Abstract
Prostate cancer (PCa) is one of the leading causes of deaths in men globally. This is a heterogeneous and complex disease that urgently warrants further insight into its pathology. Developed countries have thus far the highest PCa incidence rates, with comparatively low mortality rates. Even though PCa in the Asian population seems to have high incidence and mortality rates, the African countries are emerging as the focal center for this disease. It has also been reported that the Sub-Saharan (SSA) countries have both the highest incidence and mortality rates. To date, few studies have reported the link between PCa and African populations. Adequate evidence is still missing to fully comprehend this relationship. While it has been brought to attention that racial, geographical and socioeconomic status are contributing factors, men of African descent across the globe, irrespective of their geographical position have higher PCa incidence and mortality rates compared to their white counterparts. To date, hormone therapy is the mainstay treatment of PCa, while the dysregulation of androgen receptor (AR) signaling is a hallmark of PCa. One of the emerging problems with this therapeutic approach is resistance to antiandrogens, and that AR splice isoforms implicated in the progression of PCa lack the therapeutic ligand-binding domain (LBD) target. AR splice variants targeted therapy is emerging and in clinical trials. Leveraging PCa transcriptomics is key towards PCa precision medicine. The aim of this review is to outline the PCa epidemiology globally and in Africa, PCa associated risk factors, discuss AR signaling and PCa mechanisms, the role of dysregulated splicing in PCa as novel prognostic indicators and therapeutic targets.
Keywords: Prostate cancer (PCa), castrate resistance prostate cancer (CRPC), androgen receptor (AR), splice variants, hormone therapy, precision medicine, prostate cancer disparities
Introduction
Prostate cancer (PCa) is the second most common cancer in men globally, following lung cancer [1]. Even with high survival rates, PCa with frequent metastasis is common, leading to androgen-independent PCa that is resistant to standard therapy, hence castrate resistance PCa (CRPC) cells [2]. The prostate is a male reproductive gland, secreting the prostatic fluid important in the sperm nourishment and transport. Androgen hormonal signalling plays a fundamental role in normal prostate gland development and function. In humans, there are two main androgens-testosterone and dihydrotestosterone (DHT). Both the human androgens bind to the androgen receptor (AR). AR, a member of the nuclear receptor family, is an androgen dependent transcriptional activator. Dysregulation in AR signalling has been implicated in PCa development and progression [3,4]. Clinically, PCa is a heterogeneous disease. Some patients will present with localized disease with no signs of progression while others show aggressive disease with progression and metastasis. Developmental stages of PCa include intraepithelial neoplasia, adenocarcinoma androgen-dependent and castration-resistant or adenocarcinoma androgen-independent phases [4-6]. Men of 60 years and above, and men of African descent are at an increased risk of developing PCa, hence race, family history and age are the main PCa associated key risk factors [7]. Interestingly, men with BRCA1 and 2 and Lynch Syndrome mutations are also reported to be at an increased risk of PCa [8,9].
Prostate cancer shows a malignant neoplasm of the prostate. Many of these malignant neoplasms are carcinomas and are of epithelial origin and differentiation. There are also uncommon non-epithelial neoplasms such as the malignant mesenchymal neoplasms-the sarcomas and the lymphomas [5,10]. The Gleason grading system is the most commonly used histologic grading in PCa. This grading system is entirely based on the architectural pattern of PCa [11-16]. The histological patterns are grouped into five grades, depending on the Gleason score. This Gleason score can range from 2 to 10 by adding the primary and secondary grade patterns. The Gleason score is proportional to the cancer aggressiveness, invasion, metastasis, poor prognosis, lower survival rates and increased mortality. All primary PCa adenocarcinomas, with an exception of hormonal or radiation therapy cases, should be provided with a Gleason grade [17-19]. Compared to other cancer types, PCa presents lower mutation rates and few chromosomal gains or losses. Instead, the broad transcriptomic landscape accompanied by an active AR signaling play a role in PCa development and progression.
It has also been reported that black men in the developed and urban world show lower PCa mortality rates compared to rural men. While intra-racial and inter-and-intracultural, with other cofactors such as religion, tradition and education play a significant role in the PCa patient outcome amongst black men, it cannot be ignored that given equal opportunities such as equal access to better health facilities, increased PCa incidence and mortality rates are still evident in the black population. The sub-Saharan region is emerging as PCa hotspot. Hormonal signal transduction plays an important role in the development of the prostate. The dysregulation of AR signalling is key to the development and prognosis of PCa. Emerging in vitro reports also implicate AR ablation therapy as contributing to PCa progression. Furthermore, the dysregulation of various components of the splicing machinery that includes 7 spliceosome components (SCs) and 19 splicing factors (SFs) in poor PCa prognosis has been reported. It is evident that the knowledge gap to decipher PCa mechanisms is broad. There is therefore an urgent need to develop PCa diagnostic, prognostic and therapeutic targets. Alternative regimens are urgently needed to combat this disease and targeting the transcriptome landscape of PCa, particularly the recurrent PCa, also known as castration resistance PCa (CRPC) is a promising tool. Precision medicine holds better prognostic and therapeutic potential in the fight against PCa [20-22]. It has been reported that African American men show higher incidence and mortality rates of 1.6 fold and 2.4 fold than European Americans. Even with the calibration of socioeconomic and other non-genetic contributing factors, African American men still show greater recurrence and mortality rates [5]. This suggests the role of biological factors rather than external factors in these observed PCa disparities [23]. In the recent years, the PCa patient outcome has improved by the development of novel drugs that target AR signaling. The latest PCa drug approvals include abiraterone acetate, enzalutamide, targeting the AR signaling. Due to the resistance in the first generation of AR antagonists, there are ongoing clinical trials for second-generation AR antagonists including antagonists enzalutamide, apalutamide and darolutamide [24]. The use of the omics technologies to improve PCa patient outcome is also on the rise. Furthermore, the interest for PCa stratification and selection of treatment using genetic testing is growing. For example, patients with germline mutations in DNA damage response genes such as BRCA2 are at an increased risk of developing PCa and metastatic disease [5,8]. Although genetic testing for PCa is not yet part of routine testing in many parts of the world including Africa, this testing approach may be useful in the early determination of the risk of disease progression. The aim of this review is to outline: the PCa epidemiology globally and in Africa, PCa associated risk factors, discuss AR signalling and PCa mechanisms, the role of aberrant splicing events in PCa as novel prognostic indicators and therapeutic targets.
Epidemiology and risk factors
Prostate cancer is the second leading diagnosed cancer in men worldwide, following lung cancer. Based on the 2018 GLOBOCAN stats, Australia/New Zealand has the highest incidence rates of 87.6, accompanied by lowest mortality of 6.9. With Australia and New Zealand having incidence rates of 85.6 and 90.8 per 100000, respectively. The low mortality rates of this geographic area are due to the mortality rate in Australia and New Zealand only being around 10 per 100000. The other countries in this area have mortality rates lower than 7 per 100000.
This is followed by the geographical area with the second highest incidence rate, Northern Europe. This area includes countries with extremely high incidence rates such as Norway (ASR 106.3) and Sweden (ASR 103). In terms of mortality, these countries have a relatively high mortality rate with Norway and Sweden having a mortality rate of 16 per 100000 and 15 per 100000, respectively. The countries with the highest mortality rates in this area are Estonia and Latvia with mortality rates of 21.8 and 21 respectively. The area with the third highest incidence rate is Western Europe. The countries in this geographic area with the highest incidence rates are Ireland and France with incidence rates of 132 and 99 per 100000 respectively. In terms of mortality rates, the countries of this region have relatively low mortality rates with only that of Britain (12.7 per 100000), Ireland (11.4 per 100000) and Portugal (10.5 per 100000) exceeding mortality rates of 10 per 100000.
The area with the fourth highest incidence rate only consists of three countries Canada, USA and Mexico. The USA has the highest incidence rate of 75.7 per 100000. Canada and Mexico have comparatively low incidence rates of 38.2 and 41.6 per 100000, respectively. The mortality rates for these countries are all below 10 per 100000 individuals. Although these areas all have high incidence rates but have low mortality rates. However, the area with the fifth highest incidence rate, the Caribbean, has a relatively high mortality rate of 19.5. This is due to countries such as Martinique with an incidence rate of 158 and mortality rate of 18.7 per 100000 individuals. Barbados also has a high incidence rate of 129.3 and a mortality rate of 48 per 100000 individuals.
While most of the countries in the developed areas have higher incidence rates but lower mortality rates. However, some of the less developed regions have lower incidence rates but high mortality rates. These include areas such as Micronesia/Polynesia with an incidence rate 48.9 ad a mortality rate of 48.5 per 100000 individuals. This small difference between incidence and mortality rates in this region, indicate that prostate cancer patients have a poor chance of surviving. Another area with poor survival rates is Central and Eastern Europe. Other geographic areas with very poor survival rates include Western Asia, South East Asia, Eastern Asia and Northern Africa. (Figure 1A-C) [25].
Figure 1.
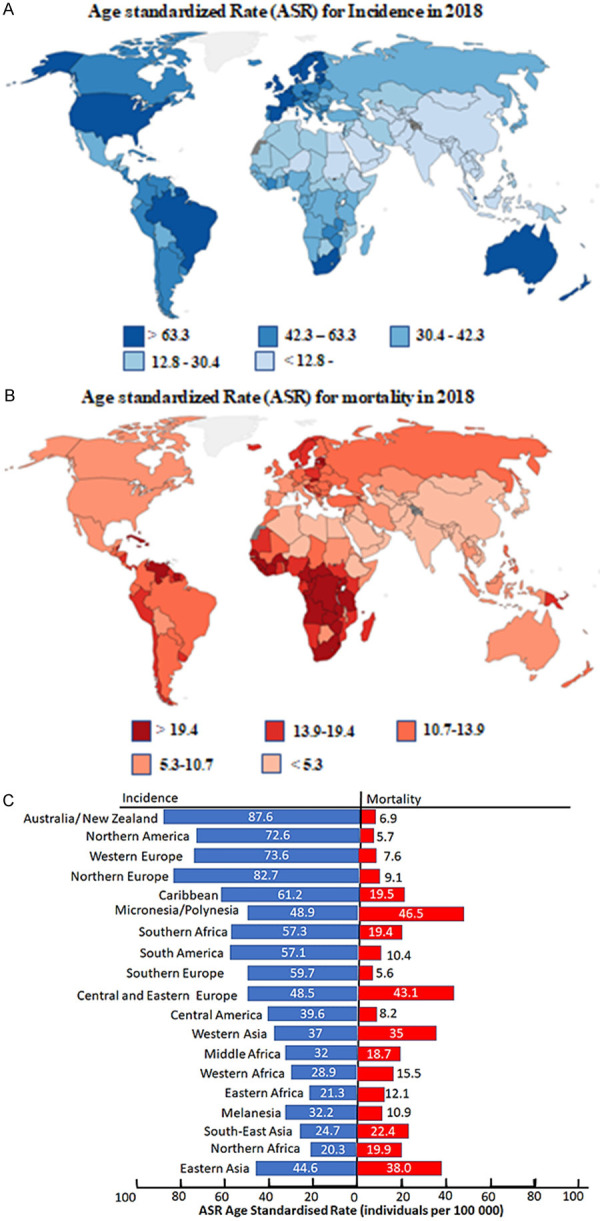
Age standardized rates (ASR) (per 100000 men) incidence and mortality for prostate cancer. (A) Worldwide age standardized rate (ASR) for incidence and for (B) mortality. (C) Incidence and mortality rates based on geographical location [25].
Age and family history are the main prostate cancer risk factors and research shows that African men have higher incidence and mortality rates of prostate cancer when compared to their white or Asian counterparts [17,26,27]. PCa age incidence rates increase significantly from 50 years and is highest in men of 90 years and above [2]. With regards to the family history risk factor, it is suggested that a man with a primary degree family member with a history of PCa, e.g. a father or a brother, has ~2.5 times higher risk in his life of being diagnosed with prostate cancer. This relative risk increases in men with more than one primary family members diagnosed with prostate cancer [28,29]. BRCA1 germline mutations and Lynch syndrome have also been reported as risk factors to PCa [8,30].
Prostate risk in the african population
Reasons for the large variation of prostate cancer in blacks within the African continent are unclear. However, these may be due to differences in medical care access, registry quality, including completeness of case ascertainment and estimates of populations at risk, screening practices, geographical location as well as lifestyle factors in subpopulations [31-33]. It is also noteworthy that improved health care systems and better reporting of cases may contribute to the rising rates in Africa [32,34]. However, it is also possible that changes in lifestyle including diet due to recent increased westernization in Africa will also play a role. It has been reported that the SSA has the highest PCa incidence rates [35]. In this region, the Southern Bantu populations represent the highest inter-and-intra population diversity in the world [36,37]. It has also been reported that South African black men are likely to present with PCa 5 years later than Americans. In addition, black men from rural parts of South Africa are more likely to present with PCa 3 years later than the one from the urban areas [20]. On average, South African rural black men will present with PCa 8 years later than American men, and this late PCa presentation is a common African problem.
Environmental exposures and African practices that are unique to the African population may help better understand PCa risk and biology. Interestingly a recent study showed an increased risk of PCa in the VhaVenda people [38]. While the genetic link could explain these observations, the role played by environmental factors cannot be ignored. For instance, the use of dichlorodiphenyltrichloroethane (DDT), with potential carcinogenic effects has been brought to attention. While banned in most countries, the pesticide use of DDT in the VhaVenda Vhembe District, Limpopo, South Africa is still in practice since 1945 [39]. In relation with the US report, linking pesticide use and higher risk of PCa, the link between pregnant mothers exposed to DDT and urogenital defects in boys was identified [39-41]. PCa would not be the first cancer to be linked to an African descent. The increased incidence rates of esophageal cancer in the South African population has also been reported. This has been associated with the brewing of traditional maize beer in iron pots [42]. Another recent study identified the 8q24 PCa risk locus in African American men [43].
Diagnosis and stratification
Generally, a multidisciplinary approach is required to manage PCa. It has been recommended by institutions such as the American Urology Association (AUA) and European Association of Urology (EAU) that PCa be managed based on risk stratification [17,44]. However, this management strategy poses challenges in the SSA countries. This is attributed to the lack of adequate resources. PCa risk stratification may include very low risk, low risk, intermediate risk and high risk. In the developed world, more than 80% of PCa is presented as localized disease, which is not the case in SSA countries, as most of PCa cases are presented already at high risk advanced stage [45].
For a successful PCa patient care, diagnosis and adequate staging play an essential role. Prostate specific antigen (PSA) blood test and digital rectal examination (DRE) remain the mainstay for screening and Magnetic Resonance Imaging (MRI) for local staging. This is followed by transrectal ultrasound (TRUS) guided biopsy. It has been reported that over 60% of PCa cases in asymptomatic patients with normal DRE and increased PSA go undiagnosed. PCa is classified into different risk-associated groups; very-low-risk PCa, low-risk PCa, Intermediate-risk PCa and high-risk PCa [17]. The very low risk PCa patient group has PSA value <10 ng/dL T1-T2a tumour and a group 1 Gleason grade [44]. Patients with very low risk PCa can be managed with active surveillance. In African countries, the incidence rates of very low risk PCa remains poorly determined due to factors indicated above. Patients with low risk PCa are considered to have PSA < 10 ng/dL, T1 - T2a tumor, Gleason grade group 1. Management of this group can still be achieved by active surveillance and focal therapy. Patients with intermediate risk PCa are further divided into favourable intermediate risk and unfavourable intermediate risk. The favourable group has PSA 10-20 with group 2 Gleason grade, while the unfavourable group has PSA 10-20, T2b-c or Gleason group grade 2 or group 3 with PSA <20 with Gleason grade group 3. CT scan or MRI are recommended for this group. Radiation therapy along with androgen therapy are recommended. The high risk localized PCa in men is life threatening. Patients with high risk PCa have PSA >20 ng/dL, T3 clinical stage and group 4-5 Gleason grade. The rate of metastatic progression is high in this group, and therefore a CT scan or MRI with bone scan is recommended. High-risk PCa patients should be treated with radiation therapy and androgen deprivation therapy [44].
AR signaling and PCa mechanisms
Similarly, to other cancer types, oncogene activation and tumour suppressor deregulation play important roles in the development and progression of PCa. For example, c myc is upregulated in PCa, while the loss of RB expression promotes CRPC. Furthermore, the PI3K/AKT/mTOR pathway is reported to be elevated in PCa and this is attributed to the loss of PTEN activity [46,47]. Additionally, elevated levels of human epidermal growth factor 2 and 3 (HER2/3) receptor tyrosine kinases are associated with poor prognosis in PCa patients [48,49].
AR signaling in the prostate gland
Both testosterone and dihydrotestosterone can bind to the AR, which is an androgen dependent transcriptional activator and a member of nuclear receptor family [24]. As a nuclear hormone receptor, AR receptor protein has three functional domains: the ligand-binding domain (LBD), the central DNA binding domain (DBD) and the NH2-terminal unstructured transcriptional activation domain. The bipartite nuclear localization signal (NLS) is harboured between the LBD and DBD, Figure 2. During androgen signal transduction, AR binds to androgen response element (ARE) as a homodimer, then both the LBD and DBD mediate dimerization [5,22,24,50,51].
Figure 2.
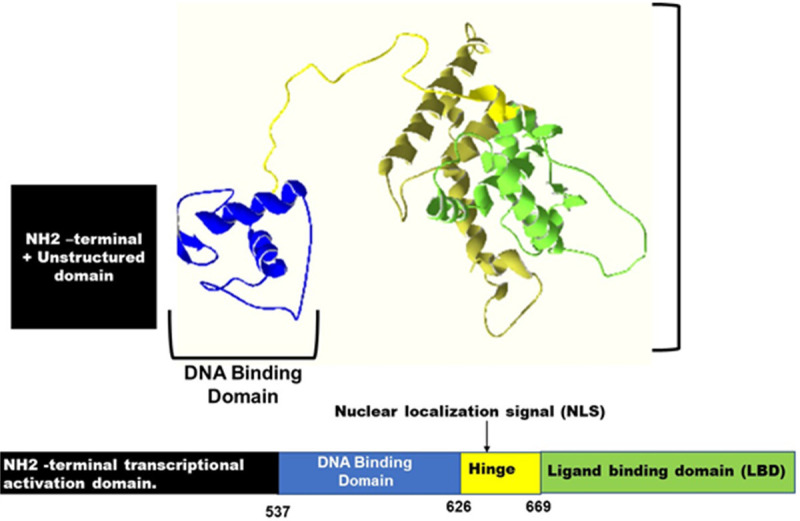
The Structure of the Androgen Receptor. The Androgen receptor consists of multiple domains including two activation regions, a DNA binding domain and a ligand binding domain.
In their inactive state, AR receptors are bound to heat shock proteins and located in the cytoplasm. Binding of androgen to AR causes conformational change and release from heat shock proteins. AR then translocates from the cytoplasm to the nucleus where it recognises ARE in the genomic DNA, recruits coactivator factors and initiates transcription of target genes such as prostate-specific antigen (PSA) and the transmembrane protease serine 2 (TMPRSS2) [24,52]. During AR signal transduction, the AR chromatin modifiers and coactivators assemble into pro-transcriptional complexes, which facilitate the transcription of AR target genes by recruiting RNA pol II to the transcription start site (TSS) [53]. FOXA1, GATA-binding protein 2 (GATA2), and homeobox B13 (HOXB13) are AR chromatin modifiers which unwind and contribute to chromatin accessibility for AR [54,55]. The recruitment of AR coactivators such as CBP (CREB binding protein) and SRC-1 (steroid receptor coactivator 1) follows the binding of AR to its ARE, initiating transcription of AR targeted genes which include genes involved in cell cycle, hormonal response signal transduction and lipid metabolism, growth and survival [53,54,56-58]. Figure 3 demonstrates AR signalling.
Figure 3.
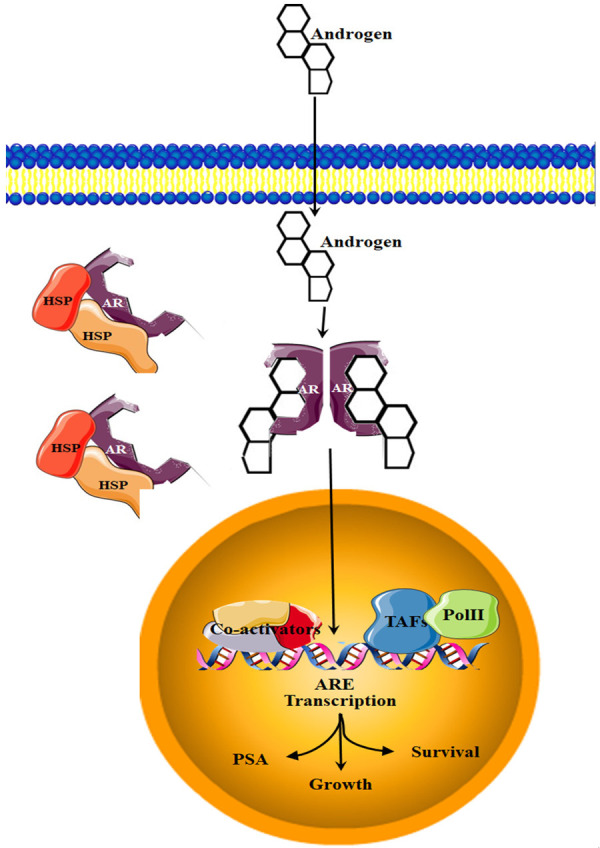
Androgen receptor (AR) signalling. When inactive, the heat shock protein bound AR is localised in the cytoplasm. Androgen hormonal signalling causes the inactive AR to dimerize, bind to the androgen and translocate to the nucleus, where it will bind to the ARE of target genes.
AR alternative splicing
Alternative splicing can lead to splice variants with antagonist functions [22]. It has also been reported that alternative splicing in tumours is ~30% upregulated compared to normal tissues [59]. In PCa, AR splice variants have been reported to impede standard therapy and are involved in PCa progression. The AR splice variants are distinct in structure and in function. Mature AR transcripts have a transcribed intron. As the majority of the AR splice variants lack the LBD, the transcribed intron region encodes a short variant peptide capable of replacing the LBD. The AR splice variants do not necessarily replace or maintain the original function of the full-length AR isoform. In relation to the CRPC, AR-V7 has been identified as one of the most abundantly expressed variants. The roles of the AR splice variants in constitutive AR signaling makes these splice variants an important therapeutic target. Furthermore, a number of factors may drive the constitutive AR signalling in CRPC, and these may include upregulated AR gene expression, AR gene mutation, amplification of AR coactivators, intra-tumour androgen synthesis aberrant activation of the kinase and constitutive expression of AR splice variants [49,50,60-63]. The AR splice variants are shown in Figure 4.
Figure 4.
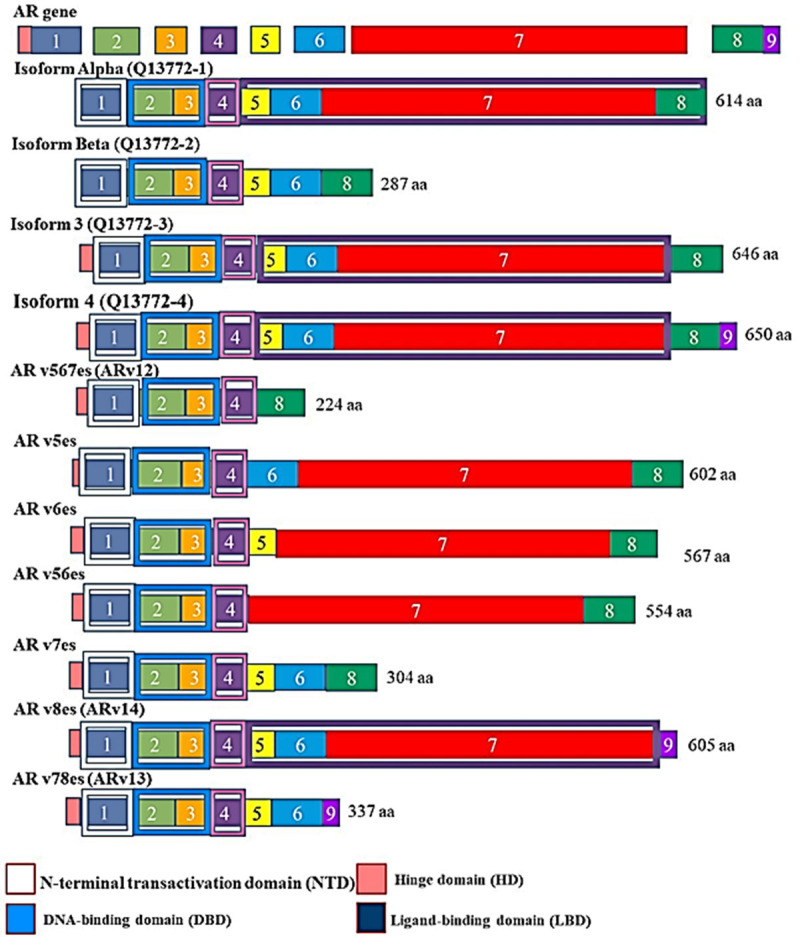
The human androgen receptor (AR) full length with its splice variants. These isoforms are named according to the number of exons they lack. For example, Arv567es lacks exons 5, 6 and 7, compared to full length (FL).
As they lack the domain targeted by traditional therapies, the LBD, most of the AR splice variants do not respond to the hormone therapy regimens [51]. It has also been reported that the deletion of LBD produces AR mutants that are androgen independent, and this might render the LBD a negative regulator of the AR transcriptional activity [64].
Interestingly, it has been reported that activated AR molecules can act as both activators and repressors of genes involved in PCa progression [65,66]. Sha et al., (2020) showed that the epithelial splicing regulator proteins (ESRP1 and ESRP2) are the facilitating splicing factors used by AR to regulate pre-mRNA splicing in PCa cells. Depending on the binding position, ESRPs regulate AR gene splicing in a position dependent manner. That is, ESRPs promote exon inclusion if it is bound in the downstream intron. Contrarily, exon skipping is promoted by ESRPs binding within or upstream from an exon, and this exon inclusion/exclusion is associated with PCa progression [67]. Shah et al., (2020) also demonstrated that the genomic or pharmacological modulation the AR signaling causes the dysregulation of splicing events of functional genes. This group also proposed the link between alternative splicing of functionally relevant genes and PCa progression.
Shah et al., (2020) reported that the clinically used antiandrogens modulate AR signalling, dysregulate AR associated splicing events, thereby unintentionally contributing to PCa progression. Understanding the PCa transcriptome landscape holds promising diagnostic, prognostic and therapeutic potentials. Several studies including Shah et al., (2020) showed that the physiological roles of AR transcription targets and AR signalling alternative splicing gene targets might differ [67-69].
This group further demonstrated that the ablation of AR signalling leads to the generation of abnormal transcripts that are translated into immunogenic peptides, resulting in immune response [70]. PCa patients on AR inhibitors may therefore benefit from immune therapy. It was demonstrated that PCa cells treatment with enzalutamide lead to unintended splice switch, which favoured the tumourigenic variant of the PLA2G2A gene [22]. Although this was an in vitro study, in vivo studies still help shed more light on AR signalling related splicing events and the progression of PCa.
Aberrant splicing and PCa pathogenesis
A spliceosome comprises of small nuclear ribonucleoproteins (SNRNPs) and core-spliceosome-associated proteins and coordinates the splicing process in eukaryotes. Additionally, the spliceosome interacts with additional proteins, the splicing factors to carry out pre-RNA splicing [71]. Jimenez-Vacas et al., (2020) reported the dysregulation in the splicing machinery components and direct link to PCa aggressiveness. These included 7 spliceosome components (SCs) and 19 splicing factors (SFs). This group reported the association between PCa recurrence and aggressiveness and the upregulated expression of SNRNP200, SRSF3 and SRRM1. The overexpression components/factors were also associated with the increased AR-7 variant. In addition to the AR splicing dysregulation, other studies have revealed differential alternative splicing (DAS) patterns of REST4, SST5TMD4, XBP1s, PKM2 to be associated with PCa development and poor prognosis [72-78]. Various studies have also demonstrated the association between increased expression of the splicing factors (SFs) RBM3, U2AF2, ESRP1, ESRP2 and NOVA1 with poor PCa prognosis [79-81].
Differential alternative splicing (DAS) compared to differential gene expression (DGE) has been shown to have a significant role in cancer pathogenesis, particularly PCa [82,83]. About 1,876 different genes including the Nuclear Factor 1 were shown to undergo DAS in African American men than European American men. Leveraging the RNA splicing landscape holds great potential to deciphering the underlying mechanisms for PCa racial disparities and open new effective therapeutic doors [82].
Targeted therapy
Decreasing AR antagonists efficiency and progression to CRPC may be attributed to AR splice variants transcriptional network [3]. The development of next generation AR antagonists that target the DBD and N-terminal domain rather than the LBD may hold promising therapeutic effects. For example, EPI-001, AR antagonist disrupts coactivator recruitment independent of ligand binding, thereby reducing AR transcriptional activity [84], Figure 5. EPI-001 is currently in preclinical testing and if clinical testing validates EPI-001 as a therapeutic agent, then EPI-001 can effectively be used to combat challenges posed by AR splice variants and should potently inhibit constitutively active AR splice variants that are not affected by traditional LBD-targeting antagonists and significantly enhance treatment effectiveness compared to first generation AR antagonist. In addition, AR antisense oligonucleotides such as EZN-4176 also downregulate AR expression and are in phase I/II clinical trials [85]. In addition to transcriptional activity, AR posttranslational modifications such as phosphorylation, acetylation and ubiquitylation also play a role in PCa progression to CRPC, therefore posttranslational AR modifications also hold promising therapeutic potential.
Figure 5.
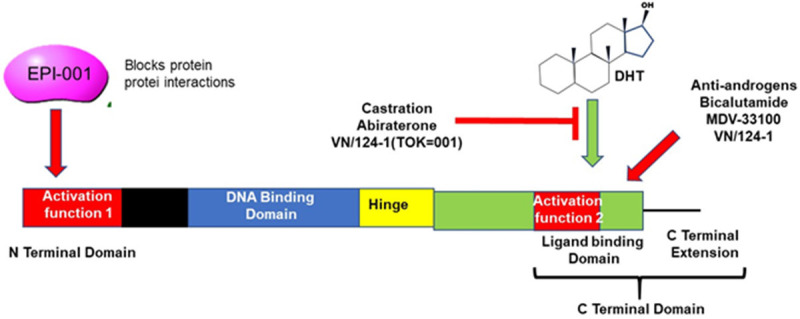
The structure of the Androgen Receptor showing binding sites of inhibitors and activators of AR signalling. The ligand for AR, DHT binds to the Activation Region 2 located in the LBD. Abiraterone can prevent this binding. The Activation region 1 is the location of protein-protein interactions and allows AR to bind to cofactors which is essential for its activity. The small molecule EPI-001 can prevent this.
Alternative splicing components as therapeutic targets
The inhibition of the spliceosome by spliceosome inhibitors such as spliceostatin-A and Pladienolide-B has been suggested as anti-cancer therapeutic targets [86,87]. The broad inhibition of the spliceosome was further reported to be less specific and with limited efficacy, while targeting of specific SCs and SFs holds novel therapeutic potential for cancer, PCa in particular [83].
Various AS targeted therapeutic compounds have been developed, and these include antisense oligonucleotides, targeting mRNAs for degradation. Despite their use to treat other diseases such as Duchenne Muscular Dystrophy, antisense oligonucleotides AZD9150 and AZD4785 targeting STAT3 and KRAS to treat solid advanced and metastatic diseases are in clinical trials [82,88-90]. In addition, small molecule inhibitors are being developed to target the dysregulated splicing factor (SF) kinases and spliceosome components [91]. In addition, natural products and their compounds have also been reported as promising potential therapeutic targets against AS [82,92].
First and second-generation antiandrogens
Medical castration has been the main treatment for advanced prostate cancer. This treatment involves the use of gonadotropin-releasing hormone (GnRH). These analogs block the production of testicular androgens by suppressing gonadotropin secretion. GnRH analogs include leuprolide, goserelin, and buserelin [24]. In combination therapy, GnRH is used in conjunction with antiandrogens to promote the survival of PCa patients [24]. Ultimately, patients develop resistance to this type of treatment. However, it was reported that even after androgen deprivation, androgen levels remained detectable and activated the AR receptor in PCa tissues with recurrent PCa. Additionally, PSA gene levels remained detectable levels in these tissues [93,94]. In light of this, it has been suggested that recurrent cancers following castration are not really androgen independent, as they still depend on AR signalling to grow and survive. These recurrent PCa have thus been classified as castration resistant PCa (CRPC) [24,95]. To overcome this challenge of castration resistance, a second generation of antiandrogens, more potent with increased binding affinity for the AR have been developed [96,97]. These include, Enzalutamide, Apalutamide, and Darolutamide.
Clonal evolution, de-differentiation of small-cell prostate cancer (SCPC)
The possibility of CRPC undergoing de-differentiation and clonal evolution has been reported. This may occur as a result of complete androgen depravation and AR degradation therapies, Figure 6. These proposed AR negative small cell PCa cells that are more CRPC will eventually lose AR expression [98,99]. It is further proposed that these AR negative small cell PCa will no longer respond to any efforts made by hormone or AR targeting therapy. This therefore highlights the urgent need to develop novel precise therapies. Interestingly, molecular analysis revealed the aberrant expression of aurora kinase A (AURKA) and N-myc proto-oncogene (MYCN) [98].
Figure 6.
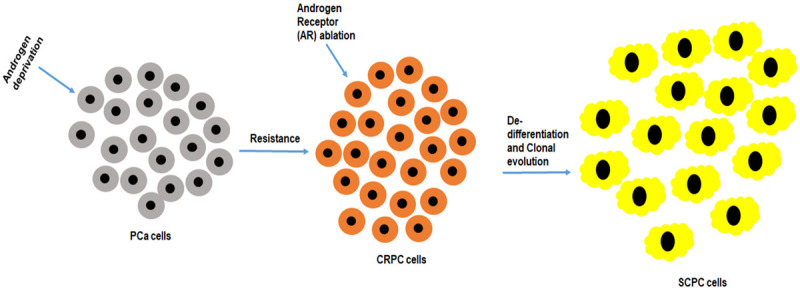
De-differentiation and clonal evolution of prostate cancer cells. The castrate resistant prostate cancer (CRPC) cells are resistant to hormonal therapy. Constitutive androgen deprivation and androgen receptor ablation therapies may lead to absolute non-response and de-differentiation of the already CRPC to SCPC and clonal evolution of this cell population.
Treatment by AR signal transduction
Efforts in pursuit of improved treatment for CRPC are ongoing. The optimum/complete ablation of the AR signalling has become point of focus. This was propelled in particular by the clinical success of the selective estrogen receptor downregulator (SERD)-Faslodex [100,101]. Similarly, selective androgen receptor downregulators (SARD) have also been developed and are currently in clinical trials [102]. This SARD compound AZD3514 causes severe AR conformational change [103]. This conformational change causes receptor degradation [102,103]. Proteolysis Targeting Chimeras (PROTACs) have also been reported to target and degrade AR. PROTACs work by binding to the target protein and the E3 ligase system, formed in a bi-partite ligand-AR molecule. Enzalutamide-derived ARCC-4 and aryloxy tetramethylcyclobutane-derived ARD-69 are AR targeting PROTACs, whose in vivo anti-prostate cancer effects remains to be confirmed [104,105]. Additionally, anti-prostate cancer epigenetic regulators are also being pursued.
Posttranscriptional AR gene silencing is another strategy to target AR signalling. For example, through microRNA (miRNA) regulation. The global dysregulation of miRNAs in cancer has been previously documented and may contribute to the development and progression of cancers. MiRNAs have been identified as targets or effectors of cancer hallmarks. These include angiogenesis, uncontrolled DNA replication and cell proliferation, resistance to cell death, suppression of growth control mechanisms, invasion and metastasis. The roles of miRNAs in the regulation of hallmarks of cancer continue to be investigated [106,107]. It is estimated that half of the mRNA transcripts are regulated by miRNAs. Furthermore, one miRNA can regulate tens of mRNA transcripts, resulting in simultaneous regulation of multiple biological pathways [108,109]. There are an increasing number of miRNA-based clinical trials in the clinical management of cancers. In clinical trials, these short non-coding RNAs are targeted as prognosticators and therapeutic targets, with the aim of using them as another means for defining the molecular and clinical heterogeneity that exist within cancers and establishing the heterogeneity of PCa can assist in the development of new treatments. In addition, the effect of miRNA differential expression on chemotherapeutic drugs has been documented. Furthermore, reports have shown evidence of the miRNAs exploiting the cell cycle in favour of tumourigenesis by either facilitating entry and progression through the cell cycle (oncogenic miRNAs) or by by-passing the cell cycle arrest (due to the loss of tumour suppressor miRNAs) [110].
Limitations and challenges
It has been reported that PCa patients are asymptomatic until disease has progressed and this poses serious challenges for clinicians [18]. Despite the current PCa management status in Africa, generally PCa is a highly prevalent disease with relatively low rates of mortality. One of the major challenges associated with PCa is early diagnosis and prognosis. Differentiating between clinically significant and insignificant PCa remains a challenge, with the current diagnostic tests limited by either false positives or false negatives. There is therefore an urgent need to address this gap. Next-generation sequencing (NGS) RNA sequencing is also being currently explored to identify molecular diagnostic and prognostic biomarkers. Furthermore, being the second most populated and second largest continent, the African population represents one of the most genetically and culturally diversified populations in the world [20]. With regards to cancer burden, prostate cancer in particular, the lack of uniform systems for reporting and monitoring are not in place [31]. With these factors highlighted, tracing and verifying the African genetic link to PCa will need broad analyses that will also include environmental factors across the African continent.
The lack of resources for PCa management in South Africa such as national PCa registries is a problem, whereas these registries exist for women cancers such as breast and cervical. The inequality between male and female cancers in South Africa is further revealed by the national funding institutions to fund female-related cancers. Other life-orientated factors highlighting the status of men in the society also negatively contribute towards the inadequate management of PCa in South Africa. For example, men (elderly men in particular) hold high esteemed leadership positions. Culturally, in black South African families, men are considered superior and heads of families. Generally, in such communities, sickness such as PCa is associated with a supernatural link. This is exacerbated by the lack of or very little background education. Presented together, all these factors negatively influence men’s attitude to seek medical help [31,111].
Resources and infrastructure is another major problem, particularly severely affecting the rural areas of South Africa. Usually patients that require a medical specialist in these settings cannot receive medical help but will rather be referred to one of the provincial hospitals that usually are miles away. The problem is compounded by the lack of staffing even in the rural provincial hospitals. The burden of infectious diseases such as HIV/AIDS and TB in the SSA and South Africa even worsens the current cancer, PCa management in these countries [95]. Efforts have been made that involve men of African origin 40 years and older undergo PSA testing, as recommended by the PCa Foundation of South Africa [38]. The cultural, linguistic, socioeconomic, geographical differences still pose as negative barriers against PCa management.
Compared to the developed world, the disproportion between the incidence and mortality rates in Africa, particularly Southern Africa is alarming. This may be due to a number of factors, of which the African ethnicity is emerging as one of the contributing factors. It has been reported that the true incidence rates of PCa in the sub-Saharan African (SSA) region is under-reported, with the majority of cases remaining undiagnosed. It has also been reported that most men in the SSA regions still cannot access standard treatment for localized PCa [17]. Despite the general decrease of PCa mortality rates, increased screening, equal access to health-care and adjusted socioeconomic status, recurrent and aggressive PCa in men of African ancestry is still linked to higher PCa mortality rates [82,112].
Conclusions
To improve the overall PCa patient care, it is obvious that PCa therapeutics will move toward precision medicine with the aid of whole genome sequencing. Insights into PCa transcriptomics hold great potential in overcoming PCa recurrence and significantly lower mortality rates particularly in vulnerable populations. Despite challenges faced in the African populations with the management of PCa, African populations hold the key to deciphering the complex biology around PCa. Although the African genetic link to PCa has been mapped, the battle against PCa management in Africa is far from over. With the emerging use of artificial intelligence (AI), NGS and precision oncology, decoding the transcriptomic landscape of CRPC is possible. The association between aberrant AS and PCa poor prognosis and drug resistance in men of African ancestry has been revealed. Previous reports such as loss of PTEN being the main contributor have been proven other in black men with PCa. While AS dysregulation in PCa remains to be fully explored, understanding the RNA splicing landscape and its impact in racial PCa racial disparities holds promising diagnostic, prognostic and therapeutic PCa targets.
Prostate cancer (PCa) is asymptomatic until it is advanced and therefore difficult to detect at early stages. Generally, an individual seeks medical attention when symptoms are evident, unless prior education is in place to teach about the deceptive nature of the specific condition. With regards to PCa, there is an urgent need for early screening and detection in at risk populations. As it is the case with hypertension or cervical cancer and breast cancer, Urologists, particularly in Africa, as advocates for men’s health are lagging behind the efforts similar to those of women’s health advocates with regards to community engagement.
Due to high prevalence of PCa in African men, it has led to the conclusion by the global urology community that PCa is more aggressive in Black men. PCa is a heterogeneous, complex and a multifactorial disease. Although still poorly understood, the genetic and environmental factors are significant risk factors in PCa and remain to be elucidated. Many studies are now being conducted to ascertain the genetic basis for this disease, and a few leads such as the dysregulation of the androgen receptor-signalling pathway in Black men have been identified, and the chromosomal mapping of the PCa risk associated genetics of African descent. However, if the urologists are waiting for black men to present PCa symptoms, then by definition the disease will be advanced. Advanced disease is characterised by a high Gleason score on biopsy. Aggressive disease is also characterised by a high Gleason score. So advanced disease and aggressive disease would histologically appear similar.
While PCa is likely to represent a major contributor to the overall burden of cancer in South African men, practices related to PCa screening, detection, diagnosis and treatment need to be addressed. There is a serious need to improve awareness/education and understanding of PCa, particularly amongst high-risk communities. On this note, barriers such as underestimated PCa’s risk, inadequate awareness, higher level of fear of loss of masculinity, embarrassment, religion, culture, lack of prioritisation of health-care may have to be overcome. In an attempt to address this public health problem, urologists, scientists, communities’ and religious leaders will have to work in synergy to combat PCa. While precision oncology holds therapeutic potential, further work is essential to ensure that awareness in high-risk communities is addressed. Encouraging a health relationship of understanding and trust amongst all stakeholders to combat PCa will also be beneficial. Furthermore, the collaboration between African countries, particularly the SSA, establishing uniform and consistent PCa management systems in these regions will aid in the decoding of the PCa transcriptome and provide adequate evidence of the relationship between PCa and African familial history. Both the intrinsic/genetic and extrinsic factors may contribute to PCa pathogenesis, understanding the PCa transcriptome may help shed light in PCa stratification, to improve diagnosis, prognosis and targeted therapy for PCa subgroups, Figure 7.
Figure 7.
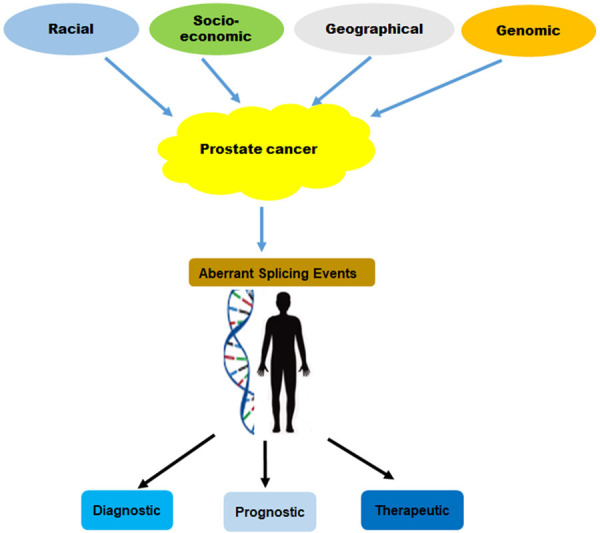
The genetic and non-genetic factors are contributing to PCa development, progression. Targeting the aberrantly splicing events holds novel potential to improving overall patient outcome.
The development of PCa novel drugs has improved patient outcome over the years. However, men of African descent still present with advanced and aggressive PCa. While PCa is highly heterogeneous and complex, there is a growing global interest in PCa stratification and treatment selection on the basis of molecular characterization. This would be beneficial to PCa patient subgroups outcome for early diagnosis, improved prognosis and personalized treatment. Although AR splice variants have been implicated in PCa progression and aggressiveness, decoding the PCa transcriptome may be useful in the understanding the underlying molecular mechanisms and the precise role of potentially aberrantly splice variants with unelucidated functions in PCa pathogenesis.
Acknowledgements
This research was funded by the South African Medical Research Council (SAMRC).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Merriel SWD, Funston G, Hamilton W. Prostate cancer in primary care. Adv Ther. 2018;35:1285–1294. doi: 10.1007/s12325-018-0766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrecengost R, Knudsen KE. Molecular pathogenesis and progression of prostate cancer. Semin Oncol. 2013;40:244–258. doi: 10.1053/j.seminoncol.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostwick DG, Liu L, Brawer MK, Qian J. High-grade prostatic intraepithelial neoplasia. Rev Urol. 2004;6:171–179. [PMC free article] [PubMed] [Google Scholar]
- 5.Testa U, Castelli G, Pelosi E. Cellular and molecular mechanisms underlying prostate cancer development: therapeutic implications. Medicines (Basel) 2019;6:82. doi: 10.3390/medicines6030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou M. High-grade prostatic intraepithelial neoplasia, PIN-like carcinoma, ductal carcinoma, and intraductal carcinoma of the prostate. Mod Pathol. 2018;31:S71–79. doi: 10.1038/modpathol.2017.138. [DOI] [PubMed] [Google Scholar]
- 7.Gann PH. Risk factors for prostate cancer. Rev Urol. 2002;4(Suppl 5):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr L, Rewhorn MJ, Longmuir M, Fraser S, Walsh S, Andrew N, Leung HY. A cohort analysis of men with a family history of BRCA1/2 and Lynch mutations for prostate cancer. BMC Cancer. 2016;16:529. doi: 10.1186/s12885-016-2573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro E, Goh CL, Eeles RA. Prostate cancer screening in BRCA and Lynch syndrome mutation carriers. Am Soc Clin Oncol Educ Book. 2013 doi: 10.14694/EdBook_AM.2013.33.e50. [DOI] [PubMed] [Google Scholar]
- 10.Kovtun IV, Cheville JC, Murphy SJ, Johnson SH, Zarei S, Kosari F, Sukov WR, Karnes RJ, Vasmatzis G. Lineage relationship of Gleason patterns in Gleason score 7 prostate cancer. Cancer Res. 2013;73:3275–3284. doi: 10.1158/0008-5472.CAN-12-2803. [DOI] [PubMed] [Google Scholar]
- 11.Mellinger GT. Prognosis of prostatic carcinoma. Recent Results Cancer Res. 1977:61–72. doi: 10.1007/978-3-642-81095-4_6. [DOI] [PubMed] [Google Scholar]
- 12.Bailar JC 3rd, Mellinger GT, Gleason DF. Survival rates of patients with prostatic cancer, tumor stage, and differentiation--preliminary report. Cancer Chemother Rep. 1966;50:129–136. [PubMed] [Google Scholar]
- 13.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowalsky AG, Ye H, Bubley GJ, Balk SP. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res. 2013;73:1050–1055. doi: 10.1158/0008-5472.CAN-12-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin MA, Girelli G, Demichelis F. Genomic correlates to the newly proposed grading prognostic groups for prostate cancer. Eur Urol. 2016;69:557–560. doi: 10.1016/j.eururo.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 16.VanderWeele DJ, Brown CD, Taxy JB, Gillard M, Hatcher DM, Tom WR, Stadler WM, White KP. Low-grade prostate cancer diverges early from high grade and metastatic disease. Cancer Sci. 2014;105:1079–1085. doi: 10.1111/cas.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassell A, Yunusa B, Jalloh M, Mbodji MM, Diallo A, Ndoye M, Kouka SC, Labou I, Niang L, Gueye SM. A review of localized prostate cancer: an African perspective. World J Oncol. 2019;10:162–168. doi: 10.14740/wjon1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Q, He B. Androgen receptor signaling in the development of castration-resistant prostate cancer. Front Oncol. 2019;9:858. doi: 10.3389/fonc.2019.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trabzonlu L, Kulac I, Zheng Q, Hicks JL, Haffner MC, Nelson WG, Sfanos KS, Ertunc O, Lotan TL, Heaphy CM, Meeker AK, Yegnasubramanian S, De Marzo AM. Molecular pathology of high-grade prostatic intraepithelial neoplasia: challenges and opportunities. Cold Spring Harb Perspect Med. 2019;9:a030403. doi: 10.1101/cshperspect.a030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes VM, Bornman MSR. Prostate cancer in southern Africa: does Africa hold untapped potential to add value to the current understanding of a common disease? J Glob Oncol. 2018;4:1–7. doi: 10.1200/JGO.2016.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Descotes JL. Diagnosis of prostate cancer. Asian J Urol. 2019;6:129–136. doi: 10.1016/j.ajur.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K, Gagliano T, Garland L, O’Hanlon T, Bortolotti D, Gentili V, Rizzo R, Giamas G, Dean M. Androgen receptor signaling regulates the transcriptome of prostate cancer cells by modulating global alternative splicing. Oncogene. 2020;39:6172–6189. doi: 10.1038/s41388-020-01429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang BD, Ceniccola K, Hwang S, Andrawis R, Horvath A, Freedman JA, Olender J, Knapp S, Ching T, Garmire L, Patel V, Garcia-Blanco MA, Patierno SR, Lee NH. Alternative splicing promotes tumour aggressiveness and drug resistance in African American prostate cancer. Nat Commun. 2017;8:15921. doi: 10.1038/ncomms15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng N, Huang J. Prostate cancer: molecular and cellular mechanisms and their implications in therapy resistance and disease progression. Asian J Androl. 2019;21:213–214. doi: 10.4103/aja.aja_31_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GLOBOCAN. Global Prostate Cancer Fact Sheet. 2018. [Available from: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf]
- 26.Odedina FT, Ogunbiyi JO, Ukoli FA. Roots of prostate cancer in African-American men. J Natl Med Assoc. 2006;98:539–543. [PMC free article] [PubMed] [Google Scholar]
- 27.Stefflova K, Dulik MC, Barnholtz-Sloan JS, Pai AA, Walker AH, Rebbeck TR. Dissecting the within-Africa ancestry of populations of African descent in the Americas. PLoS One. 2011;6:e14495. doi: 10.1371/journal.pone.0014495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiciński M, Vangronsveld J, Nawrot TS. An epidemiological reappraisal of the familial aggregation of prostate cancer: a meta-analysis. PLoS One. 2011;6:e27130. doi: 10.1371/journal.pone.0027130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd T, Hounsome L, Mehay A, Mee S, Verne J, Cooper A. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008-2010. BMC Med. 2015;13:171. doi: 10.1186/s12916-015-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fachal L, Gómez-Caamaño A, Celeiro-Muñoz C, Peleteiro P, Blanco A, Carballo A, Forteza J, Carracedo A, Vega A. BRCA1 mutations do not increase prostate cancer risk: results from a meta-analysis including new data. Prostate. 2011;71:1768–1779. doi: 10.1002/pros.21394. [DOI] [PubMed] [Google Scholar]
- 31.Chu LW, Ritchey J, Devesa SS, Quraishi SM, Zhang H, Hsing AW. Prostate cancer incidence rates in Africa. Prostate Cancer. 2011;2011:947870. doi: 10.1155/2011/947870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: cancer in indigenous Africans--burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 33.Setel PW, Macfarlane SB, Szreter S, Mikkelsen L, Jha P, Stout S, AbouZahr C. A scandal of invisibility: making everyone count by counting everyone. Lancet. 2007;370:1569–1577. doi: 10.1016/S0140-6736(07)61307-5. [DOI] [PubMed] [Google Scholar]
- 34.Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol. 2006;7:584–595. doi: 10.1016/S1470-2045(06)70759-8. [DOI] [PubMed] [Google Scholar]
- 35.Adeloye D, David RA, Aderemi AV, Iseolorunkanmi A, Oyedokun A, Iweala EE, Omoregbe N, Ayo CK. An estimate of the incidence of prostate cancer in Africa: a systematic review and meta-analysis. PLoS One. 2016;11:e0153496. doi: 10.1371/journal.pone.0153496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster SC, Miller W, Ratan A, Tomsho LP, Giardine B, Kasson LR, Harris RS, Petersen DC, Zhao F, Qi J, Alkan C, Kidd JM, Sun Y, Drautz DI, Bouffard P, Muzny DM, Reid JG, Nazareth LV, Wang Q, Burhans R, Riemer C, Wittekindt NE, Moorjani P, Tindall EA, Danko CG, Teo WS, Buboltz AM, Zhang Z, Ma Q, Oosthuysen A, Steenkamp AW, Oostuisen H, Venter P, Gajewski J, Zhang Y, Pugh BF, Makova KD, Nekrutenko A, Mardis ER, Patterson N, Pringle TH, Chiaromonte F, Mullikin JC, Eichler EE, Hardison RC, Gibbs RA, Harkins TT, Hayes VM. Complete Khoisan and Bantu genomes from southern Africa. Nature. 2010;463:943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen DC, Libiger O, Tindall EA, Hardie RA, Hannick LI, Glashoff RH, Mukerji M, Fernandez P, Haacke W, Schork NJ, Hayes VM. Complex patterns of genomic admixture within southern Africa. PLoS Genet. 2013;9:e1003309. doi: 10.1371/journal.pgen.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tindall EA, Monare LR, Petersen DC, van Zyl S, Hardie RA, Segone AM, Venter PA, Bornman MS, Hayes VM. Clinical presentation of prostate cancer in black South Africans. Prostate. 2014;74:880–891. doi: 10.1002/pros.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyk JC, Bouwman H, Barnhoorn IE, Bornman MS. DDT contamination from indoor residual spraying for malaria control. Sci Total Environ. 2010;408:2745–2752. doi: 10.1016/j.scitotenv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Cockburn M, Mills P, Zhang X, Zadnick J, Goldberg D, Ritz B. Prostate cancer and ambient pesticide exposure in agriculturally intensive areas in California. Am J Epidemiol. 2011;173:1280–1288. doi: 10.1093/aje/kwr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koutros S, Beane Freeman LE, Lubin JH, Heltshe SL, Andreotti G, Barry KH, DellaValle CT, Hoppin JA, Sandler DP, Lynch CF, Blair A, Alavanja MC. Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol. 2013;177:59–74. doi: 10.1093/aje/kws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsha T, Brink L, van Rensburg S, Hon D, Lombard C, Erasmus R. Traditional home-brewed beer consumption and iron status in patients with esophageal cancer and healthy control subjects from Transkei, South Africa. Nutr Cancer. 2006;56:67–73. doi: 10.1207/s15327914nc5601_9. [DOI] [PubMed] [Google Scholar]
- 43.Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, Penney K, Steen RG, Ardlie K, John EM, Oakley-Girvan I, Whittemore AS, Cooney KA, Ingles SA, Altshuler D, Henderson BE, Reich D. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR. Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199:990–997. doi: 10.1016/j.juro.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Hwang TH, Oseth LA, Hauge A, Vessella RL, Schmechel SC, Hirsch B, Beckman KB, Silverstein KA, Dehm SM. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31:4759–4767. doi: 10.1038/onc.2011.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad I, Patel R, Singh LB, Nixon C, Seywright M, Barnetson RJ, Brunton VG, Muller WJ, Edwards J, Sansom OJ, Leung HY. HER2 overcomes PTEN (loss)-induced senescence to cause aggressive prostate cancer. Proc Natl Acad Sci U S A. 2011;108:16392–16397. doi: 10.1073/pnas.1101263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 50.Lu C, Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol. 2013;2:178–186. doi: 10.3978/j.issn.2223-4683.2013.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu R, Denmeade SR, Luo J. Molecular processes leading to aberrant androgen receptor signaling and castration resistance in prostate cancer. Expert Rev Endocrinol Metab. 2010;5:753–764. doi: 10.1586/eem.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gritsina G, Gao WQ, Yu J. Transcriptional repression by androgen receptor: roles in castration-resistant prostate cancer. Asian J Androl. 2019;21:215–223. doi: 10.4103/aja.aja_19_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 54.Jin HJ, Zhao JC, Wu L, Kim J, Yu J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat Commun. 2014;5:3972. doi: 10.1038/ncomms4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He B, Lanz RB, Fiskus W, Geng C, Yi P, Hartig SM, Rajapakshe K, Shou J, Wei L, Shah SS, Foley C, Chew SA, Eedunuri VK, Bedoya DJ, Feng Q, Minami T, Mitsiades CS, Frolov A, Weigel NL, Hilsenbeck SG, Rosen DG, Palzkill T, Ittmann MM, Song Y, Coarfa C, O’Malley BW, Mitsiades N. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc Natl Acad Sci U S A. 2014;111:18261–18266. doi: 10.1073/pnas.1421415111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Jänne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foley C, Mitsiades N. Moving beyond the Androgen Receptor (AR): targeting AR-interacting proteins to treat prostate cancer. Horm Cancer. 2016;7:84–103. doi: 10.1007/s12672-015-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, Qiu J, Liu W, Kaikkonen MU, Ohgi KA, Glass CK, Rosenfeld MG, Fu XD. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahles A, Lehmann KV, Toussaint NC, Hüser M, Stark SG, Sachsenberg T, Stegle O, Kohlbacher O, Sander C, Rätsch G. Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Cancer Cell. 2018;34:211–224. e216. doi: 10.1016/j.ccell.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 61.Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103:14477–14482. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18:R183–196. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Djenaba JA, Soman A, Rim SH, Master VA. Recent trends in prostate cancer incidence by age, cancer stage, and grade, the United States, 2001-2007. Prostate Cancer. 2012;2012:691380. doi: 10.1155/2012/691380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- 65.Zhang Y, Pitchiaya S, Cieślik M, Niknafs YS, Tien JC, Hosono Y, Iyer MK, Yazdani S, Subramaniam S, Shukla SK, Jiang X, Wang L, Liu TY, Uhl M, Gawronski AR, Qiao Y, Xiao L, Dhanasekaran SM, Juckette KM, Kunju LP, Cao X, Patel U, Batish M, Shukla GC, Paulsen MT, Ljungman M, Jiang H, Mehra R, Backofen R, Sahinalp CS, Freier SM, Watt AT, Guo S, Wei JT, Feng FY, Malik R, Chinnaiyan AM. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat Genet. 2018;50:814–824. doi: 10.1038/s41588-018-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baniwal SK, Khalid O, Sir D, Buchanan G, Coetzee GA, Frenkel B. Repression of Runx2 by androgen receptor (AR) in osteoblasts and prostate cancer cells: AR binds Runx2 and abrogates its recruitment to DNA. Mol Endocrinol. 2009;23:1203–1214. doi: 10.1210/me.2008-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Park JW, Bebee TW, Warzecha CC, Guo Y, Shang X, Xing Y, Carstens RP. Determination of a comprehensive alternative splicing regulatory network and combinatorial regulation by key factors during the epithelial-to-mesenchymal transition. Mol Cell Biol. 2016;36:1704–1719. doi: 10.1128/MCB.00019-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miao L, Yang L, Li R, Rodrigues DN, Crespo M, Hsieh JT, Tilley WD, de Bono J, Selth LA, Raj GV. Disrupting androgen receptor signaling induces snail-mediated epithelial-mesenchymal plasticity in prostate cancer. Cancer Res. 2017;77:3101–3112. doi: 10.1158/0008-5472.CAN-16-2169. [DOI] [PubMed] [Google Scholar]
- 69.Stylianou N, Lehman ML, Wang C, Fard AT, Rockstroh A, Fazli L, Jovanovic L, Ward M, Sadowski MC, Kashyap AS, Buttyan R, Gleave ME, Westbrook TF, Williams ED, Gunter JH, Nelson CC, Hollier BG. A molecular portrait of epithelial-mesenchymal plasticity in prostate cancer associated with clinical outcome. Oncogene. 2019;38:913–934. doi: 10.1038/s41388-018-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smart AC, Margolis CA, Pimentel H, He MX, Miao D, Adeegbe D, Fugmann T, Wong KK, Van Allen EM. Intron retention is a source of neoepitopes in cancer. Nat Biotechnol. 2018;36:1056–1058. doi: 10.1038/nbt.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol. 2014;15:108–121. doi: 10.1038/nrm3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hormaechea-Agulla D, Jiménez-Vacas JM, Gómez-Gómez E, L-López F, Carrasco-Valiente J, Valero-Rosa J, Moreno MM, Sánchez-Sánchez R, Ortega-Salas R, Gracia-Navarro F, Culler MD, Ibáñez-Costa A, Gahete MD, Requena MJ, Castaño JP, Luque RM. The oncogenic role of the spliced somatostatin receptor sst5TMD4 variant in prostate cancer. FASEB J. 2017;31:4682–4696. doi: 10.1096/fj.201601264RRR. [DOI] [PubMed] [Google Scholar]
- 73.Sheng X, Nenseth HZ, Qu S, Kuzu OF, Frahnow T, Simon L, Greene S, Zeng Q, Fazli L, Rennie PS, Mills IG, Danielsen H, Theis F, Patterson JB, Jin Y, Saatcioglu F. IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat Commun. 2019;10:323. doi: 10.1038/s41467-018-08152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong N, Yan J, Ojo D, De Melo J, Cutz JC, Tang D. Changes in PKM2 associate with prostate cancer progression. Cancer Invest. 2014;32:330–338. doi: 10.3109/07357907.2014.919306. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, Lam HM, Dumpit R, Corey E, Chéry L, Lakely B, Higano CS, Montgomery B, Roudier M, Lange PH, Nelson PS, Vessella RL, Morrissey C. SRRM4 expression and the loss of REST activity may promote the emergence of the neuroendocrine phenotype in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:4698–4708. doi: 10.1158/1078-0432.CCR-15-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paschalis A, Sharp A, Welti JC, Neeb A, Raj GV, Luo J, Plymate SR, de Bono JS. Alternative splicing in prostate cancer. Nat Rev Clin Oncol. 2018;15:663–675. doi: 10.1038/s41571-018-0085-0. [DOI] [PubMed] [Google Scholar]
- 77.Kong D, Sethi S, Li Y, Chen W, Sakr WA, Heath E, Sarkar FH. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate. 2015;75:161–174. doi: 10.1002/pros.22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grupp K, Wilking J, Prien K, Hube-Magg C, Sirma H, Simon R, Steurer S, Budäus L, Haese A, Izbicki J, Sauter G, Minner S, Schlomm T, Tsourlakis MC. High RNA-binding motif protein 3 expression is an independent prognostic marker in operated prostate cancer and tightly linked to ERG activation and PTEN deletions. Eur J Cancer. 2014;50:852–861. doi: 10.1016/j.ejca.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Lu ZX, Huang Q, Park JW, Shen S, Lin L, Tokheim CJ, Henry MD, Xing Y. Transcriptome-wide landscape of pre-mRNA alternative splicing associated with metastatic colonization. Mol Cancer Res. 2015;13:305–318. doi: 10.1158/1541-7786.MCR-14-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerhauser C, Favero F, Risch T, Simon R, Feuerbach L, Assenov Y, Heckmann D, Sidiropoulos N, Waszak SM, Hübschmann D, Urbanucci A, Girma EG, Kuryshev V, Klimczak LJ, Saini N, Stütz AM, Weichenhan D, Böttcher LM, Toth R, Hendriksen JD, Koop C, Lutsik P, Matzk S, Warnatz HJ, Amstislavskiy V, Feuerstein C, Raeder B, Bogatyrova O, Schmitz EM, Hube-Magg C, Kluth M, Huland H, Graefen M, Lawerenz C, Henry GH, Yamaguchi TN, Malewska A, Meiners J, Schilling D, Reisinger E, Eils R, Schlesner M, Strand DW, Bristow RG, Boutros PC, von Kalle C, Gordenin D, Sültmann H, Brors B, Sauter G, Plass C, Yaspo ML, Korbel JO, Schlomm T, Weischenfeldt J. Molecular evolution of early-onset prostate cancer identifies molecular risk markers and clinical trajectories. Cancer Cell. 2018;34:996–1011. e1018. doi: 10.1016/j.ccell.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olender J, Lee NH. Role of alternative splicing in prostate cancer aggressiveness and drug resistance in African Americans. Adv Exp Med Biol. 2019;1164:119–139. doi: 10.1007/978-3-030-22254-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiménez-Vacas JM, Herrero-Aguayo V, Montero-Hidalgo AJ, Gómez-Gómez E, Fuentes-Fayos AC, León-González AJ, Sáez-Martínez P, Alors-Pérez E, Pedraza-Arévalo S, González-Serrano T, Reyes O, Martínez-López A, Sánchez-Sánchez R, Ventura S, Yubero-Serrano EM, Requena-Tapia MJ, Castaño JP, Gahete MD, Luque RM. Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer. EBioMedicine. 2020;51:102547. doi: 10.1016/j.ebiom.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, Watt K, Tam T, Yang YC, Bañuelos CA, Williams DE, McEwan IJ, Wang Y, Sadar MD. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Castaneda S, Dumble M, Wang M, Mileski M, Qu Z, Kim S, Shi V, Kraft P, Gao Y, Pak J, Sapra P, Bandaru R, Zhao H, Vessella RL, Horak ID, Greenberger LM. Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol Cancer Ther. 2011;10:2309–2319. doi: 10.1158/1535-7163.MCT-11-0329. [DOI] [PubMed] [Google Scholar]
- 86.Sato M, Muguruma N, Nakagawa T, Okamoto K, Kimura T, Kitamura S, Yano H, Sannomiya K, Goji T, Miyamoto H, Okahisa T, Mikasa H, Wada S, Iwata M, Takayama T. High antitumor activity of pladienolide B and its derivative in gastric cancer. Cancer Sci. 2014;105:110–116. doi: 10.1111/cas.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larrayoz M, Blakemore SJ, Dobson RC, Blunt MD, Rose-Zerilli MJ, Walewska R, Duncombe A, Oscier D, Koide K, Forconi F, Packham G, Yoshida M, Cragg MS, Strefford JC, Steele AJ. The SF3B1 inhibitor spliceostatin A (SSA) elicits apoptosis in chronic lymphocytic leukaemia cells through downregulation of Mcl-1. Leukemia. 2016;30:351–360. doi: 10.1038/leu.2015.286. [DOI] [PubMed] [Google Scholar]
- 88.Gallego-Paez LM, Bordone MC, Leote AC, Saraiva-Agostinho N, Ascensão-Ferreira M, Barbosa-Morais NL. Alternative splicing: the pledge, the turn, and the prestige: the key role of alternative splicing in human biological systems. Hum Genet. 2017;136:1015–1042. doi: 10.1007/s00439-017-1790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong D, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, Fowler N, Zhou T, Schmidt J, Jo M, Lee SJ, Yamashita M, Hughes SG, Fayad L, Piha-Paul S, Nadella MV, Mohseni M, Lawson D, Reimer C, Blakey DC, Xiao X, Hsu J, Revenko A, Monia BP, MacLeod AR. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med. 2015;7:314ra185. doi: 10.1126/scitranslmed.aac5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ross SJ, Revenko AS, Hanson LL, Ellston R, Staniszewska A, Whalley N, Pandey SK, Revill M, Rooney C, Buckett LK, Klein SK, Hudson K, Monia BP, Zinda M, Blakey DC, Lyne PD, Macleod AR. Targeting KRAS-dependent tumors with AZD4785, a high-affinity therapeutic antisense oligonucleotide inhibitor of KRAS. Sci Transl Med. 2017;9:eaal5253. doi: 10.1126/scitranslmed.aal5253. [DOI] [PubMed] [Google Scholar]
- 91.Sidarovich A, Will CL, Anokhina MM, Ceballos J, Sievers S, Agafonov DE, Samatov T, Bao P, Kastner B, Urlaub H, Waldmann H, Lührmann R. Identification of a small molecule inhibitor that stalls splicing at an early step of spliceosome activation. Elife. 2017;6:e23533. doi: 10.7554/eLife.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Effenberger KA, James RC, Urabe VK, Dickey BJ, Linington RG, Jurica MS. The natural product N-Palmitoyl-l-leucine selectively inhibits late assembly of human spliceosomes. J Biol Chem. 2015;290:27524–27531. doi: 10.1074/jbc.M115.673210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komisarof J, McCall M, Newman L, Bshara W, Mohler JL, Morrison C, Land H. A four gene signature predictive of recurrent prostate cancer. Oncotarget. 2017;8:3430–3440. doi: 10.18632/oncotarget.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 95.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–476. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 96.Rice MA, Malhotra SV, Stoyanova T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019;9:801. doi: 10.3389/fonc.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Wang Y, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wardell SE, Yllanes AP, Chao CA, Bae Y, Andreano KJ, Desautels TK, Heetderks KA, Blitzer JT, Norris JD, McDonnell DP. Pharmacokinetic and pharmacodynamic analysis of fulvestrant in preclinical models of breast cancer to assess the importance of its estrogen receptor-α degrader activity in antitumor efficacy. Breast Cancer Res Treat. 2020;179:67–77. doi: 10.1007/s10549-019-05454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, Vergote I, Erikstein B, Webster A, Morris C. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J. Clin. Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 102.Omlin A, Jones RJ, van der Noll R, Satoh T, Niwakawa M, Smith SA, Graham J, Ong M, Finkelman RD, Schellens JH, Zivi A, Crespo M, Riisnaes R, Nava-Rodrigues D, Malone MD, Dive C, Sloane R, Moore D, Alumkal JJ, Dymond A, Dickinson PA, Ranson M, Clack G, de Bono J, Elliott T. AZD3514, an oral selective androgen receptor down-regulator in patients with castration-resistant prostate cancer - results of two parallel first-in-human phase I studies. Invest New Drugs. 2015;33:679–690. doi: 10.1007/s10637-015-0235-5. [DOI] [PubMed] [Google Scholar]
- 103.Bradbury RH, Acton DG, Broadbent NL, Brooks AN, Carr GR, Hatter G, Hayter BR, Hill KJ, Howe NJ, Jones RD, Jude D, Lamont SG, Loddick SA, McFarland HL, Parveen Z, Rabow AA, Sharma-Singh G, Stratton NC, Thomason AG, Trueman D, Walker GE, Wells SL, Wilson J, Wood JM. Discovery of AZD3514, a small-molecule androgen receptor downregulator for treatment of advanced prostate cancer. Bioorg Med Chem Lett. 2013;23:1945–1948. doi: 10.1016/j.bmcl.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 104.Salami J, Alabi S, Willard RR, Vitale NJ, Wang J, Dong H, Jin M, McDonnell DP, Crew AP, Neklesa TK, Crews CM. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol. 2018;1:100. doi: 10.1038/s42003-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han X, Wang C, Qin C, Xiang W, Fernandez-Salas E, Yang CY, Wang M, Zhao L, Xu T, Chinnaswamy K, Delproposto J, Stuckey J, Wang S. Discovery of ARD-69 as a highly potent Proteolysis Targeting Chimera (PROTAC) degrader of Androgen Receptor (AR) for the treatment of prostate cancer. J Med Chem. 2019;62:941–964. doi: 10.1021/acs.jmedchem.8b01631. [DOI] [PubMed] [Google Scholar]
- 106.Manasa VG, Kannan S. Impact of microRNA dynamics on cancer hallmarks: an oral cancer scenario. Tumour Biol. 2017;39:1010428317695920. doi: 10.1177/1010428317695920. [DOI] [PubMed] [Google Scholar]
- 107.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dhawan A, Scott JG, Harris AL, Buffa FM. Pan-cancer characterisation of microRNA across cancer hallmarks reveals microRNA-mediated downregulation of tumour suppressors. Nat Commun. 2018;9:5228. doi: 10.1038/s41467-018-07657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pereira TDSF, Brito JAR, Guimarães ALS, Gomes CC, de Lacerda JCT, de Castro WH, Coimbra RS, Diniz MG, Gomez RS. MicroRNA profiling reveals dysregulated microRNAs and their target gene regulatory networks in cemento-ossifying fibroma. J Oral Pathol Med. 2018;47:78–85. doi: 10.1111/jop.12650. [DOI] [PubMed] [Google Scholar]
- 110.Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics. 2019;11:25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alexis O, Worsley A. An integrative review exploring black men of African and Caribbean backgrounds, their fears of prostate cancer and their attitudes towards screening. Health Educ Res. 2018;33:155–166. doi: 10.1093/her/cyy001. [DOI] [PubMed] [Google Scholar]
- 112.Cooperberg MR. Re-examining racial disparities in prostate cancer outcomes. J. Clin. Oncol. 2013;31:2979–2980. doi: 10.1200/JCO.2013.50.7723. [DOI] [PubMed] [Google Scholar]


