Abstract
Pasteurisation of raw milk, colostrum, dairy or colostrum‐based products must be achieved using at least 72°C for 15 s, at least 63°C for 30 min or any equivalent combination, such that the alkaline phosphatase (ALP) test immediately after such treatment gives a negative result. For cows’ milk, a negative result is when the measured activity is ≤ 350 milliunits of enzyme activity per litre (mU/L) using the ISO standard 11816‐1. The use and limitations of an ALP test and possible alternative methods for verifying pasteurisation of those products from other animal species (in particular sheep and goats) were evaluated. The current limitations of ALP testing of bovine products also apply. ALP activity in raw ovine milk appears to be about three times higher and in caprine milk about five times lower than in bovine milk and is highly variable between breeds. It is influenced by season, lactation stage and fat content. Assuming a similar pathogen inactivation rate to cows’ milk and based on the available data, there is 95–99% probability (extremely likely) that pasteurised goat milk and pasteurised sheep milk would have an ALP activity below a limit of 300 and 500 mU/L, respectively. The main alternative methods currently used are temperature monitoring using data loggers (which cannot detect other process failures such as cracked or leaking plates) and the enumeration of Enterobacteriaceae (which is not suitable for pasteurisation verification but is relevant for hygiene monitoring). The inactivation of certain enzymes other than ALP may be more suitable for the verification of pasteurisation but requires further study. Secondary products of heat treatment are not suitable as pasteurisation markers due to the high temperatures needed for their production. More research is needed to facilitate a definitive conclusion on the applicability of changes in native whey proteins as pasteurisation markers.
Keywords: alkaline phosphatase, pasteurisation, milk, colostrum, sheep, goat, indicators
Summary
Pasteurisation of raw milk, colostrum, dairy or colostrum‐based products must be achieved using heat treatment of at least 72°C for 15 s, at least 63°C for 30 min or any equivalent combination, such that the alkaline phosphatase (ALP) test immediately after such treatment gives a negative result. That is when the measured activity in cows’ milk is ≤ 350 milliunits of enzyme activity per litre (mU/L) using the ISO reference method. Following a request from the European Commission, EFSA was asked to provide scientific and technical assistance on the use of ALP and possible alternative testing to verify thermal pasteurisation of milk, colostrum, dairy and colostrum‐based products from sheep and goats. More specifically in Term of Reference 1 (ToR1), EFSA was requested to provide an overview of the scientific information available on the use and limitations of ALP testing for verifying pasteurisation in the above products derived from sheep and goats, compared to cattle. If information is available, the overview could be extended to products derived from other species such as solipeds and camelids, producing such products for human consumption. In ToR2, EFSA was requested to list the possible alternative methods to the determination of ALP activity, and their possible limitations for the verification of pasteurisation of the products immediately after such treatment in the processing plant, as well as on the end product placed on the market.
The European Commission clarified that both ToR 1 and ToR 2 should be assessed considering the relevant products immediately after pasteurisation of milk or colostrum, in the processing plant or at farm level if the adequate equipment is in place, as well as in the end products placed on the market. The pasteurisation conditions of the assessment will consider those that have been legally defined.
The overall approach to answer both ToRs was qualitative and based on using evidence extracted from the scientific literature, databases and expert knowledge. Also, a questionnaire was used to gather information about the current usage of ALP and possible alternatives to verify pasteurisation of relevant products in the EU.
Regarding ToR1, the following assessment question 1 (AQ1) was formulated to address the ToR: What is the use and what are the limitations of ALP testing to verify thermal pasteurisation of milk or colostrum from sheep and goats (and other species such as solipeds and camelids, producing such products for human consumption), compared to cattle, both immediately after such treatment, as well as for the end products placed on the market (milk or colostrum for direct human consumption and milk or colostrum‐based products such as yoghurt, cheese, ice cream, milk powder, cream, or fermented milk)?
It was concluded that one‐third of the 15 EU countries replying to the questionnaire reported using ALP testing for milk or milk products from non‐bovine species, more specifically in goats’ milk, sheep's milk, cheese from sheep's milk and cheese from goats’ milk (in descending order).
The limitations of ALP testing for verifying pasteurisation of milk and milk products from bovine species also apply to other species. It is recommended that the ALP test should be performed immediately after the heat treatment and that those factors that influence the residual ALP levels should be considered when interpreting the results.
The ALP activity in raw sheep milk appears to be about three times higher, and in caprine milk about five times lower than in bovine milk. The level in raw milk from sheep and goats is highly variable between breeds and is influenced by season, lactation stage, fat content and udder health. Further variation of basal ALP levels among non‐bovine species is expected due to greater variation in breeds of sheep, goats and equines compared to dairy cows.
Combining the information on basal ALP levels and thermal inactivation behaviour of the enzyme in the respective species would facilitate an estimation of residual ALP after pasteurisation. However, only a few studies have investigated the thermal stability of ALP in milk derived from cows, sheep and goats, with conflicting evidence. Therefore, it is not possible to estimate residual ALP levels with certainty. Assuming that the inactivation of pathogens by heat would be the same in the milk of different species, and based on the available evidence from milk samples after pasteurisation, there is 95–99% probability (extremely likely) that pasteurised goat milk and pasteurised sheep milk would have an ALP activity below a limit of 300 and 500 mU/L, respectively. Nevertheless, it is recommended to collect further data in order to conclude whether the evidence now available is representative of all situations.
For equine milk, the current test sensitivity does not allow the use of ALP testing as the basal ALP activity is very low. Camel milk also contains low basal levels and, additionally, a heat‐stable ALP, and therefore, ALP testing is not appropriate either. The data available for cheese of non‐bovine species do not allow limits to be evaluated. No data is available for colostrum or milk or colostrum‐based dairy products such as yoghurt, ice cream, milk powder, cream or fermented milk.
Regarding ToR2, the following AQ2 was formulated: What are the possible alternative methods to the determination of ALP activity, and their possible limitations, for the verification of thermal pasteurisation of milk or colostrum from sheep and goats, both immediately after such treatment, as well as for the end product placed on the market?
The main alternative methods to verify pasteurisation of these products from non‐bovine species are temperature monitoring over time during the heat treatment using data loggers and the enumeration of Enterobacteriaceae. The use of data loggers is standard practice to monitor the heat treatment applied over time but cannot detect other process failures or post‐pasteurisation contamination. Enterobacteriaceae testing is relevant for monitoring the general hygiene of milk and milk products in accordance with the process hygiene criterion but is not suitable to verify that pasteurisation conditions have been properly applied.
The assessment of different classes of heat treatment of milk can be performed by means of assaying other endogenous marker enzymes, secondary products of heat treatment or changes in whey proteins. The inactivation of some enzymes may be more suitable to verify pasteurisation conditions of milk from non‐bovine species than ALP but studies would be required to evaluate this. Due to the high temperatures needed for the production of secondary products of heat treatment, methods based on their detection are not suitable as pasteurisation markers. Changes in native whey proteins depend on their levels in milk and their variability, making it difficult to set a meaningful limit for pasteurised milk currently.
Recommendations for further studies were formulated relating to an in‐depth thermal inactivation kinetics study of ALP inactivation in milk from the various animal species. More studies are also recommended to evaluate the use and limitations of ALP testing of colostrum and milk or colostrum‐based products such as cheeses derived from goat and sheep milk and to evaluate the use of other endogenous enzyme markers for milk derived from other species such as solipeds and camelids.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
When raw milk, colostrum, dairy or colostrum‐based products from farmed animals undergo heat treatment, food business operators (FBOp) must ensure that the treatment complies with the conditions for pasteurisation or ultra‐high temperature (UHT) treatment in accordance with Part II of Chapter II to Section IX of Annex III to Regulation (EC) No 853/2004 laying down specific hygiene rules for food of animal origin.1
Pasteurisation must be achieved by a treatment involving:
a high temperature for a short time (at least 72°C for 15 s);
a low temperature for a long time (at least 63°C for 30 min); or
any other combination of time‐temperature conditions to obtain an equivalent effect,
such that the products show, where applicable, a negative reaction to an alkaline phosphatase (ALP) test immediately after such treatment.
According to Chapter II in Annex III to Commission Implementing Regulation (EU) No 2019/6272, such a test is considered to give a negative result if the measured activity in cows’ milk is not higher than 350 milliunits of enzyme activity per litre (mU/L) using the ISO reference method 11816‐1.3
While this verification method works well in products derived from cows’ milk, difficulties have been encountered when applying it to products of sheep and goat origin and no cut off value has been laid down. This has been acknowledged by the wording “where applicable” in the legal provisions laid down in Regulation (EC) No 853/2004. A presentation made by the former European Union Reference Laboratory for milk and milk products (EURL‐MMP) during its 14th Workshop of National Reference Laboratories (NRLs) in May 2011, representing the state of play at that moment on goat milk, is attached.
1.1.2. Terms of Reference (ToRs)
In accordance with Article 31 of Regulation (EC) No 178/20024, the Commission requests EFSA to provide scientific and technical assistance with an overview on the possible use of the ALP test for the above purpose in products derived from ewes and goats, and, on the availability of alternative methods. EFSA is requested to evaluate the use of ALP and possible alternative testing to verify thermal pasteurisation of milk, colostrum, dairy and colostrum‐based products (‘products’) from sheep and goats. More specifically EFSA is requested:
ToR 1: to provide an overview of the scientific information available on the use and limitations of ALP testing for verifying pasteurisation in the above products derived from sheep and goats, compared to cattle. If information is available, the overview could be extended to products derived from other species such as solipeds and camelids, producing such products for human consumption.
ToR 2: to list the possible alternative methods to the determination of ALP activity, and their possible limitations for the verification of pasteurisation of the products immediately after such treatment in the processing plant, as well as on the end product placed on the market.
1.2. Interpretation of the ToRs
The European Commission clarified that for both ToR 1 and ToR 2 the products to be assessed are milk, colostrum, dairy and colostrum‐based products (referred to as ‘relevant products’ throughout this document) and these can be derived from sheep, goats and, if possible, from other animal species such as solipeds and camelids, producing such products for human consumption. Whenever reference is made in the report to milk, colostrum or dairy products, these are derived from cows’ milk except when the animal species is specified. Cows’ milk is the major type of milk produced in the EU. In the EU‐28 in 2018, 172.2 million tonnes of milk was produced on farms, of which 96.8% was cows’ milk, 1.6% ewes’ milk, 1.3% goats’ milk and 0.1% buffaloes’ milk. In several MSs, milk other than cows’ milk contributes significantly to milk production.5
Raw milk is defined in Regulation No (EC) 853/20041 as ‘milk produced by the secretion of the mammary gland of farmed animals that has not been heated to more than 40°C or undergone any treatment that has an equivalent effect’. Dairy products are defined as ‘processed products resulting from the processing of raw milk or from the further processing of such processed products’. Colostrum is defined as ‘the fluid secreted by the mammary glands of milk‐producing animals up to 3–5 days post parturition that is rich in antibodies and minerals and precedes the production of raw milk’. Colostrum‐based products are defined as ‘processed products resulting from the processing of colostrum or from the further processing of such processed products’.
It was also clarified that both ToR 1 and ToR 2 should be assessed considering the relevant products immediately after thermal pasteurisation of milk or colostrum, in the processing plant or at farm level if the adequate equipment is in place, as well as in the end products placed on the market. The end products are the milk or colostrum for direct human consumption and any products based on those such as yoghurt, cheese, ice cream, milk powder, cream or fermented milk. ‘End products placed on the market’ should be understood as to be supplied ‘at retail’ or by ‘direct supply to the final consumer’. Thermal pasteurisation will be referred to as ‘pasteurisation’ in the remainder of this document and will consider the legally defined treatment conditions.
According to the Codex code for milk and milk products (CAC, 2004), ‘pasteurisation is the application of heat to milk and liquid milk products aimed at reducing the number of any pathogenic microorganisms to a level at which they do not constitute a significant health hazard’. It results in the elimination of the most heat‐resistant, non‐spore‐forming pathogenic bacteria and contributes to the extension of the shelf‐life. The description of pasteurisation given by the International Dairy Federation (IDF, 1986) remains very appropriate: ‘a process applied with the aim of avoiding public health hazards arising from pathogenic microorganisms associated with milk, by heat treatment which is consistent with minimal chemical, physical and organoleptic changes in the product’. This can be achieved by heating at high temperature for a short time (HTST; at least 72°C for 15 s) or at low temperature for a longer time (LTLT; at least 63°C for 30 min) or at any other combination of time–temperature (t/T) to obtain an equivalent effect, such that the ALP activity in milk is reduced to an activity not higher than 350 mU/L. The lactoperoxidase (LPO) enzyme is still active after pasteurisation; in some countries such as Switzerland (Eberhard and Gallmann, 1994), LPO negative milk is referred to as ‘highly pasteurised’ milk, but this is not a clearly defined term in the EU.
This mandate concerns the evaluation of the potential use, and limitations, of ALP activity for the verification of pasteurisation of the relevant products from sheep, goats and, if possible, from other species such as solipeds and camelids, as it is currently used for the verification of the application of pasteurisation to bovine milk. Other possible uses of this test, e.g. to assess colostrum quality or immunoglobulin G (IgG) concentration, are excluded from this mandate.
Alternative testing to verify thermal pasteurisation of the relevant products should include alternative methods to the ISO 11816‐1:2013 standard3 for the determination of ALP activity, as well as possible alternatives to the determination of ALP activity.
Based on the interpretations described above, the following assessment questions (AQs) were formulated in order to address the ToR:
AQ1: What is the use and what are the limitations of ALP testing to verify thermal pasteurisation of milk or colostrum from sheep and goats (and other species such as solipeds and camelids, producing such products for human consumption), compared to cattle, both immediately after such treatment, as well as on the end products placed on the market (milk or colostrum for direct human consumption and milk or colostrum‐based products such as yoghurt, cheese, ice cream, milk powder, cream, or fermented milk)?
AQ2: What are the possible alternative methods to the determination of ALP activity, and their possible limitations, for the verification of thermal pasteurisation of milk or colostrum from sheep and goats, both immediately after such treatment, as well as on the end product placed on the market (as above)?
1.3. Additional information
1.3.1. Study from the European Union Reference Laboratory for milk and milk products on ALP limits in goat milk
A study was carried out by the former EURL‐MMP6 on ALP testing and limits in goats’ milk from different Member States (MS). The results of this study (‘Fixation of ALP limits in goat milk, EU study’) were presented during the 14th Workshop of the NRLs in May 2011 and provided to EFSA as an addendum to this mandate. Preliminary data collected from nine countries showed that most countries complied with the legal ALP limit defined for cows’ milk of 350 mU/L, but two countries had goats’ milk samples with values higher than 350 mU/L. During the autumn of 2009, additional data were collected from another eight countries. One country partly complied with the legal ALP limit defined for cows’ milk of 350 mU/L, whereas the other seven countries fully complied with the legal limit. The fact that, overall, two countries did not comply with the legal ALP limit defined for cows’ milk triggered discussions at European Commission level regarding whether a derogation would be pertinent for certain countries, or if a higher limit should be allowed for all countries. The European Commission decided to refer this Article 31 mandate to EFSA before taking any management decisions on this topic.
1.3.2. Legal background
Part II of Chapter II of Section IX of Annex III to Regulation (EC) No 853/20041 laying down specific hygiene rules for food of animal origin describes the specific requirements for heat treatment of raw milk, colostrum and dairy or colostrum‐based products. FBOp must ensure that the treatment satisfies the requirements laid down in Chapter XI of Annex II to Regulation (EC) No 852/20047. In particular, they shall ensure, when using the following processes, that they comply with the specifications mentioned:
-
Pasteurisation is achieved by a treatment involving:
a HTST (at least 72°C for 15 s);
a LTLT (at least 63°C for 30 min); or
any other combination of t/T conditions to obtain an equivalent effect, such that the products show, where applicable, a negative reaction to an ALP test immediately after such treatment.
-
UHT treatment is achieved by a treatment:
involving a continuous flow of heat at a high temperature for a short time (not less than 135°C in combination with a suitable holding time) such that there are no viable microorganisms or spores capable of growing in the treated product when kept in an aseptic closed container at ambient temperature, and
sufficient to ensure that the products remain microbiologically stable after incubating for 15 days at 30°C in closed containers or for 7 days at 55°C in closed containers or after any other method demonstrating that the appropriate heat treatment has been applied.
When considering whether to subject raw milk and colostrum to heat treatment, FBOp must:
have regard to the procedures developed in accordance with the HACCP principles pursuant to Regulation (EC) No 852/20047; and
comply with any requirements that the competent authority (CA) may impose in this regard when approving establishments or carrying out checks in accordance with Regulation (EC) No 854/20048.
Chapter II in Annex III to Commission Implementing Regulation (EU) No 2019/6272 lays down the conditions to determine the ALP activity in pasteurised cow's milk as follows:
To determine the ALP activity in pasteurised cow's milk, the ISO standard 11816‐13 must be applied as the reference method.
The ALP activity is expressed as mU/L. One unit of ALP activity is the amount of ALP enzyme that catalyses the transformation of 1 micromole of substrate per minute.
An ALP test is considered to give a negative result if the measured activity in cows’ milk is not higher than 350 mU/L.
The use of alternative analytical methods is acceptable when they are validated against the reference method mentioned in point A in accordance with internationally accepted protocols and rules of good laboratory practice.
The Codex code for milk and milk products (CAC, 2004) established performance and process criteria for pasteurised milk and liquid milk products. ‘As C. burnettii is the most heat‐resistant non‐sporulating pathogen likely to be present in milk, pasteurisation is designed to achieve at least a 5 log reduction of C. burnettii in whole milk (4% milkfat)’ (performance criteria). In relation to the process criteria, ‘according to validations carried out on whole milk, the minimum pasteurisation conditions are those having bactericidal effects equivalent to heating every particle of the milk to 72°C for 15 s (continuous flow pasteurisation) or 63°C for 30 min (batch pasteurisation). Similar conditions can be obtained by joining the line connecting these points on a log time versus temperature graph. Processing times necessary rapidly decrease with minimal increase in temperature. Extrapolation to temperatures outside the range of 63–72°C, in particular, processing at temperatures above 72°C must be treated with the utmost caution as the ability for them to be scientifically [validated] is beyond current experimental techniques. When changes in the composition, processing and use of the product are proposed, the necessary changes to the scheduled heat treatment should be established and a qualified person should evaluate the efficiency of the heat treatment. For instance, the fat content of cream makes it necessary to apply minimum conditions greater than for milk, minimum 75°C for 15 s. Formulated liquid milk products with high sugar content or high viscosity also require pasteurisation conditions in excess of the minimum conditions defined for milk’.
Part III of Chapter I of Section IX of Annex III to Regulation (EC) No 853/20041 specifies that FBOp producing or, as appropriate, collecting raw milk and colostrum must ensure compliance with the following requirements before heat treatment;
raw cows’ milk must have a plate count at 30°C of less than 100,000 CFU per mL.
raw milk from other species must have a plate count at 30°C of less than 1,500,000 CFU per mL.
Part III of Chapter II of Section IX of Annex III to Regulation (EC) No 853/20041 lays down for FBOp manufacturing dairy products the criteria for raw cows’ milk immediately before being heat treated:
raw cows’ milk used to prepare dairy products must have a plate count at 30°C of less than 300,000 CFU per mL; and
heat treated cows’ milk used to prepare dairy products must have a plate count at 30°C of less than 100,000 CFU per mL.
In addition, milk intended for human consumption must be derived from cows and buffaloes free from brucellosis and tuberculosis (Directive 64/432/EEC9) and from sheep and goats coming from a herd free from brucellosis (Directive 91/68/EEC10).
Table 1 summarises the microbiological criteria for pasteurised milk and derived milk products, as established by Regulation (EC) No 2073/200511. These are all ‘process hygiene criteria’ indicating the acceptable functioning of the production process. Such a criterion sets an indicative contamination value above which corrective actions are required in order to maintain the hygiene of the process in compliance with food law. It is not applicable to products placed on the market.
Table 1.
Process hygiene criteria for pasteurised milk and derived milk products as defined by Regulation (EC) No 2073/200511
| Number | Food category | Microorganisms | Sampling plana | Limitsb | Analytical reference method | Stage where the criterion applies |
|---|---|---|---|---|---|---|
| 2.2.1 | Pasteurised milk and other pasteurised liquid dairy products | Enterobacteriaceae | n = 5, c = 0 | m = M = 10 CFU/mL | EN ISO 21528‐2 | End of the manufacturing process |
| 2.2.2 | Cheese made from milk or whey that has undergone heat treatment | E. coli | n = 5, c = 2 | m = 100 CFU/g M = 1,000 CFU/g | ISO 16649‐1 or 2 | At the time during the manufacturing process when the E. coli count is expected to be highest |
| 2.2.4 | […] ripened cheeses made from milk or whey that has undergone pasteurisation or stronger heat treatment | Coagulase‐positive staphylococci | n = 5, c = 2 | m = 100 CFU/g M = 1,000 CFU/g | EN/ISO 6888‐1 or 2 | At the time during the manufacturing process when the number of staphylococci is expected to be highest |
| 2.2.5 | Unripened soft cheeses (fresh cheeses) made from milk or whey that has undergone pasteurisation or a stronger heat treatment | Coagulase‐positive staphylococci | n = 5, c = 2 | m = 10 CFU/g M = 100 CFU/g | EN/ISO 6888‐1 or 2 | End of the manufacturing process |
| 2.2.7 | Milk powder and whey powder | Enterobacteriaceae | n = 5, c = 0 | m = M = 10 CFU/mL | EN ISO 21528‐2 | End of the manufacturing process |
| Coagulase‐positive staphylococci | n = 5, c = 2 | m = 10 CFU/g M = 100 CFU/g | EN/ISO 6888‐1 or 2 | End of the manufacturing process | ||
| 2.2.8 | Ice cream and frozen dairy desserts | Enterobacteriaceae | n = 5, c = 0 | m = M = 10 CFU/mL | EN ISO 21528‐2 | End of the manufacturing process |
n = number of units comprising the sample; c = number of sample units giving values between m and M.
Satisfactory if all the values observed are ≤ m, acceptable if a maximum of c/n values are between m and M, and the rest of the values observed are ≤ m, and unsatisfactory if one or more of the values observed are > M or more than c/n values are between m and M.
1.3.3. Approach to answer the ToRs
The overall approach to answer both ToRs was qualitative and based on using evidence extracted from the scientific literature, databases and expert knowledge. Also, a questionnaire was used to gather information about the current usage of ALP and possible alternatives to verify pasteurisation of relevant products in the EU.
The approach to answer the ToR was defined upfront and is described in the protocol (Annex A). It covers both the problem formulation (i.e. what the assessment aims to address) and which methods will be used for addressing the problem. Problem formulation includes: a) the clarification of the mandate (see further refined in Section 1.2) and b) the translation of each ToR into a scientifically answerable assessment question and the definition of the overall approach for the assessment. It followed the draft framework for protocol development for EFSA's scientific assessments (EFSA, 2020). The framework is a draft because it will be refined and published after the trial phase over a year.
2. Data and methodologies
2.1. Data
2.1.1. Food‐borne outbreak data in the EU
Data reported by MS on strong‐evidence food‐borne outbreaks (FBO) in the EU from 2007 to 2019 implicating, as food vehicles, milk and dairy products (including cheese) were extracted from EFSA's Zoonoses database on 28 July 2020 (Appendix A) to gather evidence on the microbiological hazards associated with the consumption of (raw) milk, colostrum, dairy and colostrum‐based products from bovine and non‐bovine species. All outbreaks implicating a multicomponent food vehicle including a non‐dairy ingredient, such as ice cream with eggs, were excluded. The data were analysed by aggregating the food‐borne outbreak vehicles; milk, cheese and dairy products (other than cheese).
2.1.2. Questionnaire on ALP and possible alternative testing
To gather information on the current usage of ALP and possible alternatives to verify pasteurisation of relevant products, a questionnaire was drafted by the WG (provided in Appendix B). The questionnaire was circulated by e‐mail to the members of the EFSA Network on Microbiological Risk Assessment (MRA) (currently including members of 25 European MS and three observer countries) in the beginning of October 2020. They were asked to forward the questionnaire to the respective contact points in their countries if they were not the appropriate point of contact. By 1 December 2020, 15 countries had responded to the questionnaire.
The responder was asked if any ALP testing data according to the ISO 11816‐1:2013 standard3 for milk and milk‐based drinks or ISO 11816‐2:201612 for cheese have been collected for relevant products from bovine as well as non‐bovine species.
If ALP data from non‐bovine species were collected, the responder was asked to further specify which products from which species had been tested and to share the respective ALP values with the WG if possible. These data were summarised by the working group. If no ALP testing data from non‐bovine species were available, the responder was asked to provide information on which alternatives were used to verify pasteurisation of the collected samples.
If no ALP testing is performed in the country, or there is restricted access to the data, the responder was further asked to indicate possible reasons for this.
2.1.3. ALP testing data from non‐bovine species
ALP testing data from samples of milk from non‐bovine species were extracted on 21 September 2020 from the database of Public Health England (PHE) from 2013 to 2020. These data related to pasteurised milk samples submitted to PHE laboratories by Local Authority Environmental Health Officers or directly from food businesses. The amount of information provided with each sample was variable, but in general, details such as the heat treatment method or conditions used were not available. Testing was performed using the ISO Fluorophos method (i.e. ISO 11816‐1:2013 standard3). These data were used to derive the ALP levels after pasteurisation for sheep and goat milk (together with the data obtained from the questionnaire, see Section 2.1.2) and to evaluate possible correlations between the Enterobacteriaceae counts and ALP levels; both measured after pasteurisation. For comparison, ALP testing data from samples of pasteurised bovine milk from 2019 to 2021 were extracted from the database on 15 January 2021.
2.1.4. Literature search
A literature search was carried out to retrieve information on the use and limitations of ALP testing for verifying pasteurisation in the relevant products derived from sheep, goats, solipeds and camelids and to provide an overview of the possible alternatives to the determination of ALP activity. The search was conducted in the Web of Science™ Core Collection (1975–present) on 6 November 2020. The search string used was TS = (milk* OR colostrum* OR cheese* OR dairy OR yoghurt* OR yogurt* OR (ice cream)) AND TS = (ALP or (alkaline phosphatase)) AND TS = (sheep* OR goat* OR soliped* OR camelid* OR horse* OR equine OR donkey* OR dromedar* OR camel* OR alpaca*). No restrictions were applied related to the document type, language or timespan.
2.1.5. Information from the European Dairy Association
The European Dairy Association (EDA)13 was contacted to establish if there are any industries in the EU producing colostrum and/or colostrum‐based products from non‐bovine species (e.g. sheep, goats, camels, horse, donkeys, etc.) intended for human consumption. If so, EFSA asked the EDA to provide any relevant contacts who may hold data/information on the use of ALP testing or alternative methods for this testing to verify pasteurisation of these products.
Although the core business of most of the members of EDA is bovine milk and dairy products, many also produce non‐bovine milk and dairy products, but this does not include colostrum from non‐bovine animals.
One company producing milk from non‐bovine species (goats) informed EFSA that ALP becomes reactivated in goat's milk after a certain amount of time, so the analyses would have to take place within 24 h after pasteurisation to obtain reliable results.
One member specified that most companies are familiar with the ALP technique and that authorities sometimes request evidence of its application to validate pasteurisation. Its use has decreased, as today all heat treatment equipment is equipped with a data logger to continuously track the operating temperature.
Another member added that there are a few suppliers of colostrum which trade on the internet, raising the possibility that most manufacturers do not have any experience with the ALP test at all as colostrum from sheep and goats is sterilised by filtration. It was claimed that ‘when colostrum is heated up the “vital substances” are destroyed’.
2.2. Methodologies
2.2.1. Use and limitations of ALP testing to verify pasteurisation of milk, colostrum, dairy and colostrum‐based products from ewes and goats (ToR 1)
Apart from the literature search, as described in Section 2.1.4, relevant documents were also identified and reviewed, based on the knowledge and expertise of the WG members. These documents included scientific papers, book chapters, non‐peer‐review papers, regulations, guidance documents, standards from national and international authorities and reports known to the experts themselves or retrieved through additional non‐systematic searches. The reference list of these documents was further screened in order to identify additional relevant publications until the coverage of the subject was considered sufficient by the WG.
These documents were used to provide an overview of different analytical methods for ALP activity determination in dairy products and to provide information on the limitations of ALP testing in milk, colostrum, dairy and colostrum‐based products from non‐bovine and/or bovine species.
Information on the use of ALP testing in milk, colostrum, dairy and colostrum‐based products from non‐bovine and/or bovine species was also obtained from the questionnaire (see Section 2.1.2) and summarised.
The initial ALP concentration in raw milk from various species (referred to as ‘basal level’ in the report) was derived by screening records from the literature. Only data using the ISO Fluorophos method were considered.
To retrieve data on the thermal stability of ALP, records derived from the literature search were screened for evidence on the destruction of ALP in milk from various species. The thermal inactivation data included in these studies were screened for their relevance against a set of criteria: (i) the type of substrate used is (raw) milk from different animal species; (ii) the inactivation of ALP was measured over time; (iii) the ALP inactivation was measured using either the ISO Fluorophos method or another quantitative and validated method; (iv) the temperature used should represent thermal inactivation (above 50°C), be measured in the substrate and conform to isothermal conditions; (v) for the D‐value calculation, the data set should include at least three data points that are above the detection limit. Many of the available studies report data with relatively few data points, which restricted the analysis to linear inactivation and the estimation of D‐values. The D‐value is reported when all the criteria were fulfilled, while the final ALP activity at the last incubation time tested was reported when one of the last two criteria could not be fulfilled.
To consider limits of ALP in pasteurised milk of different animal species, first the available quantitative data of ALP concentrations were described statistically after log10 transformation due to the non‐normal behaviour. Second, both the quantitative and semi‐quantitative data were summarised in ranges of ALP concentrations.
2.2.2. Alternative methods for the verification of thermal pasteurisation of milk, colostrum, dairy and colostrum‐based products from ewes and goats (ToR 2)
In the same way as for ToR 1, apart from the literature search as described in Section 2.1.4, relevant documents for answering ToR2 were also identified and reviewed based on the knowledge and expertise of the WG members. These documents included scientific papers, book chapters, non‐peer‐review papers, regulations, guidance documents, standards from national and international authorities and reports known to the experts themselves or retrieved through additional non‐systematic searches. The reference list of these documents was further screened in order to identify additional relevant publications until the coverage of the subject was considered sufficient by the WG.
For the alternative methods for the verification of thermal pasteurisation of milk, colostrum, dairy and colostrum‐based products from ewes and goats, the information on the alternative testing to verify pasteurisation as currently used by the MS, based on the questionnaire, was described. Then, specific descriptions of the evaluation of alternative potential methods as intrinsic time temperature integrators (TTI) were provided. For endogenous enzymes, the assessment considered their occurrence in raw milk and colostrum of various animal species, their thermal stability and presence after pasteurisation. Also, the analytical methods for testing were listed. For milk compounds, the degradation, denaturation or inactivation of heat‐labile compounds and the formation of new substances were considered.
2.2.3. Uncertainty analysis
Based on the EFSA guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a) and scientific opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018b), special attention was given to: (i) the interpretation of the ToRs, i.e. framing of the mandate and the AQs, (ii) identifying sources of uncertainty and (iii) their impact on the outcome of the assessment. The experts elicited the overall uncertainty associated with the setting of tentative limits for the ALP activity in pasteurised goats and sheep milk through expert group judgement taking into account the quantified and non‐quantified sources of uncertainty. The uncertainty was investigated in a qualitative manner following the procedures detailed in the EFSA guidance. Uncertainty has been defined as all types of limitations in available knowledge that affect the range and probability of possible answers to an AQ. It can arise from limitations in the evidence (i.e. heterogeneity, degree of relevance, degree of internal validity and/or precision) and in the methods used throughout the assessment (EFSA Scientific Committee, 2018a). The sources of the main uncertainties were identified, and for each of these, the nature or cause of the uncertainty was described (Appendix C).
3. Assessment
3.1. Heat treatment of milk and colostrum
Heat treatment of raw milk mainly aims to reduce pathogenic and spoilage microorganisms, to inactivate enzymes and to minimise chemical reactions and physical changes during storage. The most commonly used heat treatments, in order of increasing intensity, are thermisation, pasteurisation, high pasteurisation, extended shelf‐life (ESL) treatment, UHT treatment and in‐container sterilisation. The t/T conditions used for these heat treatments, and their bactericidal effect and effect on enzymes are summarised in Table 2 and further described in the following sections, based on the book by Deeth and Lewis (2017).
Table 2.
Heat treatments used for milk (in increasing order of intensity) (after Töpel (2004) and Deeth and Lewis (2017))
| Heat treatments | Time–temperature conditions | Bactericidal effect | Effect on selected enzymes | Expected shelf‐life |
|---|---|---|---|---|
| Thermisation | 57–68°C for 5–20 s | Destroys most non‐spore‐forming psychrotrophic spoilage bacteria | Does not inactivate milk ALP, lipase, LPO, plasmin or bacterial proteases/lipases | 3 days (refrigerated) |
| Pasteurisation | 63°C for 30 min (batch, LTLT) 65°C for 15 min (batch, LTLT) 72–82°C for 15–30 s (continuous, HTST) | Destroys all non‐spore‐forming pathogenic bacteria | Inactivates milk ALP and lipase but not LPO, plasmin or bacterial proteases/lipases | 2–3 weeks (refrigerated) |
| High pasteurisation | 85–127°C for 1–4 s | Destroys all non‐spore‐forming bacteria and most of the spores of psychrotrophic and mesophilic bacteria | Inactivates milk ALP, lipase and LPO but not plasmin or bacterial proteases/lipases | 4–8 weeks depending on processing conditions (refrigerated) |
| ESLa | 123–145°C for < 1–5 s | Destroys all non‐spore‐forming bacteria and most of the spores of psychrotrophic and mesophilic bacteria | Inactivates milk ALP, lipase and LPO but not plasmin or bacterial proteases/lipases | 4–13 weeks (refrigerated) |
| UHT | 138–145°C for 1–10 s | Destroys all non‐spore‐forming bacteria and all spores except highly heat‐resistant spores (rarely present) | Inactivates milk ALP, lipase, LPO; and most plasmin but not all bacterial proteases/lipases | 6–9 months (room temperature) |
| In‐container sterilisation | 115–120°C for 10–30 min (conventional) 125°C for 4 min (e.g. Shaka™ technology) | Destroys all non‐spore‐forming bacteria and all spores except highly heat‐resistant spores (rarely present) | Inactivates virtually all enzymes | 6 months (room temperature) |
ALP: alkaline phosphatase; ESL: extended shelf‐life; HTST; high temperature short time; LPO; lactoperoxidase; LTLT; low temperature long time; UHT; ultra‐high temperature.
Considering the commercial thermal processing conditions for ESL milk, as ESL milk can also be produced through non‐thermal processes such as microfiltration and bactofugation, usually combined with a final thermal pasteurisation treatment to meet regulatory requirements.
In the case of colostrum, no information about the actual heat treatment conditions being used by industry is available. It is known that its potential health benefits can be detrimentally affected by heat during pasteurisation. These health benefits can be attributed to the fact that it contains immune factors, growth factors and a variety of potentially probiotic bacteria. Many of these components such as immunoglobulins (Ig) and probiotic bacteria will be damaged by the heating.
3.1.1. Thermisation
Thermisation (also referred to as sub‐pasteurisation) involves heating milk to 57–68°C for 5–20 s. It is used to keep the quality of raw milk when the milk needs to be held chilled for some time before being further processed. It aims to reduce the growth of psychrotrophic bacteria, which may release heat‐resistant proteases and lipases into the milk if allowed to reach high levels. As these enzymes will not be totally inactivated during subsequent heat treatments, they may give rise to off‐flavours in processed milk or in subsequently manufactured cheese or milk powders and/or may significantly reduce the shelf‐life of UHT milk or milk products. Thermised milk is subsequently used for other heat‐treated milk or converted into various milk products (Deeth and Lewis, 2017). It is ALP‐positive, which distinguishes it from ALP‐negative pasteurised milks.
3.1.2. Pasteurisation
Pasteurisation is a relatively mild heat treatment which has only a small effect on the physical, chemical, nutritional and organoleptic properties of the milk (Bylund, 1995; Deeth, 2006). As well as destroying all vegetative cells of pathogenic bacteria (but not the bacterial spores), it reduces bacteria and enzymes that could cause spoilage of the product. This prolongs the shelf‐life of the milk. Pasteurised milk requires refrigeration to ensure a long shelf‐life, even in unopened packs.
The conditions used in pasteurisation are designed to inactivate the most heat‐resistant, non‐spore‐forming pathogenic bacteria in milk, Mycobacterium tuberculosis and Coxiella burnetii. According to CAC (2004), pasteurisation is designed to achieve at least a 5 log reduction of C. burnetii in whole milk. It therefore results in very substantial reduction in populations of pathogens that might be present in raw milk (Deeth and Lewis, 2017).
As mentioned previously (Section 1.3.2), the conditions of pasteurisation are legally specified and can be achieved by using HTST (at least 72°C for 15 s); LTLT (at least 63°C for 30 min); or any other combination of t/T conditions to obtain an equivalent effect. Originally, pasteurisation was performed in a batch process in which milk was heated to 63°C for 30 min (LTLT). Nowadays, pasteurisation is mostly performed by the HTST process using a heat exchanger in which the milk is heated to 72–75°C with a holding time of 15–20 s before it is cooled. It permits the use of continuous processing, regeneration of energy and long run times. The main types of indirect heat exchanger for milk are the plate heat exchanger and the tubular heat exchanger. The layout for a typical pasteurisation unit (see Figure 1) consists of the preheating of the raw milk at the regeneration step, then the heating, the holding tube, the first cooling of the pasteurised milk at the regeneration step and then the final cooling step where the milk is cooled with cold water. The regeneration step saves on heating and cooling costs.
Figure 1.
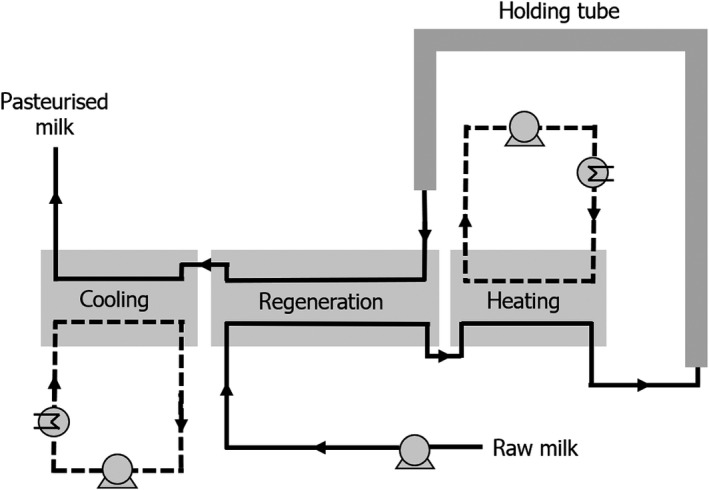
Schematic presentation of a pasteurisation unit
The ALP test was initially established based upon the finding that the naturally occurring ALP in milk had similar inactivation kinetics to the inactivation of M. tuberculosis. It can be used as an endogenous marker of correct heat treatment conditions since it is destroyed by the t/T combinations necessary for proper pasteurisation and, when inactivated to a legally defined level, it indicates that the milk has been adequately heated.
In normally pasteurised milk, the endogenous enzyme LPO must be still active. When LPO is inactivated, the milk is called ‘high pasteurised’ (see Section 1.2).
The shelf‐life for pasteurised milk varies between countries but is in the range of 5‐21 days (Deeth and Lewis, 2017).
3.1.3. High pasteurisation
Heat treatment for ‘high pasteurised’ milk is performed at temperatures between 85 and 127°C for 1–4 s (Töpel, 2004), but the heating conditions are not well defined (Deeth and Lewis, 2017). ALP and LPO activities are expected to be negative when testing these products.
3.1.4. Extended shelf‐life milk processing
ESL milk is produced by one of the two principal technologies: 1) thermal processing using more severe conditions than pasteurisation but less severe than UHT processing; and 2) non‐thermal processes such as microfiltration and bactofugation, usually combined with a final thermal pasteurisation treatment to meet regulatory requirements (Deeth, 2017). Commercial thermal processing conditions for ESL milk are in the range of 123–145°C for 1–5 s. The non‐thermal process can rely on microfiltration using a 0.8–1.4 μm pore size membrane combined with HTST heat treatment. In addition, a particularly high temperature pasteurisation of the separated fat phase can be carried out. ESL milk refers to milk which has a refrigerated shelf‐life longer than that of pasteurised milk.
3.1.5. Sterilisation
Milk can either be sterilised in bottles or other sealed containers, or by continuous UHT processing followed by aseptic packaging. UHT and in‐container sterilised products are referred to as ‘commercially sterile’ and have an extended shelf‐life without refrigeration.
In bottle or in‐container sterilisation is the original form of sterilisation, which is still used, usually at 115–120°C for 20–30 min. After fat standardisation, homogenisation and heating to about 80°C, the milk is packed in clean containers – usually glass or plastic bottles for milk, and cans for evaporated milk. The product, still hot, is transferred to autoclaves in batch production or to a hydrostatic tower in continuous production, where sterilisation at the t/T conditions above mentioned takes place.
UHT treatment is also used to sterilise milk or milk products by heating to 135–145°C for 2–5 s. It kills microorganisms and inactivates also almost all enzymes although the presence of thermoresistant proteases and/or lipases originating from psychrotrophic bacteria could shorten the shelf‐life. UHT treatment is a continuous process which takes place in a closed system that prevents the product from being contaminated by airborne microorganisms. The product passes through heating and cooling stages in quick succession. Aseptic filling, to avoid recontamination of the product, is an integral part of the process. Two alternative methods of UHT treatment are used: (i) Indirect heating and cooling in heat exchangers, (ii) Direct heating by steam injection or infusion of milk into steam and cooling by expansion under vacuum. More information can be found in Bylund (1995) and Deeth and Lewis (2017). UHT processing conditions overlap with those of ultra‐pasteurised milk being treated using at least 135°C for 2 s.
UHT processing aims to produce a product which does not contain microorganisms capable of growing under the normal conditions of storage, that is, to be ‘commercially sterile’. The rarely present bacterial contaminants of UHT milk result from either survival of heat‐resistant spores or post‐process contamination through contamination of equipment in the post‐sterilisation section of the plant. Spoilage of UHT milk by heat‐resistant spore‐forming organisms first requires activation and germination of the spores and growth of the vegetative cells (Deeth and Lewis, 2017).
3.1.6. Non‐thermal technologies
Non‐thermal technologies have been largely driven by consumer demand for minimally processed food products with the flavour and nutritive properties of fresh foods. Deeth and Lewis (2017) describe the following non‐thermal technologies alone or with some additional thermal processing for producing milk and dairy products: microfiltration, high pressure processing (HPP), pulsed electric field technology, high‐pressure homogenisation, bactofugation, UV irradiation, Gamma irradiation, carbon dioxide and high‐pressure carbon dioxide. The advantages, limitations and commercialisation status of these technologies have been reviewed by Deeth and Lewis (2017). The dairy applications of HPP, pulsed electric field technology and high‐pressure homogenisation, have been reviewed by Deeth et al. (2013).
3.2. Microbiological hazards associated with the consumption of milk, colostrum, dairy and colostrum‐based products from non‐bovine species
There is a well‐recognised association between raw milk consumption and human infection with pathogenic microorganisms (Claeys et al., 2013). Milk can be contaminated by animal pathogens directly shed into the milk within the udder or by microorganisms from a variety of environmental sources, during or after milking, including the teat apex, milking equipment and udder cloths, faeces from standing areas or bedding, air, water, feed, grass, soil and other environmental elements (EFSA BIOHAZ Panel, 2015; Parente et al., 2020).
Cows’ milk is the most commonly consumed type of milk, and FBOs linked to cows’ milk are more commonly reported than those linked to milk from other animal species. However, there have also been reports of illnesses associated with milk from other species. For example, raw goats’ milk has been implicated in outbreaks of Escherichia coli O157 (McIntyre et al., 2002), brucellosis (Ramos et al., 2008), Q Fever (Fishbein and Raoult, 1992) and tick‐borne encephalitis virus (TBEV) (Balogh et al., 2010). Moreover, an outbreak of brucellosis in Qatar was linked to the consumption of raw camel milk (Garcell et al., 2016).
In 1998, a study of 126 sheep or goats’ milk samples in England and Wales found that 6% of goats’ milk and 12% of ewes’ milk samples were contaminated with levels of Staphylococcus aureus greater than 100 CFU/mL (Little and De Louvois, 1999), but Salmonella, Campylobacter, E. coli O157 and Listeria monocytogenes were not detected in any sample. Willis et al. (2018) reported that Salmonella, Campylobacter and E. coli O157 were not detected in 269 sheep and goats’ milk samples, and only one sample of goats’ milk contained an unacceptable level of S. aureus. However, L. monocytogenes was detected in three samples of goats’ milk and one of sheep milk. Willis et al. (2018) and McLauchlin et al. (2020) both found that, while pathogens were detected in raw sheep and goats’ milk, the microbiological quality of raw cows’ milk was poorer than for other species. Verraes et al. (2014) undertook a review of the scientific literature relating to the prevalence of pathogens in raw milk of species other than cows. They did not find reports of Salmonella detection in milk from goats, horses, donkeys or buffaloes, but found a low frequency of occurrence (0–5%) in raw sheep milk. Similarly, Campylobacter detection was reported in sheep milk, but not in milk from goats, horses or buffaloes. An evaluation of the microbiological safety of donkeys’ milk in Italy (Mottola et al., 2018) found that Campylobacter coli and E. coli O157 were each detected in 1% of 90 samples.
There is little information available regarding the microbiological quality of colostrum (and little evidence of its use for human consumption). However, C. burnetii, the cause of Q Fever, was found to be shed in both the milk and colostrum of ruminants, with the prevalence in cows’ milk (14–45%) being considerably higher than in goats’ milk (2%) (Khamesipour et al., 2018). TBEV can also be excreted in the milk and colostrum of goats, sheep and cattle, and infection may then occur through consumption of the unpasteurised milk. Wallenhammar et al. (2020) demonstrated that TBEV infectivity may be preserved for several days in refrigerated milk.
In addition to raw drinking milk, products made with unpasteurised milk have also been the source of FBOs. A cluster of three cases of E. coli O157 was linked to consumption of raw cream in England in 1997 (PHLS, 1998). Outbreaks of salmonellosis (Robinson et al., 2020) and TBEV (ECDC, 2020) have been linked with the consumption of cheese made from raw goats’ milk, and a further Salmonella outbreak was reported in association with raw sheep milk cheese (Anon, 2019). Campylobacter was detected in 3 of 522 sheep milk cheeses (EFSA and ECDC, 2018). These three cheeses were all sampled from retailers in Slovakia. McLauchlin et al. (2020) found goats’ milk cheeses to be of poorer microbiological quality than those prepared from milk of other species. However, this may have been partly due to an increased proportion of soft cheeses amongst those made from goats’ milk, and it may also have been affected by re‐sampling from the same premises where previous problems had been detected.
Although pasteurisation of milk significantly reduces the risks from infectious agents, failures in pasteurisation can occur, either through failure to achieve a sufficiently high temperature for long enough to inactivate pathogens, or because there is cross‐contamination of the pasteurised milk with raw milk within the pasteurising process. For example, a Campylobacter outbreak in the UK that affected 37 people was considered to be due to a pasteurisation failure, with 17 of 22 milk samples showing ALP levels that exceeded the legal limit specified in Regulation (EU) No 2019/6272, indicating inadequate pasteurisation (Fernandes et al., 2015). It is therefore important to have rapid tests to confirm that effective heat treatment has been achieved, in order to ensure that public health is not put at risk.
Even after a heat treatment such as those described above, bacteria may still be found in milk. These may include those microorganisms that survive pasteurisation as well as those that are re‐introduced through poor hygiene or system failures during or after pasteurisation. Contamination is mainly with Gram‐negative organisms that are able to grow at refrigeration temperatures, resulting in eventual spoilage of the milk (Martin et al., 2018). Spore‐forming bacteria will not be eliminated by standard pasteurisation treatment, and therefore, spoilage due to Bacillus species e.g. can occur (Gopal et al., 2015).
Pathogens such as L. monocytogenes can survive in the post‐pasteurisation processing environment, leading to the potential for re‐contamination of pasteurised milk and dairy products (Lee et al., 2019).
Table A.11 in Appendix A provides details of 453 strong‐evidence FBOs associated with the consumption of milk and dairy products in the EU between 2007 and 2019. These included 22 outbreaks linked to raw/unpasteurised milk products of non‐bovine origin with the most common causative agents being flaviviruses (a group of viruses which includes TBEV), followed by staphylococcal enterotoxins and Salmonella spp. However, it is not clear from this data set which outbreaks were caused by milk and which were linked to dairy products such as cheese. For the majority of the FBOs (300 out of 453 outbreaks), the animal species was unknown and more specifically for raw/unpasteurised milk products, the animal species was not known for 94 outbreaks. No outbreak has been reported implicating colostrum or any colostrum‐based products intended for human consumption.
Table A.1.
Number of strong evidence food‐borne outbreaks associated with the consumption of milk and dairy products (including cheese) by causative agent, animal species of origin of the milk and heat treatment of the milk or of the dairy products, as reported by the EU Member States during the period 2007–2019
| Causative agent | Bovine species | Non‐bovine species | Unspecified animal species | All species and possible heat treatments | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pasteurised milk | Raw/unpasteurised milk | Unspecified heat treatment | Raw/unpasteurised milk | Unspecified heat treatment | Pasteurised milk | Raw/unpasteurised milk | Unspecified heat treatment | ||
| Salmonella spp. | 0 | 0 | 1 | 3 | 0 | 0 | 3 | 131 | 138 |
| Campylobacter spp. | 2 | 2 | 0 | 1 | 1 | 0 | 77 | 8 | 91 |
| Staphylococcal enterotoxins | 1 | 4 | 1 | 5 | 10 | 2 | 1 | 101 | 125 |
| Escherichia coli | 0 | 1 | 1 | 0 | 0 | 0 | 10 | 12 | 24 |
| Flavivirus | 0 | 0 | 0 | 12 | 8 | 0 | 0 | 4 | 24 |
| Bacillus spp. | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 12 | 14 |
| Escherichia coli, pathogenic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 12 |
| Histamine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 6 |
| Brucella spp. | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 5 |
| Calicivirus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 5 |
| Listeria monocytogenes | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 5 |
| Clostridium | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Cryptosporidium | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Rotavirus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Yersinia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| All causative agents | 4 | 7 | 3 | 22 | 19 | 4 | 94 | 300 | 453 |
3.3. Use and limitations of ALP testing to verify pasteurisation of milk, colostrum, dairy and colostrum‐based products from ewes and goats (ToR 1)
3.3.1. Overview of different analytical methods for ALP activity determination in milk and other dairy products
3.3.1.1. Official methods
As mentioned in Section 1.3.2, the ISO 11816‐1:2013 standard3 for milk and milk‐based drinks must be applied as reference method for milk pasteurisation when determining ALP activity with the activity expressed as mU/L. It is a fluorimetric method for raw and heat‐treated whole milk, semi‐skimmed milk, skimmed milk and flavoured milks. The method is applicable to milk and milk‐based drinks from cows, sheep and goats. It is also applicable to milk powder after reconstitution. As mentioned before, according to the Regulation (EU) No 2019/6272, an ALP test is considered to give a negative result if the measured activity in cows’ milk is not higher than 350 mU/L. This limit is equivalent to the contamination of pasteurised milk with raw milk in a percentage of approximately 0.02–0.05% according to Greenwood and Rampling (1997), 0.05% according to Punoo (2018) and below 0.1% according to Scintu et al. (2000).
The ISO 11816‐2:2016 standard12 is a fluorimetric method for cheese. The method is applicable to soft cheeses, semi‐hard and hard cheeses provided that any mould is only on the surface of the cheese and not in the inner part. It is not specified in the standard if the method is applicable to cheese from cows, sheep and goats’ milk. For cheese, no legal limit has been set as has been done for milk. However, according to the survey on ALP activities in pasteurised cheeses that was performed in France, Italy and Switzerland, a tentative target value of 10 mU/g of cheese was proposed. This target value was achieved by all tested pasteurised milk cheeses, except for blue veined cheeses and for pasta‐filata cheeses (Egger et al., 2016).
The use of alternative analytical methods to determine the ALP activity is acceptable when the methods are validated against these reference methods. Methods applied for the analysis of ALP activity in milk and dairy products can be classified according to three different measuring principles: fluorimetric, colorimetric and chemiluminescent as recently reviewed by Punoo (2018) and summarised in Table 3.
Table 3.
Overview of different analytical methods for ALP activity determination in dairy products
| Method principle | Wavelength (nm) | Substrate (S)/product (P) | Units definition | Detection limit | RSDR (%) | Scope of usea | Short description | Reference | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| Fluorimetric | Ex 440/Em > 505 | S: monophosphoric ester Fluorophos® P: Fluoro‐yellow | U = μmol/min | 0.006% raw milk | 4.4 | Part 1: liquid dairy products from cow, sheep, and goat Part 2: cheese | Measuring fluorescence in the sample with aromatic monophosphoric ester | ISO 11816‐1 | IDF 155‐1:2013 and ISO 11816‐2 | IDF 155‐2:2016 (Rocco, 1990; Shakeel‐ur‐Rehman et al., 2003) | Fluoro‐Test FML 200, Fluorophos method |
| Ex 365/Em 450 | S: 4‐methyl‐umbelli‐ferone‐phosphate P: 4‐methyl‐umbelliferone | mU = nmol/min | 0.006% raw milk | 9.5 | Fluid dairy products | Assay in a 96‐microwell plate, 4‐MUP as substrate, against a standard curve with 4‐MU of known concentration | Ziobro and McElroy (2013) | ||
| U = μmol/min | 4.1 | Dairy products (fluid and solid) | ISO/AWI TS 4985|IDF/RM 256 under development | ||||||
| NR | 0.006% raw milk | NR | Purified ALP | Reaction of purified ALP from calf with substrate for 30 min at pH 7.9 or 9.6 at 37°C | Fernley and Walker (1965) | ||||
| Ex 405/Em 519 | S: trifluoromethyl‐b‐umbelliferone phosphate P: trifluoromethyl‐b‐umbelliferone | Relative fluorescent units | 0.04% raw milk | 1.5 | Liquid and solid samples especially high fat products | Reverse micellar media (microemulsions) used for determination of enzyme activity with the non‐fluorescent substrate. | Fenoll et al. (2002) | ||
| Colorimetric | Dependent on substrate | S: phenyl phosphate, phosphate, sodium phenyl phosphate P: phenol | 1 μg phenol/min per L | 0.1–0.2% raw milk | NR | Whole milk, skim milk, chocolate milk | ALP activity equimolar with release of phosphate from substrate, reaction of phenol with colorimetric compound, Folin–Ciocalteu or 2,6‐dibromoquininechloroimide (BQC) | Shakeel‐ur‐Rehman et al. (2003) | Previous methods: Scharer (1938), ISO 3356:2009, AOAC 972.17 |
| 620 nm | S: phenyl phosphate P: phenol | μg of phenol released/h | NR | NR | Liquid samples | MFO‐3 method: kinetic measurement during 1 h with phenyl phosphate, colour reaction with phenol: CQC (2,6‐dichloroquinone‐4‐chlorimide, incubated for 15 min | MFO‐3 (1981) | Official method in Canada | |
| NA | 1 μg phenol/mL = 500 U/L | 6.7 × 10−14 mol/L Phenol = 6.31 × 10−6 μg phenol/L | 4.5 | Liquid milk | Electrochemical determination, Graphite–Teflon composite tyrosinase biosensor monitors by ALP produced phenol through reduction of o‐quinone | Serra et al. (2005) | |||
| Blue – green | S: p‐Nitrophenol phosphate P: phenol | pos/neg | NR | qualitative > 0.5 units/L | Liquid milk from buffalo, cow and goat | Visual inspection Rapid test with using this principle: on strip immobilised p‐nitrophenyl phosphate reacts with ALP and produces p‐nitrophenol that reacts with a chromogen producing a colour change | Sharma et al. (2003) | IDF 82A, 1987 and available as rapid test with Dry‐reagent strips | |
| NA | S: 5‐bromo‐4‐hloro 3‐indolyl phosphate (BCIP) P: phenol | pos/neg | % of raw milk: n.d. (0.87 U/mL based on SD) | < 5.1 | Milk | Biosensor, miniaturised: digital image colorimetry with smartphone. ALP antibody immobilised on paper, substrate BCIP | Mahato and Chandra (2019) | ||
| Chemiluminescent | 540 nm | S: 3‐(2′‐spiroadamantanane)‐4‐methoxy‐4(3″‐phosphate phenyl‐1,2 dioxetane disodium salt (Charm reagent®) AP P: adamantly1,2‐dioxetan | U = μmol/min | 0.05–0.2% raw milk | 7.5 | Liquid milk from cow, goat and sheep, flavoured drink and cream | Photo‐activation of hydrolysed product (chemiluminescent), kinetic stop reaction, Charm ALP‐Paslite, Chemi‐F‐AP | Albillos et al. (2011); ISO 22160:2007; Salter and Fitchen (2006) | Charm Test, ISO‐22160:2007, Rapid test: Chemi‐F‐AP |
ALP: alkaline phosphatase; Ex: Excitation; Em: Emission; RSDR: relative standard deviation of reproducibility; NA: not applicable; NR: not reported; neg: negative; pos: positive; SD: Standard deviation.
When species were indicated it was added to the table, otherwise there was no indication in the reference.
Different official methods are accepted in individual countries and most of them are based on spectrophotometric phenol determination after dephosphorylation of different substrates (i.e. phenyl phosphate, phenolphthalein phosphate, sodium phenyl‐phosphate, p‐Nitrophenol phosphate or 5‐bromo‐4‐chloro 3‐indolyl phosphate) by ALP (Table 3, colorimetric methods). These methods have a detection limit of 0.1–0.2% of raw cows’ milk in pasteurised milk which is above the legal limit for ALP activity after pasteurisation. The units are defined in mg of phenol released per minute per litre of milk (ISO 3356:200914; Scharer, 1938; MFO‐3, 1981; Serra et al., 2005). Some of the methods based on phenol release are only qualitative methods (Sharma et al., 2003; Mahato and Chandra, 2019).
The methods based on phenol were mostly replaced by more sensitive fluorescence methods with a detection limit of 0.006% of raw milk in pasteurised milk, such as the ISO 11816‐1:20133 and ISO 11816‐2:201612 standard, which are the current reference methods as described by Rocco (1990). These methods measure ALP activity in mU/L of milk using the specific substrate fluoro‐yellow. The reference methods (Fluorophos method, Advanced Instruments, Inc. Needham Heights, MA) as well as the chemiluminescent method (Charm Test) described below are proprietary methods and depend on instruments and consumables that are provided by one specific company.
One additional official method approved by FDA and ISO is based on chemiluminescence, with a sensitivity to detect 0.05–0.2% of raw cows’ milk. These methods are based on the substrate 3‐(2′‐spiroadamantanane)‐4‐methoxy‐4‐(3″‐phosphate)‐phenyl‐1,2 dioxetane disodium salt, of which the phenoxide intermediate emits light after being dephosphorylated by ALP. The method is approved by FDA/NCIMS and ISO/IDF, it does not need sample preparation and is suitable for liquid samples. It is a proprietary method developed and commercialised by Charm Sciences (Inc., Lawrence, MA) as Charm ALP‐Paslite, and as a rapid test called Chemi‐F‐AP test (indicated under rapid tests), respectively. The upper limit for proper pasteurisation is set at 350 mU/L. The Charm ALP‐Paslites method is published as ISO standard 22160:2007,15 enzymatic photo activated system (EPAS) method.
The ISO 3356:200914 method is the former reference test method, and it is based on determining the ALP activity of milk by considering the release of μg of phenol from disodium phenylphosphate dehydrate per mL of sample. According to the standard, a result of ≥ 2.5 μg of phenol indicates a milk that has not been properly pasteurised. The MFO‐3 method (MFO‐3, 1981) is based on the release of phenol from the substrate phenyl phosphate which is measured at 620 nm after precipitation. Methods based on phenol release are still in use in different countries, although their limit of detection is above the limit of 350 mU/L, as defined by the reference method. This method is the official method in Canada for the determination of ALP activity. According to the standard, a result of 2 mg of phenol in two out of three tested samples of the same milk indicates milk that has not properly been pasteurised. Based on the same principle is the AOAC (Association of Official Agricultural Chemists) method (US), using the substrate phenyl phosphate (16.121‐16.122, 14th Ed.; 972.13, 15th Ed) or phenolphthalein monophosphate (16.116‐16.120, 14th Ed.; 972.17, 15th Ed.). In addition, the IDF method (IDF 82A/B:198716 ) specifies two approaches to determine ALP activity in liquid samples at a limit of 0.5% a) or in reconstituted samples at a limit of 0.2% b) of raw milk contamination, respectively. The method is also based on release of p‐nitrophenol by visual inspection a) or by spectrophotometric detection at 350 nm b). Results are indicated as positive or negative. A method comparison performed by Klotz et al. (2008) showed that raw milk detection limits (DL) for the MFO‐3 method were 0.051, 0.485 and 0.023% vs. DL for the Fluorophos method of 0.007, 0.07 and 0.004% for cow, goat or sheep milk, respectively.
3.3.1.2. Alternative methods
A fluorescence method based on the dephosphorylation of 4‐methyl‐umbelliferone‐phosphate was first published by Fernley and Walker (1965) for the analysis of purified ALP activity. A method with the same substrate was later adapted for liquid dairy products by Ziobro and McElroy (2013) and has now been further improved for the analysis of liquid and solid dairy products and will be published in the near future. This microplate method will also be published within ISO as a technical specification.17 The method is capable of detecting 0.006% of raw cows’ milk in pasteurised milk. It is an open method; the substrate is commercially available and the assay can be performed with any microplate fluorimeter that is equipped with filters at the corresponding wavelength and capable of performing kinetic measurements.
There are also rapid tests available:
A rapid test called Chemi‐F-AP, based on chemiluminescence, using the same measuring principle as ALP‐Paslite (Charm Test), described under official methods; and
Dry‐reagent strips (phenyl phosphate), based on phenol release as described under official methods.
Additional alternative methods are:
Biosensor: Electrochemical graphite–teflon composite tyrosinase that detects phenol released by ALP (colorimetric method, Table 3) (Serra et al., 2005); and
Digital image colorimetry (colorimetric method, Table 3): method principle based on antibody binding and reaction with 5‐bromo‐4-chloro 3‐indolyl phosphate (BCIP) as the substrate (Mahato and Chandra, 2019).
3.3.2. Use of ALP testing in milk, colostrum, dairy and colostrum‐based products from non‐bovine and/or bovine species
Information described in this section is based on the replies received through the questionnaire from 15 countries (see Appendix B).
ALP testing data are being collected in seven countries for milk from bovine species and in eight countries for cheese from bovine species. One country only performs ALP analysis for bovine milk and dairy products when a soft cheese product is intended to be exported to the USA. Data regarding ALP activity for milk or dairy products in non‐bovine species are collected by five countries (again in one country only for the export of cheese products). The main non‐bovine products investigated are goats’ milk, sheep milk, dairy products (cheese) from sheep's milk and dairy products (cheese) from goats’ milk (in descending order of frequency of the responses).
As common reasons for the lack of information regarding ALP activity data, the countries stated a low priority and a lack of central repositories for data collection. The verification of pasteurisation is mainly considered to be the responsibility of the FBOp. During checks, they need to be able to demonstrate pasteurisation (either by ALP activity or other methods).
Two countries could only provide semi‐quantitative or qualitative data of pasteurised milk, as testing is rarely performed. Another two countries were able to share quantitative ALP data in non‐bovine milk or milk products. Additionally, some data was obtained from PHE laboratories (see Section 2.1.3) and from a study described in Berger et al. (2008). These data are described in Section 3.3.3.2.
3.3.3. Limitations of ALP testing in milk, colostrum, dairy and colostrum‐based products from non‐bovine and/or bovine species
The general differences in the composition of milk from different animal species will be described in Section 3.3.3.1. This will be followed by listing those factors that impact on the ALP testing of milk, colostrum and their products derived from bovines as compared with those from sheep and goats. Finally, it will be evaluated whether limits of ALP in products from non‐bovine species can be proposed.
The Codex code for milk and milk products (CAC, 2004) includes several considerations to be made. It was remarked that ‘ALP can be reactivated in many milk products (cream, cheese, etc.). Also, microorganisms used in the manufacture may produce microbial phosphatase and other substances that may interfere with tests for residual phosphatase. Therefore, this particular verification method must be performed immediately after the heat treatment in order to produce valid results’ and that several factors influence the residual ALP levels and should be taken into account when interpreting the results: ‘Initial concentration in milk: the “pool” of ALP present in milk varies widely between different species and within species. As pasteurisation results in a log reduction of the initial level, the post‐pasteurisation residual level will vary with the initial level in the raw milk. Consequently, different interpretation according to origin of the milk is necessary and in some cases, the use of ALP testing to verify pasteurisation may not be appropriate. Fat content of the milk: phosphatase is readily absorbed on fat globules, thus the fat content in the product subjected to pasteurisation influences the result. Application of pre‐heating: the level of ALP is decreased with heat, such as at temperatures typically applied in separation and in thermisation’.
3.3.3.1. General differences in the composition of milk from different species
Milk composition varies depending on the species (e.g. cow, goat, sheep), the animal (breed, stage of lactation, digestive tract fermentations, udder infections) and feed (grain, energy and dietary protein intake, seasonal and regional effects) (EFSA BIOHAZ Panel, 2015). This may affect the inactivation of ALP during pasteurisation. Table 4 gives an overview of the composition of mature milk from different animal species. It shows that there are striking differences between milk from different animal species.
Table 4.
Physico‐chemical parameters of mature milk from different mammalian species (Claeys et al., 2014)
| Ruminants | Non‐ruminants | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituents | Cow | Sheep | Goat | Buffalo | Yak | (Rein)deer | Camel | Llama | Horse | Donkey | |
| Total dry matter (g/L) | 118–130 | 181–200 | 119–163 | 157–172 | 135–184 | 201–271 | 119–150 | 131 | 93–116 | 88–117 | |
| Proteins (g/L) | 30–39 | 45–70 | 30–52 | 27–47 | 42–59 | 75–130 | 24–42 | 34–43 | 14–32 | 14–20 | |
| Casein/whey ratio | 4.7 | 3.1 | 3.5 | 4.6 | 4.5 | 4–5 | 2.7–3.2 | 3.1 | 1.1 | 1.28 | |
| Fat (g/L) | 33–54 | 50–90 | 30–72 | 53–90 | 53–95 | 102–215 | 20–60 | 27–47 | 3–42 | 3–18 | |
| Lactose (g/L) | 44–56 | 41–59 | 32–50 | 32–49 | 33–62 | 12–47 | 35–51 | 59–65 | 56–72 | 58–74 | |
| Ash (g/L) | 7–8 | 8–10 | 7–9 | 8–9 | 4–10 | 12–27 | 6.9–9 | 5–9 | 3–5 | 3–5 | |
| Total casein (g/L) | 24.6–28 | 41.8–46 | 23.3–46.3 | 32–40 | 34.3–45.8 | 70–80 | 22.1–26 | NA | 9.4–13.6 | 6.4–10.3 | |
| αS1‐casein (g/L) | 8–10.7 | 15.4–22.1 | 0–13.0 | 8.9 | 9.3–13.1 | NA | NA | NA | 2.4 | present | |
| αS2‐casein (g/L) | 2.8–3.4 | NA | 2.3–11.6 | 5.1 | 3.6–6.5 | NA | NA | NA | 0.2 | present | |
| β‐casein (g/L) | 8.6–9.3 | 15.6–17.6 | 0–29.6 | 12.6–20.9 | 15.0–20.6 | NA | NA | NA | 10.66 | present | |
| κ‐casein (g/L) | 2.3–3.3 | 3.2–4.3 | 2.8–13.4 | 4.1–5.54 | 4.9–8.5 | NA | NA | NA | 0.24 | present | |
| γ‐casein (g/L) | 0.8 | NA | NA | NA | NA | NA | NA | NA | present | NA | |
| Total whey protein (g/L) | 5.5–7.0 | 10.2–11 | 3.7–7.0 | 6 | NA | 13.4 | 5.9–8.1 | NA | 7.4–9.1 | 4.9–8.0 | |
| β‐lactoglobulin (g/L) | 3.2–3.3 | 6.5–8.5 | 1.5–5.0 | 3.9 | 3.4–10.1 | NA | NA | NA | 2.55 | 3.3 | |
| α‐lactalbumin (g/L) | 1.2–1.3 | 1–1.9 | 0.7–2.3 | 1.4 | 0.2–1.7 | NA | 0.8–3.5 | NA | 2.37 | 1.9 | |
| Serum albumin (g/L) | 0.3–0.4 | 0.4–0.6 | NA | 0.29 | 0.2–2.1 | NA | 7–11.9 | NA | 0.37 | 0.4 | |
| Immunoglobulin (g/L) | 0.5–1.0 | 0.7 | NA | NA | NA | NA | 1.5–19.6 | NA | 1.63 | 1.3 | |
NA: not available.
The basic composition of goats’ milk is almost similar to that of cows’ milk. While cows’ milk is fairly constant in composition, goats’ milk and sheep's milk show greater variations due to variations in feeding condition, environmental conditions, season and stage of lactation and due to breed differences. In some cases, the dry matter content of goats’ milk is higher; this is the result of a higher protein level (up to more than 5%) and/or fat content (up to more than 7%). Sheep milk is generally higher in total solids (up to 20%), especially due to a higher fat (up to 9%) and protein content (up to 7%).
The dry matter content of buffalo milk is notably higher than that of cows’ milk, mainly due to a higher casein and fat content. Reindeer milk is notable for its very high fat and protein content. Horse and donkey milk have a lower protein content with less casein. The fat content of this milk is also lower than that of ruminant milk, while the lactose content is slightly higher. In contrast to the casein content, the total whey protein content of the different species is similar. With the exception of milk from camelids, β‐lactoglobulin (β‐Lg) is the most abundant whey protein.
3.3.3.2. Initial ALP levels of raw milk from different species
The initial ALP concentration (referred to as basal level in the report) is one of several factors that influence the residual ALP levels in milk. As mentioned by the Codex code for milk and milk products (CAC, 2004) ‘the “pool” of ALP present in milk varies widely between different species and within species. Typically, raw cow's milk shows an activity much higher than goat's milk. As pasteurization results in a log reduction of the initial level, the post‐pasteurization residual level will vary with the initial level in the raw milk’.
The variation of the basal ALP level between different species and within species is dependent on breed, season and individual factors. The mean reported basal ALP levels [mU/L] in milk from different species based on the ISO Fluorophos method have been listed in Table D.1 in Appendix D and summarised in Table 5 and Figure D.1. This illustrates that raw ovine milk has the highest and raw caprine milk the lowest basal ALP content among the milk types, as reported by Assis et al. (2000), Vamvakaki et al. (2006), Klotz et al. (2008) and Lorenzen et al. (2010), comparing the ALP levels in milk from three species (cow, sheep and goat). Overall, the ALP activity in ovine milk seems to be about three times higher and in caprine milk about five times lower as compared to bovine milk.
Table D.1.
Examples of the reported basal ALP levels [mU/L] in milk from different species based on the ISO Fluorophos method
| Species | Breed | Mean | SD | Min | Max | Tested samples | Reference |
|---|---|---|---|---|---|---|---|
| Cow | NA | 330,000 | NA | NA | NA | NA | Assis et al. (2000) |
| Holstein Friesian | 774,000 | 260,000 | 328,000 | 1,155,000 | 18 | Lorenzen et al. (2010) | |
| NA | 390,000 | NA | NA | NA | 3 | Vamvakaki et al. (2006) | |
| NA | 577,511 | NA | 406,825 | 862,500 | 25 | Klotz et al. (2008) | |
| Holstein cows | 805,833 | 8,422 | NA | NA | 4 | Marchand et al. (2009) | |
| Holstein cows | 1,050,267 | 10,683 | NA | NA | 20 | Marchand et al. (2009) | |
| Holstein cows | 740,433 | 7,366 | NA | NA | 75 | Marchand et al. (2009) | |
| Holstein cows | 855,033 | 3,301 | NA | NA | 70 | Marchand et al. (2009) | |
| Mix | 764,633 | 19,092 | NA | NA | NA | Marchand et al. (2009) | |
| NA | 760,417 | NA | 546,350 | 984,700 | 3 | Rola and Sosnowski (2010) | |
| Goat | NA | 95,000 | NA | NA | NA | NA | Assis et al. (2000) |
| German Improved Fawn goats | 67,000 | 29,000 | 30,000 | 144,000 | 17 | Lorenzen et al. (2010) | |
| NA | 134,000 | NA | NA | NA | 3 | Vamvakaki et al. (2006) | |
| NA | 61,361 | NA | 29,665 | 131,494 | 21 | Klotz et al. (2008) | |
| Gemsfarbige. Gebirgsziege, Pfauenziegen, Saaneziegen, Toggenburger Ziegea | 50,211 | 92,126 | 8,983 | 421,100 | 19 | Berger et al. (2008) | |
| Gemsfarbige. Gebirgsziege, Pfauenziegen, Saaneziegen, Toggenburger Ziegeb | 341,988 | 513,933 | 35,319 | 1,785,400 | 16 | Berger et al. (2008) | |
| Gemsfarbige. Gebirgsziege, Pfauenziegen, Saaneziegen, Toggenburger Ziegec | 322,941 | 484,861 | 52,590 | 2,080,600 | 18 | Berger et al. (2008) | |
| NA | 135,861 | NA | 7,840 | 863,800 | 401 | IZSLT (2020) | |
| NA | 21,546 | NA | 21,546 | 21,546 | 2 | Rola and Sosnowski (2010) | |
| Sheep | NA | 2,200,000 | NA | NA | NA | NA | Assis et al. (2000) |
| East Friesian Dairy Sheep | 1,414,000 | 497,000 | 722,000 | 2,691,000 | 16 | Lorenzen et al. (2010) | |
| NA | 2,430,000 | NA | NA | NA | 3 | Vamvakaki et al. (2006) | |
| NA | 1,216,358 | NA | 911,800 | 1,616,600 | 20 | Klotz et al. (2008) | |
| Lacaune Schaf, Ostfriesisches. Milchschafa | 1,743,880 | 679,983 | 421,100 | 2,591,800 | 10 | Berger et al. (2008) | |
| Lacaune Schaf, Ostfriesisches. Milchschafb | 2,813,700 | 999,765 | 1,747,800 | 4,530,800 | 10 | Berger et al. (2008) | |
| Lacaune Schaf, Ostfriesisches. Milchschafc | 2,575,933 | 1,180,675 | 405,460 | 4,140,000 | 8 | Berger et al. (2008) | |
| NA | 2,685,757 | NA | 662,000 | 6,953,000 | 293 | IZSLT (2020) | |
| Buffalo | NA | 1,184,846 | NA | 71,780 | 3,434,000 | 485 | IZSLT (2020) |
| Camelids | Dromedary (Camelus dromedarius), first triald | 15,900 | 700 | 14,800 | 17,400 | 12 | Lorenzen et al. (2011a) |
| Dromedary (Camelus dromedarius), second triald | 21,000 | 1,200 | 18,900 | 22,900 | 15 | Lorenzen et al. (2011a) | |
| Dromedary (Camelus dromedarius), second triald | 24,300 | 1,600 | 22,000 | 26,000 | 15 | Lorenzen et al. (2011a) | |
| Dromedary (Camelus dromedarius), second triale | 25,000 | 2,000 | 22,000 | 29,000 | 15 | Lorenzen et al. (2011a) | |
| NA | 12,700 | 1,600 | 9,900 | 14,200 | 6 | Wernery et al. (2006) | |
| Solipeds | Donkey | 36,047 | 1,009 | NA | NA | 3 | Giacometti et al. (2016) |
| Belgian ‘Brabant’ draft horses | 8,077 | 40 | NA | NA | 20 | Marchand et al. (2009) | |
| Haflinger horses | 3,371 | 207 | NA | NA | 10 | Marchand et al. (2009) | |
| Haflinger horses | 3,517 | 94 | NA | NA | 20 | Marchand et al. (2009) | |
| New Forest Pony | 6,604 | 119 | NA | NA | 15 | Marchand et al. (2009) | |
| New Forest Pony | 20,814 | 119 | NA | NA | 13 | Marchand et al. (2009) | |
| Mix | 3,121 | 57 | NA | NA | 10 | Marchand et al. (2009) | |
| Mix | 6,079 | 49 | NA | NA | 13 | Marchand et al. (2009) | |
| Mix | 11,423 | 172 | NA | NA | 8 | Marchand et al. (2009) |
ALP: Alkaline Phosphatase; Min: minimal value; Max: maximum value; NA: not available; SD: standard deviation.
Spring.
Summer.
AUTUMN.
Analysed after thawing of frozen camel milk samples.
Analysed from fresh camel milk samples.
Table 5.
Summary of the mean reported basal ALP levels [mU/L] in milk from different species based on the ISO Fluorophos method (i.e. ISO 11816‐1:2013 standard)
| Species | ALP level [mU/L] | References | |||
|---|---|---|---|---|---|
| Mean | 95% CI | Min | Max | ||
| Cow | 704,800 | 550,200–859,500 | 330,000 | 1,050,300 | Assis et al. (2000), Vamvakaki et al. (2006), Klotz et al. (2008), Marchand et al. (2009), Lorenzen et al. (2010), Rola and Sosnowski (2010) |
| Goat | 136,700 | 46,600–226,700 | 21,500 | 342,000 | Assis et al. (2000), Vamvakaki et al. (2006), Berger et al. (2008), Klotz et al. (2008), Lorenzen et al. (2010), Rola and Sosnowski (2010), IZSLT (2020) |
| Sheep | 2,135,000 | 1,629,000–2,641,000 | 1,216,000 | 2,814,000 | Assis et al. (2000), Vamvakaki et al. (2006), Berger et al. (2008), Klotz et al. (2008), Lorenzen et al. (2010), IZSLT (2020) |
| Buffalo | 1,185,000 | NA | NA | NA | IZSLT (2020) |
| Camelids | 16,530 | 6,140–26,930 | 12,700 | 21,000 | Wernery et al. (2006), Lorenzen et al. (2011a) |
| Solipeds | 11,010 | 2,620–19,390 | 3,120 | 36,059 | Marchand et al. (2009), Giacometti et al. (2016) |
ALP: alkaline phosphatase; Mean: mean of the mean values reported in various studies; 95% CI: 95% confidence interval of the mean values reported in various studies; Min: minimum of the mean values reported in various studies; Max: maximum of the mean values reported in various studies; NA: not applicable as there is only one study considered.
Figure D.1.
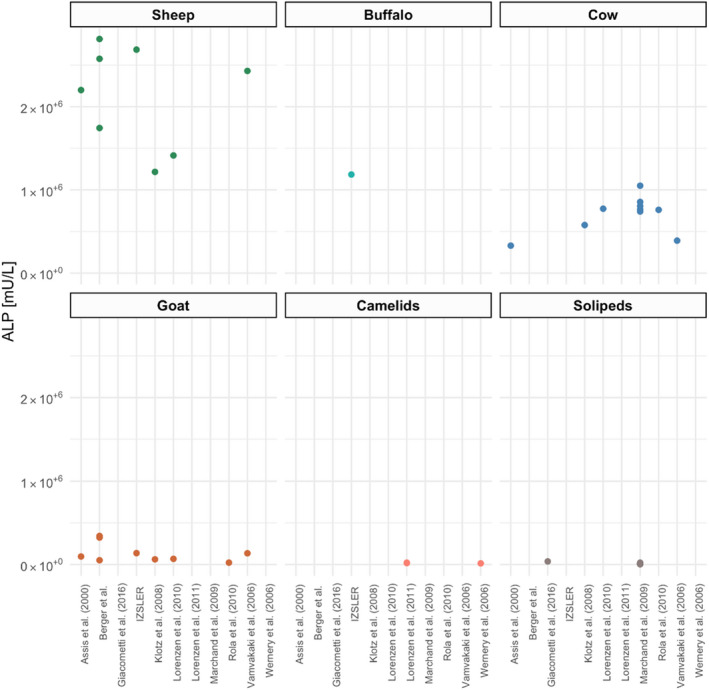
Overview of mean ALP values from studies included in the assessment (Table 5)
Berger et al. (2008) demonstrated that differences in the basal levels of ALP in raw milk from sheep and goats were variable between breeds and also during the year, being lower in spring and autumn and higher in summer. This could be due to seasonal effects as well as the stage of lactation (which influences the milk composition and yield) and can be linked to seasonal breeding practices. Ying et al. (2002) and Persson et al. (2014) reported differences in ALP in goat's milk depending on the stage of lactation, with ALP increasing in late lactation. A significant difference in ALP values depending on the lactation phase was also observed in sheep and goats’ milk, with an increase in the final months of lactation for sheep and a difference in April and May, as well as October and November, as compared to other months, for goats (IZSLT, 2020).
Vamvakaki et al. (2006) reported that the milk of individual cows can differ by as much as 40‐fold in ALP content. The presence of subclinical infection was found to increase ALP activity in sheep and goats (Katsoulos et al., 2010; Narenji Sani et al., 2018), while Patil et al. (2015) found higher ALP levels in milk from mastitic buffaloes and Ali et al. (2016) similarly found increased levels in milk from camels with mastitis infections.
3.3.3.3. Influence of milk fat
The CAC (2004) reported that ‘phosphatase is readily absorbed on fat globules, thus the fat content in the product subjected to pasteurization influence the result’. This was demonstrated by Painter and Bradley (1997) with increasing fat levels resulting in increased residual ALP activity. Since the ALP in bovine milk is reported to be associated with the milk fat globule membrane, ALP is concentrated in the cream phase (Kosikowski, 1988). In raw bovine milk, about 40% of the ALP activity is associated with the fat fraction (Painter and Bradley, 1997; Marchand et al., 2009). As a consequence, raw skimmed milk has much lower ALP activity. Sharma et al. (2009) found that ALP activity in skimmed cows’ milk was 47% of that in whole milk and 5% of that in cream, while ALP activity in skimmed goats’ milk was 56% of that in whole milk and 10% of the levels in cream. Similarly, Dumitraşcu et al. (2014) demonstrated that ALP activity in skimmed milk was 63%, 70% and 59% of that in whole milk in sheep, goats’ and cows’ milk, respectively. As a result of this difference in activity, the limit of 350 mU/L is too high for skimmed milk. On the other hand, as the ALP activity in raw cream is much higher than in raw milk, in this case the limit should be higher than 350 mU/L.
The fat content has further been shown to be positively correlated with ALP levels in ovine (Anifantakis and Rosakis, 1983; IZSLT, 2020) and pasteurised caprine milk (as provided by Dr. Gilberto Giangolini (Istituto Zooprofilattico Sperimentale del Lazio e della Toscana ‘M. Aleandri’, IZSLT) by e‐mail on 14 December 2020). Especially for ovine and caprine milk, the fat content varies with the lactation phase, similar to other milk components (see Section 3.3.3.1). This is not observed in the case of equine milk where there is no specific association of ALP with the fat fraction or milk fat globular membrane (Marchand et al., 2009).
3.3.3.4. Interfering compounds and factors
Other substances may interfere with tests for residual ALP levels as reported by CAC (2004). An overview was presented by Rankin et al. (2010). Colorimetric ALP assays, such as the methods based on phenol and phenol derivatives (see Table 3), suffer from interference by coloured dairy products such as strawberry milk. Moreover, phenolic moieties from antibiotic residues of oxytetracycline and penicillin can give false‐positive results with colorimetric tests (Manolkidis et al., 1971). False‐positive results can also be caused by additives with reactive phenolic groups, such as vanillin (when oxidised to vanillic acid), ρ‐hydroxybenzoic acid and salicylic acid (Murthy et al., 1992). In some cases, inhibition of ALP can be observed. Flavonoids, saccharides (Kuzuya et al., 1982) and ascorbic acid can inhibit ALP activity (Miggiano et al., 1983) and probably some polyphenolic compounds present in cocoa too (Murthy et al., 1992). These compounds can also be present in milk from sheep and goats and result in similar problems.
Also, the thermal stability of ALP can be affected. Sodium chloride can reduce the thermal stability of ALP (Linden, 1979), while increased lactose concentration increases the thermal stability of ALP (Sanders et al., 1954). This potential heat stabilisation effect of sugar may be of interest due to the different compositions of milk from different species (see Table 4). A high lactose content, as in mares’ milk (6.4%), could influence the heat stability of ALP in addition to genetically determined structural differences of the homologous enzyme. Very high sugar concentration, between 10% and 30%, may be associated with adverse effects leading to higher denaturation rates of ALP during heat treatment (Wijayanti et al., 2014). ALP levels were shown to be negatively correlated to lactose content in sheep milk (IZSLT, 2020).
As is the case for almost all enzymes, ALP activity is also pH dependent. In cultured milk and yoghurt, the enzyme can undergo an irreversible loss of activity at acidic pH (Murthy et al., 1992). ALP values in sheep milk were shown to be negatively correlated to pH (IZSLT, 2020).
3.3.3.5. Interference by microbial ALP
As mentioned by the CAC (2004), ‘microorganisms used in the manufacture may produce microbial phosphatase and other substances that may interfere with tests for residual phosphatase. Therefore, this particular verification method must be performed immediately after the heat treatment in order to produce valid results’. This can result in false positive results due to the presence of microbial ALP which usually has a higher thermal stability than bovine ALP.
This issue is especially relevant when milk has been stored for long periods prior to pasteurisation (IDF, 1991). To distinguish between microbial or bovine ALP, the American Public Health Association recommends the re‐pasteurisation of any positive sample (i.e. heating a portion of milk at 63°C for 30 min). If the ALP activity of the re‐pasteurised sample is not noticeably reduced (due to the activity of thermal stable ALP), it can be concluded that the original ALP assay result was due to the presence of bacterial ALP. This interference with the phosphatase test by microbial ALP, and the ability to distinguish microbial from mammalian ALP using a re‐pasteurisation process, is likely to relate to both bovine and non‐bovine milk products (provided that the ALP enzyme in non‐bovine species is less heat stable than the microbial enzyme). However, bacteria can produce both heat‐labile and heat‐stable ALP which complicates differentiating mammalian and microbial ALP (Knight and Fryer, 1989). Agarose gel electrophoresis could be used to differentiate between microbial and bovine ALP (Murthy and Kaylor, 1990).
A survey on ALP activity in the most representative cheeses from France, Italy and Switzerland showed that all pasteurised milk cheeses, except blue veined cheeses, had an activity < 10 mU/g of cheese applying the ISO 11816‐212 procedure. However, a clear distinction between raw and thermised cheeses was not possible (Egger et al., 2016). For two cheese types, ALP testing was not suitable as a pasteurisation marker. First, for blue veined cheeses because of phosphatases from moulds (Penicillium spp. and Aspergillus spp.) and second, for pasta‐filata cheeses, where the manufacturing process requires temperatures above 60°C, affecting ALP activity (Rosenthal et al., 1996; Kindstedt et al., 2004).
3.3.3.6. Reactivation of ALP
CAC (2004) stated that ‘ALP can be reactivated in many milk products (cream, cheese, etc.)… Therefore, this particular verification method must be performed immediately after the heat treatment in order to produce valid results’. This partial reactivation has been reported after UHT treatment, and therefore, although ALP may be tested as negative in just processed UHT milk, it may subsequently test positive in stored UHT milk (Deeth and Lewis, 2017). Reactivation is also frequently seen in high fat products such as cream. Lorenzen et al. (2010) demonstrated that UHT milk that was stored at ambient temperature for 148 days showed an increase in ALP activity from 20 to 454 mU/L in 3.5% fat milk and from 80 to 3,248 mU/L in 1.5% fat milk. The optimum storage temperature for reactivation is 30°C, at which reactivation can be demonstrated after 6 h and may continue to up to 7 days (Fox and Kelly, 2006).
Several authors have reported reactivation of ALP in milk or milk products after heating (Wright and Tramer, 1953; Lyster and Aschaffenburg, 1962; Murthy et al., 1976). However, this research could not establish a correlation between reactivation, t/T of pasteurisation, or post‐process storage time, but it has been concluded that milk pasteurised at temperatures higher than 71.7°C is more prone to ALP reactivation. Metallic ions (e.g. magnesium acetate) seem to play a role in the reactivation of ALP (Richardson et al., 1964; Kuzuya et al., 1982). Mg2+ and Zn2+ would stimulate ALP reactivation, whereas Co2+, Cu2+, EDTA, and Sn2+ may inhibit ALP reactivation (Sharma and Ganguli, 1974; Linden et al., 1977; Linden, 1979; Murthy and Peeler, 1979; Fox and Kelly, 2006). Rankin et al. (2010) described a test to verify whether a pasteurised product would give a false‐positive ALP assay due to reactivation. This is based on the increase in ALP activity resulting from the addition of Mg2+ to the reaction mixture, which can be used to determine whether an ALP level that exceeds the legal limit is likely to represent a genuine pasteurisation failure, or whether it is more likely to be due to reactivation. However, difficulties in the interpretation of this test may arise when applied to cream or butter.
3.3.3.7. Preheating of milk
CAC (2004) stated that ‘The level of alkaline phosphatase is decreased with heat, such as at temperatures typically applied in separation and in thermization’. Although thermised milk is ALP‐positive, the temperatures used for thermisation (57–68°C) can lead to some reduction in ALP activity, but the time is too short (5–20 s) to establish a clear effect. A similar effect can occur when centrifugal separation is used to separate milk into cream and skimmed milk. This can either be done at a temperature of about 50°C (hot milk separation) or at 10°C or lower (cold separation) but, even at the higher temperatures, the time needed for complete inactivation is not expected to be achieved.
3.3.3.8. Zonal differences in cheeses
Also, thermal treatments during processing, e.g. during scalding of cheese, may influence the results. Centripetal temperature gradients in typical raw milk big wheel cheeses may also have an influence. Examples of such cheeses are Grana Padano, Parmigiano Reggiano, Emmental, Gruyère and Sbrinz. These cheeses have in general a milk temperature for scalding above 50°C and after moulding. The heat load in the central part of the cheese will subsequently result in some decrease in ALP activity, leading to a gradient in ALP activity from the peripheral zone to the central part of the cheese. In certain cases, the ALP activity of such raw milk big wheel cheeses can even decrease below the limit for pasteurised cheeses, leading to false‐negative results (Pellegrino et al., 1997; Egger et al., 2016). In order to overcome this issue, the sample preparation of these cheeses needs to take into account these zonal differences. For example, in the ISO 11816‐212 standard, sampling of hard cheeses is precisely described and needs to focus on the peripheral zone of the cheese wheel. According to the survey on ALP activities in pasteurised cheeses that was performed in France, Italy and Switzerland, a target value of 10 mU/g of cheese was proposed. This target value was achieved by all tested pasteurised cheeses, except for blue veined cheeses and for pasta‐filata cheeses (Egger et al., 2016). The same can also be expected for cheeses made from goat or sheep milk, such as Pecorino.
3.3.3.9. Thermal stability of ALP in milk from different species
Although ALP is widely applied as an indicator of proper pasteurisation, only a few kinetic studies on thermal inactivation of ALP in raw bovine milk have been published. Thermal inactivation of ALP follows first‐order kinetics. According to Claeys et al. (2002), independently of the ALP assay applied, reported z‐values range between 5 and 6.7°C and between 8.5 and 9.5°C, depending on the source.
The thermal stability of ALP activity in raw milk derived from cows, sheep and goats has been compared in few publications. D‐values and z‐values were calculated, when relevant, to illustrate the variability between the studies on the inactivation of ALP in cows’ milk in comparison to sheep and goat milk (see Figure 2). Additional information can be found in Appendix E.
Figure 2.
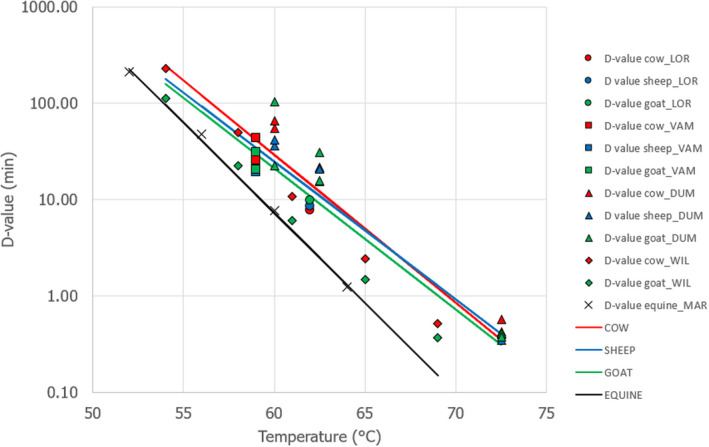
Vamvakaki et al. (2006) found that ALP inactivation was slower in bovine milk compared to that of ovine and caprine milk. They studied the decrease of ALP activity using a heat treatment at 59°C for 5–80 min of 5 mL portions of the three types of milk. Using the automated fluorimetric method (IDF 155A:1999; a currently withdrawn reference standard which has since been revised and updated18), the D59‐value was slightly higher in cows’ milk (25 min) compared to goat (20 min) and sheep milk (19 min). Using the IDF phenol method (IDF 63:1971, former reference method19), the D59 value was highest in cows’ milk (44 min) followed by goat's milk (31 min) and then sheep milk (21 min).
A study by Wilińska et al. (2007) indicated a different structure for ALP, which was reflected by a higher stability of the bovine milk enzyme compared to the caprine milk enzyme. The authors investigated the thermal inactivation of ALP in raw bovine and caprine milk in the temperature range of 54–69°C for 1–180 min using a thermostatic laboratory reactor equipped with a stirrer. On the basis of these results, D54, D58, D61, D65 and D69‐values for bovine and caprine milk could be calculated. D‐values were lower for goats’ milk (112, 22.5, 6.1, 1.5 and 0.37 min) compared to cows’ milk (233, 50.2, 10.8, 2.4 and 0.25 min) and the z‐value was higher for cows’ milk (6.0°C) compared to goat's milk (5.6°C).
Dumitraşcu et al. (2014) performed kinetic studies of ALP thermal inactivation using a fluorimetric technique in skimmed milk and whole milk derived from cows, sheep and goats using 100 μL glass capillary tubes immersed in a water bath at 60–72.5°C for 0–40 min. All experiments showed a large decrease in ALP activity with increasing temperature. At lower temperatures, the ALP inactivation was influenced by the fat content. For example, after 30 min treatment of whole milk at 60°C, the residual activity was higher in goat milk (44.8%) in comparison to sheep (21.7%) and cows’ (28.1%) milk. Applying the same heating conditions for skimmed milk, goat milk ALP presented the lowest residual activity (5.9%), followed by sheep milk (7.6%). In cows’ milk, the ALP activity was more than double (16.78%). At 72.5°C for 20 s, the ALP activity in whole and skimmed milk was reduced to 9.1 and 11.8% (sheep), to 18.5 and 19.0% (goat), and to 25.5 and 22.2% (cow). At these high temperatures, the ALP inactivation was not influenced by the fat content but ALP was shown to be more resistant in cows’ milk compared with goat milk (slightly) and sheep milk (double).
Klotz et al. (2008) evaluated the performance of the ISO Fluorophos method and the colorimetric assay (MFO‐3) for determining the ALP activity in raw and pasteurised milk derived from cows, sheep and goats. They showed a similar and dramatic reduction in ALP levels in milk of the three species at temperatures between 67.0°C and 72.5°C through pilot plant pasteurisation trials (60.0, 67.0, 72.5 and 74°C for 16 s) using 2 L samples. This confirmed the results from a study by Felipe et al. (1997) in which ALP was completely inactivated following pasteurisation of goats’ milk through a heat exchanger at 72°C at a flow rate of 500 L/h, with a holding time of 15 s, and an overall time of 2 min 30 s for warming, holding and cooling.
Lorenzen et al. (2010) compared the effects of isothermal heating of 9 mL samples in test tubes with different t/T combinations on the ALP residual activities in bovine, ovine and caprine milk. ALP showed a lower thermal stability in bovine milk than in sheep or goats’ milk. The percent of residual activity decreased after 90 s at 65°C to 2.6% in bovine milk, and to 43% and 55% in sheep's and goats’ milk, respectively. At 75°C and 85°C, only very small residual activities of ALP were found. After 75°C for 90 s, 0.06% of ALP activity was found in bovine milk, 2.7% in sheep's milk and 3.1% in goats’ milk. When the milk was heated to 85°C, residual ALP activities amounted to < 0.03% in milk from the three species. LTLT‐heating was performed in a batch process by heating 100 mL samples in a water bath at 62 ± 0.5°C for 30 min or 65 ± 0.5°C for 32 min with continuous stirring. It reduced the ALP activities in milk from the three mammals between 99.90% (62°C for 30 min) and 99.95% (65°C for 32 min). The residual ALP activities in goat milk samples were between 0.089% and 0.057% while in bovine and sheep milk were all below 0.03%. HTST‐heating (75°C for 28 s) performed in this study resulted in 99.99% inactivation of ALP in cows’ and sheep's milk, and a 99.90% inactivation in goats’ milk.
The few studies that have compared the thermal stability of ALP in milk derived from cows, sheep, and goats show conflicting evidence on whether ALP is more easily inactivated in cows’ milk compared to sheep and goat milk. In general, the results seem to illustrate that the heat inactivation of ALP from cow, goat and sheep milk is roughly similar and equine ALP is more heat sensitive (based on the trend lines in Figure 2). However, further investigation of the impacts of the expected large variations of the basal ALP levels intra‐ and inter‐species is required. Therefore, an in‐depth thermal inactivation kinetics study with different milk batches is recommended to obtain reliable data to derive the D and z values of ALP inactivation in the milk of these species. This study should be done using a method allowing a very rapid (almost instantaneous) heating to the isothermal temperature and cooling afterwards. It is recommended that D‐values are determined at five to six different temperatures in the range between 55°C and 70°C. At any chosen temperature, there should be at least five points (incubation times) on the linear part of the curve. These kinetic inactivation data, in combination with the determination of the initial enzyme activity, must provide definitive evidence on whether the marker enzyme could be used to guarantee the inactivation of pathogenic bacteria. A summary of this principle can be found in Appendix F.
Some studies have evaluated the thermal stability of ALP from camelids. For example, Lorenzen et al. (2011a) evaluated the residual activity of ALP in raw and pasteurised camel milk. The average ALP activities ranged between 15.9 and 24.3 U/L with raw milk and 5.8–10.2 U/L with pasteurised milk. Pasteurisation (75 ± 1°C, 15–30 s) was carried out using a plate heating exchanger with a capacity of 3000 L/h. The rates of inactivation due to pasteurisation were comparable with both analytical (fluorimetric and colorimetric) methods. The authors concluded that the residual activity of ALP in pasteurised milk revealed that ALP is not suitable to verify effective pasteurisation of camels’ milk.
Also, Wernery et al. (2006) concluded that ALP is not a suitable indicative endogenous marker enzyme for confirmation of camels’ milk pasteurisation using four different test systems (a fluorimetric, photometric and two colorimetric methods). For example, using the fluorimetric method, the initial ALP concentration (12.7 U/L) was reduced to 4.4 U/L after 30 s at 72°C, 4.3 U/L after 30 min at 72°C, 3.7 U/L after 30 s at 80°C and 0.1 U/L after 30 s at 90°C. All four tests showed that ALP is not completely inactivated at 72°C, the accepted temperature for HTST pasteurisation. In a later study, Wernery et al. (2008) compared the ALP inactivation in camel and cow milk, confirming that ALP cannot be used to evaluate the correct pasteurisation of camel milk as considerably greater temperatures are needed than those required to inactivate ALP in cows’ milk.
Marchand et al. (2009) evaluated the inactivation kinetics of ALP in raw whole equine milk by heating 58 μL portions in 100‐mL glass capillaries in a warm water bath. They derived the following values for D48, D52, D56, D60 and D64 in equine milk using a fluorimetric method: 998, 211, 47.8, 7.77 and 1.24 min and the z‐value was estimated as 5.31°C. The authors concluded that equine ALP is more readily inactivated in equine milk than its bovine counterpart, and, considering the rather low basal level of ALP in equine milk, that equine ALP will not be suitable as an indicator for correct pasteurisation of equine milk under the conditions currently used in the reference method for the determination of ALP in milk‐based products.
3.3.4. Limits of ALP in pasteurised milk of different animal species
The data from the two countries providing semi‐quantitative or qualitative data of pasteurised milk are first described. Using the ISO fluorimetric test, tested goats’ milk samples (total numbers unknown) had ALP activity < 350 mU/L, while the sheep milk samples (total numbers unknown) had either a value < 350 mU/L or > 1,500 mU/L (1 sample). Using a national reference method differing from the ISO fluorimetric test, one tested goat milk sample was positive and one tested whole goats’ milk sample was negative.
Another three countries were able to share quantitative ALP data for non‐bovine milk or milk products. Additionally, some data were obtained from PHE laboratories (Section 2.1.3) and from a study described in Berger et al. (2008). In total, ALP values from 2,761 cows’ milk samples, 606 goat milk, 86 sheep milk, 2 buffalo milk, 7 goat cheese and 5 sheep cheese samples tested with the ISO fluorimetric method were available for quantitative analysis. Overall, values ranged from 10 to 60,170 mU/L in bovine milk, 10 to 12,040 mU/L in non‐bovine milk and 1 to 143 mU/g in cheese. Provided data outside the limit of detection were capped for statistical analysis (737 samples < 10 mU/L are displayed as 10 mU/L and one sample > 7,000 mU/L and two samples > 20,000 are shown as 7,000 or 20,000 mU/L, respectively). A descriptive overview of the range and the distribution of the ALP values (calculated with log transformed data due to non‐normal distributions) can be found in Table 6. Figure 3 illustrates the data of the cows’, goats’ and sheep's pasteurised milk. In goat milk samples, 6 out of 606 (1%) showed an ALP activity > 350 mU/L. For sheep milk, there was a higher proportion of samples with values above this limit (21/86, 24.4%). In contrast, in the tested cows’ milk samples, this was the case for only 46 out of 2,761 samples (1.7%). The values for cheese are not presented in a figure because of the small number of samples tested. Of the few tested goat and sheep cheeses, 2/7 and 3/5 had an ALP value above the limit of 10 mU/g that was tentatively proposed for pasteurised bovine cheeses (see Section 3.3.1).
Table 6.
Testing of Alkaline Phosphatase (ALP) activity with the ISO fluorimetric method in pasteurised milk and dairy products of bovine and non‐bovine origin as provided by five different countries
| Product | Species | ALPa | n above limitb/n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CI | Min | Max | Unit | |||
| Milk | Cow | 29 | 29 | 28–31 | 10 | 60,170 | mU/L | 46/2,761 (1.67%) |
| Milk | Goat | 47 | 45 | 44–50 | 10 | 7,000 | mU/L | 6/606 (1%) |
| Milk | Sheep | 181 | 192.1 | 136–240 | 10 | 12,040 | mU/L | 21/86 (24.4%) |
| Milk | Buffalo | 29.9 | 29.9 | 16 | 56 | mU/L | 0/2 | |
| Cheese | Goat | 7.9 | 8 | 3.7–17 | 2 | 24 | mU/g | 2/7 (28.6%) |
| Cheese | Sheep | 9.5 | 11 | 0.8–111 | 1 | 143 | mU/g | 3/5 (60%) |
ALP: alkaline phosphatase; CI: confidence interval; min: minimum value; max: maximum value; n: number of samples; cut‐off: 350 mU/L for milk or 10 mU/g for cheese.
The descriptive statistics were derived after log10 transformation of the quantitative data of ALP concentrations and presented in the table after back‐transformation.
Considering for milk the legal limit of 350 mU/mL for pasteurised bovine milk and for cheese the tentatively proposed limit for pasteurised bovine cheeses of 10 mU/g.
Figure 3.
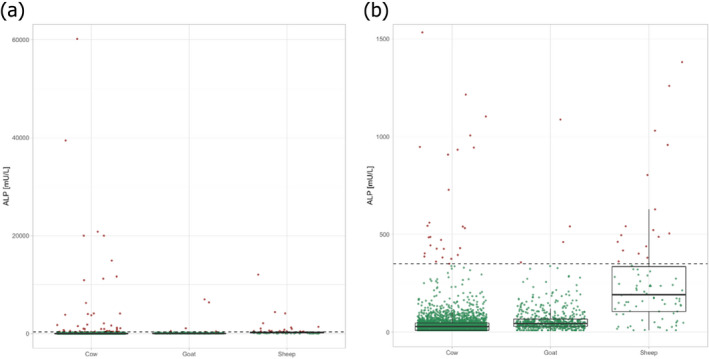
- The dashed line represents the legal limit for bovine milk of 350 mU/L.
In addition to the data obtained from MS from the questionnaire and provided by WG members, semi‐quantitative information about ALP activity in pasteurised milk and cheese from non‐bovine species has been extracted from the literature. Rola and Sosnowski (2010, 2011) tested in total 140 samples of goat milk and 31 samples of cheese products from retail stores, whereas the Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna tested raw buffalo, sheep and goat milk samples before and after pasteurisation at 63°C ± 0.5°C for 30 min (LTLT) (IZSLT, 2020). The values after pasteurisation are summarised in Tables 7 and 8. To obtain a complete overview, the quantitative data obtained were further converted into semi‐quantitative categories.
Table 7.
ALP values for pasteurised milk of various species tested with the ISO fluorimetric method as reported in the literature and as received by the different MS, presented in a semi‐quantitative way
| Species | Heating conditions | N | Percentage of samples with ALP [mU/L] values | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 100 | 100–200 | 201–300 | 301–350 | 351–400 | 401–500 | > 501–600 | 601–800 | 801–1,000 | > 1,000 | ||||
| Cow | NA | 2,761 | 93.3 | 4.1 | 0.7 | 0.2 | 0.2 | 0.3 | 0.1 | 0 | 0.1 | 0.9 | PHEc |
| Total cow | 2,761 | 93.3 | 4.1 | 0.7 | 0.2 | 0.2 | 0.3 | 0.1 | 0 | 0.1 | 0.9 | ||
| Buffalo | LTLTa | 421 | 23.0 | 50.8 | 20.9 | 4.5 | 0.7 | 0 | 0 | 0 | 0 | 0 | IZSLT (2020) |
| Buffalo | NA | 2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Questionnaire |
| Total buffalo | 423 | 23.5 | 50.8 | 20.9 | 4.5 | 0.7 | 0 | 0 | 0 | 0 | 0 | ||
| Goat | LTLTa | 157 | 55.4 | 35.7 | 7.6 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | IZSLT (2020) |
| Goat | NAb | 65 | 87.7 | 12.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Rola and Sosnowski (2010) |
| Goat | NAb | 75 | 86.7 | 13.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Rola and Sosnowski (2011) |
| Goat | NA | 606 | 85.1 | 11.4 | 2.0 | 0.5 | 0.2 | 0.2 | 0.2 | 0 | 0 | 0.5 | Questionnaire + PHEc + Berger et al. (2008)d |
| Total goat | 903 | 80.3 | 15.8 | 2.7 | 0.6 | 0.1 | 0.1 | 0.1 | 0 | 0 | 0.3 | ||
| Sheep | LTLTa | 290 | N/A | 15.9* | 50.3 | 20 | 8.62 | 4.5 | 0.7** | IZSLT (2020) | |||
| Sheep | NA | 86 | 24.4 | 27.9 | 18.6 | 4.7 | 2.3 | 7.0 | 3.5 | 1.2 | 2.3 | 8.1 | Questionnaire + PHEc + Berger et al. (2008)d |
| Total sheep | 376 | 5.6 | 18.6 | 43.1 | 16.5 | 7.2 | 5.1 | 1.3 | 0.3 | 0.5 | 1.9 | ||
NA: details about the heat treatment method or conditions used are not available; N/A: not applicable; N: number of samples tested; *< 200 mU/L; ** > 500 mU/L.
The raw milk samples collected between 2017 and 2020 have been pasteurised at laboratory scale using 63°C ± 0.5°C for 30 min.
Samples of pasteurised milk taken from retail stores.
These data are from routine samples and may contain samples that were not correctly pasteurised.
Samples have been pasteurised but the conditions used are unknown.
Table 8.
ALP values for cheese from pasteurised non‐bovine milk tested with the ISO fluorimetric method as reported in the literature and as received by the different MS, presented in a semi‐quantitative way
| Species | N | Percentage of samples with ALP [mU/g] values | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| < 5 | 5–10 | <=10 | 11–50 | 51–100 | > 100 | |||
| Goat | 14 | NA | NA | 100 | 0 | 0 | 0 | Rola and Sosnowski (2010) |
| Goat | 17 | 88 | 12 | 100 | 0 | 0 | 0 | Rola and Sosnowski (2011) |
| Goat | 7 | 14 | 57 | 71 | 29 | 0 | 0 | Questionnaire |
| Total goat | 38 | NA | NA | 94.7 | 5.3 | 0 | 0 | |
| Sheep | 5 | 40 | 0 | 40 | 40 | 0 | 20 | Questionnaire |
| Total sheep | 5 | NA | NA | 40 | 40 | 0 | 20 | |
N/A: not applicable.
Several authors confirmed that for goats’ milk, ALP activity would be useful as an indicator for correct pasteurisation (Berger et al., 2008; Klotz et al., 2008); however, the limit for proper pasteurisation most probably would need to be adapted (Banks and Muir, 2002). The same situation was observed with ovine milk, where pasteurisation reduced ALP activity, but was not below 350 mU/L after pasteurisation (Vamvakaki et al., 2006; Berger et al., 2008).
Based on distribution of the residual ALP levels after pasteurisation (63°C, 30 min), some authors (IZSLT, 2020) have suggested limits in the milk from various species.
Buffalo milk ≤ 380 mU/L
Goat milk ≤ 330 mU/L
Sheep milk ≤ 530 mU/L
This was based on the Eyeball method (‘that for buffaloes 3 out of 421 samples (0.7%) showed values higher than 350 mU/L, for goats only 2 out of 157 samples (1.2%) showed values higher than 300 mU/L and for sheep milk only 2 samples out of 290 (0.7%) recorded values higher than 500 mU/L’).
Combining the results of the IZSLT study with the additional evidence, it was confirmed that overall 1.2% and 0.6% of the samples of pasteurised goats’ milk would be expected to have residual ALP values above 300 mU/L and 350 mU/L, respectively. For sheep milk, the evidence is more variable. Combining the results of the IZSLT study with the additional evidence, 4.0% of the samples of pasteurised sheep milk would be expected to have residual ALP values above 500 mU/L and 2.7% above 600 mU/L. In comparison, in bovine milk, 1.6% of the samples had a residual ALP value above the legal limit of 350 mU/L.
Assuming that the pathogen inactivation would be the same in the milk of different species, and based only on the available evidence, there is 95–99% probability (extremely likely) that pasteurised goat milk and pasteurised sheep milk would have an ALP activity below 300 mU/L and below 500 mU/L, respectively. Nevertheless, it is recommended to gather further data from other countries and small ruminant populations, in particular for sheep milk, in order to conclude whether the evidence now available is representative of all situations. This is mainly because details regarding the actual heat treatment method or conditions used are not available for part of the evidence used. In addition, information is lacking about the different breeds of goats and sheep in the milk used in those studies and therefore also the impact of breed variability on this limit needs to be assessed.
For equine and camel milk, ALP does not appear to be a good indicator for pasteurisation, due to very low activities measured in raw milk (Marchand et al., 2009; Lorenzen et al., 2011a) and a high thermo‐stability of camel ALP (Wernery et al., 2006, 2008).
For milk of other species, the ALP limit values also need to be verified experimentally (as described in Appendix F), due to differences in species‐specific ALP abundance and heat inactivation properties.
3.3.5. Concluding remarks
-
Apart from ISO standards (ISO 11816‐1:20133 for milk and milk‐based drinks and ISO 11816‐2:201612 for cheese), alternative analytical methods for ALP activity determination are available that were either developed previously (colorimetric methods based on phenol release) or have been validated against these reference methods (chemiluminescent methods, microplate fluorescent method).
-
o
The chemiluminescent methods have been validated for liquid products, whereas the microplate method has also been validated for solid products.
-
o
The Fluorimetric methods (ISO 11816‐1:2013 standard, 11816‐2:2016 and the alternative microplate method) have a sensitivity capable of detecting 0.006% of raw bovine milk within pasteurised milk and the chemiluminescent method (ISO 22160:200715) is slightly less sensitive, with a detection limit of 0.05–0.2% of raw bovine milk. In contrast, the previously developed methods based on phenol compounds (such as IDF 63, former reference method) are less sensitive, with a detection limit of 0.1–0.2% of raw bovine milk, corresponding to the release of 1 μmol phenol per min and being above the legal ALP limit of 350 mU/L.
-
o
To date, the alternative methods (chemiluminescent and microplate methods) have not been extensively tested in milk samples from non‐bovine species and further work to validate these methods is needed.
-
o
As all enzymatic assays are substrate‐specific, the comparability between methods cannot be based on units/L of milk. The percentage of contamination of pasteurised milk with raw milk could be used instead.
Five out of 15 countries replying to the questionnaire reported using ALP testing for milk or milk products from non‐bovine species, more specifically in goats’ milk, sheep's milk, cheese from sheep's milk and cheese from goats’ milk (in descending order of frequency of the responses).
The considerations made in the Codex code for milk and milk products also apply for milk and milk products from non‐bovine species and will influence the interpretation of results of ALP testing: e.g. reactivation of ALP, interference of microbial ALP and other substances with tests for residual phosphatase, basal level of ALP in milk, fat content of the milk and application of pre‐heating.
It is recommended in the Codex code that the ALP test must be performed immediately after heat treatment to produce valid results and that several factors that influence the residual ALP levels should be considered when interpreting the results.
Overall, the ALP activity in raw sheep milk (basal level) appears to be approximately three times higher and in caprine milk about five times lower compared to bovine milk. The basal level of ALP in raw milk from goats and sheep is highly variable between breeds and is influenced by season, lactation stage, fat content and udder health. Further variation of basal ALP levels within non‐bovine animal species is expected due to a greater variation in breeds of sheep, goats and equines compared to dairy cows.
The few studies that compared the thermal stability of ALP in milk derived from cows, sheep and goats show conflicting evidence on whether ALP is more easily inactivated in cows’ milk compared to sheep and goat milk. In general, the results seem to illustrate that the heat inactivation of ALP from cow, goat and sheep milk is roughly similar and equine ALP is more heat sensitive.
An in‐depth thermal inactivation kinetics study with different milk batches is recommended to obtain reliable data to derive the D and z‐values of ALP inactivation in the milk of these species.
ALP data of pasteurised milk showed that 0.6% of the 903 samples of pasteurised goats’ milk and 16.3% of the 376 samples of pasteurised sheep's milk were above the limit of 350 mU/L as specified for cows’ milk. In a comparable data set from cows’ milk, 1.6% of 2,761 samples had ALP values above this limit.
The ALP activity values of pasteurised products of sheep origin (both milk and cheese) are higher and tend to have more variability than the respective goat products.
Assuming that the inactivation of pathogens would be the same in the milk of different species, and based on the available evidence of milk samples after pasteurisation, there is 95–99% probability (extremely likely) that pasteurised goat milk and pasteurised sheep milk would have an ALP activity below a limit of 300 mU/L and 500 mU/L, respectively. Nevertheless, it is recommended to collect further data from other countries and small ruminant populations, in particular for sheep milk, in order to conclude whether the evidence now available is representative of all situations.
For equine milk, the current test sensitivity does not allow using ALP testing as the basal ALP activity is very low, while camel milk contains a heat stable ALP form, and therefore, ALP is not appropriate either.
The procedure for the evaluation of ALP (or other endogeneous marker enzymes) as an indicator of proper pasteurisation in milk of other species than bovine, considering both pathogen and enzyme inactivation, could be used to confirm the tentative limit (see Appendix C).
Few data are available for non‐bovine cheese, so these results must be interpreted with caution. Two out of the 38 cheese samples (5.3%) made from pasteurised goats’ milk and three of the five samples (60%) made from pasteurised sheep's milk were above the proposed limit for cheese from pasteurised cows’ milk of 10 mU/g. The data available for cheese of other species do not allow limits to be evaluated.
No data is available for colostrum or other dairy derived products such as yoghurt, ice cream, milk powder, cream or fermented milk.
3.4. Alternative methods for the verification of pasteurisation of milk, colostrum, dairy and colostrum‐based products from ewes and goats (ToR 2)
In this section, first the alternative testing to verify pasteurisation as currently used by the MS (i.e. temperature monitoring of the heat treatment equipment using data loggers and Enterobacteriaceae testing) has been described (see Sections 3.4.1–3.4.3), followed by a specific description of the evaluation of alternative potential methods, such as TTI. An overview of TTI for heat treatment of milk can be found in Claeys et al. (2002). They state that the assessment of different classes of heat treatment of milk can be performed by means of endogenous marker enzymes or milk compounds being either secondary products of heat treatment, changes in whey proteins, loss of natural milk constituents (vitamin destruction) or miscellaneous parameters (colour change, surface hydrophobicity and free sulfhydryl in milk). In the context of intrinsic TTIs, the assay of degradation, denaturation or inactivation of heat‐labile compounds (e.g. enzymes as summarised in Section 3.4.4 and whey proteins as summarised in Section 3.4.5), is a suitable tool for the evaluation of low‐heat treatments and the formation of ‘new’ substances is more effective for the assessment of processes involving high temperatures (see also Section 3.4.5).
3.4.1. Alternative testing to verify pasteurisation as currently used by the MS
The main alternative method to verify pasteurisation of milk, colostrum, dairy and colostrum‐based products from non‐bovine species as indicated by the replies to the questionnaire (mainly question 3b – see Appendix B) is temperature monitoring of the heat treatment using data loggers (as specified by 9 of the 15 countries). In four countries, the enumeration of Enterobacteriaceae is performed as an alternative approach. One country occasionally checks for ALP activity, however, not with the ISO method but by using a nationally accredited standard.
To verify the pasteurisation process, FBOps can use the services of different laboratories for ALP testing or alternative methods such as milk temperature monitoring (additionally combined with flow or pressure of the pasteurisation process). The latter appears to be used by many FBOps and documentation is checked by the CA during control visits.
One country reported that they do not use ALP activity to verify pasteurisation processes due to logistical reasons, since milk samples are often several days old and ALP can be re‐activated. Another country specifically recommends that FBOps use tests other than ALP because of the differences in inactivation in sheep and goats’ milk compared to cows’ milk. Similarly, from another country, it was pointed out that there are no limits for non‐bovine milk and cheeses.
3.4.2. Temperature monitoring of the heat treatment equipment using data loggers
As required by Regulation (EU) No 852/20047, the primary responsibility for food safety lies with the FBOp. In accordance with the hazard analysis and critical control points (HACCP) guidelines and good hygienic practices (GHP), most dairy manufacturers have established and implemented effective monitoring procedures at critical control points. As mentioned in Section 3.1.2, the pasteurisation can be performed in a batch process or using a heat exchanger.
In the EU, no approval of the different constructions and types of plants or the technical requirements for recording the process parameters is required. Different data loggers for monitoring time and temperature are commercially available, but they have to be positioned correctly and calibrated regularly. Smaller dairies, where milk from other species is often processed, sometimes have difficulties in fulfilling the recommendations and standards as described e.g. by the Food and Drug Administration (FDA, 2017) for electronic recording. Monitoring of milk temperature during heating is more reliable in continuous flow equipment than in the batch process, where heat does not reach so homogeneously all spots.
The integrity of the plates or seals of the heat exchanger needs evaluation regularly as pinholes may appear in the plates of older heat exchangers. This may lead to pasteurised milk becoming recontaminated e.g. if such plates are in the regeneration section, a cracked or leaking plate may allow raw milk to contaminate already pasteurised milk. Possible recontamination of pasteurised milk with raw or partially treated product has to be avoided and checked by regularly testing the plates for pinhole leaks or by ensuring that if leaks do occur, they do so in a safe fashion, such that pasteurised milk is not contaminated with cooling water or raw milk in the regeneration section. This can be achieved by making sure that the pressure on the treated milk side (downstream of the holding tube) is higher than on the water side, or on the raw milk side in the regeneration section. The control instrumentation, diversion valves and other valves should be checked regularly (Deeth and Lewis, 2017). Process failures leading to defective pasteurisation may result in a contaminated product. High humidity and excessive condensation in dairy plants are major sources of post‐pasteurisation contamination. Pathogens may also contaminate milk after pasteurisation through microbial biofilms in distribution pipes and stainless‐steel surfaces, aerosols, unhygienic handling practices or the use of contaminated containers and other post‐pasteurisation equipment/materials (Alegbeleye et al., 2018; Martin et al., 2018).
Therefore, the use of data loggers can monitor the heat treatment applied over time and thus can detect that the required t/T profile has not been achieved. The use of data loggers cannot detect recontamination of pasteurised milk due to other process failures other than t/T profiles while ALP testing can achieve this.
3.4.3. Enumeration of bacterial hygiene indicators
The presence of elevated numbers of ‘indicator bacteria’ such as Enterobacteriaceae in pasteurised milk and dairy products may demonstrate that either pasteurisation has not been adequate or post‐pasteurisation contamination has occurred. These organisms are likely to be present in relatively high numbers in raw milk but are killed by heat processes such as pasteurisation. They are comparatively quick and easy to grow and identify in the laboratory, with a result usually available after 24–48 h. Regulation (EC) No 2073/200511 specifies a limit of 10 CFU/mL for Enterobacteriaceae in pasteurised milk and other pasteurised liquid dairy products (see Section 1.3.2).
There is not a good correlation between the presence of unsatisfactory levels of Enterobacteriaceae (> 10 CFU/mL) and elevated ALP levels in milk. Of 383 pasteurised goats’ milk samples examined in PHE laboratories between 2013 and 2020, 15 samples had ALP levels above 100 mU/L, of which none had unsatisfactory Enterobacteriaceae levels (as there were only two samples with levels above 350 mU/L, a cut‐off value of 100 mU/L was used to compare the ALP levels and Enterobacteriaceae counts). Meanwhile, 48 samples had unsatisfactory Enterobacteriaceae levels, but ALP levels of < 100 mU/L (see Figure 4). Of 11 pasteurised sheep milk samples, one had a moderately high ALP level of 238 mU/L and an unsatisfactory Enterobacteriaceae level of 500 CFU/mL, while a further four had ALP levels ≥ 100 CFU/mL, but with satisfactory Enterobacteriaceae levels. This lack of correlation between ALP and Enterobacteriaceae levels is likely to be at least partly due to post‐pasteurisation contamination of milk with Enterobacteriaceae from the dairy equipment and environment. Enterobacteriaceae levels can for FBOps be indicative for verification of correct pasteurisation if used immediately after pasteurisation before post‐contamination has occurred and are based on a comparison of counts before and after pasteurisation. However, some milk batches will have very low levels of Enterobacteriaceae present even before pasteurisation, and therefore, the absence of these bacteria in the pasteurised milk does not give a useful indication of pasteurisation adequacy.
Figure 4.
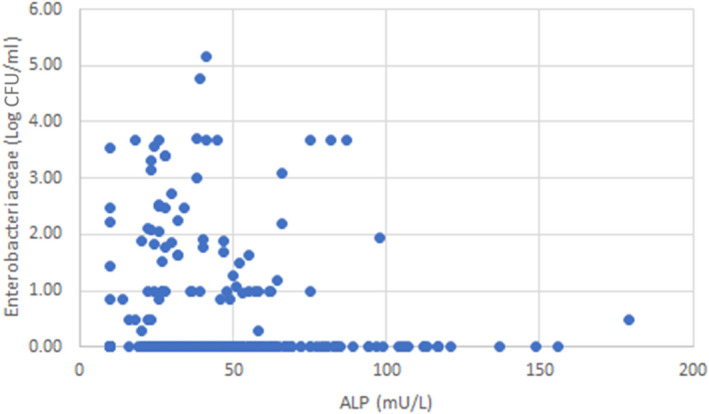
Comparison of ALP activity and Enterobacteriaceae levels in goats’ milk samples
Total bacterial counts (TBCs) can also be used as an indicator of microbiological quality in foods, including raw and pasteurised milk. An Italian study showed a significant but low positive correlation between ALP values and TBCs for sheep, goat and buffalo milk after LTLT pasteurisation (IZSLT, 2020). As described in Section 1.3.2, criteria for TBCs (or plate counts) are specified in EU legislation for determining the microbiological quality of raw milk. However, while the heat processes used in pasteurisation will destroy many of the vegetative bacteria that make up the overall plate count, some microorganisms such as spore‐forming bacteria can survive. Moreover, as with Enterobacteriaceae, recontamination of pasteurised milk may occur in some circumstances, resulting in high TBCs that are not associated with poor pasteurisation. For these reasons, TBCs are not considered to be a good alternative to ALP for monitoring pasteurisation efficacy.
3.4.4. Endogenous marker enzymes
As stated by Claeys et al. (2002), the assessment of different classes of heat treatment of milk can be performed by means of the endogenous marker enzymes ALP, γ‐glutamyl transferase (GGT) and lactoperoxidase (LPO). Other enzymes were also reviewed. These are discussed in this section.
3.4.4.1. γ‐glutamyl transferase (GGT)
γ‐glutamyl transferase (GGT) is present in raw milk from various species and in goats’ (Zarrilli et al., 2003) and ewe's colostrum (Belkasmi et al., 2019). It catalyses the transfer of γ‐glutamyl residues from glutathione and other γ‐glutamyl‐containing peptides to amino acids or peptides.
Synthetic substrates are available to monitor the activity of GGT, either by spectrophotometric analysis (g‐glutamyl‐p‐nitroanilide) (Zehetner et al., 1995) or by fluorescence measurement (L‐glutamic acid‐y‐7‐Amino‐4‐methylcoumarin) (Ziobro and McElroy, 2013).
The heat inactivation curve of this enzyme occurs in cows’ milk between 65°C and 76°C (Zehetner et al., 1995) and therefore, the inactivation properties can be used to monitor milk pasteurisation (Zehetner et al., 1995; Ziobro and McElroy, 2013). Vetsina et al. (2014) considered GGT as a useful marker for bovine milk pasteurisation. According to dos Anjos et al. (1998), GGT and ALP showed similar heat inactivation patterns. However, according to Claeys et al. (2002) and Zehetner et al. (1995), GGT has the potential to act as an indicator within the temperature region between that covered by ALP and LPO, as well as to define the limit between pasteurised and high pasteurised milk. Lorenzen et al. (2010) compared the effects of isochrone heating (35–85°C for 90 s) on the residual activities of ALP, GGT and LPO in bovine, ovine and caprine milk. They demonstrated that the heat stability of these enzymes increased in the following order: ALP < GGT < LPO. Furthermore, they showed that the residual enzyme activity in milk of ovine and caprine origin was considerably higher than in milk of bovine origin. For example, after a ‘holder pasteurisation’ (62°C for 30 min to 65°C for 32 min), the ALP activity in milk from the species was < 0.6 U/L, whereas the GGT activity remained at 70–80% after heating to 62°C for 30 min and 10–40% after heating to 65°C for 32 min relative to raw milk. Milk heated by HTST (75°C for 28 s) showed residual ALP activities of < 0.1 U/L, whereas the residual GGT activities were 6%, 40% and 11%, and the residual LPO activities were 39%, 53% and 43% relative to raw milk from cows, sheep and goats, respectively. Lombardi et al. (2000) demonstrated that in addition to ALP, GGT would be suitable as a potential marker for heat denaturation in buffalo milk, with GGT having the advantage that its concentration is higher. So far, no reactivation of GGT has been observed, making it a good candidate for monitoring milk pasteurisation, at least for cows’ milk. However, the slightly higher inactivation temperature of GGT compared to ALP could lead to residual activity when milder heat treatment is used, such as pasteurisation at 72°C for 15 s.
Although the findings of the different studies are to some extent contradictory, GGT may not be completely inactivated under pasteurisation conditions. Further experimental evaluation in milk from all species is needed to compare GGT and bacterial inactivation curves, in order to demonstrate whether GGT could be a pertinent marker for satisfactory milk pasteurisation.
3.4.4.2. Lactoperoxidase (LPO)
LPO is found in milk from different species, such as cow, buffalo, sheep, goat, llama, camel, human and mouse. For example, Lorenzen et al. (2010) found averages of 2,015, 2,796 and 5,190 U/L LPO in raw milk from cows, sheep and goats, respectively. LPO is an oxidoreductase, with its main function in milk being to oxidise molecules in the presence of hydrogen peroxide, providing an antimicrobial function (Koksal et al., 2016).
LPO has been suggested as a possible enzyme to monitor thermal processes higher than 72°C and is used to make a distinction between pasteurised and high pasteurised milk (Claeys et al., 2002). LPO is used as a marker for milk heat treatment, with an inactivation curve around 80°C in cows’ milk. The ISO/TS method 17193:201120 presenting the reference method is based on the enzymatic oxidation of ABTS (2,2′‐azino‐bis (3‐ethylbenzothiazoline‐6‐sulfonic acid)) in the presence of H2O2, yielding ABTS+ and H2O.
Dumitraşcu et al. (2012) compared the thermal inactivation of LPO in cow, sheep and goat milk. Inactivation occurred at temperatures ranging from 70°C to 77°C. When compared with goat and cow milk, LPO in sheep milk was less stable in terms of thermal denaturation. Taking into account breed‐specific differences, these authors suggested that further studies are needed in order to evaluate the possible use of this enzyme as an indicator of industrial processing of non‐bovine milk. Lorenzen et al. (2010) found that after a LTLT pasteurisation (62°C for 30 min to 65°C for 32 min), the ALP activity in milk from the same three mammals was < 0.6 U/L, whereas the residual activity of LPO activity of bovine milk remained unchanged, and that of ovine and caprine milk was reduced by about 5%. Milk heated by HTST (75°C for 28 s) conditions showed ALP activities of < 0.1 U/L, whereas the residual activities of LPO were 39%, 53% and 43% relative to raw milk from cows, sheep and goats, respectively. Also, Griffiths (1986) concluded that, by comparing the heat resistance of several endogenous bovine enzymes including ALP and LPO, ALP is the most heat‐labile of those measured (D69.8 = 15 s; z = 5.1°C), compared to LPO (D70 = 940 s; z = 5.4°C). According to Claeys et al. (2002), thermal inactivation of LPO follows first‐order kinetics, with a z‐value varying between 3.7°C and 4.3°C.
As the inactivation of LPO occurs at a higher temperature in cows’, ewes’ and goats’ milk compared to ALP (Lorenzen et al., 2010), this enzyme is not suitable as a pasteurisation marker of cows’, ewes’ or goats’ milk. This may also apply to milk from other species but has yet to be confirmed experimentally.
3.4.4.3. Other enzymes
Claeys et al. (2002) also briefly reviewed several other enzymes as potential intrinsic TTI for heat treatment of (bovine) milk: acid phosphatase, adenosine deaminase, N‐acetyl‐β‐glucosidase, catalase, α‐fucosidase, lipoprotein lipase, α‐mannosidase, phosphohexo‐isomerase, phosphodiesterase, superoxide dismutase and xanthine oxidase. For several enzymes, the kinetics are described and D‐ and z‐values are available. For example, thermal inactivation of acid phosphatase follows first‐order kinetics with a z‐value varying between 6.6 and 27.6°C and D100 = 4.8–45 s. Adenosine deaminase also follows first‐order kinetics (between 82°C and 90°C) with a z‐value of 9.2°C and D84 = 93 s. Catalase follows first‐order kinetics with a z‐value of 7.4°C and D77.5 = 15 s.
Andrews et al. (1987) determined the following residual enzyme activities in milk samples heated for 15 s at 72°C in glass capillary tubes; acid phosphatase, > 95%; α‐D‐mannosidase, 98%; xanthine oxidase, 78%; γ‐glutamyl transpeptidase (GGTP), 75%; α‐L‐fucosidase, 26%; N‐acetyl‐β‐D‐glucosaminidase, 19%; and lipoprotein lipase, 1%. It was recommended that N‐acetyl‐β‐D‐glucosaminidase could be used for more detailed studies between 65°C and 75°C and GGTP between 70°C and 80°C.
Lombardi et al. (2000) demonstrated that, in addition to ALP, lactate dehydrogenase (LDH) and GGT, but not aspartate aminotransferase (AST), were potential markers for heat denaturation in buffalo milk.
Vetsina et al. (2014) regarded xanthine oxidase as a useful marker for ultra‐pasteurisation and cream pasteurisation.
On the basis of the available data, it can be concluded that acid phosphatase, α‐D‐mannosidase, adenosine deaminase, catalase, xanthine oxidase, γ‐glutamyl transpeptidase and α‐L‐fucosidase are too heat stable to be used as an intrinsic indicator for cows’ milk pasteurisation, while N‐acetyl‐β‐D‐glucosaminidase, GGTP and lipoprotein lipase could be worthy of further investigation; however, for the other enzymes, data were insufficient to draw any conclusion. For all the mentioned enzymes, further studies are required on their basal levels and inactivation kinetics as data needed for further evaluation are lacking.
3.4.5. Milk compounds
As stated by Claeys et al. (2002), the assessment of different classes of heat treatment of milk can also be performed by means of changes in whey proteins. For the latter, the whole whey protein fraction (e.g. whey protein nitrogen index) as well as its individual components can be used for the classification of heat treatments. Pellegrino et al. (1995) refer to these as Type I indicators, including the denaturation, degradation and inactivation of heat‐labile components (these are mainly whey proteins but can also be enzymes and vitamins).
Also, changes in the secondary products of heat treatment can be investigated. Pellegrino et al. (1995) refer to these as Type II reactions, including the formation of substances which are not present, or only present in low concentration, in the unprocessed milk (i.e. hydroxymethylfurfural, lactulose, furosine).
Official methods for evaluating heat treatments relating to milk, and heat‐induced reactions, such as the determination of enzyme activities (ALP and LPO), denaturation of whey proteins (β‐Lg), Maillard reaction (MR) products (furosine) and lactose‐derived compounds (lactulose) exist, but a variety of further changes in milk composition could be determined by non‐reference methods and could be used to evaluate the heat treatment of milk of different species.
For characterisation of heat treatment, it can be useful to also apply combinations of different parameters by chemometric methods. Spectroscopic methods may be particularly suitable as rapid tests, but most of these methods applied to bovine milk are not suitable for differentiation between raw and pasteurised milk. Therefore, new approaches should be used to identify additional potential heat treatment indicators, in particular for non‐bovine milk. It is also proposed to use untargeted metabolomics or proteomics approaches (Scano et al., 2014).
3.4.5.1. Acid soluble total whey protein content
One method for rapid estimation of undenatured whey proteins, and hence of heat treatment of milk, is the measurement of acid‐soluble tryptophan by fluorescence (Birlouez‐Aragon et al., 1998). The fluorescence is measured after precipitation of casein and aggregated whey protein at pH 4.6, with an excitation wavelength of 290 nm and emission wavelength of 340 nm. For calibration, a standard solution of bovine serum albumin (BSA) is used.
Another rapid test for qualitatively assessing the extent of whey protein denaturation in milk is the Aschaffenburg turbidity test. After precipitation of casein and aggregated whey protein at pH 4.6, the clear filtrate is boiled for 5 min to denature the residual undenatured whey protein and the turbidity, which correlates with the amount of undenatured whey protein, is measured at 420 nm (Aschaffenburg, 1950). The whey protein nitrogen index (WPNI) represents the amount of heat‐undenatured whey protein nitrogen (soluble in saturated sodium chloride solution), expressed in milligrams of WPN per millilitre of milk, and is also determined by a turbidimetric method (Ritota et al., 2017). These tests can be used to estimate the extent of whey protein denaturation, as an indicator of severe heat treatment of UHT milk or for the classification of milk powder but are not suitable as indicators for milk pasteurisation.
3.4.5.2. Acid soluble individual whey protein content
In contrast to the determination of total whey protein content, the determination of individual milk proteins is a more sensitive method for evaluation of heat treatment. β‐Lg is the major whey protein in the milk of most mammals but is absent from the milk of humans, lagomorphs, rodents and camels (Uniacke‐Lowe and Fox, 2011). In contrast to casein, β‐Lg remains soluble at acidic pH. Upon heating, β‐Lg denatures and becomes insoluble and precipitates on the casein surface. An international standard was developed (ISO 13875:2005:200521) for the chromatographic determination of acid‐soluble β‐Lg, which is soluble at pH 4.6, in liquid milk. This standard specifies a method for the quantitative determination of the indicator in retail samples of Belgian drinking milk (Van Renterghem et al., 2000). The method has been tested over a range between 0 mg and 3,500 mg of β‐Lg/L of cows’ milk and is suitable for distinguishing different categories of heat‐treated liquid milk.
The study of the acid soluble β‐Lg on retail samples (Van Renterghem et al., 2000) suggests that β‐Lg is a potentially interesting indicator for the heat treatment of milk. However, as a potential marker to guarantee adequate pasteurisation, this method has serious drawbacks. First, a small overlap in β‐Lg content can be observed between thermised and pasteurised milk. Second, possible variations in the β‐Lg content of raw milk make it difficult to define limit values for the β‐Lg content of pasteurised milk and third, in many species including camelids (Shamsia, 2009) β‐Lg is absent.
More heat sensitive whey proteins (e.g. serum albumin (SA), Ig) could be considered as markers for the correct implementation of pasteurisation.
In addition to the determination of the acid soluble content of β‐Lg in milk and cheese, other major whey proteins could also be used for evaluating the heat treatment. Milk of most non‐bovine mammals contains the same main whey proteins as bovine milk, i.e. β‐Lg, α‐lactalbumin (α‐La), SA, lactoferrin (LF), Ig, but their content varies widely between ruminant and non‐ruminant milk. In camel milk, α‐LA, SA and Ig are the main whey proteins. In common with human milk, no β‐Lg can be determined. Instead, whey acidic protein (WAP) and lactoferrin can be detected in considerable amounts (Park and Haenlein, 2006). Compared with bovine milk, equine milk contains less β‐Lg and more α‐La and Ig. Mares’ milk, in particular, is rich in lysozyme and lactoferrin, which occur at low levels in bovine milk.
Thermal denaturation of whey proteins is a complex process and occurs in two main steps, conformational changes due to the unfolding of their initially folded molecules and aggregation via chemical (‐SH/S‐S exchange reactions) or physical (non‐covalent hydrophobic and electrostatic interaction) reactions. Regarding the thermal stability of milk proteins, differences between species are mainly due to differences in amino acid sequence (and number of S‐S bridges or –SH groups) and in the ‘milk environment’ (e.g. variations in pH, fat content) (Claeys et al., 2014). The whey protein aggregates by self‐association or association with other proteins, such as κ‐casein, and becomes insoluble at pH 4.6. The residual non‐aggregated whey protein can be determined by a reverse phase ‐ high‐performance liquid chromatography (RP‐HPLC)‐method according to ISO 13875:200519 or by sodium dodecyl sulfate (SDS)‐gel electrophoretic methods.
In bovine milk, the order of susceptibility of the major whey proteins to denaturation, from the most to the least heat‐sensitive, is: Ig > BSA > β‐Lg B > β‐Lg A > α‐La (Clawin‐Rädecker et al., 2000). In comparison to raw milk, no significant differences in levels of α‐La and β‐Lg can be observed in commercial HTST‐heated milk or microfiltered ESL‐milk (Table 10). The more heat sensitive whey proteins, SA, lactoferrin and Ig showed significant denaturation in HTST‐heated milk or microfiltered ESL‐milk. However, the evaluation of heating without knowledge of the corresponding contents of these proteins in raw milk is difficult, due to considerable variability in the range of the individual whey proteins (Pellegrino et al., 1996; Villamiel et al., 1999; Clawin‐Rädecker et al., 2000; Lorenzen et al., 2011b).
Table 10.
Acid soluble whey protein content of commercial milk samples (Clawin‐Rädecker and Schlimme, 1998; Lorenzen et al., 2011b)
| Heating process | N | α‐lactalbumin (mg/100 mL) | β‐lactoglobulin (mg/100 mL) | Serum albumin (mg/100 mL) | Lactoferrin (mg/100 mL) | Immunoglobulin (mg/100 mL) | β‐Lg/protein (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | min–max | Mean | min–max | Mean | min–max | Mean | min–max | Mean | min–max | Mean | min–max | ||
| Raw milk | 9 | 118.1 | 89.6–150.0 | 411.1 | 299.8–577.1 | 33.0 | 19.8–65.8 | – | – | 67.1 | 40.3–103.4 | 11.3 | 8.9–13.3 |
| HTST‐heated | 6 | 111.8 | 102.0–115.2 | 410.0 | 330.2–474.5 | 17.2 | 13.5–20.5 | 7.1 | 1.8–12.3 | 28.5 | 13.8–38.2 | 12.0 | 10.3–14.2 |
| ESL, microfiltered | 6 | 106.1 | 99.8–111.5 | 381.7 | 357.4–414.8 | 16.1 | 13.7–18.4 | 6.8 | 5.5–8.4 | 21.9 | 14.1–29.6 | 11.2 | 10.9–11.8 |
| ESL, directly heated | 8 | 100.6 | 96.6–106.6 | 208.4 | 158.9–296.8 | 6.3 | 4.6–8.8 | ND | ND | 6.0 | 4.5–8.4 | ||
| ESL, indirectly heated | 2 | 84.1 | 81.5–86.7 | 50.7 | 34.1–67.3 | ND | ND | ND | 1.4 | 1.0–1.9 | |||
| UHT | 8 | 31.4 | 12.5–57.4 | 15.3 | 6.3–29.9 | ND | ND | ND | 0.4 | 0.2–0.9 | |||
ESL: extended shelf‐life; HTST: high temperature short time; N: number of samples; ND: not detected; UHT: ultra‐high temperature; β‐Lg: β‐lactoglobulin.
The highest concentration of β‐Lg was identified in sheep milk and was about 2.5 times higher than in cows’ and goats’ milk. Therefore, the β‐Lg/α‐La ratio in sheep milk was 8.02 ± 0.71, followed by goats’ (2.30 ± 0.08) and cows’ milk (2.04 ± 0.20). In the temperature range between 72.5°C and 80°C, β‐Lg was clearly more thermosensitive in sheep and goats’ milk than in cows’ milk. Thermosensitivity of β‐Lg decreased in the following order: sheep > goat > cow. For α‐La, a change of the thermosensitivity, depending on the process temperature, was observed. Compared to cows’ milk, α‐La appears to be more heat stable in goats’ milk in the temperature range of 72.5–80°C, and more sensitive at higher temperature (90°C) in sheep and goats’ milk (Dumitraşcu et al., 2013).
The thermal stability of equine LF and BSA is comparable with that of their bovine counterparts, but equine β‐Lg and α‐La are more heat‐stable than the corresponding bovine proteins (Uniacke‐Lowe et al., 2010). Early experiments show less resistance to heat denaturation of the whey proteins of bovine milk, compared to those of buffalo or camel milk.
For a satisfactory evaluation of the suitability of the denaturation of heat‐sensitive whey proteins (LF, SA and Ig) as heat indicators for milk pasteurisation, further investigations must be carried out, in particular on the variation of the individual proteins in the raw milk of the different species and the influence of breed, environment, season and feeding practices.
3.4.5.3. 5‐Hydroxymethylfurfural
5‐Hydroxymethylfurfural (5‐HMF) is an intermediate compound of non‐enzymatic browning reactions (Morales et al., 2000). There are two main ways that 5‐HMF forms in milk. The first is via Amadori products of the MR through enolisation (in the presence of amino groups). The second, known as the Lobry de Bruyn‐Alberda van Ekenstein (LA) transformation, passes through lactose degradation and isomerisation stages (Morales et al., 2000). The relative rates of the two routes depend on a number of variables, such as pH, water activity, temperature and time. In milk, the route of HMF formation is mostly via LA transformation (Claeys et al., 2002).
Only small amounts of 5‐HMF can be detected directly in milk (free 5‐HMF). The determination of total 5‐HMF requires a preliminary digestion with 0.3 N oxalic acid at about 100°C (total 5‐HMF) and the formation of 5‐HMF depends on acid concentration and temperature. HMF concentrations can be measured by spectrophotometry, but this method has low specificity and the HMF‐thiobarbituric acid complex has also a low stability (Ritota et al., 2017). HPLC methods with UV detection at 280 nm enable a more reliable quantification, but sometimes interference between HMF and co‐eluted compounds can occur.
HMF is a recognised indicator for distinguishing in‐bottle sterilised milk from UHT‐milk (Morales et al., 2000; Claeys et al., 2002). A slight increase of HMF can be found in thermised milk and pasteurised milk compared to raw milk. However, the variation between different milk samples does not allow discrimination between raw, thermised and pasteurised milk (Morales et al., 2000).
3.4.5.4. Lactulose
The formation of lactulose (4‐O‐b‐D‐galactopyranosyl‐D‐fructo‐furanose) in heated milk due to the alkaline isomerisation of lactose catalysed by the free amino groups of casein (Richards and Chandrasekhara, 1960; Adachi and Patton, 1961) is dependent on time and temperature of heating and on the pH of the environment (Adachi, 1958; Olano et al., 1989).
An international standard was developed for the chromatographic determination of lactulose (ISO 11868:200722 ). This specifies a method for the determination of the lactulose content of heated milk, skimmed milk, partially skimmed or whole milk, by HPLC, in order to distinguish milk sterilised by UHT from in‐bottle sterilised milk. The method has been tested over a lactulose content range of 200–1,500 mg/L and is applicable to all types of heat‐treated milk. First, fat and protein are removed from the milk sample, which is then filtered. The lactulose content of the filtrate is then determined by HPLC. The result obtained for the sample is evaluated by reference to standard samples consisting of lactulose‐free skimmed milk with known amounts of added lactulose.
Also, an enzymatic method for the determination of the lactulose content was developed and described in ISO 11285:2004.23 Fat and protein are precipitated by the addition of a zinc sulfate and potassium hexacyanoferrate(II) solution and are then removed by filtration. Lactose and lactulose are hydrolysed to galactose and glucose, or galactose and fructose, respectively, in the presence of the enzyme β‐D‐galactosidase (β‐gal). The amount of liberated fructose is stoichiometric with the amount of lactulose.
Determination of the lactulose concentration allows the differentiation of UHT from in‐bottle sterilised milk, and of directly from indirectly processed UHT milk. However, an overlap between lactulose levels in UHT and in bottle sterilised milk has been reported, rendering measurement of lactulose alone insufficient as an index of heat treatment (Claeys et al., 2002). As can be seen in Table 9, lactulose concentrations in high pasteurised milk are low. The substantial overlap in lactulose concentration between high pasteurised and thermised milk makes lactulose unsuitable as an indicator for adequate pasteurisation. Since lactulose formation is species independent, this can also be generalised to other species.
Table 9.
Study of β‐lactoglobulin, lactulose and furosine concentrations in retail samples of Belgian cows’ milk (Van Renterghem et al., 2000)
| Parameter | Thermisation | (High) pasteurisation | UHT direct | UHT indirect | Sterilisation | |
|---|---|---|---|---|---|---|
| β‐lactoglobulin (mg/L) | Mean | 3,896 | 3,312 | 415 | 134 | 15 |
| Min | 3,650 | 1,767 | 68 | 65 | n.d. | |
| Max | 4,093 | 3,718 | 899 | 215 | n.d. | |
| SD | 170 | 506 | 343 | 59 | n.d. | |
| N | 5 | 14 | 8 | 5 | 7 | |
| Lactulose (mg/L) | Mean | 10.8 | 19.6 | 414 | 620 | 1,064 |
| Min | 4 | 4 | 121 | 415 | 844 | |
| Max | 15 | 42 | 689 | 797 | 1,329 | |
| SD | 4.4 | 9.7 | 261 | 188 | 193 | |
| N | 5 | 14 | 8 | 5 | 7 | |
| Furosine (mg/100 g protein) | Mean | 6.6 | 9.6 | 116 | 196 | 337 |
| Min | 4 | 6 | 63 | 150 | 273 | |
| Max | 8 | 17 | 204 | 255 | 408 | |
| SD | 1.5 | 2.9 | 54 | 46 | 46 | |
| N | 5 | 14 | 8 | 5 | 7 |
N: number of samples; SD: standard deviation; UHT: ultra‐high temperature.
3.4.5.5. Furosine
During the Maillard Reaction (MR) in milk, ε‐N‐deoxylactulosyl‐L‐lysine is formed, the main stable Amadori compound that can be partially converted by acid hydrolysis to the stable ε‐N‐2‐furoylmethyl‐L‐lysine i.e. ‘furosine’ (Finot and Mauron, 1972).
Resmini et al. (1990) proposed an ion‐pair RP‐HPLC (IP‐RP‐HPLC) method for the determination of furosine in acid‐hydrolysed dairy products. Other methods are ion‐exchange chromatography (Hartkopf and Erbersdobler, 1993) and capillary zone electrophoresis (CZE) (Tirelli and Pellegrino, 1995). Based on the method of Resmini et al. (1990), an international standard was developed (ISO 18329:200424 ). The first stable MR product formed in milk and in cheese, ε‐lactulosyl‐lysine, is partially converted by warm acid hydrolysis into furosine, the determination of which allows the extent of early stages of MR to be evaluated. The extent of MR is related to type and intensity of heat treatments applied both to raw material and in processing. The determination of furosine is performed by IP‐RP‐HPLC with UV detection at 280 nm. Quantification of furosine is obtained by reference to a standard sample of furosine. As the formation of furosine is highly dependent on protein concentration (positively correlated) it is expressed as mg/100 g protein (Montilla and Olano, 1997; Rattray et al., 1997).
As shown in Table 9, furosine concentrations in pasteurised milk are still very low. Due to the overlap in furosine concentration between thermisation and (high) pasteurisation, it cannot be considered as a suitable indicator for adequate pasteurisation. Furosine has been proposed as a useful index for heat‐induced changes in milk products; a furosine content of 8 mg/100 g protein has been suggested as an upper limit for milk after pasteurisation, 20 mg/100 g protein for high pasteurisation and 250 mg/100 g protein for UHT (Clawin‐Rädecker and Schlimme, 1995; Schlimme et al., 1996) [based on Claeys et al. (2002)].
3.4.5.6. Lysinoalanine
Lysinoalanine (LAL) is formed during protein cross‐linking through the reaction of dehydroalanine with lysine residues under severe food processing conditions such as high temperature or alkaline pH. Dehydroalanine originates from the base‐catalysed β‐elimination of one of the sulfur atoms of cysteine or of phosphate in phosphoserine. Protein cross‐linking reactions occur between dehydroalanine (DHA) and other amino acids such as lysine, histidine and cysteine reacting with DHA through their nucleophilic side chains to yield LAL, histidinoalanine (HAL) or lanthionine (LAN) cross links.
LAL can be determined by RP‐HPLC with fluorescence detection. Before analysis the acid‐hydrolysed milk samples are derivatised by 9‐fluorenyl‐methylchloro‐formate (FMOC) and purified by solid‐phase extraction on an amino cartridge (Pellegrino et al., 1996). In one study, LAL was not found in raw milk and pasteurised milk, but was present in natural Mozzarella cheese. Using a modified RP‐HPLC method with dansyl chloride derivatisation, trace amounts of LAL could also be detected in raw milk and pasteurised milk (Faist, 2000). Calabrese et al. (2009) determined LAL as FMOC derivative by liquid chromatography mass spectrometry (LCMS). They found high levels of LAL in calcium caseinate and milk powder, but it was not present in raw milk. Due to these high levels of caseinate, LAL is a suitable indicator of the use of caseinates in cheese making (Resmini et al., 2003). Even with newly developed methods such as direct quantification by liquid chromatography mass spectrometry using multiple reaction monitoring, the level of LAL was below quantification in raw and pasteurised milk (Nielsen et al., 2020). No data are available of LAL in heated milk of different species, but it is to be expected that LAL is also only formed in non‐bovine milk at the higher heat treatment levels used for UHT milk or milk powder. It is not suitable as an indicator for milk pasteurisation.
3.4.5.7. Chemometrics
To describe the effects of heat treatment in milk more efficiently, chemometric approaches based on evaluating several heat indicators have been applied. Morales et al. (2000) studied the correlation between β‐Lg, HMF and lactulose in bovine milk and applied a discriminant analysis of the thermal indices. It was possible to separate pasteurised, UHT‐treated (direct and indirect), pre‐sterilised and in‐bottle sterilised milk categories with 100% accuracy. Bulk and thermised milks could not be distinguished with confidence. No information is available on the use of chemometrics for milk of non‐bovine species.
3.4.5.8. Spectroscopy
A rapid method to determine the intermediate compounds of the advanced MR is the determination of UV absorption at 294 nm (Sun and Wang, 2009). The absorbance (Amax at 294 nm) of commercial milk after hydrolysis showed better correlation with the furosine content under mild heating conditions. Birlouez‐Aragon et al. (2002) developed a new fluorimetric FAST (fluorescence of Advanced Maillard products and Soluble Tryptophan) method, based on the quantification of protein denaturation by fluorescence measurements of tryptophan and accumulation of fluorescent Maillard products in the pH 4.6 soluble fraction of milk. The tryptophan fluorescence was measured at excitation/emission 290/340 nm and the advanced Maillard products were measured at excitation/emission wavelengths of 330/420 nm. The results were specified as FAST index, the percentage ratio between both values. A new advanced fluorescence technique, the Front‐Face fluorescence spectroscopy (FFFs), allows the measurement of the fluorescence spectra directly on the milk samples without any sample pretreatment (Ritota et al., 2017). This method was used to characterise heat‐induced changes in camel milk by the fluorescence spectra of nicotinamide adenine dinucleotide (NADH), fluorescent MR products (FMRP) and vitamin A (Kamal and Karoui, 2017). Initial results suggest that fluorescence spectroscopy could be considered as a rapid and non‐destructive screening tool for differentiating between milks according to heating intensity and time, but further investigations to determine the influence of species, breed, region or seasonal variations have to be carried out.
3.4.5.9. Untargeted metabolomics
A new analytical approach for the identification of suitable heat indicators for milk pasteurisation may be the use of untargeted metabolomics. The effects of milk pasteurisation on the low molecular weight metabolite profiles can be analysed by gas‐ or liquid chromatography‐mass spectrometry (GCMS or LCMS) or by nuclear magnetic resonance‐spectrometry (NMR). The metabolite profiles of the milk samples are analysed by a multivariate statistical approach, considering the correlations among variables. Initial results highlight substantial differences in the metabolite levels in raw and thermised ovine cheese, especially for the free amino acids and saccharides (Caboni et al., 2019). This method can also be applied to differentiate cow and buffalo mozzarella cheese (Pisano et al., 2016) or goat and cow milk (Scano et al., 2014). However, further research still needs to be done to assess the potential of the method for differentiation of raw and pasteurised milk from different animal species.
3.4.5.10. Proteomics
Proteomic‐based methods offer another promising approach to identify suitable indicators for milk pasteurisation in milk of different species. Through the combination of liquid chromatography or two‐dimensional gel electrophoresis with advanced mass spectrometry, proteomic‐based methods can play an important role for detailed and sensitive identification, characterisation and quantification of milk proteins of different animal species. These methods also allow the comprehensive detection of milk protein modifications resulting from heat treatment and storage of the products.
Through direct mass spectroscopy (MS) analysis of intact protein components and the corresponding modified forms, lactulosyllysine has been identified as the most common modification in milk proteins and has been detected in a variety of dairy products (Siciliano et al., 2013).
Meltretter et al. (2009) analysed the whey protein profiles of thermal treated milk samples with MALDI‐TOF‐MS after defatting, casein precipitation and immobilised metal affinity chromatography. Mono‐lactosylated forms of whey proteins were detected in pasteurised and high temperature milk, while di‐lactosylated forms were present in UHT milk samples. Moreover, tri‐ and quadruply‐lactosylated forms of α‐La as well as forms modified by hexose addition were observed in liquid and powdered infant formulas.
More commonly used than the MS analysis of intact protein components is the detection of modified peptides in heated milk after specific protein hydrolysis. Meltretter et al. (2014) detected 19 different structures at 26 binding sites of β‐Lg by ultrahigh‐performance liquid chromatography‐tandem mass spectrometry. The modified peptides were determined in heated milk after tryptic digestion of the milk protein and analysed by multiple reaction monitoring (MRM).
The formation kinetics of the different glycated peptides showed that the site‐specific analysis of lactuloselysine may be a more sensitive marker for mild heat treatment than its overall content. Other modified peptides (glycoxidation, oxidation and deamination products) may be good markers for more intense heat treatments. So far, only limited data is available for bovine milk and further investigations have to be done to identify the specific glycated peptides for each species. Due to possible ion suppression during electron spray ionisation (ESI), the absolute quantification of modified peptides in the different milk samples by LCMS‐based approaches may be difficult without using any isotopically labelled lactosylated peptides as internal standard compounds, and this will be necessary for validation as an official method to control milk quality.
3.4.6. Concluding remarks
The main alternative methods used in MSs to verify pasteurisation of milk, colostrum, dairy and colostrum‐based products from non‐bovine species are temperature monitoring over time during the heat treatment using data loggers, and enumeration of Enterobacteriaceae.
The use of data loggers is standard practice to monitor the heat treatment applied over time, but they have to be positioned correctly and calibrated regularly. However, data loggers cannot detect other process failures such as a cracked or leaking plate that may allow raw milk to contaminate pasteurised milk or post‐pasteurisation contamination.
Enterobacteriaceae enumeration is relevant for monitoring the general hygiene of milk and milk products in accordance with the process hygiene criterion, and total bacterial counts are relevant for monitoring the quality of raw and pasteurised milk. They are capable to detect post‐pasteurisation contamination but are not suitable to verify that pasteurisation conditions have been properly applied.
-
The assessment of different classes of heat treatment of milk can be performed by means of determining endogenous marker enzyme activities, secondary products of heat treatment or changes in whey proteins.
-
o
Some endogenous milk enzymes, i.e. GGT, N‐acetyl‐β‐D‐glucosaminidase, LDH, catalase and lipoprotein lipase, may be more suitable to verify pasteurisation conditions of milk from non‐bovine species than ALP. More studies considering their kinetic inactivation (allowing the calculation of D‐ and z‐values), the variation in their basal concentrations and possible reactivation after pasteurisation would be required to evaluate this.
-
o
Heat induced denaturation of the main whey proteins α‐La and β‐Lg is very limited during pasteurisation (< 10%). Therefore, the total acid soluble whey content (e.g. whey protein N index) or the acid soluble β‐Lg content are not sensitive indicators. For an evaluation of mild heat treatments, detection of denaturation of the heat‐sensitive minor whey proteins (LF, SA and Ig) may be more suitable. However, the evaluation of heating without knowledge of the corresponding content of these proteins in raw milk is difficult due to possible wide variations in the individual whey proteins. Further investigations are required, in particular on the variation of the individual proteins in the raw milk of the different animal species depending on breed, environment, season and feeding practices.
-
o
Due to the relatively high activation energy of heat‐induced chemical reactions, secondary products of heat treatment (HMF, lactulose, furosine, LAL) are not useful for evaluation of pasteurisation. Lactose, lactulose formation and the MR (furosine) are almost unaffected by pasteurisation in bovine milk and this can also be expected in milk of other species. No information is available on the use of chemometrics for milk of non‐bovine species.
-
o
The use of fluorescence or UV spectroscopy could be considered as a rapid and non‐destructive screening method only for confirmation of severe heat treatments. New promising technologies, such as FFF, require further research to determine the influence of species, breed, region or seasonal variations on the characterisation of heat treatments.
-
o
Metabolomic‐ or proteomic‐based methods are also promising approaches to identify suitable new heat indicators for milk pasteurisation in milk of different animal species, but further research is required to evaluate their performance and potential for routine use.
-
o
4. Conclusions
AQ1: What is the use and what are the limitations of ALP testing to verify thermal pasteurisation in milk, colostrum, dairy and colostrum‐based products from sheep and goats (and other species such as solipeds and camelids, producing such products for human consumption), compared to cattle, both immediately after such treatment as well as on the end products placed on the market (milk or colostrum for direct human consumption and milk or colostrum‐based products such as yoghurt, cheese, ice cream, milk powder, cream, or fermented milk)?
One‐third of the 15 EU countries replying to the questionnaire reported using ALP testing for milk or milk products from non‐bovine species, more specifically in goats’ milk, sheep's milk, cheese made from sheep milk and cheese from goats’ milk (in descending order).
The limitations of ALP testing for verifying pasteurisation of milk and milk products from bovine species also apply to other species. It is recommended that the ALP test should be performed immediately after the heat treatment and that those factors that influence the residual ALP levels should be considered when interpreting the results (e.g. interference of microbial ALP basal level and fat content of the milk).
The ALP activity in raw sheep milk seems to be about three times higher and in caprine milk about five times lower than in bovine milk. The level in raw milk from sheep and goats is highly variable between breeds and is influenced by season, lactation stage, fat content and udder health. Further variation of basal ALP levels among non‐bovine species is expected due to greater variation in breeds of sheep, goat and equines compared to dairy cows.
Combining the information of basal ALP levels and thermal inactivation behaviour of the enzyme in the respective species would facilitate an estimation of residual ALP after pasteurisation. However, only a few studies have investigated the thermal ALP stability in milk derived from cows, sheep and goats, with conflicting evidence. Therefore, it is not possible to estimate residual ALP levels with certainty.
Assuming that the heat inactivation of pathogens would be the same in the milk of different species, and based on the available evidence from milk samples after pasteurisation, there is 95–99% probability (extremely likely) that pasteurised goat milk and pasteurised sheep milk would have an ALP activity below a limit of 300 and 500 mU/L, respectively. Nevertheless, it is recommended to collect further data in order to conclude whether the evidence now available is representative of all situations.
For equine milk, the current test sensitivity does not allow the use of ALP testing as the basal ALP activity is very low, while camel milk contains low basal levels, and additionally a heat‐stable ALP; therefore, ALP testing is not appropriate either.
The data available for cheese of non‐bovine species do not allow limits to be evaluated.
No data is available for colostrum, or milk or colostrum‐based dairy products such as yoghurt, ice cream, milk powder, cream or fermented milk.
AQ2: What are the possible alternative methods to the determination of ALP activity, and their possible limitations for the verification of thermal pasteurisation of milk, colostrum, dairy and colostrum‐based products from sheep and goats immediately after such treatment, as well as on the end product placed on the market?.
The main alternative methods used in MSs to verify pasteurisation of milk, colostrum, dairy and colostrum‐based products from non‐bovine species are temperature monitoring over time during the heat treatment using data loggers, and the enumeration of Enterobacteriaceae.
The use of data loggers is standard practice to monitor the heat treatment applied over time but cannot detect other process failures or post‐pasteurisation contamination.
Enterobacteriaceae enumeration is relevant for monitoring the general hygiene of milk and milk products in accordance with the process hygiene criterion, but is not suitable to verify that pasteurisation conditions have been properly applied.
-
The assessment of different classes of heat treatment of milk can be performed by means of assaying other endogenous marker enzymes, secondary products of heat treatment or changes in whey proteins.
-
o
The inactivation of some enzymes, i.e. GGT, N‐acetyl‐β‐D‐glucosaminidase, LDH, catalase and lipoprotein lipase, may be more suitable to verify pasteurisation conditions of milk from non‐bovine species than ALP. More studies considering their thermal inactivation (allowing the calculation of D‐ and z‐values), the variation in their basal concentrations and possible reactivation after pasteurisation would be required to evaluate this.
-
o
Due to the high temperatures needed for the production of secondary products of heat treatment, methods based on their detection are not suitable as pasteurisation markers.
-
o
Changes in native whey proteins depend on their levels in milk and their variability, making it difficult to set a limit for pasteurised milk currently. More research is needed to reach a definitive conclusion on the applicability of changes in native whey proteins as pasteurisation markers.
-
o
5. Recommendations
An in‐depth thermal inactivation kinetics study with different milk batches is recommended to obtain reliable data to derive the D and z‐values of ALP inactivation in the milk from the various animal species. This study should be carried out using a method allowing an almost instantaneous heating to the isothermal temperature and cooling afterwards in which it is recommended that the D‐values are determined at five to six different temperatures between 55°C and 70°C. At any chosen temperature, there should be at least 5 points (incubation times) on the linear part of the curve to provide valid results.
For colostrum and milk or colostrum‐based products such as cheeses derived from goat and sheep milk, more studies are recommended to evaluate the use and limitations of ALP testing to provide indication of proper pasteurisation.
For milk derived from other species such as solipeds and camelids, studies are recommended to evaluate the use of other endogenous enzyme markers as it appears, based on the currently available evidence, that ALP testing does not provide appropriate indication of proper pasteurisation.
Glossary
Raw milk is defined in Regulation No (EC) 853/2004 as ‘milk produced by the secretion of the mammary gland of farmed animals that has not been heated to more than 40°C or undergone any treatment that has an equivalent effect’.
Dairy products are defined in Regulation No (EC) 853/2004 as ‘processed products resulting from the processing of raw milk or from the further processing of such processed products’.
Colostrum is defined in Regulation No (EC) 853/2004 as ‘the fluid secreted by the mammary glands of milk‐producing animals up to 3–5 days post‐parturition that is rich in antibodies and minerals and precedes the production of raw milk’.
Colostrum‐based products are defined in Regulation No (EC) 853/2004 as ‘processed products resulting from the processing of colostrum or from the further processing of such processed products’.
The D‐value is the time required at a given temperature to reduce the enzyme activity or a specific microbial population by a factor 10 (i.e. to reduce the enzyme activity by 90% or to kill 90% of the exposed microorganisms).
The z‐value if the temperature change required to change the D‐value by a factor of 10 (i.e. for one log10 increase or decrease in the D‐value).
Abbreviations
- α‐La
α‐lactalbumin
- β‐gal
β‐D-galactosidase
- β‐Lg
β‐lactoglobulin
- ABTS
2,2′‐azino‐bis (3‐ethylbenzothiazoline‐6-sulfonic acid)
- ALP
alkaline phosphatase
- AOAC
Association of Official Agricultural Chemists
- AQ
Assessment Question
- AST
aspartate aminotransferase
- BSA
bovine serum albumin
- CA
competent authority
- CZE
capillary zone electrophoresis
- DHA
dehydroalanine
- DL
Detection limit
- EDA
European Dairy Association
- EPAS
Enzymatic photo‐activated system
- ESI
electron spray ionisation
- ESL
extended shelf‐life
- EURL‐MMP
European Union Reference Laboratory for milk and milk products
- FAST
fluorescence of Advanced Maillard products and Soluble Tryptophan
- FBO
Food‐borne outbreak
- FBOp
Food Business Operator
- FDA
Food and Drug Administration
- FFF
Front‐face fluorescence spectroscopy
- FMOC
fluorenyl‐methylchloro‐formate
- FMRP
fluorescent MR products
- GCMS
gas chromatography‐mass spectrometry
- GGT
γ‐glutamyl transferase
- GGTP
γ‐glutamyl transpeptidase
- GHP
good hygienic practices
- HACCP
hazard analysis and critical control points
- HAL
histidinoalanine
- HMF
Hydroxymethylfurfural
- HPLC
high‐performance liquid chromatography
- HPP
high pressure processing
- HTST
high temperature short time
- IDF
International Dairy Federation
- Ig
immunoglobulin
- IgG
immunoglobulin G
- IP‐RP
ion‐pair reverse‐phase
- ISO
International Organization for Standardization
- LA
Lobry de Bruyn‐Alberda van Ekenstein
- LAL
lysinoalanine
- LAN
lanthionine
- LCMS
liquid chromatography‐mass spectrometry
- LDH
lactate dehydrogenase
- LF
lactoferrin
- LPO
lactoperoxidase
- LTLT
low temperature long time
- MR
Maillard Reaction
- MRA
Microbiological Risk Assessment
- MS
Member States
- MS
mass spectrometry
- mU/L
milliunits of enzyme activity per litre
- NMR
nuclear magnetic resonance‐spectrometry
- MRM
multiple reaction monitoring
- NADH
nicotinamide adenine dinucleotide
- NRL
National Reference Laboratory
- PHE
Public Health England
- RP‐HPLC
reverse phase ‐ high‐performance liquid chromatography
- SA
serum albumin
- SDS
sodium dodecyl sulfate
- t/T
time‐temperature
- TBC
Total bacterial count
- TBEV
tick‐borne encephalitis virus
- ToR
Terms of Reference
- TTI
time temperature integrators
- UHT
ultra‐high temperature
- UV
ultraviolet
- WAP
whey acidic protein
- WG
Working Group
- WPNI
whey protein nitrogen index
Appendix A – Strong evidence food‐borne outbreaks in the EU from 2007 to 2019 associated with the consumption of milk and dairy products
1.
Appendix B – Questionnaire on ALP and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products
1.
Many thanks for collaborating with the BIOHAZ WG on the ‘Scientific and technical assistance on the use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products’ (EFSA-Q‐2020-00331) by providing answers to the questions indicated below.
Please specify your contact point details
| Affiliation | Please specify: Competent Authority/National Laboratory |
| Reporting Country | Country |
| Address | Address |
| E‐Mail | E‐Mail |
| Name of contact person | Name |
Please check the relevant multiple choice options and fill in this questionnaire according to the available information in your country.
-
1
Do you collect any ALP testing data from milk, colostrum, dairy and colostrum‐based products from bovine species using the ‘ISO standard 11816‐1:2013 Milk and milk products – Determination of alkaline phosphatase activity – Part 1: Fluorimetric method for milk and milk‐based drinks’ to verify pasteurisation of the relevant products?
Yes ☐ No ☐
-
2
Do you collect any ALP testing data from cheese from bovine species using the ‘ISO standard 11816‐2:2016 Milk and milk products — Determination of alkaline phosphatase activity — Part 2: Fluorimetric method for cheese ‘to verify pasteurisation of the relevant products?
Yes ☐ No ☐
-
3
Have you collected any ALP testing data from milk, colostrum, dairy and colostrum‐based products from non‐bovine species (e.g. sheep, goats, solipeds, camelids etc.) using the ‘ISO standard 11816‐1:2013 Milk and milk products – Determination of alkaline phosphatase activity – Part 1: Fluorimetric method for milk and milk‐based drinks’ to verify pasteurisation of the relevant products?
Yes ☐ No ☐ -
a
If you replied yes to question 3, from which species and from which specific product(s)?
Sheep ☐ Pasteurised Milk☐ Dairy Products Please specify product Goat ☐ Pasteurised Milk☐ Dairy Products Please specify product Horse ☐ Pasteurised Milk☐ Dairy Products Please specify product Camel ☐ Pasteurised Milk☐ Dairy Products Please specify product Other Please specify if applicable -
b
If you replied no to question 3, what test do you perform to verify pasteurisation of the collected samples (e.g. enumerating Enterobacteriaceae levels)?
☐ Enterobacteriaceae enumeration ☐ Temperature monitoring of heat treatment equipment using data loggers ☐ Other ALP activity determination methods than the ISO standard 11816‐1:2013 or ISO standard 11816‐2:2016 ☐ Other Please specify if applicable -
a
-
4
Can you provide/share values for the ALP activity in milk, colostrum, dairy and colostrum‐based products from non‐bovine species using:
the ‘ISO standard 11816‐1:2013 Milk and milk products – Determination of alkaline phosphatase activity – Part 1: Fluorimetric method for milk and milk‐based drinks’ or
-
other ALP activity determination methods than the ISO standard 11816‐1:2013 or ISO standard 11816‐2:2016
Yes ☐ No ☐
If yes, please send/share the information/raw data together with the replied questionnaire attached to your email reply including details on sampled animal species, sample products and analytical methods used.
-
5
What could be the reasons for your country not performing ALP testing on milk, colostrum, dairy and colostrum‐based products from non‐bovine (e.g. sheep, goats, horses, donkeys, camelids, etc.) and/or bovine species or for not having access to ALP testing data?
Routine ALP testing data are not submitted to any central repository (e.g. NRL or other official laboratories) ☐ ALP testing data are available but not easily accessible ☐ ALP testing data are mostly collected by food business operators as own‐checks and not available to competent authorities ☐ Collection of ALP testing data isn’t considered a priority ☐ Other reasons Please specify other reasons
Appendix C – Uncertainty analysis
1.
The sources of uncertainty associated with the available data have been summarised in tabular format (Table C.1), describing the nature or cause of the uncertainties. Additional considerations about the uncertainties in the assessment and their impact on the conclusions are described below.
Table C.1.
Sources of uncertainty identified in the assessment and assessment of the impact that these uncertainties could have on the conclusion
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Data related. The type of milk used | Both whole milk and skimmed milk with various fat contents of different species were included. | Considering that the ALP activity is correlated with the milk fat content, the type of milk tested influences the results. More specific, testing of skimmed milk can lead to an underestimation of the basal ALP values. When residual ALP levels of skimmed milk products are considered, the potential limit confirming pasteurisation could be underestimated. |
| Data related. Impact of pre‐heat treatment | Usage of thermisation could negatively influence ALP basal levels. | Residual ALP values after pasteurisation could be underestimated. |
| Data related. The conditions used for milk pasteurisation | Different methods for pasteurisation of milk are available with different temperatures and holding times. | Depending on the used t/T conditions, the ALP values after pasteurisation can be over‐ or underestimated. |
| Data related. The use of correct pasteurisation of the milk | The pasteurisation process of the milk used for deriving the ALP values in the tested samples is predominantly not known. | The inclusion of milk samples not correctly pasteurised could lead to an overestimation of residual ALP levels. |
| Data related. The time‐point of ALP testing. | For most of the ALP values in pasteurised milk and cheese, there is no information on when the testing was performed. It is recommended to do the analysis directly after pasteurisation to avoid contamination or re‐activation. | The samples that are not tested immediately after the pasteurisation could lead to an overestimation of the residual ALP levels. |
| Data related. The ALP activity determination method | Various methods are available to test the ALP activity in milk which differ in sensitivity. Enzymatic assays are substrate‐specific and it is not possible to compare the results. | Depending on the sensitivity and the method tested, ALP values cannot be compared. This was overcome by evaluating the relative basal and residual ALP activities and by using only data produced with the ISO Fluorophos method. |
| Data related. Country variability | Quantitative ALP data from non‐bovine milk samples could only be obtained from three countries and one study and for non‐bovine cheese only from three countries. | Variations for ALP values in different countries could lead to over‐/underestimation of ALP levels after pasteurisation. |
| Data related. Influence of factors on basal ALP levels. | ALP basal levels seem to be more variable in non‐bovine species due to a higher variation in breeds used in Europe, the seasonal production and the different lactation stages. This information was not available in the majority of the evidence used. | All these influence factors can either under‐ or overestimate the basal ALP levels in raw milk from non‐bovine species. |
| Data related. The temperature measurement during the thermal inactivation studies | In the thermal inactivation studies, the temperature was measured in the substrate and resembled isothermal conditions. The accuracy of the temperature measurement may affect the derived D‐values | These deviations may either under or over‐estimate the level of inactivation of ALP |
| Data related. The number of data points used for D‐value calculation | The number of incubation times (time points) used for the D‐value calculation was at least three. At least 5 incubation times are recommended to indicate valid results. | These deviations may either under or over‐estimate the level of inactivation of ALP |
| Data related. Missing limit for ALP in bovine cheese products. | Although there is an ISO standard to test the ALP activity in cheese, no legal limit for distinguishing pasteurised bovine cheese has been set. | The limit of 10 mU/g was used which is based on the proposed value by the former EURL. |
| Data related. ALP values in non‐bovine cheese | Few quantitative ALP values in non‐bovine cheese are available (5 of sheep and 7 of goat only) | This may over‐/underestimation the ALP activity in non‐bovine cheeses but the uncertainty is higher compared to milk. |
| Data related. Lacking information about colostrum and colostrum products | Information about basal and residual ALP activity of pasteurised colostrum is lacking. | This cannot be evaluated. |
| Methodology related. D‐value estimation | This methodology used assumes that the ALP inactivation follows first‐order kinetics, although deviations from linearity may occur. | These deviations may either under or over‐estimate the level of inactivation of ALP depending on the presence and extent of the shoulder or the tail. |
| Methodology related. z‐value estimation | The methodology used assumes a linear relationship between log D and temperature. However, non‐linearity may exist, the rate of change of inactivation (log D) with temperature may be non‐linear. | These deviations may either under or over‐estimate the level of inactivation of ALP |
ALP: alkaline phosphatase; MS: member states; ToR: Terms of Reference; WG: working group; EURL: European Union Reference Laboratory.
Appendix D – Background info for ALP basal levels
1.
Appendix E – Background info for ALP thermal inactivation
1.
Table E.1.
Summary of studies dealing with inactivation of Alkaline Phosphatase (ALP) activity in cows’, sheep, goat and equine milk
| Reference | Matrix | ALP detection method | Heating method | Initial ALP activity | Temperature (°C) | Incubation times tested | Relevance screeninga | Final ALP activity | D‐value cow (min) | D‐value sheep (min) | D‐value goat (min) | z‐value cow (°C) | z‐value sheep (°C) | z‐value goat (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dumitraşcu et al. (2014) | Raw skimmed milk | Spectrophotometric | Glass capillary tubes were filled with 100 μL of sample, sealed, and immersed in a water bath. After thermal treatment, the tubes were immediately immersed in ice water. All samples were analysed at least in duplicate | Cow: 59,240 mU/L; sheep: 2,191,000 mU/L; goat: 52,400 mU/L | 60 | 0, 5, 10, 15, 20, 30, 40 min | Yesb | NA | 54.95 | 36.63 | 22.52 | 6.31 | 6.15 | 6.91 |
| 62.5 | 0, 1, 5, 10, 15, 20 min | Yesb | NA | 21.37 | 15.36 | 15.87 | ||||||||
| 72.5 | 0, 3, 9, 14, 20, 24 s | Yesb | NA | 0.57 | 0.35 | 0.41 | ||||||||
| Raw whole milk (3.5%) | Spectrophotometric | Cow: 376,010 mU/L; sheep: 3,458,400 mU/L; goat: 86,740 mU/L | 60 | 0, 5, 10, 15, 20, 30, 40 min | Yesb | NA | 65.79 | 41.49 | 104.17 | 5.74 | 6.10 | 5.16 | ||
| 62.5 | 0, 1, 5, 10, 15, 20 min | Yesb | NA | 21.83 | 20.66 | 31.06 | ||||||||
| 72.5 | 0, 3, 9, 14, 20, 24 s | Yesb | NA | 0.42 | 0.40 | 0.38 | ||||||||
| IZSLT (2020) | Milk | Fluorimetric | Cow: 1,184,846 mU/L; sheep: 2,685,757 mU/L; goat: 135,861 mU/L | 63 | 0, 30 min | No | Cow: 159 mU/L; sheep: 272 mU/L; goat: 105 mU/L | NA | NA | NA | NA | NA | NA | |
| Klotz et al. (2008) | Raw milk | Fluorimetric | Heating of 2 L samples in a MicroThermic UHT/HTST Lab 25EDH system with dual cooler sections, city water and ice water. The final heat‐treated product exited the pasteuriser at ~ 5°C | Cow: 743,534 mU/L; sheep: 1,458,926 mU/L; goat: 60,787 mU/L | 60 | 0, 16 s | No | Cow: 593,989 mU/L; sheep: 1,112,390 mU/L; goat: 48,032 mU/L | NA | NA | NA | NA | NA | NA |
| 67 | 0, 16 s | No | Cow: 134,206 mU/L; sheep: 175,570 mU/L; goat: 5,566 mU/L | NA | NA | NA | ||||||||
| 72.5 | 0, 16 s | No | Cow: 85.3 mU/L; sheep: 181 mU/L; goat: 223 mU/L | NA | NA | NA | ||||||||
| 74 | 0, 16 s | No | Cow: 47.3 mU/L; sheep: 132.5 mU/L; goat: 195 mU/L | NA | NA | NA | ||||||||
| Lorenzen et al. (2010) | Raw bulk milk | Fluorimetric | Holder pasteurisation (LTLT‐heating) was performed in a batch process by heating 100 mL samples in a water bath at 62 ± 0.5°C for 30 min or 65 ± 0.5°C for 32 min with continuous stirring | Cow: 1,126,000 mU/L; sheep: 1,702,000 mU/L; goat: 70,000 mU/L | 62 | 0, 30 min | No | Cow: 318 mU/L; sheep: 593 mU/L; goat: 62 mU/L | NA | NA | NA | NA | NA | NA |
| 65 | 0, 32 min | No | Cow: 42 mU/L; sheep: 269 mU/L; goat: 40 mU/L | NA | NA | NA | ||||||||
| Lorenzen et al. (2010) | Raw bulk milk | Fluorimetric | Heating of 9 mL samples in test tubes (n = 4) at temperatures from 35 to 85°C for 90 ± 5 s. All heated milk samples were immediately cooled to 5°C and stored at −18°C until measurement | Cow: 688,000 mU/L; sheep: 1,545,000 mU/L; goat: 97,000 mU/L | 55 | 0, 90 s | No | Cow: 569,000 mU/L; sheep: 1,585,000 mU/L; goat: 90,000 mU/L | NA | NA | NA | NA | NA | NA |
| 65 | 0, 90 s | No | Cow: 18,000 mU/L; sheep: 660,000 mU/L; goat: 53,000 mU/L | NA | NA | NA | ||||||||
| 75 | 0, 90 s | No | Cow: 420 mU/L; sheep: 42,000 mU/L; goat: 2,600 mU/L | NA | NA | NA | ||||||||
| 85 | 0, 90 s | No | Cow: 100 mU/L; sheep: 50 mU/L; goat: 30 mU/L | NA | NA | NA | ||||||||
| Scintu et al. (2000) | Bulk milk | Fluorimetric | A sample of 20 mL was put into a screw glass vial; a thermometer was placed into the milk to determine how long it took for the milk to reach 63°C. The milk was pasteurised at 63°C for 30 min in a circulating water bath and cooled in an ice bath after heat treatment | Sheep: 2,456 mU/L | 63 | 0, 30 min | No | Sheep: 619 mU/L | NA | NA | NA | NA | NA | NA |
| Vamvakaki et al. (2006) | Milk | Fluorimetric | Three ovine, three caprine and three bovine individual milk samples were heated at 59°C in quantities of 5 mL. Aliquots were taken after 0c, 5, 10, 20, 40 and 80 min of heating. The aliquots were immediately cooled down in a water–ice slurry | Cow: 354 μg phenol/mL; sheep: 4,618 μg phenol/mL; goat: 214 μg phenol/mL | 59 | 0, 5, 10, 20, 40, (80) min | Yes | NA | 25.25 | 19.31 | 20.28 | NA | NA | NA |
| Photometric | 59 | 0, 5, 10, 20, 40, (80) min | Yes | NA | 43.67 | 21.01 | 30.86 | NA | NA | NA | ||||
| Wilińska et al. (2007) | Milk | Photometric | Inactivation experiments were performed in a thermostatic laboratory reactor equipped with a stirrer. Raw milk was added to preheated (or not preheated, when the initial activity was determined) sterile milk. Depending on the temperature used, thermal treatment was applied for 1–180 min. At different time intervals, 1 mL of sample was taken and cooled rapidly in ice water to stop the thermal inactivation | NA | 54 | 0–160 min | Yes | NA | 232.56 | NA | 112.36 | 5.60 | NA | 6.03 |
| 58 | 0–77 min | Yes | NA | 50.25 | NA | 22.52 | ||||||||
| 61 | 0–7 min | Yes | NA | 10.85 | NA | 6.09 | ||||||||
| 65 | 0–3.5 min | Yes | NA | 2.43 | NA | 2.43 | ||||||||
| 69 | 0–0.8 min | Yes | NA | 0.52 | NA | 0.37 |
NA: not available.
Relevance screening was done against a set of criteria: (i) the type of substrate used is (raw) milk from different animal species; (ii) the inactivation of ALP was measured over time; (iii) the ALP inactivation was measured using either the ISO Fluorophos method or other quantitative and validated methods; (iv) the temperature used should represent thermal inactivation (above 50°C), be measured in the substrate and resemble isothermal conditions; (v) the number of data points used for the D‐value calculation should be more than two points above the detection limit. It is indicated as ‘yes’ and the D‐value is reported when all the criteria were fulfilled, while it was indicated as ‘no’ and the final ALP activity at the last incubation time tested was reported when one of the last two criteria could not be fulfilled.
The D‐value (z‐value) has been calculated by EFSA using the data as summarised in the paper.
The heat treatment time started when the temperature reached 59°C.
Appendix F – rocedure for the evaluation of ALP (or other endogeneous enzyme markers) as an indicator of proper pasteurisation in milk of other species than bovine
1.
To validate the ALP activity (or other endogeneous enzyme marker) in milk of other species than bovine as a suitable indicator for correct pasteurisation, the inactivation kinetics of the enzyme has to be compared with the inactivation kinetics of some well‐known heat‐resistant food‐borne pathogens such as L. monocytogenes Scott A and/or Salmonella Senftenberg 775W (Eckner, 1992) and/or inactivation kinetics of bovine ALP (it can be discussed if other heat‐resistant pathogen(s) should be used) (Marchand et al., 2009).
Determination of total initial activity (raw milk) together with detection limit (of the method) allows to identify a window of a certain number of decimal (D) reductions where activity can be measured quantitatively. The enzyme is a suitable marker for pasteurisation on the condition that a certain D‐reduction can be identified within that window that agrees with a t/T‐combination guaranteeing sufficient inactivation of pathogenic microorganisms. It requires data that has to be derived from the literature or through experimental studies. As the ALP activity of the raw milk must be known, a sample before heating needs to be tested. Then the inactivation kinetics of the enzyme must be determined by measuring the activity as a function of time and temperature in the pasteurisation area (it is recommended that D‐values are determined at 5–6 different temperatures in the range between 55°C and 70°C; at any chosen temperature, there should be at least 5 point or incubation times on the linear part of the curve). This study should be performed with great care allowing a very rapid (almost instantaneous) heating to the isothermal temperature and cooling afterwards e.g. using small (e.g. 100‐μL) glass capillaries in an accurately temperature controlled warm water bath using a calibrated thermometer for temperature registration.
The inactivation kinetics of the pathogens must be known. These can be obtained in a similar way as described above or found in the scientific literature.
The detection limit of the used ALP activity determination method must also be verified (or based on literature data).
Then, the decimal reduction time (D‐value)25 of the enzyme and pathogens for each chosen temperature must be estimated. From these D‐values the z‐value26 of the enzyme and the microbial population can be derived. Now a temperature (linear scale) – time (logarithmic scale) plot can be obtained. On this, the inactivation of the pathogens can be plotted (a 6D reduction must be sufficient).
As to the enzyme, the number of D reductions between the activity in raw milk and the minimal detectable activity (detection limit) must be determined (preferably a method with a low detection limit must be used). Plot the inactivation curve on the graph using the just determined number of D reductions of the enzyme. (In the case of bovine milk, about 3,4D reductions correspond with the legal limit of 350 mU/L).
When the enzyme curve is just on the right side (i.e. a temperature time combination slightly higher than that for pathogen inactivation) of the pathogen plots, the enzyme is validated as a good suitable marker for correct pasteurisation (see Marchand et al. (2009) for an example). When the enzyme curve is on the left side of the pathogen plots, the enzyme is not a suitable marker for correct pasteurisation. When the enzyme curve is on the far‐right side of the pathogen plots, the enzyme is not very suitable as marker for correct pasteurisation since overheating would be necessary to inactivate the enzyme (this would be the case when LPO would be used).
Annex A – Protocol for the assessment of the use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2021.6576
Supporting information
Protocol for the assessment of the use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products
Suggested citation: EFSA (European Food Safety Authority) , Clawin‐Rädecker I, De Block J, Egger L, Willis C, Da Silva Felicio MT and Messens W, 2021. The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products. EFSA Journal 2021;19(4):6576, 73 pp. 10.2903/j.efsa.2021.6576
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00331
Waiver: In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management, a waiver was granted to Charlotte (Lotti) Egger, an expert of the Working Group. Pursuant to Article 21(6) of the aforementioned Decision, the concerned expert was allowed to take part in the discussion and in the drafting phase of the EFSA Scientific Report on the use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products, and was not allowed to be, or act as, a chairman, a vice chairman or rapporteur of the BIOHAZ Working Group on ALP milk pasteurisation (EFSA‐Q‐2020‐00331). Any competing interests are recorded in the respective minutes of the meetings of that Working Group.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to acknowledge the contribution of Katrin Bote to this report for the scientific support. EFSA also wishes to thank the external reviewers Avelino Alvarez Ordóñez and Robert Davies. EFSA also wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Approved: 6 April 2021
The Outcome of the public consultation is available under the Supporting Information section .
Notes
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, p. 55–205.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) 2074/2005 as regards official controls. OJ L 131, 17.5.2019, p. 51–100.
ISO 11816‐1 [IDF 155‐1:2013]. Milk and milk products – Determination of alkaline phosphatase activity – Part 1: Fluorimetric method for milk and milk‐based drinks. International Organization for Standardization, Geneva, Switzerland.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
According to the Commission Regulation (EU) 2017/2460 of October 2017, the European Union reference laboratory for milk and milk products (EURL‐MMP) has stopped its activities on 31 December 2017.
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJ L 139, 30.4.2004, p. 1–54.
Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. OJ L 139, 30.4.2004, p. 206–320.
Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra‐Community trade in bovine animals and swine. OJ 121, 29.7.1964, p. 1977–2012.
Council Directive 91/68/EEC of 28 January 1991 on animal health conditions governing intra‐Community trade in ovine and caprine animals. OJ L 46, 19.2.1991, p. 19–36.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, p. 1–26, as amended by Commission Regulation (EC) No 2019/229 of 7 February 2019 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs as regards certain methods, the food safety criterion for Listeria monocytogenes in sprouted seeds, and the process hygiene criterion and food safety criterion for unpasteurised fruit and vegetable juices (ready‐to‐eat). OJ L 37, 8.2.2019, p. 106–110.
ISO 11816‐2 [IDF 155‐2:2016]. Milk and milk products – Determination of alkaline phosphatase activity – Part 2: Fluorimetric method for cheese. International Organization for Standardization, Geneva, Switzerland.
ISO 3356:2009 [IDF 63:2009] Milk — Determination of alkaline phosphatase. International Organization for Standardization, Geneva, Switzerland.
ISO 22160:2007 [IDF 209:2007]. Milk and milk‐based drinks – Determination of alkaline phosphatase activity – Enzymatic photo‐activated system (EPAS) method. International Organization for Standardization, Geneva, Switzerland.
IDF Standard 82A:1987 – Milk and dried milk, buttermilk and buttermilk powder, whey and whey powder. International Dairy Federation.
ISO/AWI TS 4985 [IDF/RM 256]. Milk and milk‐based drinks – Determination of alkaline phosphatase activity – Fluorimetric microplate method. Under development. International Organization for Standardization, Geneva, Switzerland.
IDF standard 155A:1999. Milk and milk‐based drinks. Determination of alkaline phosphatase activity using a fluorometric method. International Dairy Federation standard, Brussels.
IDF standard 63:1971. Milk and milk powder, buttermilk and buttermilk powder, whey and whey powder. Determination of phosphatase activity (reference method), International Dairy Federation, Brussels.
ISO/TS 17193:2011 [IDF/RM 208:2011]. Milk – Determination of the lactoperoxidase activity – Photometric method (Reference method). International Organization for Standardization, Geneva, Switzerland.
ISO 13875:2005 [IDF 178:2005]. Liquid milk — Determination of acid‐soluble beta‐lactoglobulin content — Reverse‐phase HPLC method. International Organization for Standardization, Geneva, Switzerland.
ISO 11868:2007 [IDF 147:2007]. Heat‐treated milk — Determination of lactulose content — Method using high‐performance liquid chromatography. International Organization for Standardization, Geneva, Switzerland.
ISO 11285:2004 [IDF175:2004]. Milk ‐ Determination of lactulose content ‐ Enzymatic method. International Organization for Standardization, Geneva, Switzerland.
ISO 18329:2004 | IDF193: 2004 ‐ Milk and milk products ‐ Determination of Furosine content ‐ Ion‐pair reverse‐phase high‐performance liquid chromatography method. International Organization for Standardization, Geneva, Switzerland.
The D‐value is the time required at a given temperature to reduce the enzyme activity or a specific microbial population by a factor 10 (i.e. to reduce the enzyme activity by 90% or to kill 90% of the exposed microorganisms).
The temperature change required to change the D‐value by a factor of 10 (i.e. for one log10 increase or decrease in the D‐value).
References
- Adachi S, 1958. Formation of lactulose and tagatose from lactose in strongly heated milk. Nature, 181, 840–841. 10.1038/181840a0 [DOI] [PubMed] [Google Scholar]
- Adachi S and Patton S, 1961. Presence and significance of lactulose in milk products: a review. Journal of Dairy Science, 44, 1375–1393. 10.3168/jds.S0022-0302(61)89899-8 [DOI] [Google Scholar]
- Albillos SM, Reddy R and Salter R, 2011. Evaluation of alkaline phosphatase detection in dairy products using a modified rapid chemiluminescent method and official methods. Journal of Food Protection, 74, 1144–1154. 10.4315/0362-028X.JFP-10-422 [DOI] [PubMed] [Google Scholar]
- Alegbeleye OO, Guimarães JT, Cruz AG and Sant'Ana Sant'Ana AS, 2018. Hazards of a ‘healthy’ trend? An appraisal of the risks of raw milk consumption and the potential of novel treatment technologies to serve as alternatives to pasteurization. Trends in Food Science & Technology, 82, 148–166. 10.1016/j.tifs.2018.10.007 [DOI] [Google Scholar]
- Ali F, Hussain R, Qayyum A, Gul ST, Iqbal Z and Hassan MF, 2016. Milk somatic cell counts and some hemato‐biochemical changes in sub‐clinical mastitic dromedary She‐Camels (Camelus dromedarius). Pakistan Veterinary Journal, 36, 405–408. [Google Scholar]
- Andrews A, Anderson M and Goodenough PW, 1987. A study of the heat stabilities of a number of indigenous milk enzymes. Journal of Dairy Research, 54, 237–246. 10.1017/S0022029900025371 [DOI] [Google Scholar]
- Anifantakis EM and Rosakis PS, 1983. Alkaline phosphatase activity of sheep's milk and some factors affecting it. Egyptian Journal of Dairy Science, 11, 173–182. [Google Scholar]
- dos Anjos F, Machado A, Ferro C, Otto F and Bogin E, 1998. Gamma‐glutamyltransferase as a marker for the pasteurization of raw milk. Journal of Food Protection, 61, 1057–1059. 10.4315/0362-028x-61.8.1057 [DOI] [PubMed] [Google Scholar]
- Anon , 2019. Raw sheep's milk cheese linked to French Salmonella outbreak. Food Safety News. Available online: https://www.foodsafetynews.com/2019/08/raw-sheeps-milk-cheese-linked-to-french-salmonella-outbreak/ [Google Scholar]
- Aschaffenburg R, 1950. A simple turbidity test for sterilised milk. International Journal of Dairy Technology, 3, 236–237. 10.1111/j.1471-0307.1950.tb00747.x [DOI] [Google Scholar]
- Assis G, De Bivar Roseiro ML and Barbosa M, 2000. Determination of alkaline phosphatase activity in milk from indigenous Portuguese ewe and goat breeds by the fluorometric method. International Dairy Federation Bulletin, 351, 33. [Google Scholar]
- Balogh Z, Ferenczi E, Szeles K, Stefanoff P, Gut W, Szomor KN, Takacs M and Berencsi G, 2010. Tick‐borne encephalitis outbreak in Hungary due to consumption of raw goat milk. Journal of Virological Methods, 163, 481–485. 10.1016/j.jviromet.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Banks J and Muir DD, 2002. Variability of alkaline phosphatase in goat milk in relation to its use as an effective index of pasteurisation. 175 pp. [Google Scholar]
- Belkasmi F, Madani T, Mouffok C and Semara L, 2019. Enzymatic quality of colostrum in Ouled Djellal ewes, Algeria. Biological Rhythm Research, 1–9. 10.1080/09291016.2019.1621061 [DOI] [Google Scholar]
- Berger TM, Maurer J, Schaeren W and Egger L, 2008. Alkaline phosphatase activity in Swiss ewe's and goat's milk. Available online: https://www.agroscope.admin.ch/agroscope/de/home/publikationen/suchen/reihen-bis-2013/alp-forum/_jcr_content/par/externalcontent.external.exturl.pdf/aHR0cHM6Ly9pcmEuYWdyb3Njb3BlLmNoL2RlLUNIL0VpbnplbH/B1Ymxpa2F0aW9uL0Rvd25sb2FkP2VpbnplbHB1Ymxpa2F0aW9u/SWQ9MzQwOTc=.pdf [Accessed: 9 October 2008].
- Birlouez‐Aragon I, Nicolas M, Metais A, Marchond N, Grenier J and Calvo D, 1998. A rapid fluorimetric method to estimate the heat treatment of liquid milk. International Dairy Journal, 8, 771–777. 10.1016/s0958-6946(98)00119-8 [DOI] [Google Scholar]
- Birlouez‐Aragon I, Sabat P and Gouti N, 2002. A new method for discriminating milk heat treatment. International Dairy Journal, 12, 59–67. 10.1016/s0958-6946(01)00131-5 [DOI] [Google Scholar]
- Bylund G, 1995. Dairy Processing Handbook. Tetra Pak Processing Systems AB, 436 pp. [Google Scholar]
- Caboni P, Maxia D, Scano P, Addis M, Dedola A, Pes M, Murgia A, Casula M, Profumo A and Pirisi A, 2019. A gas chromatography‐mass spectrometry untargeted metabolomics approach to discriminate Fiore Sardo cheese produced from raw or thermized ovine milk. Journal of Dairy Science, 102, 5005–5018. 10.3168/jds.2018-15885 [DOI] [PubMed] [Google Scholar]
- CAC (Codex Alimentarius Commission), 2004. Code of hygienic practice for milk and milk products. CAC/RCP 57‐2004. Food and Agriculture Organization and World Health Organization (FAO and WHO). Available online: http://www.fao.org/fileadmin/user_upload/livestockgov/documents/CXP_057e.pdf
- Calabrese MG, Mamone G, Caira S, Ferranti P and Addeo F, 2009. Quantitation of lysinoalanine in dairy products by liquid chromatography–mass spectrometry with selective ion monitoring. Food Chemistry, 116, 799–805. 10.1016/j.foodchem.2009.03.041 [DOI] [Google Scholar]
- Claeys WL, Van Loey AM and Hendrickx ME, 2002. Intrinsic time temperature integrators for heat treatment of milk. Trends in Food Science & Technology, 13, 293–311. 10.1016/s0924-2244(02)00164-4 [DOI] [Google Scholar]
- Claeys WL, Cardoen S, Daube G, De Block J, Dewettinck K, Dierick K, De Zutter L, Huyghebaert A, Imberechts H, Thiange P, Vandenplas Y and Herman L, 2013. Raw or heated cow milk consumption: review of risks and benefits. Food Control, 31, 251–262. 10.1016/j.foodcont.2012.09.035 [DOI] [Google Scholar]
- Claeys WL, Verraes C, Cardoen S, De Block J, Huyghebaert A, Raes K, Dewettinck K and Herman L, 2014. Consumption of raw or heated milk from different species: an evaluation of the nutritional and potential health benefits. Food Control, 42, 188–201. 10.1016/j.foodcont.2014.01.045 [DOI] [Google Scholar]
- Clawin‐Rädecker I and Schlimme E, 1995. Determination of furosine in pasteurised milk by use of ion‐pair reversed‐phase liquid chromatography. Kieler Milchwirtschaftliche Forschungsberichte, 47, 169–175. [Google Scholar]
- Clawin‐Rädecker I and Schlimme E, 1998. Nachweis nichtenzymatisch glycosylierter proteine in boviner kolostralmilch und reifer frauenmilch durch die bestimmung des furosingehaltes. Kieler Milchwirtschaftliche Forschungsberichte, 50, 133–145. [Google Scholar]
- Clawin‐Rädecker I, Kiesner C and Schlimme E, 2000. Analysis of the acid‐soluble contents od a‐La, b‐LG, SA and of the immunglobulin fraction in pasteurised milk. Kieler milchwirtschaftliche Forschungsberichte, 52, 323–334. [Google Scholar]
- Deeth HC, 2006. Lipoprotein lipase and lipolysis in milk. International Dairy Journal, 16, 555–562. 10.1016/j.idairyj.2005.08.011 [DOI] [Google Scholar]
- Deeth HC and Lewis MJ, 2017. High temperature processing of milk and milk products. John Wiley and Sons Ltd. 574 pp. 10.1002/9781118460467 [DOI] [Google Scholar]
- Deeth HC, Datta N and Versteeg C, 2013. Nonthermal Technologies in Dairy Processing. In: Smithers GW, Augustin MA (eds.). Advances in Dairy Ingredients, pp. 161–215. 10.1002/9781118448205.ch6 [DOI] [Google Scholar]
- Dumitraşcu L, Stănciuc N, Stanciu S and Râpeanu G, 2012. Thermal inactivation of lactoperoxidase in goat, sheep and bovine milk – a comparative kinetic and thermodynamic study. Journal of Food Engineering, 113, 47–52. 10.1016/j.jfoodeng.2012.05.028 [DOI] [Google Scholar]
- Dumitraşcu L, Moschopoulou E, Aprodu I, Stanciu S, Râpeanu G and Stănciuc N, 2013. Assessing the heat induced changes in major cow and non‐cow whey proteins conformation on kinetic and thermodynamic basis. Small Ruminant Research, 111, 129–138. 10.1016/j.smallrumres.2012.12.019 [DOI] [Google Scholar]
- Dumitraşcu L, Stănciuc N, Stanciu S and Râpeanu G, 2014. Inactivation kinetics of alkaline phosphatase from different species of milk using quinolyl phosphate as a substrate. Food Science and Biotechnology, 23, 1773–1778. 10.1007/s10068-014-0242-x [DOI] [Google Scholar]
- Eberhard P and Gallmann P, 1994. Konsummilchsorten. Agrarforschung Schweiz, 1, 456–459. [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2020. Tick borne encephalitis ‐ France ‐ 2020. Communicable Disease Threats Report. Week 23, 31 May‐6 June 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Communicable-disease-threats-report-6-June-2020-PUBLIC.pdf
- Eckner KF, 1992. Fluorometric analysis of alkaline phosphatase inactivation correlated to Salmonella and Listeria inactivation. Journal of Food Protection, 55, 960–963. 10.4315/0362‐028X-55.12.960 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Martino L, Aiassa E, Halldórsson TI, Koutsoumanis PK, Naegeli H, Baert K, Baldinelli F, Devos Y, Lodi F, Lostia A, Manini P, Merten C, Messens W, Rizzi V, Tarazona J, Titz A and Vos S, 2020. Draft framework for protocol development for EFSA's scientific assessments. EFSA supporting publication 2020:EN‐1843. 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA Journal 2015;13(1):3940, 95 pp. 10.2903/j.efsa.2015.3940 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2017. EFSA Journal 2018;16(12):5500, 262 pp. 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger L, Nicolas M and Pellegrino L, 2016. Alkaline phosphatase activity in cheese as a tracer for cheese milk pasteurization. LWT ‐ Food Science and Technology, 65, 963–968. 10.1016/j.lwt.2015.09.033 [DOI] [Google Scholar]
- Faist J, 2000. Applied physics: smaller, faster Midinfrared lasers. Science, 290, 1713–1714. 10.1126/science.290.5497.1713 [DOI] [PubMed] [Google Scholar]
- FDA (Food and Agriculture Organization), 2017. Grade “A” Pasteurized Milk Ordinance – 2017. U.S. Department of Health and Human Services, Public Health Service, Food and Drug Administration. Revision. 449 pp. Available online: http://www.fda.gov/media/114169/download
- Felipe X, Capellas M and Law AJR, 1997. Comparison of the effects of high‐pressure treatments and heat pasteurization on the whey proteins in goat's milk. Journal of Agricultural and Food Chemistry, 45, 627–631. 10.1021/jf960406o [DOI] [Google Scholar]
- Fenoll J, Jourquin G and Kauffmann JM, 2002. Fluorimetric determination of alkaline phosphatase in solid and fluid dairy products. Talanta, 56, 1021–1026. 10.1016/s0039-9140(01)00585-9 [DOI] [PubMed] [Google Scholar]
- Fernandes AM, Balasegaram S, Willis C, Wimalarathna HM, Maiden MC and McCarthy ND, 2015. Partial failure of milk pasteurization as a risk for the transmission of Campylobacter from cattle to humans. Clinical Infectious Diseases, 61, 903–909. 10.1093/cid/civ431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley HN and Walker PG, 1965. Kinetic behaviour of calf‐intestinal alkaline phosphatase with 4‐methylumbelliferyl phosphate. Biochemical Journal, 97, 95–103. 10.1042/bj0970095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finot PA and Mauron J, 1972. Blockage of lysine by Mallard's reaction. II. Chemical properties of derivatives N‐(deoxy‐1‐D‐fructosyl‐1) and N‐(deoxy‐1‐D‐lactulosyl‐1) lysine. Helvetica Chimica Acta, 55, 1153–1164. 10.1002/hlca.19720550411 [DOI] [PubMed] [Google Scholar]
- Fishbein DB and Raoult D, 1992. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. American Journal of Tropical Medicine and Hygiene, 47, 35–40. 10.4269/ajtmh.1992.47.35 [DOI] [PubMed] [Google Scholar]
- Fox PF and Kelly AL, 2006. Indigenous enzymes in milk: overview and historical aspects—part 2. International Dairy Journal, 16, 517–532. 10.1016/j.idairyj.2005.09.017 [DOI] [Google Scholar]
- Garcell HG, Garcia EG, Pueyo PV, Martin IR, Arias AV and Alfonso Serrano RN, 2016. Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. Journal of Infection and Public Health, 9, 523–527. 10.1016/j.jiph.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Giacometti F, Bardasi L, Merialdi G, Morbarigazzi M, Federici S, Piva S and Serraino A, 2016. Shelf life of donkey milk subjected to different treatment and storage conditions. Journal of Dairy Science, 99, 4291–4299. 10.3168/jds.2015-10741 [DOI] [PubMed] [Google Scholar]
- Giangolini G, 2020. Re: use of ALP to verify pasteurisation in non‐bovine species. Message to Katrin Bote. 14 December 2020. E‐mail.
- Gopal N, Hill C, Ross PR, Beresford TP, Fenelon MA and Cotter PD, 2015. The prevalence and control of Bacillus and related spore‐forming bacteria in the dairy industry. Frontiers in Microbiology, 6, 1418. 10.3389/fmicb.2015.01418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood MH and Rampling AM, 1997. Evaluation of a fluorimetric method for detection of phosphatase in milk. PHLS Microbiology Digest, 14, 216–217. [Google Scholar]
- Griffiths MW, 1986. Use of milk enzymes as indices of heat treatment. Journal of Food Protection, 49, 696–705. 10.4315/0362-028X-49.9.696 [DOI] [PubMed] [Google Scholar]
- Hartkopf J and Erbersdobler HF, 1993. Stability of furosine during ion‐exchange chromatography in comparison with reversed‐phase high‐performance liquid chromatography. Journal of Chromatography A, 635, 151–154. 10.1016/0021-9673(93)83126-d [DOI] [Google Scholar]
- IDF (International Dairy Federation), 1986. Monograph on Pasteurised Milk (Bulletin 200). IDF, Brussels, Belgium. [Google Scholar]
- IDF (International Dairy Federation), 1991. Alkaline Phosphatase Test as a Measure of Correct Pasteurisation (Bulletin 262). IDF, Brussels, Belgium. [Google Scholar]
- IZSLT (Istituto Zooprofilattico Sperimentale del Lazio e della Toscana “M. Aleandri”), 2020. Sintesi ‐ Attività della fosfatasi alcalina nel latte di pecora, capra e bufala in relazione al trattamento termico di pastorizzazione: studio sperimentale per un limite di conformità. Progetti di Ricerca Corrente 2016. N. identificativo progetto: IZSLT 06/16 RC, 3 pp. http://www.izslt.it/bpractices/wp-content/uploads/2020/05/Sintesi-6.pdf
- Kamal M and Karoui R, 2017. Monitoring of mild heat treatment of camel milk by front‐face fluorescence spectroscopy. LWT ‐ Food Science and Technology, 79, 586–593. 10.1016/j.lwt.2016.11.013 [DOI] [Google Scholar]
- Katsoulos PD, Christodoulopoulos G, Minas A, Karatzia MA, Pourliotis K and Kritas SK, 2010. The role of lactate dehydrogenase, alkaline phosphatase and aspartate aminotransferase in the diagnosis of subclinical intramammary infections in dairy sheep and goats. Journal of Dairy Research, 77, 107–111. 10.1017/S0022029909990410 [DOI] [PubMed] [Google Scholar]
- Khamesipour F, Dida GO, Anyona DN, Razavi SM and Rakhshandehroo E, 2018. Tick‐borne zoonoses in the Order Rickettsiales and Legionellales in Iran: a systematic review. PLoS Neglected Tropical Diseases, 12. 10.1371/journal.pntd.0006722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindstedt P, Carić M and Milanović S, 2004. Pasta‐filata cheeses. In: Fox PF, McSweeney PLH, Cogan TM and Guinee TP (eds.). Cheese: Chemistry, Physics and Microbiology, Vol. 2. Academic Press. pp. 251–277. 10.1016/S1874-558X(04)80047-2 [DOI] [Google Scholar]
- Klotz V, Hill A, Warriner K, Griffiths M and Odumeru J, 2008. Assessment of the colorimetric and fluorometric assays for alkaline phosphatase activity in cow's, goat's, and sheep's milk. Journal of Food Protection, 71, 1884–1888. 10.4315/0362-028x-71.9.1884 [DOI] [PubMed] [Google Scholar]
- Knight AH and Fryer SM, 1989. The development of heat‐resistant phosphatase activity in raw milk. International Journal of Dairy Technology, 42, 81–86. 10.1111/j.1471-0307.1989.tb02163.x [DOI] [Google Scholar]
- Koksal Z, Gulcin I and Ozdemir H, 2016. An important milk enzyme: lactoperoxidase. In: Gigli I (ed.). Milk proteins ‐ from structure to biological properties and health aspects. ISBN: 978‐953-51‐2537-2. 10.5772/64416 [DOI] [Google Scholar]
- Kosikowski FV, 1988. Enzyme behavior and utilization in dairy technology. Journal of Dairy Science, 71, 557–573. 10.3168/jds.S0022-0302(88)79592-2 [DOI] [Google Scholar]
- Kuzuya Y, Kanamaru Y and Tanahashi T, 1982. Purification of bovine milk alkaline phosphatase with affinity chromatography and effect of flavonoids and saccharides on the enzyme activities. Nihon Chikusan Gakkaiho, 53, 45–49. 10.2508/chikusan.53.45 [DOI] [Google Scholar]
- Lee SHI, Cappato LP, Guimarães JT, Balthazar CF, Rocha RS, Franco LT, da Cruz AG, Corassin CH and de Oliveira CAF, 2019. Listeria monocytogenes in milk: occurrence and recent advances in methods for inactivation. Beverages, 5, 14. 10.3390/beverages5010014 [DOI] [Google Scholar]
- Linden G, 1979. Biochemical study of some aspects of milk alkaline phosphatase reactivation. Milchwissenschaft, 34, 329–332. [Google Scholar]
- Linden G, Chappelet‐Tordo D and Lazdunski M, 1977. Milk alkaline phosphatase. Stimulation by Mg2+ and properties of the Mg2+ site. Biochimica et Biophysica Acta, 483, 100–106. 10.1016/0005-2744(77)90012-2 [DOI] [PubMed] [Google Scholar]
- Little CL and De Louvois J, 1999. Health risks associated with unpasteurized goats’ and ewes’ milk on retail sale in England and Wales. A PHLS Dairy Products Working Group Study. Epidemiology and Infection, 122, 403–408. 10.1017/s0950268899002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi P, Avallone L, d'Angelo A, Mor T and Bogin E, 2000. Buffalo‐milk enzyme levels, their sensitivity to heat inactivation, and their possible use as markers for pasteurization. Journal of Food Protection, 63, 970–973. 10.4315/0362-028x-63.7.970 [DOI] [PubMed] [Google Scholar]
- Lorenzen PC, Martin D, Clawin‐Rädecker I, Barth K and Knappstein K, 2010. Activities of alkaline phosphatase, γ‐glutamyltransferase and lactoperoxidase in cow, sheep and goat's milk in relation to heat treatment. Small Ruminant Research, 89, 18–23. 10.1111/j.1471-0307.2010.00656.x [DOI] [Google Scholar]
- Lorenzen PC, Wernery R, Johnson B, Jose S and Wernery U, 2011a. Evaluation of indigenous enzyme activities in raw and pasteurised camel milk. Small Ruminant Research, 97, 79–82. 10.1016/j.smallrumres.2011.01.014 [DOI] [Google Scholar]
- Lorenzen PC, Clawin‐Rädecker I, Einhoff K, Hammer P, Hartmann R, Hoffmann W, Martin D, Molkentin J, Walte HG and Devrese M, 2011b. A survey of the quality of extended shelf life (ESL) milk in relation to HTST and UHT milk. International Journal of Dairy Technology, 64, 166–178. 10.1111/j.1471-0307.2010.00656.x [DOI] [Google Scholar]
- Lyster RLJ and Aschaffenburg R, 1962. The reactivation of milk alkaline phosphatase after heat treatment. Journal of Dairy Research, 29, 21–35. 10.1017/S0022029900010955 [DOI] [Google Scholar]
- Mahato K and Chandra P, 2019. Paper‐based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. Biosensors and Bioelectronics, 128, 9–16. 10.1016/j.bios.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Manolkidis KS, Alichanidis ES and Varvoglis AG, 1971. Effects of some antibiotics on the milk phosphatase pasteurization test. Journal of Dairy Science, 54, 335–338. 10.3168/jds.S0022-0302(71)85839-3 [DOI] [PubMed] [Google Scholar]
- Marchand S, Merchiers M, Messens W, Coudijzer K and De Block J, 2009. Thermal inactivation kinetics of alkaline phosphatase in equine milk. International Dairy Journal, 19, 763–767. 10.1016/j.idairyj.2009.05.009 [DOI] [Google Scholar]
- Martin NH, Boor KJ and Wiedmann M, 2018. Symposium review: effect of post‐pasteurization contamination on fluid milk quality. Journal of Dairy Science, 101, 861–870. https://doi.org/ 10.3168/jds.2017-13339 [DOI] [PubMed] [Google Scholar]
- McIntyre L, Fung J, Paccagnella A, Isaac‐Renton J, Rockwell F, Emerson B and Preston T, 2002. Escherichia coli O157 outbreak associated with the ingestion of unpasteurized goat's milk in British Columbia, 2001. Canada Communicable Disease Report, 1, 6–8. [PubMed] [Google Scholar]
- McLauchlin J, Aird H, Elliott A, Forester E, Jorgensen F and Willis C, 2020. Microbiological quality of raw drinking milk and unpasteurised dairy products: results from England 2013–2019. Epidemiology and Infection, 148(e135), 1–12. 10.1017/S0950268820001016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltretter J, Birlouez‐Aragon I, Becker CM and Pischetsrieder M, 2009. Assessment of heat treatment of dairy products by MALDI‐TOF‐MS. Molecular Nutrition & Food Research, 53, 1487–1495. 10.1021/jf503664y [DOI] [PubMed] [Google Scholar]
- Meltretter J, Wust J and Pischetsrieder M, 2014. Modified peptides as indicators for thermal and nonthermal reactions in processed milk. Journal of Agricultural and Food Chemistry, 62, 10903–10915. 10.1002/mnfr.200900008 [DOI] [PubMed] [Google Scholar]
- MFO‐3 , 1981. Determination of Phosphatase Activity in Dairy Products. Official Methods for the Microbiological Analysis of Foods. Health Protection Brand, Government of Canada. November 1981. 7 pp. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/research-programs-analytical-methods/analytical-methods/compendium-methods/official-methods-microbiological-analysis-foods-compendium-analytical-methods.html
- Miggiano GA, Mordente A, Martorana GE, Meucci E and Castelli A, 1983. In vitro effect of ascorbic acid on bovine kidney alkaline phosphatase activity. Acta Vitaminologica et Enzymologica, 5, 153–158. [PubMed] [Google Scholar]
- Montilla A and Olano A, 1997. Effect of the protein content and dilution on the lactulose/furosine ratio in heat‐treated milk. Milchwissenschaft, 52, 506–507. [Google Scholar]
- Morales F‐J, Romero C and Jimenez‐Perez S, 2000. Characterization of industrial processed milk by analysis of heat‐induced changes. International Journal of Food Science and Technology, 35, 193–200. 10.1046/j.1365-2621.2000.00334.x [DOI] [Google Scholar]
- Mottola A, Alberghini L, Giaccone V, Marchetti P, Tantillo G and Di Pinto A, 2018. Microbiological safety and quality of Italian donkey milk. Journal of Food Safety, 38. 10.1111/jfs.12444 [DOI] [Google Scholar]
- Murthy GK and Kaylor LO, 1990. Evaluation of APHA and AOAC II methods for phosphatase in butter and differentiation of milk and microbial phosphatases by agarose‐gel electrophoresis. Journal ‐ Association of Official Analytical Chemists, 73, 681–687. https://www.ncbi.nlm.nih.gov/pubmed/2177055 [PubMed] [Google Scholar]
- Murthy GK and Peeler JT, 1979. Rapid methods for differentiating reactivated from residual phosphatase in milk and cream: collaborative study. Journal ‐ Association of Official Analytical Chemists, 62, 822–827. https://www.ncbi.nlm.nih.gov/pubmed/227830 [PubMed] [Google Scholar]
- Murthy GK, Cox S and Kaylor L, 1976. Reactivation of alkaline phosphatase in ultra high‐temperature, short‐time processed liquid milk products. Journal of Dairy Science, 59, 1699–1710. 10.3168/jds.S0022-0302(76)84427-X [DOI] [PubMed] [Google Scholar]
- Murthy GK, Kleyn DH, Richardson T and Rocco RM, 1992. Alkaline phosphatase methods. In: Marshall RT (ed..). Standard Methods for the Examination of Dairy Products. American Public Health Association, Washington, DC. pp. 413–431. [Google Scholar]
- Narenji Sani R, Hajigolikhani B, Ahmadi‐Hamedani M and Kafshdouzan K, 2018. Diagnostic evaluation of milk lactate dehydrogenase and alkaline phosphatase activities by receiver operating characteristic analysis curve in early lactation of ewes with subclinical mastitis. Veterinary Research Forum, 9, 343–348. 10.30466/vrf.2018.33077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SD, Le TT, Knudsen LJ, Rauh V, Poulsen NA and Larsen LB, 2020. Development and application of a multiple reaction monitoring mass spectrometry method for absolute quantification of lysinoalanine and lanthionine in dairy products. International Dairy Journal, 105. 10.1016/j.idairyj.2020.104693 [DOI] [Google Scholar]
- Olano A, Calvo MM and Corzo N, 1989. Changes in the carbohydrate fraction of milk during heating processes. Food Chemistry, 31, 259–265. 10.1016/0308-8146(89)90067-8 [DOI] [Google Scholar]
- Painter CJ and Bradley RL Jr, 1997. Residual alkaline phosphatase activity in milks subjected to various time‐temperature treatments. Journal of Food Protection, 60, 525–530. 10.4315/0362-028X-60.5.525 [DOI] [PubMed] [Google Scholar]
- Parente E, Ricciardi A and Zotta T, 2020. The microbiota of dairy milk: a review. International Dairy Journal, 107. 10.1016/j.idairyj.2020.104714 [DOI] [Google Scholar]
- Park YW and Haenlein GFW, 2006. In: Park YW and Haenlein GFW (eds.). Handbook of Milk of Non‐Bovine Mammals. Blackwell Publishing. 10.1002/9780470999738 [DOI] [Google Scholar]
- Patil MP, Nagvekar AS, Ingole SD, Bharucha SV and Palve VT, 2015. Somatic cell count and alkaline phosphatase activity in milk for evaluation of mastitis in buffalo. Veterinary World, 8, 363–366. 10.14202/vetworld.2015.363-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino L, De Noni I and Resmini P, 1995. Coupling of lactulose and furosine indices for quality evaluation of sterilized milk. International Dairy Journal, 5, 647–659. 10.1016/0958-6946(95)00036-3 [DOI] [Google Scholar]
- Pellegrino L, Resmini P, De Noni I and Masotti F, 1996. Sensitive determination of lysinoalanine for distinguishing natural from imitation Mozzarella cheese. Journal of Dairy Science, 79, 725–734. 10.3168/jds.S0022-0302(96)76419-6 [DOI] [Google Scholar]
- Pellegrino L, Battelli G, Resmini P, Ferranti P, Barone F and Addeo F, 1997. Effects of heat load gradient occurring in moulding on characterization and ripening of Grana Padano. Le Lait, 77, 217–228. 10.1051/lait:1997215 [DOI] [Google Scholar]
- Persson Y, Larsen T and Nyman A‐K, 2014. Variation in udder health indicators at different stages of lactation in goats with no udder infection. Small Ruminant Research, 116, 51–56. 10.1016/j.smallrumres.2013.10.004 [DOI] [Google Scholar]
- PHLS (PHLS Communicable Disease Surveillance Centre), 1998. Cases of Escherichia coli O157 infection associated with unpasteurised cream in England. Eurosurveillance, 2. 10.2807/esw.02.43.01138-en [DOI] [PubMed] [Google Scholar]
- Pisano MB, Scano P, Murgia A, Cosentino S and Caboni P, 2016. Metabolomics and microbiological profile of Italian mozzarella cheese produced with buffalo and cow milk. Food Chemistry, 192, 618–624. 10.1016/j.foodchem.2015.07.061 [DOI] [PubMed] [Google Scholar]
- Punoo HA, 2018. Validation of milk product pasteurization by alkaline phosphatase activity. Concepts of Dairy & Veterinary Sciences, 1, 78–89. 10.32474/CDVS.2018.01.000113 [DOI] [Google Scholar]
- Ramos JM, Bernal E, Esguevillas T, Lopez‐Garcia P, Gaztambide MS and Gutierrez F, 2008. Non‐imported brucellosis outbreak from unpasteurized raw milk in Moroccan immigrants in Spain. Epidemiology and Infection, 136, 1552–1555. 10.1017/S0950268807000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SA, Christiansen A, Lee W, Banavara DS and Lopez‐Hernandez A, 2010. Invited review: the application of alkaline phosphatase assays for the validation of milk product pasteurization. Journal of Dairy Science, 93, 5538–5551. 10.3168/jds.2010-3400 [DOI] [PubMed] [Google Scholar]
- Rattray W, Gallmann P and Jelen P, 1997. Influence of protein standardization and UHT heating on the furosine value and freezing point of milk. Le Lait, 77, 297–305. 10.1051/lait:1997221 [DOI] [Google Scholar]
- Resmini P, Pellegrino L and Battelli G, 1990. Accurate quantification of furosine in milk and dairy products by a direct HPLC method. Journal of Food Science, 3, 173–183. http://hdl.handle.net/2434/177459 [Google Scholar]
- Resmini P, Pellegrino L and Cattaneo S, 2003. Furosine and other heat‐treatment indicators for detecting fraud in milk and milk products. Italian Journal of Food Science, 15, 473–484. [Google Scholar]
- Richards EL and Chandrasekhara MR, 1960. Chemical changes in dried skim‐milk during storage. Journal of Dairy Research, 27, 59–66. 10.1017/s0022029900010128 [DOI] [Google Scholar]
- Richardson LA, McFarren EF and Campbell JE, 1964. Phosphatase reactivation. Journal of Dairy Science, 47, 205–210. 10.3168/jds.S0022-0302(64)88622-7 [DOI] [Google Scholar]
- Ritota M, Di Costanzo MG, Mattera M and Manzi P, 2017. New trends for the evaluation of heat treatments of milk. Journal of Analytical Methods in Chemistry, 2017, 1864832. 10.1155/2017/1864832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E, Travanut M, Fabre L, Larreche S, Ramelli L, Pascal L, Guinard A, Vincent N, Calba C, Meurice L, Le Thien MA, Fourgere E, Jones G, Fournet N, Smith‐Palmer A, Brown D, Le Hello S, Pardos de la Gandara M, Weill FX and Jourdan‐Da Silva N, 2020. Outbreak of Salmonella Newport associated with internationally distributed raw goats’ milk cheese, France, 2018. Epidemiology and Infection, 148. 10.1017/S0950268820000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco RM, 1990. Fluorometric analysis of alkaline phosphatase in fluid dairy products. Journal of Food Protection, 53, 588–591. 10.4315/0362-028X-53.6.588 [DOI] [PubMed] [Google Scholar]
- Rola JG and Sosnowski M, 2010. Determination of alkaline phosphatase activity in milk and milk products by fluorimetric method. Bulletin of the Veterinary Institute in Pulawy, 54, 537–542. [Google Scholar]
- Rola JG and Sosnowski M, 2011. Determination of alkaline phosphatase activity in goat milk and cheese by fluorimetric method as a verification of efficacy of pasteurisation process. Bulletin of the Veterinary Institute in Pulawy, 55, 705–708. [Google Scholar]
- Rosenthal I, Bernstein S and Rosen B, 1996. Alkaline phosphatase activity in Penicillium roqueforti and in blue‐veined cheeses. Journal of Dairy Science, 79, 16–19. 10.3168/jds.S0022-0302(96)76328-2 [DOI] [PubMed] [Google Scholar]
- Salter RS and Fitchen J, 2006. Evaluation of a chemiluminescence method for measuring alkaline phosphatase activity in whole milk of multiple species and bovine dairy drinks: interlaboratory study. Journal of AOAC International, 89, 1061–1070. https://www.ncbi.nlm.nih.gov/pubmed/16915846 [PubMed] [Google Scholar]
- Sanders GP, Sager OS and Hupfer JA, 1954. Factors affecting the sensitivity and accuracy of the phosphatase test. Journal of Dairy Science, 37, 698–710. 10.3168/jds.S0022-0302(54)91315-6 [DOI] [Google Scholar]
- Scano P, Murgia A, Pirisi FM and Caboni P, 2014. A gas chromatography‐mass spectrometry‐based metabolomic approach for the characterization of goat milk compared with cow milk. Journal of Dairy Science, 97, 6057–6066. 10.3168/jds.2014-8247 [DOI] [PubMed] [Google Scholar]
- Scharer H, 1938. A rapid phosphomonoesterase test for control of dairy pasteurization. Journal of Dairy Science, 21, 21–34. 10.3168/jds.S0022-0302(38)95608-5 [DOI] [Google Scholar]
- Schlimme E, Clawin‐Rädecker I, Einhoff K, Kiesner C, Lorenzen P, Martin D, Meisel H, Molkentin J and Precht D, 1996. Studies on distinguishing features for evaluating heat treatment of milk. Kieler Milchwirtschaftliche Forschungsberichte, 48, 5–36. [Google Scholar]
- Scintu MF, Daga E and Ledda A, 2000. Evaluation of spectrophotometric and fluorometric methods for alkaline phosphatase activity determination in ewe's milk. Journal Food Protection, 63, 1258–1261. 10.4315/0362-028x-63.9.1258 [DOI] [PubMed] [Google Scholar]
- Serra B, Morales MD, Reviejo AJ, Hall EH and Pingarron JM, 2005. Rapid and highly sensitive electrochemical determination of alkaline phosphatase using a composite tyrosinase biosensor. Analytical Biochemistry, 336, 289–294. 10.1016/j.ab.2004.10.039 [DOI] [PubMed] [Google Scholar]
- Shakeel‐ur-Rehman Fleming CM, Farkye NY and Fox PF, 2003. Indigenous phosphatases in milk. In: Fox PF and McSweeney PLH (eds.). Advanced Dairy Chemistry—1 Proteins: Part A/Part B. Springer, US. pp. 523–543. 10.1007/978-1-4419-8602-3_14 [DOI] [Google Scholar]
- Shamsia SM, 2009. Nutritional and therapeutic properties of camel and human milks. International Journal of Genetics and Molecular Biology, 1, 52–58. 10.5897/IJGMB.9000048 [DOI] [Google Scholar]
- Sharma R and Ganguli N, 1974. Purification and properties of reactivated alkaline phosphatase from buffalo milk. Milchwissenschaft, 29, 79–84. [Google Scholar]
- Sharma SK, Sehgal N and Kumar A, 2003. Dry‐reagent strips for testing milk pasteurization. LWT ‐ Food Science and Technology, 36, 567–571. 10.1016/s0023-6438(03)00061-6 [DOI] [Google Scholar]
- Sharma R, Kaur S, Rajput YS and Kumar R, 2009. Activity and thermal stability of indigenous enzymes in cow, buffalo and goat milk. Milchwissenschaft‐Milk Science International, 64, 173–175. [Google Scholar]
- Siciliano RA, Mazzeo MF, Arena S, Renzone G and Scaloni A, 2013. Mass spectrometry for the analysis of protein lactosylation in milk products. Food Research International, 54, 988–1000. 10.1016/j.foodres.2012.10.044 [DOI] [Google Scholar]
- Sun L and Wang D, 2009. A new method to estimate the heat treatment of milk and milk‐like systems. International Journal of Dairy Technology, 62, 321–325. 10.1111/j.1471-0307.2009.00502.x [DOI] [Google Scholar]
- Tirelli AG and Pellegrino LM, 1995. Determination of furosine in dairy products by capillary zone electrophoresis: a comparison with the HPLC method. Italian Journal of Food Science, 4, 379–385. http://hdl.handle.net/2434/197548 [Google Scholar]
- Töpel A, 2004. Chemie und Physik der Milch. Naturstoff ‐ Rohstoff – Lebensmittel. Behr's Verlag., 758 pp. ISBN: 978‐3-95468‐037-5. [Google Scholar]
- Uniacke‐Lowe T and Fox PF, 2011. Milk: Equid Milk. Encyclopedia of Dairy Sciences, 2nd Edition. Academic Press. pp. 518–529. 10.1016/B978-0-12-374407-4.00318-6 [DOI] [Google Scholar]
- Uniacke‐Lowe T, Huppertz T and Fox PF, 2010. Equine milk proteins: chemistry, structure and nutritional significance. International Dairy Journal, 20, 609–629. 10.1016/j.idairyj.2010.02.007 [DOI] [Google Scholar]
- Vamvakaki AN, Zoidou E, Moatsou G, Bokari M and Anifantakis E, 2006. Residual alkaline phosphatase activity after heat treatment of ovine and caprine milk. Small Ruminant Research, 65, 237–241. 10.1016/j.smallrumres.2005.06.025 [DOI] [Google Scholar]
- Van Renterghem R, De Block J, Grijspeerdt K, Mortier L, Hendrickx M, Ludikhuyze L and Claeys W, 2000. Intrinsic indicators for the authenticity of heat‐treated consumption milk. Final Report. DWTC. Contract nr. NP/43/033 and NP/01/034.
- Verraes C, Claeys W, Cardoen S, Daube G, De Zutter L, Imberechts H, Dierick K and Herman L, 2014. A review of the microbiological hazards of raw milk from animal species other than cows. International Dairy Journal, 39, 121–130. 10.1016/j.idairyj.2014.05.010 [DOI] [Google Scholar]
- Vetsina F, Boukidi K and Roussis I, 2014. γ‐Glutamyltransferase, xanthine oxidase and total free sulfhydryls as potential markers for pasteurization treatments in dairy technology. Journal of Food and Nutrition Research, 53, 324–332. [Google Scholar]
- Villamiel M, Arias M, Corzo N and Olano A, 1999. Use of different thermal indices to assess the quality of pasteurized milks. European Food Research and Technology, 208, 169–171. [Google Scholar]
- Wallenhammar A, Lindqvist R, Asghar N, Gunaltay S, Fredlund H, Davidsson A, Andersson S, Overby AK and Johansson M, 2020. Revealing new tick‐borne encephalitis virus foci by screening antibodies in sheep milk. Parasites & Vectors, 13, 185. 10.1186/s13071-020-04030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernery U, Maier U, Johnson B, George RM and Braun F, 2006. Comparative study on different enzymes evaluating heat treatment of dromedary milk. Milchwissenschaft‐Milk Science International, 61, 281–285. [Google Scholar]
- Wernery U, Fischbach S, Johnson B and Jose S, 2008. Evaluation of alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT) and lactoperoxidase (LPO) activities for their suitability as markers of camel milk heat inactivation. Milchwissenschaft‐Milk Science International, 63, 265–267. [Google Scholar]
- Wijayanti HB, Bansal N and Deeth HC, 2014. Stability of whey proteins during thermal processing: a review. Comprehensive Reviews in Food Science and Food Safety, 13, 1235–1251. 10.1111/1541-4337.12105 [DOI] [Google Scholar]
- Wilińska A, Bryjak J, Illeová V and Polakovič M, 2007. Kinetics of thermal inactivation of alkaline phosphatase in bovine and caprine milk and buffer. International Dairy Journal, 17, 579–586. 10.1016/j.idairyj.2006.08.008 [DOI] [Google Scholar]
- Willis C, Jorgensen F, Aird H, Elviss N, Fox A, Jenkins C, Fenelon D, Sadler‐Reeves L and McLauchlin J, 2018. An assessment of the microbiological quality and safety of raw drinking milk on retail sale in England. Journal of Applied Microbiology, 124, 535–546. 10.1111/jam.13660 [DOI] [PubMed] [Google Scholar]
- Wright RC and Tramer J, 1953. Reactivation of milk phosphatase following heat treatment. Journal of Dairy Research, 20, 177–188. 10.1017/s0022029900006828 [DOI] [Google Scholar]
- Ying C, Wang H‐T and Hsu J‐T, 2002. Relationship of somatic cell count, physical, chemical and enzymatic properties to the bacterial standard plate count in dairy goat milk. Livestock Production Science, 74, 63–77. 10.1016/s0301-6226(01)00290-1 [DOI] [Google Scholar]
- Zarrilli A, Micera E, Lacarpia N, Lombardi P, Pero ME, Pelagalli A, d'Angelo D, Mattia M and Avallone L, 2003. Evaluation of goat colostrum quality by determining enzyme activity levels. Livestock Production Science, 83, 317–320. 10.1016/s0301-6226(03)00076-9 [DOI] [Google Scholar]
- Zehetner G, Bareuther C, Henle T and Klostermeyer H, 1995. Inactivation kinetics of gamma‐glutamyltransferase during the heating of milk. Zeitschrift für Lebensmitteluntersuchung und ‐Forschung, 201, 336–338. 10.1007/BF01192728 [DOI] [PubMed] [Google Scholar]
- Ziobro GC and McElroy KM, 2013. Fluorometric detection of active alkaline phosphatase and gamma‐glutamyl transferase in fluid dairy products from multiple species. Journal of Food Protection, 76, 892–898. 10.4315/0362-028X.JFP-12-302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the assessment of the use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products


