Acute lower respiratory tract infections (ALRTI) are a key cause of mortality, morbidity, and healthcare expenditures, which affect more than 3 million individuals annually and cause more than 50,000 deaths per year in the United States alone. Worldwide, ALRTI are responsible for almost 150 million cases and 2 million deaths per year, and children are most affected (1). Pneumococcus or Streptococcus pneumoniae, a gram-positive bacterium, is the most common cause of community-acquired ALRTI (pneumonia), meningitis, otitis media, and septicemia in children and the elderly (2). Acute respiratory distress syndrome is commonly caused by bacterial ALRTI, for which S. pneumoniae is a common pathogen. Pneumococci commonly cause secondary bacterial infections in the lung after viral infections. Although current conjugate vaccines against pneumococci have inarguably lowered the incidence of pneumococcal diseases, including lung infections, challenges for these vaccines are mounting as reports of diseases caused by nonvaccine serotypes arise (3). Therefore, increased understanding of the protective immune response against pneumococci is paramount to the development of new vaccines and therapeutics. Rodent models such as mice are frequently used in studies of bacterial ALRTI or pneumonia (4, 5). In both mice and humans, S. pneumoniae infection induces lobar pneumonia with neutrophil recruitment and alveolar edema, which is due to the complex interplay between bacterial and host factors in the lung.
Necroptosis is widely recognized as a major regulatory mode of inflammatory cell death, usually occurring in the absence of caspase-8 signaling. Several pathogen- and host-derived danger signals activate RIPK-1 (receptor-interacting serine and threonine protein-kinases-1) and RIPK-3, which results in the activation and phosphorylation of MLKL (mixed lineage kinase domain-like pseudokinase) followed by cell membrane disruption (6). Necroptosis has been reported in numerous disease conditions, such as ischemia-reperfusion–induced sterile injury, neurodegenerative diseases, cancer, and viral and bacterial infections (7–9). Bacterial pore-forming toxins initiate necroptosis of lung immune and resident cells, including macrophages and epithelial cells, to dampen host defense during pneumonia. Furthermore, inhibition of the necroptosis pathway has been shown to result in beneficial outcomes (10–12). Necroptosis can actually be beneficial as well because it facilitates bacterial clearance by limiting excessive proinflammatory signals (13). Nonetheless, mechanistic insight into how the activation of the necroptotic pathway benefits host defense against certain bacterial infections is still needed.
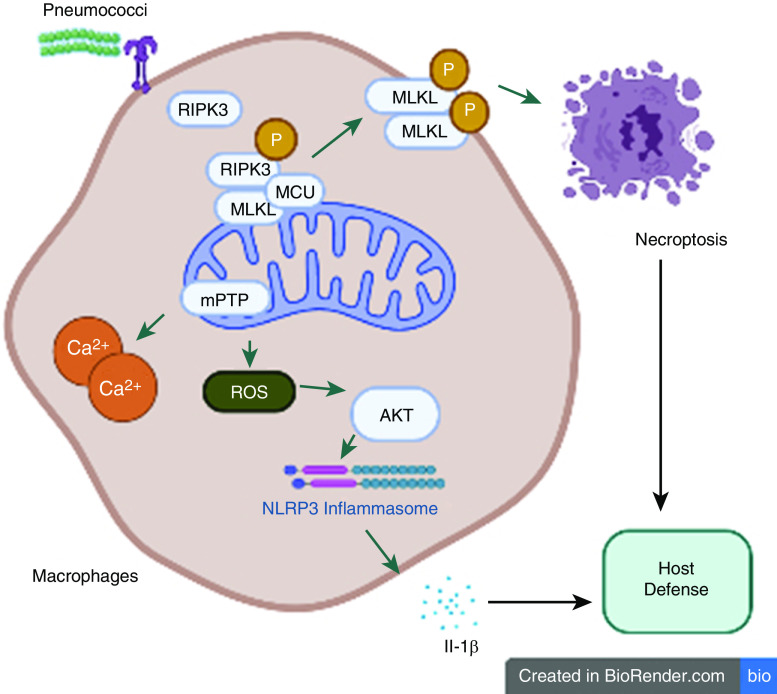
In this issue of the Journal, Huang and colleagues (pp. 579–591) outline a mechanism by which RIPK-3 modulates mitochondrial reactive oxygen species (mROS) production, thereby initiating necroptosis and NLR family pyrin domain containing 3 (NLRP3) inflammasome signaling, which is essential for host defense against pneumococcal lung infection (14). Although previous studies have established a clear role for RIPK-3 in NLRP3 inflammasome activation and regulation of mitochondrial function (15, 16), Huang and colleagues expand on these findings by investigating the role of RIPK-3 in balancing necroptosis and inflammasome activation to benefit the host in the context of streptococcal pneumonia in a mouse model. Moreover, the authors document strong evidence of elevated concentrations of RIPK-3 protein in the plasma of patients with Streptococcal ALRTI and RIPK-3/MLKL-mediated necroptosis in lungs of S. pneumoniae-infected wild-type mice. In additional experiments, Ripk-3−/− or Mlkl−/− mice exhibited increased bacterial burden, excessive inflammation, tissue damage, and death following streptococcal pneumonia. Mechanistically, using wild-type and Ripk-3−/− macrophages, the authors illustrate that RIPK3 interacts with the mitochondrial calcium uniporter together with RIPK-1/MLKL to modulate calcium uptake and mROS production during streptococcal infection. In addition, this S. pneumoniae–induced heightened mROS production led to the opening of the mitochondrial permeability transition pore, thereby triggering necroptosis. On other hand, the authors demonstrate that elevated levels of mROS lead to the activation of the AKT pathway, which is known to regulate NLRP3 inflammasome activation (17). Together, these findings suggest that RIPK-3 initiates necroptosis via mROS-mediated mitochondrial permeability transition pore opening and NLRP3 inflammasome activation via mROS–AKT signaling to provide defense against S. pneumoniae–induced lung infection.
However, identification of this previously unknown mechanism of RIPK-3–mediated activation of necroptosis and NLRP3 inflammasome signaling as an essential host defense mechanism should be included in consideration of the therapeutic targeting of RIPK-3. First, infection with another strain of pneumococci, such as virulent serotype 2 strain D39, results in different phenotypes as Ripk-3−/− or Mlkl−/− mice showed increased survival. This strain-dependent disease outcome in preclinical models makes it extremely challenging to target RIPK-3 for treatment of pneumococcal diseases caused by multiple serotypes or strains. Second, RIPK-3 can promote cell death and NLRP3 inflammasome activation without the involvement of MLKL (18), suggesting the presence of a necroptosis-independent pathway for NLRP3 activation in response to S. pneumoniae. Third, it is reported that necroptosis can be downstream of activation of certain NLRs, such as NLRC4, which regulates the necroptosis pathway in another gram-positive (Staphylococcus aureus) infection (10). Fourth, it is likely that necroptosis results in different outcomes for site-specific pneumococcal infections/diseases. For example, necroptosis has a very divergent role in determining disease outcome at two different sites of S. aureus infection (12, 13). In a murine model of S. aureus pneumonia, Ripk-3−/− mice had significant survival of alveolar macrophages resulting in improved bacterial clearance and survival (12). However, Mlkl−/− mice exhibited an increased bacterial burden and lethality in murine models of skin and/or sepsis associated with S. aureus infection (13). Fifth, it is important to understand the relative contribution of necroptotic cell death in immune cells versus resident cells. It is reported that S. pneumoniae–derived pneumolysin targets multiple cells and can induce various forms of cell death. It remains to be determined how immune cells choose necroptosis, pyroptosis, or survival in response to different pathological and physiological dangers and then execute the various cell death machineries.
In conclusion, Huang and colleagues have expanded our understanding of the mechanism of S. pneumoniae–initiated RIPK-3–dependent necroptosis leading to NLRP3 activation as an essential host defense mechanism (Figure 1). These new findings add to the evolving knowledge of immunoregulatory effects of necroptosis in bacterial infections. Furthermore, targeting RIPK-3 for therapeutic purposes may help to overcome disease burdens, including those caused by pneumococcal infections. Although necroptosis inhibitors have been developed and shown to be effective against severe inflammation, only a few have moved to clinical testing because of their extensive off-target toxicity. As the necroptosis research area is still relatively young, more studies are warranted to further explore the molecular mechanisms through which necroptosis regulates disease phenotypes.
Figure 1.
RIPK-3 (receptor-interacting serine/threonine protein-kinase-3) regulates necroptosis and NLRP3 activation in the lung. During Streptococcus pneumoniae infection, RIPK-3 initiates mitochondrial ROS production to regulate MLKL-mediated necroptosis. In addition to necroptosis, RIPK-3 activates NLRP3 inflammasome via mitochondrial ROS–AKT pathway to induce an immune response against S. pneumoniae. MCU = mitochondrial calcium uniporter; MLKL = mixed lineage kinase domain-like; mPTP = mitochondrial permeability transition pore; NLRP3 = NLR family pyrin domain containing 3; ROS = reactive oxygen species.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2021-0085ED on March 2, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H, Group WHOCHER WHO Child Health Epidemiology Reference Group. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yildirim I, Shea KM, Pelton SI. Pneumococcal disease in the era of pneumococcal conjugate vaccine. Infect Dis Clin North Am. 2015;29:679–697. doi: 10.1016/j.idc.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L387–L398. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 5.Paudel S, Baral P, Ghimire L, Bergeron S, Jin L, DeCorte JA, et al. CXCL1 regulates neutrophil homeostasis in pneumonia-derived sepsis caused by Streptococcus pneumoniae serotype 3. Blood. 2019;133:1335–1345. doi: 10.1182/blood-2018-10-878082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Zamel R, Bai XH, Lu C, Keshavjee S, Keshavjee S, et al. Ischemia-reperfusion induces death receptor-independent necroptosis via calpain-STAT3 activation in a lung transplant setting. Am J Physiol Lung Cell Mol Physiol. 2018;315:L595–L608. doi: 10.1152/ajplung.00069.2018. [DOI] [PubMed] [Google Scholar]
- 8.Orzalli MH, Kagan JC. Apoptosis and necroptosis as host defense strategies to prevent viral infection. Trends Cell Biol. 2017;27:800–809. doi: 10.1016/j.tcb.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Paudel S, Ghimire L, Jin L, Baral P, Cai S, Jeyaseelan S. NLRC4 suppresses IL-17A-mediated neutrophil-dependent host defense through upregulation of IL-18 and induction of necroptosis during Gram-positive pneumonia. Mucosal Immunol. 2019;12:247–257. doi: 10.1038/s41385-018-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G, et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 2015;11:e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitur K, Wachtel S, Brown A, Wickersham M, Paulino F, Peñaloza HF, et al. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 2016;16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HR, Cho SJ, Harris RM, Yang J, Bermejo S, Sharma L, et al. RIPK3 activates MLKL-mediated necroptosis and inflammasome signaling during Streptococcus infection. Am J Respir Cell Mol Biol. 2021;64:579–591. doi: 10.1165/rcmb.2020-0312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 2018;20:186–197. doi: 10.1038/s41556-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 16.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Moon JS, Lee S, Park MA, Siempos II, Haslip M, Lee PJ, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125:665–680. doi: 10.1172/JCI78253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.