Abstract
The Lyme disease bacterium Borrelia burgdorferi has 7–11 periplasmic flagella (PF) that arise from the cell poles and extend toward the midcell as a flat-ribbon, which is distinct from other bacteria. FlhF, a signal recognition particle (SRP)-like GTPase, has been found to regulate the flagellar number and polarity; however, its role in B. burgdorferi remains unknown. B. burgdorferi has an FlhF homolog (BB0270). Structural and biochemical analyses show that BB0270 has a similar structure and enzymatic activity as its counterparts from other bacteria. Genetics and cryo-electron tomography studies reveal that deletion of BB0270 leads to mutant cells that have less PF (4 ± 2 PF per cell tip) and fail to form a flat-ribbon, indicative of a role of BB0270 in the control of PF number and configuration. Mechanistically, we demonstrate that BB0270 localizes at the cell poles and controls the number and position of PF via regulating the flagellar protein stability and the polar localization of the MS-ring protein FliF. Our study not only provides the detailed characterizations of BB0270 and its profound impacts on flagellar assembly, morphology and motility in B. burgdorferi, but also unveils mechanistic insights into how spirochetes control their unique flagellar patterns.
Keywords: Borrelia burgdorferi, Lyme disease, motility, periplasmic flagella, signal recognition particle (SRP)-GTPase
1 |. INTRODUCTION
The flagellum is a nanomachine that propels the bacterial cells toward favorable environments or away from toxic ones [for review see (Berg, 2003; Chevance & Hughes, 2008; Macnab, 2003)]. In a plethora of bacterial pathogens, flagella and their empowered motility are directly involved in infectious processes (e.g., colonization, invasion, dissemination, tissue tropism, immune evasion and innate immunity) (Charon et al., 2012; Haiko & Westerlund-Wikstrom, 2013; Hayashi et al., 2001; Josenhans & Suerbaum, 2002; Motaleb, Liu, & Wooten, 2015; Rossez, Wolfson, Holmes, Gally, & Holden, 2015). Flagella are sophisticated and composed of more than 30 different proteins (Berg, 2003; Chevance & Hughes, 2008). These proteins are sequentially assembled into three mechanic modules: the basal body, the flagellar hook and the filament. The basal body is embedded within the cell envelope and works as a reversible rotary motor; and the flagellar hook and filament extend outward to the cell exterior, acting as a universal joint and a propeller respectively.
The basal body is a large complex consisting of several functional units: the MS/C ring (rotor), the rod (driveshaft), the L/P rings (bushings), the stator (torque generator) and the flagellar export apparatus (also known as type III secretion system, T3SS) (Erhardt, Namba, & Hughes, 2010). The motor is driven by an inward-directed electrochemical gradient of protons or sodium (Fung & Berg, 1995). The torque generated by the motor is mechanically transmitted to the filament via the rod-hook complex, leading to the rotation of the flagellar filament, which propels the bacterial cells forward. Given its complexity and exquisiteness, bacteria have evolved complex mechanisms to spatially and temporally orchestrate the process of flagellar protein synthesis and assembly. Our understanding of flagellar structure and assembly as well as its pertinent regulatory mechanisms mainly stems from three prototype bacteria, Escherichia coli, Salmonella enterica and Bacillus subtilis (Berg, 2003; Chevance & Hughes, 2008; Mukherjee & Kearns, 2014) and later extends to other bacteria species (Lertsethtakarn, Ottemann, & Hendrixson, 2011). Bacteria are very diverse but they share some common characteristics in terms of flagellar structure, assembly and regulation (Chevance & Hughes, 2008; Erhardt et al., 2010; Minamino, Imada, & Namba, 2008; Zhao, Norris, & Liu, 2014). For instance, genome comparison, sequence alignment, structural biology and cryo-electron tomography analyses have shown that bacterial flagellar structures and their pertinent proteins are highly conserved. In addition, bacteria have evolved a transcriptional cascade control mechanism to regulate their flagellar gene expression, protein stoichiometry and assembly (Aldridge & Hughes, 2002; Mukherjee & Kearns, 2014).
In contrast to the conservativeness of flagellar structure, the number and placement of flagella are diverse and vary from species to species (Boltjes, 1948; Leifson, 1951; Pallen, Penn, & Chaudhuri, 2005). While some bacteria have multiple flagella along the length of the cell, others restrict single or multiple flagella to the cell poles. The pattern of flagellar arrangement is so specific and constant that it is often used for the bacteria classification and nomenclature. Based on the types of flagellar arrangements, bacteria can be divided into the following: monotrichous (single flagellum at one pole), lophotrichous (tuft of flagella at one pole), amphitrichous (single flagellum at both poles), amphilophotrichous (tuft of flagella at both poles), and peritrichous (flagella distributed over the entire cell surface). For those that have polar flagella, an intriguing question is how those bacteria precisely control their flagellar number and placement. Studies from several polar flagellated bacteria such as Vibrio cholerae and Pseudomonas aeruginosa have demonstrated that those bacteria have evolved a specific mechanism to control their flagellar number and polarity mainly through FlhF and FlhG, two nucleotide-binding proteins [for review see (Bulyha, Hot, Huntley, & Sogaard-Andersen, 2011; Keilberg & Sogaard-Andersen, 2014; Schuhmacher, Thormann, & Bange, 2015)]. FlhF is a signal recognition particle (SRP)-like GTPase that plays a critical role in the control of flagellar number and polarity. FlhF localizes at the cell pole, although the underlying mechanism remains unknown, and controls flagellar polarity likely by targeting the MS-ring (FliF) to the cell pole. Thus, deletion of flhF often leads to mutant cells with flagella misplaced at lateral rather than at the cell pole. FlhG, also annotated as FleN in some bacterial species, is a MinD-like ATPase which when mutated results in the mutant cells with more misplaced flagella (Dasgupta, Arora, & Ramphal, 2000). Mechanistically, accumulating evidence from genetic, biochemical and structural analyses suggests that FlhF and FlhG employ a molecular switch mechanism to regulate the bacterial flagellar pattern (Bange et al., 2011; Bange, Petzold, Wild, Parlitz, & Sinning, 2007). As an SRP-GTPase, FlhF forms a GTP-dependent homodimer, which represents the “ON” state. Upon GTP-hydrolysis, the FlhF homodimer dissociates into its monomeric “OFF” state. Like FlhF, FlhG is switched-on and switched-off upon ATP binding and hydrolysis. Monomeric FlhG (“OFF” state) localizes in the cytoplasm. FlhF and FlhG interact with each other forming an active regulatory circuit, which controls, perhaps in concert with other factors as well, the flagellar polarity presumably via targeting the MS-ring protein FliF to the cell pole and precisely regulates the flagellar number through the flagellar transcription regulatory hierarchy (e.g., transcription factors FleQ, sigma28 and sigma54) (Balaban, Joslin, & Hendrixson, 2009; Correa, Peng, & Klose, 2005; Murray & Kazmierczak, 2006; Navarrete et al., 2019) and/or via controlling flagellar protein production (Gulbronson et al., 2016). It has also been speculated that during the cell division, FlhF and FlhG might interact with other effectors synchronizing the processes of cell division and flagellar biogenesis (Bulyha et al., 2011; Keilberg & Sogaard-Andersen, 2014; Schuhmacher, Thormann, et al., 2015). In support of this speculation, a deletion mutant of flhG in Campylobacter jejuni has multiple misplaced flagella and division defects, which is exemplified by the formation of minicells (Balaban & Hendrixson, 2011).
Spirochetes are a group of bacteria that can be readily recognized by their distinct cell shapes (helical or planar wave-like) and an unusual means of corkscrew-like motility, which allows them to swim in a highly viscous, gel-like medium, such as that found in connective tissue, that inhibits the motility of most other bacteria [for review see (Charon et al., 2012; Picardeau, 2017; Radolf et al., 2016; Rosa, Tilly, & Stewart, 2005)]. Spirochetes are diverse but all belong to the phylum of Spirochaetes. Based on their habitats and whether or not they cause disease, spirochetes can be classified into free-living, symbiotic and pathogenic groups. Some in the latter group cause human illnesses, such as leptospirosis, Lyme disease, relapsing fever, syphilis and yaws. Compared to other bacteria, one of the unique features of spirochetes is that they all have periplasmic flagella (PF) between the outer membrane and the peptidoglycan layer (Charon et al., 2012). The number and length of PF vary from species to species. While some spirochetes have a short single flagellum at the cell poles, others have multiple long PF that arise from the cell poles and overlap at the middle of the cells (Izard et al., 2009; Liu et al., 2010, 2009; Raddi et al., 2012; Sze et al., 2011). For instance, Leptospira interrogan, the causative agent of leptospirosis, has a single short flagellum inserted subterminally at each pole, extending toward the middle of the cell without overlapping (Raddi et al., 2012; Wunder et al., 2016). In contrast, the Lyme disease spirochete, Borrelia burgdorferi, has 7–11 long helical PF that originate from the cell poles, wrap around the cell cylinder, and extend toward the middle of the cell with overlaps (Liu et al., 2009). The syphilis spirochete, Treponema pallidum and oral spirochete, Treponema denticola (associated with periodontitis), have 2–3 long PF that overlap at the middle of the cell (Izard et al., 2009; Kurniyati et al., 2017; Liu et al., 2009). The PF pattern of spirochetes is different from other polar flagellated bacteria; however, its underlying regulatory mechanism remains largely unknown.
Borrelia burgdorferi is the causative agent of Lyme disease, which is the most prevalent tick-borne disease in the United States and Europe (Burgdorfer et al., 1982; Sanchez, Vannier, Wormser, & Hu, 2016; Steere et al., 1983, 2016). Previous studies have unveiled that motility plays a critical role in the pathogenicity of B. burgdorferi (e.g., dissemination, immune evasion, and transmission between the tick vector and mammalian hosts during the infectious cycle) (Dunham-Ems et al., 2009; Harman et al., 2012; Li, Xu, Zhang, & Liang, 2010; Motaleb et al., 2015; Sultan et al., 2013). Due to its medical importance, genetic tractability and small cell diameter suitable for cryo-electron tomography (cryo-ET) analyses, B. burgdorferi has emerged as a model organism to study the regulation, structure and assembly of PF, motility, as well as chemotaxis in spirochetes (Brisson, Drecktrah, Eggers, & Samuels, 2012; Charon et al., 2012; Liu et al., 2009; Rosa et al., 2005; Zhao et al., 2013). During the last decade, significant progress has been achieved, which confers B. burgdorferi a unique paradigm to understanding spirochete motility and chemotaxis (Charon et al., 2012; Zhao et al., 2014). The genome of B. burgdorferi encodes a homolog of FlhF (BB0270) and its role remains largely unknown. In this report, we hypothesized that BB0270 regulates the number and polarity of PFs in B. burgdorferi and tested this hypothesis by using a comprehensive approach of bioinformatics, genetics, biochemistry and cryo-ET. The results indicate that BB0270 is a GTPase that plays an essential role in the regulation of PF number and position in B. burgdorferi. The molecular mechanism involved is explored. The results obtained here shed new light into understanding the molecular mechanism by which spirochetes control their unique flagellar pattern.
2 |. RESULTS
2.1 |. Reinterpreting the flgB motility gene operon
By using DNA sequencing analysis, Ge et al identified a large motility gene cluster (21 kb) that encodes 26 open reading frames (orfs, starts from BB0294 and ends at BB0269) (Ge, Old, Saint Girons, & Charon, 1997). Transcription analyses showed that those 26 orfs are co-transcribed, forming a large motility gene operon that is initiated by a sigma70-like promoter (Figure S1a). This cluster is referred to as the flgB operon and the promoter identified upstream of flgB is designated as PflgB. Among those 26 genes, 22 are motility- and flagella-related genes, which can be found in other bacteria. The rest of the four orfs have unknown functional domains or motifs; thus, they are named as flbA (BB0287), B (BB0286), C (BB0285), and D (BB0282) (fl, for flagellum; b for Borrelia) (Fraser et al., 1997; Ge et al., 1997). Interestingly, B. subtilis has a similar large gene cluster (named fla/che) to the flgB operon (Figure S1a), though one is Gram-positive and the other is Gram-negative. Nearly all the genes in the fla/che operon have known functional domains or motifs (Guttenplan, Shaw, & Kearns, 2013). By comparing to the fla/che operon in context with protein domain search, we found that FlbC is a homolog of FliK (Figure S2a), a protein that controls the length of the flagellar hook (Erhardt, Singer, Wee, Keener, & Hughes, 2011; Shibata et al., 2007), and FlbD is a homolog of SwrD (Figure S2b), a protein that is required for the swarm motility of B. subtilis (Hall, Subramanian, Oshiro, Canzoneri, & Kearns, 2018). FlbA is present only in the Borrelia genus. We did not find any functional domain in FlbA, suggesting that it might be unique to Borrelia.
There are another five orfs downstream of flhG (BB0269) in the same orientation as other genes in the flgB operon (Figure S1a). Co-reverse transcription PCR (Co-RT-PCR) analyses using seven pairs of primers across junctions between flhG and each of these five orfs showed that they are co-transcribed with flhG, indicating that these five genes are also part of the flgB operon (Figure S1b). Among those five genes, BB0268 encodes a homolog of Hfq, a RNA chaperon (Lybecker, Abel, Feig, & Samuels, 2010); and BB0264 is DnaK, a heat shock protein of B. burgdorferi (Fraser et al., 1997). The rest of the three are hypothetical proteins (HP). Blast and protein domain search revealed that BB0267 is a homolog of flagella assembly protein A (FapA) (Figure S2c), a protein that controls Vibrio vulnificus flagellar biosynthesis and polar location (Park, Park, Lee, Kim, & Seok, 2016). There were no functional domains or motifs identified in BB0266 and BB0265. Herein, revisiting the flgB operon identifies additional five genes, extending this operon to total 26.3 kb that encodes 31 genes, and three functionally unknown orfs (BB0285, BB0282, and BB0267) that are annotated as fliK, swrD and fapA respectively. Future genetic studies will help to elucidate the role of these genes in the flagellar biosynthesis and motility of B. burgdorferi.
2.2 |. BB0270 is an FlhF-like GTPase
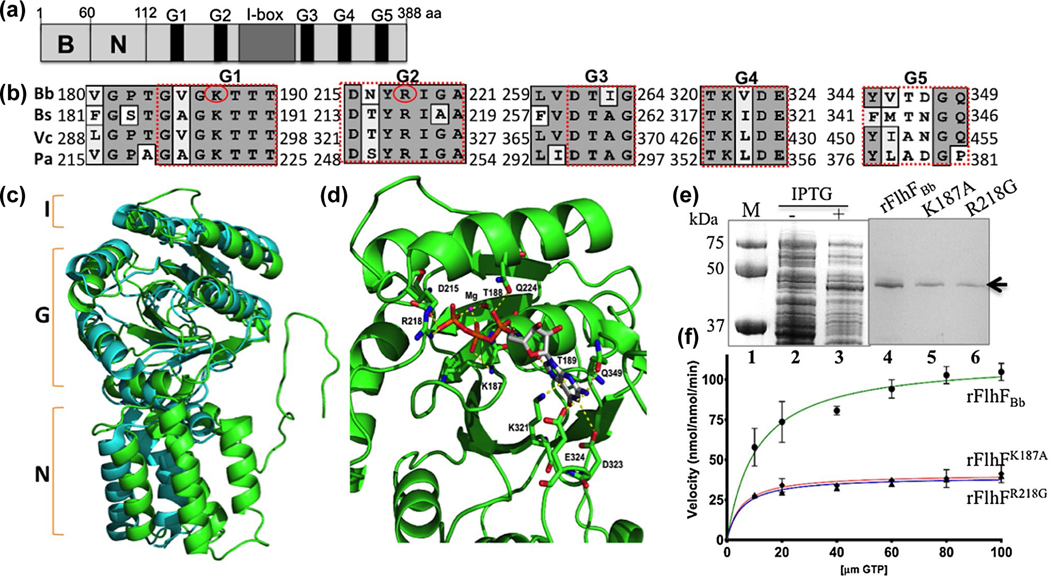
BB0270 is within the flgB operon (Figure S1) and is annotated as a flagellar biosynthesis protein FlhF (hereafter, named FlhFBb), which is composed of 388 amino acids (aa) with a theoretical isoelectric point (pI) of 8.38 and a molecular weight (MW) of 44.37 kDa (Fraser et al., 1997). Similar to its counterparts from other bacteria such as B. subtilis and V. cholerae, FlhFBb also harbors three domains: a N-terminal basic (B) domain (1–82 aa), a central N domain (83–171 aa) and a C-terminal GTPase (G) domain (172–388 aa) (Figure 1a). Blast search and sequence alignment analyses revealed that the B- and N-domains are conserved in the Borrelia genus but share minimal sequence similarity to those domains in other bacterial FlhF proteins. The G-domain of FlhFBb is conserved, for example, sharing 44% ~ 47% sequence similarity and 22% ~ 30% identity to the G-domains of FlhF in B. subtilis, V. cholerae and P. aeruginosa. Similar to other FlhF proteins, the G-domain of FlhFBb contains conserved nucleotide-binding elements (G1-G5) (Figure 1b). The crystal structure of B. subtilis FlhF (BsFlhF) is solved (Bange et al., 2011; Bange, Petzold, Wild, Parlitz, et al., 2007). Homology modeling analysis demonstrated that FlhFBb has a similar structural topology as BsFlhF: a N-terminal α-helical domain (N), an I-box insertion (I) and a GTPase domain (G) (Figure 1c). The G-domain of FlhFBb is composed of seven α-helices and eight β-sheets. The residues involved in GTP and magnesium ion (Mg2+) binding are conserved (Figure 1d). These residues include the KTTT motif in G1 (K187, T188, T189 and T190), aspartate 215 (D215) and arginine 218 (R218) in G2, lysine 321 (K321) and glutamate 323 (D323) in G4 and glutamine 349 (Q349) in G5. The residues in G1 and G2 are directly involved in GTP binding; thus, they are critical for the GTPase activity of FlhF. The last three residues in G4 and G5 stabilize the binding of GTP with FlhF proteins through forming hydrogen bonds with the purine ring of GTP. The sequence and structural similarity suggests that FlhFBb functions as a GTPase like its counterpart from other bacteria.
FIGURE 1.
BB0270 is an FlhF-like GTPase. (a) Domain structure of BB0270 (FlhFBb). The positions of the N-terminal basic (B), the central (N) domains, the conserved nucleotide-binding elements (G1-G5) as well as the I-box in FlhFBb are indicated. (b) Multiple sequence alignments of different FlhF G-domains. The numbers represent the positions of aa in the FlhF proteins of B. burgdorferi (Bb), B. subtilis (Bs), V. cholerae (Vc) and P. aeruginosa (Pa). Red circles indicate two residues that are replaced by site-directed mutagenesis. The GenBank accession numbers for these proteins: FlhFBb (WP_002556869), BsFlhF (WP_003231945), VcFlhF (WP_001881782), and PaFlhF (WP_003114281). The alignment was carried out using the program MacVector 10.6. (c) Homology modeling analysis shows that FlhFBb shares a similar structural topology to BsFlhF. The figure was generated by homology modeling using BsFlhF (PDB ID: 2PX3) as a template. Green is FlhFBb and cyan is BsFlhF. (d) FlhFBb harbors a conserved GTP and Mg2+ binding site. This is a close up view of the GTP and Mg2+ binding site of FlhFBb. The map was generated by homology modeling using BsFlhF (PDB ID: 2PX3) (Bange, Petzold, Wild, Parlitz, et al., 2007) as a template. The conserved aa involved GTP and Mg2+ binding sites were as labeled. (e) SDS-PAGE analysis of GST-FlhFBb recombinant proteins expressed in E. coli. Lane 1, molecular marker; lane 2 and lane 3, E. coli whole cell lysates induced without or with IPTG; lane 4–6, purified wild type (rFlhFBb) and two mutated (rFlhFK187A and rFlhFR218G) recombinant proteins. (f) Michaelis-Menten plots show kinetic plots for the GTPase activity of purified rFlhFBb and two variant recombinant proteins. The final data were expressed as means ± standard deviations (SD) of triplicates from three independent assays. The detail enzymatic parameters are present in Table 1
As a family of SRP-type GTPases, FlhF hydrolyzes GTP, generating GDP and free phosphate (Bange et al., 2011; Montoya, Svensson, Luirink, & Sinning, 1997). To determine if FlhFBb functions as a GTPase, we measured its enzymatic activity using recombinant proteins. Similar to other FlhF proteins, the full-length recombinant FlhFBb is insoluble (Bange, Petzold, Wild, & Sinning, 2007; Schniederberend, Abdurachim, Murray, & Kazmierczak, 2013). We then attempted to express the NG domain (80–388 aa) with different tags in various expression vectors and cell lines and found that the addition of N-terminal GST led to soluble recombinant proteins. In addition to the wild-type recombinant protein (rFlhFBb), two point-mutated proteins (rFlhFK187A and rFlhFR218G) were constructed by replacing K187 with alanine (A) and R218 with glycine (G). Previous studies indicate that these two residues are invariant and required for the GTPase activity in some bacterial FlhF proteins (Bange et al., 2011; Kusumoto, Nishioka, Kojima, & Homma, 2009; Schniederberend et al., 2013). By using the affinity and size-exclusion chromatography, the GST-tagged proteins were purified to homogeneity (Figure 1e). Their enzymatic activity was measured by monitoring the release of free phosphate as a product of GTP hydrolysis using a GTPase detection kit, as previously described (Liang & Connerton, 2018). The GTP hydrolysis activity of FlhF recombinant proteins fits single substrate steady-state kinetics (Figure 1f). Based on the Michaelis-Menten equations, their kinetic parameters were calculated (Figure 1f and Table 1). Compared to the wild-type recombinant FlhF (rFlhFBb), the two mutants have a smaller Michaelis constant (km) and maximal velocity (Vmax) and a lower rate of substrate turnover (kcat), resulting in approximately a 1.5-fold reduction in the enzymatic efficiency (kcat/km) (Figure 1e and Table 1). A similar phenotype was found in P. aeruginosa FlhF (PaFlhF), for example, PaFlhF R251G (identical to R218G in FlhFBb) has a lower km for GTP but a similar kcat to the wild type (Balaban et al., 2009; Schniederberend et al., 2013). Collectively, these results indicate that FlhFBb functions as a GTPase in a similar manner as its counterpart from other bacteria.
TABLE 1.
GTP hydrolysis activity of wild-type and mutant FlhF proteins
| FlhF constructs | km (μM) | Vmax (μmol/min) | kcat (min−1) | kcat/km(M/min) |
|---|---|---|---|---|
| rFlhFBb | 10.71 ± 2.08 | 112.6 ± 4.84 | 1.91 ± 0.08 | 178,338 |
| rFlhFK187A | 5.15 ± 0.86 | 39.39 ± 0.97 | 0.67 ± 0.02 | 130,097 |
| rFlhFR218G | 4.99 ± 1.38 | 41.03 ± 1.64 | 0.70 ± 0.03 | 140,281 |
2.3 |. A pleiotropic role of FlhFBb in cell growth, shape and motility of B. burgdorferi
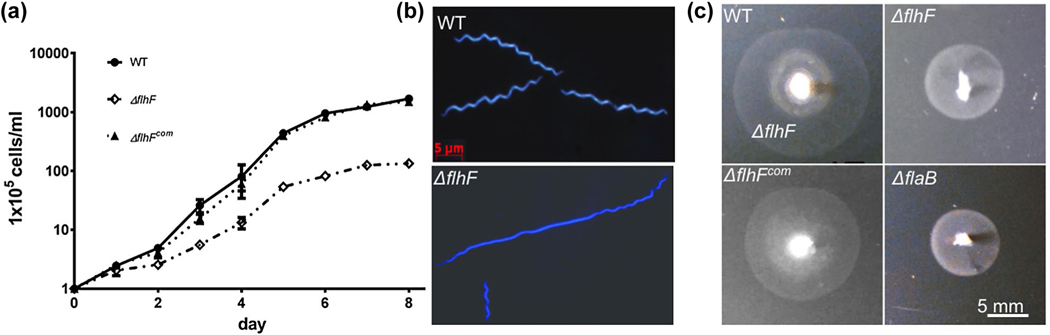
To study the role of FlhFBb in B. burgdorferi, a deletion mutant (ΔflhF) and its isogenic complemented strain (ΔflhFcom) were constructed (Figure S3a,b). Immunoblotting analyses using a specific antibody against FlhFBb (αFlhF) showed that its expression was completely abolished in the mutant and restored to the wild-type level in the complemented strain (Figure S3c). The ΔflhF mutant showed growth retardation (Figure 2a). Specifically, when grown under 34°C, at the stationary phase, the cell density of the mutant was 1.34 × 107 cells/ml, which is decreased approximately 13-fold compared to that of the wild type (1.69 × 108 cells/ml), suggesting that deletion of flhFBb impairs the growth of B. burgdorferi. The observed growth defect has not been reported in other motility mutants of B. burgdorferi. By using dark-field microscopy, we observed that the wild-type cells are wave-like and nearly homogenous in terms of cell length. The majority of mutant cells, however, became rod-shaped and grew in chains (Figure 2b). Interestingly, we found that the ΔflhF mutant underwent abnormal divisions, generating two types of cells: long rod-shaped cells (>70%, average cell length, 42.07 ± 4.48 μm, n = 14 cells) and short spiral cells (<30%, average cell length, 11.36 ± 2.17 μm, n = 6 cells) (Figure 2b). The rod-shaped cells were approximately 2-fold longer than the wild type (19.58 ± 3.48 μm, n = 15) and the short cells were only about one-half of the wild-type cells. Bacterial cell tracking analyses revealed that while the wild-type (Video 1) and complemented (Video 3) strains were highly motile in 1% methyl-cellulose, the ΔflhF mutant (Video 2) failed to displace, indicative of a defect in motility. Interestingly, a constant wave-like motion was propagating along the mutant cell body. Along with this observation, swimming plate assays further showed that the mutant failed to form swimming rings in semisolid agar as compared to the wild-type and complemented strains (Figure 2c). These results indicate that FlhFBb not only affects cell motility as reported in other bacteria, but also controls the cell growth and morphology of B. burgdorferi.
FIGURE 2.
Deletion of flhFBb affects the cell growth, shape and motility of B. burgdorferi. (a) The growth curves of WT, ΔflhF and ΔflhFcom strains. Cells were grown in BSK-II medium (pH 7.6) at 34°C for 8 days. Cell counting was repeated in triplicates in two independent experiments, and the results were expressed as means ± SD. (b) Dark-field microscopic analysis of WT and ΔflhF mutant. The cells were grown in BSK-II medium to the early stationary phase and then subjected to microscopic analysis under dark-field illumination at x 400 magnifications using a Zeiss Axiostar Plus microscope. (c) Swimming plate assays of WT, ΔflhF and ΔflhFcom strains. For this assay, B. burgdorferi cells (5 μl) were stab-inoculated into semisoft agar plates containing dPBS-diluted (10:1) BSK-II medium and 0.35% agarose, as previously described (Motaleb et al., 2000). ΔflaB, a nonmotile mutant, was used as a control to determine the initial inoculation size on the plates
2.4 |. FlhFBb controls the number and position of PF in B. burgdorferi
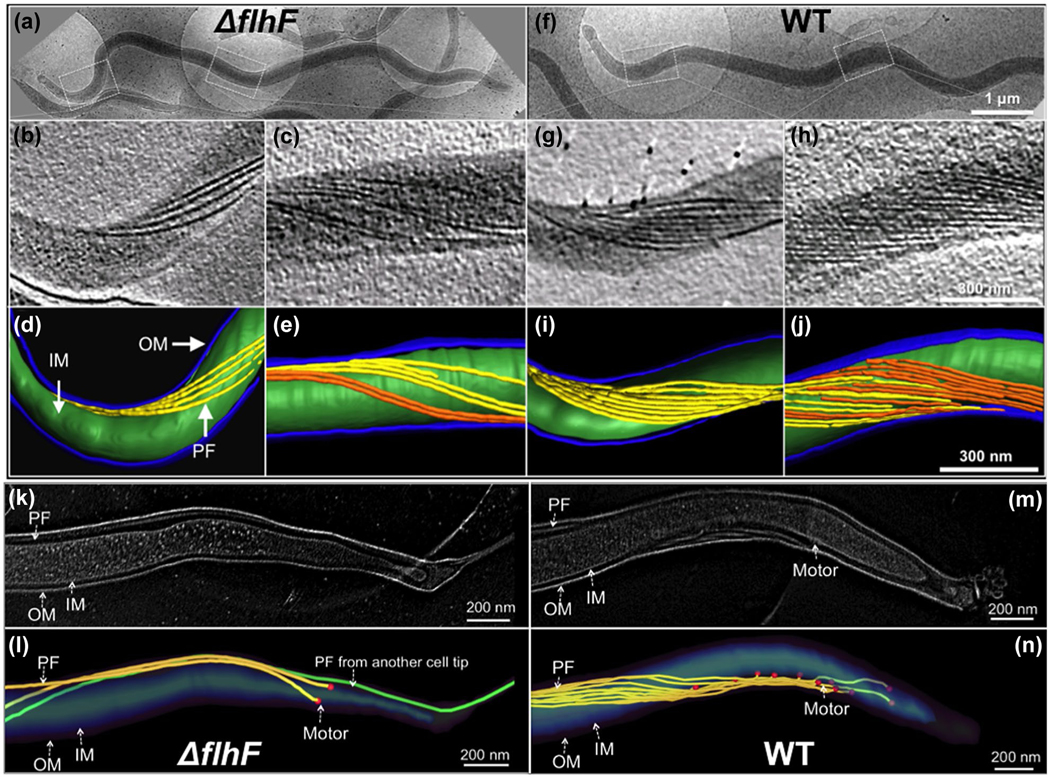
FlhF has been reported to control the flagellar number and position in several bacteria [for review see (Bulyha et al., 2011; Keilberg & Sogaard-Andersen, 2014; Schuhmacher, Thormann, et al., 2015)]. In those bacteria, flagella can be directly visualized either by light microscopy in context with fluorescence labeling or by transmission and scanning electron microscopy; however, these approaches cannot be applied to observe PF, as they reside between the outer and inner membrane in B. burgdorferi and other spirochetes. To overcome this issue, we used cryo-ET to visualize the PF in intact B. burgdorferi cells. We first found spirochete cells under low magnifications (Figure 3a,f) and then focused on the pole and central part of the cells to visualize and enumerate the flagellar motors and filaments. The wild-type cells have 7–11 PF (9 ± 2, n = 20 cells) at each of the two cell poles (Figure 5G,I) and 10–16 PF (13 ± 3, n = 20 cell) at the midcell (Figure 3h,J). In contrast, the ΔflhF mutant has only 2–6 PF (4 ± 2, n = 20 cells) at the cell poles (Figure 3B,D) and 5–7 PF (6 ± 1, n = 10) at the midcell (Figure 3C,E, Table 2). Flagellar motors were observed at the cell tips. The number of motors observed in both the wild type (9 ± 2 motors/cell tip, n = 6) and mutant (3 ± 1 motor/cell tip, n = 6) is similar to that of PF observed at the cell poles. Interestingly, in the majority of the wild-type cells, those motors are linearly distributed along the cell poles (Figure 3M,N, Video 6) and the distance between the nearest motors (DBNM) is nearly homogenous (105 ± 25 nm, n = 31 motors). However, this pattern was disrupted in the ΔflhF mutant, in which the DBNM is more random and diverse (ranging from 47 to 205 nm, average 126 ± 79 nm, n = 10 motors, Table 2) compared to that of the wild type (Figure 3K,L). In addition, the PF from one cell pole often cross the others (Video 7). Complementation of the mutant with the wild-type flhFBb gene fully restored the number and distribution of flagellar filaments and motors in the ΔflhFcom strain (9 ± 2 PF/cell tip, n = 6); however, the two flhF mutants (flhFK187A and flhFR218G) failed to do so, despite that the level of these two point-mutated proteins was restored to that of the wild type. The phenotype of these two mutants is nearly identical to that of ΔflhF mutant in terms of cell shape, motility, and the number of PF (Figure S4, Video 4, 5). Interestingly, the mutation in these two residues only led to a small decrease in the GTPase activity of FlhFBb (Figure 1e), but it had a profound impact on its biological role, suggesting that the function of FlhFBb depends on, at least in part, its GTPase activity. In addition, we also found that ectopic over-expression of flhFBb increased the number of PF (11 ± 3/tip, n = 10 cells) that were clumped at the cell pole (Figure S5). Based on these results, we infer that FlhFBb regulates the number and position of PF in B. burgdorferi.
FIGURE 3.
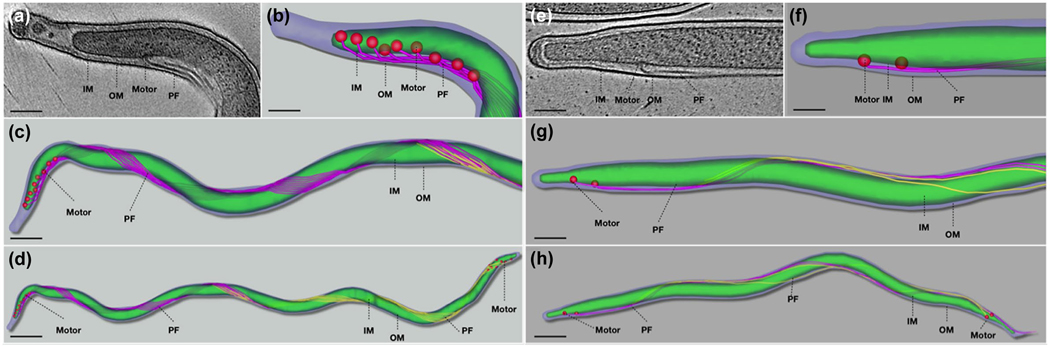
Cryo-ET analysis reveals that the ΔflhF mutant has less PF. (a) Low magnification image of a ΔflhF cell showing the overview of the cell shape. Two boxed regions in the cell tip and body were imaged by cryo-ET. (b) A section of ΔflhF cell tomogram showing four PF originated from the cell tip. (c) A section of ΔflhF midcell tomogram showing the flagellar filaments are loosely distributed along the cell body. (d, e) 3D segmentations of the ΔflhF mutant cell tip and the cell body. (k, l) High magnification image of ΔflhF cell tip tomogram and 3D segmentation. (f) Low magnification image of a WT cell. (g) A section of WT cell tomogram showing eight PF originated from the cell tip. (h) A section of WT midcell tomogram showing the flagellar filaments from both cell ends are overlapped and tightly organized into a flat-ribbon. (i, j) 3D segmentations of a WT cell tip and the cell body. (m, n) High magnification image of WT cell tip tomogram and 3D segmentation. OM: outer membrane; IM: inner membrane
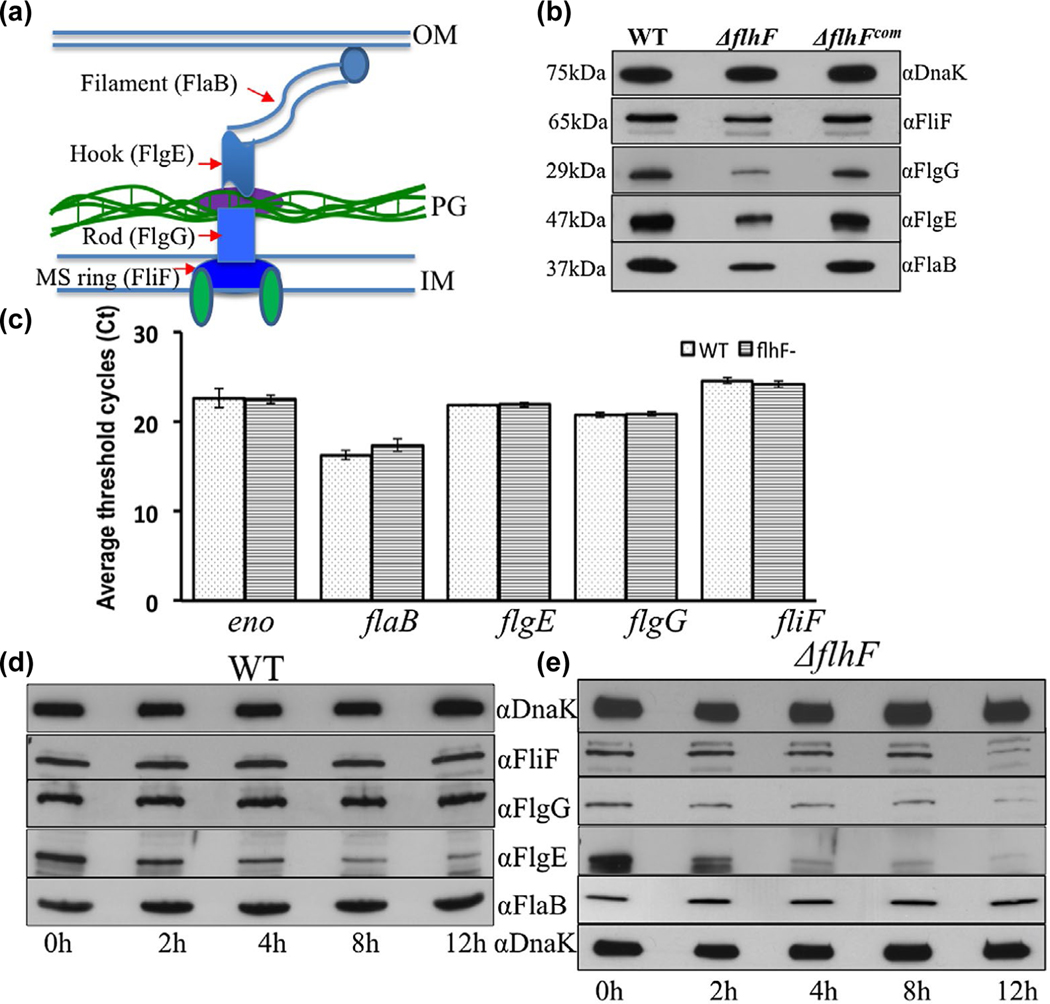
FIGURE 5.
Deletion of flhFBb affects the level and stability of flagellar proteins in B. burgdorferi. (a) A diagram illustrating the overall structure of PF. Red arrows denote four flagellar proteins that represent the MS-ring (FliF), the rod (FlgG), the hook (FlgE) and the filament (FlaB). (b) Detection of FliF, FlgG, FlgE and FlaB by immunoblotting analyses. Similar amounts of WT, ΔflhF and ΔflhFcom whole-cell lysates were subjected to SDS-PAGE and then probed with specific antibodies against these four proteins respectively. DnaK was used as a loading control. Immunoblots were developed using horseradish peroxidase secondary antibody with an ECL luminol substrate as previously described (Zhang et al., 2019). (c) Detection of four flagellar gene transcripts by qRT-PCR. The levels of flaB, flgE, flgG and fliF transcripts were measured by qRT-PCR as previously described (Sal et al., 2008; Sze et al., 2011). The transcript of enolase gene (eno) was used as an internal control to normalize the qPCR data. The results were expressed as the mean threshold cycles (CT) of triplicate samples. (d) & (e) Protein turnover assays. For this assay, protein translation was arrested by adding spectinomycin (100 μg/ml) to the cultures of WT and ΔflhF mutant, and then samples were collected at the indicated time points and subjected to immunoblotting. Of note, compared to the wild type, the loading amount of the flhF mutant was increased about 3-folds in order to detect these flagellar proteins during a period of 12 hr
TABLE 2.
Number of PF, motors, distance between nearest motors and length of PF
| No. of PF (n = 20 cells) |
No. of motors (n = 6 cells) |
|||||
|---|---|---|---|---|---|---|
| Strains | Tip | Body | aDBNM motors | Length of PF (μm) | Left | Right |
| WT | 9 ± 2 | 13 ± 3 | 9 ± 2 | 105 ± 25 (n = 10) | 7.5 ± 0.38 | 7.5 ± 0.38 |
| ΔflhF | 4 ± 2 | 6 ± 1 | 3 ± 1 | 126 ± 79 (n = 10) | b7.3, c7.0, d4.3 | b6.3, c6.6, d3.7 |
| ΔflhFcom | 9 ± 2 | 12 ± 3 | 8 ± 1 | NA | NA |
DBNM: distance between nearest motors.
Cell 1, cell length 11.7, PF (7.3 ± 0.32, 6.3 ± 0.2, n = 4).
Cell 2, cell length 8.4, PF (7.0 ± 1.1, 6.6 ± 0.0, n = 2).
Cell 3, cell length 7.8, PF (4.3 ± 0.33, 3.7 ± 0.2, n = 4).
2.5 |. Whole cell tomography reveals that deletion of flhFBb alters the flat-ribbon configuration of PF
B. burgdorferi cells average 15 μm in length (Charon et al., 2009), which imposes a technical barrier to visualize flagellar assembly and arrangement in whole cells by using cryo-ET. The above and previous cryo-ET studies only focused on one part of B. burgdorferi cells, for example, cell tips (Charon et al., 2009; Qin et al., 2018; Xu, Raddi, Liu, Charon, & Li, 2011; Zhao et al., 2013). Due to this limitation, the configuration of PF and its interaction with the cell cylinder in the periplasmic space have yet to be clearly visualized in context of a whole cell. Here, we implemented a whole cell tomography approach (See detail in Materials and Methods) to unveil the details about the arrangement and length of PF and how those PF interact with the cell cylinder. In addition, we measured PF length in situ. Moreover, it has been speculated that the two poles (i.e., one old and one new) of B. burgdorferi may be different in terms of flagellar number and arrangement (Charon et al., 2012; Li et al., 2002). To address this speculation, newly separated daughter cells were analyzed by whole cell tomography, as in those cells the end close to the division zone can be readily identified as a new pole and the opposite end as an old pole. Seven tomograms from one wild-type cell (14.7 μm in length) were combined together to generate an intact cell reconstruction. Compared to the wild type with a relative uniform cell length, the ΔflhF mutant cells were more diverse in terms of the cell length; thus, three cells were selected for tomography (Figure S6) and seven tomograms from one cell with median length (11.7 μm) were combined to generate the full cell reconstruction (Figure 4). As shown in Figure 4, the wild type had nine PF that originated from each of the cell poles and formed a flat-ribbon that tightly wrapped around the cell cylinder in a right-handed configuration (Figure 4d, Video 8). The individual flagellum in the ribbon was nearly parallel and the distance between individual flagella was almost identical. Two ribbons extended toward their opposite ends and encompassed the cell cylinder 2–3 times before they were interdigitated at the midcell (Figure 4a-d). It was extremely rare to find a ribbon across the entire cell. Interestingly, the motors at the old cell pole (left) typically formed a linear grid-like structure at the subpolar region (Figure 4b-d, Video 8). In contrast, the motors at the new cell pole (right) were randomly distributed at the subpolar region (Figure 4d). In addition, the flagella at the new pole were nearly homogenous in terms of length, but the PF at the old pole (7.5 ± 0.38 μm, n = 8) were slightly longer than the one at the new pole (6.5 ± 0.14 μm, n = 8). The ΔflhF mutant had only 2–4 PF at the cell poles and those PF seemed disengaged and were unable to form a typical flat-ribbon as observed in the wild type (Figure 4e-h, Video 9). In addition, compared to the wild type, the length of PF in the mutant was more diverse, ranging from 3.7 to 7.7 μm (Table 2). Furthermore, the PF in the mutant were less coiled and wrapped around the cell body more loosely and irregularly (Video 9); thus, the mutant was less wave-like and nearly rod-shaped (Figure 4).
FIGURE 4.
Whole cell tomography shows that deletion of flhFBb alters the configuration of PF. (a) A representative tomographic slice of the tip of a WT cell. (b) Surface view of the cell tip. The motors are colored in red. The scale bar is 100 nm. PF are colored in purple. (c) Surface views of a half of the cell. The scale bar is 250 nm. The PF from the other tip are colored in yellow. (d) Surface views of the full WT cell. The scale bar is 500 nm. (e) A representative tomographic slice of the tip of a ΔflhF cell. (f) Surface view of the mutant cell tip. The scale bar is 100 nm. (g) Surface views of a half of the mutant cell. The scale bar is 250 nm. (h) Surface views of a full ΔflhF cell. The scale bar is 500 nm
2.6 |. The level and stability of flagellar proteins are impaired in the ΔflhF mutant
To understand how FlhFBb controls the number of PF, we conducted immunoblotting analyses to measure the level of FliF, FlgG, FlgE and FlaB, four flagellar proteins that represent the MS-ring, the rod, the hook and the filament respectively (Figure 5). Compared to that of the wild type, the level of these four proteins was decreased approximately 50%–75% (Figure 5b). The attenuation of flagellar proteins correlates with the decreased flagellar number in the ΔflhF mutant, indicating that FlhFBb controls the PF number by regulating the level of flagellar proteins. In other bacteria, FlhF regulates the flagellar number transcriptionally, for example, P. aeruginosa FlhF controls the flagellin gene (fliC) transcription (Murray & Kazmierczak, 2006). To see if this is also the case in the ΔflhF mutant, we measured the transcription of these four flagellar genes by using qRT-PCR and found that there was no significant difference between the wild type and the mutant (Figure 5c), suggesting that the decrease of flagellar proteins in the ΔflhF mutant doesn’t occur at the transcription level. Then, we reasoned that FlhFBb might affect flagellar protein stability, as we previously found in other flagellar gene deletion mutants of B. burgdorferi (Sal et al., 2008; Zhang et al., 2019; Zhang, Tong, Liu, & Li, 2012). To test this hypothesis, we checked the stability of FliF, FlgG, FlgE and FlaB by using protein turnover assays. As shown in Figure 5d, FliF, FlgG and FlaB remained stable and the level of FlgE was slightly decreased in the wild type over 12 hr. Similar to the wild type, the level of FlaB in the ΔflhFBb mutant remained unchanged over 12 hr, but the level of FliF, FlgG and FlgE was significantly decreased, for example, only a trace amount of these three proteins was detected by 12 hr (Figure 5e), indicating that they were degraded in the mutant. FlaB remains stable albeit its level is decreased in the mutant (the second blot from the bottom on the right panel). We recently found that the polymerized FlaB (i.e., filaments) is resistant to protease-mediated degradation (Zhang et al., 2019) and that CsrA, a RNA-binding protein, inhibits the translation initiation of the flaB transcript (Sze et al., 2011). Thus, a conceivable explanation is that the reduction of FlaB in the ΔflhF mutant might occur at the translational level. Based on these results, we conclude that FlhFBb regulates the flagellar number mainly by controlling the flagellar protein stability and perhaps translation for the flagellin protein.
2.7 |. FlhFBb localizes to the cell poles
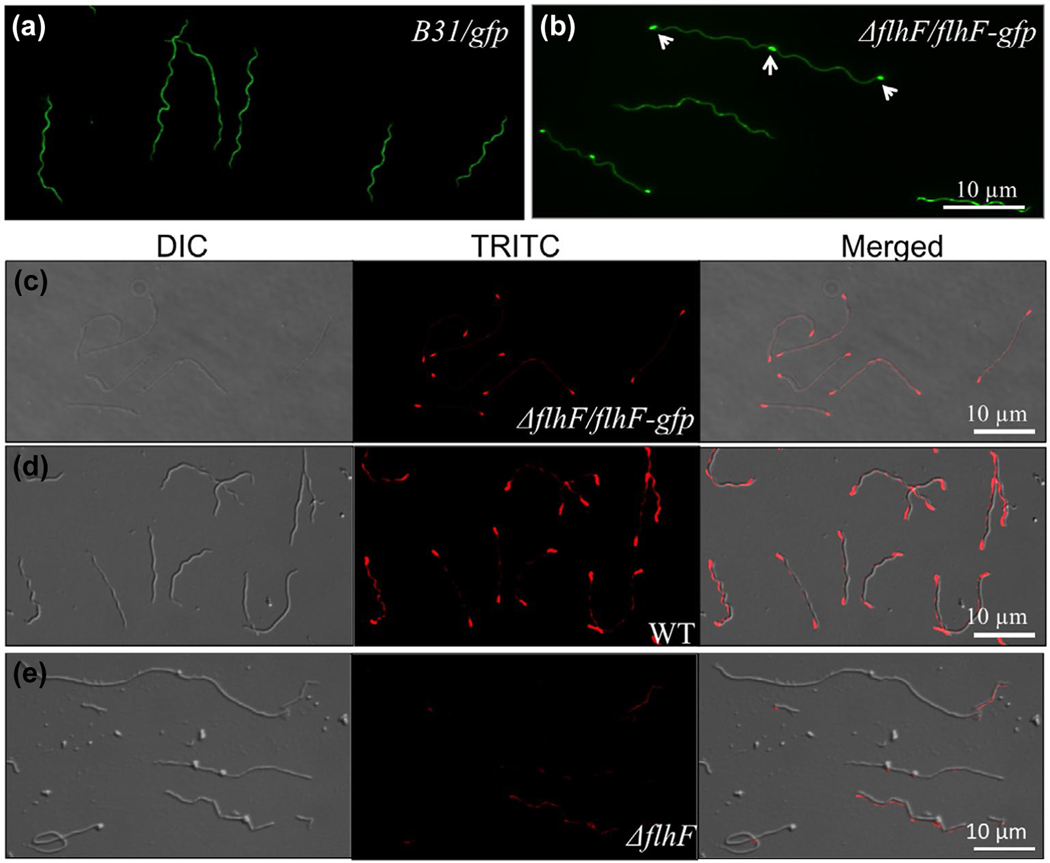
The flagellar motors in B. burgdorferi are localized at the cell poles. Thus, we hypothesized that FlhFBb resides at the cell poles, perhaps in concert with other proteins, to regulate the number and position of PF. To test this hypothesis, we first constructed a vector that expresses FlhFBb-GFP and then transformed this vector into the ΔflhF mutant to determine the cellular distribution of FlhFBb. Immunoblotting analyses using antibodies against either GFP or FlhFBb detected the full-length FlhFBb-GFP fusion protein (Figure S6a). The mutant strain that expresses FlhFBb-GFP (ΔflhF/flhF-gfp) has similar cell morphology (Figure S6b) and swimming behavior to the wild type (Video 10). Under the fluorescence microscope, we found that more than 31% of the cells (>100 cells were counted) had fluorescence puncta at the cell poles (Figure 6b). For the rest of the cells, the puncta were obscured by a high background of cytoplasmic GFP, presumably due to partial proteolysis of the fusion protein. Interestingly, for those cells that appeared to be in the process of division, as evident by their increasing length and nascent septum formation, the fluorescence puncta were also observed in the midcell between two dividing daughter cells (Figure 6b). The spatial distribution of FlhFBb-GFP was not observed in the cells that express only GFP (Figure 6a). To further confirm the polar localization of FlhFBb, we carried out immunofluorescence assay (IFA) using an FlhFBb antibody probed against the ΔflhF/flhF-gfp (Figure 6c) and the wild-type (Figure 6d) strains. The result further demonstrated that the FlhFBb protein was predominantly localized at the cell poles (Figure 6c,d). The fluorescence signal was diminished in the ΔflhF mutant (Figure 6e). Taken together, these results indicate that FlhFBb localizes at the cell pole of B. burgdorferi.
FIGURE 6.
Polar localization of FlhFBb in B. burgdorferi. (a) & (b) Fluorescence images of cells expressing GFP alone (a) or FlhFBb-GFP (b). The micrographs were taken under a fluorescence microscope (magnification, × 620) with a fluorescein isothiocyanate emission filter. (c-e) Immunofluorescence microscopic analysis of the ΔflhF/flhF-gfp (c), WT (d), and ΔflhF mutant (e) cells. Bacterial cells were fixed with methanol, stained with anti-FlhF and counterstained with anti-rat Texas Red-conjugated antibody, as previously described (Zhang, Liu, et al., 2012). The micrographs were taken under differential interference contrast (DIC) light and a fluorescence microscope with a tetramethylrhodamine isothiocyanate (TRITC) emission filter, and the resultant images were merged
2.8 |. FlhFBb affects the polar localization of FliF
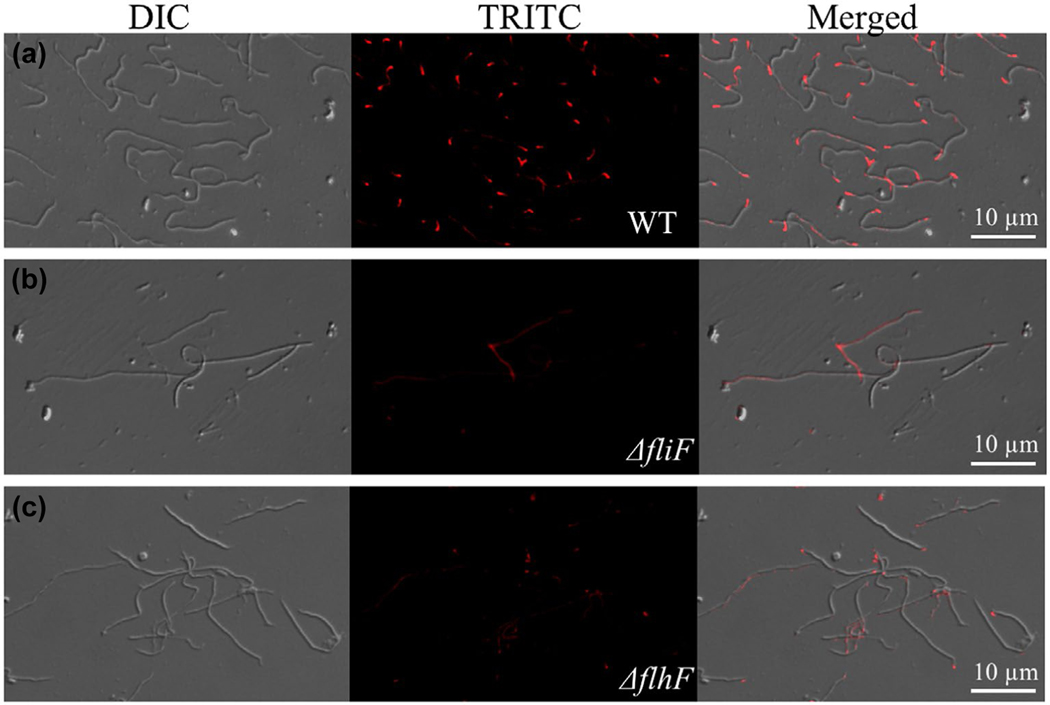
The MS-ring (FliF) anchors the flagellar basal bodies to the cytoplasmic membrane (Chevance & Hughes, 2008; Lynch et al., 2017). It has been speculated that FlhF controls the flagellar placement through recruiting FliF to the pole whereby the site of flagellar assembly is defined (Bulyha et al., 2011; Keilberg & Sogaard-Andersen, 2014; Schuhmacher, Thormann, et al., 2015). However, this speculation has yet to be tested experimentally. To test this speculation, we raised a specific antibody against the FliF protein of B. burgdorferi and then carried out IFA to determine the cellular localization of FliF. As shown in Figure 7, strong fluorescence puncta were detected at the cell poles of the wild type (Figure 7a), but not of the ΔfliF mutant cells (Figure 7b), indicating that FliF localizes to the cell pole. Compared to the wild type, less and weaker fluorescence puncta were detected in the ΔflhF mutant (Figure 7c). Of note, the FliF signal detected here is the sum of all assembled flagellar MS-rings at the cell pole because IFA is unable to visualize individual flagellar basal bodies; thus, the weaker signal implies that the assembly of flagellar basal bodies at the mutant cell poles is impaired. Along with this speculation, cryo-ET analysis revealed that the number of flagellar basal bodies in the ΔflhF mutant is less than that of the wild type (Figure 3 and Table 2). Collectively, these results indicate that FlhF not only controls the level of FliF but also affects its localization, suggesting that FlhF might act through FliF, at least in part, to control the number and placement of flagella.
FIGURE 7.
The polar localization of FliF is impaired in the ΔflhF mutant. Immunofluorescence images of the WT (a), ΔfliF (b) and ΔflhF mutant (c) cells. B. burgdorferi cells were fixed with methanol, stained with anti-FliF, and counterstained with anti-rat Texas Red-conjugated antibody, as previously described (Zhang, Liu, et al., 2012). The micrographs were taken under DIC light and a fluorescence microscope with a TRITC emission filter, and the resultant images were merged
3 |. DISCUSSION
The role of FlhF in the control of flagellar number and placement has been established primarily by using an approach of gene deletion, site-directed mutagenesis and ectopic expression in context with phenotypic characterizations [for review see (Bulyha et al., 2011; Keilberg & Sogaard-Andersen, 2014; Schuhmacher, Thormann, et al., 2015)]. By using this approach, flhF genes have thus far been investigated in at least seven bacteria, which include B. subtilis (Guttenplan et al., 2013), C. jejuni (Balaban & Hendrixson, 2011; Balaban et al., 2009; Liang & Connerton, 2018; Ren et al., 2018), P. aeruginosa (Murray & Kazmierczak, 2006; Schniederberend et al., 2013), Pseudomonas putida (Pandza et al., 2000), Shewanella putrefaciens (Gao, Shi, Ju, & Gao, 2015), V. cholera (Correa et al., 2005) and Vibrio alginolyticus (Kusumoto et al., 2006). It is believed that FlhF plays a similar role in the control of flagellar number and position; however, the phenotypes of flhF deletion mutants from these bacteria vary from species to species. For the comparison, we summarized the phenotypes of four flhF deletion mutants, which represent both polar and peritrichous bacteria (Table 3). Compared to these bacteria, the phenotype of ΔflhF mutant is different. For instance, unlike C. jejuni and V. cholera (Balaban et al., 2009; Correa et al., 2005), the flhF mutant of B. burgdorferi is still able to assemble 2–6 flagella at the cell pole and has no lateral flagella observed. Compared to the wild type, the distribution of flagellar motors (also known as basal bodies) is more random and the distance between the two nearest motors is more diverse (Table 2), which is similar to that of B. subtilis flhF mutant (Guttenplan et al., 2013). We also found that the over-expression of flhFBb increased the number of PF that were clumped at the cell pole (Figure S5). Moreover, complementation of the ΔflhFBb mutant with two variants (flhFK187A and flhFR218G), which have decreased GTPase activity, was unable to restore the wild-type phenotype (Figure S4, Videos 4 & 5), indicating that the role of FlhFBb depends on its GTPase activity. Based on these results, we conclude that FlhFBb controls the number and position of PF, at least in part, in a GTPase-dependent manner in B. burgdorferi.
TABLE 3.
The phenotypes of flhF deletion mutants in different bacteria
| Phenotype of flhF deletion mutants |
||||
|---|---|---|---|---|
| Bacteria | Flagella type | Flagellar number | Position | Motility |
| C. jejuni | Amphitrichous | Non-flagella | Lateral | Non-motile |
| P. aeruginosa | Monotrichous | Unchanged | Lateral | Fails to swarm |
| V. cholera | Monotrichous | Non- or lateral flagella | Lateral | Non-motile |
| B. subtilis | Peritrichous | Unchanged | More asymmetrical | Motile |
| B. burgdorferi | Polar PF unable to form a flat ribbon |
Less flagella | More asymmetrical | Non-motile |
How does FlhFBb control the number and position of PF in B. burgdorferi? FlhFBb localizes to the cell pole and is required for the polar localization of FliF (Figures 6 and 7) (Lynch et al., 2017; Macnab, 2003). Deletion of flhFBb decreases the accumulation of flagellar proteins such as FliF, FlgG, FlgE and FlaB (Figure 5b), which represent the four mechanical parts (MS-ring, rod, hook and filament) of a flagellum. Interestingly, the attenuation of these proteins does not occur at the transcriptional level as reported in other bacteria such as C. jejuni, P. aeruginosa and V. cholera (Balaban et al., 2009; Dasgupta et al., 2000; Navarrete et al., 2019). Instead, it mainly occurs at the posttranslational level through control of protein stability (Figure 5c-e), which supports a previous narrative that B. burgdorferi lacks a transcriptional cascade to regulate its flagellar biosynthesis. Based on these results, we propose that FlhFBb resides at the cell pole and functions as a facilitator to recruit FliF to the cell poles. Deletion of flhFBb curtails the recruitment of FliF and subsequent assembly of other flagellar proteins such as the rod, hook, and filament proteins, which leads to protein degradation; thus, fewer flagella are assembled in the mutant. This model seems conceivable and could explain the phenotype of ΔflhF mutant.
Deletion of flhFBb only alters the position of flagellar motors at the cell pole and the mutant still has polar flagella, suggesting that FlhFBb is not a determinant of flagellar polarity as reported in C. jejuni, P. aeruginosa and V. cholera, which raises an interesting question – how does B. burgdorferi restrict the PF assembled only at the cell pole? Kitaoka et al found that deletion of sflA, a novel dnaJ family gene, reverts the nonmotile phenotype of a double deletion mutant of flhF and flhG to motile in V. alginolyticus by forming peritrichous flagella, indicating that SflA is a suppressor of peritrichous flagella (Inaba, Nishigaki, Takekawa, Kojima, & Homma, 2017; Kitaoka et al., 2013). Interestingly, BLAST search using SflA as a query reveals that the genome of B. burgdorferi encodes three putative DnaJ homologs (BB0517, BB0602 and BB0655); thus, it is likely that B. burgdorferi adopts a similar mechanism as V. alginolyticus to control the flagella polarity by suppressing formation of peritrichous flagella. In addition, a recent report reveals that FapA (flagellar assembly protein A) controls the flagellar polarity and biosynthesis in V. vulnificus (Park et al., 2016). BB0267, a protein downstream of FlhFBb, belongs to the family of FapA (Figure S2c). Alternatively, B. burgdorferi may use BB0267 to control the flagellar polarity. We are currently attempting to knock out these genes and then determine if they control the flagellar polarity in B. burgdorferi.
In addition to the alteration of flagellar number and position, the cell shape and motility of ΔflhF mutant are also impaired. The mutant cells are nearly rod-shaped and deficient to swim in growth media and swim out in semi-solid agar plates (Figure 2), even though they are still able to generate wave-like motion along the cells (Video 2), indicating that the right number and correct position of PF are critical for cell morphology and motility in B. burgdorferi. The change of cell shape and loss of translational motility can be explained by a physical model previously proposed (Dombrowski et al., 2009). B. burgdorferi swims with a planar waveform, which is similar to that of eukaryotic flagella (Goldstein, Charon, & Kreiling, 1994); and the PF have both skeletal and motility functions, for example, aflagellated mutants of B. burgdorferi are rod-shaped and non-motile (Motaleb et al., 2000). To explain this phenomenon, a physical model is proposed. In this model, the spirochete cell is considered as an elastic object in which the coiled PF wrap around the rod-shaped cell cylinder, exert forces onto each other, and cause the cell to deform. Under this circumstance, the rigid PF push against the flexible cell cylinder, generating mechanical torque that propels the cell to swim with no slippage in gel-like media. Theoretically, the mechanical strength of a given PF ribbon is determined by the number and configuration of PF, for example, a ribbon with more PF should be more rigid and vice versa. Compared to the wild type, the ΔflhF mutant has only 2–6 misplaced PF at the cell pole, which might not be rigid enough to deform the cell cylinder and generate sufficient torque to propel the cell moving forward; thus, the mutant cells become less wave-like and non-motile. This elastic model explains the swimming behavior of B. burgdorferi and the impact of PF on cell morphology, and yet it has not been tested experimentally. One interesting perspective is to use FlhFBb as a tool to maneuver the number of PF and then determine its impact on the flagellar rigidity, cell shape and motility.
The other interesting perspective arising from this report is how B. burgdorferi synchronizes the assembly and elongation of PF with cell growth during division. B. burgdorferi is thin and long, approximately 0.25 μm in diameter and 15 μm in length (Charon et al., 2009; Liu et al., 2009). Unlike external flagellated bacteria such as E. coli, B. burgdorferi has the PF assembled inside the cell between the peptidoglycan and the outer membrane. During cell division, concomitant with the initiation of nucleoid separation and cell constriction, B. burgdorferi cells begin to elongate, establish septum and synthesize nascent PF (Jutras et al., 2016). Under this circumstance, the synthesis and elongation of nascent PF at the new cell pole must be spatially and temporally regulated in oscillation with the cell division and elongation. Several lines of evidence suggest that FlhFBb is involved, at least in part, in the regulation of this process. First, deletion of flhFBb disrupts the division pattern of B. burgdorferi, which is evident by growth retardation and formation of cells with different sizes in the mutant (Figure 2b). Second, in the wild type, two PF ribbons typically stop right after the midcell (Figures 3 and 4); thus, the length of PF is approximately one half of the cell length; however, this pattern is altered in the ΔflhF mutant: the PF originated from one cell pole often cross the entire cell and reach the opposite end, and sometimes even invade the neighboring sister cells, suggesting that deletion of flhFBb disrupts the oscillation between the cell and PF length. Third, GFP-fusion and IFA analyses suggest that FlhFBb is a part of the septum or at least adjacent to the division zone (Figure 6b). Lastly, FlhF is a homolog of FtsY and FlhG is a homolog of MinD, two proteins that control the cell division (Montoya et al., 1997; Ono, Takashima, Hirata, Homma, & Kojima, 2015; Schuhmacher, Rossmann, et al., 2015). It was found that FlhG negatively influence the ability of FtsZ to initiate cell division of C. jejuni. Thus, it is conceivable to speculate that FlhFBb interacts with these cell division proteins, forming septum rings that coordinate the process of cell division and flagellation. We are currently attempting to raise a specific antibody against B. burgdorferi FtsZ, a key protein of the septum ring, and construct vectors that express red fluorescent FtsZ fusion protein and then determine if FlhFBb is co-localized with FtsZ as part of the septum ring by using fluorescence microscopy.
The phylum of Spirochaetes contains at least nine genera (Rosa et al., 2005). Using FlhFBb as a query, we searched all the sequenced spirochete genomes, including the genus of Borrelia, Brachyspira, Leptospira, Leptonema, Spirochaeta, Treponema and Turneriella and found that they all have FlhF and FlhG homologs, suggesting that spirochetes might have evolved a similar mechanism as B. burgdorferi to regulate their flagellar number and position, which allows them to adapt to and swim in different niches. This report is the first attempt at understanding how spirochetes regulate their flagellar number and position by using B. burgdorferi as a representative model. The result shown here and methodology developed (e.g., GFP-fusion and whole cell tomography) open an avenue to investigate the role of FlhF in other spirochetes.
4 |. EXPERIMENTAL PROCEDURES
4.1 |. Bacterial strains and growth conditions
A high-passage Borrelia burgdorferi sensu stricto strain B31A (wild type, WT) and its isogenic mutants were grown in Barbour-Stoenner-Kelly II (BSK-II) liquid medium or on semisolid agar plates at 34°C in the presence of 3.4% carbon dioxide as previously described (Li et al., 2002). Swimming plate assays were conducted as previously described (Motaleb et al., 2000). Briefly, 5 μl of culture (108 cells/ml) was spotted onto 0.35% agarose plates containing BSK-II medium diluted 1:10 with Dulbecco’s phosphate-buffered saline (PBS) without divalent cations. Plates were incubated for 3 to 4 days at 34°C with 3.4% CO2. The diameter of the swim ring was recorded in millimeters. B31A strain was used as a positive control and a previously constructed nonmotile flaB mutant as a negative control (Motaleb et al., 2000).
4.2 |. Homology modeling
The structure of FlhFBb was built using B. subtilis FlhF (PDB ID: 2PX3) as a template (Bange et al., 2011; Bange, Petzold, Wild, Parlitz, et al., 2007). The MODELLER program was used to generate sets of initial homology models. The best model was selected on the basis of the lowest DOPE (Discrete optimized protein energy) score and the highest GA341 assessment score. The stereochemical integrity of the model and its quality was evaluated using COOT to ensure that angles and torsions are within acceptable tolerance limits based on the Rama plot (96% in favorable regions). Model refinement and visualization were performed with SYBYL-X 2.1 (Tripos, LLC). Hydrogen bonds, magnesium- and GTP-binding sites were added to the structures. Atomic charges were set with Gasteiger-Hückel, which was followed by energy minimization to a termination gradient of 0.05 kcal/(mol Å) or 10,000 iterations.
4.3 |. Generation of FlhFBb and FliF antibodies
The full-length flhFBb gene was PCR amplified (primers P19/P8) using DNA polymerase (Invitrogen, Carlsbad, CA) with engineered BamHI and PstI cut sites at its 5′ and 3′ ends respectively. The amplicon was first cloned into the pGEM-T Easy vector (Promega, Madison, WI) and then subcloned into the pQE30 expression vector (Qiagen, Valencia, CA), which encodes an N-terminal histidine (His) tag. The resultant plasmid was then transformed into M15 cells. The expression of FlhFBb was induced using 1 mM isopropyl-β-D-thiogalactoside (IPTG). The His-tagged recombinant protein (His6FlhFBb) was insoluble and thus purified under a denature condition using a nickel agarose column (Qiagen, Valencia, CA). The purified protein was dialyzed in a buffer containing 10 mM Tris-HCl at 4°C overnight. To produce an antiserum against FlhFBb, two rats were first immunized with 1 mg of His6FlhFBb during a 1-month period and then boosted (100 μg per rat) twice at weeks 6 and 7 (antiserum was manufactured by General Bioscience Corporation, Brisbane, CA). A similar method was used to express recombinant FliF and produce an antiserum against FliF.
4.4 |. Site-directed mutagenesis
Site-directed mutagenesis was performed using QuikChange site-directed mutagenesis kit (Stratagene, San Diego, CA) per the manufacturer’s instructions. The above constructed FlhFBb expression vector was used as a template for the mutagenesis. Two aa in FlhFBb (Lys187 and Arg218) were substituted with Ala and Gly respectively, using primers P20/P21 and P22/P23. The mutations were confirmed by sequencing analysis. The mutated genes were PCR amplified and subcloned into pDEST15 expression vector (Invitrogen, Carlsbad CA) to generate amino-terminal glutathione S-transferase (GST) fusion proteins, as described below.
4.5 |. Preparation of soluble GST-FlhFBb fusion proteins
His6FlhFBb is insoluble, which is not suitable for study of its enzymatic activity. To overcome this issue, different expression vectors and systems were used. We found that addition of GST tag to the N-terminus of FlhFBb increases its solubility. To express GST-FlhFBb fusion proteins, the wild-type flhFBb or two mutated genes (K187A and R218G) were PCR amplified using primers with engineered NcoI and BglII cut site at its 5′ and 3′ ends. The amplicons were cloned into pGEM-T Easy vector and then released using NcoI and BglII. The obtained flhFBb fragments were cloned into pDEST15 (Invitrogen) at sites of NcoI and BamHI. The expression vectors were then transformed into E. coli strain BL21-AI (Invitrogen) and induced with 0.2% L-arabinose for 18 hr at 16°C. Under such conditions, two liters of E. coli cultures were grown and harvested by centrifugation. The obtained cell pellets were subjected to protein purifications using Pierce™ Glutathione Magnetic Agarose Beads according to the manufacturer’s instructions (Thermo Scientific). The obtained proteins were further purified using size exclusion chromatography (SEC).
4.6 |. GTP hydrolysis assays
The concentrations of GST-FlhFBb fusion proteins were measured using a Bradford protein assay according to the manufacturer’s instructions (Bio-Rad). The GTP hydrolysis activity of recombinant FlhFBb proteins was measured by detecting the release of free phosphate (Pi) as a product of GTP hydrolysis with a colorimetric GTPase Activity Kit (Sigma-Aldrich, MO), as previously described with some modifications (Liang & Connerton, 2018). Prior to the assay, a standard curve was generated to convert the colorimetric absorbance reading into free Pi from hydrolysis reactions using the phosphate standard in the kit. To detect GTPase activity, the reactions were initiated by mixing 20 μl of assay buffer, 10 μl of fixed amount of GTP, and 10 μl of GST-FlhFBb proteins, blank (buffer alone) or negative controls (GST) in 96-well microplates, and incubated at room temperature for 30 min. And then, 200 μl of reagent buffer was added to the reactions and incubated for an additional 30 min at room temperature to generate the colorimetric products. The 96-well plates were directly subjected to a Variskan Lux microplate reader (ThermoFisher Scientific) to measure colorimetric absorbance at a wavelength of 620 nm. For kinetic assays, the reactions were conducted under the same conditions by mixing 59 nM of purified GST-FlhFBb proteins with various concentrations of GTP (0, 20, 40, 60, 80 and 100 μM). The amount of released Pi (product) was subsequently determined from OD620 readings against the standard curve. The final results were recorded by averaging triplicates of three independent assays. The initial rate of reactions was measured by plotting the amount of product (Pi) over time at a given GTP concentration; and the saturation curves were fitted to Michaelis-Menten kinetics using GraphPad Prism 7 (GraphPad Software, San Diego, CA).
4.7 |. RT-PCR and qRT-PCR
Total RNA samples were prepared as previously described (Sze et al., 2011). To produce cDNA, 300 ng of RNA was reversely transcribed using AMV reverse transcriptase (Promega, Madison, WI). For reverse transcription-PCR (RT-PCR), 1 μl of cDNA was PCR amplified with different pairs of primers using Taq DNA polymerase (Qiagen). The quantitative PCR (qPCR) analysis of fliF (primers P9/P10), flgG (primers P11/P12), flgE (primers P13/P14) and flaB (primers P15/P16) transcripts was carried out using iQ SYBR green Supermix and MyiQ thermal cycler (Bio-Rad Laboratories, Hercules, CA). The transcript of the enolase gene (eno, bb0337, primers P17/P18) was used as an internal control to normalize the qPCR data as described before (Sal et al., 2008). The results were expressed as threshold cycle (CT) value between the wild-type and mutant strains. All of the primers for RT-PCR and qRT-PCR are listed in Table S1.
4.8 |. Electrophoresis and immunoblotting analysis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting analyses were carried out as previously described (Sze et al., 2011; Zhang, Tong, et al., 2012). Briefly, B. burgdorferi cells were cultured at 34°C and harvested at approximately 108 cells/ml. Equal amounts of whole-cell lysates (10 to 30 μg) were separated on SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). Immunoblots were probed with specific antibodies against various proteins, including FlaA, FlaB, FliG2, FliF, FlgG, FlgE, FlhFBb and DnaK. DnaK was used as an internal loading control. Monoclonal antibodies to FlaB, FlaA and DnaK were kindly provided by A. Barbour (University of California, Irvine, CA), B. Johnson (Centers for Disease Control and Prevention, Atlanta, GA), and J. Benach (State University of New York, Stony Brook, NY), respectively. Polyclonal antibodies to FlgE, FlgG and FliG2 were generated in our previous studies (Li et al., 2010; Sal et al., 2008; Zhao et al., 2013). The antibodies against FliF and FlhFBb were generated in this study. Immunoblots were developed using horseradish peroxidase-labeled secondary antibodies or protein A (GE Healthcare UK Limited, Little Chalfont Buckinghamshire, United Kingdom) with Pierce ECL Western blotting substrate kit (Thermo Scientific).
4.9 |. Protein turnover assay
This assay was carried out as previously described (Motaleb, Sal, & Charon, 2004; Sal et al., 2008; Zhang et al., 2019). Briefly, B. burgdorferi strains were grown in BSK-II medium at 34°C to a density of ~108 cells/ml, and then spectinomycin (final concentration 100 μg/ml) was added to arrest protein synthesis. Following the addition of spectinomycin, B. burgdorferi cells from 5 ml of cultures were harvested at 0, 2, 4, 8 and 12 hr by centrifugation and then subjected to immunoblotting analyses against various antibodies as described above. Densitometry of immunoreactive proteins in the blots was measured using the Molecular Imager ChemiDoc™ XRS Imaging system (Bio-Rad) to determine the relative amounts of proteins as previously described (Zhang et al., 2019).
4.10 |. Construction of an flhFBb deletion mutant and its isogenic complemented strain
A previously described method was used to inactivate flhFBb (bb0270) (Li et al., 2002; Samuels, 1995; Samuels, Drecktrah, & Hall, 2018). Briefly, a DNA fragment containing the entire flhFBb gene was PCR amplified with primers P1/P2, and the resultant PCR product was cloned into the pGEM-T Easy vector (Promega). A 1.3-kb kanamycin resistance cassette (kan) was amplified by primers P3/P4 and inserted into flhFBb gene at a HindIII cut site. The final construct (flhFBb::kan) was linearized and electroporated into competent B31A cells to inactivate the targeted gene. Transformants were selected on the semisolid agar plates containing kanamycin (300 μg/ml). To complement the flhFBb mutant, the flgB promoter of B. burgdorferi (PflgB) was first PCR amplified (primers P5/P6) with engineered BamHI and NdeI cut sites at its 5′ and 3′ ends respectively. The resultant PCR fragment was cloned into the pGEM-T Easy vector, generating PflgB/pGEM-T. Meanwhile, the intact flhFBb gene was PCR amplified (primers P7/P8) with engineered NdeI and PstI cut sites at its 5′ and 3′ ends respectively, and cloned into the vector PflgB/pGEM-T at the site of NdeI, yielding PflgB-flhFBb/pGEM-T. The PflgB-flhFBb fragment was released with BamHI and PstI and subcloned into pBSV2G, a shuttle vector of B. burgdorferi, generating a vector of PflgB-flhFBb/pBSV2G. A similar method was used to delete the fliF gene of B. burgdorferi. For complementation, PflgB-flhFBb/pBSV2G was electroporated into competent flhFBb mutant cells and transformants were selected on agar plates containing both kanamycin (300 μg/ml) and gentamicin (60 μg/ml). The primers used here are listed in Table S1.
4.11 |. Construction of a plasmid that expresses FlhFBb-GFP fusion protein
This vector was constructed as previously documented (Xu et al., 2011). To make this construct, PflgB (primers P24/P25) and gfp (P26/P27) genes were first PCR amplified separately and then fused together with primers P24/P27. Of note, a BamHI and Hind III cut sites were engineered within P24 and P27 respectively; XhoI and SacII cut sites were engineered at P25 for insertion of flhFBb at the 3′ end of PflgB to create the C-terminus GFP-fusion protein. The fragment of PflgB-gfp was first cloned into pGEM-T easy vector, released, and cloned into the shuttle vector pBSV2G at the sites of BamHI and HindIII, yielding a vector of PflgB-gfp/V2G. The full-length flhFBb gene was PCR amplified with primers P28/29 in which XhoI and SacII cut sites were engineered. The resultant PCR fragment was first cloned into pGEM-T vector and then subcloned into PflgB-gfp/V2G at the site of XhoI and SacII, yielding the vector of PflgB-flhFBb-gfp/V2G. The final construct was confirmed by DNA sequencing.
4.12 |. Light and fluorescence microscopy
Cell morphology and swimming behaviors were visualized and videotaped with dark-field illumination at 100× for at least 1 min. IFA were conducted as previously described (Xu et al., 2011; Zhang, Liu, et al., 2012). Briefly, 1.5 ml of B. burgdorferi cultures were harvested, washed two times with PBS buffer (phosphate buffered saline, pH7.5), and then treated with methanol at −20°C for 1 hr. The collected cells were treated with lysozyme (1 mg/ml) in GTE buffer (50 mM glucose, 25 mM Tris, 1mM EDTA, pH 7.5) for 1 hr at room temperature, and then placed on a polylysine-coated coverslip, allowed to fully air dry. The obtained coverslips were first incubated in a blocking solution (2% BSA in PBS, pH 7.5) for 1 hr, and then incubated in blocking solution containing 1:100 diluted antibodies (i.e., anti-FlhFBb and anti-FliF) for 1 hr at room temperature. Finally, the coverslips were washed five times with PBS, incubated with secondary goat anti-rat Texas red antibody (Invitrogen) for 1 hr at room temperature, washed with PBS again, and mounted in 40% glycerol for image processing. Fluorescence images were taken using a Zeiss Axiostar plus microscope at a wavelength of 480 nm. Texas red images were taken using a Zeiss Axioimager Z1 Axiophot wide-field microscope with an excitation filter (541–569) and an emission filter (581–654 nm). The images were captured and processed using the program ZEN (Zeiss, Germany).
4.13 |. Cryo-electron tomography and 3-dimensional visualization
Frozen-hydrated B. burgdorferi specimens were prepared as described previously (Liu et al., 2009; Zhao et al., 2013). Briefly, B. burgdorferi culture was centrifuged at 5,000 × g for 5 min and the resulting pellets were suspended in PBS to achieve a cell concentration approximately 1 × 108/ml. After adding 10 nm gold marker solution, 5 μl of the cell suspension was placed on freshly glow-discharged holey carbon grid (Quantifoil Cu R2/2, 200 mesh) for 25 s. The grids were blotted with filter paper for 3 to 5 s and rapidly frozen in liquid ethane using a homemade plunger apparatus as described previously (Liu et al., 2009; Zhao et al., 2013). The specimens were imaged using a 300 kV electron microscope (Polara, FEI) with a 4K × 4K CCD (TVIPS, Germany). The microscope was operated at 300 kV with a magnification of 23,000×. Low dose single-axis tilt series were collected from each bacterium at −8 μm defocus with a cumulative dose of ~100 e−/Å2 distributed over 65 images with an angular increment of 2°, covering a range from −64° to +64°. To collect a whole cell tomogram, the frozen-hydrated specimens were visualized by using a 300 kV electron microscope (Krios, Thermo Fish Scientific) equipped with a field emission gun, a Volta Phase Plate (VPP), and a Direct Electron Detector with energy filter (Gatan K2 Summit). SerialEM was used to collect multiple tilt series along each cell. For each tilt series, a very low total dose of 40 e−/Å2 is distributed among 35 tilt images covering angles from −51° to +51° at tilt steps of 3°. For each image in the tilt series, dose-fractionated mode was used to generate 11 frames per projection image. After drift-correction by using the Motioncorr2 (Li et al., 2013), IMOD software was used to align the tilt series and tomo3d was used to generate tomograms (Kremer, Mastronarde, & McIntosh, 1996; Zhao et al., 2013). Several tomograms along each cell were combined together to generate a full cell reconstruction. For low magnification imaging analysis, cryo-tomograms were collected from 20 cells of wild type, ΔflhF and its complemented strain. For high magnification cryo-tomography, six of the tomographic reconstructions of WT cells, six of the tomographic reconstructions of ΔflhF cells, and two of the tomographic reconstructions of ΔflhF-R2G were generated to analyze the motor. For the whole cell tomography analysis, seven of the tomographic reconstructions of WT cells and five of the tomographic reconstructions of ΔflhF cells were used to generate the full cell. Segmentations of representative reconstructions from WT and ΔflhF cells were constructed using IMOD (Kremer et al., 1996). The filaments, the motors, the outer and inner membranes were manually segmented.
5 |. DESCRIPTIONS OF VIDEOS
Bacterial tracking analysis: Video 1 (WT), Video 2 (ΔflhF), Video 3 (ΔflhFcom), Video 4 (flhFK187A), Video 5 (flhFR218G) and Video 10 (ΔflhF/flhF-gfp).
Tomography videos: Video 6 (WT), Video 7 (ΔflhF), Video 8 (WT, whole cell) and Video 9 (ΔflhF, whole cell).
Supplementary Material
ACKNOWLEDGMENTS
Thank Miss Hannah Lohner for proof reading. This research was supported by Public Health Service Grants AI078958 and DE023080 to C. Li and AI087946 to J. Liu.
Funding information
National Institute of Dental and Craniofacial Research, Grant/Award Number: DE023080; National Institute of Allergy and Infectious Diseases, Grant/Award Number: AI078958 and AI087946; Public Health Service, Grant/Award Number: AI078958 and DE023080
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- Aldridge P, & Hughes KT (2002). Regulation of flagellar assembly. Current Opinion in Microbiology, 5, 160–165. 10.1016/S1369-5274(02)00302-8 [DOI] [PubMed] [Google Scholar]
- Balaban M, & Hendrixson DR (2011). Polar flagellar biosynthesis and a regulator of flagellar number influence spatial parameters of cell division in Campylobacter jejuni. PLoS Pathogens, 7, e1002420. 10.1371/journal.ppat.1002420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban M, Joslin SN, & Hendrixson DR (2009). FlhF and its GTPase activity are required for distinct processes in flagellar gene regulation and biosynthesis in Campylobacter jejuni. Journal of Bacteriology, 191, 6602–6611. 10.1128/JB.00884-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange G, Kummerer N, Grudnik P, Lindner R, Petzold G, Kressler D, … Sinning I. (2011). Structural basis for the molecular evolution of SRP-GTPase activation by protein. Nature Structural & Molecular Biology, 18, 1376–1380. 10.1038/nsmb.2141 [DOI] [PubMed] [Google Scholar]
- Bange G, Petzold G, Wild K, Parlitz RO, & Sinning I. (2007). The crystal structure of the third signal-recognition particle GTPase FlhF reveals a homodimer with bound GTP. Proceedings of the National Academy of Sciences USA, 104, 13621–13625. 10.1073/pnas.0702570104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange G, Petzold G, Wild K, & Sinning I. (2007). Expression, purification and preliminary crystallographic characterization of FlhF from Bacillus subtilis. Acta Crystallographica, Section F: Structural Biology and Crystallization Communications, 63, 449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC (2003). The rotary motor of bacterial flagella. Annual Review of Biochemistry, 72, 19–54. 10.1146/annurev.biochem.72.121801.161737 [DOI] [PubMed] [Google Scholar]
- Boltjes TY (1948). Function and arrangement of bacterial flagella. The Journal of Pathology and Bacteriology, 60, 275–287. 10.1002/path.1700600215 [DOI] [PubMed] [Google Scholar]
- Brisson D, Drecktrah D, Eggers CH, & Samuels DS (2012). Genetics of Borrelia burgdorferi. Annual Review of Genetics, 46, 515–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulyha I, Hot E, Huntley S, & Sogaard-Andersen L. (2011). GTPases in bacterial cell polarity and signalling. Current Opinion in Microbiology, 14, 726–733. 10.1016/j.mib.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, & Davis JP (1982). Lyme disease-a tick-borne spirochetosis? Science, 216, 1317–1319. 10.1126/science.7043737 [DOI] [PubMed] [Google Scholar]
- Charon NW, Cockburn A, Li C, Liu J, Miller KA, Miller MR, … Wolgemuth CW (2012). The unique paradigm of spirochete motility and chemotaxis. Annual Review of Microbiology, 66, 349–370. 10.1146/annurev-micro-092611-150145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, … Rowe N. (2009). The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. Journal of Bacteriology, 191, 600–607. 10.1128/JB.01288-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevance FF, & Hughes KT (2008). Coordinating assembly of a bacterial macromolecular machine. Nature Reviews Microbiology, 6, 455–465. 10.1038/nrmicro1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa NE, Peng F, & Klose KE (2005). Roles of the regulatory proteins FlhF and FlhG in the Vibrio cholerae flagellar transcription hierarchy. Journal of Bacteriology, 187, 6324–6332. 10.1128/JB.187.18.6324-6332.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta N, Arora SK, & Ramphal R. (2000). fleN, a gene that regulates flagellar number in Pseudomonas aeruginosa. Journal of Bacteriology, 182, 357–364. 10.1128/JB.182.2.357-364.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski C, Kan W, Motaleb MA, Charon NW, Goldstein RE, & Wolgemuth CW (2009). The elastic basis for the shape of Borrelia burgdorferi. Biophysical Journal, 96, 4409–4417. 10.1016/j.bpj.2009.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, & Radolf JD (2009). Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. Journal of Clinical Investigation, 119, 3652–3665. 10.1172/JCI39401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt M, Namba K, & Hughes KT (2010). Bacterial nanomachines: The flagellum and type III injectisome. Cold Spring Harbor Perspectives in Biology, 2, a000299. 10.1101/cshperspect.a000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt M, Singer HM, Wee DH, Keener JP, & Hughes KT (2011). An infrequent molecular ruler controls flagellar hook length in Salmonella enterica. EMBO Journal, 30, 2948–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, … Venter JC (1997). Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature, 390, 580–586. 10.1038/37551 [DOI] [PubMed] [Google Scholar]
- Fung DC, & Berg HC (1995). Powering the flagellar motor of Escherichia coli with an external voltage source. Nature, 375, 809–812. 10.1038/375809a0 [DOI] [PubMed] [Google Scholar]
- Gao T, Shi M, Ju L, & Gao H. (2015). Investigation into FlhFG reveals distinct features of FlhF in regulating flagellum polarity in Shewanella oneidensis. Molecular Microbiology, 98, 571–585. [DOI] [PubMed] [Google Scholar]
- Ge Y, Old IG, Saint Girons I, & Charon NW (1997). Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus sigma70 promoter. Journal of Bacteriology, 179, 2289–2299. 10.1128/JB.179.7.2289-2299.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SF, Charon NW, & Kreiling JA (1994). Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proceedings of the National Academy of Sciences USA, 91, 3433–3437. 10.1073/pnas.91.8.3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbronson CJ, Ribardo DA, Balaban M, Knauer C, Bange G, & Hendrixson DR (2016). FlhG employs diverse intrinsic domains and influences FlhF GTPase activity to numerically regulate polar flagellar biogenesis in Campylobacter jejuni. Molecular Microbiology, 99, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan SB, Shaw S, & Kearns DB (2013). The cell biology of peritrichous flagella in Bacillus subtilis. Molecular Microbiology, 87, 211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko J, & Westerlund-Wikstrom B. (2013). The role of the bacterial flagellum in adhesion and virulence. Biology (Basel), 2, 1242–1267. 10.3390/biology2041242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AN, Subramanian S, Oshiro RT, Canzoneri AK, & Kearns DB (2018) SwrD (YlzI) promotes swarming in Bacillus subtilis by increasing power to flagellar motors. Journal of Bacteriology, 200(2), E00529–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman MW, Dunham-Ems SM, Caimano MJ, Belperron AA, Bockenstedt LK, Fu HC, … Wolgemuth CW (2012). The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. Proceedings of the National Academy of Sciences USA, 109, 3059–3064. 10.1073/pnas.1114362109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, … Aderem A. (2001). The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature, 410, 1099–1103. 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- Inaba S, Nishigaki T, Takekawa N, Kojima S, & Homma M. (2017). Localization and domain characterization of the SflA regulator of flagellar formation in Vibrio alginolyticus. Genes to Cells, 22, 619–627. [DOI] [PubMed] [Google Scholar]
- Izard J, Renken C, Hsieh CE, Desrosiers DC, Dunham-Ems S, La Vake C, … Radolf JD (2009). Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete. Journal of Bacteriology, 191, 7566–7580. 10.1128/JB.01031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josenhans C, & Suerbaum S. (2002). The role of motility as a virulence factor in bacteria. International Journal of Medical Microbiology, 291, 605–614. 10.1078/1438-4221-00173 [DOI] [PubMed] [Google Scholar]
- Jutras BL, Scott M, Parry B, Biboy J, Gray J, Vollmer W, & Jacobs-Wagner C. (2016). Lyme disease and relapsing fever Borrelia elongate through zones of peptidoglycan synthesis that mark division sites of daughter cells. Proceedings of the National Academy of Sciences USA, 113, 9162–9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilberg D, & Sogaard-Andersen L. (2014). Regulation of bacterial cell polarity by small GTPases. Biochemistry, 53, 1899–1907. 10.1021/bi500141f [DOI] [PubMed] [Google Scholar]
- Kitaoka M, Nishigaki T, Ihara K, Nishioka N, Kojima S, & Homma M. (2013). A novel dnaJ family gene, sflA, encodes an inhibitor of flagellation in marine Vibrio species. Journal of Bacteriology, 195, 816–822. 10.1128/JB.01850-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, & McIntosh JR (1996). Computer visualization of three-dimensional image data using IMOD. Journal of Structural Biology, 116, 71–76. 10.1006/jsbi.1996.0013 [DOI] [PubMed] [Google Scholar]
- Kurniyati K, Kelly JF, Vinogradov E, Robotham A, Tu Y, Wang J, … Li C. (2017). A novel glycan modifies the flagellar filament proteins of the oral bacterium Treponema denticola. Molecular Microbiology, 103, 67–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto A, Kamisaka K, Yakushi T, Terashima H, Shinohara A, & Homma M. (2006). Regulation of polar flagellar number by the flhF and flhG genes in Vibrio alginolyticus. Journal of Biochemistry, 139, 113–121. 10.1093/jb/mvj010 [DOI] [PubMed] [Google Scholar]
- Kusumoto A, Nishioka N, Kojima S, & Homma M. (2009). Mutational analysis of the GTP-binding motif of FlhF which regulates the number and placement of the polar flagellum in Vibrio alginolyticus. Journal of Biochemistry, 146, 643–650. 10.1093/jb/mvp109 [DOI] [PubMed] [Google Scholar]
- Leifson E. (1951). Staining, shape and arrangement of bacterial flagella. Journal of Bacteriology, 62, 377–389. 10.1128/JB.62.4.377-389.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertsethtakarn P, Ottemann KM, & Hendrixson DR (2011). Motility and chemotaxis in Campylobacter and Helicobacter. Annual Review of Microbiology, 65, 389–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bakker RG, Motaleb MA, Sartakova ML, Cabello FC, & Charon NW (2002). Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proceedings of the National Academy of Sciences USA, 99, 6169–6174. 10.1073/pnas.092010499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu H, Zhang K, & Liang FT (2010). Inactivation of a putative flagellar motor switch protein FliG1 prevents Borrelia burgdorferi from swimming in highly viscous media and blocks its infectivity. Molecular Microbiology, 75, 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mooney P, Zheng S, Booth CR, Braunfeld MB, Gubbens S, … Cheng Y. (2013). Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature Methods, 10, 584–590. 10.1038/nmeth.2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, & Connerton IF (2018). FlhF(T368A) modulates motility in the bacteriophage carrier state of Campylobacter jejuni. Molecular Microbiology, 110, 616–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Howell JK, Bradley SD, Zheng Y, Zhou ZH, & Norris SJ (2010). Cellular architecture of Treponema pallidum: Novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. Journal of Molecular Biology, 403, 546–561. 10.1016/j.jmb.2010.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin T, Botkin DJ, McCrum E, Winkler H, & Norris SJ (2009). Intact flagellar motor of Borrelia burgdorferi revealed by cryoelectron tomography: Evidence for stator ring curvature and rotor/C-ring assembly flexion. Journal of Bacteriology, 191, 5026–5036. 10.1128/JB.00340-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybecker MC, Abel CA, Feig AL, & Samuels DS (2010). Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Molecular Microbiology, 78, 622–635. 10.1111/j.1365-2958.2010.07374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MJ, Levenson R, Kim EA, Sircar R, Blair DF, Dahlquist FW, & Crane BR (2017). Co-folding of a FliF-FliG split domain forms the basis of the MS: C ring interface within the bacterial flagellar motor. Structure, 25, 317–328. 10.1016/j.str.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab RM (2003). How bacteria assemble flagella. Annual Review of Microbiology, 57, 77–100. 10.1146/annurev.micro.57.030502.090832 [DOI] [PubMed] [Google Scholar]
- Minamino T, Imada K, & Namba K. (2008). Molecular motors of the bacterial flagella. Current Opinion in Structural Biology, 18, 693–701. 10.1016/j.sbi.2008.09.006 [DOI] [PubMed] [Google Scholar]
- Montoya G, Svensson C, Luirink J, & Sinning I. (1997). Crystal structure of the NG domain from the signal-recognition particle receptor FtsY. Nature, 385, 365–368. 10.1038/385365a0 [DOI] [PubMed] [Google Scholar]
- Motaleb MA, Corum L, Bono JL, Elias AF, Rosa P, Samuels DS, & Charon NW (2000). Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proceedings of the National Academy of Sciences USA, 97, 10899–10904. 10.1073/pnas.200221797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Liu J, & Wooten RM (2015). Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease. Current Opinion in Microbiology, 28, 106–113. 10.1016/j.mib.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaleb MA, Sal MS, & Charon NW (2004). The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. Journal of Bacteriology, 186, 3703–3711. 10.1128/JB.186.12.3703-3711.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, & Kearns DB (2014). The structure and regulation of flagella in Bacillus subtilis. Annual Review of Genetics, 48, 319–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TS, & Kazmierczak BI (2006). FlhF is required for swimming and swarming in Pseudomonas aeruginosa. Journal of Bacteriology, 188, 6995–7004. 10.1128/JB.00790-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete B, Leal-Morales A, Serrano-Ron L, Sarrio M, Jimenez-Fernandez A, Jimenez-Diaz L, … Govantes F. (2019). Transcriptional organization, regulation and functional analysis of flhF and fleN in Pseudomonas putida. PLoS ONE, 14, e0214166. 10.1371/journal.pone.0214166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Takashima A, Hirata H, Homma M, & Kojima S. (2015). The MinD homolog FlhG regulates the synthesis of the single polar flagellum of Vibrio alginolyticus. Molecular Microbiology, 98, 130–141. [DOI] [PubMed] [Google Scholar]
- Pallen MJ, Penn CW, & Chaudhuri RR (2005). Bacterial flagellar diversity in the post-genomic era. Trends in Microbiology, 13, 143–149. 10.1016/j.tim.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Pandza S, Baetens M, Park CH, Au T, Keyhan M, & Matin A. (2000). The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Molecular Microbiology, 36, 414–423. 10.1046/j.1365-2958.2000.01859.x [DOI] [PubMed] [Google Scholar]
- Park S, Park YH, Lee CR, Kim YR, & Seok YJ (2016). Glucose induces delocalization of a flagellar biosynthesis protein from the flagellated pole. Molecular Microbiology, 101, 795–808. 10.1111/mmi.13424 [DOI] [PubMed] [Google Scholar]
- Picardeau M. (2017). Virulence of the zoonotic agent of leptospirosis: Still terra incognita? Nature Reviews Microbiology, 15, 297–307. 10.1038/nrmicro.2017.5 [DOI] [PubMed] [Google Scholar]
- Qin Z, Tu J, Lin T, Norris SJ, Li C, Motaleb MA, & Liu J. (2018). Cryo-electron tomography of periplasmic flagella in Borrelia burgdorferi reveals a distinct cytoplasmic ATPase complex. PLoS Biology, 16, e3000050. 10.1371/journal.pbio.3000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddi G, Morado DR, Yan J, Haake DA, Yang XF, & Liu J. (2012). Three-dimensional structures of pathogenic and saprophytic Leptospira species revealed by cryo-electron tomography. Journal of Bacteriology, 194, 1299–1306. 10.1128/JB.06474-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Deka RK, Anand A, Smajs D, Norgard MV, & Yang XF (2016). Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nature Reviews Microbiology, 14, 744–759. 10.1038/nrmicro.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Lei T, Song Z, Yu T, Li Q, Huang J, & Jiao XA (2018). Could FlhF be a key element that controls Campylobacter jejuni flagella biosynthesis in the initial assembly stage? Microbiological Research, 207, 240–248. 10.1016/j.micres.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Rosa PA, Tilly K, & Stewart PE (2005). The burgeoning molecular genetics of the Lyme disease spirochaete. Nature Reviews Microbiology, 3, 129–143. 10.1038/nrmicro1086 [DOI] [PubMed] [Google Scholar]
- Rossez Y, Wolfson EB, Holmes A, Gally DL, & Holden NJ (2015). Bacterial flagella: Twist and stick, or dodge across the kingdoms. PLoS Pathogens, 11, e1004483. 10.1371/journal.ppat.1004483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sal MS, Li C, Motalab MA, Shibata S, Aizawa S, & Charon NW (2008). Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure. Journal of Bacteriology, 190, 1912–1921. 10.1128/JB.01421-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS (1995). Electrotransformation of the spirochete Borrelia burgdorferi. Methods in Molecular Biology, 47, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS, Drecktrah D, & Hall LS (2018). Genetic transformation and complementation. Methods in Molecular Biology, 1690, 183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E, Vannier E, Wormser GP, & Hu LT (2016). Diagnosis, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: A review. JAMA, 315, 1767–1777. 10.1001/jama.2016.2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schniederberend M, Abdurachim K, Murray TS, & Kazmierczak BI (2013). The GTPase activity of FlhF is dispensable for flagellar localization, but not motility, in Pseudomonas aeruginosa. Journal of Bacteriology, 195, 1051–1060. 10.1128/JB.02013-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher JS, Rossmann F, Dempwolff F, Knauer C, Altegoer F, Steinchen W, … Bange G. (2015). MinD-like ATPase FlhG effects location and number of bacterial flagella during C-ring assembly. Proceedings of the National Academy of Sciences USA, 112, 3092–3097. 10.1073/pnas.1419388112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher JS, Thormann KM, & Bange G. (2015). How bacteria maintain location and number of flagella? FEMS Microbiology Reviews, 39, 812–822. 10.1093/femsre/fuv034 [DOI] [PubMed] [Google Scholar]
- Shibata S, Takahashi N, Chevance FF, Karlinsey JE, Hughes KT, & Aizawa S. (2007). FliK regulates flagellar hook length as an internal ruler. Molecular Microbiology, 64, 1404–1415. 10.1111/j.1365-2958.2007.05750.x [DOI] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, … Malawista SE (1983). The spirochetal etiology of Lyme disease. New England Journal of Medicine, 308, 733–740. 10.1056/NEJM198303313081301 [DOI] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, … Mead PS (2016). Lyme Borreliosis. Nature Reviews Disease Primers, 2, 16090. 10.1038/nrdp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW, & Motaleb MA (2013). Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infection and Immunity, 81, 2012–2021. 10.1128/IAI.01228-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CW, Morado DR, Liu J, Charon NW, Xu H, & Li C. (2011). Carbon storage regulator A (CsrA(Bb)) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Molecular Microbiology, 82, 851–864. 10.1111/j.1365-2958.2011.07853.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder EA Jr, Figueira CP, Benaroudj N, Hu B, Tong BA, Trajtenberg F, … Ko AI (2016). A novel flagellar sheath protein, FcpA, determines filament coiling, translational motility and virulence for the Leptospira spirochete. Molecular Microbiology, 101, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Raddi G, Liu J, Charon NW, & Li C. (2011). Chemoreceptors and flagellar motors are subterminally located in close proximity at the two cell poles in spirochetes. Journal of Bacteriology, 193, 2652–2656. 10.1128/JB.01530-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Liu J, Tu Y, Xu H, Charon NW, & Li C. (2012). Two CheW coupling proteins are essential in a chemosensory pathway of Borrelia burgdorferi. Molecular Microbiology, 85, 782–794. 10.1111/j.1365-2958.2012.08139.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Qin Z, Chang Y, Liu J, Malkowski MG, Shipa S, … Li C. (2019). Analysis of a flagellar filament cap mutant reveals that HtrA serine protease degrades unfolded flagellin protein in the periplasm of Borrelia burgdorferi. Molecular Microbiology, 111, 1652–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Tong BA, Liu J, & Li C. (2012). A single-domain FlgJ contributes to flagellar hook and filament formation in the Lyme disease spirochete Borrelia burgdorferi. Journal of Bacteriology, 194, 866–874. 10.1128/JB.06341-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Norris SJ, & Liu J. (2014). Molecular architecture of the bacterial flagellar motor in cells. Biochemistry, 53, 4323–4333. 10.1021/bi500059y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhang K, Boquoi T, Hu B, Motaleb MA, Miller KA, … Liu J. (2013). Cryoelectron tomography reveals the sequential assembly of bacterial flagella in Borrelia burgdorferi. Proceedings of the National Academy of Sciences USA, 110, 14390–14395. 10.1073/pnas.1308306110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.