Abstract
Alcohol use disorder (AUD) and methamphetamine use disorder (MUD) are prevalent and have high adverse impacts on both the individual and society. Current treatment strategies for these disorders are ineffective at a population level. Lorcaserin, a 5‐HT2C receptor agonist, has shown potential at reducing the symptoms of substance use disorder. This pilot study (initiated prior to market withdrawal) examined feasibility and safety of lorcaserin treatment in people undergoing residential detoxification and treatment for AUD and MUD. This was an open label pilot study of lorcaserin where participants (n = 10 AUD; n = 8 MUD) received 10‐mg lorcaserin daily for 4 days then twice daily for 1 month. Primary outcome measures included recruitment and retention rate, incidence of treatment‐emergent events, incidence of methamphetamine or alcohol withdrawal‐related events, heart rate, and blood pressure. Secondary measures included pharmacokinetic data and self‐reported alcohol or methamphetamine use, craving, and psychological distress. AUD participants were recruited faster and had a greater retention rate compared with MUD participants. Lorcaserin did not alter vital signs, was well tolerated, and had a similar pharmacokinetic profile to individuals with obesity. Lorcaserin reduced self‐reported alcohol and amphetamine‐type substance use and craving in AUD and MUD participants, respectively. Self‐reported psychological health also improved over the treatment period for all participants. Despite the pilot nature of this study, our data support the notion of 5‐HT2C receptors as a therapeutic target for drug and alcohol abuse.
Keywords: 5‐HT2C agonist, alcohol, craving, lorcaserin, methamphetamine, serotonin
Abbreviations
- AUD
Alcohol use disorder
- BMI
body mass index
- FDA
Food and Drug Administration
- MUD
methamphetamine use disorder
1. INTRODUCTION
Alcohol use disorder (AUD) poses a major social and economic burden to society, accounting for ~5% of deaths worldwide in 2016 and costing upward of $36 billion/year in Australia. 1 With regard to illicit drug use, methamphetamine is the second most regularly used drug following cannabis in Australia, and in the United States, there was a threefold increase in the number of methamphetamine‐associated deaths between 2010 and 2015. 2 , 3 As a result, the recreational use and abuse of drugs continues to be a major global public health issue. Importantly, poor retention and frequent relapse remain serious obstacles for the treatment of substance use disorders with continuous and intense cravings persisting both during and following treatment. 4 , 5 There are three Food and Drug Administration (FDA)‐approved pharmacotherapies for AUD, disulfiram, naltrexone, and acamprosate. Current pharmacotherapeutic treatments for AUD remain ineffective at a population level due to a combination of limited effectiveness and under‐prescribing. 6 , 7 There are no FDA‐approved pharmacotherapies specifically for methamphetamine use disorder (MUD). In a recent review, most medications evaluated were found to not have a statistically significant benefit. 8 Clearly, there is need for improved pharmacotherapeutic treatments aimed at reducing drug‐related craving for both AUD and MUD.
The neural circuitry surrounding craving and relapse to drug use involves several brain structures including the ventral tegmental area, striatal complex, amygdala, hippocampus, and the prefrontal cortex. 9 , 10 Serotonin‐containing cells (5‐hydroxytryptamine [5‐HT]) are located predominantly in the raphe nuclei of the mid/hind‐brain, including the dorsal and median raphe. 11 From here, 5‐HT fibers widely innervate the central nervous system including multiple nodes of reward‐related circuitry. 12 Indeed, the serotonergic system has been implicated in substance use disorder and relapse for several decades and may represent an avenue for future pharmacological interventions. 13 , 14 , 15 , 16 , 17 , 18 There are 14 known subtypes of 5‐HT receptors, and serotonin signaling is well known to modulate dopamine activity. 19 Specifically, the 5‐HT2C receptor is expressed in the hippocampus, striatum, and amygdala in both rat and human. 20 , 21 , 22
5‐HT2C receptor signaling has been implicated in the development and maintenance of AUD and MUD. For example, the 5‐HT2C receptor agonist, Ro60‐0175, decreased alcohol consumption in rats whereas the 5‐HT2C receptor antagonist, SB‐242 084, increased alcohol consumption. 23 However, Ro60‐0175 reduced both alcohol (gel solution) and vehicle (plain gel containing polycose) operant self‐administration in rats suggesting possible nonspecific effects on caloric intake. 24 5‐HT2C receptor signaling is also involved in methamphetamine use; for example, Ro60‐0175 reversed methamphetamine self‐administration‐induced decreases in nucleus accumbens shell excitability. 25 Additionally, methamphetamine‐induced behavioral sensitization is associated with a functional upregulation of 5‐HT2C receptors in the ventral pallidum. 26 Together, these preclinical studies highlight a role for the 5‐HT2C receptor in both alcohol and methamphetamine use.
The development of therapeutic drugs that selectively target individual 5‐HT2 receptor subtypes is difficult. Indeed, most 5‐HT2C receptor agonists also bind to 5‐HT2A and/or 5‐HT2B receptors. 27 Lorcaserin is a selective serotonin 2C (5‐HT2C) receptor agonist with a 3‐benzazepine scaffold, 28 developed as an anti‐obesity medication. Notably, lorcaserin reduces the consumption of alcohol in rats and methamphetamine use in rhesus monkeys, 29 , 30 implicating the 5‐HT2C receptor as a potential treatment target for alcohol and substance use disorders. 15 Until recently, lorcaserin (Belviq®) was FDA‐approved as an anti‐obesity medication 31 but was withdrawn from the market after a safety trial indicated an increased occurrence of cancer, where 7.7% of participants receiving drug developed cancer relative to 7.1% in the placebo arm. 31 Here, we carried out a pilot study, prior to market withdrawal, to evaluate the ability of lorcaserin to suppress alcohol and methamphetamine craving and consumption in treatment‐seeking AUD or MUD participants.
2. MATERIALS AND METHODS
2.1. Study design and participants
This was an open label pilot study. The protocol and amendments were approved by the Human Research Ethics Committee of St Vincent's Hospital Melbourne (HREC 031/17). Eligible participants were males and females over the age of 18 years, diagnosed with alcohol or methamphetamine substance use disorder (DSM5). Exclusion criteria included pregnant (urine βHCG positive) or breastfeeding; highly dependent on medical care for co‐existing conditions; other medical treatments for substance dependence including anti‐craving (e.g., acamprosate and naltrexone), aversive (e.g., disulfiram), or substitution (e.g., atomoxetine, dexamphetamine, and methylphenidate) treatments; known allergy to lorcaserin; already receiving lorcaserin; severe liver impairment (Child Pugh C); severe renal impairment (creatinine clearance <30 ml/min); hypertension; unstable diabetes; history of serotonin syndrome; low body mass index (BMI <20); and unstable mental state (including active psychosis or schizophrenia). Written informed consent was obtained from all participants.
The study protocol specified the recruitment of 10 AUD participants and 10 MUD participants. Recruitment was ceased when the initial FDA alert was issued in January 2020, resulting in the final recruitment of 10 alcohol‐ and 8 methamphetamine‐dependent participants. Note that the HREC was advised immediately by the Chief Investigators of the original FDA alert and the subsequent product withdrawal. All participants were advised in writing of the product withdrawal and the health risks associated with their participation in the study.
2.2. Procedures
Participants were treated with the lowest effective dose of lorcaserin (immediate release) used in the treatment of obesity. Participants received lorcaserin 10 mg once daily for 4 days then twice daily for 1 month. All participants received symptomatic treatment using the St Vincent's Hospital standard protocol. Withdrawal was actively managed as either an outpatient or, if required, in a residential setting. Alcohol withdrawal was managed in accordance with Clinical Institute Withdrawal Assessment of Alcohol (CIWA‐Ar). 32 Prescribed medications consisted of 5–20 mg of diazepam 2 hourly with a maximum of 80 mg per 24‐h period, anti‐emetics for nausea, and paracetamol and nonsteroidal anti‐inflammatories for aches. All medications were used only as required and ceased by Day 7 following participants’ last use of alcohol or methamphetamine and the commencement of lorcaserin.
2.3. Pharmacokinetic sampling
On Day 7 of treatment, 4‐ml EDTA blood samples were taken for assessment of plasma concentration levels of the IP (lorcaserin) at predose (time 0), 2, 4, and 8 h after treatment. Bloods were refrigerated at ≤4°C and centrifuged at 2000g for 10 min within 12 h after blood sampling. Immediately after centrifugation, plasma were stored in two labeled polypropylene tubes and stored at ≤−20°C for plasma concentration analysis. All plasma concentration analyses were performed after all participants had completed the final visit.
2.4. Measures
Measures were assessed over five treatment time points (baseline, Day 7, Day 14, Day 21, and Day 28). A baseline researcher‐administered questionnaire assessed demographic and clinical characteristics and determined eligibility. Liver function and blood glucose were assessed every second week (baseline, Day 14, and Day 28).
The Obsessive Compulsive Drinking Scale was used to assess alcohol craving. 33 A total craving score was calculated by summing the 14 items of this questionnaire, which were ranked on a Likert scale ranging from 1 to 7. Summing Items 1–6 calculated the obsessive subscale, and summing Items 7–14 calculated the Compulsive Subscale. A brief, 10‐item Methamphetamine Craving Questionnaire, based on the Cocaine Craving Questionnaire, was used to assess methamphetamine craving over time. Scores across each item were averaged. 34 , 35
The Kessler Psychological Distress Scale (K10) yielded a global measure of distress, which was the sum of all 10 items.
The Australian Treatment Outcomes Profile (ATOP), a validated Australian version of the UK Treatment Outcome Profile, was used to assess self‐reported drug use and health and well‐being. 36 Higher scores on the substance use questions, measured using the timeline follow‐back method, reflected more days of use whereas higher scores on health and well‐being questions indicated greater self‐rated health outcomes.
2.5. Outcomes
Primary endpoints were prespecified. Feasibility endpoints were recruitment rate and retention in treatment at Days 7, 14, 21, and 28. Safety endpoints were incidence of treatment‐emergent adverse events (AEs); incidence of methamphetamine or alcohol withdrawal‐related treatment‐emergent events; heart rate; and blood pressure.
Secondary endpoints were methamphetamine use (self‐report, saliva screen, and urine drug screen) on Study Days 0, 7, 14, 21, and 28; alcohol use (self‐report, breath alcohol, blood testing, and urine drug screen) on Study Days 0, 7, 14, 21, and 28; craving measures—Obsessive Compulsive Scale for Drinking (AUD group) and Cocaine Craving Questionnaire (MUD group) on Study Days 0, 7, 14, 21, and 28; and K10 psychological distress and ATOP measures on Study Days 0, 7, 14, 21, and 28.
A final outcome was to assess the pharmacokinetics of lorcaserin in a clinical population with normal BMI: AUC 0–8 h, C max, t ½, t max.
2.6. Pharmacokinetic analysis
Pharmacokinetic data were analyzed at the Clinical Pharmacology Laboratory, School of Medicine and Public Health, University of Newcastle, Australia. Plasma samples were analyzed using a validated liquid chromatography with tandem mass spectrometry (LCMSMS) method for lorcaserin. Plasma samples (50 µl) were prepared by adding twice the volume of acetonitrile, samples were vortexed and then centrifuged, and the supernatant was transferred to a vial and injected onto the LCMSMS. The LCMSMS system consisted of a Shimadzu 8060 LCMS using a Kinetex C18 column and a gradient of 0.1% formic acid and acetonitrile. Lorcaserin was linear over the range of 10–500 ng/ml. Using the concentration–time profiles determined by the analysis of the plasma samples, the pharmacokinetic parameters, maximum plasma concentration (C max), time of C max (T max), area under the plasma concentration time curve (AUC 0–12), and plasma half‐life (t ½), were determined using PKSolver. 37 The data were fitted using the NCA Extravascular module, with AUC0 − t calculated using the log‐linear trapezoidal method. The 12‐h time point for each individual was predicted with a one compartment model using PKSolver.
2.7. Statistical analysis
Unless otherwise stated, data were analyzed separately for AUD and MUD participants.
Demographic data were summarized as numbers and proportions for categorical data and mean (±standard error of mean [SEM]) for continuous data using Microsoft Office Excel 2016. Clinical characteristics and vital signs were analyzed using a repeated measures ANOVA. Due to the high dropout rate of participants with MUD, either three (baseline to Day 14) or five time points (baseline to Day 28) were assessed. The oral fluid test and urinary drug screen were analyzed using the Friedman nonparametric test. For participants with AUD, baseline and Day 21 oral fluid substance results were not detected for one participant and baseline blood glucose levels were missing for another participant. For participants with MUD, Day 7 blood pressure data were missing for one participant, baseline weight data were missing for two participants, and Day 7 weight data were missing for one participant. To allow for the inclusion of these participants in statistical analyses, a mean imputation of missing values was conducted in SPSS. For MUD participants, one participant had all liver function and blood glucose data missing and three participants had their urine drug screen data missing and were thus excluded from analyses. The Obsessive Compulsive Drinking Scale, Methamphetamine Craving Questionnaire, and K10 Scale were analyzed using a repeated measures ANOVA as the data were normally distributed and ANOVA assumptions were not violated. A repeated measures ANOVA was used to analyze the ATOP data. For participants with AUD, the ATOP data for the number of days participants drank alcohol and the typical quantity of alcohol consumed were not normally distributed and thus assessed using the Friedman nonparametric test. Two participants had data missing from the health and well‐being questions and were excluded from this analysis. Only two to three participants with MUD completed the ATOP self‐report questionnaire for the duration of the study (baseline to Day 28); thus, data were also analyzed from baseline to Day 14, with five participants completing the questionnaire at these time points. All analyses were performed using SPSS v27 (α = .05). Data are presented as mean ± SEM.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 38 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 39
3. RESULTS
3.1. Demographic information
The participants recruited with AUD were predominantly male (8/10). The mean age was 48.1 years (SEM = 2.1). Mean typical quantity of alcohol consumed on day used was 21.8 standard drinks (SEM = 4.4). The participants recruited with MUD were predominantly male (6/8) with a mean age (M = 36.6 years, SEM = 2.9) of more than 10 years younger than the AUD group. The mean typical quantity of methamphetamine on a day used was 0.66 g (SEM = 0.22).
Table 1 describes further characteristics at baseline for both participant groups. Age of use characteristics were younger for AUD participants compared with MUD participants. AUD participants had experienced more withdrawal symptoms compared with MUD participants. The majority of AUD participants (90%) had at least one previous treatment for dependence compared with 50% for MUD participants. The types of treatments for AUD were both pharmacotherapies and nonpharmacotherapies, but only nonpharmacotherapies for MUD participants.
TABLE 1.
Participant characteristics at baseline
| Characteristic | Number (%) | |
|---|---|---|
| Alcohol use disorder (n = 10) | Methamphetamine use disorder (n = 8) | |
| Country of birth | ||
| Australia | 8 (80.0) | 6 (75.0) |
| Other | 2 (20.0) (Yugoslavia, United Kingdom) | 2 (25.0) (Scotland, South Africa) |
| Indigenous Australian | 1 (10.0) | 0 |
| Living status | ||
| Alone | 4 (40.0) | 2 (25.0) |
| Partner (with at least 1 child) | 3 (30.0) | 2 (25.0) |
| Partner (no children) | 2 (20.0) | 1 (12.5) |
| Relationship status | ||
| Single | 4 (40.0) | 5 (62.5) |
| Living with spouse (including married, de facto, life partner) | 4 (40.0) | 3 (37.5) |
| Separated but not divorced | 2 (20.0) | 0 |
| Accommodation | ||
| Private accommodation | 10 (100.0) | 8 (100.0) |
| Employment | ||
| Employed | 4 (40.0) | 4 (80.0) |
| Disability support pension | 2 (20.0) | 1 (12.5) |
| Currently unemployed | 1 (10.0) | 1 (10.0) |
| Highest level of education | ||
| Secondary (years 7–10) | 2 (10.0) | 3 (37.5) |
| Secondary (years 11–12) | 1 (10.0) | 3 (37.5) |
| Tertiary | 6 (60.0) | 1 (12.5) |
| Post graduate | 1 (10.0) | 1 (12.5) |
| Medical history | ||
| Current conditions | ||
| Depression | 4 (40.0%) | 3 (37.5%) |
| Anxiety | 4 (40.0%) | 3 (37.5%) |
| High cholesterol | 2 (20.0%) | 0 |
| Alcohol‐related conditions | ||
| None | 7 (70.0%) | 6 (75.0%) |
| Seizures | 2 (20.0%) | 0 |
| Alcohol or methamphetamine use history | Alcohol use | Methamphetamine use |
|---|---|---|
| Estimated amount (standard drinks) | Estimated amount (g) | |
| Mean (SE) | 21.8 (4.4) | 0.66 (0.22) |
| Age at first drink (years) | Age at first use (years) | |
| Mean (SE) | 16.3 (0.4) | 24.0 (3.1) |
| Age at daily drinking (years) | Age at problematic use (years) | |
| Mean (SE) | 31.6 (2.8) | 25.7 (3.4) |
| Duration since daily drinking (years) | Duration since problematic use (years) | |
| Mean (SE) | 15.8 (2.5) | 10.4 (2.5) |
| Previous detoxifications | ||
| At least 1 detoxification | 9 (90.0%) | 4 (50.0%) |
| Previous withdrawal symptoms | ||
| Sweating | 9 (15.0%) | 1 (1.7%) |
| Anxiety | 8 (13.3%) | 3 (5.0%) |
| Tremor | 7 (11.7%) | 1 (1.7%) |
| Nausea | 5 (8.3%) | 0 |
| Agitation | 5 (8.3%) | 3 (5.0%) |
| Seizures | 2 (3.3%) | 0 |
| Vomiting | 3 (5.0%) | 0 |
| Diarrhea | 2 (3.3%) | 0 |
| Delirium tremens | 1 (1.7%) | 0 |
| Previous treatment for dependence | ||
| At least 1 treatment | 9 (90.0%) | 4 (50.0%) |
| Previous types of treatment (often multiple) | ||
| Counseling | 9 (100.0%) | 4 (100.0%) |
| Acamprosate maintenance | 9 (100.0%) | 0 |
| Naltrexone maintenance | 8 (88.9%) | 0 |
| Alcoholics anonymous (AA) | 7 (77.8%) | 0 |
| Residential rehabilitation activities | 4 (44.4%) | 1 (25.0%) |
| Disulfiram maintenance | 2 (22.2%) | 0 |
| Other | 3 (33.3%) (recovery and support program, 2× baclofen) | 1 (25.0%) (The Rewired Program—self‐administered) |
3.2. Primary endpoints
3.2.1. Feasibility
The first participant was recruited into the clinical trial on the 24th of July 2018, and the last participant was recruited on the 2nd of December 2019, equating to a total of just over 16 months (496 days) to recruit the 18 participants. For the alcohol group (n = 10), the median time between recruiting each participant was just over 1 month (median = 37 days, interquartile range = 16–59 days), that is, a rate of 0.82 AUD participants per month. For the MUD group (n = 8), it took longer between participants to be recruited (median = 73 days, interquartile range = 44–98 days), that is, a rate of 0.42 participants per month. For the AUD participants, the retention in treatment rate at Day 7 was 100%, and 80% for each weekly visit thereafter. For the MUD participants, the retention rate at Days 7, 14, 21, and 28 was 87.5%, 62.5%, 37.5%, and 50%, respectively. The main reason for participants discontinuing the trial was due to failing to attend appointments. Note that one patient missed Day 21 appointment but represented for Day 28, and this accounts for the dip in the retention rates over time at Day 21. Each was deemed “lost to follow‐up” after multiple unsuccessful attempts by a clinician to contact the participant.
3.2.2. Safety
Treatment‐emergent AEs
Eight AUD participants (80%) reported at least one AE, and seven MUD participants (87.5%). Of these, seven of the AUD participants (87.5%), and five MUD participants (71.4%), reported at least one AE that was considered by a study doctor to be related to lorcaserin. These included ratings of definitely related, probably related, or possibly related (Table 2). Of all AEs (n = 28), 19 were considered to be treatment emergent (67.9%).
TABLE 2.
Number of treatment‐emergent adverse events (n = 19) (definitely related, probably related, or possibly related to lorcaserin) grouped by System Organ Class and preferred term, for both groups of participants
| Event (System Organ Class/preferred term) | Alcohol use disorder (n = 10) | Methamphetamine use disorder (n = 8) |
|---|---|---|
| Number of participants with ≥1 medication‐related adverse event | 7 | 5 |
| Gastrointestinal disorders | ||
| Diarrhea | 0 | 1 |
| Dry mouth | 2 | 1 |
| Lip dry | 1 | 0 |
| Nausea | 0 | 1 |
| General disorders and administration site conditions | ||
| Lethargy | 3 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 3 | 1 |
| Nervous system disorders | ||
| Dizziness | 2 | 0 |
| Headache | 2 | 2 |
The most frequently occurring treatment‐emergent AEs were “decreased appetite” (21.1%, 4 of 19) and headache (21.1%, 4 of 19). These occurred throughout the 4 weeks of study medication administration. The second most frequently occurring treatment‐emergent AEs were “lethargy” (15.8%, 3 of 19) and “dry mouth” (15.8%, 3 of 19), again occurring through the duration of the trial. None of the treatment‐emergent AEs was classified as “severe.” One was classified as “moderate” severity. This event of headache reported at Day 21 lasted 2 days and resolved spontaneously. The remaining 18 treatment‐emergent AEs were classified as “mild” severity. None of the treatment‐emergent AEs was a serious AE.
Withdrawal‐related treatment‐emergent AEs (n = 1)
One event was “probably related” to lorcaserin. An AUD participant experienced ongoing fatigue. They dropped out at Day 9, and no further follow‐up by the study staff was achieved.
Vital signs
For participants with AUD, temperature, pulse, systolic and diastolic blood pressure, and weight did not significantly change over time (Table S1). Respiratory rate (breaths/min) significantly increased over the treatment period (Table S1). For participants with MUD, temperature, pulse, respiratory rate, systolic and diastolic blood pressure, and weight did not significantly change over time (Table S2).
3.3. Secondary endpoints
3.3.1. Substance use
Alcohol use
Self‐report
For the AUD group, the number of days drinking alcohol in the past week did not change between baseline and Day 28 (Table 3); however, the typical quantity of alcohol consumed on any given day did decrease over time (Table 3).
TABLE 3.
ATOP self‐report data from baseline to Day 28 for participants with alcohol use disorder and methamphetamine use disorder
| ATOP self‐report | F statistic | χ 2 | p value | n |
|---|---|---|---|---|
| Alcohol use disorder participants | ||||
| Number of days drank alcohol in the last 7 days | 5.756 | .218 | 8 | |
| Typical quantity (standard drinks) of alcohol consumed on day used | 12.860 | .012 | 8 | |
| Psychological health status | 16.139 | <.001 | 6 | |
| Physical health status | 2.828 | .052 | 6 | |
| Quality of life | 5.689 | .003 | 6 | |
| Methamphetamine use disorder participants | ||||
| Number of days used amphetamine‐type substance in the last 7 days | 4.214 | .040 | 3 | |
| Psychological health status | 51.000 | .001 | 2 | |
| Physical health status | 1.370 | .384 | 2 | |
| Quality of life | 1.190 | .435 | 2 | |
Abbreviation: ATOP, Australian Treatment Outcomes Profile.
Clinical characteristics
For the AUD group, breath alcohol content, liver function, blood glucose levels, and urinary drug screen results did not change over the treatment period (Table S3).
Methamphetamine use
Self‐report
For the MUD group, from baseline to Day 14, the number of days using an amphetamine‐type substance in the past week did not change (Table S4). From baseline to Day 28, the number of days using an amphetamine‐type substance in the past week decreased (Table 3).
Clinical characteristics
For the MUD group, liver function, blood glucose levels, urinary drug screen results, and oral fluid test results did not change over time (Table S5).
3.3.2. Craving
Total craving scores for participants with AUD decreased over time (F 4, 28 = 7.299, p < .001; Figure 1A). Scores on the obsessive subscale (F 4, 28 = 5.200, p = .003) and on the compulsive subscale (F 4, 28 = 7.689, p < .001) also significantly decreased over time (Figure 1A).
FIGURE 1.
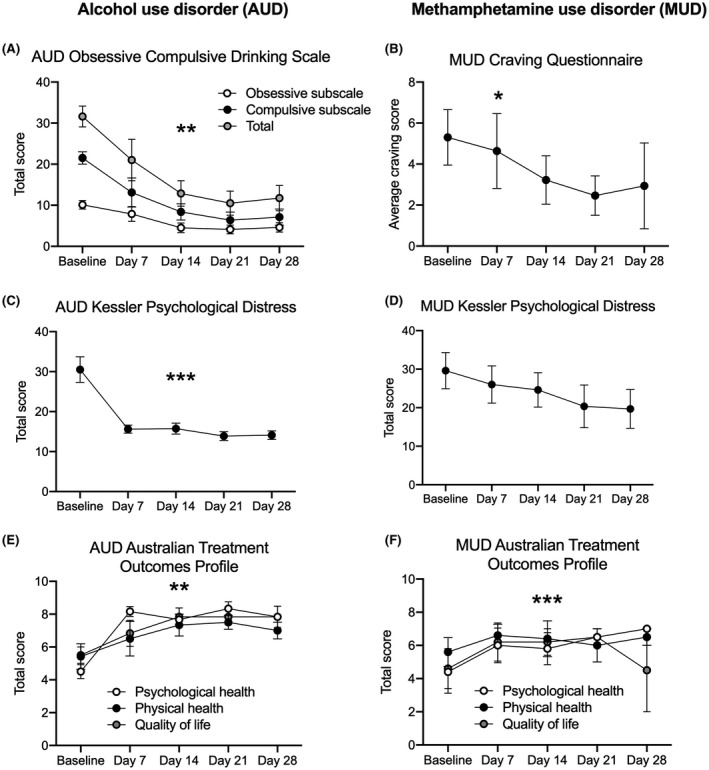
Self‐reported craving, psychological distress, and health and well‐being after lorcaserin treatment in participants with alcohol use disorder (AUD) and methamphetamine use disorder (MUD). Total obsessive compulsive drinking score over the 28‐day treatment period. Total n = 8. **p < .01 for each craving measure (A). Average craving score over the 28‐day treatment period. n = 3–5. *p < .05 baseline to Day 14 (B). Kessler Psychological Distress self‐report data over the 28‐day treatment period. Total n = 8 for AUD participants, n = 3–5 for MUD participants. ***p < .001 (C and D). Psychological health status and quality of life increased over the treatment period; however, there was no significant change in physical health status for AUD participants. Total n = 8. **p < .01 for psychological health status and quality of life (E). Psychological health status increased over the treatment period; however, there was no significant change in physical health status or quality of life. n = 2–5. ***p = 0.001 for psychological health status (F). All data are presented as mean ± SEM
Methamphetamine craving for participants with MUD significantly decreased from baseline to Day 14 (F 2, 8 = 5.200, p = .036) and approached significance from baseline to Day 28 (F 4, 8 = 2.880, p = .095; Figure 1B).
3.3.3. K10 psychological distress
K10 psychological distress scores significantly decreased over time for participants with AUD (F 4, 28 = 20.629, p < .001; Figure 1C).
K10 psychological distress scores did not change between baseline and Day 14 (F 2, 8 = 4.193, p = .057) or between baseline and Day 28 for participants with MUD (F 4, 8 = 1.537, p = .280; Figure 1D).
3.3.4. ATOP self‐report health and well‐being data
For the AUD participants, psychological health status and quality of life significantly increased across the experimental period between baseline and Day 28, and physical health status approached a significant improvement (Table 3; Figure 1E).
For the MUD participants, from baseline to Day 14, the perceived health and well‐being in the past week did not change (Table S4). From baseline to Day 28, the psychological health status in the past week increased; however, physical health status and quality of life did not change (Table 3; Figure 1F).
3.4. Pharmacokinetics of lorcaserin
Plasma concentration time profiles (0, 2, 4, 8, and 12 h) for six participants are shown in Figure 2. The pharmacokinetic profile followed an oral absorption profile (Figure S1) with a T max at 2 h and a C max of 102 ± 25 ng/ml before following a one phase exponential decay, resulting in a t 1/2 of 7.9 ± 2 h. Pharmacokinetic parameters are summarized in Table 4.
FIGURE 2.
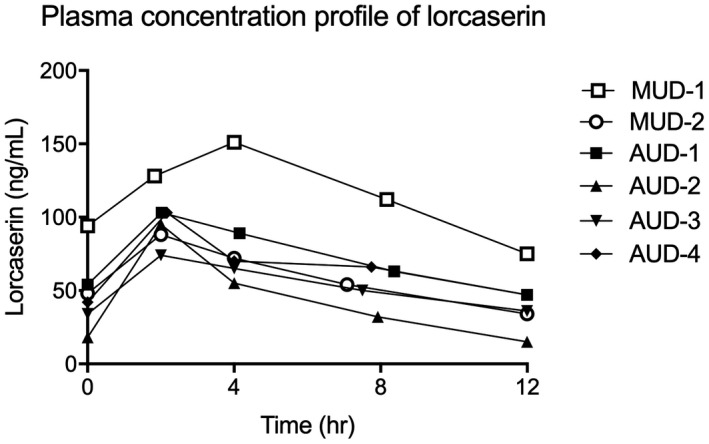
Plasma concentration time profiles (0, 2, 4, 8, and 12 h) after lorcaserin was administered. The pharmacokinetic profile of lorcaserin followed a standard oral absorption profile for the six participants examined. The 12‐h time point for each individual was predicted with a one compartment model using PKSolver. 37 AUD, alcohol use disorder; MUD, methamphetamine use disorder
TABLE 4.
Pharmacokinetic parameter summary data
| Parameter | Unit | Mean | SD | CV (%) |
|---|---|---|---|---|
| t 1/2 | h | 7.9 | 2.0 | 26 |
| T max | h | 2.4 | 0.8 | 34 |
| C max | ng/ml | 102 | 26 | 26 |
| AUC 0–12 | ng/ml·h | 824 | 311 | 38 |
| Css | ng/ml | 69 | 26 | 38 |
4. DISCUSSION
The aim of our pilot study was to examine the safety and efficacy of the 5‐HT2C receptor agonist lorcaserin in people undergoing residential withdrawal and seeking treatment for AUD and MUD. There are limited data on the pharmacokinetics of lorcaserin, and in general, this has been observed in an obese population with a T max of 1.5 h, C max of 80 ng/ml with a variability of 26%, and a t ½ of 11.9 ± 1.5 h. 40 In AUD and MUD participants, we observed a slightly longer T max of 2.4 h, a higher C max of 102 ng/ml with similar variability of 26%, and a shorter t ½ of 7.9 ± 2 h. With the small sample sizes and similar variability of approximately 30% in both studies, it is unlikely that there is a different pharmacokinetic profile between an obese and AUD/MUD populations.
In the present study, AUD participants were recruited faster and had a greater retention rate compared with MUD participants. There were no significant changes in vital signs over the treatment period except respiratory rate did increase for AUD participants. Importantly, lorcaserin was well tolerated, having few, if any, side effects. Lorcaserin was not anorectic and had no cardiovascular side effects. We also tested the effect of lorcaserin on alcohol and Methamphetamine craving and consumption. Although number of drinking days did not change, self‐reported alcohol consumption per session decreased for AUD participants and amphetamine‐type substance use decreased for those MUD participants who we retained. However, there was no change in clinically monitored alcohol or amphetamine content, respectively. Notably, lorcaserin treatment resulted in decreased craving over time for both AUD and MUD participants. Mean baseline craving scores for AUD participants in this study were high compared with those reported for other similar populations 41 and decreased by more than 50% at Day 28. MUD participants’ craving scores trended toward significance but showed considerable interparticipant variability. Self‐reported psychological distress scores decreased for AUD participants, but there was no change in psychological distress for MUD participants. Nevertheless, self‐reported psychological health status significantly improved over the treatment period for both AUD and MUD participants. K10 scores for AUD participants dropped to the Australian national average by Day 7. 42 Overall, and despite the pilot nature of this study, our data do indicate clinically significant improvements on key outcomes and support the notion of 5‐HT2C receptors as a therapeutic target for drug and alcohol abuse. Clinically, 28 days is a short duration of treatment for both alcohol and Methamphetamine dependence. Longer treatment duration may have seen improved outcomes on a number of physiological variables that were trending positively (e.g., GGT levels).
Lorcaserin has shown promise at reducing symptoms of substance use disorder in other, recent clinical trials. For example, lorcaserin reduced cannabis intake compared with placebo controls. 43 Lorcaserin, in combination with counseling, increased abstinence rates in individuals with nicotine use disorder compared with those treated with smoking cessation counseling alone. 44 However, lorcaserin did not reduce cocaine or oxycodone use compared with placebo controls. 45 , 46 Lorcaserin also did not improve extended‐release naltrexone induction rates in an outpatient sample of individuals with opioid use disorder compared with placebo. 47 There are some discrepancies regarding the effectiveness of lorcaserin at improving treatment outcomes for those with substance use disorder, but the reasons for these discrepancies are currently unknown and may be due to the mechanistic impact of lorcaserin and the interaction with the drug of choice based on the neural circuits driving the behavior. Nevertheless, at least for alcohol, nicotine, and cannabis, there is supporting evidence.
Our findings are also somewhat consistent with preclinical literature showing reductions in alcohol consumption in rats following chronic lorcaserin treatment. 30 Lorcaserin can also decrease self‐administration of methamphetamine and cocaine in rhesus monkeys. 29 , 48 , 49 Similarly, lorcaserin decreased heroin self‐administration and cue‐induced reinstatement in rhesus monkeys. 50 , 51 Finally, both acute and chronic administration of lorcaserin plus pimavanserin, a selective 5‐HT2A receptor antagonist, decreased cocaine relapse‐like behavior in rats, suggesting that perhaps the actions of the 5‐HT2C receptor alone are not straightforward. 52
Although lorcaserin was recently withdrawn from the market following a safety concern, we contend the involvement of 5‐HT2C receptors in substance use disorder are relevant and targeting these receptors is still a viable avenue for treatment, particularly given our current findings and those discussed above. The 5‐HT2C receptor has been implicated in both compulsive‐ and impulsive‐like behaviors in rodents, cardinal features of substance use disorder. 53 Additionally, 5‐HT2C receptor activity can modulate dopamine function. 54 , 55 Of note however is the complexity of the 5‐HT system, particularly regarding the interaction between 5‐HT2C and 5‐HT2A receptors and their relationship to impulsive‐like behavior. 56
4.1. Methodological considerations and future research
A methodological limitation of the current study is that all participants received lorcaserin treatment; that is, there was no placebo control. Additionally, this study was open label, limiting the interpretation of our findings. Although overall sample size was small, particularly for MUD participants, those that completed the study seemingly benefitted from lorcaserin. The differential outcomes between AUD and MUD participants go some way to mitigate against these shortfalls; however, further studies are clearly warranted.
MUD participants were more difficult to retain and had lower retention in treatment. The observed difficulty in recruiting and maintaining MUD participants in the trial could possibly be because methamphetamine users seek treatment reluctantly due to a lack of perceived effective treatments. It could also simply reflect that there are more people with AUD than MUD. There was no difference in the rates of AEs between the two groups, so this is unlikely to be a factor. It is difficult to draw firm conclusions due to the small number of participants in the study. Any future study should include design elements to improve recruitment rates and mitigate against attrition. For example, contingency management approaches have been shown to be effective in the treatment of many substance use disorders and hold promise for MUD. 57 , 58
5. CONCLUSION
In summary, our data suggest that the 5‐HT2C system is still a viable treatment option for substance use disorders, particularly AUD. However, given the current FDA withdrawal of lorcaserin, other 5‐HT2C agonists with high selectivity may be beneficial. Alternatively, due to the conserved nature of the orthosteric binding site for serotonin at 5‐HT2 family receptors, selective positive allosteric modulators, analogous to those for acetylcholine muscarinic receptors, 59 may provide a fruitful line of enquiry and enable more selective targeting. Another promising therapeutic target may be the use of psychedelics, targeting the serotonin system, for substance use disorder treatment. 60 , 61 However, animal studies have recently questioned the efficacy of psychedelics in models of AUD. 62 Nevertheless, our findings add to the literature supporting the 5‐HT2C receptor as a therapeutic target for AUD and MUD.
DISCLOSURE
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
AJL and YB conceived and designed research; YB, AP, LC, and AN collected the data; EJC analyzed data; PG and JJ conducted and analyzed pharmacokinetic data; EJC and AJL interpreted data and wrote the first draft of the manuscript; all authors contributed to, edited, and approved the final manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
We acknowledge the Victorian State Government Operational Infrastructure Scheme.
Erin J. Campbell and Yvonne Bonomo are co‐first authors.
Funding information
AJL is a NHMRC Principal Fellow (1116930). YB received a Seeding Grant from SVHM Research Endowment Fund (REF81803).
Contributor Information
Yvonne Bonomo, Email: yvonne.bonomo@svha.org.au.
Andrew J. Lawrence, Email: andrew.lawrence@florey.edu.au.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- 1. World Health Organization . Global status report on alcohol and health. 2018.
- 2. Australian Institute of Health and Welfare . National drug strategy household survey 2016: Detailed findings. 2017.
- 3. National center for health statistics/centers for disease control; U.S. Department of Justice; Drug Enforcement Administration . National drug threat assessment. 2017.
- 4. Campbell EJ, Lawrence AJ, Perry CJ. New steps for treating alcohol use disorder. Psychopharmacology. 2018;235(6):1759‐1773. [DOI] [PubMed] [Google Scholar]
- 5. Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218(1):69‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018;320(8):815‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leggio L, Falk DE, Ryan ML, Fertig J, Litten RZ. Medication development for alcohol use disorder: a focus on clinical studies. Handbook Exp Pharmacol. 2020;258:443‐462. [DOI] [PubMed] [Google Scholar]
- 8. Chan B, Freeman M, Kondo K, et al. Pharmacotherapy for methamphetamine/amphetamine use disorder ‐ a systematic review and meta‐analysis. Addiction. 2019;114(12):2122‐2136. [DOI] [PubMed] [Google Scholar]
- 9. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481‐1489. [DOI] [PubMed] [Google Scholar]
- 10. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinbusch HW. Distribution of serotonin‐immunoreactivity in the central nervous system of the rat‐cell bodies and terminals. Neuroscience. 1981;6(4):557‐618. [DOI] [PubMed] [Google Scholar]
- 12. Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72(1):165‐229. [DOI] [PubMed] [Google Scholar]
- 13. Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross‐fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38(8):861‐868. [DOI] [PubMed] [Google Scholar]
- 14. Cloninger CR, Sigvardsson S, von Knorring AL, Bohman M. The Swedish studies of the adopted children of alcoholics: a reply to Littrell. J Stud Alcohol. 1988;49:500‐509. [Google Scholar]
- 15. Collins GT, Gerak LR, France CP. The behavioral pharmacology and therapeutic potential of lorcaserin for substance use disorders. Neuropharmacology. 2018;142:63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ezaki N, Nakamura K, Sekine Y, et al. Short allele of 5‐HTTLPR as a risk factor for the development of psychosis in Japanese methamphetamine abusers. Ann N Y Acad Sci. 2008;1139:49‐56. [DOI] [PubMed] [Google Scholar]
- 17. Gilligan SB, Reich T, Cloninger CR. Etiological heterogeneity in alcoholism. Genet Epidemiol. 1987;4:395‐414. [DOI] [PubMed] [Google Scholar]
- 18. Johnson BA, Elkashef AM, Seneviratne C, et al.; Methamphetamine Study, G . Association between genotype of the serotonin transporter‐linked polymorphic region of the serotonin transporter gene and age of onset of methamphetamine use: a preliminary analysis. Front Psychiatry. 2010;1:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costall B, Naylor RJ, Marsden CD, Pycock CJ. Serotonergic modulation of the dopamine response from the nucleus accumbens. J Pharm Pharmacol. 1976;28(6):523‐526. [DOI] [PubMed] [Google Scholar]
- 20. Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M. Localization of the 5‐hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology. 1995;34(12):1635‐1645. [DOI] [PubMed] [Google Scholar]
- 21. Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5‐HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39(1):123‐132. [DOI] [PubMed] [Google Scholar]
- 22. Pasqualetti M, Ori M, Castagna M, Marazziti D, Cassano GB, Nardi I. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92(2):601‐611. [DOI] [PubMed] [Google Scholar]
- 23. Tomkins DM, Joharchi N, Tampakeras M, Martin JR, Wichmann J, Higgins GA. An investigation of the role of 5‐HT(2C) receptors in modifying ethanol self‐administration behaviour. Pharmacol Biochem Behav. 2002;71(4):735‐744. [DOI] [PubMed] [Google Scholar]
- 24. Kasper J, Tikamdas R, Kim MS, et al. The serotonin‐2 receptor modulator, (‐)‐trans‐PAT, decreases voluntary ethanol consumption in rats. Eur J Pharmacol. 2013;718(1–3):98‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graves SM, Clark MJ, Traynor JR, Hu XT, Napier TC. Nucleus accumbens shell excitability is decreased by methamphetamine self‐administration and increased by 5‐HT2C receptor inverse agonism and agonism. Neuropharmacology. 2015;89:113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Napier TC, Istre ED. Methamphetamine‐induced sensitization includes a functional upregulation of ventral pallidal 5‐HT2A/2C receptors. Synapse. 2008;62(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 27. Wold EA, Wild CT, Cunningham KA, Zhou J. Targeting the 5‐HT2C receptor in biological context and the current state of 5‐HT2C receptor ligand development. Curr Top Med Chem. 2019;19(16):1381‐1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith BM, Smith JM, Tsai JH, et al. Discovery and structure‐activity relationship of (1R)‐8‐chloro‐2,3,4,5‐tetrahydro‐1‐methyl‐1H‐3‐benzazepine (Lorcaserin), a selective serotonin 5‐HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008;51(2):305‐313. [DOI] [PubMed] [Google Scholar]
- 29. Gerak LR, Collins GT, France CP. Effects of lorcaserin on cocaine and methamphetamine self‐administration and reinstatement of responding previously maintained by cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2016;359(3):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rezvani AH, Cauley MC, Levin ED. Lorcaserin, a selective 5‐HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol Biochem Behav. 2014;125:8‐14. [DOI] [PubMed] [Google Scholar]
- 31. Food and drug administration . https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐requests‐withdrawal‐weight‐loss‐drug‐belviq‐belviq‐xr‐lorcaserin‐market. Accessed July 10, 2020.
- 32. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). Br J Addict. 1989;84(11):1353‐1357. [DOI] [PubMed] [Google Scholar]
- 33. Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psych. 1996;53(3):225‐231. [DOI] [PubMed] [Google Scholar]
- 34. Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233‐237. [DOI] [PubMed] [Google Scholar]
- 35. Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug and alcohol dependence. Drug Alc Dep. 1993;34(1):19‐28. [DOI] [PubMed] [Google Scholar]
- 36. Ryan A, Holmes J, Hunt V, et al. Validation and implementation of the Australian Treatment Outcomes Profile in specialist drug and alcohol settings. Drug Alc Rev. 2014;33(1):33‐42. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add‐in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306‐314. [DOI] [PubMed] [Google Scholar]
- 38. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2019: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alexander SPH, Christopoulos A, Davenport AP, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. Br J Pharmacol. 2019;176(S1):S21‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Christopher R, Morgan M, Ferry J, et al. Single‐ and multiple‐dose pharmacokinetics of a lorcaserin extended‐release tablet. Clin Ther. 2016;38(10):2227‐2238. [DOI] [PubMed] [Google Scholar]
- 41. Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self‐rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alc Clin Exp Res. 1995;19(1):92‐99. [DOI] [PubMed] [Google Scholar]
- 42. Andrews G, Slade T. Interpreting scores on the Kessler psychological distress scale (K10). Aust N Z J Pub Health. 2001;25(6):494‐497. [DOI] [PubMed] [Google Scholar]
- 43. Arout CA, Cooper ZD, Reed SC, et al. 5HT‐2C agonist lorcaserin decreases cannabis self‐administration in daily cannabis smokers. Addict Biol. 2021:e12993. 10.1111/adb.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez‐Kam M. Lorcaserin for smoking cessation and associated weight gain: a randomized 12‐week clinical trial. Nicotine Tob Res. 2017;19(8):944‐951. [DOI] [PubMed] [Google Scholar]
- 45. Brandt L, Jones JD, Martinez S, et al. Effects of lorcaserin on oxycodone self‐administration and subjective responses in participants with opioid use disorder. Drug Alcohol Depend. 2020;208:107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pirtle JL, Hickman MD, Boinpelly VC, Surineni K, Thakur HK, Grasing KW. The serotonin‐2C agonist Lorcaserin delays intravenous choice and modifies the subjective and cardiovascular effects of cocaine: a randomized, controlled human laboratory study. Pharmacol Biochem Behav. 2019;180:52‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levin FR, Mariani JJ, Pavlicova M, et al. Lorcaserin treatment for extended‐release naltrexone induction and retention for opioid use disorder individuals: a pilot, placebo‐controlled randomized trial. Drug Alc Dep. 2021;219:108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Collins GT, France CP. Effects of lorcaserin and buspirone, administered alone and as a mixture, on cocaine self‐administration in male and female rhesus monkeys. Exp Clin Psychopharmacol. 2018;26(5):488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Collins GT, Gerak LR, Javors MA, France CP. Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(1):85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gerak LR, Collins GT, Maguire DR, France CP. Effects of lorcaserin on reinstatement of responding previously maintained by cocaine or remifentanil in rhesus monkeys. Exp Clin Psychopharmacol. 2019;27(1):78‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kohut SJ, Bergman J. Lorcaserin decreases the reinforcing effects of heroin, but not food, in rhesus monkeys. Eur J Pharmacol. 2018;840:28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anastasio NC, Sholler DJ, Fox RG, et al. Suppression of cocaine relapse‐like behaviors upon pimavanserin and lorcaserin co‐administration. Neuropharmacology. 2020;168:108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Higgins GA, Brown M, St John J, MacMillan C, Silenieks LB, Thevarkunnel S. Effects of 5‐HT2C receptor modulation and the NA reuptake inhibitor atomoxetine in tests of compulsive and impulsive behaviour. Neuropharmacology. 2020;170:108064. [DOI] [PubMed] [Google Scholar]
- 54. Di Giovanni G, Di Matteo V, Di Mascio M, Esposito E. Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin(2C/2B) receptor agonists: a combined in vivo electrophysiological and microdialysis study. Synapse. 2000;35(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 55. Millan MJ, Dekeyne A, Gobert A. Serotonin (5‐HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5‐HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37(7):953‐955. [DOI] [PubMed] [Google Scholar]
- 56. Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5‐HT2A and 5‐HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5‐HT depletion. Psychopharmacology. 2004;176(3–4):376‐385. [DOI] [PubMed] [Google Scholar]
- 57. Brown HD, DeFulio A. Contingency management for the treatment of methamphetamine use disorder: a systematic review. Drug Alc Dep. 2020;216:108307. [DOI] [PubMed] [Google Scholar]
- 58. Roll JM. Contingency management: an evidence‐based component of methamphetamine use disorder treatments. Addiction. 2007;102:114‐120. [DOI] [PubMed] [Google Scholar]
- 59. Walker LC, Berizzi AE, Chen NA, et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: converging evidence from humans and rodents. Biol Psychiatry. 2020;88(12):898‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andersen KAA, Carhart‐Harris R, Nutt DJ, Erritzoe D. Therapeutic effects of classic serotonergic psychedelics: a systematic review of modern‐era clinical studies. Acta Psychiatr Scand. 2021;143(2):101‐118. [DOI] [PubMed] [Google Scholar]
- 61. Nutt D. Psychedelic drugs‐a new era inpsychiatry? Dialogues Clin Neurosci. 2019;21(2):139‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Meinhardt MW, Gungor C, Skorodumov I, Mertens LJ, Spanagel R. Psilocybin and LSD have no long‐lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology. 2020;45(8):1316‐1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


