Through a collaborative effort, multiple data sets were combined to reveal significant delays in infants and toddlers with an FMR1 gene change.
Abstract
Video Abstract
BACKGROUND:
Children with FMR1 gene expansions are known to experience a range of developmental challenges, including fragile X syndrome. However, little is known about early development and symptom onset, information that is critical to guide earlier identification, more accurate prognoses, and improved treatment options.
METHODS:
Data from 8 unique studies that used the Mullen Scales of Early Learning to assess children with an FMR1 gene expansion were combined to create a data set of 1178 observations of >500 young children. Linear mixed modeling was used to explore developmental trajectories, symptom onset, and unique developmental profiles of children <5 years of age.
RESULTS:
Boys with an FMR1 gene full mutation showed delays in early learning, motor skills, and language development as young as 6 months of age, and both sexes with a full mutation were delayed on all developmental domains by their second birthday. Boys with a full mutation continued to gain skills over early childhood at around half the rate of their typically developing peers; girls with a full mutation showed growth at around three-quarters of the rate of their typically developing peers. Although children with a premutation were mostly typical in their developmental profiles and trajectories, mild but significant delays in fine motor skills by 18 months were detected.
CONCLUSIONS:
Children with the FMR1 gene full mutation demonstrate significant developmental challenges within the first 2 years of life, suggesting that earlier identification is needed to facilitate earlier implementation of interventions and therapeutics to maximize effectiveness.
What’s Known on This Subject:
Most children with an FMR1 full mutation (fragile X syndrome) have impairments in all areas of development, and children with a premutation also are at risk for developmental differences.
What This Study Adds:
This study is the largest examination of emerging symptoms in infants and toddlers with an FMR1 gene change.
Knowing the early sequelae of a disorder is essential to determine the age of symptom onset, identify early presenting signs, and provide baseline data against which the efficacy of new treatments can be compared. But understanding the early natural history of rare disorders is problematic, primarily because of the challenge of early identification. Such is the case with fragile X syndrome (FXS), the most common inherited form of intellectual disability and the leading known single-gene cause of autism spectrum disorder.1 FXS occurs when the trinucleotide cytosine-guanine-guanine (CGG) repeat on the X-linked FMR1 gene expands to >200 repeats (full mutation), silencing FMR1 transcription and reducing or eliminating fragile X mental retardation protein (FMRP) needed for normal brain development.2 Although not obvious at birth, symptoms of FXS have been reported within the first year of life in children with a full mutation. In small studies of infants and toddlers, researchers have documented developmental differences in boys with a full mutation as young as 6 months,3 with a subset demonstrating visual processing issues,4 autistic features,5 and atypical physiologic responses6,7 by 12 months. Atypical motor behavior, language delays, and sensory sensitivities are the most common first signs reported by parents in retrospective studies.7
Individuals with an FMR1 premutation have 55 to 200 CGG repeats on at least 1 allele. Premutation alleles, particularly at larger CGG repeat lengths, can cause subtle but significant FMRP deficits and elevated FMR1 messenger RNA, resulting in reduced neuronal function and central nervous system dysregulation.8–11 Adults with a premutation are at risk for 2 well-documented adult-onset conditions, namely, fragile X–associated tremor ataxia syndrome and fragile X–associated primary ovarian insufficiency, along with other medical (eg, hypertension, thyroid disease), emotional (eg, anxiety12), and cognitive (eg, executive function) challenges.13 Risks associated with early development are also apparent in a subset of children with a premutation, as evidenced by parent reports of elevated rates of mild developmental delays, autistic features, and anxiety.14–16 Early signs of visual processing, social-emotional, and sensory processing challenges have been reported in infants with a premutation.14,15 Understanding the prevalence and impact of these early developmental differences is critical to understanding the lifetime impact of having a premutation. In addition, although research to date suggests that only approximately one-third of individuals with a premutation will experience ≥1 of these fragile X–associated conditions, biomarkers that can provide a more definitive prognosis have yet to be discovered.
Despite these early indicators, the average age of FXS diagnosis is 3 years for boys.17 Girls with a full mutation are often diagnosed with FXS later16 because they typically have a less severe phenotype.18 Children with a premutation are seldom identified except through cascade testing or family history studies of an individual with FXS. Delayed diagnoses can reduce early access to interventions and significantly limit the ability to conduct prospective infant studies to describe the symptom progression. Studies of older children and retrospective parent reports have aided in a well-described phenotype for boys with FXS, but there remains a significant need for natural history data during the early years.
To address questions about early development across the range of FMR1 gene expansions, we conducted a secondary data analysis combining extant data sets using the Mullen Scales of Early Learning (hereafter referred to as the Mullen).19 Guiding research questions are as follows:
What is the nature and pace of development for boys and girls with an FMR1 gene expansion?
At what age do infants and toddlers with an FMR1 gene expansion first differ from typically developing infants?
Are there distinct patterns of developmental strengths and weaknesses for boys and girls with a premutation or full mutation?
Methods
Participants
Eight research studies across the United States contributed 1178 observations from 508 individuals with an FMR1 premutation or full mutation. Objectives of the studies varied, but all researchers focused on describing developmental or behavioral outcomes for children with FMR1 expansions. Studies that enrolled children with a premutation included a pilot newborn screening study for FXS that also identified infants with a premutation, as well as several studies that included premutation siblings of children with FXS. Most studies required an existing diagnosis, with confirmation via genetic report; for those without genetic confirmation, studies provided molecular testing to confirm. Participant characteristics are shown in Table 1. Most (86%) had a full mutation. On average, children with a premutation were 23 months old, and children with a full mutation were 34 months old at the initial evaluation. There were similar numbers of girls and boys in the premutation group, but there were more boys than girls (78% vs 22%) in the full mutation group. Although current prevalence numbers suggest the full mutation is more common in boys, because of the fact that several of the contributing studies focused exclusively on boys with a full mutation, the male-to-female ratio in this data set is larger than would be expected, likely because FXS is most often clinically ascertained. In contrast, none of the studies that included children with a premutation were sex specific. Approximately half of all children had missing data for race. For those that were reported, most were white.
TABLE 1.
Sample Description (N = 508; Observations = 1178)
| Premutation | Full Mutation | |||
|---|---|---|---|---|
| No. Children | No. Observations | No. Children | No. Observations | |
| n = 69 | n = 167 | n = 439 | n = 1011 | |
| Sex, n (%) | ||||
| Female | 34 (49.3) | 106 (63.5) | 94 (21.4) | 226 (22.4) |
| Male | 35 (50.7) | 61 (36.5) | 345 (78.6) | 785 (77.6) |
| Race, n (%) | ||||
| White | 26 (37.7) | 77 (46.1) | 171 (39.0) | 459 (45.4) |
| Black | 6 (8.7) | 19 (11.4) | 11 (2.5) | (31 (3.1) |
| Asian | 1 (1.4) | 1 (0.6) | 5 (1.1) | 13 (1.3) |
| Hispanic | 2 (2.9) | 9 (5.4) | 4 (0.9) | 5 (0.5) |
| Other | 3 (4.3) | 9 (5.4) | 16 (3.6) | 54 (5.3) |
| Missing | 31 (44.9) | 52 (31.1) | 232 (52.8) | 449 (44.4) |
| Age at baseline, mo, mean (SD) | 23.0 (14.2) | 23.4 (16.1) | 34.8 (14.5) | 33.7 (17.9) |
| No. observations, mean (SD) | 2.4 (1.6) | — | 2.3 (1.4) | — |
| No. with 1 visit, n (%) | 29 (42.0) | — | 163 (37.1) | — |
| Test-retest interval, in mo | ||||
| Mean (SD) | — | 9.9 (8.4) | — | 12.1 (7.6) |
| Minimum–maximum | — | 1–49 | — | 1–45 |
—, not applicable.
Measure
All participants were administered the Mullen at least once, and up to 8 times, between 2 and 68 months of age. The Mullen is a standardized direct assessment of early verbal, nonverbal, visual reception, and motor functioning for children birth through 68 months of age.19 Five scales are available for children up through 38 months of age: gross motor, fine motor, receptive language, expressive language, and visual reception. For participants >38 months, the gross motor scale is not administered. Raw scores, age-normed t scores (X = 50; SD = 10), and age equivalents are obtained to describe individual performance. Individual scale scores are combined to determine a standard score for the early learning composite (ELC) (X = 100; SD = 15). The Mullen has well-established internal consistency, test-retest reliability (gross motor = 0.96, cognitive scales = 0.76–0.84), interscorer reliability (0.91–0.99), construct validity, and concurrent validity19 with other measurements of early development.20
Procedures
Data from 8 sites were combined, with the following requirements for inclusion: (1) administration of the Mullen at least once between birth through 68 months of age in individuals with confirmed premutation or full mutation, (2) access to the raw scores across Mullen scales, and (3) known information on age and sex for all participants. Confirmation of premutation or full mutation was obtained through review of genetic reports. Duplicates were removed whenever possible and are believed to be minimal on the basis of participant demographics from the studies included; however, given that the data were deidentified per ethics board requirements, including removal of date of birth information, duplicates in the sample are possible.
Statistical Analyses
Descriptive analyses used frequencies for categorical descriptive variables, whereas means and SDs were used for continuous variables. Individual growth curves based on the standard scores for the ELC, along with age-equivalent scores and t scores of the 5 domains, were plotted to characterize growth patterns by sex and mutation status. Although standard scores and t scores based on the norming sample are used for some analyses, these had a strong floor effect, especially for the full mutation samples. For example, the majority of boys with a full mutation scored at the floor of the test on all domains by age 3. We considered calculating developmental quotients, as has been done in other studies using the Mullen.21,22 However, this approach does not account for varying levels of functional significance across different ages of early development. For example, a 12-month-old child with a 6-month delay would receive the same developmental quotient as a 48-month-old child with a 2-year delay. In contrast, age-equivalent scores provide a metric by which to compare a given child’s score on a domain to the expected performance for a child that same age and have been used to characterize development in children with severe developmental delays.23 Therefore, age equivalents were used in the regression models to describe the extent of delay as well as trajectories of development. To ensure each unit of age had adequate information (and aid in model convergence), age was collapsed into 3-month groupings. A sensitivity test was then conducted by running the model after excluding those with only 1 observation and found that the results of the significance tests were the same with the exception of the gross motor domain; therefore, the full sample was used for all domains except for gross motor.
Linear mixed modeling was used to examine fixed and random effects of growth by group. Four groups based on sex and mutation status were compared with the Mullen norming sample: girls with a premutation, boys with a premutation, girls with a full mutation, and boys with a full mutation. A series of nested models were tested for each domain on the Mullen, and the Akaike information criterion and Bayesian information criterion24 were used to determine the best fit. Results were plotted for a visual presentation of group differences. Although a curvilinear function of time may be apparent for some plots (eg, fine motor development among boys with a full mutation), we tested only for a linear function because of insufficient repeated measurements per group to justify a higher-order transformation of time.
To test group differences in overall mean ELC score and slope, pairwise testing was conducted by using least-squares means of group and slope interaction fixed effects, with Bonferroni adjustment for multiple comparisons. An identical approach was conducted to compare relative domain strengths and weaknesses across domains within groups. Means and SEs were plotted by group to examine relative strengths and weaknesses across domains within group. The SAS PROC MIXED procedure (SAS Institute, Inc, Cary, NC) was used to fit the linear mixed model to the data.
Results
Early Developmental Trajectories
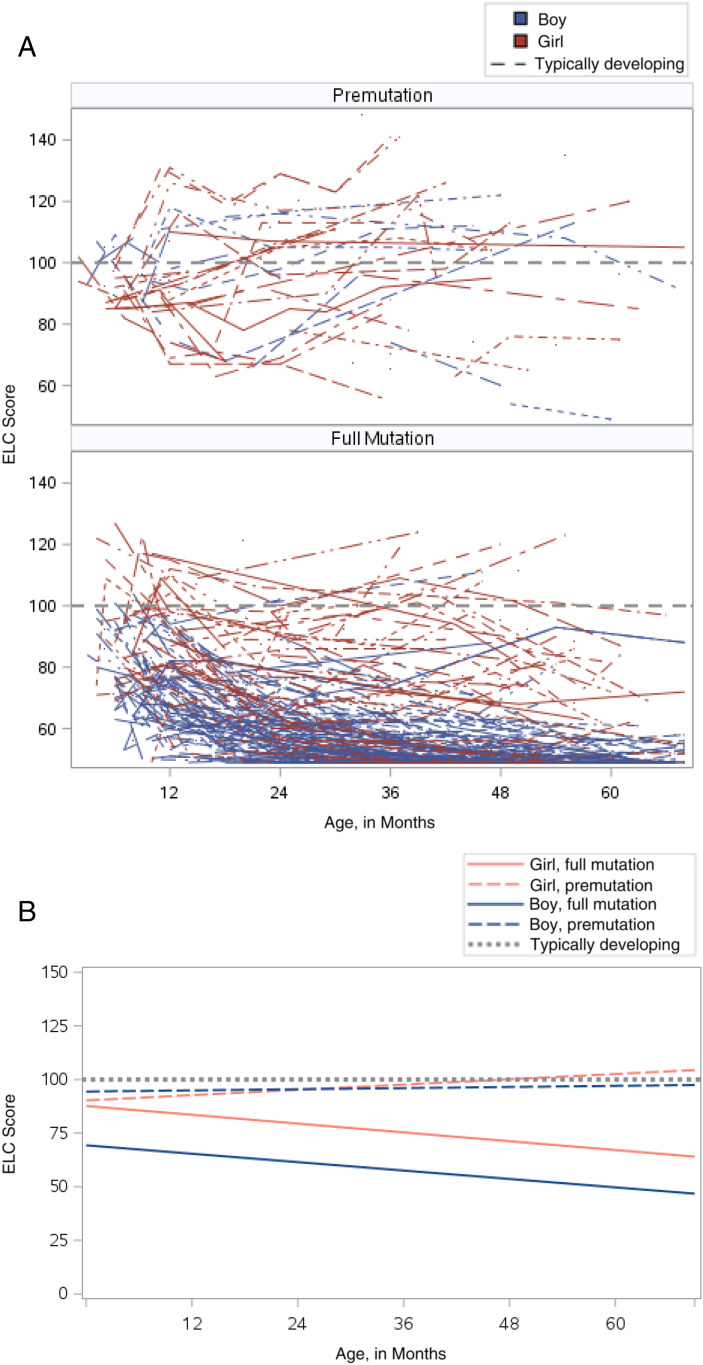
Regression models comparing Mullen scores to the norming sample, by group, are presented in Table 2, and results are illustrated in Figs 1 and 2. Data from the norming sample are shown with the dotted line in each figure. Average ELC scores for boys (97.8; SE = 1.47) and girls (98.5; SE = 1.17) with a premutation were higher than for those with a full mutation and not significantly different from the norming sample at 36 months of age. Average ELC scores were 57 (SE = 0.41) for boys with a full mutation and 77 for girls with a full mutation (SE = 0.69), which were statistically lower than the norming sample. Across all 5 domains, boys and girls with a full mutation had statistically lower scores than the norming sample (see Table 2). Boys with a premutation had statistically lower scores only on the fine motor domain at 36 months of age (x = −1.8; P < .001).
TABLE 2.
Parameter Estimates (and SE) Comparing Age-Equivalent Scores to the Norming Sample Within Domain by Group
| ELC | Gross Motor | Visual Receptive | Fine Motor | Receptive Language | Expressive Language | |
|---|---|---|---|---|---|---|
| Fixed effects | ||||||
| Initial status | ||||||
| Intercept (centered, ref) | 100.00 (0.22)* | 18.00 (0.06) | 36.00 (0.11)* | 36.00 (0.08)* | 36.00 (0.12)* | 36.00 (0.11)* |
| Girl with a premutation | −1.50 (1.17) | −0.36 (0.21) | 0.63 (0.57) | −0.45 (0.46) | −0.00 (0.63) | −1.21 (0.63) |
| Boy with a premutation | −2.24 (1.47) | −0.24 (0.38) | −0.08 (0.72) | −1.84 (0.58)* | 0.43 (0.81) | −0.20 (0.80) |
| Girl with an FM | −23.00 (0.69)* | −2.54 (0.16)* | −5.67 (0.34)* | −8.28 (0.27)* | −7.86 (0.38)* | −8.75 (0.38)* |
| Boy with an FM | −43.01 (0.41)* | −5.67 (0.11)* | −14.93 (0.20)* | −16.05 (0.16)* | −16.40 (0.22)*, | −18.87 (0.22)* |
| Rate of change, mo, centered | ||||||
| Time (ref) | −0.00 (0.05) | 1.00 (0.00)* | 1.00 (0.03)* | 1.00 (0.03)* | 1.00 (0.03)* | 1.00 (0.04)* |
| Time by girl with a premutation | 0.11 (0.10) | −0.05 (0.02) | −0.01 (0.06) | −0.07 (0.05) | −0.01 (0.06) | −0.01 (0.07) |
| Time by boy with a premutation | −0.04 (0.11) | −0.03 (0.05) | −0.05 (0.06) | −0.10 (0.06) | −0.03 (0.07) | −0.05 (0.08) |
| Time by girl with an FM | −0.33 (0.07)* | −0.20 (0.02)* | −0.27 (0.04)* | −0.41 (0.04)* | −0.28 (0.05)* | −0.32 (0.05)* |
| Time by boy with an FM | −0.34 (0.06)* | −0.44 (0.01)* | −0.54 (0.03)* | −0.64 (0.03)* | −0.53 (0.04)* | −0.56 (0.04)* |
| Random effects | ||||||
| Time, mo | 0.13 (0.02)* | — | 0.05 (0.01)* | 0.05 (0.00)* | 0.07 (0.01)* | 0.09 (0.01)* |
| Residual | 66.25 (2.18)* | 2.40 (0.08)* | 15.37 (0.51)* | 9.64 (0.32)* | 18.67 (0.63)* | 18.07 (0.61)* |
Age is centered at 36 mo for each domain except for gross motor, which is centered at 18 mo for interpretation of the intercept. Cells that share the same alphabetic superscript within domains are not statistically different from each other. Data are presented as parameter estimates (SE). FM, full mutation; ref, reference group (norming sample); —, not applicable.
P < .001.
FIGURE 1.
ELC score plots. A, Raw ELC line plots. B, Model-based ELC plots.
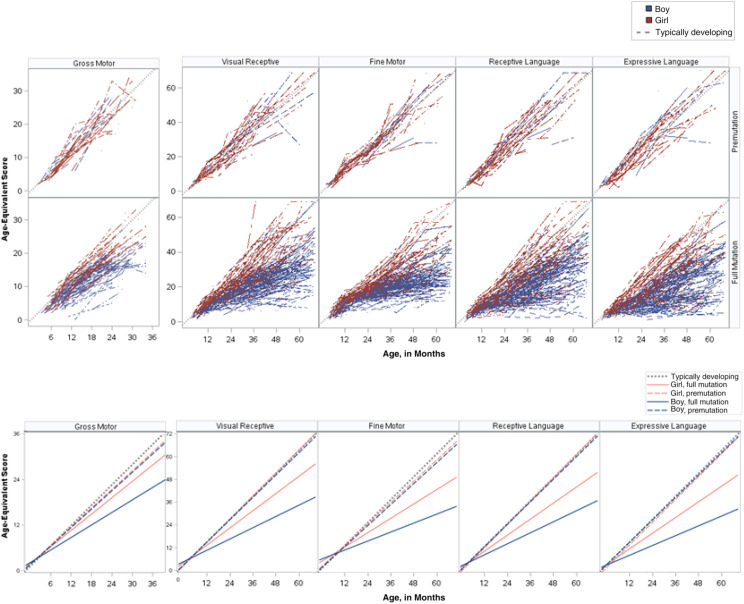
FIGURE 2.
Age-equivalent plots. A, Raw line plots. B, Model-based plots.
Similarly, the rate of growth for boys with a full mutation was significantly slower on all developmental domains compared with the norming sample and all other groups (see Table 2). Relative to the norming sample and the premutation groups, boys with a full mutation demonstrated growth at half the rate on all developmental domains. The rate of growth on the ELC score, however, was similar between boys and girls with a full mutation (see Supplemental Table 5).
For girls with a full mutation, the rate of growth was approximately three-quarters of the rate of the norming sample. Similar trajectories were found across developmental domains, with growth estimated at approximately two-thirds of the rate of the norming sample on visual reception, receptive language, and expressive language and nearly half that of the norming sample on fine motor (see Table 2).
Age When Developmental Differences First Appear
Shown in Table 3 is the age at which each of the FMR1 groups demonstrate significant delays across domains on the basis of standard ELC scores or domain t scores. Neither boys or girls with a premutation exhibited clinically significant delays at any of the tested ages on the ELC or any of the subdomains, with 1 exception. Boys with a premutation exhibited significant delays in fine motor skills by 36 months of age, a finding that persisted through the oldest ages in this sample.
TABLE 3.
Age of Significant Negative Deviation From Norming Sample by Using Standardized Scores (ELC and t Scores)
| Age, mo | ||||||
|---|---|---|---|---|---|---|
| 6 | 12 | 18 | 24 | 30 | 36 | |
| ELC, mean (SD) | ||||||
| Girl with a premutation | −7.60 (1.06) | −6.20 (0.93) | −4.39 (0.88) | −4.54 (0.96) | −3.98 (1.04) | −1.50 (1.17) |
| Boy with a premutation | −3.17 (1.34) | −3.10 (1.21) | −2.47 (1.23) | −3.42 (1.25) | −3.86 (1.31) | −2.24 (1.47) |
| Girl with an FM | −10.76 (0.84) | −13.74 (0.74) | −16.59 (0.71)a | −18.82 (0.72)a | −20.96 (0.68)a | −23.00 (0.69)a |
| Boy with an FM | −29.27 (0.59)a | −32.01 (0.53)b | −35.17 (0.49)b | −37.39 (0.49)b | −39.67 (0.44)b | −43.01 (0.41)b |
| Gross motor | ||||||
| Girl with a premutation | −3.71 (1.00) | −2.72 (0.72) | −1.73 (0.70) | −0.74 (0.95) | 0.25 (1.33) | — |
| Boy with a premutation | −4.49 (1.58) | −3.21 (1.07) | −1.94 (1.25) | −0.66 (1.93) | 0.62 (2.77) | — |
| Girl with an FM | −9.10 (0.82) | −9.51 (0.59)a | −9.92 (0.54)a | −10.34 (0.70)a | −10.75 (0.98)a | — |
| Boy with an FM | −15.10 (0.61)a | −17.61 (0.44)a | −20.12 (0.35)b | −22.63 (0.40)b | −25.14 (0.56)b | — |
| Visual receptive, mean (SD) | ||||||
| Girl with a premutation | −1.90 (0.82) | −1.29 (0.71) | −0.60 (0.66) | −1.01 (0.70) | −0.93 (0.76) | 0.06 (0.87) |
| Boy with a premutation | −1.03 (1.04) | −1.05 (0.92) | −0.72 (0.90) | −1.61 (0.90) | −2.13 (0.94) | −1.42 (1.07) |
| Girl with an FM | −4.03 (0.65) | −5.24 (0.57) | −6.57 (0.53) | −7.97 (0.52) | −9.16 (0.49) | −10.33 (0.51)a |
| Boy with an FM | −14.49 (0.46)a | −16.18 (0.40)a | −18.11 (0.36)a | −19.82 (0.36)b | −21.46 (0.32)b | −23.66 (0.30)b |
| Fine motor, mean (SD) | ||||||
| Girl with a premutation | −1.42 (0.91) | −1.15 (0.84) | −1.45 (0.72) | −1.75 (0.68) | −0.36 (0.70) | −0.90 (0.80) |
| Boy with a premutation | −3.96 (1.14) | −3.86 (1.08) | −4.25 (0.93) | −4.64 (0.87) | −3.41 (0.92) | −4.32 (1.00) |
| Girl with an FM | −3.65 (0.71) | −6.70 (0.68) | −8.79 (0.58) | −10.88 (0.51)a | −13.23 (0.47)a | −15.38 (0.48)a |
| Boy with an FM | −15.24 (0.50)a | −17.99 (0.48)a | −19.76 (0.41)b | −21.54 (0.35)b | −24.47 (0.29)b | −26.20 (0.28)b |
| Receptive language, mean (SD) | ||||||
| Girl with a premutation | −4.78 (0.81) | −4.00 (0.70) | −3.10 (0.65) | −2.85 (0.69) | −2.41 (0.75) | −1.29 (0.86) |
| Boy with a premutation | −0.12 (1.02) | −0.51 (0.91) | −0.49 (0.90) | −0.46 (0.90) | −0.70 (0.95) | −0.04 (1.08) |
| Girl with an FM | −7.58 (0.64) | −9.14 (0.56) | −10.41 (0.52)a | −11.17 (0.52)a | −12.02 (0.49)a | −12.69 (0.50)a |
| Boy with an FM | −16.12 (0.45)a | −17.76 (0.40)a | −19.43 (0.36)a | −20.59 (0.36)b | −21.90 (0.32)b | −23.62 (0.30)b |
| Expressive language, mean (SD) | ||||||
| Girl with a premutation | −7.43 (0.72) | −6.30 (0.62) | −5.04 (0.58) | −4.42 (0.62) | −3.65 (0.67) | −2.00 (0.76) |
| Boy with a premutation | −1.70 (0.91) | −1.46 (0.81) | −1.37 (0.79) | −1.76 (0.80) | −1.91 (0.84) | −1.05 (0.95) |
| Girl with an FM | −8.69 (0.57) | −9.99 (0.50)a | −11.19 (0.46)a | −11.84 (0.47)a | −12.63 (0.44)a | −13.30 (0.45)a |
| Boy with an FM | −18.61 (0.40)a | −19.88 (0.35)b | −21.34 (0.32)b | −22.36 (0.32)b | −23.45 (0.28)b | −25.03 (0.27)b |
ELC has mean of 100 and SD of 15. t score has mean of 50 and SD of 10. FM, full mutation; —, not applicable.
t score <1 SD from mean.
t score <2 SD from mean.
Girls with a full mutation scored ∼11 points lower than the norming sample on the ELC at 6 months, which is still considered within the average range for the test. However, by 18 months, they were scoring nearly 17 points lower than the norming sample, and by 36 months, the gap was 23 points lower, which is 1.5 SDs below the average. Boys with a full mutation showed significant delays on the ELC by 6 months, with the extent of delays becoming larger as they got older. At 6 months, boys with a full mutation scored 29 points lower than the norming sample on the ELC, and by 36 months, this difference grew to 43 points, which is nearly 3 SDs below average.
Girls with a full mutation showed clinically significant delays in gross motor and expressive language skills by 12 months of age. Fine motor and receptive language skills were significantly delayed by 18 months of age, and visual reception skills were delayed by their second birthday (Table 3). All delays for girls with a full mutation were between 1 and 2 SDs from average.
Boys with a full mutation showed significant delays by 6 months of age on all developmental domains (Table 3) with the extent of delays becoming larger as they got older. By 3 years of age, boys with a full mutation had delays that were 2.5 SDs from average on all developmental domains.
Patterns of Developmental Strengths and Weaknesses
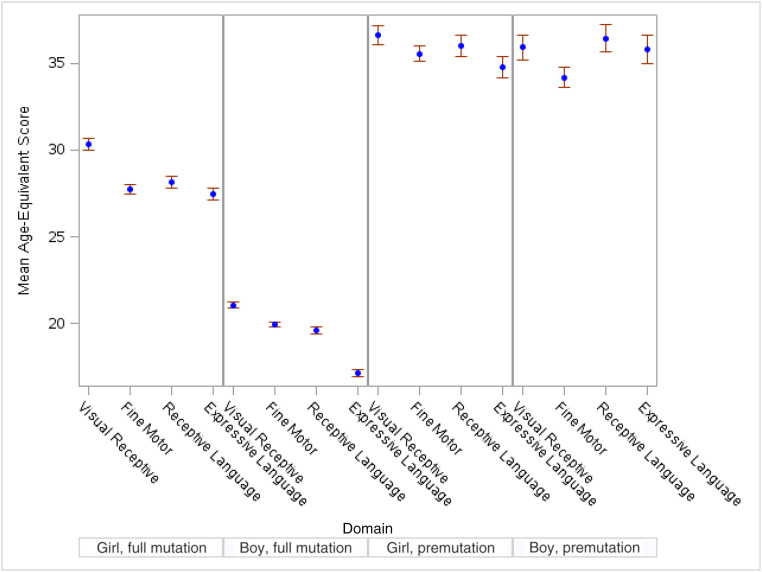
Presented in Table 4 are regression models comparing Mullen scores across domains, within groups, and in Fig 3, these scores are depicted visually. Although age-equivalent scores were similar across domains for girls with a premutation, receptive language had a faster rate of development than fine motor skills (b = 1.05 compared with b = 0.94; P < .05) (see Table 4 and Supplemental Table 6). No significant patterns of developmental strengths or weaknesses in domain scores were found for boys with a premutation at 36 months of age (see Table 4).
TABLE 4.
Parameter Estimates (and SE) Comparing Age-Equivalent Scores to the Norming Sample Within Groups by Domain
| Premutation | Full Mutation | |||
|---|---|---|---|---|
| Girl | Boy | Girl | Boy | |
| Fixed effects | ||||
| Initial status | ||||
| Intercept (centered, ref) | 36.00 (0.59)***,a | 36.00 (0.67)***,a | 36.00 (0.84)***,a | 36.00 (0.68)***,a |
| Expressive language | −1.17 (1.04)a | −0.41 (1.27)a | −9.77 (1.11)*** | −18.86 (0.75)*** |
| Fine motor | −1.71 (1.04)a | −2.31 (1.27)a | −8.99 (1.11)*** | −16.00 (0.75)*** |
| Receptive language | −0.03 (1.04)a | −0.43 (1.29)a | −8.91 (1.11)*** | −16.36 (0.75)*** |
| Visual receptive | −0.11 (1.04)a | −0.54 (1.27)a | −6.76 (1.11)*** | −14.96 (0.75)*** |
| Rate of change, mo (centered, ref) | ||||
| Time | 1.00 (0.02)***,a | 1.00 (0.03)***,a | 1.00 (0.04)***,a | 1.00 (0.03)***,a |
| Time by expressive language | 0.02 (0.04)a | −0.04 (0.07)a | −0.30 (0.05)*** | −0.56 (0.03)*** |
| Time by fine motor | −0.06 (0.04)a | −0.07 (0.07)a | −0.37 (0.05)*** | −0.64 (0.03)*** |
| Time by receptive language | 0.05 (0.04)a | −0.03 (0.07)a | −0.27 (0.05)*** | −0.52 (0.03)*** |
| Time by visual receptive | 0.00 (0.04)a | −0.12 (0.07)a | −0.20 (0.05)*** | −0.52 (0.03)*** |
| Random effects | ||||
| Time, mo | 0.01 (0.00)*** | 0.04 (0.01)*** | 0.08 (0.02)*** | 0.04 (0.00)*** |
| Domain | 20.81 (2.35)*** | 27.26 (3.36)*** | 41.77 (3.64)*** | 27.79 (1.30)*** |
| Time x domain | 0.01 (0.00)*** | 0.03 (0.00)*** | 0.01 (0.00)*** | 0.01 (0.00)*** |
| Residual | 1.65 (0.06)*** | 0.44 (0.02)*** | 3.40 (0.12)*** | 4.73 (0.15)*** |
ref, reference group (norming sample).
Cells that share the same alphabetic superscript within domains are not statistically different from each other.
P < .001
FIGURE 3.
Differences in means (at 36 months of age) by group across domains.
Developmental profiles of girls with a full mutation suggest significantly better visual reception than expressive language scores at 36 months of age (age-equivalent x = 29.2 for visual reception compared with x = 26.2 for expressive language; P < .05) (see Table 4 and Supplemental Table 6). They also showed better rates of growth in visual reception skills relative to expressive language and fine motor skills (b = 0.80 compared with b = 0.70, P < .01; b = 0.63, P < .001), and better rates of growth in receptive language than in fine motor skills (b = 0.73 compared with b = 0.63; P < .01) (see Table 4 and Supplemental Table 6).
Boys with a full mutation had a significant weakness relative to all other domains of development in expressive language at 36 months of age (see Supplemental Table 6). Fine motor skills developed at a significantly slower rate than other domains (P < .001).
Discussion
Our multisite collaboration represents the largest exploration of early development in young children with an FMR1 gene expansion to date. With data on >500 children and nearly 1200 observations, our findings expand and contribute to the understanding of the natural history of the FMR1 expansions found in earlier direct-assessment studies,3,25,26 parent reports,17 and results from imaging studies.22 Our findings confirm the emergence of developmental differences in young children with a full mutation within the first 6 to 12 months of life, and patterns of performance by 3 years of age mimic those found in older children and adults with a full mutation, a significant contribution to the holistic understanding of development in FXS. Additionally, our findings contribute to the limited understanding of the early developmental trajectory and potential differences observed in a subsample of those with a premutation.
Estimated trajectories from this sample indicate boys with a full mutation were delayed on all developmental domains by 6 months of age. This confirms previous retrospective reports by parents who have noted first concerns for their boys with FXS at ∼6 months of age.17 Imaging studies of infants and toddlers with FXS have also indicated differences in white matter tracts as young as 6 months of age, with implications for limbic system function and visual processing.22 The primary implication of these findings is that the impact of reduced FMRP on brain functioning is significant early in development, manifesting in observable delays in all areas of development. However, white matter is a highly plastic brain target that can be amenable to intervention, suggesting intensive early intervention may reduce the downstream negative outcomes for children with FXS.27
Girls with a full mutation had more variability in development across domains, with delays in language and gross motor skills emerging earlier (at ∼1 year of age) than delays in fine motor and visual reception skills (emerging between 18 and 24 months). Both boys and girls with a full mutation had delays in expressive language by their first birthday and demonstrated a relative weakness in expressive language relative to other domains at age 3. This is consistent with previous retrospective parent-report studies highlighting language delays as one of the earliest reported concerns by parents of children with FXS, with the average age of first words for boys nearly a year behind typical expectations.7,28 Furthermore, Brady et al29 observed nearly half of the children with FXS in their sample to still be nonverbal at age 3, suggesting language delays may be one of the most significant and pervasive developmental challenges for infants and toddlers with a full mutation.
Our study also confirms previous reports that infants with a premutation do not show signs of significant developmental differences as measured by standard developmental measures. However, we did find that, as a group, boys with a premutation had significant delays in fine motor skill development at age 3. Although the delays were mild relative to those of their counterparts with a full mutation, this finding is significant, especially given that in previous reports of deviations from typical development in infants with a premutation, researchers also found motor-related concerns, specifically with visual-motor14 and sensory-motor processing.15 Motor coordination and sequencing are considered neurologic soft signs for neural dysfunction, suggesting motoric development as an important potential target of future premutation-focused research.
Implications for Identification
In our sample, delays were present as early as 6 months of age in all domains for boys with a full mutation. In addition, girls with a full mutation, who are generally believed to be not as severely impacted as their male counterparts, showed delays in expressive language and gross motor skills by 12 months and demonstrated significant delays across all domains by their second birthday. Early intervention programs can be used to address early difficulties and promote better developmental outcomes; however, to receive early intervention, children must have a delay identified, which can take many months to document. Unfortunately, the average age of diagnosis for boys with a full mutation is at ∼36 months,17 which suggests that infants with a full mutation may show signs of a delay upward of 2.5 years before obtaining an accurate diagnosis. Without an established diagnosis, these early delays may be overlooked or dismissed until they are more problematic or major milestones are missed. Because delays are present before a formal diagnosis, most toddlers with FXS, especially boys, will receive early intervention, usually by 18 to 24 months of age. However, this reflects a potential of up to 2 years of missed therapeutic intervention, time that would not be lost with earlier identification and an “established condition” for early intervention eligibility.
Facilitation of early identification has been a goal of FXS researchers and clinicians for several decades.30–32 Preconception carrier testing, newborn screening, and systematic universal developmental screening of infants and toddlers are among the top efforts proposed, with significant challenges accompanying each of these options.33 Currently, the most accessible and common practice implemented is the recommendation for a microarray and FXS testing for those who screen positive for a developmental delay through universal screening in pediatric settings34 Unfortunately, implementation of screening and referring to genetic testing are inconsistently implemented, and even when delay is identified, families are often told the child will “catch up,” resulting in delays in access to intervention and often a long diagnostic journey for families. Public health initiatives like newborn screening hold great potential for early identification of genetic disorders; however, the inclusion of disorders like FXS, particularly without actionable medical intervention, is limited. Although parents have indicated that they are willing to consent and engage in this type of work,35 moving these initiatives forward has proven challenging. Programs like Early Check,36 which provides expanded newborn screening options for FXS under a research protocol, have the potential to make a significant change in this field by simultaneously contributing further to the natural history of developmental profiles from birth and beyond, adding to data necessary to move consideration for nomination to the Recommended Universal Newborn Screening Panel forward and providing an innovative model of second-tier screening for FXS, leading to a more accessible model of identification.
Implications for Treatment
On the basis of standard scores, children with a full mutation move farther away from the developmental trajectory of their neurotypical peers over time. However, similar to previous reports,37 we found that children with a full mutation continue to gain skills over their early childhood years on the basis of age-equivalent scores. For boys with a full mutation, this progress was approximately half the pace of their typically developing peers, whereas girls with a full mutation demonstrated a gain in skills at approximately three-quarters of the pace of expected development. This is a helpful metric by which to measure the early natural history of the full mutation because it provides a marker by which early treatment can be shown to have efficacy. For example, even if standard scores are not found to change significantly, a new therapeutic medication or targeted early intervention program that was able to shift this trajectory so that the rate of developmental skill attainment was more similar to neurotypical rates would have a tremendous impact on outcomes for individuals with FXS.
Disease-modifying therapeutics are currently being explored for FXS.38 These have the potential to dramatically change long-term outcomes for individuals with FXS and their families. However, to date, clinical trials in FXS have not succeeded in significantly altering cognitive or behavioral outcomes. Therefore, behavioral interventions are still the primary treatment mechanism for FXS. Although we do not have data to report on the extent to which children in this sample received early intervention services, we can assume on the basis of federal eligibility criteria, that most children with FXS would have been eligible for these services. Therefore, these findings can be considered to reflect the natural history of early development in FXS, including access to standard care, suggesting a more targeted set of intervention strategies may be necessary. Although unlikely to have a curative effect sought through medical therapeutics, targeted behavioral intervention strategies can make significant differences, especially when implemented early and consistently. Parent-mediated interventions are especially promising because they focus on enhancing parental efficacy and skills, which can then be implemented within the family’s daily routines. Several case studies have shown promise for this approach39–41; future intervention trials are needed to demonstrate true efficacy.
Limitations
Two key limitations should be noted, primarily because of the fact that data had already been collected under different study protocols at each site. First, although we could confirm the diagnosis, we were not able to include specific genetic information (eg, CGG repeat, FMRP levels) about each child. Second, we lack information on co-occurring diagnoses (eg, autism spectrum disorder, seizures), family variables such as race and/or ethnicity and socioeconomic status, and treatment history. However, despite these limitations, we believe that these data provide a robust and informative characterization of early development associated with FMR1 gene expansions.
Conclusions
With our study, we demonstrate the power of collaborative efforts in understanding rare disorders. By combining multiple data sets, we were able to characterize early developmental patterns and show that many children with FXS have significant delays beginning in the first year of life. These data suggest that gene therapy or other innovative therapeutics may be most effective if administered early in life.
Acknowledgments
We thank the many individuals with FXS and their families for participating in this and other research studies.
Glossary
- CGG
cytosine-guanine-guanine
- ELC
early learning composite
- FMRP
fragile X mental retardation protein
- FXS
fragile X syndrome
- NS
norming sample
Footnotes
Dr Wheeler contributed to the conceptualization and drafted the majority of the manuscript; Dr Gwaltney conducted all analysis; Dr Raspa contributed to the conceptualization and supported analysis; Dr Okoniewski managed data acquisition; Drs Berry-Kravis, Budimirovic, Hazlett, Hessl, Losh, Martin, Rivera, and Roberts contributed data from various studies; Dr Bailey contributed to the conceptualization and provided data; and all authors edited previous drafts, approved of the final submitted manuscript, and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Dr Bailey has received contributed reagents and equipment from Asuragen; Dr Berry-Kravis has received funding from Acadia, Alcobra, AMO Pharmaceuticals, Asuragen, BioMarin, Cydan, Fulcrum, GeneTx, GW Pharmaceuticals, Ionis, Lumos, Marinus, Neuren, Neurotrope, Novartis, Ovid, Roche, Seaside Therapeutics, Ultragenyx, Vtesse/Sucampo/Mallinckrodt, Yamo, and Zynerba Pharmaceuticals to consult on clinical trial design, run clinical trials, or develop testing standards or biomarkers, all of which is directed to Rush University Medical Center in support of rare disease programs; Dr Budimirovic has received funding from Seaside, Roche, Neuren, Pfizer, Shire, Lundbeck, Forest, Sunovion, SyneuRX, Alcobra, Akili, Medgenics, Purdue, and Supernus as a main sub-investigator, and Ovid and Zynerba Pharmaceuticals as an investigator on clinical trials and/or to consult on clinical trial outcome measures (Seaside, Ovid), and Asuragen/National Institutes of Health to develop molecular biomarkers, all of which has been directed to Kennedy Krieger Institute/the Johns Hopkins Medical Institutions; Dr Hessl has received compensation for consulting with Autifony, Ovid, and Zynerba pharmaceutical companies; Drs Wheeler and Raspa have received funding from Ovid; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Preparation of this article was provided by the John Merck Fund. Collection of data included in this study came from subjects screened for the Network for Excellence in Neuroscience Clinical Trials NN107 FX-LEARN (funded by U01NS096767 to Dr Berry-Kravis), participants recruited under National Institutes of Health (NIH) grant 1R01HD056031 (to Dr Rivera), participants recruited under NIH grant R01HD038819 (to Dr Losh and Dr Martin) and additional clinical assessment and data processing supported by R01MH091131 (to Dr Losh), participants recruited under NIH grants R01MH1075732 and R01MH90194 (to Dr Roberts), participants recruited under NIH grant P30-HD003110 (to Dr Bailey), and participants recruited under Office of Special Education Programs grant H324B010041 (to Dr Wheeler). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Farzin F, Koldewyn K. Fragile X Syndrome and Autism. In: Patel VB, Preedy VR, Martins CR, eds. Comprehensive Guide to Autism. New York, NY: Springer; 2014:2743–2754 [Google Scholar]
- 2.Malter HE, Iber JC, Willemsen R, et al. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15(2):165–169 [DOI] [PubMed] [Google Scholar]
- 3.Roberts JE, McCary LM, Shinkareva SV, Bailey DB Jr.. Infant development in Fragile X syndrome: cross-syndrome comparisons. J Autism Dev Disord. 2016;46(6):2088–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farzin F, Whitney D, Hagerman RJ, Rivera SM. Contrast detection in infants with fragile X syndrome. Vision Res. 2008;48(13):1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts JE, Tonnsen BL, McCary LM, Caravella KE, Shinkareva SV. Brief report: autism symptoms in infants with fragile X syndrome. J Autism Dev Disord. 2016;46(12):3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonnsen BL, Shinkareva SV, Deal SC, Hatton DD, Roberts JE. Biobehavioral indicators of social fear in young children with fragile X syndrome. Am J Intellect Dev Disabil. 2013;118(6):447–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinton R, Budimirovic DB, Marschik PB, et al. Parental reports on early language and motor milestones in fragile X syndrome with and without autism spectrum disorders. Dev Neurorehabil. 2013;16(1):58–66 [DOI] [PubMed] [Google Scholar]
- 8.Cao Z, Hulsizer S, Tassone F, et al. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21(13):2923–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sellier C, Rau F, Liu Y, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29(7):1248–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sellier C, Freyermuth F, Tabet R, et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013;3(3):869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Sharma MR, Shi X, Agrawal RK, Joseph S. Fragile X mental retardation protein regulates translation by binding directly to the ribosome. Mol Cell. 2014;54(3):407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider A, Johnston C, Tassone F, et al. Broad autism spectrum and obsessive-compulsive symptoms in adults with the fragile X premutation. Clin Neuropsychol. 2016;30(6):929–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler AC, Bailey DB Jr., Berry-Kravis E, et al. Associated features in females with an FMR1 premutation. J Neurodev Disord. 2014;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego PK, Burris JL, Rivera SM. Visual motion processing deficits in infants with the fragile X premutation. J Neurodev Disord. 2014;6(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler AC, Sideris J, Hagerman R, Berry-Kravis E, Tassone F, Bailey DB Jr.. Developmental profiles of infants with an FMR1 premutation. J Neurodev Disord. 2016;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordeiro L, Abucayan F, Hagerman R, Tassone F, Hessl D. Anxiety disorders in fragile X premutation carriers: preliminary characterization of probands and non-probands. Intractable Rare Dis Res. 2015;4(3):123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey DB Jr., Raspa M, Bishop E, Holiday D. No change in the age of diagnosis for fragile X syndrome: findings from a national parent survey. Pediatrics. 2009;124(2):527–533 [DOI] [PubMed] [Google Scholar]
- 18.Bartholomay KL, Lee CH, Bruno JL, Lightbody AA, Reiss AL. Closing the gender gap in fragile X syndrome: review on females with FXS and preliminary research findings. Brain Sci. 2019;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullen EM. Mullen Scales of Early Learning: AGS Edition. Minneapolis, MN: Pearson (AGS); 1995 [Google Scholar]
- 20.Nordahl-Hansen A, Kaale A, Ulvund SE. Language assessment in children with autism spectrum disorder: concurrent validity between report-based assessments and direct tests. Res Autism Spectr Disord. 2014;8(9):1100–1106 [Google Scholar]
- 21.Clarkson T, LeBlanc J, DeGregorio G, et al. Adapting the Mullen Scales of Early Learning for a standardized measure of development in children with Rett syndrome. Intellect Dev Disabil. 2017;55(6):419–431 [DOI] [PubMed] [Google Scholar]
- 22.Swanson MR, Wolff JJ, Shen MD, et al.; Infant Brain Imaging Study (IBIS) Network . Development of white matter circuitry in infants with Fragile X syndrome. JAMA Psychiatry. 2018;75(5):505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delaney KA, Rudser KR, Yund BD, Whitley CB, Haslett PAJ, Shapiro EG. Methods of neurodevelopmental assessment in children with neurodegenerative disease: Sanfilippo syndrome. JIMD Rep. 2014;13:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003 [Google Scholar]
- 25.Roberts JE, Hatton DD, Long AC, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. J Autism Dev Disord. 2012;42(6):937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caravella KE, Roberts JE. Adaptive skill trajectories in infants with Fragile X syndrome contrasted to typical controls and infants at high risk for autism. Res Autism Spectr Disord. 2017;40:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts JE, Mirrett P, Anderson K, Burchinal M, Neebe E. Early communication, symbolic behavior, and social profiles of young males with Fragile X syndrome. Am J Speech Lang Pathol. 2002;11:295–304 [Google Scholar]
- 29.Brady N, Skinner D, Roberts J, Hennon E. Communication in young children with fragile X syndrome: a qualitative study of mothers’ perspectives. Am J Speech Lang Pathol. 2006;15(4):353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey DB Jr. Newborn screening for fragile X syndrome. Ment Retard Dev Disabil Res Rev. 2004;10(1):3–10 [DOI] [PubMed] [Google Scholar]
- 31.Hill MK, Archibald AD, Cohen J, Metcalfe SA. A systematic review of population screening for fragile X syndrome. Genet Med. 2010;12(7):396–410 [DOI] [PubMed] [Google Scholar]
- 32.Riley C, Wheeler A. Assessing the Fragile X syndrome newborn screening landscape. Pediatrics. 2017;139(suppl 3):S207–S215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okoniewski KC, Wheeler AC, Lee S, et al. Early identification of fragile X syndrome through expanded newborn screening. Brain Sci. 2019;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeschler JB, Shevell M; American Academy of Pediatrics Committee on Genetics . Clinical genetic evaluation of the child with mental retardation or developmental delays. Pediatrics. 2006;117(6):2304–2316 [DOI] [PubMed] [Google Scholar]
- 35.Bailey DB Jr., Berry-Kravis E, Gane LW, et al. Fragile X newborn screening: lessons learned from a multisite screening study. Pediatrics. 2017;139(suppl 3):S216–S225 [DOI] [PubMed] [Google Scholar]
- 36.Bailey DB Jr., Gehtland LM, Lewis MA, et al. Early Check: translational science at the intersection of public health and newborn screening. BMC Pediatr. 2019;19(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey DB Jr., Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. J Autism Dev Disord. 2000;30(1):49–59 [DOI] [PubMed] [Google Scholar]
- 38.Berry-Kravis EM, Lindemann L, Jønch AE, et al. Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nat Rev Drug Discov. 2018;17(4):280–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winarni TI, Schneider A, Borodyanskara M, Hagerman RJ. Early intervention combined with targeted treatment promotes cognitive and behavioral improvements in young children with fragile X syndrome. Case Rep Genet. 2012;2012:280813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oakes A, Ma M, McDuffie A, Machalicek W, Abbeduto L. Providing a parent-implemented language intervention to a young male with fragile X syndrome: brief report. Dev Neurorehabil. 2015;18(1):65–68 [DOI] [PubMed] [Google Scholar]
- 41.Vismara LA, McCormick CEB, Shields R, Hessl D. Extending the parent-delivered Early Start Denver Model to young children with fragile X syndrome. J Autism Dev Disord. 2019;49(3):1250–1266 [DOI] [PubMed] [Google Scholar]