Abstract
Glioblastoma is one of the most common malignant tumors in the central nervous system. Due to the high plasticity, heterogeneity and complexity of the tumor microenvironment, these tumors are resistant to almost all therapeutic strategies when they reach an advanced stage. Along with being a unique and effective way to kill cancer cells, tumor-treating fields (TTFields) has emerged as a breakthrough among glioblastoma therapies since the advent of temozolomide (TMZ), and the combination of these treatments has gradually been promoted and applied in the clinic. The combination of TTFields with other therapies is particularly suitable for this type of “cold” tumors and has attracted a large amount of attention from clinicians and researchers in the era of cancer cocktail therapy. Here, we introduced the current treatment regimen for glioblastoma, highlighting the unique advantages of TTFields in the treatment of glioblastoma. Then, we summarized current glioblastoma clinical trials that combine TTFields and other therapies. In addition, the main and potential mechanisms of TTFields were introduced to further understand the rationale for each combination therapy. Finally, we focused on the most advanced technologies applied in glioblastoma research and treatment and the prospect of their combination with TTFields. This review provides a unique overview of glioblastoma treatment.
Keywords: Glioblastoma, tumor-treating fields, cocktail therapy, clinical application, basic research
Introduction
Glioblastoma (GBM; grade IV glioma) is one of the most common and aggressive types of primary malignant brain tumors in adults [1]. Even though various treatments have been widely applied (Figure 1), the prognosis remains poor, with a median overall survival of 14-17 months. The poor therapeutic effect is mainly attributed to several causes, including high tumor invasiveness and cellular heterogeneity, which lead to incomplete surgical resection and monodrug resistance [2]. In addition, the existence of glioma stem cells (GSCs) and the immunosuppressive tumor microenvironment in situ and in the peripheral blood also worsen the prognosis of GBM patients [3,4]. In addition, the blood-brain barrier (BBB) reduces therapeutic efficacy. This physical barrier can hinder drug delivery, and the antitumor effect is greatly weakened [5]. The above reasons cause GBM to be characterized as a “cold” tumor. Recently, tumor cocktail therapy has become a popular concept for cancer treatment and is especially suitable for GBM because it mainly acts through the combination of a variety of drugs to inhibit tumor growth at multiple, such as combining nano- or immunotherapy drugs to target the abnormal tumor microenvironment (TME) and prevent immune escape or cancer cell growth to the greatest extent. In a broad sense, it can also be described as a combination of multiple therapeutic regimens, and each treatment has a unique mode of action or mechanism. Since 2005, maximal safe surgical resection following radiotherapy, concurrent temozolomide (TMZ) and adjuvant TMZ chemotherapy have become accepted as the standard treatment strategy for GBM [6]. With the development of a number of clinical trials and basic research, targeted therapy represented by bevacizumab and immunotherapy, including PD-1/PD-L1 and CAR-T cells, have also become popular in clinical treatment. However, no treatments have provided revolutionary advances for the treatment of glioblastoma after the advent of TMZ until the advent of TTFields. The Food and Drug Administration (FDA) approved TTFields for the adjuvant treatment of recurrent and primary GBMs in 2011 and 2015, respectively. More recently, the National Comprehensive Cancer Network (NCCN) guidelines recommend the TTFields strategy for glioblastoma and listed it as having category 1 evidence. In contrast to the traditional antitumor mechanism, TTFields can inhibit the mitosis of tumor cells by changing the intracellular electric field, which may effectively overcome chemoradiotherapy resistance and sensitize several moderately effective therapies, and it is expected to bring greater hope to GBM patients. As research advances, TTFields combined with chemoradiotherapy has become considered more effective than radiotherapy and chemotherapy alone, and this has been confirmed in many clinical trials [7]. Currently, many other existing therapies are becoming more effective when combined with TTFields. Therefore, it is necessary to summarize the clinical trials based on TTFields therapy in GBM, together with potential mechanisms of TTFields to further understand the rationality of the corresponding therapeutic combinations. Additionally, also it is essential to propose their combination with current advanced treatment or diagnosis technologies.
Figure 1.
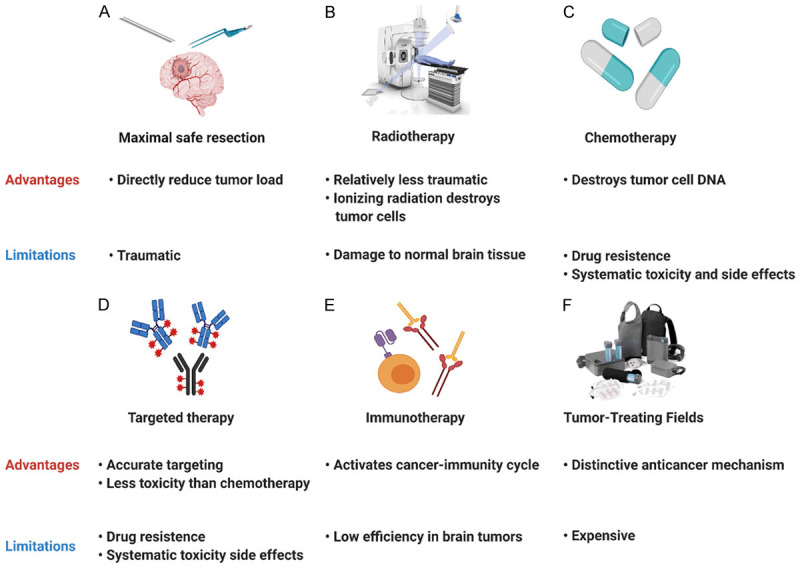
Advantages and disadvantages of current clinical therapies for glioblastoma. A. Surgical resection; B. Radiotherapy; C. Chemotherapy; D. Targeted therapy; E. Immunotherapy; F. Tumor-treating fields.
Current common treatment strategy in glioblastoma
Traditional treatment
Surgical resection
Maximal safe surgical resection is the foundation of GBM treatment, as it can not only clarify the tumor pathology but also reduce the tumor load. It also helps to improve the curative effect of postoperative adjuvant therapy. The application of advanced technology and equipment makes surgery less invasive, such as intraoperative MRI, neuronavigation systems, real-time ultrasound-MRI multimodal fusion virtual navigation systems (UMNSs), awake craniotomy with motor and speech mapping through intraoperative cortical electrodes, electrophysiological monitoring and fluorescence-guided resection with 5-aminolevulinic acid (5-ALA) [8-11]. Surgeons can accurately identify the boundary of the tumor and achieve maximum safe resection. However, due to the high invasiveness and inevitable residual tumor, simple surgical resection is not sufficient, and patients without postoperative adjuvant therapy may suffer from tumor recurrence after a short period. Postoperative adjuvant therapy can target residual tumor cells or destroy the environment needed for recurrence to delay recurrence. Moreover, a rational combination of postoperative adjuvant therapy based on individualized tumor genetic characteristics can reduce the toxicity and side effects of treatment and inhibit tumor progression to the greatest possible extent.
Chemotherapy
Chemotherapy is one of the most critical preoperative or postoperative adjuvant therapies for cancers. In glioblastoma, high blood-brain barrier permeability and low toxicity or side effects had caused TMZ to become the most widely used chemotherapeutic drug, and its concurrent use with RT for at least 6 cycles has become a standard adjuvant therapy for GBM patients. In recent years, lomustine has shown a powerful antiglioblastoma effect in several clinical trials [12]. The 2020 central nervous system tumors NCCN guidelines also recommended lomustine as a treatment for GBM. Although chemotherapy plays a critical role in killing cancer cells, it has little effect on the tumor immune microenvironment (TIME), which is closely related to recurrence. Therefore, we should focus on cancer cells as well as the TIME and explore drugs that target immune cells and key tumor-promoting molecules or pathways.
Radiotherapy
Radiotherapy (RT) can produce ionizing radiation and damage the DNA of cancer cells, thus controlling local tumor progression and delaying tumor recurrence after surgery. The standard radiotherapy regimen for GBM in adults is 60 Gy divided equally into 30 fractions after surgery. In elderly GBM patients, hypofractionation with a 45-Gy (> 2 Gy fractions) dose is also recommended. In addition to whole-brain radiotherapy, stereotactic radiotherapy and gamma knife are approved by the FDA [13]. More recently, FLASH radiotherapy, ultrahigh dose rate radiation, has exhibited considerable potential and is expected to produce the same radiation effect and reduce radiation-induced toxicities [14]. Tumor molecular pathology plays an important role in determining the mode of radiotherapy and affects the efficacy of radiotherapy. For anaplastic gliomas with 1p19q codeletion, the NCCN guidelines recommend combined standard RT, while for those without 1p19q codeletion and a poor KPS score (< 60), combined hypofractionated RT is preferred. GBM patients with unmethylated or indeterminate MGMT promoters may consider RT alone. Patients with IDH1/2 gene mutations are more sensitive to radiation than those with wild-type IDH. In addition, radiosensitizers, including poly-(ADP-ribose)-DNA polymerase (PARP) inhibitors, DNA-PK inhibitors, and ATM/ATR inhibitors, can also impede DNA repair pathways and enhance the efficacy of radiation [15]. Although progress has been made, radiotherapy resistance should not be ignored. It is especially obvious for GSCs, which may be the source of recurrence. Therefore, it is necessary to combine radiotherapy with antineoplastic drugs or other treatment techniques.
Immunotherapy and targeted therapy
Immunotherapy has achieved favorable therapeutic effects in many solid tumors, which has triggered unprecedented research on this treatment for GBM [16]. Immunotherapy mainly kills cancer cells by activating cytotoxic T lymphocytes (CTLs) or increasing exogenous CTLs to target cancer cells directly. There are four main types of immunotherapies for GBM, including immune checkpoint inhibitors (PD1/PDL1, and CTLA-4), CAR-T cells (EGFRvIII and IL-13Rα2), vaccine therapy (DC/peptide vaccines) and oncolytic viruses. More recently, CAR-NK immunotherapy has also been reported. However, the treatment outcomes of immunotherapy in GBM are not as favorable as those in other malignancies because of the suppressive TIME [17]. Therefore, reversing this unique characteristic is key to achieving favorable immunotherapeutic effects. In addition, targeted therapies are also novel therapeutic regimens that have achieved promising curative effects in glioblastoma, especially for recurrent patients. The most commonly used targeted drug is bevacizumab (Bev), which is a monotarget antagonist of VEGF-A that controls abnormal tumor angiogenesis to some extent. However, due to high inter- and intracellular heterogeneity, single targeted therapies have a short therapeutic effect duration, and cancer cells soon achieve immune escape [18]. Thus, promoting the normalization of the anomalous TIME with simultaneous multitarget targeted therapy (also called cocktail therapy) may be a future treatment direction.
Tumor-treating fields
TTFields is a unique treatment modality that utilizes alternating electric fields to deliver therapy. By acting on tubulin in proliferating cancer cells, it interferes with mitosis, causing the apoptosis of affected cancer cells and inhibiting tumor growth. It may also effectively reverse the issues of a suppressive TIME and drug resistance. A number of clinical trials have shown that when TTFields is combined with surgical resection and chemoradiotherapy, GBM patients can achieve a better prognosis than with surgery and chemoradiotherapy. Moreover, TTFields has been recommended as a treatment for GBM in the NCCN guidelines since 2016 and was upgraded to a category 1 recommendation in 2018. Therefore, TTFields is a reasonable supplement to GBM surgery and chemoradiotherapy. Traditional therapy combined with TTFields therapy may kill tumor cells via multiple mechanisms to maximize the antitumor benefits.
Developments in TTFields-based cocktail therapy in GBM
TTFields-based cocktail therapies have shown special promise in GBM therapy in many studies. Furthermore, preclinical studies and clinical trials are currently advancing the combination of TTFields with chemoradiotherapy, targeted therapy, immune therapy, small molecular inhibitors, skull remodeling surgery (SR surgery) and even two or more of these therapies simultaneously (Table 1).
Table 1.
A summary of preclinical studies and clinical trials on TTFields-based cocktail therapies
| Type of Study | Authors or NCT Number | Disease | Intervention | Outcomes | Enrollment | Completion Date |
|---|---|---|---|---|---|---|
| Basic Research | Kirson et al. | GBM | Drug: Temozolomide | TMZ efficacy and sensitivity were increased by two orders of magnitude with adjuvant TTFields | U-118-MG | January 2009 [20] |
| Procedure: TTFields | ||||||
| Basic Research | Clark et al. | GBM | Drug: Temozolomide | Combination of TMZ and TTFields performed well regardless of the MGMT promoter status | GSCs | February 2017 [21] |
| Procedure: TTFields | ||||||
| Basic Research | Jo et al. | GBM | Drug: Sorafenib | Sorafenib sensitized glioblastoma cells to TTFields | U373/U87-MG | November 2018 [30] |
| Procedure: TTFields | ||||||
| Basic Research | Groves et al. | GBM | Drug: Cytostatic Agents | TTFields in combination with cytostatic agents led to enhanced inhibitory effect on glioma cells | U-118-MG/U87-MG | November 2016 [32] |
| Procedure: TTFields | ||||||
| Basic Research | Kessler et al. | GBM | Drug: MPS1-IN-3 | Mitotic checkpoint inhibition augmented effects of TTFields on glioblastoma cells | U-87MG/GaMG | July 2018 [33] |
| Procedure: TTFields | ||||||
| Basic Research | Chang et al. | GBM | Drug: Withaferin A | TTFields and withaferin A synergistically inhibited proliferation in glioblastoma | GBM2/GBM39/U87-MG | September 2017 [35] |
| Procedure: TTFields | ||||||
| Case Report | Stein et al. | ndGBM | Drug: Temozolomide | Complete radiological response was observed 1 year after the end of radiation therapy | 1 patient | April 2020 [27] |
| Radiation | ||||||
| Procedure: TTFields | ||||||
| Case Report | Meletath et al. | ndGBM | Drug: Dabrafenib | TTFields in combination with dabrafenib yielded a remarkable clinical and radiologic response in a BRAF V600-mutated high-grade glioma patient | 1 patient | November 2016 [31] |
| Procedure: TTFields | ||||||
| Case Report | Elzinga et al. | rGBM | Drug: Bevacizumab | The GBM cyst and most of the cerebral edema in the surrounding brain were reduced after 6 cycles of add-on TTFields therapy | 1 patient | April 2014 [28] |
| Procedure: TTFields | ||||||
| Retrospective Study | Lu et al. | rGBM | Drug: Bevacizumab | The triple-drug regimen demonstrated efficacy with no unexpected toxicities | 48 patients | January 2019 [29] |
| Drug: Irinotecan | ||||||
| Drug: Temozolomide | ||||||
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT04397679 | ndGBM | Drug: Temozolomide | Ongoing | 10 patients | September 2022 |
| Drug: Chloroquine | ||||||
| Radiation | ||||||
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT01925573 | rGBM | Drug: Bevacizumab | Ongoing | 7 patients | December 2026 |
| Radiation | ||||||
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT03477110 | GBM | Drug: Temozolomide | Ongoing | 35 patients | September 2021 |
| Radiation | ||||||
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT03705351 | GBM | Drug: Temozolomide | Ongoing | 30 patients | November 2025 |
| Radiation | ||||||
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT02903069 | ndGBM | Drug: Marizomib | Ongoing | 66 patients | October 2020 |
| Drug: Temozolomide | ||||||
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT02893137 | rGBM | Procedure: SR surgery | Ongoing | 15 patients | May 2019 |
| Procedure: TTFields | ||||||
| Phase 1 Clinical Trial | NCT03223103 | GBM | Drug: Poly-ICLC | Ongoing | 20 patients | May 2023 |
| Procedure: TTFields | ||||||
| Biological: Peptides | ||||||
| Phase 2 Clinical Trial | NOA09/CeTeG | ndGBM | Drug: Lomustine | TTFields/lomustine/temozolomide is safe and feasible | 16 patients | March 2020 [23] |
| Drug: Temozolomide | PFS: 20 months | |||||
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT03780569 | GBM | Drug: Temozolomide | PFS: 8.9 months | 10 patients | January 2019 [26] |
| Radiation | Skin toxicity was reported in eight (80%) patients | |||||
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT01894061 | rGBM | Drug: Bevacizumab | Ongoing | 25 patients | July 2019 |
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT02743078 | rGBM | Drug: Bevacizumab | Ongoing | 3 patients | October 2019 |
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT02663271 | rGBM | Drug: Bevacizumab | Ongoing | 18 patients | March 2021 |
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT03687034 | GBM | Drug: Bevacizumab | Ongoing | 21 patients | December 2020 |
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT02343549 | ndGBM | Drug: Bevacizumab | Ongoing | 46 patients | June 2021 |
| Drug: Temozolomide | ||||||
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT03430791 | rGBM | Drug: Nivolumab | Ongoing | 60 patients | August 2021 |
| Drug: Ipilimumab | ||||||
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT03405792 | ndGBM | Drug: Temozolomide | Ongoing | 29 patients | February 2023 |
| Drug: Pembrolizumab | ||||||
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT04221503 | GBM | Drug: Niraparib | Ongoing | 30 patients | December 2025 |
| Procedure: TTFields | ||||||
| Phase 2 Clinical Trial | NCT04223999 | rGBM | Procedure: SR surgery | Ongoing | 70 patients | March 2024 |
| Procedure: TTFields | ||||||
| Phase 3 Clinical Trial | NCT00916409 | ndGBM | Drug: Temozolomide | OS: 20.5 vs 15.6 months; PFS6: 56% vs 37% | 695 patients | March 2017 [22] |
| Procedure: TTFields | No significant increase in systemic AEs with TTFields compared with TMZ alone (48 vs 44%, respectively; P = 0.58) | |||||
| Phase 3 Clinical Trial | NCT04218019 | ndGBM | Drug: Temozolomide | Ongoing | 68 patients | February 2023 |
| Radiation | ||||||
| Procedure: TTFields | ||||||
| Phase 3 Clinical Trial | NCT04471844 | GBM | Drug: Temozolomide | Ongoing | 950 patients | August 2026 |
| Radiation | ||||||
| Procedure: TTFields |
GBM: glioblastoma, ndGBM: newly diagnosed glioblastoma, rGBM: recurrent glioblastoma, MGMT: O6-methylguanine-dna methyltransferase, GSC: glioma stem cell, SR surgery: skull remodeling surgery, OS: overall survival, PFS: progression-free survival, PFS6: progression-free survival at 6 months, AE: adverse effect.
TTFields combined with chemotherapy
Chemoresistance occurs in nearly all GBM patients due to mechanisms such as DNA damage repair pathway activation, enhanced cell plasticity, and glioma stem cell (GSC) development [19]. Aiming to overcome these obstacles, Kirson et al. attempted to combine TTFields with TMZ in glioma cell lines and discovered that the effectiveness and sensitivity of TMZ could be increased by adjuvant TTFields [20]. Further, by using patient-derived GBM stem-like cells (GSCs), including MGMT-expressing and non-MGMT-expressing lines, Clark et al. verified that the combination of TMZ and TTFields functions well regardless of the MGMT promoter status [21]. EF-14, as the first phase 3 clinal trial to study the effect of combining TMZ and TTFields in GBM, also verified the additive effects of TTFields. It enrolled 695 patients, and the results showed that TTFields plus TMZ significantly improved progression-free survival while increasing the overall survival by 5 months compared with TMZ alone [22]. The updated results of EF-14 showed that adding TTFields to TMZ resulted in significantly improved 5-year overall survival compared with TMZ alone [7]. In addition to TMZ, Lazaridis et al. indicated that the combination of TTFields and lomustine was safe and feasible, and the observed survival outcomes showed potential benefits in GBM patients [23]. More recently, chloroquine, which is mainly used to treat malaria, has been researched in combination with TTFields (NCT04397679). It is likely that more drugs will be used in combination with TTFields because studies have reported that TTFields can enhance the BBB permeability of chemotherapeutic drugs.
TTFields combined with radiotherapy
Radiotherapy is an effective combination treatment with TTFields because it slows DNA damage repair [24]. To test whether TTFields and radiotherapy interfere with each other when applied synchronously, Stachelek et al. performed a study that simulated the radiation plans with TTFields on a skull model, optimized the anatomical structure of the model and studied the effect of TTFields on planning target volume (PTV). Their results demonstrated that the placement of TTFields arrays did not affect the target volume coverage of radiotherapy [25]. To date, many studies have suggested the safety and efficacy of TTFields-based cocktail therapy containing chemoradiotherapy. A pilot study that enrolled 10 patients (NCT03780569) verified the feasibility and safety of combining TTFields treatment with initial radiotherapy and TMZ therapy in newly diagnosed GBM. The results demonstrated a median PFS of 8.9 months and low-severity local dermatological complications in 80% of patients [26]. In addition, Stein et al. reported a case of complete radiological response of thalamic GBM after treatment with proton therapy followed by TMZ and TTFields [27]. Furthermore, several ongoing clinical trials are trying to determine the safety and efficacy of the combination of TTFields with chemoradiotherapy (NCT03477110, NCT04471844 and NCT03705351) and are studying the optimal timing for TTFields in combination with chemoradiotherapy (NCT04218019). These investigations are likely to encourage more efforts to develop a combination of radiotherapy and TTFields for GBM therapy with higher efficacy and fewer adverse effects.
TTFields combined with immunotherapy
Based on the mechanisms by which TTFields affects cancer immunity, many researchers are exploring the combined effects of these two therapies. Among the ongoing clinal trials, peptide vaccines and immune checkpoint inhibitors are being combined with TTFields. A phase 1 clinical trial (NCT03223103) used precision medicine in the form of a vaccine, a mutation-derived tumor antigen vaccine (MTA-based vaccine), in combination with TTFields during the maintenance phase of TMZ. The other two phase 2 clinical trials combined pembrolizumab (a PD-1 monoclonal antibody) plus TMZ for newly diagnosed GBM and nivolumab (a PD-1 monoclonal antibody) plus ipilimumab (a CTLA-4 monoclonal antibody) for recurrent GBM with TTFields (NCT03405792 and NCT03430791). The results of these clinical studies may be of great significance for clinicians for helping to design a more flexible and effective therapeutic schedule. More importantly, these combination strategies can also help researchers better understand the TME.
TTFields combined with targeted therapy
Studies have indicated that TTFields-based cocktail therapy containing targeted drugs has synergistic effects. Among them, bevacizumab is the most widely used, and many phase 2 clinical trials are trying to determine the effects of bevacizumab with TTFields in both newly developed GBMs and recurrent GBMs (NCT01894061, NCT02743078, NCT02663271, NCT03687034, and NCT02343549). To date, several clinical results have shown the efficacy of bevacizumab combined with TTFields. For example, Elzinga et al. reported that a patient with recurrent cystic GBM had an insignificant response to single-agent bevacizumab; after 6 cycles of add-on TTFields therapy, the GBM cyst and cerebral edema were significantly relieved [28]. Another retrospective study analyzed the potential effect of three drugs, including bevacizumab, irinotecan, and TMZ, plus TTFields for recurrent GBM; this study also reported an obvious improvement in PFS and OS with the combination regimen [29]. In addition, some other targeted drugs are also being explored in combination with TTFields. Yunhui et al. found that sorafenib sensitized GBM cells to TTFields. TTFields-based cocktail therapy with sorafenib accelerated apoptosis via reactive oxygen species (ROS) generation and inhibited cancer cell motility, invasiveness and angiogenesis [30]. In addition, Meletath et al. described a case in which TTFields-based cocktail therapy with dabrafenib yielded a remarkable clinical and radiologic response in a BRAF V600-mutated high-grade glioma patient [31]. All of the above results show that TTFields may strengthen the therapeutic effects of various targeted agents regardless of their targets. Further, studies are not limited to simply combining targeted drugs with TTFields, and they tested bevacizumab for treating recurrent GBM in combination with TTFields and hypofractionated stereotactic irradiation simultaneously (NCT01925573). Overall, clinical trials of targeted drug combinations with TTFields or even more therapeutic regimens simultaneously are expected to bring more hope for the treatment of primary or recurrent GBM.
TTFields combined with small molecule inhibitors
Small molecule inhibitors are often used to inhibit the function of specific enzymes or proteins that have been proven to greatly increase cancer aggressiveness. The feasibility of combining them with TTFields is also being researched. In preclinical studies, Groves et al. found that TTFields in combination with several cytostatic agents, including mefloquine, metformin, bumetanide, minocycline and ganciclovir, led to enhanced inhibitory effects on glioma cells [32]. Some inhibitors, such as MPS1-IN-3 (a mitotic checkpoint inhibitor), can also synergistically augment the effect of TTFields [33]. In terms of clinical trials, niraparib, a PARP inhibitor, was shown to effectively reduce cell viability and proliferation [34]; it can also induce DNA damage and sensitize cells to radiotherapy. A phase 2 clinical trial is trying to determine whether it can enhance the effect of TTFields in patients with GBM (NCT04221503). Recently, Chang et al. observed the synergistic anticancer effect of withaferin A and TTFields on glioma cells, which laid the foundation for the treatment of cancer with natural products [35]. Marizomib, which is a type of brain osmotic proteasome inhibitor, is being used to treat recurrent GBM with TTFields and TMZ in North America to assess the improvements in PFS and OS (NCT02903069). Therefore, molecule inhibitors combined with TTFields could help to progress future anti-GBM therapy.
TTFields combined with SR surgery
Minor craniectomy or distributed burr holes is a surgical skull remodeling approach designed for individual patients. Preclinical modeling results suggested that such procedures enhance the induced electrical field strength by up to ~100% and thereby potentially improve the clinical outcome of treated patients to a significant extent [36]. The burr holes are approximately 15 mm in diameter. Theoretically, the burr holes can increase the electric current in the tumor by funneling the electricity through the path of least resistance, since bone hinders the electricity. Previously, a phase 1 clinical trial of this combination treatment (NCT02893137) with 15 trial participants showed safety and promising results by increasing the overall survival of trial participants. Optimal TTF-2 is an ongoing trial testing a new potential treatment, skull-remodeling surgery combined with TTFields, for patients with the first recurrence of GBM. In addition, the direct implantation of electrodes into the brain or around the tumor may theoretically have better antitumor efficacy and reduce the inconvenience caused by long-term wearing, which may represent the future trend of TTFields.
Potential mechanisms of TTFields-based cocktail therapy
Alternative electric fields have mutually independent biological effects determined by the frequency, such as the membrane depolarization of low-frequency fields (under 1 kHz) and heating effect of high-frequency fields (above 1 MHz). TTFields, as intermediate-frequency (100-300 kHz) alternating electric fields, was initially thought to have no biological effect on cells. However, in later experiments, exposure to TTFields at 200 kHz was proven to exert a remarkable growth inhibitory effect on GBM cell lines but to have little impact on normal brain cells [37,38]. Therefore, TTFields is currently applied after surgery or radiation and often concomitantly with chemotherapy agents to obtain better therapeutic effects. Furthermore, a variety of potential mechanisms induced by TTFields beyond inhibiting the cell cycle have been discovered and proposed in recent years and have become the theoretical foundation for new combination therapies (Figure 2).
Figure 2.
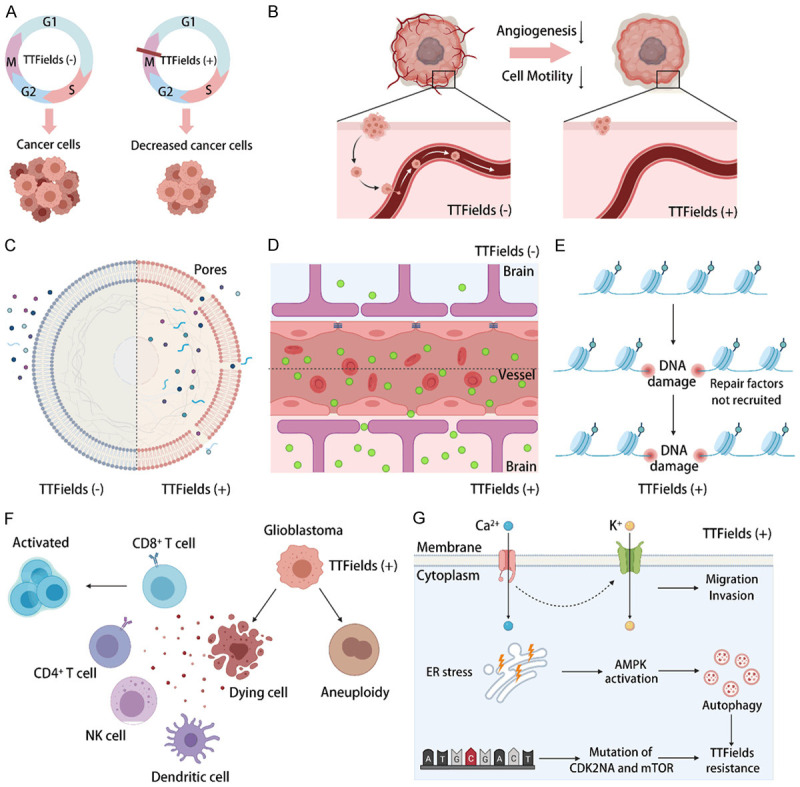
Mechanisms of TTFields. Summary of existing and potential mechanisms that have been discovered and proposed in recent years. A. Generation of cell cycle-specific effects; B. Reduction of cancer cell motility and angiogenesis; C. Increase in cancer cell membrane permeability; D. Increase in blood-brain barrier (BBB) permeability; E. Delay in DNA damage repair; F. Regulation of the anticancer immune response; G. Induction of resistance to TTFields.
Generation of cell cycle-specific effects
At the subcellular level, the delayed formation of the mitotic spindle and dielectrophoresis-induced death are two widely acknowledged mechanisms that cause cell cycle-specific effects on cancer cells. TTFields contributes to cell cycle arrest and apoptosis mainly by affecting metaphase, anaphase and telophase in mitosis. During metaphase, TTFields restrains mitotic spindle formation and the tubulin polymerization process, leading to improper chromosome segregation and the caspase-dependent apoptosis of daughter cells [37,39]. During anaphase, the malfunction of septin protein complexes in the presence of TTFields leads to a failure to stabilize the contractile apparatus, which causes aberrant mitotic exit [40]. During telophase, a higher-intensity electric field at the furrow region of daughter cells that are about to separate induces dielectrophoretic forces, compromising normal cytokinesis and leading to cell death [37]. In addition, large biological molecules such as certain proteins are dipolar particles (those with a positive and negative charge) that are responsive to electric fields; when placed within a nonuniform electric field, dipolar particles will move toward the area of a higher intensity field. During cytokinesis, two daughter cells present a cellular morphology resembling an hourglass shape and form a higher-density intracellular electric field at the furrow under TTFields exposure. This nonuniform field exerts a force on polar macromolecules and organelles, moving them toward the narrow neck and separating the newly formed daughter cells, which we refer to as dielectrophoresis [11,41].
Reduction of cancer cell motility and angiogenesis
Wound healing assays and Transwell invasion assays validated that the migration and invasion were significantly reduced in GBM cell lines treated by TTFields compared to untreated cell lines. Additionally, cell adhesion assays and cell deadhesion assays demonstrated that cell adhesion to the substrate was significantly reduced and that the cell deadhesion process took a significantly longer time with trypsinization upon exposure to TTFields. In these cases, epithelial-to-mesenchymal transition-related genes such as vimentin and E-cadherin were dysregulated, and the NF-κB, MAPK, PI3K/AKT signaling pathways were inhibited, suppressing the potential mobility of cancer cells [42,43]. In addition, endothelial tube formation assays showed that vessel numbers were decreased with exposure to TTFields. Regarding further mechanisms, researchers suggested that TTFields can suppress the expression of VEGF and HIF1α in GBM cell lines, which are two classical key molecules in angiogenesis [42]. This provides a solid theoretical basis for the superiority of TTFields combined with corresponding targeted therapy.
Increase in cancer cell membrane and blood-brain barrier (BBB) permeability
Researchers found that TTFields can increase the uptake of some membrane-penetrating reagents, such as dextran-FITC, ethidium D and 5-aminolevulinic (5-ALA), in GBM cell lines but not in normal cell lines. Scanning electron microscopy (SEM) showed that the number of membrane pores increased and the pore size increased upon TTFields exposure, leading to increased membrane permeability, and the altered membrane morphology always disappeared by 24 h after the discontinuation of TTFields [44]. Based on these findings, we can infer that the application of preoperative TTFields may help to delineate cancer boundaries and improve the surgical excision of GBM; meanwhile, TTFields increases the intracellular drug concentration and has the potential to treat drug-resistant cancer cells that overexpress ABC transporters [45]. On the other hand, TTFields upregulated the amount of chemical chromogenic agents (Evans blue and TRITC-dextran), immunoglobulin G (IgG) and gadolinium contrast agent (Gd-DTPA) in rat brains after intravenous injections. Frozen section staining results demonstrated that the vascular structure of rat brains became dispersed by the application of TTFields, and this effect was also reversible [44]. The application of TTFields increases the membrane permeability of cancer cells and the blood-brain barrier simultaneously, which has the potential to make it easier for various therapeutic drugs to enter intracranial GBM cells. Therefore, combination therapy with TTFields can increase the antitumor effect of therapeutic drugs.
Delay of DNA damage repair
A comet assay was performed in GBM cell lines for the simple evaluation of cellular DNA damage, and the results showed that the majority of the initial DNA damage was repaired within 24 h after radiotherapy, while more than 40% of the initial DNA damage remained unrepaired when TTFields was subsequently applied. TTFields decrease total ataxia telangiectasia mutated (ATM) expression and its phosphorylation, which is one of the earliest activators triggered in response to DNA double-strand breaks [46]. In addition, some other mechanisms that delay DNA damage repair have recently been verified in NSCLC cell lines, which suggested that TTFields reduces DNA double-strand break (DSB) repair capacity by downregulating BRCA1 signaling [47]. Recently, TTFields was shown to not only reduce the rejoining of radiation-induced DNA DSBs but also induce the generation of replication stress, causing DNA DSBs directly [24]. This new information suggests that using TTFields as a neoadjuvant therapy should be considered to take advantage of the vulnerabilities generated by prior or concomitant TTFields exposure.
Regulation of the anticancer immune response
TTFields-induced cell death is characterized by upregulated cell surface exposure of calreticulin and the release of HMGB1, as well as the secretion of adenosine triphosphate (ATP) by dying cells, which are all hallmarks of immunogenic cell death (ICD). Meanwhile, TTFields causes micronuclei structures to be released into the cytoplasm and activates micronuclei-dsDNA sensor complexes (AIM2 and interferon-inducible protein cGMP-AMP synthase), activating innate immunity, such as via the STING pathway and pyroptosis [48]. Thus, cancer cells under TTFields can induce an anticancer immune response, such as DC maturation and leukocyte recruitment, leading to the enhancement of the anti-PD-1 therapeutic effect [49]. However, another study showed that TTFields hindered the proliferation of anti-GBM T cells, regardless of peripheral blood-derived or GBM-infiltrated cells [50]. TTFields-induced aneuploidy has been proven to be a marker of immune evasion and to be accompanied by a reduced response to immunotherapy [51]. Although the effect of TTFields on tumor immunity is still controversial, with the promotion of preclinical and clinical trials, more potential mechanisms will be found, and a more reasonable combination will be developed in the near future.
Induction of resistance to TTFields
Cancer cells with different genetic backgrounds behave differently when treated with TTFields, which means that there are some ways to resist the anticancer effects of TTFields. To date, several resistance mechanisms have been reported. One is the activation of voltage-gated Ca2+ (Cav) Channels. TTFields has been demonstrated to evoke intracellular Ca2+ signals that may be involved in particular Cav channels expressed by GBM cells [52]. Potential downstream targets of Cav are Ca2+-activated K+ (KCa3.1) channels, which contribute to the cell migration or therapeutic resistance of GBM cells [53,54]. Cav channel activity results in a cellular stress response to TTFields, and Cav inhibition may augment TTFields effects. Another resistance mechanism is AMP-activated protein kinase (AMPK)-dependent autophagy. Autophagy was found to be upregulated in glioma cells treated with TTFields and to desensitize cells to the treatment; pathway analysis demonstrated that the TTFields-induced upregulation of autophagy was dependent on AMPK activation. Thus, combining TTFields with an autophagy inhibitor may result in a more efficient reduction in cancer development than TTFields alone [55]. Additionally, molecular alterations after TTFields therapy were detected in a recurrent GBM patient with next-generation sequencing technology. The acquisition of a widespread deletion of CDK2NA and an activating mutation in mTOR (V2006I) were found to play key roles in TTFields resistance [56]. Therefore, a rational combination therapy is expected to overcome the potential drug resistance mechanism of TTFields to achieve a greater antitumor effect.
Current challenge in tumor-treating fields
Although TTFields has broad application prospects, there are still some issues and challenges. These mainly include the following aspects. First, the mechanism of TTFields in glioma still needs to be further studied, which is not limited to tumor cells but also includes the effects of TTFields on vascular endothelial cells, stromal cells, and even the whole tumor immune microenvironment. In addition, more preclinical or clinical studies are needed to validate combination strategies with TTFields and different treatments. Furthermore, there are some side effects and economic problems in the clinical application of TTFields. For example, skin adverse events (AEs) are the most commonly reported AEs, occurring in 35% of primary and 20% of recurrent GBM patients [57]. Many patients report scalp AEs caused by the direct contact of the array with the scalp, including contact dermatitis, hyperhidrosis, dryness or itching, as well as relatively rare skin erosion/ulcers and infections [58]. The treatment measures include antibiotics, topical corticosteroids and moisturizers. It is also expected that adding a buffer protector or protective device between the array and scalp or achieving nondirect contact wearing may solve this problem. A long wearing time is an important cause of reduced patient compliance and has limited the popularization of electric fields. According to the EF-14 clinical trial, the recommended wearing time is at least 18 h a day, and longer wearing times were associated with better antitumor effects [7,59]. However, wearing it for too long greatly affects the quality of life of patients. Safely improving the working efficiency of electric field equipment may shorten the wearing time in the future. In addition, the high price also hinders the popularization of electric field therapy. The average cost of TTFields therapy is approximately 20000 US dollars per month, which places a very large economic burden on cancer patients [60]. However, with the development of technology and the reduction of equipment costs, the high treatment costs are expected to be gradually reduced.
Current advanced technologies in glioblastoma and their potential application in TTFields
In recent years, a number of advanced diagnostic and therapeutic technologies have emerged in the field of brain tumor research and therapy, such as glioma organoids, 3D bioprinting models, liquid biopsy, single-cell sequencing, spatial transcriptome, nanotechnology, and CRISP-cas9, known as “gene scissors”, whose developers won the Nobel Prize in chemistry in 2020. These advanced technologies have greatly benefited glioma treatment, and ways to effectively apply these technologies to TTFields therapy and research on GBM is worthy of further study (Figure 3).
Figure 3.
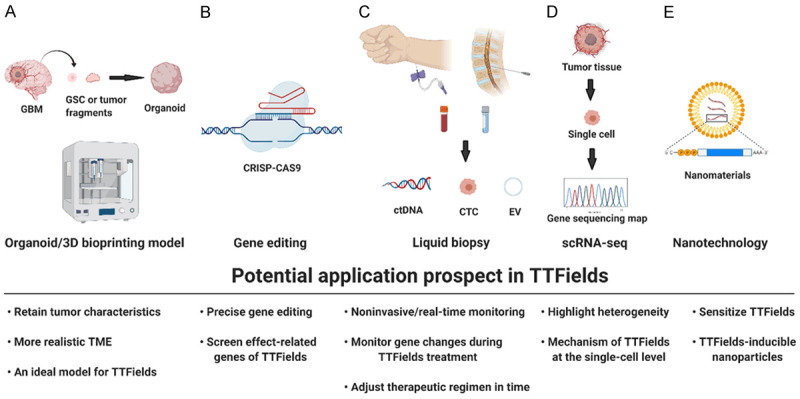
Potential application prospects of TTFields. TTFields has the potential to be widely implemented in the latest research technologies. A. Organoid/3D bioprinting models; B. Gene editing; C. Liquid biopsy; D. Single-cell RNA sequencing; E. Nanotechnology.
Preclinical research models
Human organoids are three-dimensional (3D) models that are derived from human stem cells, have the ability to self-organize and can simulate both the structure and function of primary human organs [61]. Compared with cell lines and animal models, tumor organoids are better at preserving the characteristics of primary tumors and replicate the stereoscopic tumor microenvironment (TME) [62,63]. Instead of isolating glioma stem cells, Jacob and colleagues directly cultured small tumor fragments in vitro to produce organoids [64]. This method saves a considerable amount of time, and it only takes approximately two weeks to generate GBM organoids. Moreover, these GBM organoids can solve the problem of a single cell type to some extent, but only in the early stage of culture. There are other new ways to generate glioma organoids, including genetic engineering and coculturing. The former method introduces oncogenic mutations into cerebral organoids via transposon- and CRISPR/Cas9-mediated mutagenesis [65-67]. As they contain tumor stem cells, primary tumor characteristics, a tumor hypoxic microenvironment, and the ability to develop living organism banks in a limited time, organoid-based models of malignancy may be a more effective preclinical model to evaluate the clinical efficacy and toxicity of TTFields. In addition, 3D bioprinting models are also potential 3D ex vivo tumor research models. Compared with organoids, they can effectively solve the problems of lacking blood vessels and single cell types [68,69]. Therefore, both organoid and 3D bioprinting can provide preclinical models that are more similar to parental tumors, which is essential for the translation of basic cancer research into novel therapeutic regimens for patients with brain tumors. We can use this model to more accurately explore the therapeutic effect of TTFields and its mechanism on cancer cells.
Liquid biopsy
Liquid biopsy is mainly used to diagnose or monitor tumor progression by extracting bodily fluids from patients. The main indicators include circulating tumor cells (CTCs), circulating DNA (ctDNA), extracellular vesicles (EVs), etc. [70-72]. Compared with histopathological detection, liquid biopsy differs from local tissue, which cannot reflect all the genetic characteristics of tumors due to tumor heterogeneity. In addition, due to the relatively noninvasive characteristics of liquid biopsy, it can allow multiple time point monitoring of tumor gene mutations and more rational symptomatic treatment. In 2019, Miller and his colleague detected ctDNA in the cerebrospinal fluid (CSF) of 85 preoperative glioma patients. They found that 42 (49.4%) of 85 patients had tumor-derived DNA in the CSF, which was associated with disease burden and adverse outcomes. Furthermore, the genome map of glioma in cerebrospinal fluid (CSF) contains a wide range of genetic changes and is very similar to the genome of a tumor biopsy, such as the codeletion of chromosome arms 1p and 19q (1p/19q codeletion) and mutations in the metabolic genes isocitrate dehydrogenase 1 (IDH1) or IDH2 [73]. Therefore, we can monitor the progression of tumors after surgery with the results of liquid biopsy and further study the mutation of tumor genes in the process of TTFields treatment to adjust the electric field intensity and even the time and frequency of TTFields therapy more accurately according to tumor gene mutations during clinical treatment.
Single-cell RNA sequencing (scRNA-seq)
High-throughput data analysis of the genetic characteristics of cancer cells is part of mainstream glioma research. From first-generation sequencing to the present single-cell sequencing and spatial transcriptomics analysis, revolutionary leaps have been made in the exploration of glioma gene alterations [74,75]. Single-cell RNA sequencing (scRNA-seq) has recently emerged as a vital tool for identifying and characterizing cell types, states, lineages and circuitry [76]. By combining single-cell RNA sequencing and time-of-flight mass spectrometry (TOF-MS), Sankowski discovered previously neglected transcriptional status profiles in microglia. Their transcriptional status is determined by their spatial distribution and changes with age and pathological changes in brain tumors [77]. Goswami and his colleagues performed single-cell sequencing analysis on GBM patients with poor responses to PD-1/CTLA-4 immunotherapy and found that CD73 is a specific immunotherapy target that can improve the antitumor immune response to immune checkpoint therapy in glioblastoma [78]. More recently, Pine compared the single-cell sequencing maps of glioma stem cells in different models, and the results showed that a glioblastoma cerebral organoid (GLICO) model reproduced the cell state and plasticity of the corresponding primary tumor. These results highlight the importance of the TME and tumor host cell interactions [79]. Thus, scRNA-seq can help to obtain a large number of genetic and biological characteristics of a single cell. When applied to TTFields and other new treatment studies, it can help to obtain high-throughput data of single cells after treatment and a more comprehensive understanding of tumor genetic profiles.
Nanotechnology
Nanotechnology refers to the manipulation or design of materials and structures with required characteristics within a size of 1-1000 nm [80]. Rapid progress has been made in the field of cancer treatment in recent years, especially for brain tumors, which have a natural barrier, the blood-brain barrier (BBB). Nanomaterials can assist drugs in penetrating the blood-brain barrier. In addition, nanomaterials used in drug encapsulation have the advantages of targeted transmission and specific tumor microenvironment response and release. More importantly, in preclinical studies, TTFields reversibly increased tumor cell-specific membrane permeability [44]. Recently, barium titanate nanoparticles were shown to sensitize refractory breast cancer to the effect of TTFields [81]. Therefore, the combination of nanotechnology could be immeasurable in cancer treatment.
Conclusion
TTFields is gradually being incorporated in the standardized treatment of glioblastoma. With the promotion of preclinical and clinical research, cocktail therapy based on TTFields has shown great potential and prospects for GBM treatment. The application of advanced technology will speed up the in-depth study of the mechanism of TTFields and help to develop a more rational combination therapy strategies. This novel and multidimensional treatment strategy is expected to effectively treat “cold” tumors in the near future.
Acknowledgements
We would like to thank everyone who participated in writing the text and creating figures with BioRender.com. Funding information: this work was supported by the Hainan Provincial Natural Science Foundation of China (819QN351), the National Natural Science Foundation of China (grants 81272778 and 81974390 to Dr X. Jiang), the Fundamental Research Funds for the Central Universities (grant 2020kfyXGYJ010 to Dr X. Jiang), and the Key Research and Development Projects of Hainan Province (ZDYF2019173).
Disclosure of conflict of interest
None.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:v1–v100. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, Dewitt J, Gritsch S, Perez EM, Gonzalez Castro LN, Lan X, Druck N, Rodman C, Dionne D, Kaplan A, Bertalan MS, Small J, Pelton K, Becker S, Bonal D, Nguyen QD, Servis RL, Fung JM, Mylvaganam R, Mayr L, Gojo J, Haberler C, Geyeregger R, Czech T, Slavc I, Nahed BV, Curry WT, Carter BS, Wakimoto H, Brastianos PK, Batchelor TT, Stemmer-Rachamimov A, Martinez-Lage M, Frosch MP, Stamenkovic I, Riggi N, Rheinbay E, Monje M, Rozenblatt-Rosen O, Cahill DP, Patel AP, Hunter T, Verma IM, Ligon KL, Louis DN, Regev A, Bernstein BE, Tirosh I, Suvà ML. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178:835–849. e821. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couturier CP, Ayyadhury S, Le PU, Nadaf J, Monlong J, Riva G, Allache R, Baig S, Yan X, Bourgey M, Lee C, Wang YCD, Wee Yong V, Guiot MC, Najafabadi H, Misic B, Antel J, Bourque G, Ragoussis J, Petrecca K. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat Commun. 2020;11:3406. doi: 10.1038/s41467-020-17186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abadi B, Ahmadi-Zeidabadi M, Dini L, Vergallo C. Stem cell-based therapy treating glioblastoma multiforme. Hematol Oncol Stem Cell Ther. 2020;14:1–15. doi: 10.1016/j.hemonc.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Haumann R, Videira JC, Kaspers GJL, van Vuurden DG, Hulleman E. Overview of current drug delivery methods across the blood-brain barrier for the treatment of primary brain tumors. CNS Drugs. 2020;34:1121–1131. doi: 10.1007/s40263-020-00766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unsgård G, Lindseth F. 3D ultrasound-guided resection of low-grade gliomas: principles and clinical examples. Neurosurg Focus. 2019;47:E9. doi: 10.3171/2019.9.FOCUS19605. [DOI] [PubMed] [Google Scholar]
- 9.Liang C, Li M, Gong J, Zhang B, Lin C, He H, Zhang K, Guo Y. A new application of ultrasound-magnetic resonance multimodal fusion virtual navigation in glioma surgery. Ann Transl Med. 2019;7:736. doi: 10.21037/atm.2019.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub D, Hyde J, Dogra S, Nicholson J, Kirkwood KA, Gohel P, Loftus S, Schwartz TH. Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis. J Neurosurg. 2020:1–15. doi: 10.3171/2019.12.JNS191203. [DOI] [PubMed] [Google Scholar]
- 11.Gesperger J, Lichtenegger A, Roetzer T, Salas M, Eugui P, Harper DJ, Merkle CW, Augustin M, Kiesel B, Mercea PA, Widhalm G, Baumann B, Woehrer A. Improved diagnostic imaging of brain tumors by multimodal microscopy and deep learning. Cancers (Basel) 2020;12:1806. doi: 10.3390/cancers12071806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann RD, Krex D, Grauer O, Goldbrunner R, Schnell O, Bähr O, Uhl M, Seidel C, Tabatabai G, Kowalski T, Ringel F, Schmidt-Graf F, Suchorska B, Brehmer S, Weyerbrock A, Renovanz M, Bullinger L, Galldiks N, Vajkoczy P, Misch M, Vatter H, Stuplich M, Schäfer N, Kebir S, Weller J, Schaub C, Stummer W, Tonn JC, Simon M, Keil VC, Nelles M, Urbach H, Coenen M, Wick W, Weller M, Fimmers R, Schmid M, Hattingen E, Pietsch T, Coch C, Glas M. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393:678–688. doi: 10.1016/S0140-6736(18)31791-4. [DOI] [PubMed] [Google Scholar]
- 13.Angelopoulou E, Paudel YN, Shaikh MF, Piperi C. Fractalkine (CX3CL1) signaling and neuroinflammation in Parkinson’s disease: potential clinical and therapeutic implications. Pharmacol Res. 2020;158:104930. doi: 10.1016/j.phrs.2020.104930. [DOI] [PubMed] [Google Scholar]
- 14.Harrington KJ. Ultrahigh dose-rate radiotherapy: next steps for FLASH-RT. Clin Cancer Res. 2019;25:3–5. doi: 10.1158/1078-0432.CCR-18-1796. [DOI] [PubMed] [Google Scholar]
- 15.Ott M, Kassab C, Marisetty A, Hashimoto Y, Wei J, Zamler D, Leu JS, Tomaszowski KH, Sabbagh A, Fang D, Gupta P, Priebe W, Zielinski RJ, Burks JK, Long JP, Kong LY, Fuller GN, DeGroot J, Sulman EP, Heimberger AB. Radiation with STAT3 blockade triggers dendritic cell-T cell interactions in the glioma microenvironment and therapeutic efficacy. Clin Cancer Res. 2020;26:4983–4994. doi: 10.1158/1078-0432.CCR-19-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20:12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20:1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 18.Wildes TJ, Dyson KA, Francis CP, Wummer BM, Yang C, Yegorov O, Shin D, Grippin AJ, DiVita-Dean B, Abraham RS, Pham CD, Moore G, Kuizon C, Mitchell DA, Flores CT. Immune escape after adoptive T cell therapy for malignant gliomas. Clin Cancer Res. 2020;26:5689–5700. doi: 10.1158/1078-0432.CCR-20-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hombach-Klonisch S, Mehrpour M, Shojaei S, Harlos C, Pitz M, Hamai A, Siemianowicz K, Likus W, Wiechec E, Toyota BD, Hoshyar R, Seyfoori A, Sepehri Z, Ande SR, Khadem F, Akbari M, Gorman AM, Samali A, Klonisch T, Ghavami S. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol Ther. 2018;184:13–41. doi: 10.1016/j.pharmthera.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Kirson ED, Schneiderman RS, Dbalý V, Tovarys F, Vymazal J, Itzhaki A, Mordechovich D, Gurvich Z, Shmueli E, Goldsher D, Wasserman Y, Palti Y. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields) BMC Med Phys. 2009;9:1. doi: 10.1186/1756-6649-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark PA, Gaal JT, Strebe JK, Pasch CA, Deming DA, Kuo JS, Robins HI. The effects of tumor treating fields and temozolomide in MGMT expressing and non-expressing patient-derived glioblastoma cells. J Clin Neurosci. 2017;36:120–124. doi: 10.1016/j.jocn.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 23.Lazaridis L, Schäfer N, Teuber-Hanselmann S, Blau T, Schmidt T, Oster C, Weller J, Tzaridis T, Pierscianek D, Keyvani K, Kleinschnitz C, Stuschke M, Scheffler B, Deuschl C, Sure U, Herrlinger U, Kebir S, Glas M. Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J Cancer Res Clin Oncol. 2020;146:787–792. doi: 10.1007/s00432-019-03106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karanam NK, Ding L, Aroumougame A, Story MD. Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: implications for cancer therapy. Transl Res. 2020;217:33–46. doi: 10.1016/j.trsl.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Stachelek GC, Grimm J, Moore J, Huang E, Spoleti N, Redmond KJ, Lim M, Bettegowda C, Kleinberg L. Tumor-treating field arrays do not reduce target volume coverage for glioblastoma radiation therapy. Adv Radiat Oncol. 2020;5:62–69. doi: 10.1016/j.adro.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bokstein F, Blumenthal D, Limon D, Harosh CB, Ram Z, Grossman R. Concurrent tumor treating fields (TTFields) and radiation therapy for newly diagnosed glioblastoma: a prospective safety and feasibility study. Front Oncol. 2020;10:411. doi: 10.3389/fonc.2020.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein M, Dohmen H, Wölk B, Eberle F, Kolodziej M, Acker T, Uhl E, Jensen A. Case report of complete radiological response of a thalamic glioblastoma after treatment with proton therapy followed by temozolomide and tumor-treating fields. Front Oncol. 2020;10:477. doi: 10.3389/fonc.2020.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elzinga G, Wong ET. Resolution of cystic enhancement to add-on tumor treating electric fields for recurrent glioblastoma after incomplete response to bevacizumab. Case Rep Neurol. 2014;6:109–115. doi: 10.1159/000362264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu G, Rao M, Zhu P, Liang B, El-Nazer RT, Fonkem E, Bhattacharjee MB, Zhu JJ. Triple-drug therapy with bevacizumab, irinotecan, and temozolomide plus tumor treating fields for recurrent glioblastoma: a retrospective study. Front Neurol. 2019;10:42. doi: 10.3389/fneur.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo Y, Kim EH, Sai S, Kim JS, Cho JM, Kim H, Baek JH, Kim JY, Hwang SG, Yoon M. Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int J Mol Sci. 2018;19:3684. doi: 10.3390/ijms19113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meletath SK, Pavlick D, Brennan T, Hamilton R, Chmielecki J, Elvin JA, Palma N, Ross JS, Miller VA, Stephens PJ, Snipes G, Rajaram V, Ali SM, Melguizo-Gavilanes I. Personalized treatment for a patient with a BRAF V600E mutation using dabrafenib and a tumor treatment fields device in a high-grade glioma arising from ganglioglioma. J Natl Compr Canc Netw. 2016;14:1345–1350. doi: 10.6004/jnccn.2016.0145. [DOI] [PubMed] [Google Scholar]
- 32.Groves M, Schneiderman R, Zeevi E, Voloshin T, Giladi M, Kirson E, Weinberg U. Cytostatic agents combined with tumor treating fields (TTFields) in glioma cell lines. Neuro Oncol. 2016;18:vi133. [Google Scholar]
- 33.Kessler AF, Frömbling GE, Gross F, Hahn M, Dzokou W, Ernestus RI, Löhr M, Hagemann C. Effects of tumor treating fields (TTFields) on glioblastoma cells are augmented by mitotic checkpoint inhibition. Cell Death Discov. 2018;4:12. doi: 10.1038/s41420-018-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chornenkyy Y, Agnihotri S, Yu M, Buczkowicz P, Rakopoulos P, Golbourn B, Garzia L, Siddaway R, Leung S, Rutka JT, Taylor MD, Dirks PB, Hawkins C. Poly-ADP-ribose polymerase as a therapeutic target in pediatric diffuse intrinsic pontine glioma and pediatric high-grade astrocytoma. Mol Cancer Ther. 2015;14:2560–2568. doi: 10.1158/1535-7163.MCT-15-0282. [DOI] [PubMed] [Google Scholar]
- 35.Chang E, Pohling C, Beygui N, Patel CB, Rosenberg J, Ha DH, Gambhir SS. Synergistic inhibition of glioma cell proliferation by withaferin A and tumor treating fields. J Neurooncol. 2017;134:259–268. doi: 10.1007/s11060-017-2534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikic N, Korshoej AR. Improving tumor-treating fields with skull remodeling surgery, surgery planning, and treatment evaluation with finite element methods. In: Makarov SN, Noetscher GM, Nummenmaa A, editors. Brain and Human Body Modeling 2020: Computational Human Models Presented at EMBC 2019 and the BRAIN Initiative® 2019 Meeting. Copyright 2021, The Author(s); Cham (CH): Springer; 2021. pp. 63–77. [PubMed] [Google Scholar]
- 37.Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.can-04-0083. [DOI] [PubMed] [Google Scholar]
- 38.Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S, Gurvich Z, Schneiderman R, Wasserman Y, Salzberg M, Ryffel B, Goldsher D, Dekel E, Palti Y. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giladi M, Schneiderman RS, Voloshin T, Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A, Cahal S, Shteingauz A, Chaudhry A, Kirson ED, Weinberg U, Palti Y. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:18046. doi: 10.1038/srep18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gera N, Yang A, Holtzman TS, Lee SX, Wong ET, Swanson KD. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One. 2015;10:e0125269. doi: 10.1371/journal.pone.0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci. 2013;1291:86–95. doi: 10.1111/nyas.12112. [DOI] [PubMed] [Google Scholar]
- 42.Kim EH, Song HS, Yoo SH, Yoon M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7:65125–65136. doi: 10.18632/oncotarget.11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneiderman R, Zeevi E, Voloshin T, Shteingauz A, Giladi M, Porat Y, Munster M, Kirson E, Weinberg U, Palti Y. CBIO-30. Tumor treating fields (TTFields) inhibit cancer cell migration and invasion by inducing reorganizing of the actin cytoskeleton and formation of cell adhesions. Neuro Oncol. 2017;19:vi38. [Google Scholar]
- 44.Chang E, Patel CB, Pohling C, Young C, Song J, Flores TA, Zeng Y, Joubert LM, Arami H, Natarajan A, Sinclair R, Gambhir SS. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018;4:113. doi: 10.1038/s41420-018-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneiderman RS, Shmueli E, Kirson ED, Palti Y. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010;10:229. doi: 10.1186/1471-2407-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giladi M, Munster M, Schneiderman RS, Voloshin T, Porat Y, Blat R, Zielinska-Chomej K, Hååg P, Bomzon Z, Kirson ED, Weinberg U, Viktorsson K, Lewensohn R, Palti Y. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12:206. doi: 10.1186/s13014-017-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karanam NK, Srinivasan K, Ding L, Sishc B, Saha D, Story MD. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017;8:e2711. doi: 10.1038/cddis.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen D, Thomas N, Tran DD. Abstract 3280: TTFields induces immunogenic cell death and STING pathway activation through cytoplasmic double-stranded DNA in glioblastoma cells. Cancer Res. 2019;79:3280. [Google Scholar]
- 49.Voloshin T, Kaynan N, Davidi S, Porat Y, Shteingauz A, Schneiderman RS, Zeevi E, Munster M, Blat R, Tempel Brami C, Cahal S, Itzhaki A, Giladi M, Kirson ED, Weinberg U, Kinzel A, Palti Y. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020;69:1191–1204. doi: 10.1007/s00262-020-02534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simchony H, Diamant D, Ram Z, Volovitz I. Evaluation of the compatibility of electric tumor treating fields with key anti-tumoral T-cell functions. Isr Med Assoc J. 2019;21:503. [PubMed] [Google Scholar]
- 51.Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355:eaaf8399. doi: 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catacuzzeno L, Franciolini F. Role of KCa3.1 channels in modulating Ca(2+) oscillations during glioblastoma cell migration and invasion. Int J Mol Sci. 2018;19:2970. doi: 10.3390/ijms19102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stegen B, Butz L, Klumpp L, Zips D, Dittmann K, Ruth P, Huber SM. Ca2+-activated IK K+ channel blockade radiosensitizes glioblastoma cells. Mol Cancer Res. 2015;13:1283–1295. doi: 10.1158/1541-7786.MCR-15-0075. [DOI] [PubMed] [Google Scholar]
- 55.Shteingauz A, Porat Y, Voloshin T, Schneiderman RS, Munster M, Zeevi E, Kaynan N, Gotlib K, Giladi M, Kirson ED, Weinberg U, Kinzel A, Palti Y. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to tumor treating fields (TTFields) Cell Death Dis. 2018;9:1074. doi: 10.1038/s41419-018-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robins HI, Nguyen HN, Field A, Howard S, Salamat S, Deming DA. Molecular evolution of a glioblastoma controlled with tumor treating fields and concomitant temozolomide. Front Oncol. 2018;8:451. doi: 10.3389/fonc.2018.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinberg U, Perez S, Grewal J, Kinzel A. INNV-04. Safety and adverse event profile of tumor treating fields in glioblastoma a global post-market surveillance analysis. Neuro Oncol. 2018;20:vi139. [Google Scholar]
- 58.Lacouture ME, Anadkat MJ, Ballo MT, Iwamoto F, Jeyapalan SA, La Rocca RV, Schwartz M, Serventi JN, Glas M. Prevention and management of dermatologic adverse events associated with tumor treating fields in patients with glioblastoma. Front Oncol. 2020;10:1045. doi: 10.3389/fonc.2020.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toms SA, Kim CY, Nicholas G, Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol. 2019;141:467–473. doi: 10.1007/s11060-018-03057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18:1129–1136. doi: 10.1093/neuonc/now102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ballard DH, Boyer CJ, Alexander JS. Organoids - preclinical models of human disease. N Engl J Med. 2019;380:1981–1982. doi: 10.1056/NEJMc1903253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behjati S, Huch M, van Boxtel R, Karthaus W, Wedge DC, Tamuri AU, Martincorena I, Petljak M, Alexandrov LB, Gundem G, Tarpey PS, Roerink S, Blokker J, Maddison M, Mudie L, Robinson B, Nik-Zainal S, Campbell P, Goldman N, van de Wetering M, Cuppen E, Clevers H, Stratton MR. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature. 2014;513:422–425. doi: 10.1038/nature13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin MZ, Han RR, Qiu GZ, Ju XC, Lou G, Jin WL. Organoids: an intermediate modeling platform in precision oncology. Cancer Lett. 2018;414:174–180. doi: 10.1016/j.canlet.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, Liu DA, Qian X, Petrov D, Lucas T, Chen HI, Dorsey JF, Christian KM, Binder ZA, Nasrallah M, Brem S, O’Rourke DM, Ming GL, Song H. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180:188–204. e122. doi: 10.1016/j.cell.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogawa J, Pao GM, Shokhirev MN, Verma IM. Glioblastoma model using human cerebral organoids. Cell Rep. 2018;23:1220–1229. doi: 10.1016/j.celrep.2018.03.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA. Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15:631–639. doi: 10.1038/s41592-018-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, Reich B, Cohen-Gould L, Storaska A, Nakayama Y, Schenkein E, Singhania R, Cirigliano S, Magdeldin T, Lin Y, Nanjangud G, Chadalavada K, Pisapia D, Liston C, Fine HA. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 2019;26:3203–3211. e3205. doi: 10.1016/j.celrep.2019.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yi HG, Jeong YH, Kim Y, Choi YJ, Moon HE, Park SH, Kang KS, Bae M, Jang J, Youn H, Paek SH, Cho DW. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat Biomed Eng. 2019;3:509–519. doi: 10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- 69.Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater. 2017;16:303–308. doi: 10.1038/nmat4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lucero R, Zappulli V, Sammarco A, Murillo OD, Cheah PS, Srinivasan S, Tai E, Ting DT, Wei Z, Roth ME, Laurent LC, Krichevsky AM, Breakefield XO, Milosavljevic A. Glioma-derived miRNA-containing extracellular vesicles induce angiogenesis by reprogramming brain endothelial cells. Cell Rep. 2020;30:2065–2074. e2064. doi: 10.1016/j.celrep.2020.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Indira Chandran V, Welinder C, Månsson AS, Offer S, Freyhult E, Pernemalm M, Lund SM, Pedersen S, Lehtiö J, Marko-Varga G, Johansson MC, Englund E, Sundgren PC, Belting M. Ultrasensitive immunoprofiling of plasma extracellular vesicles identifies syndecan-1 as a potential tool for minimally invasive diagnosis of glioma. Clin Cancer Res. 2019;25:3115–3127. doi: 10.1158/1078-0432.CCR-18-2946. [DOI] [PubMed] [Google Scholar]
- 72.Zachariah MA, Oliveira-Costa JP, Carter BS, Stott SL, Nahed BV. Blood-based biomarkers for the diagnosis and monitoring of gliomas. Neuro Oncol. 2018;20:1155–1161. doi: 10.1093/neuonc/noy074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N, Zheng Y, Skakodub A, Mehta SA, Campos C, Hsieh WY, Selcuklu SD, Ling L, Meng F, Jing X, Samoila A, Bale TA, Tsui DWY, Grommes C, Viale A, Souweidane MM, Tabar V, Brennan CW, Reiner AS, Rosenblum M, Panageas KS, DeAngelis LM, Young RJ, Berger MF, Mellinghoff IK. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–658. doi: 10.1038/s41586-019-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tirosh I, Suvà ML. Dissecting human gliomas by single-cell RNA sequencing. Neuro Oncol. 2018;20:37–43. doi: 10.1093/neuonc/nox126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venteicher AS, Tirosh I, Hebert C, Yizhak K, Neftel C, Filbin MG, Hovestadt V, Escalante LE, Shaw ML, Rodman C, Gillespie SM, Dionne D, Luo CC, Ravichandran H, Mylvaganam R, Mount C, Onozato ML, Nahed BV, Wakimoto H, Curry WT, Iafrate AJ, Rivera MN, Frosch MP, Golub TR, Brastianos PK, Getz G, Patel AP, Monje M, Cahill DP, Rozenblatt-Rosen O, Louis DN, Bernstein BE, Regev A, Suvà ML. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355:eaai8478. doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanay A, Regev A. Scaling single-cell genomics from phenomenology to mechanism. Nature. 2017;541:331–338. doi: 10.1038/nature21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sankowski R, Böttcher C, Masuda T, Geirsdottir L, Sagar , Sindram E, Seredenina T, Muhs A, Scheiwe C, Shah MJ, Heiland DH, Schnell O, Grün D, Priller J, Prinz M. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci. 2019;22:2098–2110. doi: 10.1038/s41593-019-0532-y. [DOI] [PubMed] [Google Scholar]
- 78.Goswami S, Walle T, Cornish AE, Basu S, Anandhan S, Fernandez I, Vence L, Blando J, Zhao H, Yadav SS, Ott M, Kong LY, Heimberger AB, de Groot J, Sepesi B, Overman M, Kopetz S, Allison JP, Pe’er D, Sharma P. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat Med. 2020;26:39–46. doi: 10.1038/s41591-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pine AR, Cirigliano SM, Nicholson JG, Hu Y, Linkous A, Miyaguchi K, Edwards L, Singhania R, Schwartz TH, Ramakrishna R, Pisapia DJ, Snuderl M, Elemento O, Fine HA. Tumor microenvironment is critical for the maintenance of cellular states found in primary glioblastomas. Cancer Discov. 2020;10:964–979. doi: 10.1158/2159-8290.CD-20-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X, Yang H, Yang W, Chen X, Gao J, Gong X, Wang H, Duan Y, Wei D, Chang J. Nanoparticle-based diagnostic and therapeutic systems for brain tumors. J Mater Chem B. 2019;7:4734–4750. doi: 10.1039/c9tb00860h. [DOI] [PubMed] [Google Scholar]
- 81.Yoon YN, Lee DS, Park HJ, Kim JS. Barium titanate nanoparticles sensitise treatment-resistant breast cancer cells to the antitumor action of tumour-treating fields. Sci Rep. 2020;10:2560. doi: 10.1038/s41598-020-59445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


