Abstract
In utero exposure to the ubiquitous plasticizer, bisphenol A (BPA) is associated with offspring obesity. As food intake/appetite is one of the critical elements contributing to obesity, we determined the effects of in vivo maternal BPA and in vitro BPA exposure on newborn hypothalamic stem cells which form the arcuate nucleus appetite center. For in vivo studies, female rats received BPA prior to and during pregnancy via drinking water, and newborn offspring primary hypothalamic neuroprogenitor (NPCs) were obtained and cultured. For in vitro BPA exposure, primary hypothalamic NPCs from healthy newborns were utilized. In both cases, we studied the effects of BPA on NPC proliferation and differentiation, including putative signal and appetite factors. Maternal BPA increased hypothalamic NPC proliferation and differentiation in newborns, in conjunction with increased neuroproliferative (Hes1) and proneurogenic (Ngn3) protein expression. With NPC differentiation, BPA exposure increased appetite peptide and reduced satiety peptide expression. In vitro BPA-treated control NPCs showed results that were consistent with in vivo data (increase appetite vs satiety peptide expression) and further showed a shift towards neuronal versus glial fate as well as an increase in the epigenetic regulator lysine-specific histone demethylase1 (LSD1). These findings emphasize the vulnerability of stem-cell populations that are involved in life-long regulation of metabolic homeostasis to epigenetically-mediated endocrine disruption by BPA during early life.
Keywords: Neuroprogenitor cells; proliferation, differentiation; obesity; perinatal exposures; epigenetic
INTRODUCTION
Bisphenol A (BPA) is a monomer plasticizer used in the manufacture of common household goods including polycarbonate plastics (e.g. food and drink containers), paints and adhesives(Vandenberg et al., 2007). As an estrogen endocrine disrupter chemical, BPA has been associated with a range of adverse perinatal, childhood, and adult health outcomes (Rochester, 2013), including reproductive and developmental effects (Kim et al., 2011), neurogenesis (Kim et al., 2009), neurological behaviour (Palanza et al., 2008), and metabolic disease (Teppala et al., 2012). BPA exposure has been linked to childhood and adult obesity and likely contributes to the on-going obesity epidemic (Di Ciaula and Portincasa, 2017; Janesick and Blumberg, 2012). In rodents, maternal BPA exposure increases postnatal body weights and growth rates, with some studies showing greater susceptibility to BPA-increased adiposity in female as compared to male offspring (Richter et al., 2007; Rubin and Soto, 2009; Somm et al., 2009). Critically, fetal exposures to BPA at levels equivalent to, or below the established daily human safe-dose (50μg BPA/kg body weight/day) not only increase body weight and postnatal growth rate, but also alter body composition in later life (Alonso-Magdalena et al., 2006; Alonso-Magdalena et al., 2010; Richter et al., 2007; Rubin and Soto, 2009; vom Saal et al., 2012)
One of the critical determinants of energy balance include energy (calorie) intake (Hill et al., 2012). The arcuate nucleus (ARC) of the hypothalamus is the key regulator of appetite, containing both orexigenic (neuropeptide Y, NPY; agouti-related peptide, AgRP) and anorexigenic (pro-opiomelanocortin, POMC) neurons involved in central regulation of food intake (Blevins et al., 2002). Orexigenic and anorexigenic neurons develop before birth, in preparation for extra-uterine life, (Kagotani et al., 1989) however functional projections are established during the early postnatal period in rodents (Grove et al., 2001; Nilsson et al., 2005; Padilla et al., 2010; Walsh and Brawer, 1979). Studies including those by our laboratory have shown prenatal nutrition-mediated effects on ARC neurogenesis resulting in a shift from satiety to appetite neurons in association with offspring hyperphagia and obesity (Staples et al., 2017; Val-Laillet et al., 2017). We have further shown that hypothalamic neuroprogenitor cell (NPC) proliferation (self-renewal) and differentiation (generation of neurons/glial cells) are vulnerable to endocrine disruption, with potential long-term consequences for appetite regulation and energy balance (Desai et al., 2011a; Desai et al., 2011b). Notably, BPA has been shown to influence neurogenesis in humans (Preciados et al., 2016) and animal models (Kim et al., 2009). In mice, prenatal exposure to BPA increases neurogenesis and neuronal migration (Nakamura et al., 2006) resulting in altered brain structure (Nakamura et al., 2007) and function (Nakamura et al., 2012).
Neurogenesis is regulated, in part, by basic-helix-loop-helix (bHLH) genes including differentiation repressor genes (e.g., Hes1) that maintain the NPC population, and activator genes (e.g. Math3; Mash1; Neurogenin, Ngn), which accelerate neurogenesis and differentiation (Kageyama et al., 2008; Masica et al., 1971; Ohtsuka et al., 2001). Maternal BPA up-regulates Math3 and Ngn2 in mouse embryos, (Nakamura et al., 2006) and accelerated neurogenesis due to BPA exposure may reduce the population of NPCs in fetal (e14.5) mice (Komada et al., 2012; Nakamura et al., 2006). However, the effects of perinatal BPA on rat hypothalamic NPC cell proliferation and differentiation have not been determined.
We studied the effects of maternal BPA exposure during pregnancy on cultured hypothalamic NPCs from 1 day old newborns and examined development of appetite/satiety neurons (Desai et al., 2014). To more fully explore the mechanisms of BPA-mediated effects, we then utilized established models of newborn rat primary hypothalamic NPCs (which ultimately form appetite/satiety neurons), exploring both proliferative (i.e., trophic) and differentiation effects of BPA (Desai et al., 2011a; Desai et al., 2011b). We further explored putative signal factors which explain, in part, NPC responses, and underlying epigenetic mechanisms mediated by BPA. Our results demonstrate marked effects of BPA on hypothalamic progenitor cell proliferation as well as differentiation. These findings emphasize the vulnerability of stem-cell populations that are involved in life-long regulation of metabolic homeostasis to endocrine disruption by BPA during early life.
MATERIALS AND METHODS
BPA Models
In Vivo Maternal BPA Exposure:
Studies were approved by the Animal Care Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA and were in accordance with the American Accreditation Association of Laboratory Care. All animals were treated humanely and with regard for alleviation of suffering. Virgin Sprague Dawley female rats (Charles River Laboratories, Hollister, CA) were housed in an animal facility with controlled 12/12 hour light/dark cycles, constant temperature and humidity conditions and ad libitum access to chow diet (Lab Diet 5001; Brentwood, Missouri). To avoid potential BPA contamination, polypropylene cages and purified water in glass bottles were utilized. Female rats were randomly assigned to Control (n=6) or BPA (n=6) group. Control rats had access to purified drinking water, whereas the BPA group received purified drinking water containing BPA (5mg/L; BPA Sigma-Aldrich, purity ≥ 99%, CAS no. 80-05-7) for two weeks prior to mating and throughout pregnancy (Table 1). Among studies administering BPA to pregnant rodents via drinking water, a concentration of 10 mg/l water (consumption of ~1.2 mg/kg BW/day) (Mendoza-Rodriguez et al., 2011) produced BPA tissue concentrations of 10-25 ng/g tissue (Kabuto et al., 2004; Nakajima et al., 2012) consistent with that of human samples (Schonfelder et al., 2002). A gavage dose five-fold higher (6 mg/kg BW/day) achieved a significant increase in maternal serum BPA concentration (Yoshida et al., 2004), whereas a water concentration of only 1 mg/l resulted in low maternal plasma free BPA levels (0.84 ng/ml) (Patisaul et al., 2012). Our dose was selected based upon our confirmation (pilot study) of maternal and newborn serum levels within the lower range of demonstrated human levels with normal BPA exposure.
Table 1:
Study Design of BPA Exposure
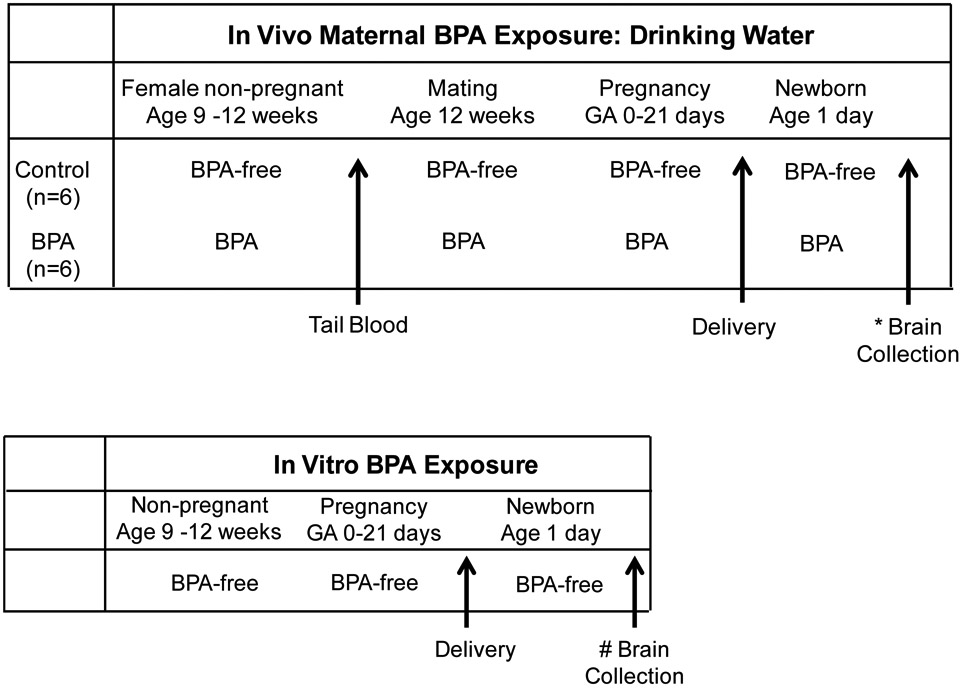 |
In Vivo Maternal BPA Exposure: Non-pregnant female rats at 9 weeks of age were allowed drinking water that was BPA-free (Control group) or contained BPA (BPA group). At 12 weeks of age, tail blood was obtained for BPA analysis and all females were mated and continued on same drinking water regimen throughout pregnancy. Brains were collected from their newborns for cultures of hypothalamic NPCs in BPA free media.
In Vitro BPA Exposure: Non-pregnant and pregnant dams had access to BPA-free drinking water. Brains were collected from their newborns for cultures of hypothalamic NPCs in media containing BPA.
Maternal blood prior to mating was obtained via tail bleed and newborn blood was collected in BPA-free tubes for BPA analysis. We did not obtain blood samples during pregnancy as blood collection via tail vein is known to induce stress, resulting in fetal resorption (Weinstock, 2017). Further, maternal stress has been demonstrated to be an independent risk factor for offspring obesity and for impacting brain development (Hohwu et al., 2014; Moog et al., 2018; Mueller and Bale, 2006). Free (unconjugated) BPA levels were measured using GC/MS (NMS Labs, PA) with assay sensitivity of 0.25 ng/ml. Insufficient plasma volume from maternal and newborns necessitated pooling of samples and hence only mean values are reported.
Dams gave birth spontaneously and at 1 day of age, four males from one litter were sacrificed, hypothalamus dissected and samples pooled (representing N=1) for primary NPC culture studies (described below). A total of 6 litters of Control and 6 litters of BPA group were studied.
In Vitro BPA Exposure:
An additional four control litters were studied for in vitro effects of exogenous BPA on NPCs. From each litter, hypothalamus was dissected from 1 day old Control males (four pooled samples representing N=1) for cell culture studies (Table 1). Passage 2 NPCs cultured in complete medium were treated with DMSO (control) or BPA (1, 10, 20 μM) for 5 days. For NPC differentiation studies, passage 2 cells were treated with 10μM BPA (see below). The total number studied for NPC cultures was N=4 from 4 litters.
Primary Cultures
Hypothalamic NPC Cultures:
NPCs were isolated and grown as neurospheres, as described previously in detail (Desai et al., 2011a). Briefly, hypothalamus was dissected in DMEM/F12 medium, cells dissociated by trypsin, centrifuged and cells seeded (~5×104 cells/ml) in complete medium [NeurobasalTM Medium containing 1% anti-anti (Invitrogen), 2% B27 (GIBCO, Cat# 17504-044), 20ng/ml FGF2 (Sigma), 20ng/ml EGF (Sigma), 1μg/ml heparin (Lylli), and 2.5μg/ml L-glutamine (Invitrogen)]. After 8 days in culture (passage 0), centrifuged neurospheres were dissociated into single-cell by trypsinization and reseeded (passage 1) in complete medium. For induction of differentiation, dissociated cells were re-suspended in differentiating medium (in absence of FGF2, EGF and heparin) and seeded in culture dishes pre-coated with 0.01% poly-L-lysine (Sigma). Cells in complete and differentiated medium were harvested and protein extracted for analysis as described under Western Blot.
Analysis
NPC Proliferation Assay:
NPC (complete medium) proliferation was determined as previously reported (Desai et al., 2011a) using MTT colorimetric assay (Mosmann, 1983).
Western Blot:
Protein was extracted and Western Blotting performed as described previously (Desai et al., 2008). For NPCs, protein expression analysis included NPC marker (Nestin, Sigma); neuroproliferative and (Hes1, 35 kDa, Santa Cruz), proneurogenic (Mash1, 30 kDa, Santa Cruz; Ngn3, 23 kDa, abcam) factors; markers of neuron (Tuj1, 50 kDa) and astrocyte (GFAP, 46 kDa, Dako), orexigenic (AgRP, 14 kDa, Santa Cruz; NPY) and anorexigenic (POMC, 30 kDa, Santa Cruz) neuropeptides, and; epigenetic regulators DNA methyltransferase 3a (DNMT3a, 120 kDa, Santa Cruz) and lysine (K)-specific histone demethylase 1A (LSD1, 110 kDa, Cell Signaling).
Immunostaining:
Staining of cultured NPC has been previously described (Desai et al., 2011a; Desai et al., 2011b). Briefly, disassociated neurosphere cells in complete or differentiating medium were fixed in 4% paraformaldehyde in PBS and stained with rabbit anti-nestin (Sigma), anti-Hes1(Santa Cruz) or anti-Ngn3 (abcam). Secondary antibodies were donkey anti-rabbit IgG-Alexa 488 or donkey anti-mouse IgG-Alexa 568.
Statistical Analyses:
In vivo responses between BPA and control offspring were compared by unpaired t-test. In vitro responses between BPA exposed and untreated (DMSO) Control cells were compared by unpaired t-test or analysis of variance (ANOVA) with Dunnett’s post-hoc test, as appropriate. P values ≤ 0.05 were considered significant.
RESULTS
Maternal BPA Effects
Plasma BPA Levels:
The average water consumption over the course of pregnancy was similar in BPA and Control dams (BPA=47.4±3.0 ml/day; Control = 46.4±3.7 ml/day). Prior to BPA administration, the pooled maternal plasma BPA value was 0.46 ng/ml. The amount of BPA consumed by dams via drinking water was approximately 500-900 μg/kg/day during pregnancy. Newborns of BPA dams had higher plasma BPA levels (0.62 ng/ml) as compared to undetectable levels in newborns of Control dams.
Maternal BPA Effects on Offspring Hypothalamic NPCs:
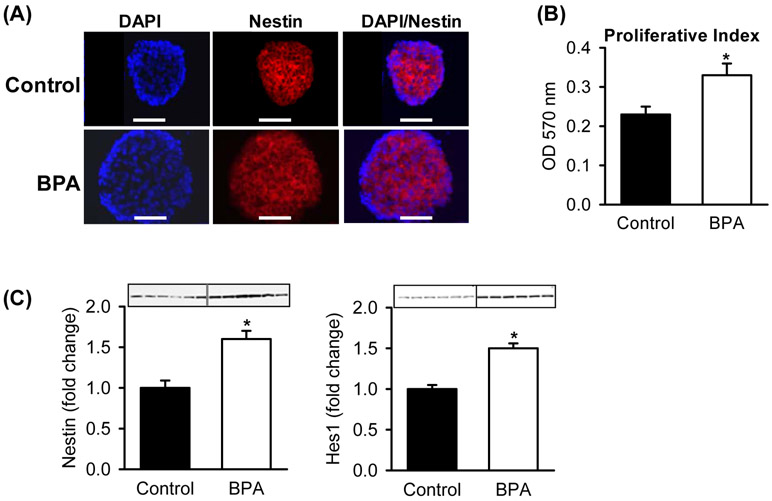
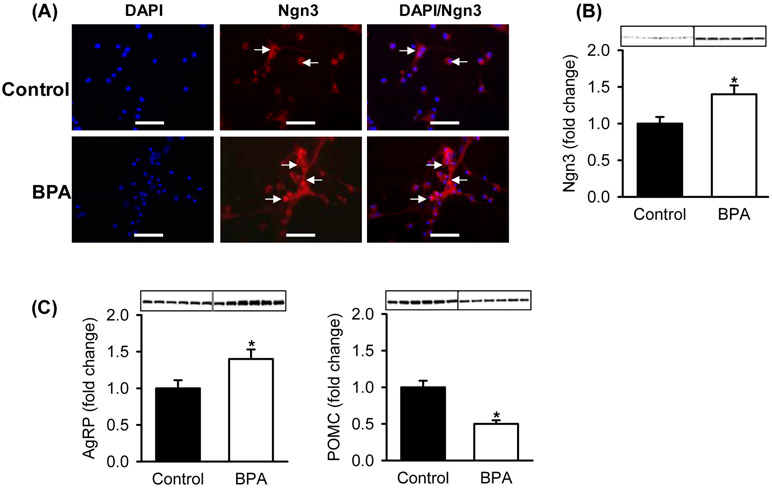
At 1 day of age, neurospheres from BPA males cultured in complete media had increased NPC proliferation and increased expression of the NPC marker (Nestin). The bHLH proliferative factor Hes1 was increased in BPA-exposed as compared to Control NPCs (Figure 1). In differentiation media, BPA NPCs showed increased expression of the pro-differentiation neurogenic factor Ngn3 by both immunostaining and protein expression as compared to Controls (Figures 2A, B). Importantly, BPA NPCs exhibited enhanced differentiation toward appetite as compared to satiety neurons, as evident by increased protein expression of AgRP and decreased expression of POMC (Figure 2C).
Figure 1: Maternal BPA Effects on Offspring NPC Proliferation.
Hypothalamic NPCs from Control and BPA 1 day old male newborns were cultured in complete media. (A) Double immunostained images of (x40; scale bar = 50μm) of DAPI (blue, nuclear stain) and Nestin (red, NPC marker). (B) Proliferative index measured at 570 OD. (C) Protein expression of NPC marker (Nestin) and neuroproliferative factor (Hes1). Values are fold change (mean±SE) of n=6 of pooled hypothalami from each of 6 litters per group. * P< 0.05 BPA (□) vs. Control (■).
Figure 2: Maternal BPA Effects on Offspring NPC Differentiation and Neuropeptides.
Hypothalamic NPCs from Control and BPA 1 day old male newborns were cultured in differentiation media. (A) Double immunostained images of (x40; scale bar = 50μm) of DAPI (blue, nuclear stain) and Ngn3 (red, proneurogenic factor). (B) Protein expression of Ngn3. (C) Protein expression of appetite (AgRP) and satiety (POMC) neuropeptides. Values are fold change (mean±SE) of n=6 of pooled hypothalami from each of 6 litters per group. * P< 0.05 BPA (□) vs. Control (■).
In Vitro BPA Effects
NPCs:
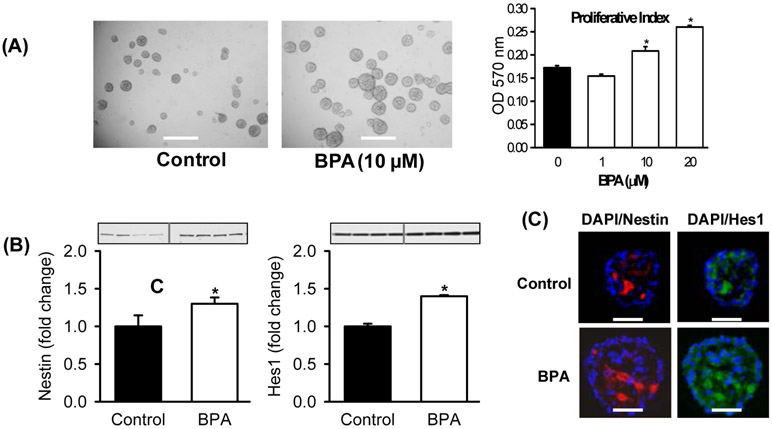
We also examined the effects of in vitro exposure to BPA on cultures from Control hypothalamic tissue. Control hypothalamic NPCs cultured in complete media with BPA showed dose-dependent increased NPC proliferation (Figure 3A), consistent with increased expression of the NPC marker Nestin and the proliferation bHLH factor Hes1 (Figure 3B), confirmed by double immunostaining (Figure 3C). In differentiation media, BPA promoted NPC differentiation toward increased neuronal (Tuj1) as compared to glial (GFAP) cell fate (Figure 4A), in conjunction with increased expression of the differentiation bHLH factors Mash1 and Ngn3. Consistent with the in vivo results (above), BPA enhanced differentiation toward appetite as compared to satiety neurons, as evident by increased protein expression of AgRP/NPY and decreased expression of POMC (Figure 4B).
Figure 3: In Vitro BPA Effects on NPC Proliferation.
Hypothalamic NPCs from 1 day old Control newborns were cultured in complete media and treated with DMSO (Control) or BPA (10 μM unless otherwise specified) for 5 days. (A) Images of NPCs (x20; scale bar = 50μm) and proliferative index measured at 570 OD of NPCs treated with BPA (1, 10, 20 μM). (B) Protein expression of Nestin (NPC marker) and bHLH proliferative (Hes1). (C) Immunostained images of (x40) Nestin (red), Hes1 (green) and DAPI (blue, nuclear stain). Values are fold change (mean±SE) of n=4 of pooled NPCs from each of 4 litters. * P< 0.05 BPA (□) vs. Control (■).
Figure 4: In Vitro BPA Effects on NPC Differentiation.
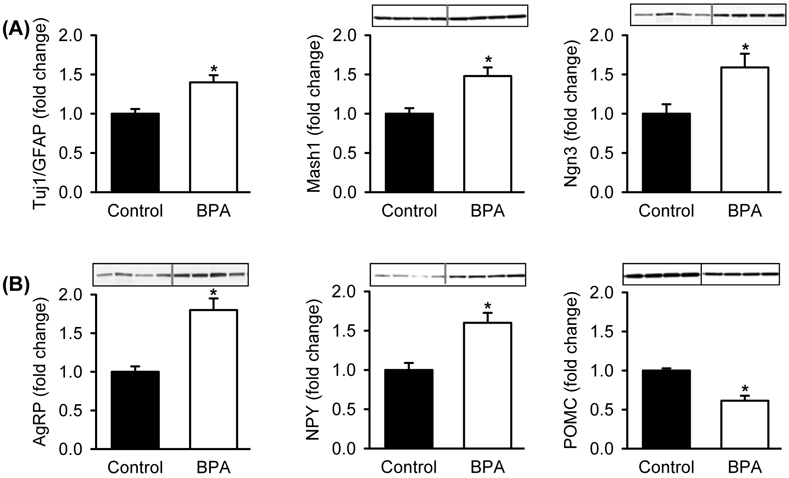
Hypothalamic NPCs from 1 day old Control newborns were cultured in differentiation media and treated with DMSO (Control) or BPA (10 μM,) for 5 days. (A) Protein expression of bHLH proneurogenic factors (Mash1, Ngn3) and neuronal (Tuj1) and astrocyte (GFAP) markers. (B) Protein expression of appetite (AgRP, NPY) and satiety (POMC) neuropeptides .Values are fold change (mean±SE) of n=4 of pooled NPCs from each of 4 litters; * P< 0.05 BPA (□) vs. Control (■).
Epigenetic Factors:
BPA treated NPCs in complete media demonstrated no change in DNMT3, but significantly increased LSD1 (Figure 5).
Figure 5: In Vitro BPA Effects on NPC Epigenetic Factors.
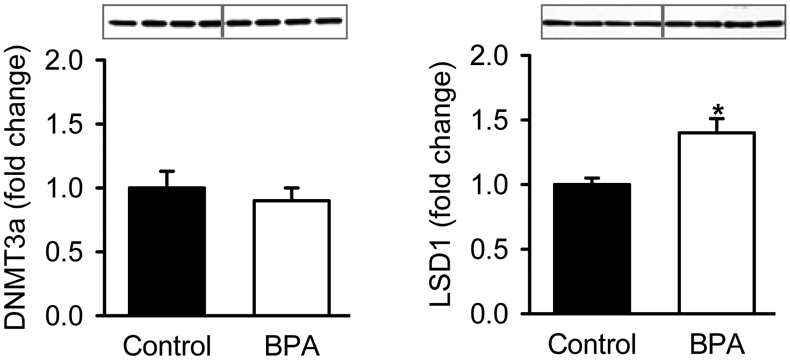
Hypothalamic NPCs from 1 day old Control newborns were cultured in complete medium and treated with DMSO (Control) or BPA (10 μM,) for 5 days and protein expression of DNMT3a and LSD1 analyzed. Values are fold change (mean±SE) of of pooled cells n=4 from each of 4 litters; * P< 0.05 BPA (□) vs. Control (■).
DISCUSSION
The effects of prenatal BPA exposure on offspring hypothalamic NPC proliferation and differentiation, and the potential underlying mechanism involving regulatory transcription factors have not been previously explored. The results of the present study suggest that BPA-induced dysregulation of hypothalamic NPC proliferation and differentiation may influence appetite regulation and contribute to obesity.
Measurable BPA levels are seen in adults and children, including breast milk (1.1 ng/ml), maternal (0.2- >10 ng/ml) and fetal/newborn serum (0.2-9.2 ng/ml). More importantly, the higher levels reported in amniotic fluid (8.3-8.7 ng/ml) and placental tissues (1.0-104.9 ng/ml) (Kosarac et al., 2012; Padmanabhan et al., 2008), suggest the continued exposure of the fetus to BPA throughout development. BPA levels are higher in infants and children than in adults (Welshons et al., 2006) and notably, are associated with increased adiposity (Harley et al., 2013; Rochester, 2013). Animal studies confirm the association of BPA with adiposity and note that it is low (≤500 μg/kg/day) rather than high dose (>5,000 μg/kg/day) of maternal BPA that is effective in promoting offspring weight gain (Angle et al., 2013; Somm et al., 2009). Increased male and female postnatal growth is seen at maternal BPA doses between 2.4-500 μg/kg/day (Richter et al., 2007; van Esterik et al., 2014; Wei et al., 2011) with sex-specific effects seen in food intake. Males though not females exhibit age-related increased food intake (Angle et al., 2013).
As BPA-mediated adiposity is dependent, in part, on enhanced food intake (suckling) (Dyer and Rosenfeld, 2011; Garza and Butte, 1990; Ojha et al., 2013), we investigated the effects of in vivo and in vitro BPA exposure on hypothalamic NPCs that ultimately produce appetite and satiety neurons (Miller and Gauthier, 2007; Sousa-Ferreira et al., 2011). Exposure to BPA in vivo and in vitro increased both the proliferation and differentiation of hypothalamic NPCs, consistent with previous studies of BPA-effects on rat embryonic neural stem cell cultures (Okada et al., 2008; Tiwari et al., 2014), and murine neurogenesis in vivo (Itoh et al., 2012; Kim et al., 2009; Komada et al., 2012; Nakamura et al., 2006). Specifically, maternal BPA exposure induced a marked trophic effect on the hypothalamic NPCs from 1 day old offspring, as indicated by increased NPC proliferation index and increased NPC protein expression of the NPC marker, Nestin. Further, BPA increased the protein expression of the bHLH proliferation transcription factor Hes1, which suppresses neuronal differentiation by inhibiting proneurogenic bHLH factors Mash1 and Ngn3 (Kageyama et al., 2008). In differentiating medium, BPA exposure increased Ngn3, indicating increased neuronal differentiation. More importantly, Mash1 and Ngn3 are required for development of POMC/NPY neurons (McNay et al., 2006; Pelling et al., 2011) and notably, BPA NPCs showed increased appetite (AgRP) versus satiety (POMC) peptide expression.
In vitro BPA exposure complemented the in vivo exposure findings and further demonstrated increased expression of Mash1 and Ngn3, indicating that there was increased neurogenesis as opposed to astrogliogenesis (increased Tuj1/GFAP ratio). These data are consistent with accelerated neurogenesis, consistent with BPA effects on murine neocortical and hippocampal structure (Jang et al., 2012; Komada et al., 2012; Kunz et al., 2011; Nakamura et al., 2007; Nakamura et al., 2006) synaptogenesis (Kagotani et al., 1989; Xu et al., 2013) and cerebellar granule neuron development (Mathisen et al., 2013). In addition, studies of embryonic zebrafish demonstrate that low dose BPA causes increased neurogenesis at birth (Kinch et al., 2015). Notably, endogenous neurodifferentiation factors may preferentially direct NPC differentiation towards neuronal (e.g., leptin) or astrocyte fate (e.g., insulin) (Desai et al., 2011a; Garza et al., 2008; Machida et al., 2012). Whether BPA exposure alters the relative expression of neuronal to glial cells in vivo (Okada et al., 2008) is unknown, but of concern. However, a recent study by MacKay et al (MacKay et al., 2017) demonstrates in vivo BPA effects on specific hypothalamic pathways involving melanocortin circuitry. Young adult offspring exposed to prenatal BPA exhibited a delayed postnatal leptin surge with leptin resistance, and showed a reduced density of POMC projections into the hypothalamic paraventricular nucleus (PVN). Notably daily injections of supplemental leptin in BPA exposed pups, rescued POMC projections into the PVN.
The mechanism underlying BPA-induced enhanced NPC proliferation and differentiation may involve epigenetic modifications (Bastos et al., 2013; Kundakovic and Champagne, 2011), particularly altered methylation of gene Hes1 (Lillycrop et al., 2015). Methylation and demethylation is catalyzed by enzymes DNMT (DNA methyltransferase) and LSD1 (lysine (K)-specific histone demethylase), respectively, both of which have been implicated in determining stem cell proliferation (self-renewal) and differentiation (Adamo et al., 2011; Wu et al., 2012). For example, Dnmt3a or Lsd1 knockout mice demonstrate impaired neuronal production coupled with increased astrogliogenesis and reduced NPC proliferation, respectively (Sun et al., 2010; Wu et al., 2012). Although we demonstrated no change in DNMT3a, the increased protein expression of LSD1 in response to BPA is consistent with an epigenetically-mediated shift toward neurogenesis. Previous studies show that perinatal BPA exposure alters brain DNMT3a which is region specific with increased mRNA levels of Dnmt3a seen in the cerebral cortex and no changes evident in the hippocampus (Kumar and Thakur, 2017) Dnmt1 and Lsd1 are highly interrelated and rely mechanistically on each other for normal chromatin function in vivo. Targeted deletion of the gene encoding Lsd1 in embryonic stem cells induces progressive loss of DNA methylation. This loss correlates with a decrease in DNMT1 protein, as a result of reduced Dnmt1 stability (Wang et al., 2009). However, it is unclear whether the effects of Lsd1 deficiency are mediated through an inability of Dnmt3a to catalyse 5mC, or via direct effects on the maintenance methyltransferase Dnmt (Rose and Klose, 2014).
Notably, LSD1 interacts with Notch pathway (Lopez et al., 2016), which is involved in neurogenesis and regulation of Hes1 expression (Imayoshi et al., 2010), and shown to specifically target the expression of bHLH gene HEYL (Hirano and Namihira, 2016a; Hirano and Namihira, 2016b). While the current data suggest BPA alters transcriptional regulation of genes involved in proliferation and differentiation by demethylation within hypothalamic NPCs, further studies of site-specific epigenetic modification of specific genes are required to fully elucidate BPA-induced changes in neurogenesis.
Developing brain is more vulnerable to BPA due to its lipophilic chemical structure that allows it to easily cross the blood-placental and blood-brain barriers, impacting neurogenesis and thereby brain physiology. Although BPA mimics estradiol effects (Rubin, 2011), it may exert its action via differing pathways. Estradiol upregulates neurogenesis (Barker and Galea, 2008; Tanapat et al., 1999) and exerts its actions primarily through the genomic pathway involving nuclear estrogen receptors (Quaedackers et al., 2001). In contrast, BPA has higher affinity for membrane-bound G protein-coupled estrogen receptors (Thomas and Dong, 2006). Thus, BPA may act through both pathways in promoting NPC (Okada et al., 2008) proliferation. BPA interferes with the dimorphic development of the neuronal networks controlling brain functions (Delfosse et al., 2014; Wolstenholme et al., 2011) and alters dimorphic feeding behaviour (Liang et al., 2002; Negri-Cesi, 2015; Titolo et al., 2006). Specifically, estrogen can modulate the production of NPY and AgRP (Titolo et al., 2006) and mediate anorectic properties (Liang et al., 2002) by influencing POMC neurons in the ARC (Gao et al., 2007). The activity of this neuronal circuitry is gender specific, with females showing responsiveness to various anorectic inputs different from that of males (Mackay et al., 2013).
CONCLUSION
These data confirm that primary neuroprogenitor cells are vulnerable to endocrine disruption by BPA resulting in altered proliferation and differentiation, independent of systemic influences. Enhanced proliferation coupled with increased differentiation of NPCs to appetite as compared to satiety neurons indicate the potential for maternal/fetal BPA exposure to program an increased risk of offspring obesity (Ding et al., 2014; Miyawaki et al., 2007; Perreault et al., 2013; Somm et al., 2009). Of equal importance, the marked shift in NPC differentiation to neuronal versus glial fates may adversely impact cerebral development (e.g., cognition, behaviour) in regions beyond the appetite network. Future study that addresses long-term effect of altered neurogenesis and whether similar changes are evident in females should overcome the limitation of the present study
Acknowledgements:
The authors thank Stacy Behare for animal assistance.
Funding Sources:
This work was supported by the National Institute of Environmental Health Sciences (R21ES023112-01; MD, MGR), National Center for Advancing Translational Sciences UCLA- CTSI (Grant U11TR000124; MD), National Institute on Minority Health and Health Disparities (5U54MD007598-06 (MGF) and Flora Foundation (MD, MGR).
Abbreviations
- ARC
Arcuate nucleus
- bHLH
Basic-helix-loop-helix
- LSD1
Demethylase enzyme
- NPY
Neuropeptide Y
- POMC
Pro-opiomelanocortin
- AgRP
Agouti-related peptide
- BPA
Bisphenol A
- Ngn
Neurogenin
- NPCs
Neuroprogenitor cells
Footnotes
Ethical Approval on Animal Research: Studies were approved by the Animal Research Committee of the Los Angles Biomedical Research Institute at Harbor-UCLA Medical Center and were conducted in strict accordance with guidelines provided by the American Accreditation Association of Laboratory Care and the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and conform to the principles and regulations as described in the Editorial by Grundy (Grundy, 2015). All animals were treated humanely and with regard for alleviation of suffering. Virgin Sprague Dawley female rats (Charles River Laboratories, Hollister, CA) were housed in an animal facility with controlled 12/12 hour light/dark cycles, constant temperature and humidity conditions and ad libitum access to chow diet (Lab Diet 5001; Brentwood, Missouri).
Literature Cited
- Adamo A, et al. , 2011. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat.Cell Biol 13, 652–659. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, et al. , 2006. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ.Health Perspect 114, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, et al. , 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ.Health Perspect 118, 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle BM, et al. , 2013. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 42, 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Galea LA, 2008. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 152, 888–902. [DOI] [PubMed] [Google Scholar]
- Bastos SL, et al. , 2013. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol.In Vitro 27, 1634–1643. [DOI] [PubMed] [Google Scholar]
- Blevins JE, et al. , 2002. Peptide signals regulating food intake and energy homeostasis. Can.J.Physiol Pharmacol 80, 396–406. [DOI] [PubMed] [Google Scholar]
- Cobellis L, et al. , 2009. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed Chromatogr. 23, 1186–90. [DOI] [PubMed] [Google Scholar]
- Delfosse V, et al. , 2014. Nuclear receptor profiling of bisphenol-A and its halogenated analogues. Vitam Horm. 94, 229–51. [DOI] [PubMed] [Google Scholar]
- Desai M, et al. , 2008. Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod.Sci 15, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, et al. , 2014. Programmed hyperphagia secondary to increased hypothalamic SIRT1. Brain Res. 1589, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, et al. , 2011a. Fetal Hypothalamic Neuroprogenitor Cell Culture: Preferential Differentiation Paths Induced by Leptin and Insulin. Endocrinology. 152, 3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, et al. , 2011b. Hypothalamic neurosphere progenitor cells in low birth-weight rat newborns: neurotrophic effects of leptin and insulin. Brain Res. 1378, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciaula A, Portincasa P, 2017. Diet and contaminants: driving the rise to obesity epidemics? Curr Med Chem. [DOI] [PubMed] [Google Scholar]
- Ding S, et al. , 2014. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J Endocrinol. 221, 167–179. [DOI] [PubMed] [Google Scholar]
- Dyer JS, Rosenfeld CR, 2011. Metabolic imprinting by prenatal, perinatal, and postnatal overnutrition: a review. Semin.Reprod Med 29, 266–276. [DOI] [PubMed] [Google Scholar]
- Gao Q, et al. , 2007. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 13, 89–94. [DOI] [PubMed] [Google Scholar]
- Garza C, Butte NF, 1990. Energy intakes of human milk-fed infants during the first year. J Pediatr. 117, S124–S131. [DOI] [PubMed] [Google Scholar]
- Garza JC, et al. , 2008. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol.Chem 283, 18238–18247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, et al. , 2001. Chronic maternal nicotine exposure alters neuronal systems in the arcuate nucleus that regulate feeding behavior in the newborn rhesus macaque. J.Clin.Endocrinol.Metab 86, 5420–5426. [DOI] [PubMed] [Google Scholar]
- Grundy D, 2015. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Exp Physiol. 100, 755–8. [DOI] [PubMed] [Google Scholar]
- Harley KG, et al. , 2013. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ.Health Perspect 121, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JO, et al. , 2012. Energy balance and obesity. Circulation. 126, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Namihira M, 2016a. LSD1 Mediates Neuronal Differentiation of Human Fetal Neural Stem Cells by Controlling the Expression of a Novel Target Gene, HEYL. Stem Cells. 34, 1872–82. [DOI] [PubMed] [Google Scholar]
- Hirano K, Namihira M, 2016b. New insight into LSD1 function in human cortical neurogenesis. Neurogenesis (Austin). 3, e1249195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwu L, et al. , 2014. Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: a Danish National Cohort Study. PLoS One. 9, e97490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, et al. , 2010. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 30, 3489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, et al. , 2012. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology. 32, 447–457. [DOI] [PubMed] [Google Scholar]
- Janesick A, Blumberg B, 2012. Obesogens, stem cells and the developmental programming of obesity. Int.J Androl 35, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, et al. , 2012. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 296, 73–82. [DOI] [PubMed] [Google Scholar]
- Kabuto H, et al. , 2004. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 74, 2931–40. [DOI] [PubMed] [Google Scholar]
- Kageyama R, et al. , 2008. Roles of Hes genes in neural development. Dev Growth Differ. 50 Suppl 1, S97–103. [DOI] [PubMed] [Google Scholar]
- Kagotani Y, et al. , 1989. Development of the neuronal system containing neuropeptide Y in the rat hypothalamus. Int.J.Dev.Neurosci 7, 359–374. [DOI] [PubMed] [Google Scholar]
- Kim K, et al. , 2009. Potencies of bisphenol A on the neuronal differentiation and hippocampal neurogenesis. J Toxicol.Environ.Health A 72, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Kim ME, et al. , 2011. Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem Toxicol. 49, 3383–3389. [DOI] [PubMed] [Google Scholar]
- Kinch CD, et al. , 2015. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc.Natl.Acad.Sci U.S.A 112, 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, et al. , 2012. Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology. 295, 31–38. [DOI] [PubMed] [Google Scholar]
- Kosarac I, et al. , 2012. A novel method for the quantitative determination of free and conjugated bisphenol A in human maternal and umbilical cord blood serum using a two-step solid phase extraction and gas chromatography/tandem mass spectrometry. J Chromatogr.B Analyt.Technol.Biomed.Life Sci 898, 90–94. [DOI] [PubMed] [Google Scholar]
- Kumar D, Thakur MK, 2017. Effect of perinatal exposure to Bisphenol-A on DNA methylation and histone acetylation in cerebral cortex and hippocampus of postnatal male mice. J Toxicol Sci. 42, 281–289. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA, 2011. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav.Immun 25, 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz N, et al. , 2011. Developmental and metabolic brain alterations in rats exposed to bisphenol A during gestation and lactation. Int.J Dev Neurosci 29, 37–43. [DOI] [PubMed] [Google Scholar]
- Liang YQ, et al. , 2002. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 26, 1103–9. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, et al. , 2015. Association between perinatal methylation of the neuronal differentiation regulator HES1 and later childhood neurocognitive function and behaviour. Int.J Epidemiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CI, et al. , 2016. The chromatin modifying complex CoREST/LSD1 negatively regulates notch pathway during cerebral cortex development. Dev Neurobiol. 76, 1360–1373. [DOI] [PubMed] [Google Scholar]
- Machida M, et al. , 2012. The insulin regulatory network in adult hippocampus and pancreatic endocrine system. Stem Cells Int. 2012, 959737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay H, et al. , 2017. Perinatal Exposure to Low-Dose Bisphenol-A Disrupts the Structural and Functional Development of the Hypothalamic Feeding Circuitry. Endocrinology. 158, 768–777. [DOI] [PubMed] [Google Scholar]
- Mackay H, et al. , 2013. Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology. 154, 1465–75. [DOI] [PubMed] [Google Scholar]
- Masica DN, et al. , 1971. Fetal feminization and female gender identity in the testicular feminizing syndrome of androgen insensitivity. Arch.Sex Behav 1, 131–142. [DOI] [PubMed] [Google Scholar]
- Mathisen GH, et al. , 2013. Prenatal exposure to bisphenol A interferes with the development of cerebellar granule neurons in mice and chicken. Int.J Dev Neurosci 31, 762–769. [DOI] [PubMed] [Google Scholar]
- McNay DE, et al. , 2006. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol.Endocrinol 20, 1623–1632. [DOI] [PubMed] [Google Scholar]
- Mendoza-Rodriguez CA, et al. , 2011. Administration of bisphenol A to dams during perinatal period modifies molecular and morphological reproductive parameters of the offspring. Reprod Toxicol. 31, 177–83. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS, 2007. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 54, 357–369. [DOI] [PubMed] [Google Scholar]
- Miyawaki J, et al. , 2007. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler.Thromb 14, 245–252. [DOI] [PubMed] [Google Scholar]
- Moog NK, et al. , 2018. Intergenerational Effect of Maternal Exposure to Childhood Maltreatment on Newborn Brain Anatomy. Biol Psychiatry. 83, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T, 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J.Immunol.Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL, 2006. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav. 88, 605–614. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, et al. , 2012. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health. 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, et al. , 2012. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev. 34, 57–63. [DOI] [PubMed] [Google Scholar]
- Nakamura K, et al. , 2007. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neuroscience Letters. 420, 100–105. [DOI] [PubMed] [Google Scholar]
- Nakamura K, et al. , 2006. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J Neurosci.Res 84, 1197–1205. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, 2015. Bisphenol A Interaction With Brain Development and Functions. Dose Response. 13, 1559325815590394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I, et al. , 2005. Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Brain Res Dev Brain Res. 155, 147–154. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, et al. , 2001. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J Biol.Chem 276, 30467–30474. [DOI] [PubMed] [Google Scholar]
- Ojha S, et al. , 2013. Excess nutrient supply in early life and its later metabolic consequences. Clin.Exp.Pharmacol.Physiol 40, 817–823. [DOI] [PubMed] [Google Scholar]
- Okada M, et al. , 2008. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed.Res 29, 163–170. [DOI] [PubMed] [Google Scholar]
- Padilla SL, et al. , 2010. Pome-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat.Med 16, 403–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, et al. , 2008. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 28, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, et al. , 2008. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 108, 150–7. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, et al. , 2012. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 7, e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelling M, et al. , 2011. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev.Biol 349, 406–416. [DOI] [PubMed] [Google Scholar]
- Perreault L, et al. , 2013. Bisphenol A impairs hepatic glucose sensing in C57BL/6 male mice. PLoS.ONE 8, e69991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preciados M, et al. , 2016. Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases. Int J Mol Sci. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedackers ME, et al. , 2001. 4-hydroxytamoxifen trans-represses nuclear factor-kappa B activity in human osteoblastic U2-OS cells through estrogen receptor (ER)alpha, and not through ER beta. Endocrinology. 142, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Richter CA, et al. , 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR, 2013. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 42, 132–155. [DOI] [PubMed] [Google Scholar]
- Rose NR, Klose RJ, 2014. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta. 1839, 1362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, 2011. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem.Mol.Biol 127, 27–34. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Soto AM, 2009. Bisphenol A: Perinatal exposure and body weight. Mol.Cell Endocrinol 304, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano S, et al. , 2015. Bisphenol-A plasma levels are related to inflammatory markers, visceral obesity and insulin-resistance: a cross-sectional study on adult male population. J Transl Med. 13, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfelder G, et al. , 2002. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ.Health Perspect 110, A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E, et al. , 2009. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ.Health Perspect 117, 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Ferreira L, et al. , 2011. Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and Orexin-A and differentiate to functional neurons. PLoS.ONE 6, e19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples MC, et al. , 2017. Dietary restriction reduces hippocampal neurogenesis and granule cell neuron density without affecting the density of mossy fibers. Brain Res. 1663, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, et al. , 2010. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol.Cell Biol 30, 1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, et al. , 1999. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 19, 5792–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppala S, et al. , 2012. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int.J Endocrinol 2012, 598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Dong J, 2006. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem.Mol.Biol 102, 175–179. [DOI] [PubMed] [Google Scholar]
- Titolo D, et al. , 2006. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol Endocrinol. 20, 2080–92. [DOI] [PubMed] [Google Scholar]
- Tiwari SK, et al. , 2014. Inhibitory Effects of Bisphenol-A on Neural Stem Cells Proliferation and Differentiation in the Rat Brain Are Dependent on Wnt/beta-Catenin Pathway. Mol.Neurobiol [DOI] [PubMed] [Google Scholar]
- Val-Laillet D, et al. , 2017. A maternal Western diet during gestation and lactation modifies offspring's microbiota activity, blood lipid levels, cognitive responses, and hippocampal neurogenesis in Yucatan pigs. FASEB J. 31, 2037–2049. [DOI] [PubMed] [Google Scholar]
- van Esterik JC, et al. , 2014. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology. 321, 40–52. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2007. Human exposure to bisphenol A (BPA). Reprod Toxicol. 24, 139–177. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, et al. , 2012. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol.Cell Endocrinol 354, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RJ, Brawer JR, 1979. Cytology of the arcuate nucleus in newborn male and female rats. J Anat. 128, 121–133. [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. , 2009. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 41, 125–9. [DOI] [PubMed] [Google Scholar]
- Wei J, et al. , 2011. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 152, 3049–3061. [DOI] [PubMed] [Google Scholar]
- Weinstock M, 2017. Prenatal stressors in rodents: Effects on behavior. Neurobiol Stress. 6, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, et al. , 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 147, S56–S69. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, et al. , 2011. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 59, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, et al. , 2012. Dnmt3a regulates both proliferation and differentiation of mouse neural stem cells. J Neurosci.Res 90, 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. , 2013. Perinatal exposure to bisphenol-A inhibits synaptogenesis and affects the synaptic morphological development in offspring male mice. Chemosphere. 91, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Yoshida M, et al. , 2004. Maternal exposure to low doses of bisphenol a has no effects on development of female reproductive tract and uterine carcinogenesis in Donryu rats. J Reprod Dev. 50, 349–60. [DOI] [PubMed] [Google Scholar]