Abstract
Introduction:
Opioid use disorder (OUD) is common among people in jail and is effectively treated with medications for OUD (MOUD). People with OUD may have an incomplete or inaccurate understanding of OUD and MOUD, and of how to access care. We evaluated an OUD treatment decision making (TDM) intervention to determine whether the intervention increased MOUD initiation post-release.
Methods:
We conducted an observational retrospective cohort study of the TDM intervention on initiation of MOUD, individuals with records data indicating confirmed or suspected OUD incarcerated in four eligible jails were eligible to receive the intervention. Time-to-event analyses of the TDM intervention were conducted using Cox proportional hazard modeling with MOUD as the outcome.
Results:
Cox proportional hazard modeling, with the intervention modeled as having a time-varying effect due to violation of the proportionality assumption, indicated that those receiving the TDM intervention (n = 568) were significantly more likely to initiate MOUD during the first month after release from jail (adjusted hazard ratio 6.27, 95 % C.I. 4.20–9.37), but not in subsequent months (AHR 1.33 95 % C.I. 0.94–1.89), adjusting for demographics, prior MOUD, or felony or gross misdemeanor arrest in the prior year compared to those not receiving the intervention (n = 3174).
Conclusion:
The TDM intervention was associated with a significantly higher relative hazard of starting MOUD, specifically during the first month after incarceration. However, a minority of all eligible people received any MOUD. Future research should examine ways to increase initiation on MOUD immediately after (or ideally during) incarceration.
Keywords: Opioid, Jail, Decision-making, Treatment, Buprenorphine, Methadone, Naltrexone
1. Introduction
Due to criminalization of substance use in the U.S., persons with opioid use disorder (OUD) are frequently incarcerated, and as a result, jails house many individuals with OUD (Green et al., 2014; Magura et al., 2009) and many people with opioid use disorder are frequently incarcerated (Banta-Green et al., 2018; Jenkins et al., 2011). For instance, previous research indicates that one in five prison inmates report a history of heroin use (Mumola and Karberg, 2006). In Washington State, among people surveyed at syringe exchanges in 2017, 39 % had been incarcerated in the prior year (Banta-Green et al., 2018). The time after release from incarceration is very high risk for relapse to opioid use (Fox et al., 2015a) as well as opioid involved overdoses, with former inmates having approximately a 10-fold higher overdose mortality rate compared to non-institutionalized people (Binswanger et al., 2013; Merrall et al., 2010). Incarcerations as short as five days are associated with significantly increased risk for non-fatal opioid overdoses (Jenkins et al., 2011).
OUD treatment with methadone and buprenorphine has been shown to lead to improved quality of life, reduced costs, reduced arrests, improved health care utilization including infectious disease treatment, and decreased mortality (Dolan et al., 2005; Hser et al., 2016; Johnson et al., 2000; Saxon et al., 2013; Tsui et al., 2014). These medications have been found to be effective for those with OUD involving heroin as well as pharmaceutical opioids (Banta-Green et al., 2009; Noe, 2012). Initial evidence for a newer medication, long-acting-naltrexone, indicates an association with decreased illicit opioid use, however initiation and retention rates are significantly lower than for buprenorphine on average (Larochelle et al., 2018; Lee et al., 2018, 2015; Morgan et al., 2018).
Based on surveys of syringe exchange clients in Washington State, most people with OUD do not want to be using illicit opioids and a majority of them are interested in medications for OUD (MOUD) (Banta-Green et al., 2018; Frost et al., 2018) yet are not accessing them. Barriers to initiating MOUD may include inaccurate information about the nature of OUD and MOUD as well as inaccurate or out-of-date information about care setting options to receive MOUD (Kelly et al., 2012; Schwartz et al., 2008) which are the targets of the intervention tested in this analysis. Other very important structural, financial, geographic, cultural and other barriers exist to initiating MOUD that were not within the scope of the intervention (Fox et al., 2015b; Walley et al., 2008).
The effectiveness of motivational enhancement interventions for increasing treatment entry among those with substance use disorders is mixed and warrants further study across care settings (Saitz et al., 2010). Research findings for motivational interventions specifically focused on MOUD uptake among those incarcerated do not appear to have been published. A randomized trial for people incarcerated in jail with a broad array of substance use disorders, a minority with OUD, did not find an impact on subsequent substance use or treatment engagement for all participants or for those with OUD though their intervention was not focused on MOUD (Prendergast et al., 2017). A qualitative study of jail inmates with alcohol use disorder explored perceptions of helpful components of brief interventions concluding that “Findings align with established approaches for working with marginalized groups, namely… shared decision-making models for treatment” (Owens et al., 2018). To address these potential knowledge, self-efficacy, and motivation barriers, a treatment decision making (TDM) intervention was developed and assessed for feasibility in a previous study with a population recently released from prison to community corrections (i.e. probation or parole) in Washington State (Banta-Green et al., 2019). TDM extends the process of shared decision making for other health conditions (Elwyn et al., 2012) moving beyond clinical settings and medical providers and with a focus on OUD and MOUD. The previous TDM intervention included an extensive discussion facilitated by trained research staff/treatment navigators with each participant about their perspective on the pros and cons of treatment options including social support, outpatient, inpatient and each of the three FDA approved medications. The treatment navigator then worked to connect the person to their preferred treatment service(s) in the community and provided ongoing treatment navigation services for six months. This small feasibility study found acceptability among participants for the process, modest interest in medications immediately upon release with increasing interest over time, and substantial implementation challenges due to the logistics of locating potential research participants post-prison release at community corrections offices. Based on the potential of this TDM intervention, the Washington State Department of Corrections (WADOC) chose to roll it out on a pilot basis at four jails in Washington State beginning in September 2017. Resource constraints prevented the inclusion of ongoing care navigation. The goal of this study was to evaluate the impact of the TDM intervention on initiating MOUD post-release.
2. Methods
2.1. Intervention
The person-centered TDM intervention: 1) included education on OUD and MOUD (using a 2-page educational brochure “Medications for Opioid Use Disorder” available at www.learnabouttreatment.org); 2) explored people’s perceptions and history of use of MOUD (See Fig. 1); 3) provided a motivational-interviewing-informed approach to evaluating the pros and cons of each medication, given a person’s preferences and life circumstances; and 4) helped identify specific next steps towards initiating MOUD, if selected as the desired treatment. Staff from the University of Washington Alcohol and Drug Abuse Institute provided initial training on the TDM intervention to WADOC re-entry staff. Study interventionists from the previous feasibility study did an onsite observation of each WADOC staff person implementing the refined TDM process for the intervention analyzed in this analysis to confirm general adherence to the TDM approach; ongoing formal fidelity monitoring was not conducted.
Fig. 1.
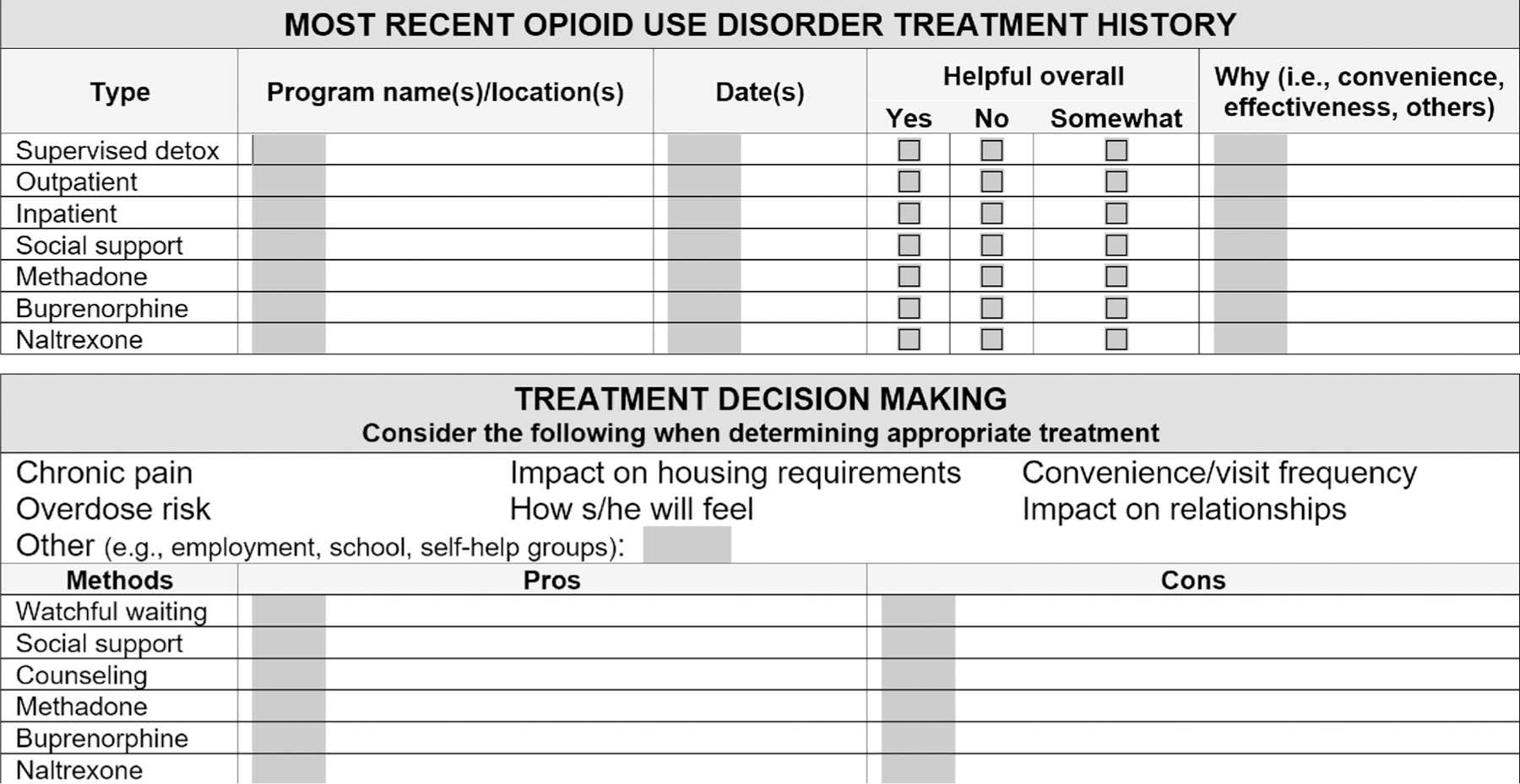
Treatment decision making tool.
Four WADOC re-entry staff delivered the TDM intervention at four different jails to which they were assigned, where people under WADOC supervision were incarcerated for violating the terms of their community supervision (i.e. probation or parole). The intervention was a one-time session delivered individually. WADOC re-entry staff searched WADOC databases for people approaching their release date at the facility to which they were assigned and then attempted to meet with potential participants prior to release. Staff approached as many people as possible for the intervention, but due to time and staffing constraints, not all potentially eligible people were approached. This became the pool from which we selected comparators.
Funding for the intervention was provided to WADOC by the Washington State Health Care Authority, Division of Behavioral Health and Recovery, with grant funding provided by the Substance Abuse and Mental Health Services Administration-State Targeted Response (SAMHSA-STR) grant.
2.2. Study design
We conducted an observational retrospective cohort study to evaluate the impact of the intervention on time to initiating MOUD. The intervention group consisted of those identified by WADOC as having been screened as potentially eligible and enrolled in the intervention. Those in the intervention arm may or may not have decided to complete the intervention or selected MOUD as their preferred treatment, but using an intent-to-treat approach all were considered to have received the intervention. The comparison group was constituted of people incarcerated at the same four facilities where the intervention was delivered, who had similar characteristics as the enrolled group, as documented in WADOC records, but were not enrolled in the intervention. This study was reviewed by the Washington State Institutional Review Board and was determined to be exempt from human subjects’ regulations.
2.3. Data sources
Data were processed initially by the Washington State Department of Social and Health Services-Research and Data Analysis (DSHS-RDA) group, who created linked and merged datasets using a unique study identification number; records were subsequently stripped of all potentially identifying information before release to the research team. DSHS-RDA had maintained a list of TDM intervention participants in order to provide data support for WA State’s SAMHSA-STR grant. The analyses utilized data for those eligible for the TDM intervention between September 1, 2017 and August 31, 2018
DSHS-RDA provided data from a client database they manage including arrests and associated crime types for felonies and gross misdemeanors which were provided as counts per calendar month from an original dataset administered by the Washington State Patrol. For MOUD, buprenorphine and naltrexone prescription data from Medicaid were provided as counts of prescriptions per month and methadone used for OUD in an opioid treatment program (data from State treatment authority) was provided as an indicator of any utilization in a month. MOUD treatment data, used to construct the outcome of interest, were available through January 31, 2019.
WADOC Research and Data Analytics provided data to DSHS-RDA on all stays at local jail facilities during the evaluation window in which the individual was on community supervision violation status; these are stays of days to weeks, generally less than 30 days. Data elements indicative of possible opioid use disorder were utilized and are detailed below.
2.4. Eligibility and recruitment
For the analysis, we designed a set of inclusion and exclusion criteria for the intervention and comparison groups based on data that were available for research which excluded access to some additional computer notes fields and historical urine drug testing data results not available in secondary data. Some individuals known to have received the intervention, but for whom secondary data did not explicitly document eligibility, were excluded from this study in the interest of creating the most comparable groups since those individuals could not be identified in a manner equivalent to the comparison group.
Eligibility for the analysis included having data elements indicative of possible opioid use disorder: a documented diagnosis of OUD, self-reported opioid use, or urine drug tests indicating opiate, buprenorphine, oxycodone, and methadone. Those with urine drug test results positive for methadone or buprenorphine were excluded if the person had recorded publicly-funded dispensing or prescription for the same medication in the month of or the month prior to the urinalysis as this was considered indicative of current authorized MOUD use. People with documented MOUD in the calendar month prior to entering the facility were similarly excluded.
A given person could be ineligible (no data indicating possible OUD, perhaps due to no recent opioid use) for one visit but eligible upon a subsequent visit. Episodes were coded as having received the intervention if an intervention enrollment date for a particular study ID fell within the start and end date of a stay with the same study ID.
Fig. 2 shows how various trajectories of visits during and after the intervention window affect group assignment and follow-up. The figure shows one month prior to implementation, the 12-month intervention period, and then the five month window that just contributed follow up time. The shading indicates months for which follow up data were utilized for the episode, enrolled or not. The first example has one month of available follow-up for the first visit, during which the person was enrolled, subsequent episodes of incarceration occur, but due to the restriction that non-enrollments could not be included as comparison episodes if the person had been enrolled previously (since they received the TDM intervention), these episodes are not included in the analysis. Example 2 contributes two enrollment episodes, but the third possible episode, during which they were not enrolled, is censored due to incarceration. In contrast, example 3 has three episodes during the intervention window in which they were not enrolled, all included. Example 4 shows a person not enrolled for one episode and enrolled in a subsequent visit and then censored due to incarceration. For example 5, incarceration during the last month of the intervention window is followed until the end of the available data, while example 6, like example 3, is censored by re-incarceration (for violation of community supervision) after the intervention window.
Fig. 2.
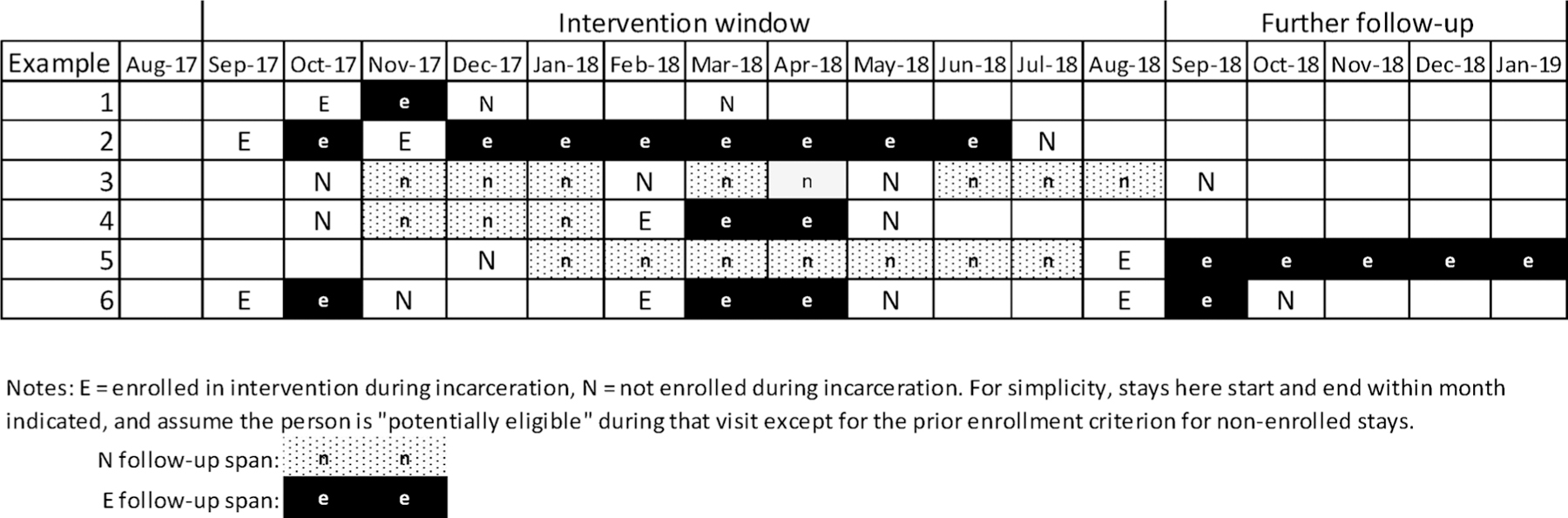
Examples of episode of care construction.
2.5. Variables
2.5.1. Main outcome/dependent variable
The MOUD initiation outcome was constructed by identifying the first post-release month in which any MOUD was observed. Individuals could have received multiple kinds of MOUD after release, but only the first prescription or dispensing was used in the time-to-event analyses. Episodes were censored as of the month of re-incarceration. Any incarceration stay without a release date was assumed to be ongoing as of the end of the window and removed from analyses. Contiguous days, in which the end date of one was on the beginning date of another, were combined into a single episode. These may represent recorded transfers, additional sanctions and time added subsequent to initiating the current stay, or literally release and re-incarceration in the same day. These cannot be differentiated, but all represent stays in which the individual could not have any follow-up time after the first stay, so they were merged into a single episode.
2.5.2. Main predictor/independent variable
TDM intervention participation was based upon a log maintained by DSHS-RDA in their role as the data collector for the SAMHSA-STR grant.
2.5.3. Covariates
WADOC data consisted of the facility name, admission date, and release date. Standardized data elements used by WADOC re-entry staff to identify people in need of assistance with potential opioid use disorder (OUD) were utilized. Needs assessments were completed upon incarceration that documented self-reported drug use, specifically heroin and prescription drugs. Responses indicating opioid use (from an open-ended follow-up to the prescription drug question) were classified as indicating opioid use, based upon a text string search. Urine drug test results for various types of opioids were utilized. Finally, indicators of whether the person had a recorded diagnosis of OUD were documented.
Age, gender, and ethnicity (collapsed to white-only versus any minority, including Latino/Hispanic) were documented in administrative datasets. Other covariates were aggregated over the 12 available months prior to incarceration, including indicators for prior experience with MOUD (any documented buprenorphine, combination buprenorphine-naloxone, naltrexone, or methadone treatment), and prior arrest history for felony and gross misdemeanors.
2.6. Statistical analysis
An episode based analysis was utilized to account for people having multiple incarcerations in the evaluation window—people may have been enrolled in the intervention during one or more stays and not enrolled during others. Follow-up was conducted post jail release until an event of interest or censoring occurred. Individuals were censored when a subsequent community supervision violation stay was initiated, or when the observation period ended on January 31, 2019. Among non-intervention stays, any involving an individual previously receiving the intervention were excluded to avoid contamination since that individual had previously been exposed to the intervention. Follow-up time was measured in months until either the event of interest or censoring occurs, with the month of exit being considered month 0.
We used Cox proportional hazard models to identify differences in time to outcome for episodes enrolled in TDM versus similar episodes among those not enrolled, controlling for covariates described above. Analyses were conducted with Stata 14. Cox models were estimated by stratifying on entry facility (modeling as a fixed effect), with robust standard errors clustered on unique individuals.
3. Results
Descriptive statistics for the 3174 non-enrolled episodes and 568 enrolled episodes are provided in Table 1, these data represent 2388 unique individuals. Intervention recipients were slightly more likely to be white only, younger, and to have been arrested overall, for both felonies and gross misdemeanors, in the prior year.
Table 1.
Descriptive statistics for intervention and non-intervention episodes.
| No Intervention |
Intervention |
Total |
||||
|---|---|---|---|---|---|---|
| (n = 3174) |
(n = 568) |
(n = 3742) |
||||
| % or Mean | SD | % or Mean | SD | % or Mean | SD | |
| Female | 20 % | 0.4 | 21% | 0.41 | 20 % | 0.4 |
| White only | 53 % | 0.5 | 58 % | 0.49 | 53 % | 0.5 |
| Incarceration duration | 14.4 | 12.3 | 22.3 | 7.3 | 15.6 | 12.0 |
| Age | 35.9 | 10.4 | 34.5 | 9.2 | 35.7 | 10.3 |
| 18–29 | 34% | 39 % | 35 % | |||
| 30–39 | 36 % | 38 % | 37 % | |||
| 40–49 | 18 % | 16 % | 18 % | |||
| 50+ | 12 % | 8 % | 12 % | |||
| MOUD prior yeara | 6 % | 0.25 | 9% | 0.29 | 7 % | 0.25 |
| Felony prior year | 54 % | 0.50 | 58 % | 0.49 | 55% | 0.5 |
| Gross misdemeanor prior year | 26 % | 0.44 | 32% | 0.47 | 27 % | 0.44 |
| Any arrest prior year | 62 % | 0.49 | 67 % | 0.47 | 62 % | 0.48 |
MOUD medications for opioid use disorder- methadone, buprenorphine or naltrexone.
There were 349 occurrences of receiving publicly-funded MOUD, the outcome of interest. Fig. 3 describes documented MOUD utilization before and after release by medication type. Incarcerations episodes where people received the intervention were more likely to have had buprenorphine prior to the violation visit.
Fig. 3.
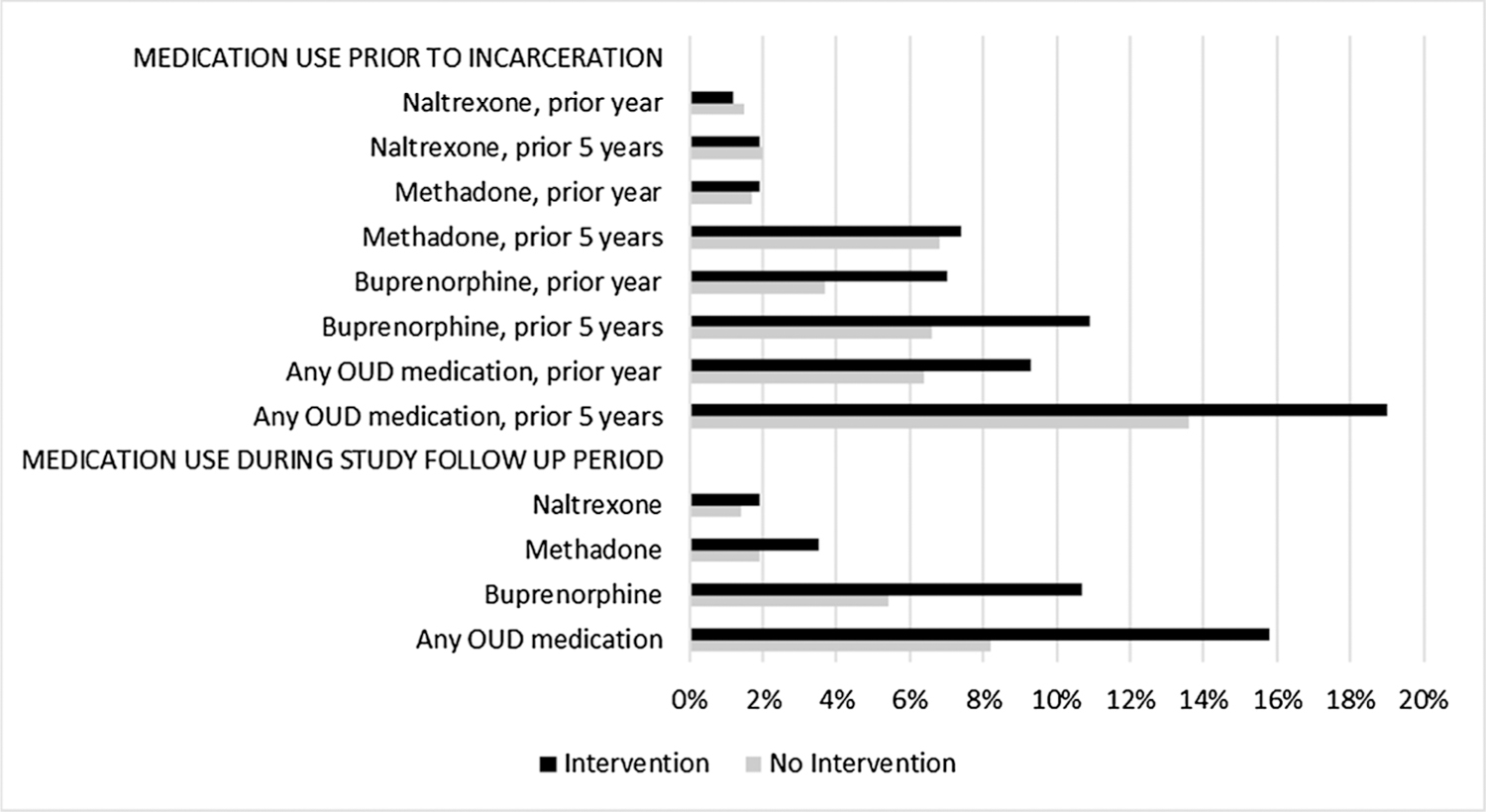
Types of medications used before and after incarceration.
Kaplan-Meier survival curves (Fig. 4) are presented by group (TDM intervention v. no intervention), indicating MOUD was initiated by 16 % of intervention recipients compared to 8 % of the comparison group, not adjusting for demographics, arrest or medication history.
Fig. 4.
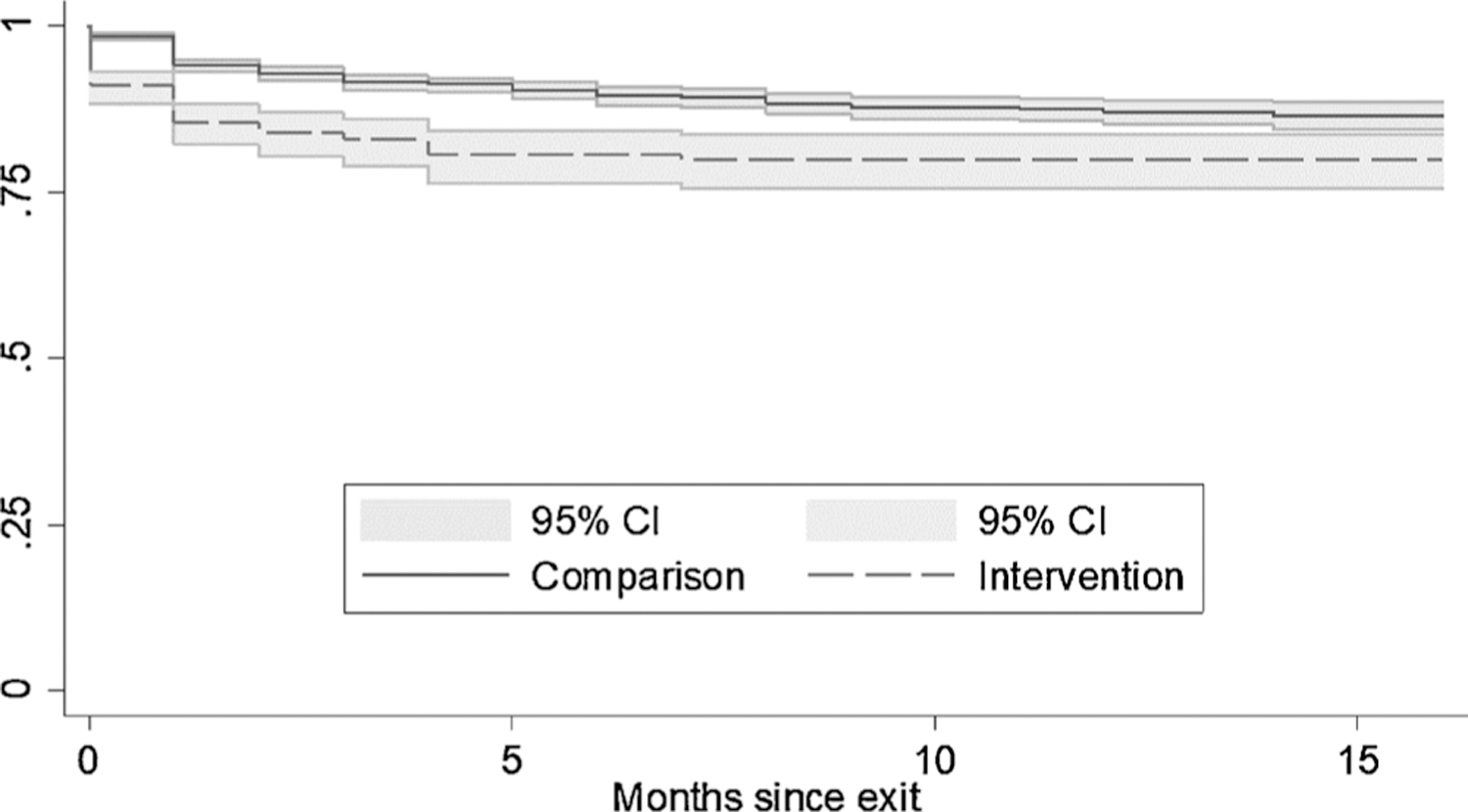
Kaplan-Meier survival curves with 95 % confidence intervals, time to starting medications for opioid use disorder.
The initial Cox proportional hazard model testing the effect of the intervention on MOUD initiation adjusting for demographics, arrest and MOUD history was found to violate the proportional hazards globally (χ2 = 35.85, df = 8, p < 0.001) and with respect to the key variable, enrolled (χ2 = 11.37, df = 1, p < 0.001). To address this, intervention enrollment was modelled as having a time-varying effect (using the Stata -tvc- option). Models with continuous time in months and with indicators for time less than one (i.e. in the exit month only), less than two, and less than three months were estimated. The model fit was best for month < 1, the exit month, per both Akaike and Bayesian Information Criteria (Claeskens and Hjort, 2010). (Model fit statistics available upon request.)
Intervention enrollment is associated with significantly increased uptake of MOUD in the exit month (HR 6.27 95 % C.I. 4.20–9.37) and not associated after the first month onward (HR 1.33 95 % C.I. 0.94–1.89) adjusting for demographics, MOUD and arrest history (Table 2).
Table 2.
Impact of intervention on time to MOUD initiation-Cox proportional hazard result.
| Hazard Ratio | SE | z | p | 95 % Confidence Interval | ||
|---|---|---|---|---|---|---|
| Intervention impact during exit month | 6.27 | 1.29 | 8.95 | 0.000 | 4.20 | 9.37 |
| Intervention impact month 1 & onward | 1.33 | 0.24 | 1.59 | 0.112 | 0.94 | 1.89 |
| Female | 1.73 | 0.21 | 4.55 | 0.000 | 1.37 | 2.19 |
| White only | 1.61 | 0.18 | 4.23 | 0.000 | 1.29 | 2.01 |
| Age, 18–29 as reference | … | |||||
| 30–39 | 0.94 | 0.11 | −0.53 | 0.596 | 0.74 | 1.19 |
| 40–49 | 0.75 | 0.13 | −1.73 | 0.084 | 0.53 | 1.04 |
| 50+ | 0.67 | 0.15 | −1.84 | 0.066 | 0.44 | 1.03 |
| Any MOUD in prior yeara | 4.45 | 0.62 | 10.67 | 0.000 | 3.38 | 5.85 |
| Any arrest in prior year | 1.01 | 0.11 | 0.05 | 0.962 | 0.80 | 1.26 |
MOUD medications for opioid use disorder- methadone, buprenorphine or naltrexone.
4. Discussion
A significant, but short-lived, effect of the TDM intervention on MOUD initiation was found. Given the high rates of relapse, multiple stressors, and substance use reinforcers upon release (Clark et al., 2014, 2011; Magura et al., 2009) it is perhaps not surprising that if people who were offered the intervention did not start MOUD within the first month, they were no more likely than the group who did not get the intervention to initiate MOUD after a month had elapsed. Challenges during the period immediately following re-entry are numerous including: busy schedules with probation/parole visits, job interviews, reconnecting with family, child care obligations, searching for housing, and obtaining health insurance and identification (Johnson, 2007; Rose et al., 2008; Travis et al., 2005). Although there was a statistically significant impact of the intervention on MOUD initiation, only 16 % of people initiated MOUD during the follow-up period, among those determined to potentially had OUD. More needs to be done to create care that is accessible for people with OUD, particularly those with more chaotic lives who may not be able to engage or stay in care in an opioid treatment program or primary care setting either because of their own behaviors and circumstances or MOUD programs’ unwillingness to serve them (Kourounis et al., 2016). The time in incarceration and the available infrastructure are increasingly utilized to initiate people on MOUD prior to release and this should be widely implemented given the need and the evidence (Brinkley-Rubinstein et al., 2018; Green et al., 2018; Kinlock et al., 2009; Magura et al., 2009).
Descriptive analyses indicated that the study population was mostly male, a slight majority white only, and relatively young, with a mean age of 35.6. Few had been on MOUD in the prior year (7 %) while a majority had committed a felony in the prior year. Given that MOUD has been found to decrease arrests (Ettner et al., 2006), these results showing very low MOUD utilization and very high arrest rates suggest important barriers to access care and much potential to improve outcomes.
Time to event analyses indicated that women and those identified as white only were significantly more likely to initiate MOUD post-release. This is an important and concerning finding given that 80 % of those studied were male and 47 % were people of color. Previous research indicates that women and whites generally have better retention rates including findings from a multi-side study of Medicaid enrollees (Samples et al., 2018) and that hospitalized people with OUD were less likely to initiate buprenorphine in the community if they were non-white(Lee et al., 2017). Any MOUD in the prior year was strongly associated with higher likelihood of MOUD initiation post release, which indicates the importance of exploring in future research how to enhance medication uptake among those without a previous history of utilizing MOUD. Given that most people had not been on MOUD previously, this represents an important population to address given the currently unrealized potential for improved outcomes if effective engagement approaches could be identified. Arrest in the prior year was not uniquely associated with time to medication initiation, indicating that there may be no reason to consider those with a recent arrest record any less likely to be successfully started on MOUD.
4.1. Strengths and limitations
The study design and data sources allowed for a relatively rapid analysis of a new intervention on an important topic for a population at high risk for relapse to opioid use disorder and overdose. The study design, a retrospective cohort study, does not allow for the results to be interpreted as showing a causal relationship between the intervention and the outcome. We chose to exclude some people who received the intervention, but who we could not identify as eligible from secondary records data to enhance the comparability of the groups, therefore the proportion of who received any MOUD may be underestimate.
Pre-incarceration demographic and arrest histories were very similar for the intervention and comparison groups, although the intervention group was somewhat more likely to have received buprenorphine in past. These characteristics were adjusted for in the final model to account for differences between groups. However, there remains the possibility of unmeasured confounding and differences between the groups that could have impacted the study findings. The potential direction of this bias is not easily determined and should be kept in mind when interpreting the results.
Eligibility was based upon potential OUD, however a formal diagnosis of OUD was not present for many of those categorized as eligible for the intervention. It is possible that some who were eligible for the study did not have OUD, therefore the proportion who got MOUD among those who actually had OUD may be higher than the proportion we report among those who potentially had OUD.
Unlike the screening conducted by re-entry staff conducting the intervention to identify potentially eligible people, who could see all recorded historical urinalysis results, only urinalysis results within the evaluation window were available for constructing the comparison group. Some of the episodes excluded from analysis as intervention episodes may be due to this data issue.
Those in the intervention arm may or may not have decided to complete the intervention or selected MOUD as their preferred treatment, but using an intent-to-treat approach all were considered to have received the intervention. Although not technically a study limitation, this could result in an under-estimate of the impact of the intervention. Comparison episodes were substantially shorter than intervention episodes on average. WADOC intervention staff aimed to talk with people several days prior to release, so it is possible that not receiving the intervention is related to the shorter incarcerations. The possible direction of bias this introduces is not known, however the comparison group having shorter episodes suggests that efforts to provide the intervention to those with shorter stays may need to be improved.
For those approached to be enrolled in the intervention, many of those marked as eligible were not reached, whether they left before the counselor could contact them or otherwise were not available on the given day. Some secondary screening occurred by re-entry staff, which may have eliminated from the intervention group those with recent behavior problems, work details, recent transfers to other facilities, etc. which could also be related to the likelihood of initiating MOUD.
Data on MOUD utilization were limited to publicly funded care. This could have impacted the results in several ways. Both historical and prospective utilization of MOUD are potentially under-estimated. In addition, our process of excluding episodes with urine drug tests positive for methadone or buprenorphine for people who were known to be on those treatment medications during the same month, may have misidentified use as representing illicit use and therefore included people who were ineligible. This would be an issue only to the extent such individuals did not also meet other criteria (i.e. self-reported illicit use or an OUD diagnosis).
Data utilized were for receipt of a MOUD during a particular month. More detailed data on the number of days of care or doses received would provide important additional information and more fine-tuned temporal data would provide more subtle insights into the timing of medication initiation than available in the month-based data available for these analyses.
5. Conclusion
The TDM intervention was found to be associated with a modest, but statistically significant, increase in MOUD uptake during the first month after release, but there was no significant difference after the first month. Given that the intervention was a brief one-time intervention, the impact on MOUD initiation is encouraging; however, more robust interventions to increase uptake further need to be developed and tested. In parallel, efforts to increase medication initiation prior to release, if possible, and post-release, if that is all that is feasible should be dramatically expanded. The short-term nature of the effect found here supports efforts to determine how to support people in staying on MOUD as long as they wish to continue. There are important medical and ethical imperatives to deliver MOUD to incarcerated people and we need to learn how to provide meaningful access to all people, (Beletsky et al., 2015; Wakeman, 2017) particularly marginalized populations at high risk for overdose due to frequent incarcerations. Substantial opportunities to further increase uptake on MOUD remain, and–given the seriousness of untreated OUD in this population–this work warrants rapid action on research, policy, and practice fronts.
Acknowledgements
The authors would like to thank Vasiliki Georgoulas-Sherry, Paige Harrison, Dawn Williams, and Linda Barker of the Washington State Department of Corrections, along with Jim Mayfield of the Washington State Department of Social and Health Services Research and Data Analysis division for their support of this project.
Role of funding source
This study was supported by funds from Arnold Ventures in 2016–2019. The views expressed in this report are the authors’ and do not necessarily reflect the views of the funder.
Footnotes
Declaration of Competing Interest
None.
References
- Banta-Green CJ, Floyd AS, Vick K, Arthur J, Hoeft TJ, Tsui JI, 2019. Opioid use disorder treatment decision making and care navigation upon release from prison: a feasibility study. Subst. Abuse Rehabil 10, 57–67. 10.2147/SAR.S192045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta-Green CJ, Maynard C, Koepsell TD, Wells EA, Donovan DM, 2009. Retention in methadone maintenance drug treatment for prescription-type opioid primary users compared to heroin users. Addiction 104, 775–783. [DOI] [PubMed] [Google Scholar]
- Banta-Green CJ, Newman A, Kingston S, 2018. Washington State Syringe Exchange Health Survey: 2017 Results [WWW Document]. Alcohol Drug Abus Institute, Univ. Washingt. Webpage. URL http://adai.uw.edu/pubs/pdf/2017syringeexchangehealthsurvey.pdf (accessed 5.5.18). [Google Scholar]
- Beletsky L, Lasalle L, Newman M, Tochka A, 2015. Prisoners’ rights in the modern era individuals newly released from incarceration. Northeast. Univ. Law J 7. [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, Stern MF, 2013. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann. Intern. Med 159, 592–600. 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley-Rubinstein L, McKenzie M, Macmadu A, Larney S, Zaller N, Dauria E, Rich J, 2018. A randomized, open label trial of methadone continuation versus forced withdrawal in a combined US prison and jail: findings at 12 months post-release. Drug Alcohol Depend 184, 57–63. 10.1016/j.drugalcdep.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeskens G, Hjort NL, 2010. The Bayesian information criterion. Model Selection and Model Averaging Cambridge University Press, Cambridge, pp. 70–98. 10.1017/CBO9780511790485.004. [DOI] [Google Scholar]
- Clark RE, Baxter JD, Barton BA, Aweh G, O’Connell E, Fisher WH, 2014. The impact of prior authorization on buprenorphine dose, relapse rates, and cost for Massachusetts Medicaid beneficiaries with opioid dependence. Health Serv. Res 49, 1964–1979. 10.1111/1475-6773.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Samnaliev M, Baxter JD, Leung GY, 2011. The evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff. (Millwood) 30, 1425–1433. 10.1377/hlthaff.2010.0532. [DOI] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD, 2005. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction 100, 820–828. 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, Edwards A, Barry M, 2012. Shared decision making: a model for clinical practice. J. Gen. Intern. Med 27 (10), 1361–1367. 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettner SL, Huang D, Evans E, Rose Ash D, Hardy M, Jourabchi M, Hser Y-I, 2006. Benefit-cost in the California treatment outcome project: does substance abuse treatment “pay for itself”. Health Serv. Res 41, 192–213. 10.1111/j.1475-6773.2005.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AD, Chamberlain A, Sohler NL, Frost T, Cunningham CO, 2015a. Illicit buprenorphine use, interest in and access to buprenorphine treatment among syringe exchange participants. J. Subst. Abuse Treat 48, 112–116. 10.1016/j.jsat.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AD, Maradiaga J, Weiss L, Sanchez J, Starrels JL, Cunningham CO, 2015b. Release from incarceration, relapse to opioid use and the potential for buprenorphine maintenance treatment: a qualitative study of the perceptions of former inmates with opioid use disorder. Addict. Sci. Clin. Pract 10, 2. 10.1186/s13722-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MC, Williams EC, Kingston S, Banta-Green CJ, 2018. Interest in getting help to reduce or stop substance use among syringe exchange clients who use opioids. J. Addict. Med 1. 10.1097/ADM.0000000000000426. [DOI] [PubMed] [Google Scholar]
- Green TC, Bowman SE, Ray M, McKenzie M, Lord SE, Rich JD, 2014. Development of an incarceration-specific overdose prevention video: staying alive on the outside. Health Educ. J 74, 627–637. 10.1177/0017896914550321. [DOI] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, Marshall BDL, Alexander-Scott N, Boss R, Rich JD, 2018. Postincarceration fatal overdoses after implementing medications for addiction treatment in a statewide correctional system. JAMA Psychiatry 75, 405. 10.1001/jamapsychiatry.2017.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, Liu D, Wakim P, Matthews AG, Hatch-Maillette M, Jelstrom E, Wiest K, Mclaughlin P, Ling W, 2016. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 111, 695–705. 10.1111/add.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LM, Banta-Green CJ, Maynard C, Kingston S, Hanrahan M, Merrill JO, Coffin PO, 2011. Risk factors for nonfatal overdose at seattle-area syringe exchanges. J. Urban Health 88, 118–128. 10.1007/s11524-010-9525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE, 2000. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N. Engl. J. Med. 343, 1290–1297. 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Johnson SE, 2007. Post-Incarcerated Mothers’ Perceptions of Their Relationships with Their Children and Patterns of Re-Offense. ProQuest Diss. Theses
- Kelly SM, Brown BS, Katz EC, O’Grady KE, Mitchell SG, King S, Schwartz RP, 2012. A comparison of attitudes toward opioid agonist treatment among short-term buprenorphine patients. Am. J. Drug Alcohol Abuse 38, 233–238. 10.3109/00952990.2011.643983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE, 2009. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J. Subst. Abuse Treat 37, 277–285. 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourounis G, Richards BDW, Kyprianou E, Symeonidou E, Malliori MM, Samartzis L, 2016. Opioid substitution therapy: lowering the treatment thresholds. Drug Alcohol Depend 161, 1–8. 10.1016/j.drugalcdep.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for opioid use disorder after non-fatal opioid overdose and association with mortality. Ann. Intern. Med 169 (3), 137–145. 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Liebschutz JM, Anderson BJ, Stein MD, 2017. Hospitalized opioid-dependent patients: exploring predictors of buprenorphine treatment entry and retention after discharge. Am. J. Addict 26, 667–672. 10.1111/ajad.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, Gourevitch MN, 2015. Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction 110, 1008–1014. 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, Shmueli-Blumberg D, Stablein D, Subramaniam G, Rotrosen J, 2018. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet (London, England) 391, 309–318. 10.1016/S0140-6736(17)32812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, Rosenblum A, 2009. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend 99, 222–230. 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, Bird SM, 2010. Meta-analysis of drug-related deaths soon after release from prison. Addiction 105, 1545–1554. 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY, 2018. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J. Subst. Abuse Treat 85, 90–96. 10.1016/j.jsat.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumola CJ, Karberg JC, 2006. Drug use and dependence, state and federal prisoners, 2004. Bur. Justice Stat. Spec. Rep doi:NCJ 1728171.
- Noe SR, 2012. Office-based Buprenorphine Treatment: Identifying Factors That Promote Retention in Opioid Dependent Patients pp. 41. http://search.ebscohost.com/login.aspx?direct=true&site=eds-live&db=rzh&AN=109861025. Accessed December 16, 2019. [DOI] [PubMed]
- Owens MD, Kirouac M, Hagler K, Rowell LN, Williams EC, 2018. Being able to speak: what individuals in jail perceived as helpful about participating in alcohol-related brief interventions. Subst. Abus 39, 342–347. 10.1080/08897077.2017.1393034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast ML, McCollister K, Warda U, 2017. A randomized study of the use of screening, brief intervention, and referral to treatment (SBIRT) for drug and alcohol use with jail inmates. J. Subst. Abuse Treat 74, 54–64. 10.1016/j.jsat.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DR, Michalsen V, Wiest DR, 2008. Women, Re-Entry and Everyday Life: Time to Work? Women’s Prison Association, New York. [Google Scholar]
- Saitz R, Alford DP, Bernstein J, Cheng DM, Samet J, Palfai T, 2010. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J. Addict. Med 4, 123–130. 10.1097/ADM.0b013e3181db6b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples H, Williams AR, Olfson M, Crystal S, 2018. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J. Subst. Abuse Treat 95, 9–17. 10.1016/j.jsat.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Hser YI, Woody G, Ling W, 2013. Medication-assisted treatment for opioid addiction: methadone and buprenorphine. J. Food Drug Anal 21 (4), S69–S72. 10.1016/j.jfda.2013.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Peterson JA, Reisinger HS, Agar MH, Brown BS, 2008. Attitudes toward buprenorphine and methadone among opioid-dependent individuals. Am. J. Addict 17, 396–401. 10.1080/10550490802268835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J, McBride EC, Solomon AL, 2005. Families left behind: the hidden costs of incarceration and reentry Children. Available online at: http://webarchive.urban.org/publications/310882.html. Accessed October 1, 2019. [Google Scholar]
- Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K, 2014. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern. Med 174, 1974–1981. 10.1001/jamainternmed.2014.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman SE, 2017. Why it’s inappropriate not to treat incarcerated patients with opioid agonist therapy. AMA J. Ethics 19, 922–930. 10.1001/journalofethics.2017.19.9.stas1-1709. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP, 2008. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J. Gen. Intern. Med 23, 1393–1398. 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


