2006, 7(8), 1041–1053. [DOI] [PubMed] [Google Scholar]
- [99].Rosenberg B; VanCamp L; Trosko JE; Mansour VH Platinum compounds: A new class of potent antitumour agents. Nature, 1969, 222(5191), 385–386. [DOI] [PubMed] [Google Scholar]
- [100].Jamieson ER; Lippard SJ Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev, 1999, 99(9), 2467–2498. [DOI] [PubMed] [Google Scholar]
- [101].Dhar S; Lippard S, In: Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy, Bonetti A.; Leone R.; Muggia F.; Howell S., Eds. Humana Press; 2009; pp. 135–147. [Google Scholar]
- [102].Dhar S; Lippard SJ, In: Bioinorganic Medicinal Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA; 2011; pp. 79–95. [Google Scholar]
- [103].Galanski M; Jakupec MA; Keppler BK Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr. Med. Chem, 2005, 12(18), 2075–2094. [DOI] [PubMed] [Google Scholar]
- [104].Avgoustakis K; Beletsi A; Panagi Z; Klepetsanis P; Karydas AG; Ithakissios DS PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J. Control. Release, 2002, 79(1-3), 123–135. [DOI] [PubMed] [Google Scholar]
- [105].Fujiyama J; Nakase Y; Osaki K; Sakakura C; Yamagishi H; Hagiwara A Cisplatin incorporated in microspheres: development and fundamental studies for its clinical application. J. Control. Release, 2003, 89(3), 397–408. [DOI] [PubMed] [Google Scholar]
- [106].Karve S; Werner ME; Sukumar R; Cummings ND; Copp JA; Wang EC; Li CX; Sethi M; Chen RC; Pacold ME; Wang AZ Revival of the abandoned therapeutic wortmannin by nanoparticle drug delivery. Proc. Natl. Acad. Sci. USA, 2012, 109(21), 8230–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Orjuela P; Gonzalez I; Osorio L Combination therapy as a strategy to prevent antimalarial drug resistance. Biomedica, 2004, 24(4), 423–437. [PubMed] [Google Scholar]
- [108].de Gaetano Donati K; Rabagliati R; Iacoviello L; Cauda R HIV infection, HAART, and endothelial adhesion molecules: current perspectives. Lancet Infect. Dis, 2004, 4(4), 213–222. [DOI] [PubMed] [Google Scholar]
- [109].Suarez-Pinzon WL; Power RF; Yan Y; Wasserfall C; Atkinson M; Rabinovitch A Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes, 2008, 57(12), 3281–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jia J; Zhu F; Ma X; Cao Z; Li Y; Chen YZ Mechanisms of drug combinations: interaction and network perspectives. Nat. Rev. Drug Discov, 2009, 8(2), 111–128. [DOI] [PubMed] [Google Scholar]
- [111].Aryal S; Hu CMJ; Zhang LF Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol. Pharmaceut, 2011, 8(4), 1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Marrache S; Choi JH; Tundup S; Zaver D; Harn DA; Dhar S Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr. Biol, 2013, 5(1), 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Roger VL; Go AS; Lloyd-Jones DM; Benjamin EJ; Berry JD; Borden WB; Bravata DM; Dai S; Ford ES; Fox CS; Fullerton HJ; Gillespie C; Hailpern SM; Heit JA; Howard VJ; Kissela BM; Kittner SJ; Lackland DT; Lichtman JH; Lisabeth LD; Makuc DM; Marcus GM; Marelli A; Matchar DB; Moy CS; Mozaffarian D; Mussolino ME; Nichol G; Paynter NP; Soliman EZ; Sorlie PD; Sotoodehnia N; Turan TN; Virani SS; Wong ND; Woo D; Turner MB; American Heart Association Statistics, C.; Stroke Statistics, S. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation, 2012, 125(1), e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Libby P; Theroux P Pathophysiology of coronary artery disease. Circulation, 2005, 111(25), 3481–3488. [DOI] [PubMed] [Google Scholar]
- [115].Biana G; Jason HS; Rita ES; Alessandro G; Ali B; Mauro F Review: Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol. Sci, 2010, 81, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kim KS; Khang G; Lee D Application of nanomedicine in cardiovascular diseases and stroke. Curr. Pharma. Design, 2011, 17(18), 1825–1833. [DOI] [PubMed] [Google Scholar]
- [117].Bauters C; Banos JL; Van Belle E; Mc Fadden EP; Lablanche JM; Bertrand ME Six-month angiographic outcome after successful repeat percutaneous intervention for in-stent restenosis. Circulation, 1998, 97(4), 318–321. [DOI] [PubMed] [Google Scholar]
- [118].Ganaha F; Kao EY; Wong H; Elkins CJ; Lee J; Modanlou S; Rhee C; Kuo MD; Yuksel E; Cifra PN; Waugh JM; Dake MD Stent-based controlled release of intravascular angiostatin to limit plaque progression and in-stent restenosis. J. Vasc. Interv. Radiol, 2004, 15(6), 601–608. [DOI] [PubMed] [Google Scholar]
- [119].Reimers B; Moussa I; Akiyama T; Tucci G; Ferraro M; Martini G; Blengino S; Di Mario C; Colombo A Long-term clinical follow-up after successful repeat percutaneous intervention for stent restenosis. J. Am. Coll. Cardiol, 1997, 30(1), 186–192. [DOI] [PubMed] [Google Scholar]
- [120].Popma JJ; Califf RM; Topol EJ Clinical trials of restenosis after coronary angioplasty. Circulation, 1991, 84(3), 1426–1436. [DOI] [PubMed] [Google Scholar]
- [121].Bauters C; Isner JM The biology of restenosis. Prog. Cardiovasc. Dis, 1997, 40(2), 107–116. [DOI] [PubMed] [Google Scholar]
- [122].Chorny M; Fishbein I; Golomb G Drug delivery systems for the treatment of restenosis. Crit. Rev. Ther. Drug, 2000, 17(3), 249–284. [PubMed] [Google Scholar]
- [123].Bhargava B; Karthikeyan G; Abizaid AS; Mehran R New approaches to preventing restenosis. BMJ, 2003, 327(7409), 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Klugherz BD; Jones PL; Cui X; Chen W; Meneveau NF; DeFelice S; Connolly J; Wilensky RL; Levy RJ Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat. Biotechnol, 2000, 18(11), 1181–1184. [DOI] [PubMed] [Google Scholar]
- [125].Perlstein I; Connolly JM; Cui X; Song C; Li Q; Jones PL; Lu Z; DeFelice S; Klugherz B; Wilensky R; Levy RJ DNA delivery from an intravascular stent with a denatured collagen-polylactic-polyglycolic acid-controlled release coating: mechanisms of enhanced transfection. Gene Ther., 2003, 10(17), 1420–1428. [DOI] [PubMed] [Google Scholar]
- [126].Mei L; Sun H; Jin X; Zhu D; Sun R; Zhang M; Song C Modified paclitaxel-loaded nanoparticles for inhibition of hyperplasia in a rabbit arterial balloon injury model. Pharm Res, 2007, 24(5), 955–962. [DOI] [PubMed] [Google Scholar]
- [127].Margolis J; McDonald J; Heuser R; Klinke P; Waksman R; Virmani R; Desai N; Hilton D Systemic nanoparticle paclitaxel (nab-paclitaxel) for in-stent restenosis I (SNAPIST-I): a first-in-human safety and dose-finding study. Clin. Cardiol, 2007, 30(4), 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].McDowell G; Slevin M; Krupinski J Nanotechnology for the treatment of coronary in stent restenosis: a clinical perspective. Vasc. Cell, 2011, 3(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Patel T; Zhou J; Piepmeier JM; Saltzman WM Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev, 2012, 64(7), 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Vauthier C; Labarre D; Ponchel G Design aspects of poly(alkylcyanoacrylate) nanoparticles for drug delivery. J. Drug Target, 2007, 15(10), 641–663. [DOI] [PubMed] [Google Scholar]
- [131].Muller RH; Lherm C; Herbort J; Couvreur P In vitro model for the degradation of alkylcyanoacrylate nanoparticles. Biomaterials, 1990, 11(8), 590–595. [DOI] [PubMed] [Google Scholar]
- [132].Kattan J; Droz JP; Couvreur P; Marino JP; Boutan-Laroze A; Rougier P; Brault P; Vranckx H; Grognet JM; Morge X; et al. Phase I clinical trial and pharmacokinetic evaluation of doxorubicin carried by polyisohexylcyanoacrylate nanoparticles. Invest. New Drugs, 1992, 10(3), 191–199. [DOI] [PubMed] [Google Scholar]
- [133].Bazile DV; Ropert C; Huve P; Verrecchia T; Marlard M; Frydman A; Veillard M; Spenlehauer G Body distribution of fully biodegradable [14C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials, 1992, 13(15), 1093–1102. [DOI] [PubMed] [Google Scholar]
- [134].Moghimi SM; Hunter AC; Murray JC Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev, 2001, 53(2), 283–318. [PubMed] [Google Scholar]
- [135].Vinogradov SV; Batrakova EV; Kabanov AV Nanogels for oligonucleotide delivery to the brain. Bioconjugate Chem., 2004, 15(1), 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Aktas Y; Yemisci M; Andrieux K; Gursoy RN; Alonso MJ; Fernandez-Megia E; Novoa-Carballal R; Quinoa E; Riguera R; Sargon MF; Celik HH; Demir AS; Hincal AA; Dalkara T; Capan Y; Couvreur P Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjugate Chem., 2005, 16(6), 1503–1511. [DOI] [PubMed] [Google Scholar]



