Abstract
A 42-year-old male presented with a history of headaches for the previous 2 weeks. Magnetic resonance imaging of the brain showed a 3 cm-sized well-defined, enhancing mass in the atrium of the right lateral ventricle. The tumor comprised two heterogeneous components: approximately one-third of the tumor exhibited complex and delicate papillary fibrovascular cores lined with uniform cuboidal-to-columnar epithelial cells, whereas the remaining part was seen as a solid sheet comprising ovoid-to-spindle cells with plump cytoplasm, which occasionally had a whorling pattern. Further, immunohistochemical staining with cytokeratin 7 (CK7) and epithelial membrane antigen (EMA) clearly demarcated each component: the CK7+/EMA- choroid plexus papilloma and CK7-/EMA+ meningioma. This report provides a description of an unusual case of concomitant choroid plexus papilloma and ventricular meningioma presenting as a single mass, along with a review of relevant literature.
Keywords: Choroid plexus papilloma, meningioma, intraventricular tumor
Introduction
Intraventricular tumors account for 2%-7% of all intracranial neoplasms [1]. They are classified according to their pathologic origins, which may include the walls of the ventricular system, the septum pellucidum, and the choroid plexus [2]. Based on the composition and patient’s age, differential diagnosis primarily reveals choroid plexus papilloma (CPP) in patients under the age of 10 years, low-grade glioma in patients aged 10-40 years, and metastases, lymphoma, and meningioma (MG) after the fourth decade of life [3].
MG is the most common intracranial tumor, accounting for approximately 20% of all primary brain neoplasms [4]. They typically arise in the supratentorial compartment, and intraventricular MG constitutes approximately 0.5%-2% of all intracranial MGs [4,5]. CPP is a rare benign neoplasm of the central nervous system, representing 0.3%-0.8% of all brain neoplasms and <1% of all intracranial neoplasms in adults [6]. CPP is commonly found surrounded by normal choroid plexus structures such as the lateral ventricle [1,6]. In childhood, CPP usually develops in the lateral ventricle with its atrial level as the most common site [7]. In adults, it frequently occurs in the fourth ventricle and/or lateral recess [8].
The occurrence of brain tumors with different pathologies in a single mass is rare [9]. In particular, the simultaneous presentation of a CPP and an MG as a single mass in the intraventricular system has never been reported. Herein, we describe an unusual case of concomitant CPP and MG in the lateral ventricle, supplemented with a review of pertinent literature.
Case presentation
Clinical features
A 42-year-old male presented with a history of headache for the previous 2 weeks. The pain was described as dull, intermittent, and not localized to one side. Neurological examination showed no signs of sensory, motor, or cranial nerve abnormalities. He had a history of hepatitis B virus-associated liver cirrhosis, but no history of surgery, stroke, or a familial history of stroke or cancer. Magnetic resonance imaging (MRI) of the brain showed a well-defined and enhancing mass in the atrium of the right lateral ventricle, measuring 3.3 × 2.8 × 2.6 cm (Figure 1A). Obstructive dilatation of the occipital and temporal horn of the right lateral ventricle was observed. Based on radiologic examination, possible differential diagnoses included intraventricular MG, CPP, and exophytic intra-axial tumors. Cerebral angiography revealed that the mass was supplied by the right anterior and posterior choroidal arteries. Tumor embolization was technically difficult because of the thin wall of the supplying blood vessel. Therefore, the mass was surgically resected.
Figure 1.
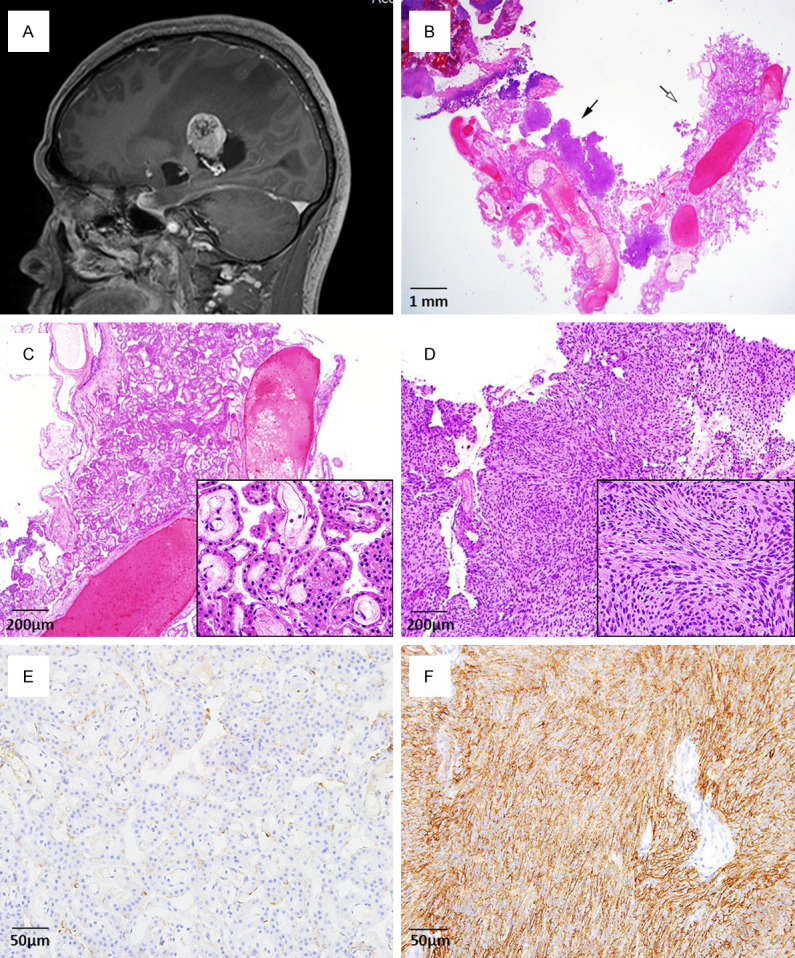
(A) Brain MRI shows a well-defined and intense enhancing mass in the atrium of the right lateral ventricle. (B) The ventricular tumor comprises two distinct components: a choroid plexus papilloma (white arrow) and meningioma (black arrow). (C) In the choroid plexus papilloma, complex and delicate papillary fibrovascular fronds are covered by a single layer of uniform cuboidal-to-columnar epithelial cells. (D) Meningioma cells are arranged in lobules and are packed together in short fascicles, forming whorls and syncytial structures. (E) In the choroid plexus papilloma, tumor cells are negative for immunohistochemical staining of the epithelial membrane antigen (EMA, 1:100, clone GP1.4, Leica Biosystems: Novocastra, UK). (F) In contrast, tumor cells are diffusely and strongly positive for EMA in the meningioma component. (B-D, Hematoxylin and eosin; B, Scan view; C, D, × 40; inset, × 400; E, F, EMA, × 200).
Pathologic findings
Microscopic examination of the mass revealed two different tumors: CPP and MG (Figure 1B). The CPP was composed of complex and delicate papillary fibrovascular cores covered by uniform cuboidal-to-columnar epithelial cells. Tumor cells were round to oval with monotonous, basally located nuclei and plump eosinophilic pale cytoplasm (Figure 1C). The border of the CPP was partially intermingled with the MG component, which was arranged in lobules with occasional whorling patterns. Tumor cells had ovoid-to-spindle shaped nuclei with pink cytoplasm and indistinct cell membranes (Figure 1D). In both the CPP and MG, cytologic atypia was minimal and mitotic figures were absent.
Immunohistochemical staining
Immunohistochemical staining of the CPP was strong and diffuse positive for cytokeratin 7 (CK7, 1:200, clone OV-TL, Dako, Glostrup, Denmark) and was negative for the epithelial membrane antigen (EMA, 1:100, clone GP1.4, Leica Biosystems: Novocastra, UK) (Figure 1E). In contrast, tumor cells in the MG were diffusely positive for EMA and negative for CK7 (Figure 1F). The proliferative labeling index measured by Ki-67 expression (1:100, clone MIB-1, Dako, Glostrup, Denmark) was less than 2% for both tumors.
Clinical course
The patient was discharged on postoperative day 13 without complications. Follow-up brain imaging revealed residual linear or nodular enhancement along the right choroid plexus and right ventricular wall with hemorrhage, indicative of a residual tumor at the surgical site. Three months later, gamma knife radiosurgery was performed on the suspected residual tumor. The patient was alive without tumor recurrence 5 months after surgery.
Discussion
Here, we report an unusual case of a CPP and an MG constituting a single mass in the lateral ventricle. To date, only two cases with coincidental CPP and MG have been reported in the English literature (Table 1) [10,11]. In Case 1 (Table 1), a 45-year-old female experienced headache for 2 months, accompanied by sensory and motor impairments due to extensive involvement of the lower cranial nerves and tumor adherence to the medullary pia. The CPP and MG were separate but both tumors had developed in the extraventricular area. In Case 2 (Table 1), a 45-year-old female complained of progressive confusion and altered sensory and motor functions. The CPP was filling the fourth ventricle, whereas the MG was distinctly located at the craniocervical junction. Unlike these cases, the present case showed an admixture of CPP and MG within a single mass in the intraventricular space. As the tumor was located in the ventricle, clinical presentation was mainly characterized by obstructive hydrocephalus and functional deficits in the neurological systems were absent.
Table 1.
Summary of cases with coincidental choroid plexus papilloma and meningioma
| Case (Ref.) | Age/Sex | Clinical presentation | Tumor location and size | Tumor continuity |
|---|---|---|---|---|
| 1 (10) | 45/F | Headache for 2 months | CPP: Petrotentorial junction, 2.5 cm | Noncontiguous |
| Sensory and motor deficit | MG: Cervicomedullary junction, 5 cm | |||
| 2 (11) | 45/F | Progressive confusion for 2 months | CPP: Fourth ventricle, 4.4 cm | Noncontiguous |
| Sensory and motor deficit | MG: Craniocervical junction, 2.3 cm | |||
| Present case | 42/M | Headache for 2 weeks | CPP and MG: Lateral ventricle, 3 cm | Contiguous |
| No sensory and motor deficit |
Abbreviations: CPP, choroid plexus papilloma; MG, meningioma.
Patients with two distinct primary intracranial neoplasms are very rare [12,13]. In particular, it is extremely rare for multiple primary intracranial neoplasms to develop concurrently in patients without prior intracranial radiotherapy or underlying phacomatoses such as neurofibromatosis-2 or tuberous sclerosis [14]. Approximately, fewer than 100 cases of multiple concurrent primary intracranial neoplasms have been reported, and the majority of coincidental intracranial neoplasms are MGs and gliomas [14,15]. Of all primary intracranial neoplasms, MGs are well-known to occur concurrently with other types of tumors [16,17]. This may be due to the indolent course of such tumors, which allows its prolonged clinical evolution before diagnosis. Further, detection of MGs is primarily incidental [13,18].
An admixture of CPP and MG within a single mass may complicate diagnosis during pathologic examinations. The main histologic differential diagnoses for such tumor samples include papillary MG, papillary ependymoma, and metastatic carcinoma with papillary features [1]. Papillary MG is an MG in which the perivascular pseudopapillary pattern involves most of the tumor [20]. The detection of high-grade histologic features with aggressive presentation is sufficient for a conclusive diagnosis of a papillary MG [1]. Papillary ependymomas exhibit finger-shaped processes lined with a single layer of cuboidal cell with smooth surfaces as well as glial fibrillary acidic protein-positive tumor cell processes. CPP can be distinguished from a papillary ependymoma by bumpy and hobnail cellular surfaces that are not characterized by extensive glial fibrillary acidic protein immunoreactivity [1].
The pathophysiology of the simultaneous development of multiple primary intracranial neoplasms at the same location remains unclear, especially the occurrence of intermingled components of CPP and MG. Various mechanisms have been proposed to explain concurrent primary intracranial tumors [20-28]. First, the concurrent occurrence of various tumor types at the same anatomical site may be due to the collision of two discrete tumors [20-22]. Second, the first tumor may act as a stimulant and cause excessive growth or metaplasia, leading to the development of a second tumor [23]. The slow growth of benign early tumors is generally considered to be a stimulus that leads to the production of microenvironmental factors, which may induce the growth of other de novo tumors [24-26]. Third, development of the mixed tumor may be associated with the independent development of two elements or bidirectional differentiation from common progenitor cells [27,28]. Fourth, in rare situations, commonly occurring tumors may accidently present at the same site [24-26]. Despite some evidence for the aforementioned theories, plausible underlying mechanisms have not been conclusively proven. Further research and additional case studies are needed to develop a comprehensive understanding of the mechanisms that drive the simultaneous occurrence of multiple intracranial neoplasms.
In our patient, both CPP and MG were present in the right ventricle and could be removed using one surgical approach. A small remnant of the CPP remained attached to the pia of the medulla due to adherence. This tumor remnant was observed at the 3-month follow-up MRI scan. Equivocal growth was seen in serial imaging, following which gamma knife radiosurgery was successfully applied.
In conclusion, this is the first case report of coincident CPP and MG at the same locus of the lateral ventricle. This rare presentation of intraventricular tumors suggests that an admixture of tumors with papillary and lobular growth patterns in a single intraventricular mass may be due to the coincidental development of a CPP and MG at the same site.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT, and Future Planning (NRF-2020R1G1A1003692).
Informed consent was obtained from the patient described in this study.
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathologica. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Koeller KK, Sandberg GD. From the archives of the AFIP. Cerebral intraventricular neoplasms: radiologic-pathologic correlation. Radiographics. 2002;22:1473–505. doi: 10.1148/rg.226025118. [DOI] [PubMed] [Google Scholar]
- 3.de Castro FD, Reis F, Guerra JG. Intraventricular mass lesions at magnetic resonance imaging: iconographic essay - part 1. Radiologia Brasileira. 2014;47:176–81. doi: 10.1590/0100-3984.2013.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14:2161–77. doi: 10.2217/fon-2018-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, Vecht C. Meningioma. Crit Rev Oncol Hematol. 2008;67:153–71. doi: 10.1016/j.critrevonc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Sethi D, Arora R, Garg K, Tanwar P. Choroid plexus papilloma. Asian J Neurosurg. 2017;12:139–41. doi: 10.4103/1793-5482.153501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz Montoya JE, Maldonado Moran MA, Santamaria Rodriguez P, Toro Lopez S, Perez Cataño CS, Luque Suarez JC. Choroid plexus papilloma of the fourth ventricle: a pediatric patient. Asian J Neurosurg. 2019;14:585–8. doi: 10.4103/ajns.AJNS_301_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura M, Takayasu M, Suzuki Y, Negoro M, Nagasaka T, Nakashima N, Sugita K. Primary choroid plexus papilloma located in the suprasellar region: case report. Neurosurgery. 1992;31:563–6. doi: 10.1227/00006123-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Kim SI, Kim SK, Kim IO, Park SH. A mixed choroid plexus papilloma and ependymoma. Brain Tumor Pathol. 2016;33:147–50. doi: 10.1007/s10014-015-0242-4. [DOI] [PubMed] [Google Scholar]
- 10.McIver JI, Link MJ, Giannini C, Cohen-Gadol AA, Driscoll C. Choroid plexus papilloma and meningioma: coincidental posterior fossa tumors: case report and review of the literature. Surg Neurol. 2003;60:360–5. doi: 10.1016/s0090-3019(03)00157-5. [DOI] [PubMed] [Google Scholar]
- 11.Khayal HB, Abograra A, Lashhab M. Fourth ventricle papilloma and cranio-cervical junction meningioma: coincidental tumors: a case report and review of the literature. Curr Opin Neurol Sci. 2018;2:463–7. [Google Scholar]
- 12.Lee EJ, Chang CH, Wang LC, Hung YC, Chen HH. Two primary brain tumors, meningioma and glioblastoma multiforme, in opposite hemispheres of the same patient. J Clin Neurosci. 2002;9:589–91. doi: 10.1054/jocn.2002.1086. [DOI] [PubMed] [Google Scholar]
- 13.Maiuri F, Cappabianca P, Iaconetta G, Esposito F, Messina A. Simultaneous presentation of meningiomas with other intracranial tumours. Br J Neurosurg. 2005;19:368–75. doi: 10.1080/02688690500305548. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Yang Y, Zhang K, Zhuang J, Shao F, Liu H, Xing Y, Xu S. Collision tumor of glioblastoma and meningioma: case report and literature review. World Neurosurg. 2018;117:137–41. doi: 10.1016/j.wneu.2018.05.246. [DOI] [PubMed] [Google Scholar]
- 15.Gordon AS, Fallon KE, Riley KO. Meningioma interdigitated with primary central nervous system B-cell lymphoma: a case report and literature review. Surg Neurol Int. 2011;2:181. doi: 10.4103/2152-7806.90716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honegger J, Buchfelder M, Schrell U, Adams EF, Fahlbusch R. The coexistence of pituitary adenomas and meningiomas: three case reports and a review of the literature. Br J Neurosurg. 1989;3:59–69. doi: 10.3109/02688698909001027. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Momota H, Tonooka A, Noguchi H, Yamamoto K, Wanibuchi M, Minamida Y, Hasegawa T, Houkin K. Glioblastoma simultaneously present with adjacent meningioma: case report and review of the literature. J Neurooncol. 2010;99:147–53. doi: 10.1007/s11060-009-0109-9. [DOI] [PubMed] [Google Scholar]
- 18.Slowik F, Jellinger K. Association of primary cerebral lymphoma with meningioma: report of two cases. Clin Neuropathol. 1990;9:69–73. [PubMed] [Google Scholar]
- 19.Wang DJ, Zheng MZ, Gong Y, Xie Q, Wang Y, Cheng HX, Mao Y, Zhong P, Che XM, Jiang CC, Huang FP, Zheng K, Li SQ, Gu YX, Bao WM, Yang BJ, Wu JS, Xie LQ, Tang HL, Zhu HD, Chen XC, Zhou LF. Papillary meningioma: clinical and histopathological observations. Int J Clin Exp Pathol. 2013;6:878–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Dohrmann GJ, Collias JC. Choroid plexus carcinoma. J Neurosurg. 1975;43:225–32. doi: 10.3171/jns.1975.43.2.0225. [DOI] [PubMed] [Google Scholar]
- 21.Karami KJ, Poulik J, Rabah R, Krass J, Sood S. Simultaneous choroid plexus carcinoma and pilocytic astrocytoma in a pediatric patient. J Neurosurg Pediatr. 2010;5:104–12. doi: 10.3171/2009.8.PEDS09117. [DOI] [PubMed] [Google Scholar]
- 22.Iyer V, Sanghvi D, Shenoy A, Goel A. Three distinct co-existent primary brain tumors in a patient. J Can Res Ther. 2009;5:293–6. doi: 10.4103/0973-1482.59914. [DOI] [PubMed] [Google Scholar]
- 23.Spallone A, Santoro A, Palatinsky E, Giunta F. Intracranial meningiomas associated with glial tumours: a review based on 54 selected literature cases from the literature and 3 additional personal cases. Acta Neurochir (Wien) 1991;110:133–9. doi: 10.1007/BF01400681. [DOI] [PubMed] [Google Scholar]
- 24.Hokari M, Hida K, Ishii N, Seki T, Iwasaki Y, Nakamura N. Associated meningioma and neurofibroma at the same cervical level without clinical signs of neurofibromatosis: case report. No Shinkei Geka. 2002;30:953–7. [PubMed] [Google Scholar]
- 25.Nakamizo A, Suzuki SO, Shimogawa T, Amano T, Mizoguchi M, Yoshimoto K, Sasaki T. Concurrent spinal nerve root schwannoma and meningioma mimicking single-component schwannoma. Neuropathology. 2012;32:190–5. doi: 10.1111/j.1440-1789.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 26.Oichi T, Chikuda H, Morikawa T, Mori H, Kitamura D, Higuchi J, Taniguchi Y, Matsubayashi Y, Oshima Y, Tanaka S. Concurrent spinal schwannoma and meningioma mimicking a single cervical dumbbell-shaped tumor: case report. J Neurosurg Spine. 2015;23:784–7. doi: 10.3171/2015.3.SPINE141315. [DOI] [PubMed] [Google Scholar]
- 27.Tunthanathip T, Kanjanapradit K, Ratanalert S, Phuenpathom N, Oearsakul T, Kaewborisutsakul A. Multiple, primary brain tumors with divers origins and different localizations: case series and review of the literature. J Neurosci Rural Pract. 2018;9:593–607. doi: 10.4103/jnrp.jnrp_82_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deen HG, Laws ER. Multiple primary brain tumors of different cell types. Neurosurgery. 1981;8:20–5. doi: 10.1227/00006123-198101000-00005. [DOI] [PubMed] [Google Scholar]


