Abstract
Purpose: The effect of resveratrol on subchondral bone in osteoarthritis was explored by constructing a mouse model of osteoarthritis and giving resveratrol as intervention. Methods: The degree of proteoglycan loss in articular cartilage was assessed by safranine fast green staining. The expressions of Lubricin and Aggrecan, COLX, and MMP-13, the co-expression of CD31 and Endomucin, and the expression of angiogenesis-related factors were determined by immunohistochemistry. TRAP stain and immunostaining were used to assess abnormal subchondral bone resorption and bone formation. Angiography was employed to analyze the effect of resveratrol on the proliferation of subchondral bone vessels. Results: Resveratrol inhibited cartilage thickening and the increase of COLX and MMP-13 expression, delayed the loss of proteoglycan, Lubricin, and Aggrecan, and inhibited osteoclast differentiation by up-regulating osteoprotegerin (OPG) and down-regulating the expression of RANKL. Angiography showed that resveratrol can reduce the abnormally elevated number and volume of blood vessels in the subchondral bone. Immunostaining showed that resveratrol inhibited CD31hiEmcnhi angiogenesis and high expression of VEGFA and Angiopoietin-1. Conclusion: Resveratrol inhibits osteoclast differentiation and reduces active bone resorption by regulating the OPG/RANKL/RANK pathway, and inhibits the abnormal proliferation of CD31hiEmcnhi blood vessels by downregulating the expression of VEGFA and Angiopoiein-1, thereby eliminating the pathologic coupling mechanism of osteogenesis and vascularization, and delaying the progression of osteoarthritis.
Keywords: Resveratrol, osteoarthritis, OPG/RANKL/RANK, CD31hiEmcnhi
Introduction
Osteoarthritis is a chronic joint disease caused by a variety of factors. The pathologic characteristics are mainly the degenerative changes of articular cartilage and secondary bone hyperplasia around the joints, which mostly involve the joints with heavy loads and abundant movement, such as knee joints and hips joints, spine and other parts. Hand joints are also one of the most common foci of osteoarthritis. The main clinical manifestations are chronic joint pain, morning stiffness, joint swelling, weakness, and dysfunction. Osteoarthritis can have primary and secondary pathogenic factors. The cause of primary osteoarthritis is not fully understood. Its occurrence and development are a long-term, chronic, and gradual pathologic process. It is generally believed to be caused by the interaction of a variety of pathogenic factors, including mechanical and biologic factors. Among them, age is the main high-risk factor. Others include cartilage nutrition and metabolic abnormalities, biomechanical stress balance disorders, biochemical changes, abnormal degradation of cartilage matrix by enzymes, cumulative minor traumas, obesity, and increased joint load. The incidence in women is relatively higher, and it increases significantly after menopause, which may be related to estrogen receptors in articular cartilage. Studies have found that in osteoarthritis patients with high fat and low muscle, the severity of the disease is linearly related to BMI. In female patients, regardless of body weight, there is a correlation between knee joints and low muscle content [1-3].
The osteoprotegerin (OPG) OPG/RANKL/RANK signaling pathway is a key regulator of the activity and function of osteoclasts. Under normal stress, RANK ligand (RANKL), secreted in large quantities by osteoblasts, osteocytes, and chondrocytes, combines with RANK on the osteoclast precursor cell membrane, so that TRAF6 in the cytoplasm gathers on the cytoplasmic side of RANK and activates it, then it activates NF-κB and the MAPK (p38, ERK, JNK) signaling pathways. OPG secreted by osteoblasts acts as an inducible ligand of RANKL and prevents osteoclast precursor cell differentiation by combining with RANKL. When the NF-κB and MAPK signaling pathways are activated, the relevant molecules of each pathway enter the nucleus through phosphoric acid to connect to specific gene sites, perform transcription and translation procedures, and promote the transformation of osteoclast precursor cells into mature osteoclasts [4,5]. Mature osteoclasts cling to the surface of the subchondral bone plate and trabecular bone, and dissolve the bone matrix by releasing catabolic enzymes and acid products, thereby releasing potential TGF-β. The activated TGF-β induces bone marrow mesenchymal stem cells to migrate to bone resorption sites and differentiate into osteogenic precursor cells, which ultimately balances the resorption and formation of subchondral bone [6,7]. In osteoarthritis, the large amounts of RANKL secreted by the above-mentioned cells combine with RANK to promote the over-differentiation of osteoclast precursor cells into mature osteoclasts, which continuously erode the subchondral bone matrix and release a large amount of potential TGF-β. This in turn promotes the migration and differentiation of MSCs into osteogenic precursor cells, and finally forms abnormal bone islands in the subchondral medullary cavity, causing the destruction of the subchondral bone microstructure, and simultaneously changing the distribution of joint stress, which ultimately leads to cartilage degeneration and damage [8,9]. In addition, a large number of activated osteoclast precursor cells can abnormally secrete platelet-derived growth factor-BB (PDGF-BB) cytokines, promote the occurrence of abnormal vascularization of subchondral bone, and constitute the pathologic coupling mechanism of abnormal blood vessel formation and ectopic bone formation, eventually leading to subchondral bone sclerosis and cystic changes [10].
Methods
Establishment and grouping of mouse model of osteoarthritis
10-week-old C57BL/6J mice were obtained. The anterior cruciate ligament was cut off by micro-scissor under direct microscopy. After the anterior drawer test, it was obvious that the proximal tibia was displaced relative to the distal femur, suggesting the anterior cruciate ligament was completely severed. The patella was reset, the joint capsule, medial femoral muscle, subcutaneous fat layer and connective tissue were sutured with absorbable sutures, and the skin was sutured with non-absorbable sutures. Experimental groups are as follows: A, the Sham operation group, in which the mice were given normal saline intraperitoneal injection for 60 days. B, the Model group, in which the mice were given normal saline intraperitoneal injection for 60 days, and the anterior cruciate ligament dissection was combined with placebo. C, the resveratrol group, in which the mice were given intraperitoneal injection of 50 mg/kg resveratrol for 60 days, and anterior cruciate ligament dissection was combined with resveratrol. The procedures for the care and use of animals have been approved by the Ethics Committee of the First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, and all applicable institutions and government regulations regarding the ethical use of animals have been complied with.
Safranin solid green dyeing
According to the instructions, sectioning, dewaxing and gradient ethanol hydration, staining with hematoxylin, fast green, safranine O dyeing, and dehydration were performed. Vitrification, mounting, and microscopy were conducted.
TRAP stain
The procedures were performed according to instructions: sectioning, deparaffinization and gradient ethanol hydration, TRAP staining, hematoxylin staining, color separation, dehydration, vitrification, mounting, and image collection under an optical microscope.
Immunohistochemical staining
According to the instructions, the following procedures were conducted: sectioning, dewaxing and gradient ethanol hydration, washing, endogenous peroxidase inactivation, antigen retrieval, serum blocking, secondary antibody incubation, color development, hematoxylin staining, color separation, reverse blue, gradient ethanol dehydration, vitrification, mounting, and picture collection under optical microscope.
Micro-CT scan
Micro-CT scans were used to analyze the microvascular changes in the subchondral bone of each group of mice at 60 days postoperatively. The 3D reconstruction of angiography was completed with CT Vol v2.0. The vessel volume (VV) and vessel number (VN) were analyzed.
Statistical analysis
The experimental data was analyzed using SPSS 17.0. The histogram was constructed using GraphPad Prism 7.0. The descriptive statistics are expressed in the form of mean ± standard deviation. The data were compared by one-way analysis of variance (one-way ANOVA). The corresponding test method was chosen according to the test result of the homogeneity of variance between the two groups. If the variance was uniform, the LSD method was chosen; if the variance was not uniform, the Dunnett’s T3 method was applied. The χ2 test was used to compare the count data. P < 0.05 was considered a significant difference.
Results
The effect of resveratrol on articular cartilage in mice
Safranin and fast green staining result revealed that the proteoglycan in the Model group was obviously lost, the number of cartilage cells was reduced, the cells were in a hypertrophic pathologic state, and fibrous cracks appeared on the cartilage layer. Under the intervention of resveratrol, the loss of cartilage proteoglycan components was alleviated, the number of cells increased, mast cells and fibrous fissures were reduced, and the structure of cartilage was relatively complete, indicating that resveratrol prevented the loss of proteoglycans in cartilage, preserved the complete structure of articular cartilage, and delayed cartilage degeneration during osteoarthritis, as shown in Figure 1.
Figure 1.
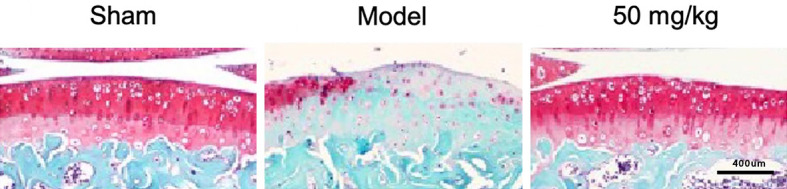
Protective effect of resveratrol on articular cartilage, scale: 1:400 mm (×400).
Resveratrol maintains the metabolic balance of cartilage
Articular cartilage is always in a dynamic balance of synthesis and catabolism. In the course of osteoarthritis, cartilage degenerates. The expression of factors involved in catabolism, such as MMP-13 and COLX, increases, while the expression of factors involved in anabolism, such as Lubricin and Aggrecan, decreases. In this study, the expressions of Lubricin, Aggrecan, MMP-13, and COLX in articular cartilage were evaluated by immunohistochemical staining 60 days after the anterior cruciate ligament was cut. The experimental results showed that, compared with the Sham group, the expressions of Lubricin and Aggrecan were down-regulated in the Model group, while the expressions of MMP-13 and COLX were up-regulated. Under the intervention of resveratrol, the high expression of MMP-13 and COLX was significantly inhibited, while the low expression of Lubricin and Aggrecan was significantly improved, as shown in Figure 2.
Figure 2.
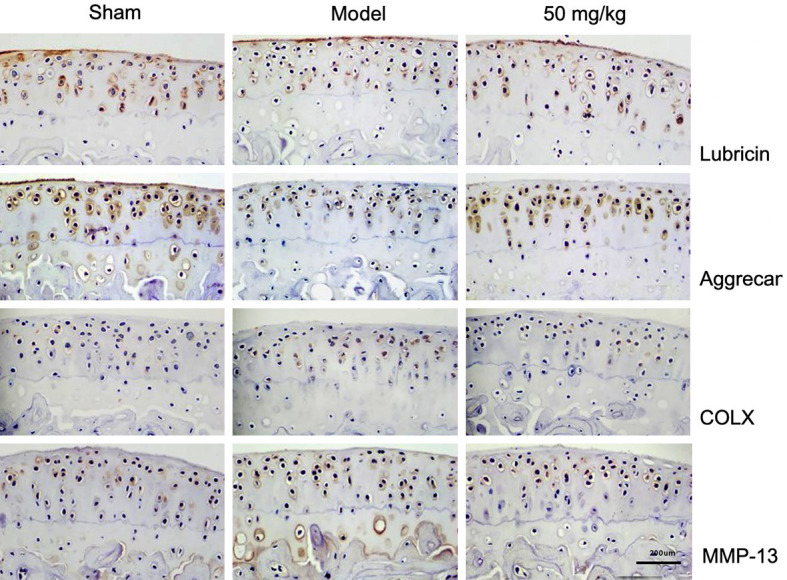
Resveratrol maintains the metabolic balance of cartilage, scale: 1:200 mm (×200).
Resveratrol reconstructs the normal coupling mechanism of osteoclasts and osteogenesis in subchondral bone
TRAP staining results showed that, at 60 days after surgery, compared with the Sham group, TRAP+ osteoclasts in the Model group were significantly increased. However, under the intervention of resveratrol, compared with the Model group, TRAP+ osteoclasts were significantly reduced. The results of immunohistochemical staining showed that, compared with Sham at 60 days postoperatively, osterix+ osteoblasts in the Model group were significantly increased, and the cells were transferred from the bone surface to the marrow cavity. Under the intervention of resveratrol, compared with the Model group, osterix+ osteoblasts were significantly reduced and mostly attached to the bone surface. The above experimental results showed that resveratrol maintains the normal microstructure by reconstructing the normal bone coupling mechanism of the subchondral bone, and then restores the shock absorption and support function of the subchondral bone, thereby protecting the articular cartilage covering it.
VEGFA is a heparin-binding growth factor specific for vascular endothelial cells. It stimulates vascular endothelial cells to sprout and promote growth in the early stage of neovascularization by binding to the VEGFR-2 receptor. The formation of new blood vessels provides favorable conditions for the migration of MSCs and the transport of inflammatory factors. Therefore, inhibiting the expression of VEGFA can effectively prevent the occurrence and development of abnormal vascular proliferation.
In the experiment, immunohistochemical staining was used to evaluate the expression of VEGFA in subchondral bone 60 days after surgery and the inhibitory effect of resveratrol on this factor. The results showed that, compared to the Sham group, the VEGFA expression in the Model group was significantly higher. However, under the intervention of resveratrol, the expression of VEGFA was significantly reduced, suggesting resveratrol prevents the initial stage of angiogenesis by inhibiting the expression of VEGFA.
Angiopoietin-1 (Ang-1) is a family of secreted growth factors that can enhance the circumferential proliferation of vascular endothelial cells and induce a unique pattern of vasodilation, which is mainly responsible for the stabilization and remodeling of new blood vessels. In the experiment, immunohistochemical staining was used to evaluate the expression level of Ang-1 in subchondral bone 60 days after surgery and the inhibitory effect of resveratrol on this factor. The results showed that, under the intervention of resveratrol, the expression of Ang-1 was significantly decreased, indicating that resveratrol inhibits the later remodeling and stabilization of microvessels by inhibiting the abnormal expression of Ang-1, thereby effectively inhibiting vascular proliferation (Figure 3).
Figure 3.
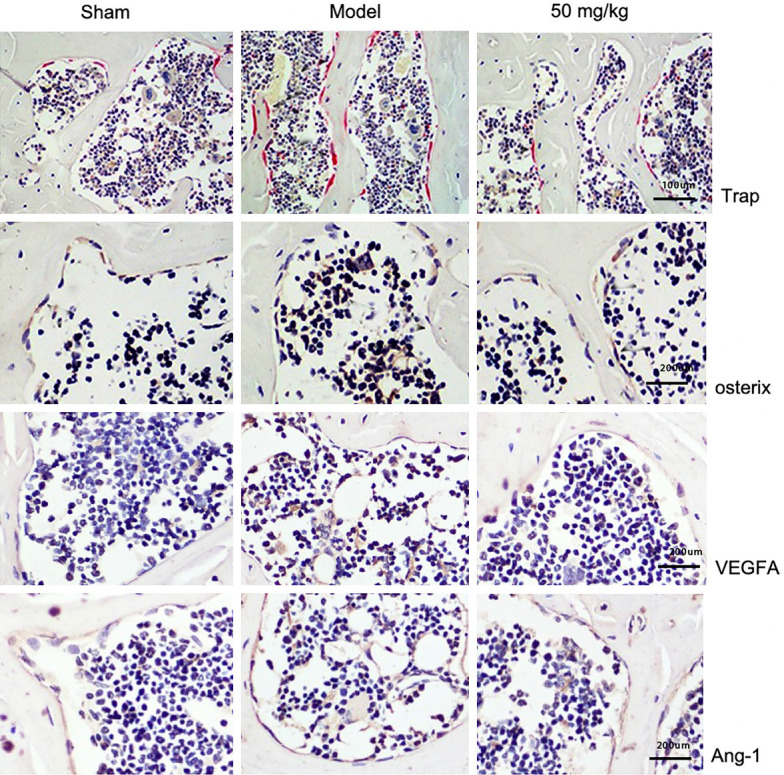
Resveratrol reconstructs subchondral bone osteoclasts, osteogenesis, and the expressions of VEGFA and angiopoietin-1 (Ang-1), scale: Trap: 1:100 mm, osterix 1:200 mm, VEGFA: 1:200 mm, Ang-1: 1:200 mm (Trap ×100, osterix ×200, VEGFA ×200, Ang-1 ×200).
Immunofluorescence results
Resveratrol inhibits osteoclast differentiation by regulating the OPG/RANKL/RANK pathway
The OPG/RANKL/RANK signaling pathway plays a decisive role in the process of osteoclast differentiation. The combination of RANKL and RANK aggregates and activates TRAF6 in the cytoplasm, which in turn activates the NF-κB and MAPK signaling pathways, and promotes the differentiation of osteoclast precursor cells. However, OPG, as an inducible ligand of RANKL, prevents osteoclast precursor cell differentiation through combining with RANKL. In the experiment, immunofluorescence staining was used to evaluate the inhibitory effect of resveratrol on the differentiation of osteoclast precursor cells 60 days after surgery. The results of immunofluorescence staining showed that, compared with Sham, the expression of OPG in the Model group was decreased while the expressions of RANKL and TRAF6 were increased. However, under the intervention of resveratrol, the expression of OPG was increased and the expressions of RANKL and TRAF6 were decreased in comparison with the Model group (Figure 4).
Figure 4.
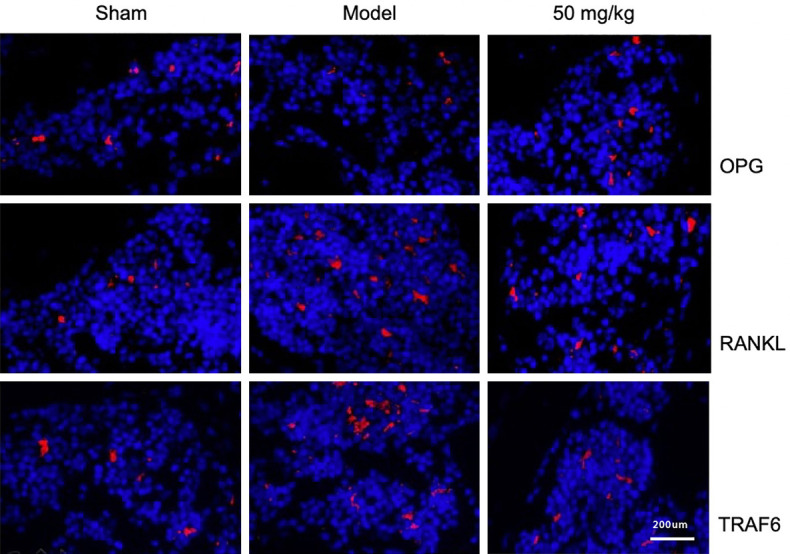
Effect of resveratrol on the expressions of OPG, RANKL, and TRAF6 in subchondral bones, 1:200 mm (×200).
Effect of resveratrol on the angiogenesis of CD31hi Endomucinhi in subchondral bone
There are a large number of ectopic new bone islands in the subchondral bone of osteoarthritis. It is still unclear whether the formation of these bone islands has a coupling mechanism with H-type blood vessels and whether resveratrol has an inhibitory effect on the blood vessels. In the experiment, immunofluorescence staining was used to evaluate the expression of H-type blood vessels at 60 days postoperatively and the inhibitory effect of resveratrol on H-type blood vessels. The results showed that, compared with the Sham group, the angiogenesis of CD31hi Endomucinhi in the Model group was significantly increased, and the intervention of resveratrol significantly inhibited its pathologic changes. This suggests that resveratrol can inhibit the abnormal proliferation of the blood vessels, and at the same time inhibit osteoclast differentiation and maturation by regulating the OPG/RANKL/RANK signaling pathway, thereby inhibiting the release of TGF-β and preventing it from inducing MSC to differentiate into vascular precursor cells (Figure 5).
Figure 5.
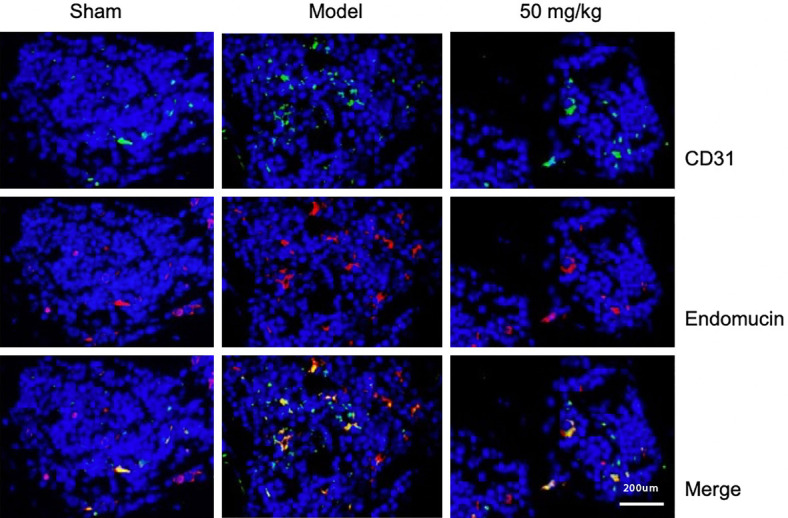
The effect of resveratrol on the expressions of CD31 and Endimucin in subchondral bone, 1:200 mm (×200).
The effect of resveratrol on the abnormal proliferation of microvessels in the subchondral bone
Angiography was performed 60 days postoperatively. Three-dimensional images of subchondral bone micro-vessels were constructed through micro-CT scanning. The volume and number of hyperplastic blood vessels were analyzed. The results showed that, compared with the Sham group, the vascular network in the Model group was denser and the blood vessel diameter was longer. However, in the resveratrol group, the blood vessels were sparse, and the diameter of the tube was shorter, which tended to be similar to the Sham group, and the volume and number of blood vessels increased significantly. Under the intervention of resveratrol, compared with the Model group, the volume and number of blood vessels were significantly reduced, as shown in Figure 6.
Figure 6.
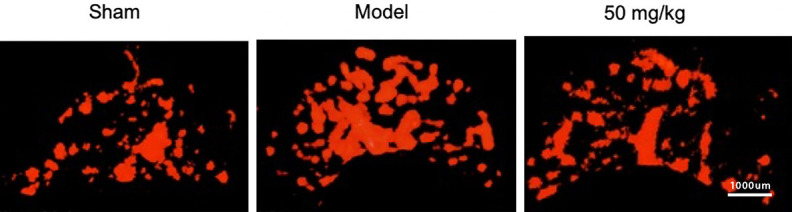
The effect of resveratrol on changes in subchondral bone vessels, 1:1000 mm (×1000).
Discussion
Normal bone remodeling is a necessary condition for maintaining the dynamic balance of bone tissue, and is regulated by the mutual adjustment of osteoblasts and osteoclasts in time and space. Active bone resorption caused by the activation of osteoclasts is a hot spot in the study of various bone diseases, such as osteoporosis, rheumatoid arthritis, and bone tumors. Recently, its role in the pathogenesis of early osteoarthritis has attracted attention [11].
At present, the temporal relationship between cartilage and subchondral bone in the pathogenesis of early osteoarthritis has become the focus of scholars. Some scholars believe that cartilage inflammation and mechanical stress are the direct causes of cartilage destruction. On this basis, the mechanical load transmitted to the subchondral bone changes, which in turn causes abnormal bone remodeling and vascularization of the subchondral bone [12]. However, other scholars believe that, due to the changes in stress load, the subchondral bone is the first to undergo abnormal bone remodeling, resulting in ectopic new bone in the marrow cavity and accompanying vascular proliferation. When the stress acts on the cartilage again, the subchondral bone is unable to absorb and cushion the shock, so that the stress load acting on the cartilage rises sharply, which ultimately leads to cartilage damage [13].
Bone remodeling is a process of bone damage and repair. Osteoclasts remove the damaged bone tissue and replace it with osteoblasts. In the normal bone remodeling process, the activation of osteoclasts induced by RANKL is the most important first step in bone remodeling. These osteoclasts are derived from early or late peripheral blood precursor cells adjacent to the bone surface in the bone marrow cavity. After it is positioned on the surface of the damaged bone tissue, it begins to perform an osteolysis procedure, and degrades bone tissue by secreting osteolytic enzymes and acidic metabolites. Subsequently, the osteolytic lacuna is filled by osteoblasts through osteogenesis to maintain the dynamic balance of bone structure and morphology [14,15]. The vascular network in the subchondral bone is composed of endothelial cells and pericytes, like other capillaries. In the process of blood vessel germination, cell migration and proliferation, blood vessel maturation, and the interconnection of newly formed blood vessels, endothelial cells and pericytes interact delicately, forming a highly branched pipe network system, which makes fluid and nutrients, gases, hormones, and circulating cells that can be transported in vertebrates [16]. In the course of osteoarthritis, the abnormally activated signal transduction pathway consumes a lot of nutrients and energy. Therefore, new blood vessels are required to continuously provide sufficient nutrients and remove metabolic waste. At the same time, the remodeling and proliferation of blood vessels provide more a convenient environment for MSC migration. Thus, inhibiting the vascular proliferation and remodeling of subchondral bone can be regarded as a therapeutic target to improve the process of osteoarthritis [17,18].
In the subchondral bone of osteoarthritis, the capillaries are stimulated by abnormal mechanical stress, and the molecular balance between promoting and inhibiting angiogenesis is broken, tending to promote angiogenesis molecules. This pathologic change of angiogenesis can stimulate the surrounding cells to express a variety of angiogenic factors, including VEGFA, platelet-derived growth factors (PDGFs), hemolytic phosphate (LPA), and angiopoietins (Angs) [19]. VEGF and Angs can activate matrix degrading enzymes, including plasminogen activators (PAs) and matrix metalloproteinases (MMPs), to relax the matrix and allow endothelial cells to migrate and germinate. In osteoarthritis subchondral bone, MSC migration and angiogenesis are coupled with bone formation, and this specific micro-vessel is an H-type blood vessel. CD31 and Endomucin are highly expressed in this blood vessel [20,21].
Conclusions
It was found that, after the anterior cruciate ligament was cut in adult male C57BL/6J mice, a large number of osteoclasts and active bone resorption were seen in the subchondral bone. At the same time, a large number of osteogenic precursor cells appeared in the medullary cavity and ectopic new bone islands. Resveratrol could effectively inhibit osteoclasts and osteogenic precursor cells, thereby restoring the normal bone remodeling mechanism and maintaining the stability of the subchondral bone microstructure. The OPG/RANKL/RANK signaling pathway is a key pathway for osteoclast precursor cell differentiation and maturation. Resveratrol can regulate this pathway and inhibit the mature differentiation and function of osteoclast precursor cells. Endomucinhi blood vessels are coupled with osteogenic precursor cells to promote the formation of ectopic bone islands in subchondral bone. Resveratrol not only effectively reduces the volume and number of CD31hi Endomucinhi blood vessels, but also reduces the expression of CD31 and Endomucin, thereby interrupting the pathologic coupling mechanism of abnormal vascularization and ectopic bone formation in the subchondral bone and maintaining the microscopic stability. The undecided structure ensures the normal stress distribution of the articular cartilage.
Disclosure of conflict of interest
None.
References
- 1.Menon J, Mishra P. Health care resource use, health care expenditures and absenteeism costs associated with osteoarthritis in US healthcare system. Osteoarthritis Cartilage. 2018;26:480–484. doi: 10.1016/j.joca.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Saito T, Tanaka S. Molecular mechanisms underlying osteoarthritis development: notch and NF-κB. Arthritis Res Ther. 2017;19:94. doi: 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018;149:325–341. doi: 10.1007/s00418-018-1636-2. [DOI] [PubMed] [Google Scholar]
- 4.Mu W, Xu B, Ma H, Li J, Ji B, Zhang Z, Amat A, Cao L. Halofuginone attenuates osteoarthritis by rescuing bone remodeling in subchondral bone through oral gavage. Front Pharmacol. 2018;9:269. doi: 10.3389/fphar.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei CM, Liu Q, Song FM, Lin XX, Su YJ, Xu J, Huang L, Zong SH, Zhao JM. Artesunate inhibits RANKL-induced osteoclastogenesis and bone resorption in vitro and prevents LPS-induced bone loss in vivo. J Cell Physiol. 2018;233:476–485. doi: 10.1002/jcp.25907. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19:248. doi: 10.1186/s13075-017-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Das P, Kumar VL. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem Biol Interact. 2017;278:84–91. doi: 10.1016/j.cbi.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Zeng C, Wei J, Li H, Yang T, Zhang Y, Xiong YL, Gao SG, Li YS, Lei GH. The associations of serum uric acid level and hyperuricemia with knee osteoarthritis. Rheumatol Int. 2016;36:567–573. doi: 10.1007/s00296-015-3418-7. [DOI] [PubMed] [Google Scholar]
- 9.Amar S, Smith L, Fields GB. Matrix metalloproteinase collagenolysis in health and disease. Biochim Biophys Acta Mol Cell Res. 2017;1864:1940–1951. doi: 10.1016/j.bbamcr.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan T, Shi X, Chen H. Geniposide suppresses interleukin-1β-induced inflammation and apoptosis in rat chondrocytes via the PI3K/Akt/NF-κB signaling pathway. Inflammation. 2018;41:390–399. doi: 10.1007/s10753-017-0694-2. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Zhong S, Kong R. Paeonol alleviates interleukin-1β-induced inflammatory responses in chondrocytes during osteoarthritis. Biomed Pharmacother. 2017;95:914–921. doi: 10.1016/j.biopha.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Feng Z, Zheng W, Li X. Cryptotanshinone protects against IL-1β-induced inflammation in human osteo-arthritis-chondrocytes and ameliorates the progression of osteoarthritis in mice. Int Immunopharmacol. 2017;50:161–167. doi: 10.1016/j.intimp.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Hong X, Lin D, Luo X, Zhu M, Mo H. Artesunate influences Th17/Treg lymphocyte balance by modulating Treg apoptosis and Th17proliferation in a murine model of rheumatoid arthritis. Exp Ther Med. 2017;13:2267–2273. doi: 10.3892/etm.2017.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez C, Bay-Jensen AC, Pap T, Dvir-Ginzberg M, Quasnichka H, Barrett-Jolley R, Mobasheri A, Henrotin Y. Chondrocyte secretome: a source of novel insights and exploratory biomarkers of osteoarthritis. Osteoarthritis Cartilage. 2017;25:1199–1209. doi: 10.1016/j.joca.2017.02.797. [DOI] [PubMed] [Google Scholar]
- 15.Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. 2019;53:212–223. doi: 10.1016/j.cellsig.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Mu W, Xu B, Ren J, Wahafu T, Wuermanbieke S, Ma H, Gao H, Liu Y, Zhang K, Amat A, Cao L. Artesunate, an anti-malaria agent, attenuates experimental osteoarthritis by inhibiting bone resorption and CD31hi Emcn hi vessel formation in subchondral bone. Front Pharmacol. 2019;10:685. doi: 10.3389/fphar.2019.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Wang S, Zhan S. The prevalence of symptomatic knee osteoarthritis in China: results from the china health and retirement longitudinal study. Arthritis Rheumatol. 2016;68:648–653. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 18.Salmon JH, Rat AC, Sellam J. Economic impact of lower-limb osteoarthritis worldwide: a systematic review of cost-of-illness studies. Osteoarthritis Cartilage. 2016;24:1500–1508. doi: 10.1016/j.joca.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Javaheri B, Caetano-Silva SP, Kanakis I, Bou-Gharios G, Pitsillides AA. The chondro-osseous continuum: is it possible to unlock the potential assigned within. Front Bioeng Biotechnol. 2018;6:28. doi: 10.3389/fbioe.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MK, Wang LC, Hu FL. Value of serum matrix metalloproteinase 3 in the assessment of early rheumatoid arthritis. Beijing Da Xue Xue Bao Yi Xue Ban. 2018;50:981–985. [PubMed] [Google Scholar]
- 21.Teja KV, Ramesh S, Priya V. Regulation of matrix metalloproteinase-3 gene expression in inflammation: a molecular study. J Conserv Dent. 2018;21:592–596. doi: 10.4103/JCD.JCD_154_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


