Abstract
Background
Meningioma is the most common primary intracranial tumor and surgery is the main treatment modality. As death from lack of tumor control is rare, other outcome measures like anxiety, depression and post‐operative epilepsy are becoming increasingly relevant. In this nationwide registry‐based study we aimed to describe the use of antiepileptic drugs (AED), antidepressants and sedatives before and after surgical treatment of an intracranial meningioma compared to a control population, and to provide predictors for continued use of each drug‐group two years after surgery.
Methods
All adult patients with histopathologically verified intracranial meningiomas were identified in the Swedish Brain Tumor Registry and their data were linked to relevant national registries after assigning five matched controls to each patient. We analyzed the prescription patterns of antiepileptic drugs (AED), antidepressants and sedative drugs in the two years before and the two years following surgery.
Results
For the 2070 patients and 10312 controls identified the use of AED, antidepressants and sedatives was comparable two years before surgery. AED use at time of surgery was higher for patients than for controls (22.2% vs. 1.9%, p < 0.01), as was antidepressant use (12.9% vs. 9.4%, p < 0.01). Both AED and antidepressant use remained elevated after surgery, with patients having a higher AED use (19.7% vs. 2.3%, p < 0.01) and antidepressant use (14.8% vs. 10.6%, p < 0.01) at 2 years post‐surgery. Use of sedatives peaked for patients at the time of surgery (14.4% vs. 6.1%, p < 0.01) and remained elevated at two years after surgery with 9.9% versus 6.6% (p < 0.01). For all the studied drugs, previous drug use was the strongest predictor for use 2 years after surgery.
Conclusion
This nationwide study shows that increased use of AED, antidepressants and sedatives in patients with meningioma started perioperatively, and remained elevated two years following surgery.
Keywords: antidepressant, anti‐epileptic drugs, neurosurgery, primary brain tumor, quality of life
By coupling a nationwide cancer registry of 2070 patients with data on purchased drugs and diagnoses, it was shown that after meningioma surgery the use of antiepileptics, antidepressants and sedatives was distinctly increased compared to controls. As this may be an often‐overlooked factor in deciding whether to perform surgery, predictors for identifying especially vulnerable patients are explored.

1. INTRODUCTION
Meningiomas are the most common primary intracranial tumors. 1 , 2 , 3 Surgical treatment has been shown to increase survival 4 and quality of life, 5 while more extensive resection is related to a lower recurrence rate. 6 However, as complications and neurological deterioration related to tumor resection are not trivial, 2 , 7 , 8 careful risk‐benefit considerations are essential for optimal management.
Epilepsy is a common presenting symptom of meningioma, 8 and seizure control is an important outcome measure after surgery. 9 While patients may experience seizure remission after meningioma surgery, the reported chance for this varies widely (38%–72%) between studies. 9 , 10 , 11 , 12 There is also a considerable risk of new onset seizures in previously seizure free patients, varying between 6 and 19% between studies. 9 , 10 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Such a cross‐over of symptoms is likely to mask both the positive and negative impacts of surgery. However, interpretation of available studies is difficult as the time‐point of follow‐up varies considerably between studies and is sometimes vaguely defined. To better understand these cross‐over events, we need to study the exact time‐patterns with a high resolution.
It is unclear if patients undergoing meningioma surgery have an increased rate of depression preoperatively. While preoperative depression seems related to impaired outcome, 19 surgery may 20 or may not 21 improve depressive symptoms. Also, patients treated with psychotropic medication could undergo a disproportionate rate of brain imaging, with increased risk of incidental meningioma diagnosis and possible overtreatment. 22 , 23 Even less is known about use of sedative drugs in patients with meningioma.
In this nationwide registry‐based study we aimed to describe the use of AED, antidepressants and sedatives before and after surgical treatment of an intracranial meningioma compared to a control population, and to provide predictors for continued use of each drug‐group two years after surgery.
2. MATERIALS AND METHODS
We combined data from several nationwide Swedish registries, linked through the unique personal identification numbers given to all Swedish citizens. The methods have been described in detail previously, 24 but the procedure is described in short below.
2.1. Linking of registries
The Swedish Brain Tumor Registry (SBTR) is a nationwide registry of adult (≥18 years) patients with primary brain tumors, with a surgically acquired pathohistological diagnosis. In SBTR we identified all patients with a first‐time histological diagnosis of intracranial meningioma according to the 2007 WHO classification of brain tumors, and a day of surgery/index date between April 1st 2009 and December 31st 2015. 25 Patients with radiologically suspected meningioma without histological diagnosis were not included in the present study. As a criterion to be included the annual rate of registration per region needed to be above 80% as compared to the compulsory National Cancer Registry. This was done to provide population‐based data. Therefore, for one region, we included data only from January 1st 2012 to December 31st 2013 while for all other regions patients were included for the entire study period.
Statistics Sweden (www.scb.se) provided data on educational level and income for the patients included in the analyses. Furthermore, a control population of five individuals for each patient was obtained from the general population. The control population was matched with respect to year of birth, sex, municipality of residence and educational level. For 18 patients the number of controls per patient was incomplete. This rendered a patient population of 2070 and a control population of 10312 individuals. A flow‐chart of the inclusion and exclusion of patients and controls is available in Figure 1.
FIGURE 1.
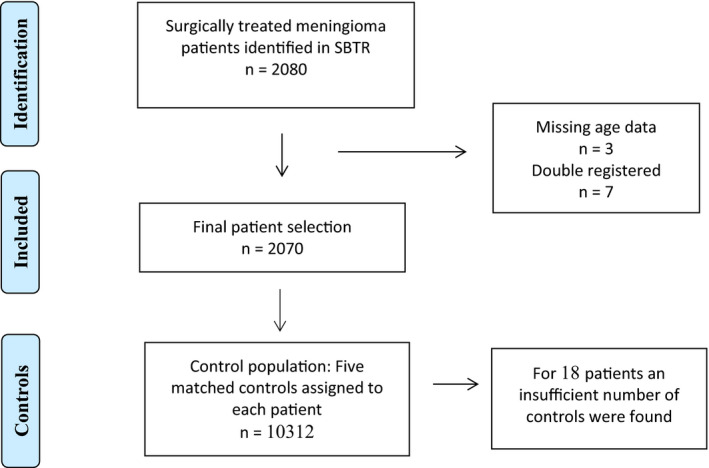
Flow chart of patient selection
From the Swedish National Board of Health and Welfare we received data from the National Patient Registry (NPR) and the National Prescription Registry (NPrP). The NPR included information on ICD‐code and date of inpatient and outpatient visits, including diagnostic and procedural codes, in the period 2007–2016. It included private and public hospitals, but no primary care contacts. The ICD‐10 codes were used to classify comorbidity according to the Elixhauser comorbidity index. 26 , 27 The prescription registry provided information on date of dispensing and type of drug according to the Anatomical Therapeutic Chemical (ATC) classification system, in the period from 2007 through 2017. Data on drug use were based on purchase of prescription drugs by the patient, please see Table 1 for ATC‐based definitions of drug groups. Active use of a prescription group was defined as having purchased a drug of this prescription group in the prior 90 days. For sedatives, the patient was considered an active user only for 30 days after a purchase since sedatives are more often used as a short‐term drug. When calculating active users as percentage of the population, only living individuals were considered. The registries under The National Board of Health and Welfare were accessed on March 16th 2018.
TABLE 1.
Definition of variables, and the source of data used for calculating each variable
| Variable | Definition | Source of data |
|---|---|---|
| Index date | Date of surgery for patients. Controls received the same index date as their respective cases. | SBTR |
| Radiotherapy | Registered as “yes” if indicated in SBTR and/or if procedure codes in NPR indicated administration of radiotherapy. | SBTR |
| Elixhauser comorbidity index | According to index. The conditions removed from the index due to possible association with diagnosis of meningioma were: G40 Epilepsy, G41 Status epilepticus, R56 Convulsions, R47 Dysphasia/aphasia, C70‐72: Malignant tumor in central nervous system. With this index both cases and controls were provided with a score from 0–30 based upon comorbid categories present or not. We report as 0, 1, 2, ≥3 categories present. The ICD−10 data used to classify comorbidity were from NPR the 2 years prior to index date | NPR |
| Prescription group |
All drugs with a common ATC‐code. Groups were defined as follows:
|
NPrR |
| Active use | Active use of a prescription group was defined as having purchased a drug of this prescription group in the prior 90 days. For the prescription group “Sedatives” the patient was considered an active user only for 30 days after a purchase. When calculating the percentage of the population that is active users, only alive individuals were considered. | NPrR |
2.2. Statistics
Data from the registries were imported into corresponding tables in a mySQL database. Data on purchased prescription drugs use were individually analyzed for each patient (date and ATC‐code) and each control and was then combined with clinical data using Python as described earlier. 28 Definitions regarding index date, active use, and prescription groups are provided in Table 1.
Other data derivations were done using mySQL. R version 2.13.1 was used for statisticasl analyses. For each day, from two years (730 days) prior through two years after index date, the percentage of all alive patients and controls that were users of the defined prescription groups was analyzed. Continuous variables were summarized using the median, first and third quartiles and compared between cases and controls using the Mann‐Whitney U test. Categorical variables were summarized using counts and proportions and compared between cases and controls using the Fisher's exact test.
To identify relevant predictors for use of AED, antidepressants and sedatives at end of follow‐up, we performed multivariable regression analyses, see Table S1–S3. Covariates were chosen based upon presumed relevance.
3. RESULTS
3.1. Demographic data
Baseline characteristics of the 2070 patients included in this study are presented in Table 2. A comparison between patients and controls regarding socioeconomic variables and comorbidities is presented in Table 3.
TABLE 2.
Baseline and treatment characteristics for patients with meningioma. (n = 2070)
| Variable |
Meningioma patients (n = 2070) |
|---|---|
| Age, median (First quartile(Q1), Third quartile(Q3)) | 60 (49, 69) |
| Female, n (%) | 1449, (70.0) |
| Asymptomatic, n (%) | 294 (14.2) |
| WHO functional status, n (%) | |
| 0, fully active | 933 (45.1) |
| 1, light work possible | 566 (27.3) |
| 2, cares for self | 305 (14.7) |
| 3, limited self care | 144 (7.0) |
| 4, disabled, confined to bed | 20 (1.0) |
| Missing | 102 |
| Tumor laterality, n (%) | |
| Left | 736 (35.6) |
| Right | 856 (41.4) |
| Bilateral | 123 (5.9) |
| Missing | 355 |
| Localization, n (%) | |
| Skull‐base | 290 (14.0) |
| Not Skull‐base | 1780 (86.0) |
| Tumor size, n (%) | |
| <4 cm | 924 (52.8) |
| 4–6 cm | 597 (34.1) |
| >6 cm | 230 (13.1) |
| Missing | 319 |
| Simpson grade, n (%) | |
| Grade I‐III | 1587 (86.5) |
| Grade IV‐V | 247 (13.5) |
| Missing | 236 |
| New deficit after surgery, n (%) | 310 (15.0) |
| Missing | 4 |
| Reoperation due to complication, n (%) | 103 (5.0) |
| Missing | 3 |
| WHO grade, n (%) | |
| Grade I | 1803 (87.1) |
| Grade II‐III | 267 (12.9) |
| Oncological treatment planned, n (%) | 120 (5.9) |
| Missing | 27 |
| Wait time from radiological diagnosis (surgery date‐radiological diagnosis), weeks median (Q1,Q3) | 10 (4, 24) |
TABLE 3.
Socioeconomic variables and comorbidities of patients and controls
| Meningioma n = 2070 |
Controls, n = 10,312 |
p‐value | |
|---|---|---|---|
| Educational level, at index year, n (%) | |||
| Basic to high‐school | 1375 (69.2) | 7019 (69.2) | 0.98 |
| Higher education | 612 (30.8) | 3131 (30.8) | |
| Missing | 83 | 162 | |
| Disposable income, n (%) | 180 k (132 k, | 199 k (137 k, | <0.001 |
| Median (Q1, Q3) | 256 k) | 277 k) | |
| Elixhauser comorbidities at index date, n (%) | |||
| 0 | 1067 (51.5) | 7126 (69.1) | <0.001 |
| 1 | 523 (25.3) | 1671 (16.2) | |
| 2 | 254 (12.3) | 754 (7.3) | |
| 3 or more | 226 (10.9) | 761 (7.4) | |
3.2. Drug use patterns
The percentage of patients and controls with an active use of each of the defined prescription groups are presented for the index date and 2 years after surgery in Table 4, and for the entire time interval from two years before until two years following index date in Figure 2A‐C.
TABLE 4.
Drug use of patients and controls
| Meningioma n = 2070 | Controls, n = 10312 | p‐value | |||
|---|---|---|---|---|---|
| n (%) | 95% CI | n (%) | 95% CI | ||
| Use of AED at index date, n (%) | 455 (22.0) | 20.2–23.8 | 200 (1.9) | 1.7–2.2 | <0.001 |
| Use of antidepressants at index date, n (%) | 266 (12.9) | 11.4–14.4 | 968 (9.4) | 8.8–10.0 | <0.001 |
| Use of sedatives at index date, n (%) | 298 (14.4) | 12.9–16.0 | 627 (6.1) | 5.6–6.6 | <0.001 |
| Alive at 2 years after index date, n (%) | 1957 (94.5) | 93.5–95.5 | 10082 (97.8) | 97.5–98.0 | <0.001 |
| AED use at 2 years after index date, n (% of alive) | 386 (19.7) | 18.0–21.6 | 231 (2.3) | 2.0–2.6 | <0.001 |
| Use of antidepressants at 2 years after index date, n (% of alive) | 289 (14.8) | 13.2–16.4 | 1072 (10.6) | 10.0–11.3 | <0.001 |
| Sedatives use at 2 years after index date, n (% of alive) | 193 (9.9) | 8.6–11.3 | 698 (6.9) | 6.4–7.4 | <0.001 |
FIGURE 2.
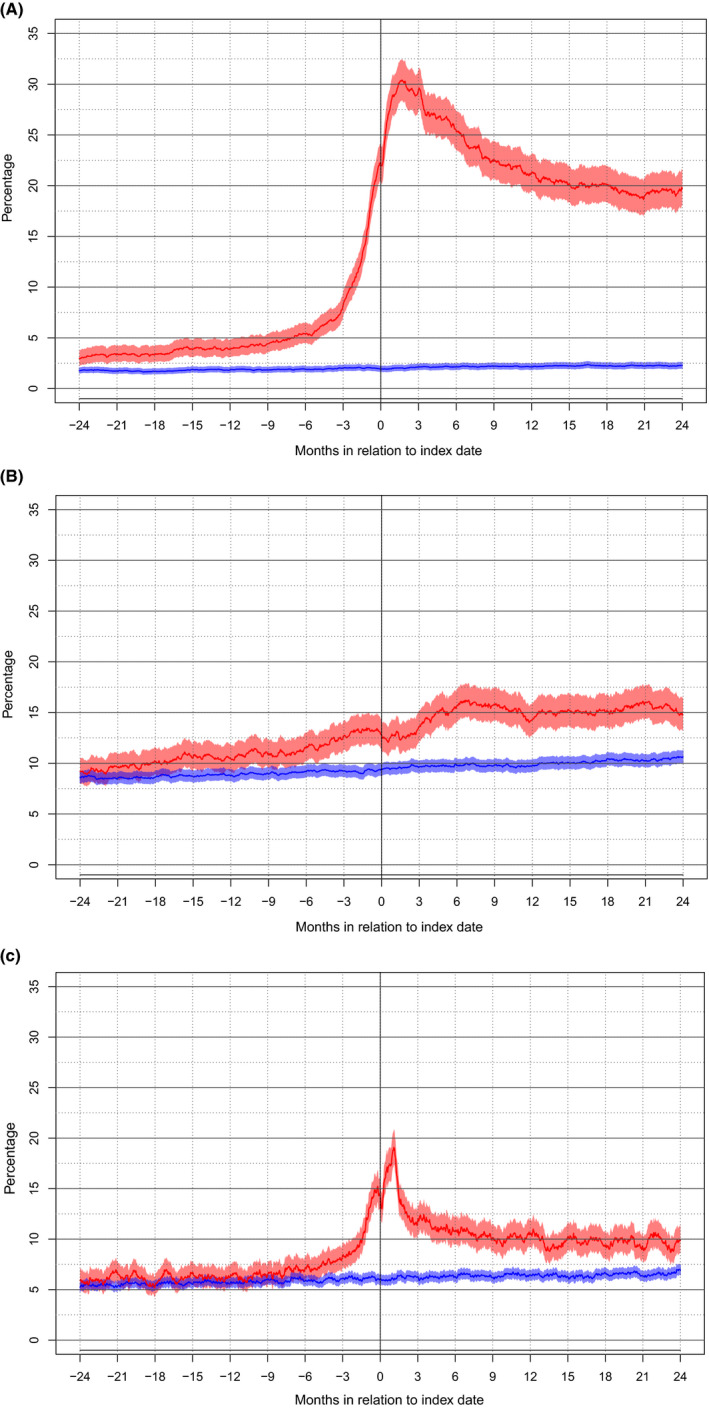
(A‐C) Graph representing the proportions (95% CI) of patients (red) and controls (blue) with active use of the following prescription groups two years prior to index date through two years following index date: (A) AED, (B) Antidepressants, (C) Sedatives
As seen in Figure 2A the rate of AED use for patients at the date of surgery was 22.2% compared to 1.9% for controls (p < 0.01). The rate for patients remained high with 18.6% of patients and 2.2% of controls using AED at two years after index date (p < 0.01). As seen in Figure 2B, the corresponding rate of antidepressant use was 12.9% for patients and 9.4% of controls at index date (p < 0.01). However, while AED use decreased over time, antidepressant use increased during follow‐up for both patients with 14.0% use at two years after index date and controls with 10.4% use at two years after index date (p < 0.01). As seen in Figure 2C the use of sedatives was elevated for patients compared to controls with 14.2% versus 6.0% use at index date (p < 0.01) and remained somewhat elevated at two years after surgery with 9.9% versus 6.6% (p < 0.01). Concerning symptom cross‐over (e.g. seizure remission and induced seizures) a summary of changes in use of AED, antidepressants and sedatives at index date and at two years after surgery is provided in Tables 5, 6, 7.
TABLE 5.
Pattern of change in AED treatment, for patients and controls
| Group (n) |
Use of AED at index date |
N (% of group) | Use of AED at 2‐year follow‐up | N (% of subgroup) |
|---|---|---|---|---|
| Patients (2070) | Yes | 455 (22.0) | Yes | 219 (48.1) |
| No | 219 (48.1) * | |||
| Dead | 17 (3.7) | |||
| No | 1615 (78.0) | Yes | 167 (10.3) b | |
| No | 1352 (83.7) | |||
| Dead | 96 (5.9) | |||
| Controls (10,312) | Yes | 200 (1.9) | Yes | 137 (68.5) |
| No | 54 (27.0) a | |||
| Dead | 9 (4.5) | |||
| No | 10112 (98.1) | Yes | 94 (0.9) b | |
| No | 9797 (96.9) | |||
| Dead | 221 (2.2) |
Use of AED at index date but no use of AED at 2‐year follow‐up was 48.1% for patients and 27.0% for controls (p < 0.001).
Use of AED at 2‐year follow‐up but not at index date occurred for 10.3% of patients and 0.9% of controls (p < 0.001).
TABLE 6.
Pattern of change in antidepressant treatment, for patients and controls
| Group (n) |
Use of antidepressant at index date |
N (% of group) | Use of antidepressant at 2‐year follow‐up | N (% of subgroup) |
|---|---|---|---|---|
| Patients (2070) | Yes | 264 (12.8%) | Yes | 133 (50.4%) |
| No | 113 (42.8%) a | |||
| Dead | 18 (6.8%) | |||
| No | 1806 (87.2%) | Yes | 156 (8.6%) b | |
| No | 1555 (86.1%) | |||
| Dead | 95 (5.3%) | |||
| Controls (10,312) | Yes | 963 (9.3%) | Yes | 647 (67.2%) |
| No | 266 (27.6%) a | |||
| Dead | 50 (5.2%) | |||
| No | 9349 (90.7%) | Yes | 425 (4.5%) b | |
| No | 8744 (93.6%) | |||
| Dead | 180 (1.9%) |
Use of antidepressants at index date but no use of antidepressants at 2‐year follow‐up was 42.8% for patients and 27.6% for controls (p < 0.001).
Use of antidepressants at 2‐year follow‐up but not at index date occurred for 8.6% of patients and 4.5% of controls (p < 0.001).
TABLE 7.
Pattern of change in sedative treatment, for patients and controls
| Group (n) |
Use of sedatives at index date |
N (% of group) | Use of sedatives at 2‐years after index date | N (% of subgroup) |
|---|---|---|---|---|
| Patients (2070) | Yes | 298 (14.4) | Yes | 87 (29.2) |
| No | 188 (62.6) a | |||
| Dead | 23 (7.8) | |||
| No | 1772 (85.6) | Yes | 106 (6.0) b | |
| No | 1576 (88.9) | |||
| Dead | 90 (5.1) | |||
| Controls (10,312) | Yes | 627 (6.1) | Yes | 309 (49.3) |
| No | 278 (44.3) a | |||
| Dead | 40 (6.4) | |||
| No | 9685 (93.9) | Yes | 389 (4.0) b | |
| No | 9106 (94.0) | |||
| Dead | 190 (2.0) |
Use of sedatives at index date but no use of sedatives at 2‐year follow‐up was 62.6% for patients and 44.3% for controls (p < 0.001).
Use of sedatives at 2‐year follow‐up but not at index date occurred for 6.0% of patients and 4.0% of controls (p < 0.001).
3.3. Predictors of drug use
A logistic regression model was created to identify potential predictors for active use of AED at the end of follow‐up (Table S1). Previous AED use, more comorbidities, a new neurological deficit after surgery, larger tumor size, worse WHO functional status, and planned postoperative oncological treatment (i.e. radiation) were associated with an increased risk of being on active use of AED at two years after surgery. Higher level of education and longer wait time from radiological diagnosis to surgery decreased the risk of AED use at two years after surgery.
For antidepressants, use at two years after surgery was predicted by previous use of antidepressants and worse WHO functional status (Table S2). For sedatives, use at two years after surgery was predicted by previous sedative use, higher age, more comorbidities, a new neurological deficit after surgery and worse WHO functional status (Table S3).
4. DISCUSSION
This nationwide registry‐based study demonstrates that use of AED, antidepressant drugs and to a lesser degree also sedative drugs, is elevated in patients with meningioma compared to a matched control group, both at the time of surgery and two years after. Furthermore, there was no favorable trend postoperatively with less use of AED, antidepressant drugs or sedative drugs as compared to preoperative use, although a substantial shift in AED and antidepressant users was seen.
4.1. Antiepileptics
There was a marked increase in the use of AED, starting approximately six months prior to surgery, whereas controls had stable AED use (around 2%) for the entire studied period. A previous meta‐analysis based on 4709 patients reported a mean preoperative seizure rate of 29%. 17 This is in line to our finding of 22% of patients with AED use at index date. The two year rate of AED use in our study of 19.7% is comparable to several studies reporting post‐operative seizure rates of 15%–30%. 9 , 10 , 12 , 29 , 30
The rate of new‐onset AED use in previously AED naïve patients was 10% in our study, and in line with several previous studies reporting new onset seizure rates of 6%–19%. 9 , 10 , 12 , 13 , 14 , 15 , 16 , 17 Interestingly, this does not seem to have improved much with microsurgical techniques as historical case series indicate a similar rate (6%) as far back as 1935. 12 It is also important to determine to what extent patients with seizures become seizure free after surgery. There is considerable variability in the degree of seizure relief that is reported after meningioma surgery (38%–72%) between different studies, 9 , 10 , 11 , 12 In our material, 52% of the patients using AED at the date of surgery were no longer active users at the end of our two‐year follow up. This figure is somewhat lower than the rate of seizure relief of 69% reported by the above mentioned meta‐analysis. 17 It is worth noting however that a higher pre‐operative seizure rate was also reported and they registered seizure freedom rather than AED use. Some patients in our material with AED use before surgery, on the other hand, may have continued active use of AED for the entire two‐year follow‐up period without having any seizure during this time. Thus, the difference is probably to some extent related to a different study design and differences in definition, for example, if a seizure during the first 7 days should be included. Also of note, primary prevention with AED is generally avoided in Sweden for patients with primary brain tumors. Differences in guidelines concerning preventive AED could affect the reported postoperative use of AED and seizure rate, but such primary prophylaxis is generally considered not to affect the long‐term development of epilepsy. 31
Identifying risk factors for postoperative seizures is of particular interest for daily clinical practice, as there are indications that AED use among patients with meningioma has a negative impact on quality of life. 32 STAMPE2 is a prognostic index developed to predict the risk of postoperative seizures after meningioma surgery. 9 Our results only partially concur with the STAMPE2 scoring system for predicting postoperative seizures. New onset neurological deficits and preoperative epilepsy were useful predictors in both STAMPE2 and our study. However, we were surprised to find that age and reoperation due to complication were not significant predictors in our model. However, according to our model it seems like complex cases in terms of more comorbidity, lower functional status and larger tumors have an increased risk of postoperative AED use.
4.2. Antidepressants
A prescription of an antidepressant is an indicator that a patient has some form of mental distress, the main indication is depression. 33 In patients with meningioma preoperative depression has been negatively associated to clinical outcome, including survival. 19 In our study, the use of antidepressants was not different between cases and controls at two years before surgery but was significantly elevated from around one year prior to surgery. Since the increase in use of antidepressants preceded the median wait time from diagnosis to surgery, it seems unlikely that the only cause of the increase in antidepressant use is the psychological reaction to the meningioma diagnosis. It can, conversely, be speculated that patients with depression are more likely to receive a CT or MRI of the brain, increasing the probability of discovering an otherwise asymptomatic meningioma. In line with this notion, short‐term antidepressant use is associated with an increased risk of being diagnosed with meningioma. 34
It has been reported that depression is common prior to surgery but declines again following surgery to a level comparable to the general population. 20 However, this observation was not reproduced at group level in our study where there was a consistent increase in use of antidepressants postoperatively compared to matched controls. Thus, our findings are in line with a cross sectional study, demonstrating that depressive symptoms and anxiety were common in patients with meningioma at an average of 33 months postoperatively. 21 Interestingly, 43% of patients with preoperative antidepressant use did not require antidepressant treatment two years after surgery, i.e. considerably more than controls for whom this rate was 28% (p < 0.01).
4.3. Sedatives
Sedatives can provide effective short‐term symptom relief, but long‐term use is problematic. Benzodiazepine use exceeding 1–2 weeks increase the risk for dependency 35 and falls. 36 It is therefore discouraged.
The baseline level of 5%–7% use of sedatives among both patients and controls two years before surgery is comparable to previous reports. 37 Sedative use for patients peaked around the time of surgery with 14.4% versus 6.1% for controls (p < 0.01). This may have been caused by anxiety and sleep disturbances following meningioma surgery. A similar pattern was shown in a Norwegian study from 2016 on prescriptions after total hip arthroplasty. 38 More worrisome given the side effects of this class of drugs is that there is also an increased use two years after surgery among patients with 9.9% vs 6.9% for controls (p < 0.01). In the multivariable regression model, use of sedatives at the time of surgery was a strong predictor of continued use 2 years after surgery (p < 0.01). It is worth noting that higher age, while not being a predictor for continued AED or antidepressant use, was a predictor for continued use of sedatives. Hence, we recommend keeping the prescriptions of sedatives at a minimum, especially for elderly patients.
4.4. Clinical implications
Our findings can guide clinicians by shedding light on common problems for meningioma patients around the time of surgery. Increased awareness can aid in developing screening strategies to effectively identify issues and address them. The search for predictors may add further knowledge, in tailoring the surveillance to particular risk‐groups. Also, particularly for oligosymptomatic patients with limited tumor burden, knowledge of how softer end‐points are likely to develop after surgery may be of interest when considering the timing of surgical intervention.
4.5. Limitations of this study
When comparing large groups of drugs together, differences and nuances in prescription are unavoidably lost. It is difficult to interpret what a drug prescription means in terms of morbidity, and even harder to make causal conclusions. Therefore, in an effort to address this, we decided during study planning to exclude the drugs N03AX12 (Gabapentin) and N03AX16 (Pregabalin) from the AED group, as they are mainly used for the indication of pain relief in Sweden. Our interpretation of the results is that the main indication for antidepressants in this population was depressive symptoms, and that the main indication for sedatives was anxiety or sleep disturbances, but we have not measured the mood, anxiety level or quality of sleep of the patients directly.
Our method requires patients to purchase the drug in order to be counted as active users. A patient that receives AED in a hospital setting would be counted as a non‐user until this patient has purchased the AED in a store. This may have led to an underreporting of AED use in the weeks after surgery, but it is highly unlikely to have caused underreporting in patients with longer‐term AED use. It may also be considered a strength of the study that we used actual purchase, and not prescriptions, as a measure of drug use, since bias from non‐compliance is considerably lower when patients have purchased the drug. Indeed, the purchase of a prescribed drug is considered the definition of compliance in many studies. 39
Disease registers have limited resolution over time and often lack details concerning risk factors when trying to explain findings or specific patterns. Therefore, we have chosen a descriptive pattern to avoid excessive speculation. We have studied only patients undergoing surgical resection of meningioma. Hence, we cannot compare against other modalities (e.g. radiotherapy) or simply conservative management as case selection clearly plays an important role.
4.6. Strengths of this study
Compared to other studies on the subject the 2070 patients included in this study is a very large cohort of surgically treated meningioma patients with both drug‐use data and high‐quality surgical data. We have used matched controls to compare our results to a baseline level in the general population. As we have studied use of drugs rather than prescriptions, bias from non‐adherence has been reduced. By organizing the drug use data using exact dates on an individual basis, we have achieved a time‐resolution uncommon for this type of study.
5. CONCLUSION
This nationwide study shows that use of antidepressants, AED, and sedatives was comparable between meningioma patients and controls two years before surgery but was increased for meningioma patients perioperatively and remained elevated during the entire studied period of two years after surgery.
CONFLICT OF INTERESTS
None declared.
ETHICS STATEMENT
This study was approved by the regional ethical committee in Västra Götaland region (Dnr: 363–17). The need for informed consent was waived by the ethical committee.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
This project was made possible by the continuous work of the Swedish Brain Tumor Registry (SBTR).
Øyvind Salvesen and Asgeir Store Jakola are shared last authorship/contributed equally.
Statistical Analysis was conducted by Øyvind Salvesen, University of Trondheim and Erik Thurin, Gothenburg University.
Funding information
This project was funded by research grant from the Swedish Research Council (2017‐00944). The authors report no disclosures.
DATA AVAILABILITY STATEMENT
Due to restrictions from the registry holders, raw data cannot be shared.
REFERENCES
- 1. Kruchko C, Ostrom QT, Boscia A, Truitt G, Gittleman H, Barnholtz‐Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1‐iv86. 10.1093/neuonc/noy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartek J, Sjåvik K, Förander P, et al. Predictors of severe complications in intracranial meningioma surgery: a population‐based multicenter study. World Neurosurg. 2015;83(5):673‐678. [DOI] [PubMed] [Google Scholar]
- 3. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383‐e391. [DOI] [PubMed] [Google Scholar]
- 4. Cahill KS, Claus EB. Treatment and survival of patients with nonmalignant intracranial meningioma: results from the surveillance, epidemiology, and end results program of the national cancer institute. J Neurosurg. 2011;115(2):259‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jakola AS, Gulati M, Gulati S, Solheim O. The influence of surgery on quality of life in patients with intracranial meningiomas: a prospective study. J Neuro Oncol. 2012;110(1):137‐144. [DOI] [PubMed] [Google Scholar]
- 6. Nanda A, Bir SC, Maiti TK, Konar SK, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence‐free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126(1):201‐211. [DOI] [PubMed] [Google Scholar]
- 7. van Alkemade H, de Leau M, Dieleman EMT, et al. Impaired survival and long‐term neurological problems in benign meningioma. Neuro‐oncology. 2012;14(5):658‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corell A, Thurin E, Skoglund T, et al. Neurosurgical treatment and outcome patterns of meningioma in Sweden: a nationwide registry‐based study. Acta Neurochir. 2019;161(2):333‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wirsching H‐G, Morel C, Gmür C, et al. Predicting outcome of epilepsy after meningioma resection. Neuro Oncol. 2016;18(7):1002‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xue H, Sveinsson O, Bartek J, et al. Long‐term control and predictors of seizures in intracranial meningioma surgery: a population‐based study. Acta Neurochir. 2018;160(3):589‐596. [DOI] [PubMed] [Google Scholar]
- 11. Islim AI, Ali A, Bagchi A, et al. Postoperative seizures in meningioma patients: improving patient selection for antiepileptic drug therapy. J Neurooncol. 2018;140(1):123‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groff RA. The meningioma as a cause of epilepsy. Ann Surg. 1935;101(1):167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamasaki T, Yamada K, Yano S, et al. Higher incidence of epilepsy in meningiomas located on the premotor cortex: a voxel‐wise statistical analysis. Acta Neurochir. 2012;154(12):2241‐2249. [DOI] [PubMed] [Google Scholar]
- 14. Lieu AS, Howng SL. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors. Epilepsy Res. 2000;38(1):45‐52. [DOI] [PubMed] [Google Scholar]
- 15. Musluman AM, Yilmaz A, Tufan Canseve R, Cavusoglu H, Kahyaoglu O, Aydin Y. Unilateral frontal interhemispheric transfalcial approaches for the removal of olfactory groove meninjiomas. Turk Neurosurg. 2012;22(2):174‐182. [DOI] [PubMed] [Google Scholar]
- 16. Chan RC, Thompson GB. Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg. 1984;60(1):52‐60. [DOI] [PubMed] [Google Scholar]
- 17. Englot DJ, Magill ST, Han SJ, Chang EF, Berger MS, McDermott MW. Seizures in supratentorial meningioma: a systematic review and meta‐analysis. J Neurosurg. 2016;124(6):1552‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen WC, Magill ST, Englot DJ, et al. Factors associated with pre‐ and postoperative seizures in 1033 patients undergoing supratentorial meningioma resection. Neurosurgery. 2017;81(2):297‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bunevicius A, Deltuva VP, Tamasauskas A. Association of pre‐operative depressive and anxiety symptoms with five‐year survival of glioma and meningioma patients: a prospective cohort study. Oncotarget. 2017;8(34):57543‐57551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goebel S, Mehdorn HM. Development of anxiety and depression in patients with benign intracranial meningiomas: a prospective long‐term study. Supportive Care Cancer. 2013;21(5):1365‐1372. [DOI] [PubMed] [Google Scholar]
- 21. van der Vossen S, Schepers VP, Berkelbach van der Sprenkel JW, Visser‐Meily JM, Post MW. Cognitive and emotional problems in patients after cerebral meningioma surgery. J Rehabil Med. 2014;46(5):430‐437. [DOI] [PubMed] [Google Scholar]
- 22. Islim A, Rathi N, Mills S, Brodbelt AR, Jenkinson M. Management and outcomes of incidental meningiomas: is routine follow‐up required? Neuro Oncol. 2018;20(suppl_5):v346. 10.1093/neuonc/noy129.008. [DOI] [Google Scholar]
- 23. Ratcliffe GE, Enns MW, Jacobi F, Belik SL, Sareen J. The relationship between migraine and mental disorders in a population‐based sample. Gen Hosp Psychiatry. 2009;31(1):14‐19. [DOI] [PubMed] [Google Scholar]
- 24. Thurin E, Corell A, Gulati S, et al. Return to work following meningioma surgery: a Swedish nationwide registry‐based matched cohort study. Neuro Oncol Practice. 2020;7(3):320‐328. 10.1093/nop/npz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 28. Gulati S, Solheim O, Carlsen SM, et al. Risk of intracranial hemorrhage (RICH) in users of oral antithrombotic drugs: nationwide pharmacoepidemiological study. PLoS One. 2018;13(8):e0202575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Studeniak T, Smolanka V, Borovik OI. The effect of the presence of epileptic attacks on the clinical duration of supratentorial brain meningiomas. Wiad Lek. 2020;73(3):541‐545. [PubMed] [Google Scholar]
- 30. Ali A, Bagchi A, Mills S, et al. Risk factors for developing post‐operative seizures following meningioma resection. Neuro Oncol. 2018;20(suppl_1):i1. 10.1093/neuonc/nox237.001. [DOI] [Google Scholar]
- 31. Islim AI, McKeever S, Kusu‐Orkar TE, Jenkinson MD. The role of prophylactic antiepileptic drugs for seizure prophylaxis in meningioma surgery: a systematic review. J Clin Neurosci. 2017;43:47‐53. [DOI] [PubMed] [Google Scholar]
- 32. Tanti MJ, Marson AG, Jenkinson MD. Epilepsy and adverse quality of life in surgically resected meningioma. Acta Neurol Scand. 2017;136(3):246‐253. [DOI] [PubMed] [Google Scholar]
- 33. Aarts N, Noordam R, Hofman A, Tiemeier H, Stricker BH, Visser LE. Self‐reported indications for antidepressant use in a population‐based cohort of middle‐aged and elderly. International journal of clinical pharmacy. 2016;38(5):1311‐1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cea‐Soriano L, Wallander M‐A, García Rodríguez LA. Epidemiology of meningioma in the United Kingdom. Neuroepidemiol. 2012;39(1):27‐34. 10.1159/000338081 [DOI] [PubMed] [Google Scholar]
- 35. Uzun S, Kozumplik O, Jakovljević M, Sedić B. Side effects of treatment with benzodiazepines. Psychiatria Danubina. 2010;22(1):90‐93. [PubMed] [Google Scholar]
- 36. Cumming RG, Le Conteur DG. Benzodiazepines and risk of hip fractures in older people. CNS Drugs. 2003;17(11):825‐837. [DOI] [PubMed] [Google Scholar]
- 37. Roehrs T, Roth T. ‘Hypnotic'prescription patterns in a large managed‐care population. Sleep Med. 2004;5(5):463‐466. [DOI] [PubMed] [Google Scholar]
- 38. Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants, and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2016;157(3):643‐651. [DOI] [PubMed] [Google Scholar]
- 39. Hansen DG, Vach W, Rosholm JU, Søndergaard J, Gram LF, Kragstrup J. Early discontinuation of antidepressants in general practice: association with patient and prescriber characteristics. Fam Pract. 2004;21(6):623‐629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
Due to restrictions from the registry holders, raw data cannot be shared.


