Abstract
The metabolic syndrome is prevalent in developed nations and accounts for the largest burden of non-communicable diseases worldwide. The metabolic syndrome has direct effects on health and increases the risk of developing cancer. Lifestyle factors that are known to promote the metabolic syndrome generally cause pro-inflammatory alterations in microbiota communities in the intestine. Indeed, alterations to the structure and function of intestinal microbiota are sufficient to promote the metabolic syndrome, inflammation and cancer. Among the lifestyle factors that are associated with the metabolic syndrome, disruption of the circadian system, known as circadian dysrhythmia, is increasingly common. Disruption of the circadian system can alter microbiome communities and can perturb host metabolism, energy homeostasis and inflammatory pathways, which leads to the metabolic syndrome. This Perspective discusses the role of intestinal microbiota and microbial metabolites in mediating the effects of disruption of circadian rhythms on human health.
Non-communicable diseases are the leading cause of death globally1. The most common non-communicable disease is the metabolic syndrome, which is a condition characterized by obesity, insulin resistance, hypertension and hyperlipidaemia1. The metabolic syndrome can lead to the development of diseases, including type 2 diabetes mellitus (T2DM), cardiovascular disease, non-alcoholic steatohepatitis and cancer2,3. Therefore, it is imperative that we improve our understanding of the pathological mechanisms so that we can develop effective strategies to combat the causes and consequences of the metabolic syndrome.
An increase in the prevalence of the metabolic syndrome was first observed in ‘westernized’ nations that follow a Western lifestyle. The Western lifestyle is characterized by adverse lifestyle habits, including consumption of diets high in fat and red meat, and low in fibre, a sedentary lifestyle and circadian dysrhythmia. All of these habits are postulated to dysregulate the metabolic axis and lead to chronic inflammation and the metabolic syndrome4. Compelling evidence indicates that the Western lifestyle promotes the metabolic syndrome1. For example, in the USA, rates of obesity rose from 13% to 38% in less than five decades5. Furthermore, in populations that have adopted the Western lifestyle, such as those of urban China, the Middle East, India and Brazil, there has been an emergence of the metabolic syndrome6,7.
Of the lifestyle factors that can lead to the metabolic syndrome, circadian disruption has received an increasing amount of attention within the scientific community. Circadian rhythms allow organisms to synchronize behaviour and physiology with regular and predictable routines in sleep–wake cycles and times of food consumption8. One of the most obvious behavioural consequences of circadian rhythms is sleep–wake cycles in which sleep is regulated by the circadian-controlled production of melatonin. At the molecular level, circadian rhythms are driven by the molecular circadian clock, which is found in nearly every cell in the body. The molecular clock includes the core transcription factors BMAL1, CLOCK, CRY1 and CRY2, and PER1, PER2 and PER3, as well as other components that fine-tune the core clock including REV–ERB and SIRT9. The ‘central’ circadian clock located in the suprachiasmatic nucleus in the hypothalamus is regulated by light exposure and acts as a conductor to regulate subordinate circadian clocks in the rest of the body. However, ‘peripheral’ clocks can also be regulated by factors other than the central clock. For example, time of food consumption regulates circadian clocks in the intestine and liver10,11. Alterations in time of light exposure and food consumption can disrupt circadian rhythms resulting in cellular and organ dysfunction, including metabolic dysfunction9. Shift work, rotating shift work, bright street lights, the use of light-emitting devices at night, social jet lag and even late night eating can all promote circadian disruption9,12,13. Circadian dysrhythmia is associated with the metabolic syndrome and a high risk of developing cancer14,15. Understanding the interaction between circadian rhythms and host metabolic function could provide opportunities to lessen the prevalence and consequences of the metabolic syndrome.
One mechanism by which circadian dysrhythmia can influence the development of the metabolic syndrome is via the intestinal microbiome. A wide variety of microorganisms inhabit the gastrointestinal tract, including bacteria, fungi and viruses. The bacteria that inhabit the gastrointestinal tract are the most studied among the human microbiome constituents and are known to contribute to the regulation of health and disease16. For the purpose of this Perspective, we operationally define microbiome and microbiota as the bacterial communities in the gastrointestinal tract. In this Perspective, we examine the link between circadian disruption, the gastrointestinal tract microbiome, metabolism, the metabolic syndrome and cancer.
Circadian rhythms and metabolism
The link between the molecular circadian clock and metabolism is well established17,18. For example, studies in rodents have shown that whole animal genetic disruption of the molecular circadian clock (that is, perturbations in Bmal1, Clock or Rev-erb), which disrupts circadian rhythms both centrally and peripherally, is associated with features of the metabolic syndrome, including adipocyte hypertrophy, fatty liver, abnormal lipid profile, hypoinsulinaemia, hyperglycaemia and obesity19–22. Similarly, manipulations of light–dark cycles to induce central circadian disruption predisposes rodents to glucose intolerance, reduced insulin sensitivity and pancreatic β-cell dysfunction23,24. These findings in rodents have been largely recapitulated in humans. Humans with variations and polymorphisms in CRY2 and PER2 demonstrate metabolic dysfunction, namely increased blood levels of glucose25,26.
Most individuals, however, do not have abnormalities in the molecular circadian clock. Instead, they have common lifestyle factors that are known to cause circadian disruption centrally (such as shift work, jet lag and sleep deprivation) or peripherally (including late-night or irregular times of food consumption)27,28. These lifestyle factors are often associated with metabolic dysfunction and the metabolic syndrome29–31. It should be noted that not all clinical studies have found an association between circadian disruption and the metabolic syndrome. However, carefully controlled studies demonstrate that consuming calories closer to the onset of melatonin production (that is, near sleep onset) is associated with increased body adiposity, whereas consumption of higher calories (carbohydrate and protein) after ~1900 hours (~4 h before sleep onset) is associated with being less lean, which indicates that eating later in the day (which probably induces peripheral circadian dysrhythmia) is associated with obesity32,33. In addition, monitoring melatonin production (a gold standard to assess central circadian rhythms) demonstrates that central circadian disruption (low melatonin levels) is associated with insulin resistance, T2DM and obesity34. Taken together, compelling evidence links circadian dysrhythmia to metabolic dysfunction in both rodents and humans.
A close relationship exists between circadian rhythms and metabolism. Metabolic function is thought to be regulated by circadian rhythms. For example, many metabolic functions demonstrate diurnal rhythmicity (suggesting they are directly or indirectly controlled by circadian rhythms), including enzymes (expression and function) that are important for the regulation of cholesterol, other lipids, glucose and amino acids35,36. In addition, melatonin receptors (an output of central circadian rhythms) are involved in regulating gluconeogenesis, pancreatic β-cell signalling and insulin resistance37,38. Thus, low melatonin associated with central circadian disruption might be one mechanism by which circadian disruption promotes the metabolic syndrome. Indeed, studies have shown that humans with a polymorphism in a melatonin receptor tend to have increased fasting blood levels of glucose and HbA1c, as well as an increased incidence of gestational diabetes and T2DM39. Conversely, evidence also indicates that metabolism can influence the function of the molecular circadian clock. For example, SIRT function (deacetylase activity) depends on the availability of cellular metabolites (such as NAD+ and fatty acids) and it is in this way that metabolism can influence the function of the molecular circadian clock40. Taken together, a high degree of crosstalk between the circadian clock and metabolism is present.
Metabolic syndrome and cancer
Similar to the metabolic syndrome, cancer rates are on the rise globally, including in populations that have adopted the Western lifestyle (for example, populations in China and the Middle East), suggesting that cancer in these countries could be a consequence of the metabolic syndrome and/or that there are overlapping mechanisms contributing to the development of these conditions41–43. The hypothesis that cancer is a consequence of the metabolic syndrome is supported by data demonstrating that the metabolic syndrome is associated with an increased risk of developing several types of cancer, including colorectal cancer3,44. Data that support the idea of there being overlapping mechanisms that contribute to the development of the metabolic syndrome and cancer demonstrate that circadian dysrhythmia promotes the development of the metabolic syndrome or cancer in rodent models and humans45–50. In either case, metabolic pathways are likely a converging mechanism.
Altered cellular metabolism is a key feature associated with cancer; therefore, it is possible that circadian dysrhythmia-induced metabolic dysfunction promotes carcinogenesis and the development of cancer. Numerous pathways that contribute to metabolic survival of cancer cells exhibit circadian oscillations47,51, and it is hypothesized that circadian disruption might promote survival of cancerous cells by making them more proficient at utilizing available energy sources and performing lipogenesis52. Consistent with this hypothesis, circadian dysrhythmia disrupts hepatic circadian gene expression and alters metabolism, resulting in activation of β-catenin and hepatocellular carcinoma transformation48. It is possible that altered metabolism resulting from circadian disruption could activate oncoproteins and tumour growth53, while at the same time those oncoproteins are able to further disrupt cellular circadian rhythms, which initiates a feedforward system by affecting cellular metabolism54. In addition to metabolism, a variety of other cancer-relevant cellular processes are under circadian control, but this is beyond the scope of this article. Taken together, these findings implicate metabolic signalling as a linchpin that connects circadian disruption to cancer.
Microbiota and circadian rhythms
Microbiota has circadian rhythmicity.
A diverse bacterial community (that is, the microbiota) thrives in the gastrointestinal tract, where there is a readily available supply of nutrients. The microbiota contributes to the mucosal integrity of the intestinal epithelial barrier, and food digestion and absorption, and also produces a number of hormones, making the microbiota an auxiliary ‘metabolic organ’55,56. Since the early 1970s, it has been known that the function of the gastrointestinal tract has circadian rhythms57, but newly emerging data demonstrate that the microbiome also exhibits diurnal variations58,59. In vitro data demonstrate that bacteria exhibit diurnal fluctuations in behaviour (for example, swarming activity of Enterobacter aerogenes in vitro)60 and that the number of bacteria in the epithelial mucous layer varies during light and dark periods (for example, Mucispirillum schaedleri)61. Evidence suggests that microbial rhythms are tightly interrelated to the host circadian rhythm.
The specific mechanisms are still not well understood but are probably a combination of circadian clock-dependent and clock-independent mechanisms. For example, bacterial rhythms typically have a period of ~24 h, and can be regulated by melatonin and temperature (which are also regulators of circadian rhythms in mammalian cells)60,62 and the microbiome diurnal pattern is lost in the absence of core molecular circadian clock genes (such as a Clock mutation) even when behavioural rhythms in the host are normal59. Altered microbiota rhythmicity has been found to be associated with other Clock network mutations, including both positive (such as Bmal1 knockout) and negative (for example, Per1 and Per2 double mutant) loop components61,63. These data suggest that the fluctuations are dependent on an intact host circadian clock gene network. However, reinstating rhythms in the intestine of Clock-mutant mice by regulating the time of food consumption also reinstates robust microbial rhythms, which is consistent with food being a dominant cue for bacterial rhythms, independent of the Clock network64,65. Clearly additional studies are necessary to tease out the details of this relationship as fluctuations in bacterial communities and function have important biological consequences, including bacterial metabolite production, nutrient digestion and energy harvest, with both intestinal and extra-intestinal metabolic consequences for the host66.
Based on these data, it is clear that signals from the host influence diurnal fluctuations in the gastrointestinal tract microbiota, but the microbiota can also regulate host circadian and metabolic homeostasis. The production of bacterial metabolites is rhythmic61,67, which is critical because these metabolites can regulate host circadian rhythms and metabolism68. Indeed, ablation of the gastrointestinal tract microbiota disrupts the rhythmic expression of genes in the gastrointestinal tract61. This relationship remains rather mysterious but studies from 2019 suggest that the host diurnal rhythms might be driven by bacterial antigens or their metabolites69,70. For example, dietary choline is converted to trimethylamine by the intestinal microbiota and is converted to trimethylamine N-oxide (TMAO) in the liver71,72, which might influence the expression of circadian clock genes (including Clock and Bmal1) in endothelial cells73. Thus, there is a dynamic interplay between the host circadian system and the microbiota. Disruptions in this relationship have important consequences for host metabolism and could be one mechanism by which the microbiota is involved in the metabolic syndrome and related pathologies (FIG. 1).
Fig. 1 |. The bidirectional bacteria–host relationship.
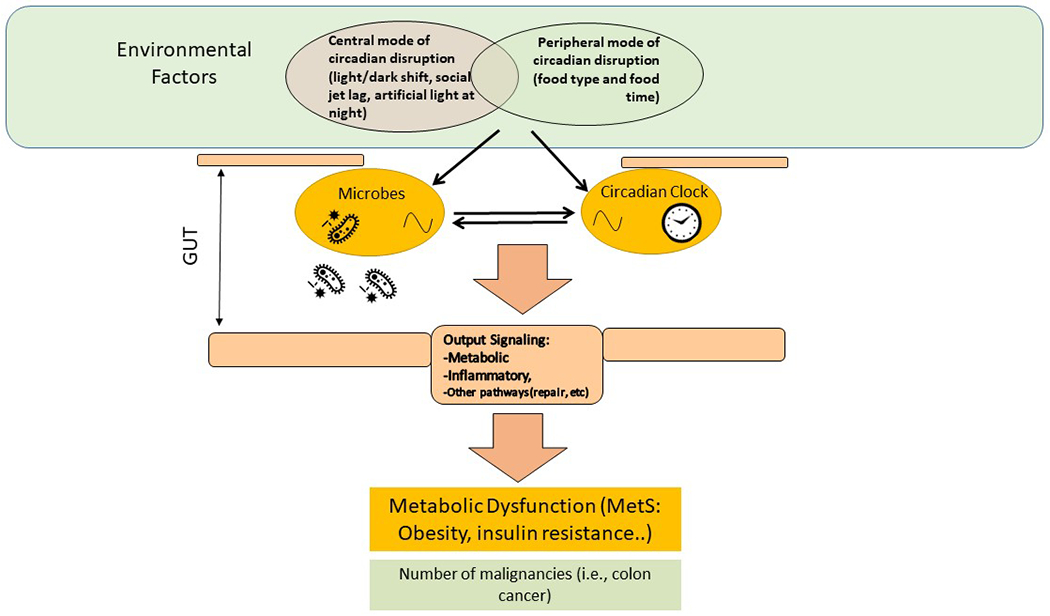
Host circadian rhythms influence intestinal microbiota structure and function, and microbial rhythms influence circadian rhythms in the digestive system; both systems being intact is key to maintaining host metabolic homeostasis. Modern lifestyle factors that lead to circadian rhythm disruption (for example, artificial light exposure at night, irregular sleep–wake cycles, eating late at night) interrupt this homeostasis by affecting the microbiota and the host circadian clock, shifting metabolism towards increased calorie extraction and decreased energy expenditure, providing an environment that promotes the metabolic syndrome (MetS), chronic inflammation and a number of malignancies.
Development of the metabolic syndrome.
A variety of lifestyle habits that are risk factors for the metabolic syndrome alter the composition of the microbiota16. Indeed, a plethora of literature demonstrates that the metabolic syndrome is associated with intestinal microbiota dysbiosis. Obesity and the metabolic syndrome are associated with microbiota alterations, including an increase in the ratio of Firmicutes to Bacteroidetes and an increase in the relative abundance of Proteobacteria74–77 as well as alterations in specific bacteria such as Lactobacillus and Clostridium78–80. Conversely, manipulations that improve features of the metabolic syndrome (for example, anti-obesity surgery, faecal microbiota transplant) are associated with changes in bacterial communities such as reduced abundance of Proteobacteria, Streptococcus, Clostridium and Enterococcaceae, and increased Akkermansia and Bifidobacteriaceae81–83. Thus, the microbiota can both negatively and beneficially affect features of the metabolic syndrome. Studies demonstrate that changes in the gastrointestinal tract microbiota are sufficient to induce or mitigate features of the metabolic syndrome16,58,59,68. For example, obesity-prone (129S6) mice lose their phenotype when their microbiome is altered84 and inoculation of microbiota from obese-prone rodents into a germ-free, non-obese recipient transfers the obese phenotype into the recipient85. Specific bacteria are associated with metabolism. For instance, Akkermansia muciniphila is negatively correlated with obesity, T2DM and hypertension86–88 and administration of Akkermansia or purified components of Akkermansia have beneficial metabolic effects89,90. Indeed, dietary supplementation of A. muciniphila significantly improves insulin sensitivity, reduces insulinaemia and decreases total cholesterol89,90. Overall, it is clear that the gastrointestinal tract microbiota affects the host metabolism.
In both experimental models and humans, circadian disruption alters the gastrointestinal tract microbiota91,92, which might increase the host’s susceptibility to metabolic dysfunction49,50,93. For example, genetic modification of the circadian clock (such as in mice with modified Clock) increases susceptibility to metabolic dysfunction and is associated with a concurrent change in the gastrointestinal tract microbiota91,94. Experimental models have demonstrated that the metabolic syndrome induced by circadian disruption is (at least in part) mediated by the intestinal microbiome. Indeed, transfer of stool from circadian-disrupted rodents (with metabolic dysfunction) into non-disrupted rodents is sufficient to induce metabolic dysfunction in the recipients and microbial interventions can improve the metabolic syndrome59,64. Furthermore, in mice that consumed alcohol, colon carcinogenesis from eating at the ‘wrong’ time (that is, when the mice should have been resting) could be ameliorated by manipulating the microbiota composition50. Therefore, it is highly plausible that circadian disruption-induced effects on the intestinal microbiome can influence the development of the metabolic syndrome.
Mechanistic links
The microbiome can affect host circadian rhythms and metabolism through a variety of mechanisms, including contact-dependent and contact-independent mechanisms as well as changes in intestinal barrier integrity16. These mechanisms are discussed in this section and are partly summarized in FIG. 2.
Fig. 2 |. Microbiota signalling to the host circadian and metabolic systems occurs via both contact-dependent mechanisms and contact-independent mechanisms.
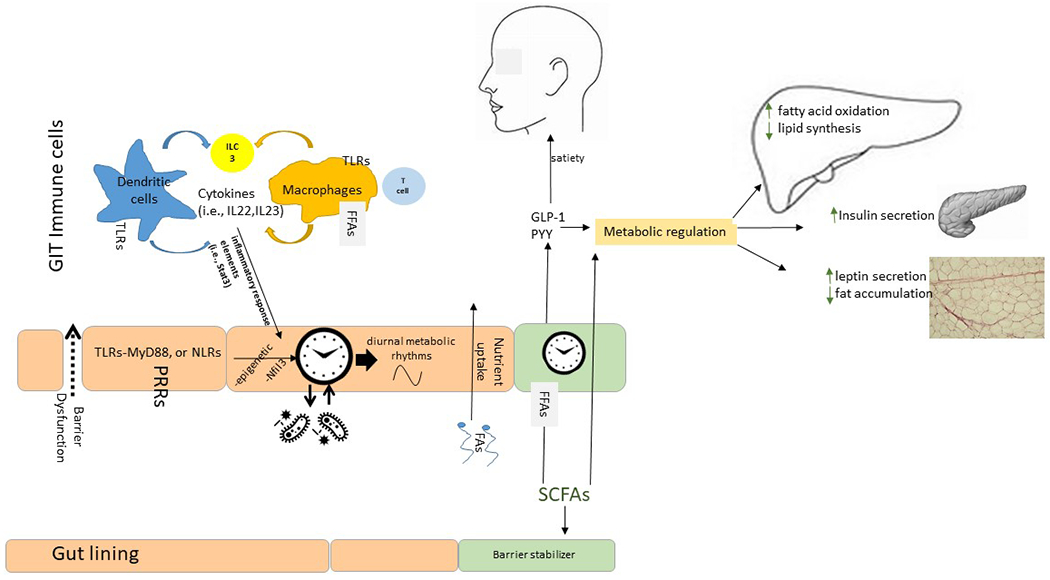
Pattern recognition receptors (PRRs) on intestinal epithelial cells and immune cells represent a contact-dependent signalling pathway where the activation of PRRs eventually leads to alterations in gene expression via nuclear receptors (such as nuclear factor, IL-3-regulated (NFIL3)), epigenetic alterations or other mechanisms (contact-dependent mechanism). Bacterial products (for example, metabolites) are responsible for contact-independent mechanisms; of these, bacterial fermentation of fibre and the production of short-chain fatty acids (SCFAs) regulate satiety and the function of metabolic organs (liver, pancreas and adipose tissue). FFARs, free fatty acid receptors; GLP1, glucagon-like peptide 1; MyD88, myeloid differentation primary response protein 88; NLRs, NOD-like receptors; PYY, peptide YY; TLRs,Toll-like receptors.
Contact-dependent mechanisms.
Contact-dependent mechanisms require a direct interaction between the bacteria and the host cells. Epithelial cells that line the gastrointestinal tract and immune cells in the gastrointestinal mucosa express pattern recognition receptors (PRRs), which recognize conserved bacterial features. Through this direct contact mechanism, bacteria can influence the host circadian rhythms and metabolism. For example, segmented filamentous bacteria regulate rhythmic gene expression in the intestinal epithelium via PRRs61,95 and animals with deficient PRR function (that is, deficient function of MyD88) have abnormal rhythms in metabolic pathways in the small intestine70. Examples of PRRs include NOD-like receptors (NLRs) and Toll-like receptors (TLRs). NLRs are intracellular PRRs and activation of NLRs (for example, NOD1 and NOD2) modulates metabolic function with effects on obesity, fatty liver disease, adipocyte differentiation and adipogenicity96,97. TLRs are transmembrane receptors and require downstream components such as MyD88 (REF.98. A large body of evidence supports the idea that PRRs mediate the effects of the microbiota on the host clock and metabolism. Microbiota via TLRs signals to the epithelium clock in the small intestine, and subsequently regulates numerous genes associated with metabolic processes99. In this study, loss of microbiota signalling via TLRs disrupted the clock network, induced downstream corticosterone overproduction, and led to a pre-diabetic condition in mice99.
Research on TLRs has provided compelling evidence that they affect metabolism. Activation of TLR4 initiates a series of events culminating in the production of pro-inflammatory factors that modulate insulin resistance, glucose uptake and fatty acid storage, as well as carcinogenesis100,101. Mice deficient in TLR2 have less adipose inflammation and less metabolic dysfunction than wild-type animals102. Furthermore, knocking out TLR4 protects against the development of diet-induced obesity103,104. In addition, TLR5-deficient animals demonstrate less metabolic dysfunction than their wild-type counterparts105. Importantly, knocking out TLR5 is associated with an altered microbiome, and transfer of stool from the metabolically improved TLR5-deficient mice into germ-free wild-type mice improves metabolic function105. Studies in humans support the observation that TLRs are important in metabolism wherein insulin resistance is associated with increased tissue expression of TLR2 and TLR4 (REF.106). Thus, compelling evidence indicates that bacterial signalling through PRRs can influence metabolism.
One consequence of stimulation of PRRs is inflammasome activation, and evidence implicates the NLR family pyrin domain containing 3 (NLRP3) inflammasome in mediating the effects of PRR on metabolism. Data demonstrate that NLRP3 activation contributes to insulin resistance via mechanisms including production of cytokines, such as IL-18 and IL-1β, that cause adipose inflammation and impair pancreatic β-cell function107. Compared with wild-type mice, Nlrp3-knockout mice demonstrated reduced adipose inflammation and enhanced insulin signalling108. While these data are compelling, the potential role of the NLRP3 inflammasome as a microbial clock sensor in coordinating the host circadian rhythmicity, inflammation and metabolism requires further investigation.
Contact-independent mechanisms: short-chain fatty acids.
The bacteria in the gastrointestinal tract are metabolically active and metabolites secreted from these bacteria can affect host circadian rhythms and metabolism via contact-independent mechanisms. Specifically, many investigations have focused on bacterial production of short-chain fatty acids (SCFAs), such as butyrate, acetate and propionate. SCFAs are metabolic byproducts of certain bacteria that are able to ferment dietary fibre, and they regulate a wide variety of processes that have metabolic benefits, including food and energy intake (via satiety), energy expenditure, hepatic metabolism, glucose uptake, gluconeogenesis and adiposity109. SCFAs act either through G protein-coupled receptors (GPCRs) or by entering cells via transporters with subsequent epigenetic consequences via histone deacetylase activity110.
Studies have demonstrated that SCFAs regulate satiety via GPCR41 and GPCR43 (REF.111). Specifically, SCFAs decrease appetite by stimulating secretion of ‘satiety’ peptides such as peptide YY (PYY) and glucagon-like peptide 1 (GLP1) from intestinal cells with endocrine functions (enteroendocrine cells) via the GPCRs109,112. SCFAs also increase adipocyte expression and plasma levels of leptin, another satiety hormone, in part via GPCR41 and GPCR43 (REFS113,114). SCFAs are increasingly recognized as important players in the microbiota–gut–brain axis and circadian rhythms and act by multiple mechanisms, including modulating the host metabolism115.
SCFAs also influence energy expenditure. Specifically, SCFAs are associated with increased oxygen consumption and fat oxidation116, as well as reduced lipid synthesis and lipid accumulation in liver and adipose tissue117. These effects seem to be mediated through GPCR41 and GPCR43 as reduced energy expenditure has been observed in GPCR41 and GPCR43 knockout mice117,118. Finally, SCFA-stimulated production of GLP1 also stimulates fatty acid oxidation, reduces the amount of lipid in the liver and increases insulin secretion from pancreatic β-cells (hence the use of GLP1 agonists for the treatment of T2DM)119.
Indirect effects of SCFAs on metabolism can be mediated via effects on the intestinal barrier. The intestinal barrier separates the pro-inflammatory contents inside the gastrointestinal tract from the mucosa and systemic circulation where they can initiate profound inflammation. The barrier is maintained by contacts between adjacent epithelial cells. Several factors can affect the intestinal barrier, including the intestinal microbiota directly and bacterial metabolites, such as SCFAs120. Intestinal epithelial cells preferentially use SCFA butyrate as an energy source, and abundant SCFAs fortify intestinal barrier integrity. Reduced SCFA production is commonly associated with barrier dysfunction, systemic inflammation and altered metabolism111. Specifically, barrier dysfunction is linked to obesity, hyperglycaemia, insulin resistance, T2DM and the metabolic syndrome121.
Despite compelling evidence in rodents supporting the beneficial role of SCFAs in metabolic function, data from humans are inconsistent. For example, while increased dietary fibre consumption (and subsequent increases in SCFAs) improves metabolic function in humans, including by reducing blood pressure, improving lipid profile, reducing weight and increasing satiety122,123, it has also been reported that individuals with obesity have increased circulating levels of SCFAs, which is inconsistent with a beneficial effect of SCFAs on metabolism16,98. However, this paradoxical increase in SCFAs in individuals with obesity could be secondary to bacterial overgrowth, increased microbial energy harvest, or a compensatory homeostatic mechanism as a sensor for excessive dietary energy16,98. This is just one example, but, broadly speaking, inconsistency in the findings of studies in humans regarding the effects of fibre consumption on metabolism could be explained by variations in fibre type, dose, and host genetic and microbial composition, among other factors109. Taking these findings together, SCFA signalling is a credible mechanism connecting the microbiota to metabolic dysfunction and the metabolic syndrome.
Other contact-independent mechanisms.
Consumption of fat initiates the release of bile acids into the small intestine; however, key enzymes in bile acid biosynthesis are regulated by circadian rhythms12,124 and circadian disruption is associated with an abnormal bile acid profile12. Bile acids are recognized as important regulators of host metabolic function. For example, several primary bile acids improve metabolism by promoting GLP1 secretion, which induces satiety, stimulates fatty acid oxidation, reduces levels of lipid in the liver, and increases insulin secretion from pancreatic β-cells, via a mechanism that is TGR5-dependent or FXR-dependent125–128. Studies have shown that bile acids are involved in blunting the deleterious effects of high-fat diets in mice undergoing time-restricted feeding58. Other mechanisms might also be important. For example, sulfur in red meat is metabolized by sulfur-reducing bacteria to produce hydrogen sulfide, which can promote insulin resistance via inhibition of insulin secretion and sensitivity129,130. Hydrogen sulfide could have diurnal oscillations in the mouse intestine while in vitro could blunt the rhythmic expression of core clock genes68. Red meat also contains choline, which, upon microbial metabolism, is converted to TMAO in the liver, which, as described previously, might influence the expression of circadian clock genes (including Clock and Bmal1) in endothelial cells71,72. These are just a few of the contact-independent mechanisms by which microbiota signalling can regulate the host circadian rhythms and control metabolism.
Limitations and future directions
We are just starting to learn about the complex interactions among circadian rhythms, the gastrointestinal tract microbiota and host metabolism, and how these interactions affect human health. As these studies are still in their infancy, there are several limitations in extrapolating from the data and in the conclusions that can be drawn.
Circadian limitations.
Many challenges and limitations to studying circadian rhythms currently exist: accurate assessment of circadian rhythms in humans is labour intensive and requires controlled environments that can only be achieved in a laboratory, participant-to-participant variability is high and evaluating circadian rhythms in the human gastrointestinal tract is challenging. While the assessment of central circadian rhythms in humans is fairly straightforward (such as via melatonin and body temperature), studies have to be conducted in tightly controlled environments that limit exposure to light and regulate time of food consumption and meal size (factors known to alter rhythms). Additionally, there is a high degree of variability among humans due to genetics, age, sex and diet, all of which influence circadian rhythms and make robust signals in data difficult to discern. However, the biggest challenge for the purpose of this Perspective is lack of feasibility in evaluating circadian rhythms in the gastrointestinal tract. Frequent tissue sampling, while an option in rodents, is not possible in humans. In this context, the use of intestinal organoids to assess circadian rhythms ex vivo could be promising. Organoids harbour phenotypic and genotypic characteristics of the intestinal tissue from which they are extracted and they exhibit robust circadian rhythms ex vivo, which means that organoids could be utilized for indirect measurement of the circadian rhythmicity in the gastrointestinal tract131. In summary, while studies in rodents are fairly straightforward, studies in humans are challenging and the use of new technologies, such as intestinal organoids, might aid in conducting careful studies of circadian rhythms in the gastrointestinal tract.
Microbiota limitations.
The studies discussed in this Perspective had several limitations. Firstly, the majority of the studies discussed (including those that investigated microbiota rhythmicity) used 16S ribosomal RNA gene sequencing. While this technique is adequate to broadly assess bacterial populations, studies using this technique cannot address other components of the microbiome, such as viruses and fungi. It is possible that these other components also exhibit a diurnal oscillation, are affected by circadian disruption and are critical regulators of metabolism. Future studies should utilize shotgun metagenomic sequencing, which will provide additional resolution (to the species level), allow characterization of non-bacterial components of the microbiome and also provide critical information about function132. RNA sequencing might also be a useful technique to fully elucidate how gene expression is altered diurnally, by circadian disruption and in the metabolic syndrome.
Secondly, most of the data available on gastrointestinal tract microbiota (and bacterial rhythms) come from analysis of stool; however, there are several niches inside the gastrointestinal tract and stool is only one. For example, mucosal-associated bacteria represent a niche that can be critically important for host interactions. In addition, microbial populations and functions vary among the different regions of the gastrointestinal tract (for example, small intestine versus colon), and certainly this regionality could have important implications for metabolism, circadian dynamics and bacteria–host interactions133.
Finally, with the exception of a few characterizations99,134, studies have yet to carefully evaluate how manipulation of the gastrointestinal tract microbiota influences the host epithelium, including circadian rhythms. Additional studies are necessary to precisely define the mechanisms that contribute to the complex interaction between the microbiome and the host. In conclusion, much is yet to be learned and the coming years are likely to be highly informative.
Experimental models.
Most of the mechanistic evaluations of circadian rhythms and microbiota have been performed in mouse models. Although there are numerous advantages to using rodents (low cost, easy environmental control and/or manipulation, and availability of genetic models), there are limitations in the translatability of murine data to humans. Besides the cross-species microscopic and macroscopic differences in the intestine, the microbial composition is different between mice and humans, making direct translation of data from mice to humans a challenge135. In addition, the influence of specific bacteria or bacterial populations on host pathology is often assessed in gnotobiotic models, such as germ-free mice, but such assessments are fraught with problems, which include the abnormal immune function of these models. To move the field closer to the bedside, the development of human-based ex vivo systems wherein bacteria (that is, enterotype)–host (that is, organotype) interactions could be studied has been long awaited. In fact, organ-on-a-chip is a new technological advance that will open doors in this area of research. Several gut-on-a-chip platforms, such as HuMiX and OrganoPlate, are currently being developed, and the availability of such platforms will allow integrated analysis of host–microbiota interactions ex vivo136,137. Inclusion of host non-epithelial components in such systems, although technically challenging, could allow information to be obtained on the role of the cues from the microenvironment. In conclusion, extrapolating rodent model data to humans is challenging; however, continued development of ex vivo approaches in the future are expected to lead to an improved understanding of the host–microbiota interaction.
Conclusions
Epidemiological data suggest that the ‘Western lifestyle’ might contribute to the prevalence of non-communicable diseases. Chief among these lifestyle factors are lack of physical activity, poor diet, sleep disturbances and circadian rhythm disruption. It is interesting that all of these lifestyle factors are known to alter microbiome communities in the gastrointestinal tract, but of these factors, circadian rhythms have only started being appreciated for their important effects on health and disease in the past decade. Circadian rhythms are critical for optimal function of a host, and disruption of rhythms contributes to susceptibility and development of pathology and disease. Circadian rhythms are influenced by environmental factors, such as light exposure and time of food consumption; therefore, light exposure at night (from light-emitting devices and light pollution), irregular sleep–wake schedules (through jet lag, social jet lag and shift work) disrupt central circadian rhythms, and eating food late at night or at irregular times disrupts circadian rhythms in the gastrointestinal tract. An increasing number of studies have demonstrated that circadian disruption negatively influences microbiota communities in the gastrointestinal tract, which may be associated with metabolic dysfunction and might contribute to the development of the metabolic syndrome and cancer.
A dynamic crosstalk exists between microbiota communities in the gastrointestinal tract and the host, and disruption of this complex interaction is associated with inflammation and metabolic dysfunction. Therefore, it is possible that circadian-directed interventions might beneficially alter gastrointestinal tract microbiome communities to promote host resilience and prevent or delay the onset of chronic metabolic and inflammatory disorders. As proof of concept, light therapy (a circadian-based strategy (known as chronotherapy) that can increase the robustness of central circadian rhythms) improves features of the metabolic syndrome, such as insulin sensitivity138,139. While clinical trials (such as NCT03777722) to evaluate the utility of light therapy as a treatment for the metabolic syndrome are ongoing, it will be critical to interrogate microbiota composition before and after light treatment, which could provide mechanistic insights about the role of the microbiota. Another circadian treatment approach to increasing the robustness of circadian rhythms in the intestine and/or liver is optimization of time of food consumption. Therefore, limiting food intake to only daylight hours (10–12 hours food availability, 12–14 hours of fasting) has the potential to optimize circadian homeostasis and microbiota community structure and/or function by improving healthy metabolic function in the host. We propose that these circadian-directed interventions have the promise of preventing the metabolic syndrome and its pro-inflammatory consequences, such as cancer, in high-risk populations. The addition of microbiota-directed interventions such as prebiotic or probiotic supplements (and/or consumption of primarily plant-based diets) could be considered to further optimize the microbiota community and circadian rhythms, especially in high-risk populations such as shift workers.
This Perspective summarizes compelling evidence to support the concept that circadian disruption negatively effects metabolism through a mechanism involving (at least in part) the gastrointestinal tract microbiome. Therefore, the use of circadian and/or microbiota-directed interventions might be able to ameliorate metabolic dysfunction associated with the Western lifestyle. Well-designed randomized clinical trials are needed to establish the therapeutic effectiveness of such approaches to decrease the burden of the metabolic syndrome and its associated pathologies.
Acknowledgements
The authors acknowledge the support provided by NIAAA AA025387 and Rush Translational Sciences Consortium/Swim Across America Organization to F.B., NIAAA AA026801 to A.K. and R.M.V., NIAAA AA023417 and AA026801 to A.K, and NIA AG056653 to R.M.V.. The authors are grateful for the support of the Brinson Foundation, Barbara and Larry Field, Ellen and Philip Glass, and Marcia and Silas Keehn.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Endocrinology thanks J. Cryan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.World Health Organization. Global Health Estimates 2016: Disease burden by Cause, Age, Sex, by Country and by Region, 2000–2016 (WHO, 2018). [Google Scholar]
- 2.Scholze J et al. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public. Health 10, 529 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pothiwala P, Jain SK & Yaturu S Metabolic syndrome and cancer. Metab. Syndr. Relat. Disord 7, 279–288 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 15, 913–924 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Fryar CD & Flegal KM Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 219, 1–8 (2015). [PubMed] [Google Scholar]
- 6.Misra A & Khurana L Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab 93, S9–S30 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Moreira GC, Cipullo JP, Ciorlia LA, Cesarino CB & Vilela-Martin JF Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS One 9, el05056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohawk JA, Green CB & Takahashi JS Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci 35, 445–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokkan KA, Yamazaki S, Tei H, Sakaki Y & Menaker M Entrainment of the circadian clock in the liver by feeding. Science. 291,490–493 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Hoogerwerf WA et al. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133,1250–1260 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Bishehsari F, Levi F, Turek FW & Keshavarzian A Circadian rhythms in gastrointestinal health and diseases. Gastroenterology 151, e1–e5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touitou Y, Touitou D & Reinberg A Disruption of adolescents’ circadian clock: the vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J. Physiol. Paris 110, 467–479 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Mota MC, Silva CM, Balieiro LCT, Fahmy WM & Crispim CA Social jetlag and metabolic control in non-communicable chronic diseases: a study addressing different obesity statuses. Sci. Rep 7, 6358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kervezee L, Kosmadopoulos A & Boivin DB Metabolic and cardiovascular consequences of shift work: the role of circadian disruption and sleep disturbances. Eur. J. Neurosci 51, 396–412 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Moossavi S & Bishehsari F Microbes: possible link between modern lifestyle transition and the rise of metabolic syndrome. Obes. Rev 20, 407–419 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Bass J & Takahashi JS Circadian integration of metabolism and energetics. Science 330, 1349–1354 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panda S et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Rudic RD et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcheva B et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turek FW et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308, 1043–1045 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho H et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 485, 123–127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale JE et al. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythm 26,423–433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian J, Block GD, Colwell CS & Matveyenko AV Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 62, 3469–3478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappa KI et al. Circadian clock gene expression is impaired in gestational diabetes mellitus. Gynecol. Endocrinol 29, 331–335 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Rios A et al. Beneficial effect of CLOCK gene polymorphism rs 1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol. Int 31,401–408 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Bracci M et al. Rotating-shift nurses after a day off: peripheral clock gene expression, urinary melatonin, and serum 17-β-estradiol levels. Scand. J. Work. Env. Health 40, 295–304 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Wehrens SMT et al. Meal timing regulates the human circadian system. Curr. Biol 27, 1768–1775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leproult R, Holmback U & Van CE Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morikawa Y et al. Effect of shift work on body mass index and metabolic parameters. Scand. J. Work. Env. Health 33, 45–50 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Bo S et al. Consuming more of daily caloric intake at dinner predisposes to obesity. A 6-year population-based prospective cohort study. PLoS ONE 9, e108467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHill AW et al. Caloric and macronutrient intake differ with circadian phase and between lean and overweight young adults. Nutrients 11,587 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHill AW et al. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr 106, 1213–1219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMullan CJ, Curhan GC, Schernhammer ES & Forman JP Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am. J. Epidemiol 178, 231–238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bass J Circadian topology of metabolism. Nature 491,348–356 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Panda S Circadian physiology of metabolism. Science 354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faria JA et al. Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am. J. Physiol. Endocrinol. Metab. 305, E230–E242 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Bazwinsky-Wutschke I, Wolgast S, Muhlbauer E, Albrecht E & Peschke E Phosphorylation of cyclic AMP-response element-binding protein (CREB) is influenced by melatonin treatment in pancreatic rat insulinoma β-cells (INS-1). J. Pineal Res 53, 344–357 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Bonnefond A et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat. Genet 44, 297–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masri S Sirtuin-dependent clock control: new advances in metabolism, aging and cancer. Curr. Opin. Clin. Nutr. Metab. Care 18, 521–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Global Burden of Disease Cancer Collaboration, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 4, 1553–1568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R & Mariani-Costantini R Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol 20, 6055–6072 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng RM, Zong YN, Cao SM & Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 39, 22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renehan AG, Tyson M, Egger M, Heller RF & Zwahlen M Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Schernhammer ES et al. Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. J. Natl Cancer Inst 95, 825–828 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Carter BD, Diver WR, Hildebrand JS, Patel AV & Gapstur SM Circadian disruption and fatal ovarian cancer. Am. J. Prev. Med 46, S34–S41 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Altman BJ Cancer clocks out for lunch: disruption of circadian rhythm and metabolic oscillation in cancer. Front. Cell Dev. Biol 4, 62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kettner NM et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bishehsari F et al. Light/dark shifting promotes alcohol-induced colon carcinogenesis: possible role of intestinal inflammatory milieu and microbiota. Int. J. Mol. Sci 17, 2017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bishehsari F et al. Abnormal eating patterns cause circadian disruption and promote alcohol-associated colon carcinogenesis. Cell. Mol. Gastroenterol. Hepatol 9, 219–237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masri S, Kinouchi K & Sassone-Corsi P Circadian clocks, epigenetics, and cancer. Curr. Opin. Oncol 27, 50–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sulli G et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papagiannakopoulos T et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altman BJ et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonnenburg JL & Sonnenburg ED Vulnerability of the industrialized microbiota. Science 366, eaaw9255 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Bishehsari F & Keshavarzian A Microbes help to track time. Science 365, 1379–1380 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Furuya S & Yugari Y Daily rhythmic change of L-histidine and glucose absorptions in rat small intestine in vivo. Biochim. Biophys. Acta 343, 558–564 (1974). [DOI] [PubMed] [Google Scholar]
- 58.Zarrinpar A, Chaix A, Yooseph S & Panda S Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 20, 1006–1017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thaiss CA et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Paulose JK, Wright JM, Patel AG & Cassone VM Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS ONE 11, e0146643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thaiss CA et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell 167, 1495–1510 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Paulose JK, Cassone CV, Graniczkowska KB & Cassone VM Entrainment of the circadian clock of the enteric bacterium Klebsiella aerogenes by temperature cycles. iScience 19, 1202–1213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang X, Bushman FD & FitzGerald GA Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl Acad. Sci. USA 112, 10479–10484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaix A, Zarrinpar A, Miu P & Panda S Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaix A, Lin T, Le HD, Chang MW & Panda S Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 29, 303–319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nobs SP, Tuganbaev T & Elinav E Microbiome diurnal rhythmicity and its impact on host physiology and disease risk. EMBO Rep. 20, e47129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tahara Y et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci. Rep 8, 1395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leone V et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, 681–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parkar SG, Kalsbeek A & Cheeseman JF Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms 7, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuang Z et al. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 365, 1428–1434 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang WH et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med 368, 1575–1584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu X et al. Regulation of circadian rhythms by NEAT1 mediated TMAO-induced endothelial proliferation: a protective role of asparagus extract. Exp. Cell Res 382, 111451 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Ley RE et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Backhed F et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad. Sci. USA 101, 15718–15723 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Verdam FJ et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 21, E607–E615 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Karlsson FH et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Fei N & Zhao L An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 7, 880–884 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cortes-Martin A, Iglesias-Aguirre CE, Meoro A, Selma MV & Espin JC There is no distinctive gut microbiota signature in the metabolic syndrome: contribution of cardiovascular disease risk factors and associated medication. Microorganisms 8, 416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liou AP et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl Med 5, 178ra41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vrieze A et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Kootte RS et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–619 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Fujisaka S et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J. Clin. Invest 126, 4430–4443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janssen AW & Kersten S The role of the gut microbiota in metabolic health. FASEB J. 29, 3111–3123 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Zhang X et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 8, e71108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yassour M et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 8, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Depommier C et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med 25, 1096–1103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Plovier H et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med 23, 107–113 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Voigt RM et al. Circadian disorganization alters intestinal microbiota. PLoS ONE 9, e97500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosselot AE, Hong CI & Moore SR Rhythm and bugs: circadian clocks, gut microbiota, and enteric infections. Curr. Opin. Gastroenterol 32, 7–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Voigt RM, Forsyth CB & Keshavarzian A Circadian disruption: potential implications in inflammatory and metabolic diseases associated with alcohol. Alcohol Res. Curr. Rev 35, 87–96 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Voigt RM et al. The circadian clock mutation promotes intestinal dysbiosis. Alcohol. Clin. Exp. Res 40, 335–347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramanan D & Cadwell K Intrinsic defense mechanisms of the intestinal epithelium. Cell Host Microbe 19, 434–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henao-Mejia J et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Purohit JS et al. The effects of NOD activation on adipocyte differentiation. Obesity 21, 737–747 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Tremaroli V & Backhed F Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Mukherji A, Kobiita A, Ye T & Chambon P Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153, 812–827 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Rogero MM & Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 10, 432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Semlali A et al. Expression and polymorphism of toll-like receptor 4 and effect on NF-κB mediated inflammation in colon cancer patients. PLoS ONE 11, e0146333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Himes RW & Smith CW Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 24, 731–739 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim F et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res 100, 1589–1596 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Saberi M et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vijay-Kumar M et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmad R et al. Elevated expression of the toll like receptors 2 and 4 in obese individuals: its significance for obesity-induced inflammation. J. Inflamm 9, 48 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Masters SL et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol 11,897–904 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vandanmagsar B et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med 17, 179–188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Byrne CS, Chambers ES, Morrison DJ & Frost G The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes 39, 1331–1338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ganapathy V, Thangaraju M, Prasad PD, Martin PM & Singh N Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol 13, 869–874 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Tan J et al. The role of short-chain fatty acids in health and disease. Adv. Immunol 121, 91–119 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Frost G et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun 5, 3611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong YH et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005). [DOI] [PubMed] [Google Scholar]
- 114.Zaibi MS et al. Roles of GPCR41 and GPCR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 584, 2381–2386 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Teichman EM, O’Riordan KJ, Gahan CGM, Dinan TG & Cryan JF When rhythms meet the blues: circadian interactions with the microbiota-gut-brain axis. Cell Metab. 31,448–471 (2020). [DOI] [PubMed] [Google Scholar]
- 116.Gao Z et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bellahcene M et al. Male mice that lack the G-protein-coupled receptor GPCR41 have low energy expenditure and increased body fat content. Br. J. Nutr 109, 1755–1764 (2013). [DOI] [PubMed] [Google Scholar]
- 118.Kimura I et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPCR43. Nat. Commun 4, 1829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yaribeygi H, Sathyapalan T & Sahebkar A Molecular mechanisms by which GLP-1 RA and DPP-4i induce insulin sensitivity. Life Sci. 234, 116776 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Takiishi T, Fenero CIM & Camara NOS Intestinal barrier and gut microbiota: shaping our immune responses throughout life. Tissue Barriers 5, e1373208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hotamisligil GS Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Fechner A, Kiehntopf M & Jahreis G The formation of short-chain fatty acids is positively associated with the blood lipid-lowering effect of lupin kernel fiber in moderately hypercholesterolemic adults. J. Nutr 144, 599–607 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Talati R, Baker WL, Pabilonia MS, White CM & Coleman CI The effects of barley-derived soluble fiber on serum lipids. Ann. Fam. Med 7, 157–163 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferrell JM & Chiang JY Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell. Mol. Gastroenterol. Hepatol 1 , 664–677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhai H et al. Takeda G protein-coupled receptor 5-mechanistic target of rapamycin complex 1 signaling contributes to the increment of glucagon-like peptide-1 production after Roux-en-Y gastric bypass. EBioMedicine 32, 201–214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pathak P et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chavez-Talavera O, Tailleux A, Lefebvre P & Staels B Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–1694 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Albaugh VL et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 156, 1041–1051 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang G, Zhang L, Yang G, Wu L & Wang R Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 56, 533–541 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Szabo C Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal 17, 68–80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moore SR et al. Robust circadian rhythms in organoid cultures from PERIOD2::LUCIFERASE mouse small intestine. Dis. Model. Mech 7, 1123–1130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jovel J et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol 7, 459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heintz-Buschart A & Wilmes P Human gut microbiome: function matters. Trends Microbiol. 26, 563–574 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Wang Y et al. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 357, 912–916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nguyen TL, Vieira-Silva S, Liston A & Raes J How informative is the mouse for human gut microbiota research? Dis. Model. Mech 8, 1–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vargason AM & Anselmo AC Clinical translation of microbe-based therapies: current clinical landscape and preclinical outlook. Bioeng. Transl. Med 3, 124–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baydoun M et al. An interphase microfluidic culture system for the study of ex vivo intestinal tissue. Micromachines 11, 150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brouwer A et al. Light therapy for better mood and insulin sensitivity in patients with major depression and type 2 diabetes: a randomised, double-blind, parallel-arm trial. BMC Psychiatry 15, 169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brouwer A et al. Effects of light therapy on mood and insulin sensitivity in patients with type 2 diabetes and depression: results from a randomized placebo-controlled trial. Diabetes Care 42, 529–538 (2019). [DOI] [PubMed] [Google Scholar]


