Abstract
Hypothalamic AgRP and POMC neurons are conventionally viewed as the yin and yang of the body’s energy status, since they act in an opposite manner to modulate appetite and systemic energy metabolism. However, although AgRP neurons’ functions are comparatively well understood, a unifying theory of how POMC neuronal cells operate has remained elusive, probably due to their high level of heterogeneity, which suggests that their physiological roles might be more complex than initially thought. In this Perspective, we propose a conceptual framework that integrates POMC neuronal heterogeneity with appetite regulation, whole-body metabolic physiology and the development of obesity. We highlight emerging evidence indicating that POMC neurons respond to distinct combinations of interoceptive signals and food-related cues to fine-tune divergent metabolic pathways and behaviours necessary for survival. The new framework we propose reflects the high degree of developmental plasticity of this neuronal population and may enable progress towards understanding of both the aetiology and treatment of metabolic disorders.
Since the discovery of the hormone leptin and its powerful metabolic effects1, the field of neuroendocrinology has made enormous progress in understanding how the brain orchestrates appetite and peripheral metabolism, and a population of neurons expressing the peptidergic precursor pro-opiomelanocortin (POMC) has emerged as a key piece of this puzzle.
POMC neuronal cells are mostly located in the hypothalamus and release bioactive molecules (melanocortins) as a result of the post-translational cleavage of POMC, which signals via specialized brain metabolic receptors (melanocortin receptor type 4 (MC4R)) to modulate food intake and systemic energy metabolism2.
POMC neurons are switched off under conditions of low energy availability while being stimulated by hormonal and nutrient-related signals of positive energy. Their activation in response to energy supply, such as after a meal, promotes maintenance of a stable body weight by reducing food intake and increasing energy dissipation3,4.
A second neuronal population co-expressing neuropeptide Y (NPY) and the agouti-related protein (AgRP) is instead activated by negative energy balance. Following activation, NPY/AgRP neurons inhibit POMC neurons5,6 and antagonize central MC4R signalling via the release of AgRP7, ultimately stimulating food intake and reducing energy expenditure.
This simple neurobiological model has offered a foundation for understanding how the brain regulates whole-body energy handling, and a mechanistic rationale explaining why loss-of-function mutations affecting the POMC or MC4R gene cause severe forms of obesity8–11. In fact, synthetic MC4R agonists are now being scrutinized for the treatment of obesity12–14.
The canonical view whereby AgRP and POMC neurons behave as yin–yang partners, however, may still be incomplete, and perhaps too simplistic. On the basis of recent chemogenetic (ligand-based) and optogenetic (light-based) approaches, which provide real-time information on how neuronal activities translate into behavioural or metabolic outputs, POMC neuronal activation under specific conditions can produce similar, rather than opposite, behavioural effects relative to NPY/AgRP neuronal stimulation, including the promotion of feeding15. Moreover, rapid in vivo activation of NPY/AgRP neurons promotes hunger independently of POMC neuronal activity16–18, suggesting that these supposedly antagonistic populations do not always influence appetite in an interdependent manner.
One possible explanation for these differences lies in the heterogeneous nature of POMC neurons. More than 10 years ago, ex vivo studies addressing the electrochemical properties of hypothalamic POMC neurons surprisingly revealed that POMC neurons are electrophysiologically diverse, as they respond differently to neurotransmitters and hormones19,20. Now that molecular profiling of hypothalamic neurons at a single-cell resolution is increasingly used21–23, the field can no longer ignore the fact that POMC neurons form several, molecularly distinct clusters21–23. This molecular complexity probably translates into functionally divergent effects and might explain why an overarching theory on the mode(s) of action of these neurons has not yet been achieved.
In this Perspective, we propose that the physiological purpose of POMC neurons is broader and more complex than was originally predicted, owing to mechanisms of intracellular and intercellular plasticity that have yet to be fully explored. Thus, we present new models that interrogate how the heterogeneous nature of POMC neurons can be linked with appetite regulation, metabolic control and obesity pathophysiology.
One population, multiple effects: changing POMC neurons’ dogma
According to the prevailing view, POMC neuronal activity is modulated by the body’s energy status. Indeed, Pomc messenger RNA (mRNA) expression, POMC peptide release and POMC neuronal activation (as assessed by the marker c-Fos) decrease when systemic energy levels drop (fasting) and increase when the body’s energy levels recover (feeding)24–27.
An often-underappreciated aspect, however, is that only 20–50% of all POMC neurons show activity changes in response to nutritional variations26–29, which may be the result of cellular heterogeneity (see below) but may also be due to one intrinsic technical caveat of these observations: c-Fos, the neuronal activation marker used in the majority of these studies, does not provide temporal information. Neurons with rapid/transitory activation in response to the stimulus (food) may thus not show c-Fos immunoreactivity by the time the marker is assessed, which is typically within 1–2 h after the stimulus.
The use of fibre photometry and optetrode electrophysiology, which record real-time neuronal activity in awake mice, has provided unexpected insights into POMC neuronal regulation. Rapid activation is detected after the presentation of regular chow or palatable food, before the food is actually consumed, and in association with food-related sensory cues25,30,31. When food is presented to a mouse, the animal starts eating and continues to eat avidly, sometimes well after the initial and sustained POMC neuronal activation32, which appears to contradict the established model that POMC neurons encode a satiety signal32.
In fact, POMC neuronal activation for several hours does not influence food intake16,33,34; rather, chronic neuronal activation (from 24 h to multiple days) is required in order to observe the classic appetite-reducing effect16,33–35. Under certain conditions, POMC neurons can even promote food intake. Cannabinoid-dependent hyperphagia, for example, is amplified by chemogenetic stimulation of hypothalamic POMC neurons15, due to the release of the less studied hunger-promoting POMC-derived opioid β-endorphin15.
Taken together, in vivo manipulations and recordings of POMC neurons belie their proposed role as a satiety signal. Instead, these neurons appear to operate in ways that are reminiscent of the dog in Pavlov’s famous experiment, which salivates in response to the ringing of a bell that predicts food availability. Accordingly, changes in molecular pathways implicated in intracellular nutrient sensing (for example, the mechanistic target of rapamycin pathway) are observed in the liver of fasted mice immediately after food presentation, and this response is primed by POMC neuronal activation25.
Thus, in addition to affecting feeding behaviour and other metabolic outputs (see below) in response to the body’s metabolic needs, POMC neurons contribute to anticipatory (or cephalic) mechanisms, which use environmental cues associated with the energy source, such as its smell or sight, to prepare the body for the imminent consumption of a meal36.
Dismantling the bulk: multiple subsets and multiple purposes
Spatial heterogeneity.
Within the brain, POMC neurons are mainly located in the hypothalamic arcuate nucleus (ARC), a region with a leaky blood–brain barrier where blood-borne signals can rapidly enter and come into contact with the local network37. In the ARC, POMC neurons receive broad and dense inputs from other brain regions and target multiple forebrain centres38.
Whereas cells positioned in the rostral ARC project mainly to autonomic areas39, caudal POMCARC neurons have mostly, but not exclusively38, axonal connections within the hypothalamus40. This complex organization explains how such a relatively small population (~9,000 cells41) can respond to a plethora of extracellular signals, which are all implicated in whole-body metabolic control.
A small fraction of POMC neurons are also present outside the hypothalamus, in the brainstem nucleus of the solitary tract (NTS)42, a hub within the hindbrain that is sensitive to gastrointestinal hormones that modulate food intake43. POMCARC and POMCNTS subpopulations share similarities while also being different. Both clusters are equipped with specific receptors that allow responding to signals implicated in appetite and energy balance regulation, such as leptin or somatostatin42 (see below). However, whereas acute activation of POMCNTS produces an immediate inhibitory feeding response34, POMCARC neurons need to be stimulated over a few hours to days to suppress food intake16,34.
Spatially segregated POMC neuronal subpopulations may therefore operate at different time scales, possibly via divergent yet coordinated neurophysiological mechanisms, highlighting the close link between spatial and functional heterogeneity.
Heterogeneous expression of receptors.
Several extracellular messengers, including insulin, leptin and serotonin (among others) may affect systemic energy balance by acting through different POMC neuronal subpopulations. Based on single-cell transcriptomic data, leptin receptor (Lepr)-expressing POMC cells form a molecularly distinct cluster relative to POMC neurons expressing the serotonin receptor Htr2c or the insulin receptor (Insr)21,22. These subpopulations are also spatially and electrophysiologically segregated19,20,44.
Of note, transgenic mice with deletion of the Lepr protein product (LepR) or the Insr protein product (InsR) in POMC neurons during embryonic life have broad variations in their metabolic phenotypes45–51, which provides further evidence of functional heterogeneity. POMC-expressing progenitors, however, share developmental origins with other hypothalamic cell types52, and developmental compensation or LepR/InsR deletion from non-POMC neurons may represent a confounding factor in these models (see also Box 1).
Box 1 |. Methodological limitations due to POMC neuronal developmental plasticity.
Two types of genetically modified mouse strain have been generated and used in a large number of studies that investigate the role of POMC neurons in energy balance. The first is a transgenic mouse model with enhanced green fluorescent protein (eGFP) reporters driven by Pomc promoter elements (Pomc-eGFP)5, which has been extensively employed for ex vivo electrophysiological recordings. A second Pomc-Cre bacterial artificial chromosome transgenic strain was instead developed to restrict genetic manipulations to POMC neurons and thus evaluate their in vivo function47. The use of Pomc-eGFP mice is limited by the fact that POMC neuron visualization will happen only if the neuron expresses a sufficient amount of Pomc mRNA. Hence, clusters with negligible Pomc mRNA levels, or transient expression of this marker, are likely to be missed during the analysis.
In contrast, when using the Pomc-Cre transgene to manipulate the expression of a certain molecular factor in POMC neurons, off-target Cre-mediated recombination may occur in non-POMC-expressing populations121, since POMC (and therefore Cre) expressing cellular progenitors can generate multiple cell types across development52. These off-target effects represent an important caveat when interpreting the phenotype observed, especially if one considers that POMCARC neurons have differential effects on food intake regulation relative to ARC neurons derived from POMC-expressing progenitors65. Notwithstanding, a transgenic line that allows temporal (tamoxifen-based) Cre-mediated recombination in POMC neurons is also available54 and overcomes these obstacles.
Mice with postnatal LepR ablation also have alterations in systemic glucose control and impaired systemic leptin production53 without changes in body weight or food intake53. Conversely, postnatal ablation of the Htr2c protein product (5-HT2CRs) in POMC neurons promotes hyperphagia, increases the release of the hyperglycaemic hormone glucagon and favours diet-induced obesity (DIO)54. These observations suggest that LepR- and 5-HT2CR-positive POMC neuron populations probably mediate different physiological outputs.
Distinct neuronal clusters respond to the concomitant action of multiple hormonal signals; for example, co-modulation by insulin and leptin21. Transgenic mice with enhanced LepR and InsR signalling in POMC neurons show better glucose handling, increased energy expenditure and adipose tissue browning (that is, the conversion of white fat cells into energy-burning brown adipocytes)55. Hence, neuronal subgroups responsive to multiple hormonal actions are particularly relevant for body weight control and obesity pathophysiology.
Heterogeneous expression of neuropeptides and neurotransmitters.
In adult mice, not every POMC neuron expresses high levels of the main functional marker POMC. Certain subsets (~27%) present low Pomc mRNA levels and high levels of the appetite-promoting neuropeptides Agrp and Npy21. This raises the question of whether these subsets have functional similarity to NPY/AgRP neurons.
In addition, different groups of POMC neurons with specific spatial localization can express either the inhibitory neurotransmitter GABA, the excitatory neurotransmitter glutamate or both21,56–61. Given the opposing neurobiological actions of GABA and glutamate, these subtypes could have distinct physiological consequences.
Interestingly, mice carrying a loss-of-function mutation for the Pomc gene are obese. When the Pomc gene is selectively re-expressed during postnatal life in POMC/GABAergic neurons of these animals, food intake is reduced, which completely reverses the obese phenotype62. Conversely, non-selective reactivation of Pomc in all POMC cells promotes only negligible anti-obesity effects62. Hence, POMC/GABAergic cells may act through yet-to-be-defined mechanisms that promote food intake and, possibly, body weight gain. Such a working model is supported by the recent observation that random activation of GABAergic hypothalamic ARC neurons results in obesity63. Accordingly, pharmacological inhibition of the mechanistic target of rapamycin pathway in mice, which leads to hyperphagia by mimicking a condition of low cellular energy levels in POMC neurons, activates POMC/GABAergic neurons64. Other POMC neuronal clusters (perhaps the glutamatergic ones) possibly govern opposing effects.
Additional layers of plasticity also seem to exist within each molecularly defined POMC neuron subpopulation. For instance, POMC neurons expressing the cannabinoid receptor type 1 are activated by the action of cannabinoids and promote food intake, by preferentially producing β-endorphin rather than α-melanocyte-stimulating hormone (α-MSH)15. Thus, following activation, certain POMC neuronal clusters can release various neuropeptides with opposing functions, possibly due to mitochondrial-related mechanims15. Other studies have shown that specific neuropeptide production may also be related to POMC cell ontogeny65.
Collectively, several studies, involving both unbiased and biased methods (Fig. 1), suggest that different POMC neurons influence divergent metabolic responses as a result of their intrinsic cellular properties, including the expression of specific sets of receptors, neurotransmitters and neuropeptides implicated in metabolic control. Nevertheless, unbiased methods of classification, such as single-cell mRNA sequencing (scRNA-seq), rely on ex vivo information obtained from dissociated cells, which may not be representative of the in vivo context in which these cells normally reside. In addition, the extent to which a certain molecular profile overlaps with a specific neuroanatomical organization and functional state is not yet fully understood. Certain subsets could also operate independently of cell-intrinsic properties (for example, under the influence of heterogeneous afferent signals). A LepR-negative POMC neuron, for instance, might still respond to leptin via changes in the inhibitory inputs from leptin-sensitive NPY/AgRP neurons.
Fig. 1 |. Current classification methods used to identify POMC neuron subtypes.
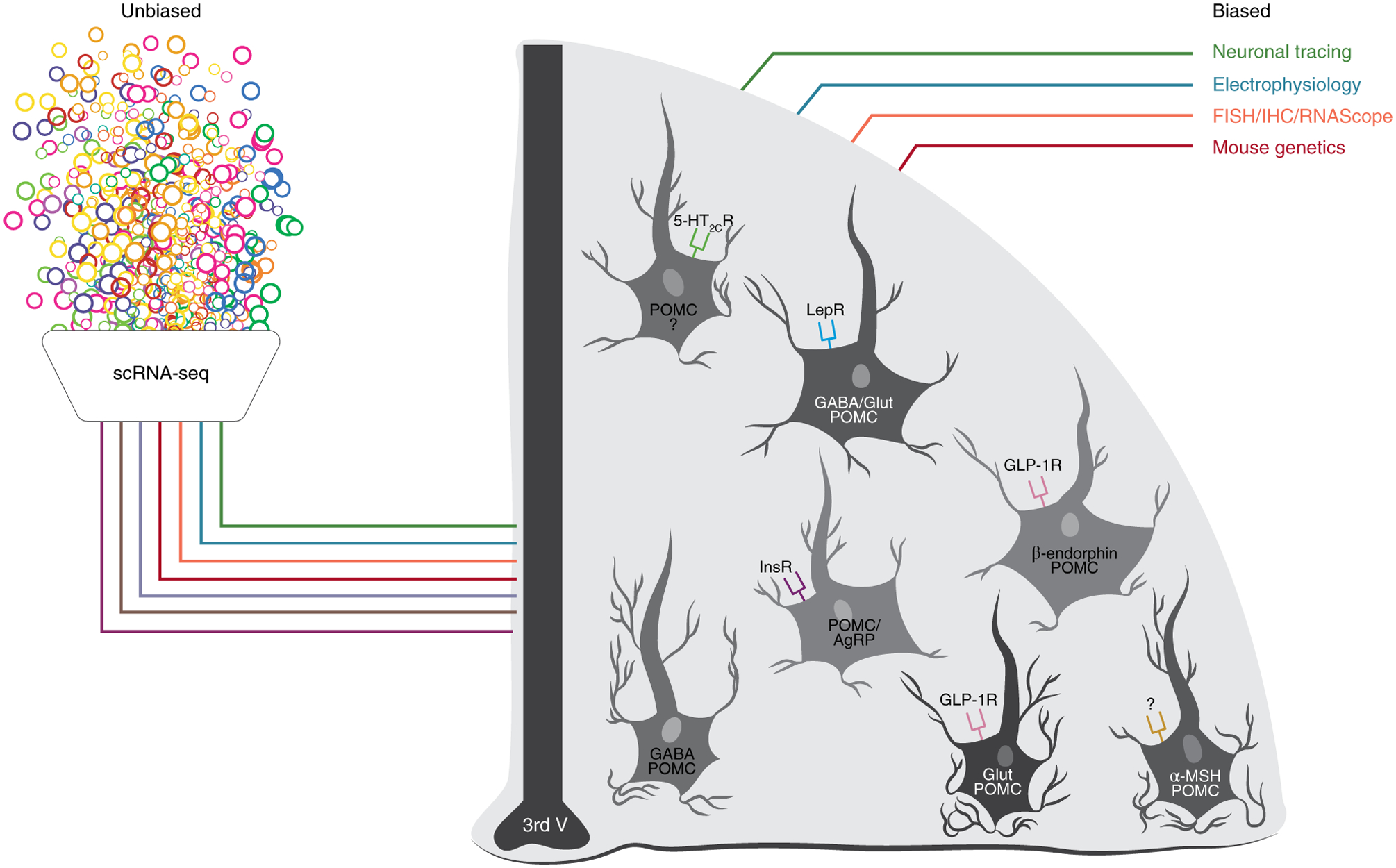
Schematic of the unbiased (scRNA-seq analysis) and biased methods (neuronal tracing, electrophysiology, cell labelling (fluorescence in situ hybridization (FISH), immunohistochemistry (IHC) and RNAScope for molecular marker, neurotransmitter and neuropeptide expression) and mouse genetics) used to date for identifying and characterizing POMC neurons. Note that the expression of receptors, neuropeptides and neurotransmitters at the level of the cells is only representative. The question marks represent receptors or other molecular markers that are still to be discovered. 3rd V: third ventricle; Glut, glutamate. Figure adapted with permission from C. Padgett.
Thus, only a holistic approach that integrates the intrinsic cellular properties of POMC neurons with their spatial position in the brain and their sensitivity to afferent signals can uncover how the activity of specific POMC neuronal subsets translates into specific behavioural or metabolic effects. To effectively and rapidly establish this concept, the development of new tools is required, including methodologies that align a specific molecular profile with a defined morphological, electrophysiological and functional state (see Box 2)66.
Box 2 |. New tools for studying POMC neuronal heterogeneity.
Recent progress has been made in the development of spatial transcriptomics methods that exploit transcriptional cell-type classifications and map their spatial distributions122–124. This approach allows the investigation of specific cell types in situ, preserving the spatial context and overall brain circuit architecture.
A key recent advance in neuroscience research is the implementation of a system called INTRSECT (intronic recombinase sites enabling combinatorial targeting), which combines multiple recombinase-dependent engineered viral vectors with specific recombinase-expressing transgenic animals125. INTRSECT allows the targeting of particular cell types with multiple (dual/triple) defined features126 and could therefore be used to elucidate the function of diverse POMC neuronal subsets.
The generation of new intersectional viruses might enable the application of electrophysiology, optogenetics, chemogenetics, calcium imaging or circuit mapping in specific clusters of POMC neurons. For instance, calcium imaging combined with two-photon microscopy127 could be employed in association with INTERSECT to image single neuronal cells in immobilized animals. A microendoscopic calcium imaging approach that utilizes a head-mounted, miniaturized microscope128, or a different and recently established technique that involves optetrode electrophysiology30, might instead be used to assess specific subsets at single-cell resolution in freely moving mice.
Scope of heterogeneity: multiple subsets, but same purpose?
Apart from regulating food intake, POMC neuronal activity has been linked to the modulation of multiple peripheral metabolic endpoints3, although the data related to the underlying mechanisms involved are somewhat contradictory. For instance, several studies support the idea that POMC neurons influence the release and utilization of glucose in peripheral organs33,34,67–69, independently of changes in body weight or food intake3, and possibly in response to the sensing of extracellular glucose fluctuations69. However, not all studies have found that POMC neurons are sensitive to glucose70.
Moreover, when POMC neuronal glucose sensing is disrupted via different approaches, including alterations in ATP-sensitive potassium channel function, perturbations in specific pathways or modifications in mitochondria dynamics69,71–74, disparate energy and glucose homeostasis phenotypes are observed69,71–74. In this context, chemogenetic inhibition of POMC neuronal activity in normoglycaemic mice reduces systemic blood glucose33, which is surprising given that activation of POMC neurons in response to leptin prevents hyperglycaemia48,49.
Cellular heterogeneity might explain these inconsistencies. On the basis of ex vivo electrophysiological investigations, certain POMC neuronal clusters increase their activity when extracellular glucose concentrations are reduced, whereas others decrease it, or exhibit a biphasic response75. Similarly, different POMC neuronal subsets can be inhibited or activated or can remain unaffected by the glucoregulatory hormone insulin67. Given that the above-mentioned genetics-based studies involve approaches that target the entire population of POMC neurons, the observed changes (or lack thereof) may reflect the modulation of specific subpopulations with divergent (that is, atypical) effects.
But what is the advantage of having different subpopulations that respond in a heterogeneous manner to the same fuel substrate? In light of the dynamic mode of action of these neurons (see previous sections), divergent mechanisms of glucose sensing may allow rapid accommodation of metabolic responses to defend euglycaemia.
During fasting, for instance, when both glucose and insulin levels drop, certain POMC neuronal subsets could either be activated or inhibited, working towards the same goal of increasing plasma blood glucose or reducing glucose uptake into peripheral organs, to maintain constant systemic glucose levels. Conversely, after a meal, these same neuronal subtypes operate in an opposite manner, but always to preserve glucose homeostasis.
Heterogeneous yet convergent mechanisms can also link POMC neuronal activity with the control of systemic lipid metabolism. For example, POMC neurons were shown to influence circulating cholesterol levels76 and triglyceride synthesis77, possibly in response to cell-specific mechanisms of fatty acid sensing78,79.
Thus, heterogeneity and synergy probably represent two sides of the same coin (Fig. 2), with different POMC neuronal subsets adjusting to changes in the body’s energy status and fuel availability, not unlike the individual components of a car engine, which work in concert to regulate speed in response to movements in the brake and gas pedals.
Fig. 2 |. Mode(s) of action of different subsets of POMC neurons for the regulation of energy balance.
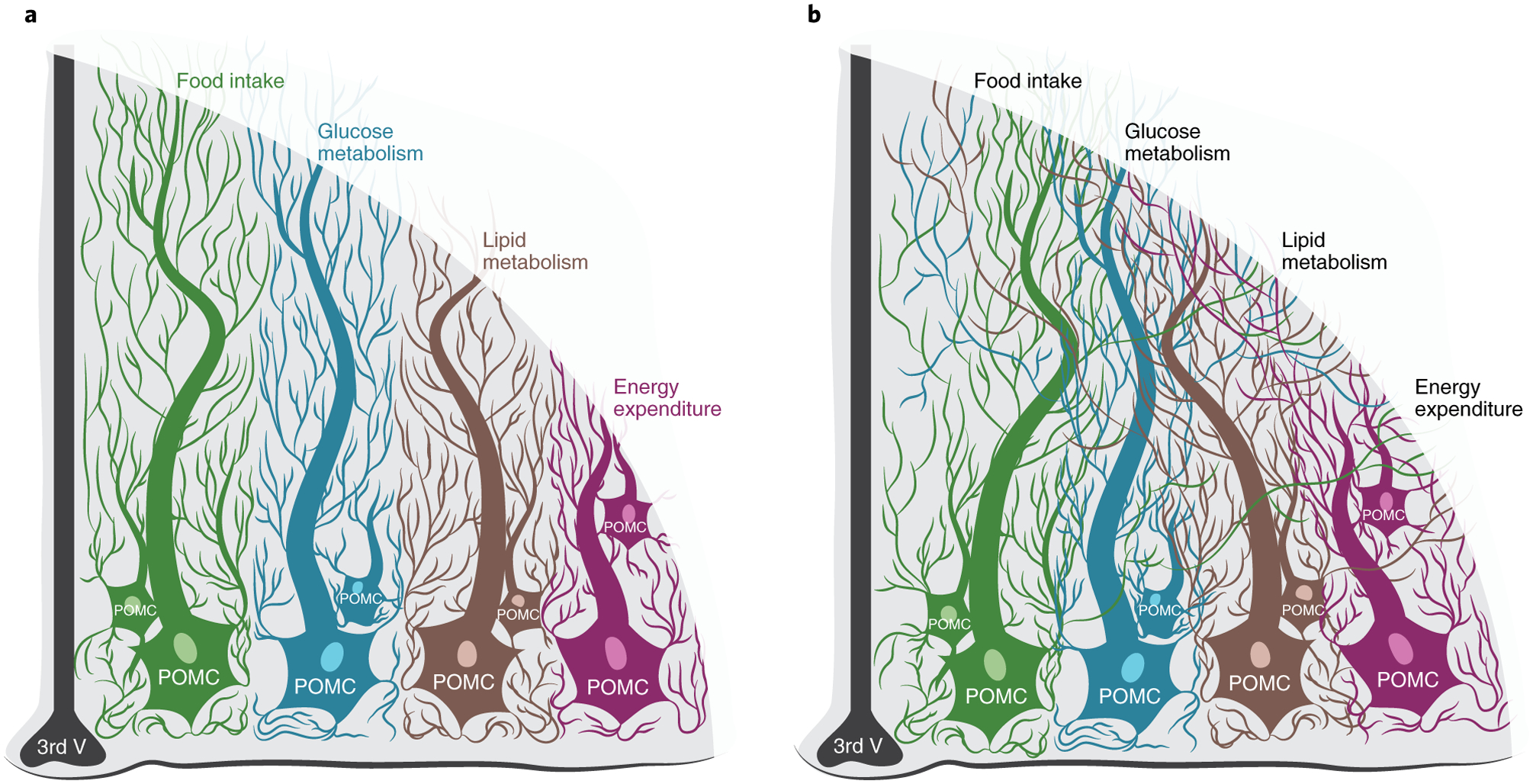
a, Different subsets of hypothalamic POMC neurons can modulate different outputs. b, Different subsets of POMC neurons can also converge to modulate the same output and/or the same subsets of cells can act on multiple outputs. Figure adapted with permission from C. Padgett.
How and when is POMC neuronal heterogeneity established?
Embryonic plasticity.
Pomc expression starts at embryonic day 10.5, when this gene is transiently expressed by the vast majority of cells in the developing ventral hypothalamus52. During gestation, Pomc-expressing immature cells can switch off POMC production and give rise to NPY/AgRP neurons or alternative cell types52,80. POMC and other neurons of ARC ontogeny are initiated and maintained during early embryonic development by transcriptional programmes launched by specific transcription factors, including Dlx1/2, Otp and neurogenin 3 (refs.81,82), among others83. Several microRNAs can also modulate these processes84,85, highlighting the importance of epigenetic mechanisms.
Neonatal plasticity.
While the developmental identity of the majority of hypothalamic neurons is considered locked after early embryonic development in rodents, POMC neurons may retain plasticity during neonatal life, when the formation of axonal projections occurs, under the influence of hormonal signals86,87. Processes regulating these maturation steps play a critical role in programming whole-body energy balance in adulthood. Metabolic perturbations during this maturation period (for instance, those induced by maternal high-fat diet feeding) impair the POMC neuron translatome and neurocircuit development, predisposing the offspring to metabolic disorders88–90.
Interestingly, at postnatal day 1, mice possess a significant proportion (40%) of POMC/glutamatergic neurons, whose levels progressively decline to 8% as the animals approach 8 weeks of age91. Conversely, a low proportion (just 8%) of POMC/GABAergic neurons are present at postnatal day 1, and their numbers progressively increase to 46% in adults91. The exact nature of the intracellular signals that maintain POMC neuronal identity plasticity after embryonic development is not fully known, but recent advances have identified islet-1 (ref.92), certain microRNAs84 and the transcription factor T-box gene 3 (Tbx3)26 as crucial regulators.
Plasticity in adulthood.
Loss of Tbx3 function in hypothalamic neurons alters the differentiation state and functional identity of ~50% of POMC neuronal cells during developmental maturation, ultimately leading to obesity and glucose intolerance26. Intriguingly, similar cellular and physiological perturbations are observed after loss of Tbx3 in fully differentiated hypothalamic neurons of adult animals26. Hence, specific POMC neuronal subsets may maintain intracellular programmes that possibly confer identity plasticity, even after terminal differentiation. This hypothesis is supported by the observation that some mature POMC neurons are enmeshed by perineuronal nets93—a condensed form of extracellular matrix implicated in neuronal developmental programming94.
A certain fraction of POMC neuronal cells co-express high levels of Agrp and Npy mRNA in adult mice21 (see also previous section). These subsets with such a mixed peptidergic identity and low Pomc expression could be embryonically derived cells where Pomc transcription is never fully silenced during postnatal life, even after differentiation into NPY/AgRP neurons. Alternatively, they might represent newly committed NPY/AgRP neurons from residing neuronal stem cells, in which Pomc expression is yet to be fully suppressed. Indeed, hypothalamic neurogenesis can occur in adult mice95, although its contribution to energy balance regulation is still controversial96.
In conclusion, POMC neuronal diversity reflects the high degree of developmental plasticity of this cellular population during both embryonic and postembryonic life. It is worth noting that such plasticity may represent a conceptual obstacle when trying to decipher the postnatal functional role of these neurons using commonly available reporter mouse models (Box 1).
POMC neurons’ heterogeneity beyond metabolism
The Pomc gene arose approximately 500 million years ago (ref.2), in an environment characterized by intermittent food availability, where being able to forage for supplies was imperative for survival. Our ancestors had to choose between staying immobile, safe and starving or moving and encountering potential threats or stressful/painful situations; thus, neurobiological mechanisms that orchestrate competing emotional states had to develop to overcome periods of famine97,98. In this evolutionary context, POMC neuronal heterogeneity may have originated as an adaptive mechanism, given that the activity of POMC neurons can affect behavioural and physiological responses associated with the evolutionary survival of our species, including stress2,68,99,100, pain101,102, fear99,103 and locomotion48,104.
Chronic restraint stress, or just an acute injection of a vehicle solution in mice, activates POMCARC neurons31,100 and translates into inhibition of dopamine neurons located in the ventral tegmental area100. Photo-inhibition of this POMCARC–ventral tegmental area circuit in chronically stressed mice increases body weight and food intake and reduces depression-like behaviours and anhedonia (that is, a deficit in the ability to experience pleasure)100. Certain POMCARC neurons project to the nucleus accumbens38, and α-MSH-mediated activation of MC4R in this brain area is required for chronic stress-elicited anhedonia, which can be prevented by inhibiting MC4R signalling105. Thus, striatal melanocortin signalling is critical for assigning negative motivational valence to harmful stimuli106. Other brain areas, such as the dorsal raphe nucleus, may further bridge POMC neuronal activity with the control of food intake and emotional states. Chemogenetic inhibition of dorsal raphe nucleus MC4R neurons induces depression, anxiety and reduced appetite, whereas chemogenetic activation reverses these effects107.
Hence, one might speculate that POMC neuronal activation during stressful conditions is responsible for eliciting or amplifying negative emotional states that compete with hunger, although this contrasts with the observation that postnatal loss of function (ablation) of POMC neurons in mice leads to anxiety-like behaviours68.
Heterogeneity, once again, may be at the core of these inconsistencies. If the ultimate physiological goal of POMC neuronal heterogeneity is to influence appetite and energy balance, different POMC neuronal clusters may have been programmed by evolution to override potential anxiogenic feelings and painful or even fearful situations that stop locomotion and undermine food acquisition.
Effective foraging requires a proficient cardiovascular system that supports rapid and intense physical activity and subsequent oxygen delivery to internal organs during food processing. Certain POMC neuronal subsets are specialized in the modulation of cardiovascular response, due to their ability to control the sympathetic nervous system tone108.
Accordingly, mice lacking LepR in POMC neurons do not respond to the hypertensive and sympathomimetic action of leptin108,109. These LepR-positive POMC cells may therefore operate via top-down pathways to increase blood pressure and heart rate in response to leptin action. Other subpopulations, instead, may have opposing cardiovascular effects, as chronic chemogenetic activation of POMC neurons reduces blood pressure110, possibly reflecting the action of yet-to-be-identified subtypes.
Thus, multiple subsets and divergent mechanisms may allow integration of emotional, cardiovascular and behavioural outputs linked with food foraging. While reconciling conflicting data around this topic, overall, this evidence suggests that POMC neurons may have been programmed through mammalian evolution to coordinate a broader number of biological responses beyond energy balance (Fig. 3).
Fig. 3 |. Physiological roles of POMC neurons beyond energy balance.
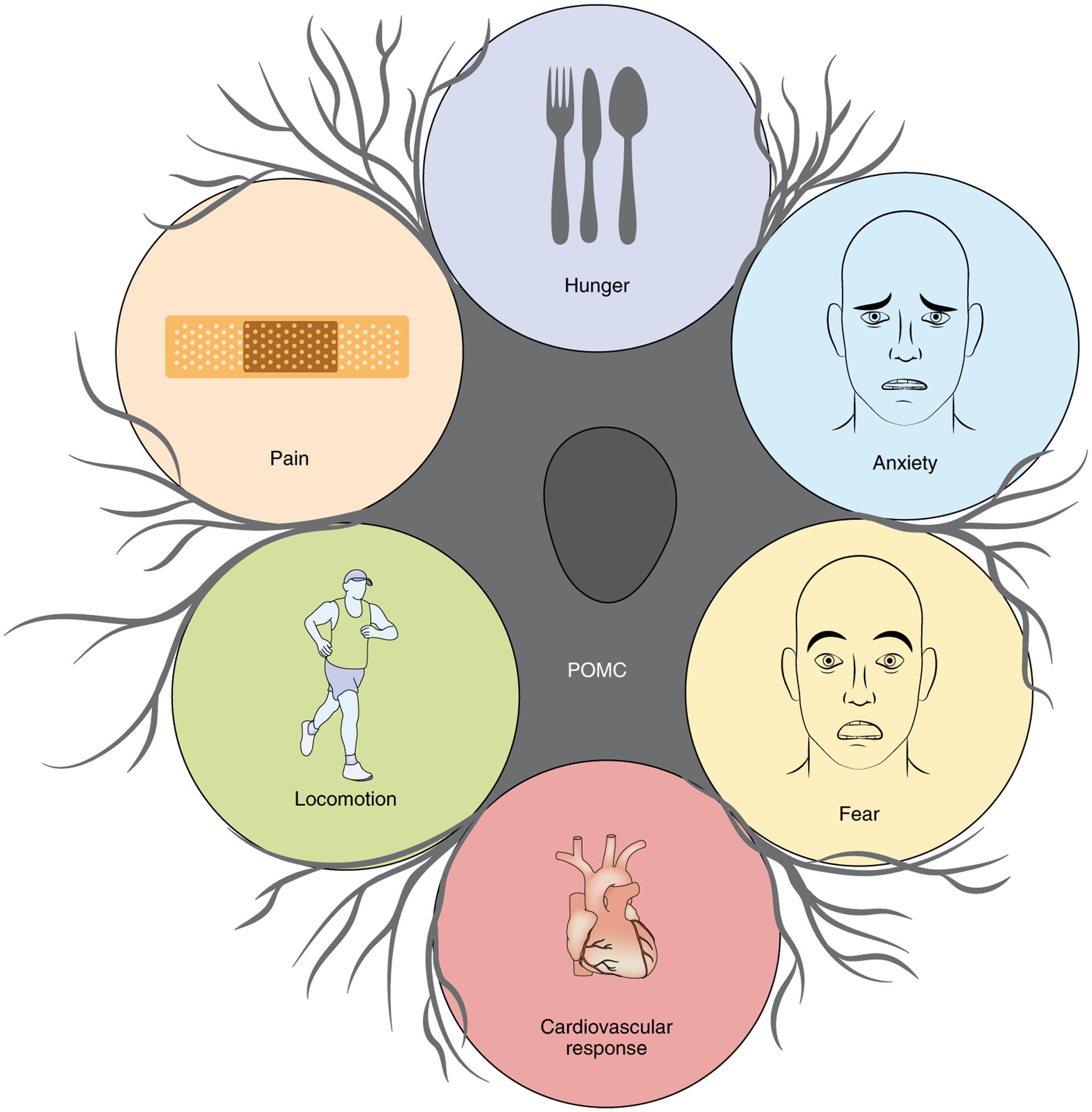
Beyond a critical role in energy balance, POMC neurons orchestrate behavioural and physiological responses necessary for survival, including modulation of pain, stress/anxiety, fear, locomotion and cardiovascular responses. Figure adapted with permission from C. Padgett.
POMC neuronal heterogeneity in obesity
Available evidence.
The increasing worldwide prevalence of obesity is the result of genetic and epigenetic factors that interact with the environment and our lifestyle111. Sedentary behaviours and energy-rich/highly palatable foods promote weight gain in metabolically prone individuals. If POMC neuronal heterogeneity is the result of adaptive evolutionary mechanisms, obesity may then derive from alterations in the heterogeneous behaviour of these neuronal clusters since this disease is accompanied by POMC neuronal dysfunction3,8–11. This concept leads to two relevant questions: (1) are certain POMC neuronal subsets more or less susceptible to drive DIO; and (2) does POMC neuronal heterogeneity contribute to obesity and its associated cardiovascular or metabolic sequelae?
A final answer is not yet available, but several observations point to the existence of a complex link between POMC neuronal activity and obesity. Obesity is only observed after >80% of POMC neurons in the ARC are ablated34, suggesting a large degree of redundancy34, or that certain POMC cell clusters may somehow take over the role of dysfunctional cells during metabolic stress.
Only partial signs of POMC neuronal alterations are observed in animal models of DIO, which often involve a sub-fraction of cells. The number of inactive POMC neurons with no spontaneous action potential firing is increased in DIO mice by only ~20%112 and long-term (8 months) exposure to hypercaloric diets leads to only 20–50% loss of POMC-expressing hypothalamic neurons113,114. In DIO mice, increased inflammatory and potentially dangerous interactions between POMC neurons and the adjacent glial cells occur in a small subset (~10%) of neurons114, resulting in synaptic derangements that are implicated in altered body weight regulation115.
Notably, genetic approaches aimed at resolving or counteracting POMC neuronal dysfunction in DIO mice have led to divergent phenotypic outcomes. DIO activates inflammatory molecular pathways that potentially undermine neuronal activity116, including the pathway formed by the proinflammatory protein nuclear factor κB and its upstream activator IκB kinase-β (IKK-β)116. POMC neuronal-specific IKK-β ablation does not prevent DIO, but it ameliorates obesity-induced hypertension117. Since certain POMC subsets are implicated in modulating the cardiovascular system, DIO-linked alterations in the nuclear factor κB/IKK-β pathway may involve specific subpopulations controlling cardiac outputs.
Under obesogenic conditions, perturbations in intracellular factors that are part of the InsR and LepR signalling cascade in POMC neurons (such as suppressor of cytokine signalling-3, protein tyrosine phosphatase 1B or T-cell protein tyrosine phosphatase, among others3) contribute to the pathogenesis of DIO. These molecular derangements promote a condition of hormonal inflexibility whereby POMC neurons are incapable of adapting their activity to extracellular hormonal signals3. Transgenic mice in which these common intracellular nodes have been specifically manipulated in POMC neurons to augment both insulin and leptin cellular sensitivity are protected from DIO55, suggesting that neuronal subsets responsive to multiple hormonal signals may be particularly susceptible to DIO-induced neuronal dysfunction.
Missing evidence.
The number and exact identity of POMC neuronal subtypes affected by chronic metabolic stress may be influenced by several variables, including diet composition, length of exposure to overfeeding, genetic/epigenetic background and sex.
Understanding the potential impact of sex is particularly relevant given that obesity is growing at an alarming rate in both sexes118. DIO mice may have sex-specific effects on POMC neuronal dysfunction. Indeed, protein tyrosine phosphatase 1B deficiency in POMC neurons attenuates weight gain and fat mass accumulation in response to a high-fat diet in both sexes, but improves glucose tolerance and reduces hepatic lipid accumulation only in male mice119. Male and female mice have phenotypic differences in physical activity, energy expenditure and DIO susceptibility, and this is in part driven by subpopulations of POMC neurons expressing 5-HT2CRs120.
Independently of how sex or other variables influence POMC neuronal dysfunction in obesity, which will require additional studies, unravelling whether and how metabolic stress alters POMC neuronal heterogeneity may significantly improve our comprehension of the neurobiology of this disease. Addressing this question may also pave the way to novel treatments that target specific POMC neuronal subtypes to safely ameliorate obesity, while bypassing potential emotional or cardiovascular side effects.
Conclusions and future directions
For those who have been working in the metabolic field for a long time, the story of how POMC neurons affect energy balance has been, and continues to be, an intriguing tale full of twists, turns and surprises.
POMC neurons were thought to decode a satiety signal in response to the internal energy state and to mainly behave as antagonistic functional partners of NPY/AgRP neurons, a model clearly too simplistic given the high degree of intercellular and intracellular plasticity discussed herein. Thus, the time is ripe for revising the conventional yin–yang action of POMC and NPY/AgRP as opposite partners in the regulation of energy balance. The molecular and functional diversity of POMC neurons now offers a foundation for understanding paradoxical effects on metabolism or non-canonical actions on emotional states and cardiovascular outputs.
Our understanding of the different POMC neuronal subsets and their various roles is still emerging, with several big questions remaining unresolved (Box 3). Addressing these questions is a priority as it may enable progress towards our understanding of both the aetiology and treatment of metabolic disorders.
Box 3 |. Outstanding questions.
Heterogeneity or heterogeneities?
Different POMC neuronal subsets have been programmed by evolutionary selection to orchestrate metabolic and behavioural endpoints necessary for survival. However, can one molecularly distinct POMC subtype switch its identity and adopt multiple phenotypes? For instance, POMC neurons can release both α-MSH and β-endorphin15,65, neuropeptides with opposing effects on food intake regulation. The release of one neuropeptide rather than the other certainly depends on specific input signals, but the cellular underpinnings have yet to be clearly elucidated. Addressing this question will also require thorough analysis of the function of specific POMC neuron subpopulations in response to changing metabolic needs or different behavioural patterns, or during DIO.
Which subtype controls which circuit?
Beyond contributing to energy balance, POMC neurons affect cardiovascular responses, pain, fear, anxiety and locomotion. Their widespread spatial projection profile38 probably explains such functional diversity. However, the exact identity and mode(s) of action of the multiple brain circuits controlled by different POMC neuronal subtypes are substantially unknown.
Does obesity alter only specific POMC neuronal subpopulations?
DIO may selectively alter certain POMC neurons, but what defines the susceptibility of these cells to metabolic stress? A comprehensive investigation of the effects of hypercaloric diets at single-cell resolution is still missing. scRNA-seq21–23 or other emerging spatial transcriptomic approaches (see Box 2) could be used to address this key open question. These efforts may represent a first step leading to the development of precision therapeutic tools targeting selective POMC subtypes and safely ameliorating obesity.
How does sex impact the function of POMC neurons?
Sexual dimorphism exists in energy balance and obesity pathogenesis, hampering the efficacy of anti-obesity strategies in both sexes. The underlying mechanisms are still unclear, but the female sex hormone oestrogen has long been considered a contributor129. Female mice have more POMC neurons in the hypothalamus than males130 and present phenotypic differences in physical activity, energy expenditure and DIO susceptibility120, which are partly driven by a specific POMC neuronal subpopulation120. Uncovering the molecular and functional identity of these POMC subtypes implicated in sex dimorphism may favour the development of novel anti-obesity treatments effective in both sexes.
Can specific POMC neuron subpopulations be targeted pharmacologically?
Obesity is a heterogeneous disease that requires combined pharmacological strategies to correct multiple metabolic pathways. Multi-agonists that concomitantly activate glucagon-like peptide-1 receptors (GLP-1Rs) and additional metabolic receptors are under scrutiny given their exceptional preclinical efficacy131,132. POMC neuronal activity partially mediates the anti-obesity effects of GLP-1R agonists133,134 and the weight-lowering drug lorcaserin135. It is likely that different POMC neuronal subpopulations expressing GLP-1Rs or 5-HT2CRs21 mediate these effects. Thus, uncovering the mode(s) of action of POMC neuronal subtypes expressing GLP-1Rs, 5-HT2CRs or other druggable metabolic receptors may spur the development of potent and safe pharmacological approaches against obesity that target specific POMC neuronal clusters.
Acknowledgements
We acknowledge support by INSERM (D.C. and C.Q.), Nouvelle Aquitaine Region (D.C.) and Agence Nationale de la Recherche (LabEX BRAIN (ANR-10-LABX-43), OPTOPATH (ANR-10-EQX-008-1), BABrain (ANR-17-CE14-0007) and MitObesity (ANR-18-CE14-0029) to D.C. and neuroIDobese (ANR-20-CE14-0046) to C.Q.). C.Q. is also supported by the Société Française d’Endocrinologie (Pfizer-SFE Prix de Recherche en Endocrinologie), Société Française de Nutrition and Société Francophone du Diabète. M.C. is supported by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement 725004) and CERCA Programme/Generalitat de Catalunya. L.M.Z. is supported by the Russell Berrie and Klarman Family foundations, 1R01 DK125094, 1R01 MH113353 and 2P01 AG032959. K.W.W. is supported by NIH R01 DK119169 and DK119130-5830. S.D. is supported by NIH DK097566, DK105571, DK107293 and DK120321. G.S.H.Y. is supported by the Medical Research Council Metabolic Diseases Unit (MC_UU_00014/1). J.C.B. received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 266408 (SYNEME). We thank C. Padgett for artwork.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information Nature Metabolism thanks Michael Krashes and Qingchun Tong for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt.
References
- 1.Friedman JM & Halaas JL Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Harno E, Gali Ramamoorthy T, Coll AP & White A POMC: The physiological power of hormone processing. Physiol. Rev 98, 2381–2430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quarta C, Fioramonti X & Cota D POMC neurons dysfunction in diet-induced metabolic disease: hallmark or mechanism of disease? Neuroscience 447, 3–14 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Andermann ML & Lowell BB Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowley MA et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Tong Q, Ye C-P, Jones JE, Elmquist JK & Lowell BB Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci 11, 998–1000 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ollmann MM et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Huszar D et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Lee YS et al. A POMC variant implicates β-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 3, 135–140 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Krude H et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet 19, 155–157 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Biebermann H et al. A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 3, 141–146 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Kühnen P et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med 375, 240–246 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Kievit P et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 62, 490–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collet T-H et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab 6, 1321–1329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch M et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519, 45–50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aponte Y, Atasoy D & Sternson SM AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci 14, 351–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atasoy D, Betley JN, Su HH & Sternson SM Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krashes MJ et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest 121, 1424–1428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams KW et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci 30, 2472–2479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn J-W et al. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71, 488–497 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam BYH et al. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol. Metab 6, 383–392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell JN et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci 20, 484–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Wu X, Jiang L & Zhang Y Single-cell RNA-seq reveals hypothalamic cell diversity. Cell Rep. 18, 3227–3241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuno TM et al. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 47, 294–297 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Brandt C et al. Food perception primes hepatic ER homeostasis via melanocortin-dependent control of mTOR activation. Cell 175, 1321–1335. e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quarta C et al. Functional identity of hypothalamic melanocortin neurons depends on Tbx3. Nat. Metab 1, 222–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q et al. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology 155, 840–853 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Fekete C et al. Activation of anorexigenic pro-opiomelanocortin neurones during refeeding is independent of vagal and brainstem inputs. J. Neuroendocrinol 24, 1423–1431 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Knight ZA et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandelblat-Cerf Y et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4, e07122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Lin Y-C, Kuo T-W & Knight ZA Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeley RJ & Berridge KC The hunger games. Cell 160, 805–806 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Üner AG et al. Role of POMC and AgRP neuronal activities on glycaemia in mice. Sci. Rep 9, 13068 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan C et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci 33, 3624–3632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenselau H et al. A rapidly-acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci 20, 42–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods SC The eating paradox: how we tolerate food. Psychol. Rev 98, 488–505 (1991). [DOI] [PubMed] [Google Scholar]
- 37.Clasadonte J & Prevot V The special relationship: glia–neuron interactions in the neuroendocrine hypothalamus. Nat. Rev. Endocrinol 14, 25–44 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Wang D et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat 9, 40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elias CF et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Baker RA & Herkenham M Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J. Comp. Neurol 358, 518–530 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Lemus MB et al. A stereological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology 156, 1701–1713 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Georgescu T et al. Neurochemical characterization of brainstem pro-opiomelanocortin cells. Endocrinology 161, bqaa032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grill HJ & Hayes MR Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y et al. TrpC5 mediates acute leptin and serotonin effects via Pomc neurons. Cell Rep. 18, 583–592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Könner AC et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5, 438–449 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Shin AC et al. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes 66, 1560–1571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balthasar N et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Huo L et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 9, 537–547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berglund ED et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Invest 122, 1000–1009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang H et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat. Neurosci 15, 1391–1398 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556, 505–509 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Padilla SL, Carmody JS & Zeltser LM Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med 16, 403–405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caron A et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. eLife 7, e33710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berglund ED et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest 123, 5061–5070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodd GT et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dicken MS, Tooker RE & Hentges ST Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J. Neurosci 32, 4042–4048 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hentges ST, Otero-Corchon V, Pennock RL, King CM & Low MJ Proopiomelanocortin expression in both GABA and glutamate neurons. J. Neurosci 29, 13684–13690 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hentges ST et al. GABA release from proopiomelanocortin neurons. J. Neurosci 24, 1578–1583 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarvie BC & Hentges ST Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J. Comp. Neurol 520, 3863–3876 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wittmann G, Hrabovszky E & Lechan RM Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J. Comp. Neurol 521, 3287–3302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atasoy D, Aponte Y, Su HH & Sternson SM A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci 28, 7025–7030 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trotta M et al. Hypothalamic Pomc expression restricted to GABAergic neurons suppresses Npy overexpression and restores food intake in obese mice. Mol. Metab 37, 100985 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu C et al. Profound and redundant functions of arcuate neurons in obesity development. Nat. Metab 2, 763–774 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saucisse N et al. POMC neurons functional heterogeneity relies on mTORC1 signaling. Preprint at bioRxiv 10.1101/2020.03.25.007765 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Wei Q et al. Uneven balance of power between hypothalamic peptidergic neurons in the control of feeding. Proc. Natl Acad. Sci. USA 115, E9489–E9498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng H & Sanes JR Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci 18, 530–546 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Dodd GT et al. Insulin regulates POMC neuronal plasticity to control glucose metabolism. eLife 7, e38704 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenman Y et al. Postnatal ablation of POMC neurons induces an obese phenotype characterized by decreased food intake and enhanced anxiety-like behavior. Mol. Endocrinol 27, 1091–1102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parton LE et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449, 228–232 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Fioramonti X et al. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 56, 1219–1227 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Claret M et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest 117, 2325–2336 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levin BE Neuronal glucose sensing: still a physiological orphan? Cell Metab. 6, 252–254 (2007). [DOI] [PubMed] [Google Scholar]
- 73.Santoro A et al. DRP1 suppresses leptin and glucose sensing of POMC neurons. Cell Metab. 25, 647–660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramírez S et al. Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab. 25, 1390–1399.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Hu J, Jiang L, Low MJ & Rui L Glucose rapidly induces different forms of excitatory synaptic plasticity in hypothalamic POMC neurons. PLoS ONE 9, e105080 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez-Tilve D et al. Melanocortin signaling in the CNS directly regulates circulating cholesterol. Nat. Neurosci 13, 877–882 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nogueiras R et al. The central melanocortin system directly controls peripheral lipid metabolism. J. Clin. Invest 117, 3475–3488 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jo Y-H, Su Y, Gutierrez-Juarez R & Chua S Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J. Neurophysiol 101, 2305–2316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michael NJ & Watt MJ Long chain fatty acids differentially regulate sub-populations of arcuate POMC and NPY neurons. Neuroscience 451, 164–173 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Sanz E et al. Fertility-regulating Kiss1 neurons arise from hypothalamic Pomc-expressing progenitors. J. Neurosci 35, 5549–5556 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pelling M et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol 349, 406–416 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Lee B et al. Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat. Commun 9, 2026 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeltser LM Developmental influences on circuits programming susceptibility to obesity. Front. Neuroendocrinol 39, 17–27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croizier S, Park S, Maillard J & Bouret SG Central Dicer-miR-103/107 controls developmental switch of POMC progenitors into NPY neurons and impacts glucose homeostasis. eLife 7, e40429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Messina A et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci 19, 835–844 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Bouret SG, Draper SJ & Simerly RB Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Steculorum SM et al. Neonatal ghrelin programs development of hypothalamic feeding circuits. J. Clin. Invest 125, 846–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuente-Martín E et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J. Clin. Invest 122, 3900–3913 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogt MC et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156, 495–509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haddad-Tóvolli R et al. Pro-opiomelanocortin (POMC) neuron translatome signatures underlying obesogenic gestational malprogramming in mice. Mol. Metab 36, 100963 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dennison CS, King CM, Dicken MS & Hentges ST Age-dependent changes in amino acid phenotype and the role of glutamate release from hypothalamic proopiomelanocortin neurons. J. Comp. Neurol 524, 1222–1235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasif S et al. Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc. Natl Acad. Sci. USA 112, E1861–E1870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirzadeh Z et al. Perineuronal net formation during the critical period for neuronal maturation in the hypothalamic arcuate nucleus. Nat. Metab 1, 212–221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reichelt AC, Hare DJ, Bussey TJ & Saksida LM Perineuronal nets: plasticity, protection, and therapeutic potential. Trends Neurosci. 42, 458–470 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Li J, Tang Y & Cai D IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol 14, 999–1012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoo S & Blackshaw S Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol 170, 53–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piazza PV, Cota D & Marsicano G The CB1 receptor as the cornerstone of exostasis. Neuron 93, 1252–1274 (2017). [DOI] [PubMed] [Google Scholar]
- 98.Sutton AK & Krashes MJ Integrating hunger with rival motivations. Trends Endocrinol. Metab 31, 495–507 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Mandela P, Yan Y, LaRese T, Eipper BA & Mains RE Elimination of Kalrn expression in POMC cells reduces anxiety-like behavior and contextual fear learning. Horm. Behav 66, 430–438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu N et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol. Psychiatry 25, 1006–1021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubinstein M et al. Absence of opioid stress-induced analgesia in mice lacking β-endorphin by site-directed mutagenesis. Proc. Natl Acad. Sci. USA 93, 3995–4000 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cerritelli S, Hirschberg S, Hill R, Balthasar N & Pickering AE Activation of brainstem pro-opiomelanocortin neurons produces opioidergic analgesia, bradycardia and bradypnoea. PLoS ONE 11, e0153187 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Comeras LB, Herzog H & Tasan RO Neuropeptides at the crossroad of fear and hunger: a special focus on neuropeptide Y. Ann. NY Acad. Sci 1455, 59–80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He Z et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab 18, 107–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lim BK, Huang KW, Grueter BA, Rothwell PE & Malenka RC Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klawonn AM et al. Motivational valence is determined by striatal melanocortin 4 receptors. J. Clin. Invest 128, 3160–3170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruschetta G, Jin S, Liu Z-W, Kim JD & Diano S MC4R signaling in dorsal raphe nucleus controls feeding, anxiety, and depression. Cell Rep. 33, 108267 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Bell BB et al. Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol. Metab 8, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Do Carmo JM et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 57, 918–926 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang J, Morgan DA, Cui H & Rahmouni K Activation of hypothalamic AgRP and POMC neurons evokes disparate sympathetic and cardiovascular responses. Am. J. Physiol. Heart Circ. Physiol 10.1152/ajpheart.00411.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Van der Klaauw AA & Farooqi IS The hunger genes: pathways to obesity. Cell 161, 119–132 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Paeger L et al. Energy imbalance alters Ca2+ handling and excitability of POMC neurons. eLife 6, e25641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thaler JP et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest 122, 153–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yi C-X et al. TNFα drives mitochondrial stress in POMC neurons in obesity. Nat. Commun 8, 15143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim JD, Yoon NA, Jin S & Diano S Microglial UCP2 mediates inflammation and obesity induced by high-fat feeding. Cell Metab. 30, 952–962.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jais A & Brüning JC Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest 127, 24–32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Purkayastha S, Zhang G & Cai D Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med 17, 883–887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med 377, 13–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aberdein N et al. Role of PTP1B in POMC neurons during chronic high-fat diet: sex differences in regulation of liver lipids and glucose tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol 314, R478–R488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burke LK et al. Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons. Mol. Metab 5, 245–252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Padilla SL, Reef D & Zeltser LM Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 153, 1219–1231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lein E, Borm LE & Linnarsson S The promise of spatial transcriptomics for neuroscience in the era of molecular cell typing. Science 358, 64–69 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Moffitt JR et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hanchate NK et al. Connect-seq to superimpose molecular on anatomical neural circuit maps. Proc. Natl Acad. Sci. USA 117, 4375–4384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fenno LE et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fenno LE et al. Comprehensive dual- and triple-feature intersectional single-vector delivery of diverse functional payloads to cells of behaving mammals. Neuron 107, 836–853.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grienberger C & Konnerth A Imaging calcium in neurons. Neuron 73, 862–885 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Betley JN et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu Y et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 29, 1232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang C et al. TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nat. Commun 9, 1544 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Müller TD, Clemmensen C, Finan B, DiMarchi RD & Tschöp MH Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol. Rev 70, 712–746 (2018). [DOI] [PubMed] [Google Scholar]
- 132.Quarta C et al. Molecular integration of incretin and glucocorticoid action reverses immunometabolic dysfunction and obesity. Cell Metab. 26, 620–632.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 133.He Z et al. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons—implications for energy balance and glucose control. Mol. Metab 28, 120–134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Secher A et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest 124, 4473–4488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.D’Agostino G et al. Nucleus of the solitary tract serotonin 5-HT2C receptors modulate food intake. Cell Metab. 28, 619–630.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


