Abstract
Over the last several decades, our understanding of regulated-cell-death (RCD) pathways has increased dramatically. In addition to apoptosis and accidental cell death (primary necrosis), a diverse spectrum of RCD pathways has been delineated. In the lung, airway macrophages are critical for maintaining the functionality of airways via the clearance of inhaled particles, cell debris, and infectious agents. Exposure of these cells to pathogenic organisms or particles can induce a variety of RCD pathways that promote the release of danger signals into the lung. These responses have evolved to trigger the innate and adaptive arms of the immune system and thus offer protection against pathogens; yet they can also contribute to the development of lung injury and pathogenic immune responses. In this review, we discuss recent studies that suggest a critical role for airway-macrophage RCD pathways in promoting the release of pulmonary danger signals in health and disease.
Keywords: macrophages, regulated cell death, danger signals, innate immunity, adaptive immunity
Clinical Relevance
Over the last several decades, a spectrum of novel regulated-cell-death pathways has been characterized. This review summarizes current knowledge on regulated cell death in airway macrophages that have shown they play important roles in resistance to infection but also contribute to the pathogenesis of acute lung injury and the development of immune-mediated disease. The work serves to inform researchers of areas where further research is needed and to inform the development of novel therapies to target pathogenic cell-death pathways in the lung.
The balance among cellular survival, proliferation, and death is tightly controlled in multicellular organisms to maintain healthy tissue physiology. In the lung, regulation of cell death is especially critical for repair and renewal of the airway epithelial cells (AECs) and capillary endothelial cells required for gas exchange. Apoptosis was the first regulated-cell-death (RCD) pathway to be identified, but in the last few decades, over 10 other distinct RCD modalities have been characterized (Table 1) (1). The induction of RCD in different cell types has profound impacts on lung health and disease in a variety of contexts (2), and defining the cells and molecular pathways involved may allow the development of novel therapeutic strategies.
Table 1.
RCD Modalities and Their Characterization in AMs, Other Macrophages, or in Other Lung Cells
| Death Modality | Unique Molecular Requirements | Other Lung Cells |
|---|---|---|
| Lung-resident macrophages (AMs)* | ||
| Apoptosis | Apoptotic caspases (caspases 3, 7–10) | AECs, fibroblast endothelial cells |
| Lysosome-dependent cell death | LMP, cathepsins, and other lysosomal enzymes | AECs, fibroblasts |
| Pyroptosis | Inflammatory caspases (caspases 1, 4, 5, 11) and GSDMD | AECs |
| Necroptosis | RIP1K, RIP3K activity, and MLKL | AECs |
| Secondary necrosis | Lack of efferocytosis, caspase 3 and GSDME | AECs |
| Other macrophages (e.g., BMDMs, cell lines)† | ||
| Ferroptosis | Free iron, ROS, membrane lipid peroxidation | AECs |
| Parthanatos | PARP1, AIF- and MIF-dependent DNA degradation | AECs |
| MPT-driven necrosis | Cyclophilin D induced inner membrane permeability | AECs |
| Other cell types‡ | ||
| Anoikis | Loss of integrin-dependent anchorage | Myofibroblasts |
| Lung cancer cells and cell lines | ||
| Autophagy-dependent cell death | Autophagic machinery | Lung cancer cells and cell lines |
| Autosis | Plasma membrane Na+/K+ ATPase | Lung cancer cells and cell lines |
| Entotic cell death | Actomyosin-dependent cell-in-cell internalization | Lung cancer cells and cell lines |
| Mitotic death | Induced in tumor cells after mitotic catastrophe | Lung cancer cells and cell lines |
Definition of abbreviations: AEC = airway epithelial cell; AIF = apoptosis inducing factor; AM = airway macrophage; BMDM = bone marrow–derived macrophage; GSDMD = gasdermin D; GSDME = gasdermin E; LMP = lysosomal membrane permeability; MIF = macrophage migration inhibitory factor; MLKL = mixed-lineage kinase domain–like pseudokinase; MPT = mitochondrial permeability transition; PARP = polyADP-ribose polymerase; RIP1K = receptor-interacting serine/threonine-protein kinase 1; RIP3K = receptor-interacting serine/threonine-protein kinase 3; RCD = regulated cell death; ROS = reactive oxygen species.
RCD modalities that have been well characterized in lung-resident macrophages, primarily in AMs.
RCD modalities that have been well characterized in other myeloid cells/macrophages, including BMDMs, macrophage cell lines, and recruited macrophages.
RCD modalities that have been well characterized only in other lung cell types (tumor cells, somatic cells), listed under “Other Lung Cells” column.
RCD pathways in macrophages are tightly associated with their sentinel and innate immune functions (3). Apoptosis, pyroptosis, necroptosis, and lysosome-dependent cell-death pathways have been well characterized in pulmonary macrophages, particularly in those present in the lumen of the airways (airway macrophages [AMs]). In some cases, these RCD pathways promote clearance of intracellular pathogens and release danger signals that alert the immune system to respond to the invasion (4). AMs clear apoptotic cells (including apoptotic AECs and AMs) via efferocytosis (5). Thus, AM cell death can directly result in the release of danger signals but can also indirectly cause the accumulation of danger signals from other dying cells. In this article, we will discuss recent insights in lung-macrophage biology, what is currently known regarding the regulation of cell death in AMs, and the downstream effects of these pathways on the innate and adaptive arms of the immune system in health and disease.
Macrophage Heterogeneity and Plasticity in the Lung
In healthy lung tissue (steady state), there is a heterogeneous population of tissue-resident macrophages. Although most AMs reside in the surfactant layer that covers the luminal-side alveolar epithelium (alveolar macrophages), a small population of phenotypically indistinguishable bronchial macrophages resides in the lumen of the conducting airways (6). In contrast, interstitial macrophages (IMs) reside in the connective tissue that surrounds the bronchial airways (7). Although IMs and pulmonary dendritic cells (DCs) arise from infiltrating monocytes, AMs are embryonically derived and self-renew (8). During inflammation, monocytes are recruited into the interstitial space and airways, and some monocytes differentiate into macrophages. These cells, referred to as “recruited macrophages,” initially have a phenotype different from that of the resident macrophages upon their arrival to the lung; however, tissue factors can promote their differentiation into macrophages with similar characteristics as resident macrophages.
The vast majority of published studies related to pulmonary macrophage RCD have been performed in mouse or human AMs. Whether or not IMs undergo RCD during respiratory infection, in response to pathogenic substances or in different disease states has not been well studied. Therefore, this review will primarily focus on describing what is known about the role of RCD pathways in resident AMs. Although the RCD pathways discussed here have been studied in both mouse and human primary AMs in vitro, the downstream effects of these pathways on the immune response and in various disease models have been primarily characterized in mice.
Under steady-state conditions, AMs are critical for catabolism of airway surfactants and clearance of debris and are kept in a relatively quiescent state by healthy AECs (3). AMs can be activated to perform a spectrum of different functions depending on their ontology and receptor signaling in their microenvironment (9). RCD pathways are induced in steady-state or activated AMs in response to cellular or mechanical stress, exposure to damaging particles, toxic molecules, epithelial injury, and/or infectious agents (4, 10).
RCD Modalities Induced in AMs
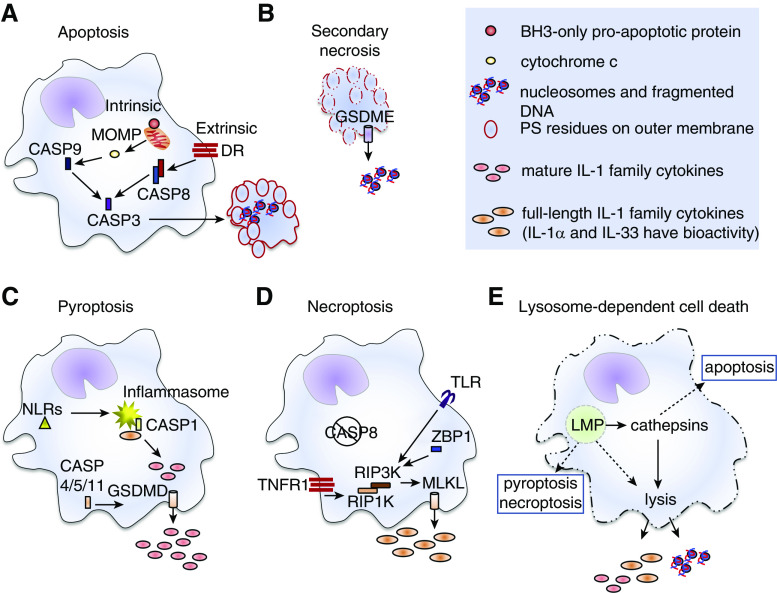
RCD modalities that have been well characterized in AMs include apoptosis, secondary necrosis, pyroptosis, necroptosis, and lysosome-dependent cell death (Figure 1) (1). Apoptosis (Figure 1A) is mediated by apoptotic caspases and in healthy lungs, efficient efferocytosis of apoptotic bodies by other AMs restricts the release of their cellular contents to lysosomes, where they are degraded (11). In the absence of sufficient scavengers, apoptotic AMs undergo secondary necrosis and release danger signals into the airways (Figure 1B). Pyroptosis is induced by the activation of proinflammatory caspases, which cleave and activate GSDMD (gasdermin D), leading to their assembly into pore-like structures in the plasma membrane. AMs that have been primed or “classically activated” by innate receptors express the full-length forms of IL-1 family cytokines in their cytosol. In these AMs, activation of cytosolic NLR-family (NOD-like receptor family) proteins by pathogens or cellular stress promotes assembly of the inflammasome and activation of caspase 1, which cleaves the full-length cytokines into the mature, more biologically active forms. GSDMD pores then allow the release of these cytokines from the cells, promote disruption of the integrity of the plasma membrane, and promote pyroptotic cell death (1). Apoptosis may be impaired by pathogens that express caspase inhibitors (2). In this context, necroptosis is an alternate RCD pathway and is driven by RIP3K (receptor-interacting serine/threonine-protein kinase 3)–mediated phosphorylation of MLKL (mixed-lineage kinase domain–like pseudokinase). Similar to GSDMD and GSDME (gasdermin E) proteins, phosphorylated MLKL assembles into pore-like structures in the plasma membrane and promotes cell lysis (Figure 1D). Lysosome-dependent cell death is induced in response to particles or pathogens that induce lysosome membrane permeabilization (LMP) and is driven by the activity of lysosomal cathepsin enzymes that have leaked into the cytosol (Figure 1E) (1).
Figure 1.
Pulmonary macrophage regulated-cell-death modalities. (A) Intrinsic apoptosis is induced by intracellular or extracellular alterations to a cell’s microenvironment that lead to activation of BH3 (Bcl2 homology domain 3)–only proapoptotic proteins and mitochondrial outer-membrane permeabilization (MOMP). Release of cytochrome c drives activation of CASP9 (caspase 9). Extrinsic apoptosis is induced by receptors on the plasma membranes of cells, including death receptors (DR) in the TNF superfamily. Engagement of these receptors promotes activation of CASP8. Active CASP8 and/or CASP9 activate the executioner CASP3 enzyme that initiates the apoptotic program, leading to DNA fragmentation, flipping of phosphatidylserine (PS) residues to the outer leaflet of the plasma membrane (red outline), breakdown of the nuclear membrane, and packaging of cellular contents into apoptotic bodies. Apoptotic cells and bodies are either cleared by other phagocytes via efferocytosis and die within lysosomes (not shown) or (B) undergo secondary necrosis in which the plasma membranes fall apart (dashed red outline) and damage-associated molecular patterns (DAMPs) are released. Secondary necrosis has been associated with nucleosome release, and may involve CASP3-mediated activation of GSDME (gasdermin E), which forms pore-like structures in the plasma membrane (cylinder with arrow). (C) Pyroptosis results after activation of NLRs (Nod-like receptors, yellow triangle) that promote assembly of the inflammasome (yellow starburst) and activation of CASP1. Other inflammatory caspases (CASP4, CASP5 and/or CASP11) can be activated by intracellular pathogens independently of the inflammasome. In macrophages that express full-length precursor forms of certain members of the IL-1 family, active CASP1 cleaves them into the mature cytokines. This enhances their biological activity and is essential for IL-1β and IL-18 bioactivity. The active forms of proinflammatory caspase enzymes also promote activation of GSDMD (gasdermin D), which forms pore-like structures (cylinder with arrow) in the plasma membrane. Mature IL-1 family cytokines are released through these pores and plasma membrane integrity (solid cell outlines) is weakened. (D) Necroptosis can be induced as an alternative cell-death pathway when apoptotic CASP8 activity is impaired (negation symbol). It can be induced by TLR (Toll-like receptor), ZBP1 (Z conformation DNA [Z-DNA]-binding protein 1), or TNFR1 (TNF receptor 1) signaling events that activate RIP3K (receptor-interacting serine/threonine-protein kinase 3), an enzyme that cleaves mixed-lineage kinase domain–like pseudokinase (MLKL). Cleaved MLKL proteins self-oligomerize and form pore-like structures in the plasma membrane (cylinder with arrow) and thus promote release of small DAMPs, like IL-1 family members, and promote influx of ions into the cells leading to disruption of the plasma membrane. (E) Lysosome-dependent cell death occurs after lysosome membrane permeabilization (LMP). A defining feature of lysosome-dependent cell death is the release of cathepsin enzymes into the cytosol that promote (solid arrows) catastrophic rupture of the cells (dashed cell outline). Depending upon the degree of LMP, other overlapping cell death pathways or cathepsin-independent cell lysis (dashed arrows) can be induced. RIP1K = receptor-interacting serine/threonine-protein kinase 1.
Caspase 8 can inhibit necroptosis and there is thus often a rigid distinction between extrinsic apoptosis and necroptosis (2). In contrast, the distinction among other RCD pathways is less strict. For example, RIP3K and MLKL can each independently activate the NLRP3 (NLR family pyrin domain–containing 3) inflammasome, and RIP3K can induce pyroptotic cell death in the absence of MLKL (12). In addition, induction of multimodal cell death is induced in AMs in a variety of contexts. For example, induction of LMP and lysosome-dependent cell death is often associated with parallel initiation of other RCD programs, including apoptosis and pyroptosis and/or necroptosis (1).
Initiation of RCD Pathways in AMs
Resident macrophages in the lung undergo apoptosis during growth and development under steady-state conditions, and recruited macrophages undergo apoptosis during the resolution of inflammation. Analysis of macrophage turnover in animal models suggests that although both IMs and AMs undergo apoptosis, IMs are shorter lived and undergo a higher rate of apoptosis under steady-state conditions (13). AMs constantly phagocytose and degrade surfactant proteins and innocuous inhaled particles. Thus, AMs must use autophagy, an intracellular system of recycling and repair, to salvage nutrients and limit cell death in the face of the stress induced by their phagocytic function (14). In addition, damage to lysosomes in AMs induced by ingestion of pathogens or toxic particles can trigger lysophagy, a specialized type of autophagy that promotes recycling of damaged lysosomes. Failure of macrophages to complete autophagy in the face of excessive LMP can lead to other forms of RCD as discussed below.
AMs are triggered to undergo various modes of RCD after phagocytosing toxic particles, drugs, pollutants, vaccine adjuvants, or infectious agents (15). A number of different “pathogenic” particles, including silica, uric acid, and asbestos can induce LMP in AMs, and the degree of LMP induction depends on particle size, surface area, and mechanism of entry. Our work has shown that phagocytosis of soluble crystalline metal hydroxide salts by AMs induces LMP that can lead to lysosome-dependent cell death and the release of immunogenic danger signals, whereas noncrystalline forms of the same metal do not (16). Macrophage ontogeny may impact the modality of RCD initiated after phagocytosis of a pathogenic particle. For example, phagocytosis of crystalline aluminum vaccine adjuvants by peritoneal and monocyte-derived macrophages promotes activation of the NLRP3 inflammasome and release of IL-1β from pyroptotic peritoneal and monocyte-derived macrophages but leads to necroptotic cell death in AMs (17, 18).
AM Release of Danger Signals and Their Effects on the Immune System
Danger signals released by AMs include alarmins and damage-associated molecular patterns (DAMPs). Although these two types of danger signals are often referred to interchangeably, we define alarmins here (as others previously have) as danger signals that are actively secreted or released from living cells. In contrast, we define DAMPs as those released passively from dead cells upon loss of plasma-membrane integrity (19). Some examples of alarmins include reactive oxygen species (ROS) and TNFα, both of which are elevated and released by AMs within minutes to hours of their exposure to pathogenic particles, microbes, and/or LMP (19, 20). Some examples of DAMPs released by AMs upon cell death include full-length and mature IL-1–family cytokines, nucleosomes, nucleic acids, histones, HMGB1 (high-mobility group box 1 protein), HSPs (heat shock proteins), uric acid, and ATP (19).
Most danger signals can activate the innate immune system and induce inflammation, but not all are strong activators of immunogenic function in DCs or of adaptive immune responses. Immunogenic danger signals include those that directly activate DCs or adaptive immune cells (Table 2). They promote migration of immunogenic DCs from the lung to the lung-draining lymph nodes where they initiate the expansion and survival of effector and memory T cells (16). IL-1 family cytokines are immunogenic, and different members of the IL-1 family may differently impact DC cytokine production and thus may differentially impact the quality of CD4+ effector-T-cell responses that develop (19).
Table 2.
Recognition and Effects of Immunogenic and Accessory Danger Signals
| Danger Signals | Target(s) | Effects |
|---|---|---|
| Immunogenic danger signals | ||
| Endosomal nuDNA, mtDNA, nucleosomes | TLR9 | ↑ DC costim, IL-12, type I IFNs, migration to LN, Th1 responses, B-cell responses |
| IL-1α (FL or mature), IL-1β (mature) | IL-1R1 and IL-1R3 | ↑ DC costim, migration to LN, T cell responses, B-cell recruitment, iBALT formation |
| IL-18 (mature) | IL-18Rα and IL-18Rβ | ↑ BMDC costim, IL-12, Th1 responses |
| IL-33 (FL or mature) | ST2 and IL-1R3 | ↑ BMDC costim, CCR7 expression, Th2 and Th17 responses |
| IL-36α, β, or γ (mature) | IL-36R and IL-1R3 | ↑ BMDC costim, IL-12, IL-23, Th1 and Th17 responses |
| HMGB1 | RAGE | ↑ DC costim, IL-23, Th17 responses |
| Accessory danger signals | ||
| TNFα | TNFR1 | ↑ Intracellular expression of FL IL-1 family cytokines, apoptosis, nuDNA fragmentation |
| ROS | NLRP3 | ↑ Inflammasome activation, pyroptosis |
| HMGB1 | ↑ Amounts of disulfide form of HMGB1 | |
| PAD2 and/or PAD4 | ↑ ET formation (e.g., neutrophils) | |
| Cytosolic DNA | ZBP1 | ↑ Necroptosis, type I IFNs |
| AIM2 | ↑ Activation of the inflammasome, pyroptosis | |
| HMGB1 | TLR9, TLR2, TLR4 | ↑ Sensitivity of TLRs for TLR ligands |
| Histones | TLR2 | |
| Heat shock proteins | TLR4 | |
| Uric acid | Lysosome membrane | ↑ LMP, lysosome-dependent cell death |
| NLRP3 | ↑ Inflammasome activation, pyroptosis | |
| ATP | P2X7R | ↑ Pyroptosis |
| Histones, ETs | Nonselective | ↑ Necrosis |
| Ca2+ channels |
Definition of abbreviations: ↑ = indicates enhancement; AIM2 = absent in melanoma 2; BMDC = bone marrow–derived dendritic cell; costim = costimulatory molecules; DC = pulmonary dendritic cell; ET = extracellular trap; FL = full-length precursor form; HMGB1 = high-mobility group box 1; iBALT = induced bronchoalveolar lymphoid tissue; IL-1R1 = IL-1 receptor 1; LMP = lysosome membrane permeabilization; LN = lymph node; mtDNA = mitochondrial DNA; NLRP3 = Nod-like receptor family pyrin domain–containing 3; nuDNA = nuclear DNA; P2X7R = P2X purinoceptor 7; PAD = peptidylarginase deiminase enzyme; RAGE = receptor for advanced glycation end products; ROS = reactive oxygen species; ST2 = IL-33 binding subunit (originally named after a cardiac biomarker); Th = T-helper cell; TLR = Toll-like receptor; TNFR1 = TNFα receptor 1; ZBP1 = Z conformation DNA (Z-DNA)-binding protein 1.
Although most danger signals are proinflammatory (activating the innate immune response), there are only some danger signals that enhance adaptive immune responses (immunogenic). Examples of known immunogenic and accessory danger signals are shown together with the receptors that recognize them and the effects they induce.
Immunogenic danger signals are powerful, as they are required to promote immune-mediated protection against infection or toxins but can also trigger hypersensitivity and autoimmune disease in genetically susceptible individuals (21, 22). Accessory danger signals promote inflammation but can also amplify the intracellular availability, biological activity, or recognition of immunogenic DAMPs (19, 20) (Table 2). Others promote more necrotic death and DAMP release, creating a positive feedback loop referred to as “necroinflammation” that can exacerbate and prolong tissue injury and inflammation (23).
Several small-molecule inhibitors for master regulators of different RCD pathways have been characterized, a few of which are being developed for possible future clinical use (24). Currently, only caspase, cathepsin, and RIP1K (receptor-interacting serine/threonine-protein kinase 1) inhibitors have been deemed safe enough to enter clinical studies (Table 3); however, these inhibitors may not be highly effective at blocking RCD, particularly when used separately, because of the existence of alternate and overlapping RCD pathways (24) Specific and potent inhibitors of RIP3K- and MLKL-induced necroptosis have been identified and are likely to be more effective at blocking necroptosis than RIP1K inhibitors but are associated with greater toxicity (24). Several other “antiinflammatory” therapies that are clinically approved for use in various inflammatory and autoimmune diseases can nonspecifically inhibit RCD pathways (Table 3) (25–27).
Table 3.
Clinical Development of Novel RCD Inhibitors and Therapies Currently in Use That Target RCD Pathways
| Drug | Target | RCD Pathways Impacted | Clinical Status |
|---|---|---|---|
| Small-molecule inhibitors | |||
| Z-VAD-FMK*† | Caspases | Apoptosis, pyroptosis | Preclinical |
| IDN‐6556 | Phase I/II (I,R) | ||
| VX-765 | Caspase 1 | Pyroptosis | Phase I/II trials (RA) |
| Necrostatin 1*‡ | RIP1K | Necroptosis (TNFR1) | Preclinical |
| GSK2982772 | Phase I/II trials (RA, P, UC) | ||
| DNL747, DNL788 | Phase I (RA, MS, ALS, AD) | ||
| GSK′872* | RIP3K | Necroptosis (TNFR1, TLR, ZBP1) | Preclinical |
| Pyroptosis (RIP3K-mediated) | |||
| NSA* | MLKL | Necroptosis (TNFR1, TLR, ZBP1) | Preclinical |
| Pyroptosis (RIP3K-mediated) | |||
| RWJ-445380 | Cathepsins | Lysosome-dependent cell death | Phase II (RA) |
| MDL-28170*† | Calpains | Lysosome-dependent cell death | Preclinical |
| Lysomotrophic agents | |||
| Chloroquine (and derivatives) | Lysosome | Lysosome-dependent cell death | Used as an immunosuppressive therapy in autoimmunity (lupus, RA) and as an antimicrobial (e.g., malaria) |
| Azithromycin | Lysosomal membrane | Lysosome-dependent cell death | Used as an antimicrobial and antiinflammatory therapy during respiratory infection |
| Monoclonal antibodies | |||
| Infliximab Adalimumab, Certolizumab, Golimumab | TNFα | TNFR1-dependent apoptosis, pyroptosis, and necroptosis | Used as an antiinflammatory in various inflammatory and autoimmune diseases (e.g., lupus, RA) |
Definition of abbreviations: AD = Alzheimer disease; ALS = amyotrophic lateral sclerosis; IDN-6556 = Emricasan; IFNR = type I IFN receptor; MS = multiple sclerosis; NSA = necrosulfonamide; P = psoriasis; RA = rheumatoid arthritis; RCD = regulated cell death; RIP1K = receptor-interacting serine/threonine-protein kinase 1; RIP3K = receptor-interacting serine/threonine-protein kinase 3; TNFR = TNF receptor; UC = ulcerative colitis; VX-765 = Belnacasan; Z-VAD-FMK = z-Val-Ala-Asp-(OMe)-fluoromethyl ketone.
Pharmacotherapeutic agents in development or in use for the treatment of diseases in which RCD pathways promote disease pathogenesis are shown.
Inhibitors for which clinical development has been discontinued because of high toxicity.
Inhibitors for which clinical development has been discontinued because of undesirable off target side effects are indicated with superscript numbers.
Inhibitors for which clinical development has been discontinued because of low potency.
Alarmins and RCD Pathways Modulate the Spectrum of DAMPs Released from AMs
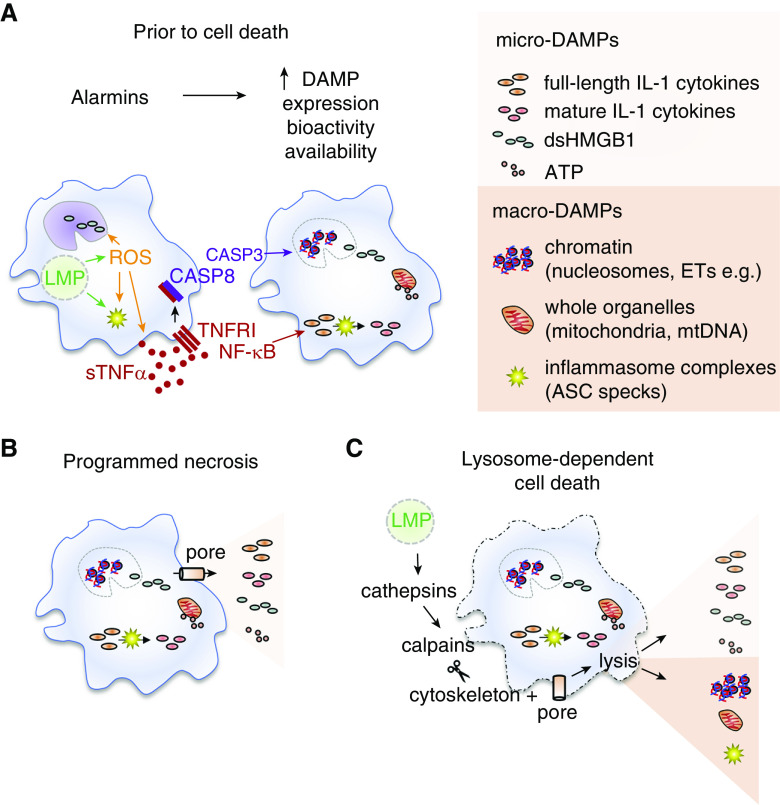
Not all forms of cell death are the same, and the events that occur before cell death modulate DAMP release. Steady-state AMs do not release immunogenic DAMPs if they undergo primary necrosis (20). In contrast, AMs can be preconditioned to release immunogenic DAMPs by alarmins, which elevate the intracellular expression of certain DAMPs, enhance their biological activity, and/or promote structural changes that allow them to be more efficiently released from the cells upon their death. For example, TNFα and ROS can precondition steady-state AMs to release immunogenic DAMPs (Figure 2A). Exposure of AMs to pathogenic particles rapidly induces LMP, followed by elevated production and release of ROS and/or TNFα (4, 20). ROS can further amplify TNFα release and augment activation of the NLRP3 inflammasome in AMs, leading to caspase 1–mediated processing of full-length IL-1β and IL-18 into their biologically active, mature forms (4). ROS can oxidize HMGB1 and thus enhance its biological activity (28). TNFα primes steady-state AMs to release immunogenic DAMPs via TNFR1 (TNF superfamily receptor 1A)–signaling pathways after exposure to pathogenic particles (20). Initially, TNFR1 signaling in AMs boosts intracellular amounts of full-length IL-1 cytokines. In response to LMP, these effects are followed by internalization of the TNFR1 complex, activation of caspase 8, initiation of apoptosis, and fragmentation of chromatin into nucleosomes (Figure 2A). If these events are interrupted by cell lysis (via programmed necrosis [pyroptosis or necroptosis] or lysosome-dependent cell death), both of these DAMPs are efficiently released into the airways (18, 20).
Figure 2.
Alarmins and regulated-cell-death pathways modulate the release of DAMPs from airway macrophages (AMs). (A) Alarmins are a class of danger signals that are actively secreted from cells before they die, and some can precondition AMs to boost intracellular concentrations of biologically active DAMPs available for efficient release. Exposure to pathogenic particles or pathogens can induce LMP, which can induce activation of the inflammasome and oxidative stress (solid green arrows). Reactive oxygen species (ROS) boost inflammasome activation, and the rapid release of TNFα from AMs, and promote formation of dsHMGB1 (immunostimulatory disulfide form of high-mobility group box 1) (solid orange arrows). TNFα binds to membrane-associated TNFR1, leading to the activation of NF-κB (solid red arrows), a transcription factor that induces expression of full-length IL-1 family cytokine precursors, which are retained in the cytosol or cleaved into their mature forms by active CASP1. In stressed cells, TNFR1 is internalized and activates CASP8 and CASP3 (solid black arrow). CASP3 initiates the apoptotic program (solid purple arrow), leading to fragmentation of nuclear chromatin into nucleosomes, disruption of the nuclear membrane, and alterations in mitochondrial function and permeability. (B) Small DAMPs (micro-DAMPs) are easily released by AMs after pore insertion in the plasma membrane induced by secondary necrosis, pyroptosis, or necroptosis (programmed necrosis). In the absence of additional mechanical stressors, AMs are able resist lysis and efficient release of larger DAMPs via this pathway alone (solid cell boundary). (C) LMP can induce parallel induction of programmed necrosis culminating in pore insertion (cylinder with black arrow) and lysosome-dependent cell death that can induce osmotic lysis of AMs and the release of larger, more immunogenic DAMPs (macro-DAMPs). Cathepsins released into the cytosol from leaky lysosomes activate cytosolic calpains (solid black arrows) to break down the cytoskeleton. This further destabilizes the plasma membrane in AMs and allows pore insertion to result in rupture of the plasma membrane (dashed cell boundary) and efficient release of both micro- and macro-DAMPs (solid black arrows). ASC = apoptosis-associated speck-like protein containing CARD; ET = extracellular trap; mtDNA = mitochondrial DNA.
DNA can be also be efficiently released from macrophages in the form of extracellular traps (ETs), unfragmented strands of chromatin that are decorated with cellular proteins. The release of ETs was initially described in neutrophils and also can occur upon cellular death of macrophages and eosinophils (29). ET release from AMs has not been well characterized; although in one study, human AMs released ETs in response to intracellular infection with Hemophilus influenza (30). The ETs released from the infected AMs were different from those released by neutrophils in that they were decorated with metalloproteinase 12 instead of with neutrophil elastase or myeloperoxidase. A clearer understanding of ET formation and release from AMs and IMs is needed to understand the molecular mechanisms involved and the biological significance of this pathway in the lung.
The mode of cell death has profound impacts on the spectrum of DAMPs released upon plasma-membrane permeabilization and/or rupture. Previous dogma supported a model in which apoptosis is always immunologically “silent” and is associated with both limited DAMP release and limited activation of immunogenic DCs. However, in vivo, apoptotic macrophages release “find-me” signals that recruit phagocytic cells, promoting a limited level of inflammation (5). We have found that phagocytosis of crystalline particles in AMs and initiation of apoptosis was associated with release of high amounts of fragmented nuclear DNA upon lysosome-dependent cell lysis (20). Paradoxically, nucleosomal DNA released upon lysis of cells undergoing apoptosis is highly immunogenic (16). This may be in part due to the fact that nucleosomal DNA released from AMs is efficiently taken up into the endosomes of DCs and activates them via TLR9, whereas purified DNA requires liposomal delivery to endosomes and is less immunostimulatory (16).
Programmed necrosis via necroptosis or pyroptosis of AMs results in the release of distinct DAMP profiles and promotes different types of immune responses. Some of these differences may be related to the regulation of the release of specific members of the IL-1 family (19). Our work and that of other groups suggests that AM necroptosis is associated with enhanced release of members of the IL-1 family that do not require processing of the full-length protein for biological activity (IL-1α and IL-33) (18, 31). The release of these cytokines in the absence of other members of the IL-1 family was associated with exacerbated T-helper cell type 2 (Th2) immune responses, reduced regulatory T cell (Treg) responses, and features of allergic asthma. AMs express very low amounts of full-length IL-1α and IL-33 precursors compared with AECs, but the cytosolic concentrations of these DAMPs are amplified by certain alarmins or infectious agents (20, 32). In contrast, apoptotic caspases and LMP-induced activation of autophagy promote the degradation of the aforementioned cytosolic DAMPs (14). Pyroptosis is uniquely associated with activation of the inflammasome and release of mature IL-1β and IL-18 and is associated with development of Th1 and Th17 responses (19).
Programmed necrosis in the form of pyroptosis or necroptosis culminates in the formation of pores in the plasma membrane and the release of small micro-DAMPs from AMs (Figure 2B); however, recent evidence suggests that the cytoskeleton in macrophages makes them resistant to subsequent cell lysis and release of larger, more immunogenic macro-DAMPs (33). In lysosome-dependent cell death, LMP induces parallel activation of pyroptosis, GSDMD pore formation and the activation of calpain enzymes that promote disassembly of the cytoskeleton, rupture of the plasma membrane, and release of both micro- and macro-DAMPs into the extracellular space (Figure 2C) (33).
The Role of AM RCD during Infection with Respiratory Pathogens
During infection, RCD in AMs can be protective or pathogenic, depending on the pathogen and timing of cell death (34). Although certain viruses, bacteria, and protozoans have evolved to evade their antimicrobial functions, AMs are important innate immune cells for intracellular pathogens. In some cases, AMs can serve as cellular niches for intracellular pathogens, allowing them to replicate in a nutrient-rich environment sequestered from the humoral immune system. Execution of AM cell death before pathogen replication can drastically dampen infection, and many successful pathogens express molecules that suppress or delay RCD pathways (35). In contrast, execution of AM cell death after pathogen replication can promote pathogen dissemination, either as a result of pathogen- or RCD-induced cell lysis or as a result of release of pathogen-filled apoptotic bodies that are rapidly taken up by other AMs in the lung (36). Efferocytosis of apoptotic bodies by AMs can limit their ability to function as effective innate immune cells because of the induction of IL-10 and TGFβ (transforming growth factor β) (5).
Pyroptosis in AMs triggers strong protective innate and adaptive immune responses via parallel activation of the inflammasome and release of IL-1β and IL-18. This pathway is inhibited by many pathogens, including certain strains of influenza A (36). In addition, pyroptosis is associated with direct lysis of bacteria via GSDMD and/or release of intracellular bacteria in membrane enclosed structures, also called “pore-induced intracellular traps” (37). Subsequent efferocytosis of pore-induced intracellular traps leads to lysosomal degradation of the encapsulated microorganisms. IL-1β initiates the innate response by inducing rapid expression of chemokines from type II AECs that promote influx of other innate myeloid cells (neutrophils and monocytes) from the blood into the lung (34). Neutrophils and recruited monocytes and macrophages can offer additional innate protection against microbes via phagocytosis, production of antimicrobial substances, release of enzymes that can promote caspase 1-dependent processing of IL-1 family members, and release of ETs to control extracellular bacteria (38). At later stages, recruited innate cells act protectively by clearing extracellular debris, promoting a tissue-healing phenotype in AMs, and by replenishing depleted macrophage and DC populations in the lung (38). Infections with bacteria that express toxins (e.g., Staphylococcus aureus) induce necroptosis in AMs. Inhibiting this pathway improved pathogen clearance and reduced markers of pneumonia (39). Parallel induction of necroptosis and pyroptosis has been observed in AMs infected by other pore-forming bacteria, and synergy of these pathways was associated with a higher incidence of pneumonia (40). These studies suggest that timed targeting of RCD pathways in AMs could limit development of life-threatening outcomes during infection with certain respiratory pathogens.
The Role AM RCD in the Pathogenesis of Lung Disease
RCD of AMs is induced in different models of acute lung injury (ALI) and plays an important role in the development of its most serious form, acute respiratory distress syndrome (ARDS) (41). An increased risk of developing ALI and/or ARDS is associated with certain respiratory infections, toxic chemical inhalation, sepsis, hemorrhagic shock, and trauma (41). ARDS is initiated by cell death, danger signals, and excessive systemic release of inflammatory cytokines (cytokine storms) that drive severe pulmonary inflammation. Further death of inflammatory cells like neutrophils amplifies damage to the pulmonary epithelium and/or endothelium, leading to pulmonary edema, respiratory insufficiency, hypoxemia, and death (42).
In animal models of ALI, systemic treatment with inhibitors of RCD pathways and the use of genetic knockout animals suggest that RIP3K-mediated activation of the inflammasome is associated with ARDS pathogenesis. Although RIP3K is required for the development of ALI, MLKL is not required, suggesting that pyroptosis, rather than necroptosis, plays an important role in ARDS (43). AM pyroptosis aggravates lung inflammation by promoting the release of inflammatory cytokines, including IL-1β and HMGB1. In contrast, RCD in other cell types, such as neutrophils, is important for resolution of acute inflammation, and the death and renewal of infected or damaged AECs may also be important for the lung to return to a healthy state (41). These data suggest that targeted disruption of these RCD pathways in AMs may be a valid approach in ALI and/or ARDS. These RCD pathways have also been implicated in the pathogenesis of chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, and pulmonary arterial hypertension (2). A clearer understanding of the pathogenic role of RCD pathways in specific cell types (e.g., macrophages, neutrophils, epithelial cells, endothelial cells) in the pathogenesis of these diseases is needed.
The Role of AM RCD in the Development of Pathogenic Immune Responses
The release of danger signals after RCD of AMs in response to fine particulates may enhance the development of inappropriate adaptive immune responses to innocuous antigens (immune hypersensitivity or allergy) and/or self-antigens (autoimmunity). Although central tolerance mechanisms result in the depletion or functional inactivation of autoreactive B and T cells, steady-state DCs maintain peripheral tolerance by deleting or inactivating T cells specific for presented self or foreign antigens (44). Addition costimulatory signals provided by “immunogenic” DCs are required for survival of the expanding T cells. Immunogenic danger signals drive the “activation” of immunogenic DCs and license them to break peripheral tolerance (Table 2). Conversely, regulation of these pathways by endogenous nuclease activity, clearance of DAMPs, and regulatory cells and/or cytokines that initiate tissue repair are critical for preventing development of autoimmunity during acute inflammation (21).
Exposures to pathogenic particles, chemicals, or viruses that induce death of AMs have been linked to exacerbated development of hypersensitivity and associated lung disease. Exposures that lead to DAMP release from necroptotic AMs and AECs have been associated with the development and exacerbation of type 2 delayed hypersensitivity and airway reactivity in models of allergic asthma (18, 31). We recently found a role for AM TNFR1 signaling in the development of type 1 delayed hypersensitivity and autoreactivity in a mouse model of chronic beryllium disease (CBD) (20). CBD is a rare but serious occupational lung disease mediated by CD4+ Th1 cells (45). Beryllium ions (Be) alter the topography of self-peptide antigen presentation by HLA-DP2 (human leukocyte antigen DP2*0201) molecules in a manner that alters their structure and results in the presentation of certain self-peptide in a conformation that appears foreign to T cells (Be/self-peptide neoantigens) “neoantigens” to the immune system (Be/self-peptides) (45). Beryllium-sensitized individuals have elevated numbers of Be/self-peptide–specific CD4+ Th1 cells in the lung that orchestrate chronic granulomatous inflammation that can progress to pulmonary fibrosis, respiratory dysfunction, and death. Using a novel preclinical HLA-DP2 transgenic murine model of CBD (46), we showed that lysosome-dependent cell death of AMs in beryllium particle-exposed mice and their release of IL-1α and DNA were essential for beryllium sensitization (Figure 3) (20). We found that TNFα blockade of beryllium-exposed mice did not prevent AM cell death but did prevent their release of IL-1α and DNA. In HLA-DP2 transgenic mice treated with TNFα-neutralizing antibodies, Be/self-peptide neoantigens were presented by tolerogenic DCs and beryllium sensitization was prevented. These data suggest that targeted blockade of TNFR signaling or of particle-induced LMP in AMs may promote tolerance and prevent and/or reverse pathogenic T-cell responses. A small study of patients with CBD treated with infliximab (humanized TNFα-neutralizing antibody therapy) showed that after treatment, Be/self-peptide–reactive T cells exhibited reduced secretion of type 1 cytokines (47), but whether this reflects a short- or long-term tolerogenic effect in this small study is not known.
Figure 3.
AM cell death is a critical early signal that leads to activation of pulmonary dendritic cells (DCs) and breaks peripheral tolerance in chronic beryllium disease, a model of hypersensitivity and autoimmunity. In the lungs, 1) inhalation of beryllium dust (containing beryllium crystalline particles and beryllium ions [Be]) is engulfed by live AMs, 2) induces LMP, and 3) induces rapid secretion of TNFα. 4) TNFα initially promotes TNFR1 signaling at the plasma membrane via complex I (red), which activates NF-κB and induces expression of full-length IL-1α protein in the cytosol of AMs (solid red arrow). 5) At later time points, TNFR1 is internalized (dashed black arrow) and promotes activation of CASP8, apoptosis, and fragmentation of nuclear chromatin into nucleosomes (solid purple arrow). 6) AMs undergo lysosome-dependent cell death (solid black arrow) that lyses the cell (dashed cell outline), interrupts the apoptotic program, and results in the release of nucleosomal DNA (nsDNA) and biologically active full-length IL-1α into the airways. 7) nsDNA is internalized into immature DCs and engages TLR9, whereas IL-1α engages IL-1R1 (IL-1 receptor 1) on the plasma membrane. These damage-associated recognition receptors induce increased expression of costimulatory molecules (CD80) that are required for immunogenic function in DCs and accelerate the migration of DCs from the lung to the lymph nodes, where naive T cells circulate. In the lymph nodes, 8) immunogenic DCs present HLA-DP2 (human leukocyte antigen DP2*0201)/Be/self-peptide complexes as neoantigens and costimulatory signals to T cells. T cells that recognize the neoantigen complex via their TCR receive survival signals proliferate (solid black arrow) and are primed to develop into Be/self-peptide–specific T-helper cell type 1 (Th1) effector cells or effector memory CD4+ T cells (TEM) (beryllium sensitization). 9) Be/self-peptide–specific CD4+ TEM cells reenter the circulation (solid black arrow) and express adhesion molecules and chemokine receptors that allow them to enter inflamed lung tissue. In the lung, they respond to local presentation of Be/self-peptide neoantigen and secrete cytokines (IFNγ and TNFα) that orchestrate granulomatous inflammation (not shown). Conversely, in the absence of TNFα, the dying AMs in mice exposed to beryllium dust do not release sufficient levels of DAMPs to break peripheral tolerance, and thus, exposure to beryllium is tolerated and does not result in beryllium sensitization or development of granulomatous lung disease.
Pulmonary exposures to toxic substances that induce AM RCD have been associated with development of other autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (21). Although immune-mediated joint damage is the defining feature of RA, several studies have suggested that it may be initiated in the lung and that many patients with RA develop interstitial lung disease (48). Circulating autoantibodies targeted against RCD-related proteins can be detected years before joint disease begins and are positively associated with development of RA. Pulmonary exposures to cigarette smoke and respiratory pathogens are also risk factors for development of RA and can enhance RCD in AMs and amplify the release of danger signals in the lung (48). Pulmonary exposure to fine particles that are toxic to AMs and impair efferocytosis are also associated with enhanced disease activity in patients with SLE (49). Elevated macrophage cell death is positively associated with autoantibody formation and tissue damage in SLE (50), and many autoantibodies in SLE are targeted against extracellular nuclear antigens released after lytic RCD (21). Further study is needed to understand the relationship between pulmonary exposures to certain particles or pathogens, AM cell death, and the development and exacerbation of local and systemic immune-mediated diseases.
Conclusions
Many studies suggest that AM RCD plays a central role in regulating pulmonary danger signals, activating innate immune responses, and promoting immunogenic function in pulmonary DCs. The regulation of RCD pathways in AMs can impact outcomes after respiratory infection, the development of ALI and/or ARDS, and the development and/or exacerbation of immune-mediated disease. RCD pathways are involved in the pathogenesis of other lung diseases like chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis and in the development of hypersensitivities and autoimmune disease. Defining the relative role of RCD in AMs versus other cell types (e.g., IMs, AECs, endothelial cells) in the pathogenesis of these diseases is an important unaddressed problem in the field. In addition, a clearer understanding of how systemic, lung-targeted, or macrophage-targeted delivery of RCD inhibitors impacts pulmonary DC function and or disease pathogenesis in animal models of ALI and/or ARDS, hypersensitivity, and autoimmunity is also needed. Although the systemic use of the most effective RCD inhibitors has been associated with a high degree of toxicity in preclinical studies, defining critical cells and molecular pathways and characterizing the downstream effects on innate and adaptive immune responses may promote the development of cell-targeted strategies or other precision-based approaches to more safely and effectively modulate danger signals in the lung.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank members of the Fontenot Laboratory and Peter Henson for continued discussion and intellectual input related to this topic.
Footnotes
Supported by U.S. National Institutes of Health grants HL126736 (A.S.M.) and HL136137, ES025534, HL102245, and HL152756 (A.P.F).
Originally Published in Press as DOI: 10.1165/rcmb.2020-0420TR on December 17, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauler M, Bazan IS, Lee PJ. Cell death in the lung: the apoptosis-necroptosis axis. Annu Rev Physiol. 2019;81:375–402. doi: 10.1146/annurev-physiol-020518-114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 4.Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L. Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019;26:101239. doi: 10.1016/j.redox.2019.101239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henson PM. Cell removal: efferocytosis. Annu Rev Cell Dev Biol. 2017;33:127–144. doi: 10.1146/annurev-cellbio-111315-125315. [DOI] [PubMed] [Google Scholar]
- 6.Puttur F, Gregory LG, Lloyd CM. Airway macrophages as the guardians of tissue repair in the lung. Immunol Cell Biol. 2019;97:246–257. doi: 10.1111/imcb.12235. [DOI] [PubMed] [Google Scholar]
- 7.Patel VI, Metcalf JP. Airway macrophage and dendritic cell subsets in the resting human lung. Crit Rev Immunol. 2018;38:303–331. doi: 10.1615/CritRevImmunol.2018026459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanz AB, Sanchez-Niño MD, Izquierdo MC, Gonzalez-Espinoza L, Ucero AC, Poveda J, et al. Macrophages and recently identified forms of cell death. Int Rev Immunol. 2014;33:9–22. doi: 10.3109/08830185.2013.771183. [DOI] [PubMed] [Google Scholar]
- 11.Boada-Romero E, Martinez J, Heckmann BL, Green DR. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21:398–414. doi: 10.1038/s41580-020-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Núñez G, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol. 2014;192:2821–2829. doi: 10.4049/jimmunol.1302269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulos C, Kravic B, Meyer H. Repair or lysophagy: dealing with damaged lysosomes. J Mol Biol. 2020;432:231–239. doi: 10.1016/j.jmb.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Wong J, Magun BE, Wood LJ. Lung inflammation caused by inhaled toxicants: a review. Int J Chron Obstruct Pulmon Dis. 2016;11:1391–1401. doi: 10.2147/COPD.S106009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade MF, Collins MK, Richards D, Mack DG, Martin AK, Dinarello CA, et al. TLR9 and IL-1R1 promote mobilization of pulmonary dendritic cells during beryllium sensitization. J Immunol. 2018;201:2232–2243. doi: 10.4049/jimmunol.1800303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee AS, Marrack P. Old and new adjuvants. Curr Opin Immunol. 2017;47:44–51. doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda E, Ozasa K, Temizoz B, Ohata K, Koo CX, Kanuma T, et al. Inhaled fine particles induce alveolar macrophage death and interleukin-1α release to promote inducible bronchus-associated lymphoid tissue formation. Immunity. 2016;45:1299–1310. doi: 10.1016/j.immuni.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016;283:2599–2615. doi: 10.1111/febs.13775. [DOI] [PubMed] [Google Scholar]
- 20.Collins MK, Shotland AM, Wade MF, Atif SM, Richards DJ, Torres-Llompart M, et al. A role for TNF-α in alveolar macrophage damage-associated molecular pattern release. JCI Insight. 2020;5:e134356. doi: 10.1172/jci.insight.134356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EH, Wong SW, Martinez J. Programmed necrosis and disease: we interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 2019;26:25–40. doi: 10.1038/s41418-018-0179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee AS, Fontenot AP. Interplay of innate and adaptive immunity in metal-induced hypersensitivity. Curr Opin Immunol. 2016;42:25–30. doi: 10.1016/j.coi.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazawa D, Marschner JA, Platen L, Anders HJ. Extracellular traps in kidney disease. Kidney Int. 2018;94:1087–1098. doi: 10.1016/j.kint.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang C, Chen F. Small-molecule inhibitors of necroptosis: current status and perspectives. J Med Chem. 2020;63:1490–1510. doi: 10.1021/acs.jmedchem.9b01317. [DOI] [PubMed] [Google Scholar]
- 25.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 26.Persson HL, Vainikka LK, Sege M, Wennerström U, Dam-Larsen S, Persson J. Leaky lysosomes in lung transplant macrophages: azithromycin prevents oxidative damage. Respir Res. 2012;13:83. doi: 10.1186/1465-9921-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-α agents: comparison among therapeutic TNF-α antagonists. Cytokine. 2018;101:56–63. doi: 10.1016/j.cyto.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74–82. doi: 10.1111/imr.12601. [DOI] [PubMed] [Google Scholar]
- 29.Doster RS, Rogers LM, Gaddy JA, Aronoff DM. Macrophage extracellular traps: a scoping review. J Innate Immun. 2018;10:3–13. doi: 10.1159/000480373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King PT, Sharma R, O’Sullivan K, Selemidis S, Lim S, Radhakrishna N, et al. Nontypeable Haemophilus influenzae induces sustained lung oxidative stress and protease expression. PLoS One. 2015;10:e0120371. doi: 10.1371/journal.pone.0120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shlomovitz I, Erlich Z, Speir M, Zargarian S, Baram N, Engler M, et al. Necroptosis directly induces the release of full-length biologically active IL-33 in vitro and in an inflammatory disease model. FEBS J. 2019;286:507–522. doi: 10.1111/febs.14738. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani N, Nabe T, Yoshino S. Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice. Immunology. 2013;139:205–218. doi: 10.1111/imm.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis MA, Fairgrieve MR, Den Hartigh A, Yakovenko O, Duvvuri B, Lood C, et al. Calpain drives pyroptotic vimentin cleavage, intermediate filament loss, and cell rupture that mediates immunostimulation. Proc Natl Acad Sci USA. 2019;116:5061–5070. doi: 10.1073/pnas.1818598116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow SH, Deo P, Naderer T. Macrophage cell death in microbial infections. Cell Microbiol. 2016;18:466–474. doi: 10.1111/cmi.12573. [DOI] [PubMed] [Google Scholar]
- 36.Atkin-Smith GK, Duan M, Chen W, Poon IKH. The induction and consequences of Influenza A virus-induced cell death. Cell Death Dis. 2018;9:1002. doi: 10.1038/s41419-018-1035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213:2113–2128. doi: 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizgerd JP. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am J Respir Crit Care Med. 2012;186:824–829. doi: 10.1164/rccm.201206-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Juarbe N, Bradley KM, Riegler AN, Reyes LF, Brissac T, Park SS, et al. Bacterial pore-forming toxins promote the activation of caspases in parallel to necroptosis to enhance alarmin release and inflammation during pneumonia. Sci Rep. 2018;8:5846. doi: 10.1038/s41598-018-24210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan EKY, Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir Res. 2018;19:50. doi: 10.1186/s12931-018-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auriemma CL, Zhuo H, Delucchi K, Deiss T, Liu T, Jauregui A, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46:1222–1231. doi: 10.1007/s00134-020-06010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siempos II, Ma KC, Imamura M, Baron RM, Fredenburgh LE, Huh JW, et al. RIPK3 mediates pathogenesis of experimental ventilator-induced lung injury. JCI Insight. 2018;3:e97102. doi: 10.1172/jci.insight.97102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ardouin L, Luche H, Chelbi R, Carpentier S, Shawket A, Montanana Sanchis F, et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity. 2016;45:305–318. doi: 10.1016/j.immuni.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Fontenot AP, Falta MT, Kappler JW, Dai S, McKee AS. Beryllium-induced hypersensitivity: genetic susceptibility and neoantigen generation. J Immunol. 2016;196:22–27. doi: 10.4049/jimmunol.1502011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack DG, Falta MT, McKee AS, Martin AK, Simonian PL, Crawford F, et al. Regulatory T cells modulate granulomatous inflammation in an HLA-DP2 transgenic murine model of beryllium-induced disease. Proc Natl Acad Sci USA. 2014;111:8553–8558. doi: 10.1073/pnas.1408048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maier LA, Barkes BQ, Mroz M, Rossman MD, Barnard J, Gillespie M, et al. Infliximab therapy modulates an antigen-specific immune response in chronic beryllium disease. Respir Med. 2012;106:1810–1813. doi: 10.1016/j.rmed.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikuls TR, Payne JB, Deane KD, Thiele GM. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: the spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol. 2016;137:28–34. doi: 10.1016/j.jaci.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Bernatsky S, Fournier M, Pineau CA, Clarke AE, Vinet E, Smargiassi A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE) Environ Health Perspect. 2011;119:45–49. doi: 10.1289/ehp.1002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J Immunol. 2006;176:2095–2104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.